Abstract
Apicomplexan parasites employ multiple adhesive ligands for recognition and entry into host cells. The Duffy binding-like (DBL) and the Reticulocyte binding protein-like (RBL) families are central to the invasion of erythrocytes by the malaria parasite. These type-1 transmembrane proteins are composed of large ectodomains and small conserved cytoplasmic tail domains. The cytoplasmic tail domain of the micronemal DBL protein EBA-175 is required for a functional ligand-receptor interaction, but not for correct trafficking and localization. Here we focus on the cytoplasmic tail domain of the rhoptry-localized Plasmodium falciparum RBL PfRh2b. We have identified a conserved sequence of six amino acids, enriched in acidic residues, in the cytoplasmic tail domains of RBL proteins from Plasmodium spp. Genetic analyses reveal that the entire cytoplasmic tail and the conserved motif within the cytoplasmic tail are indispensable for invasion P. falciparum. Site-directed mutagenesis of the conserved moiety reveals that changes in the order of the amino acids of the conserved moiety, but not the charge of the sequence, can be tolerated. Shuffling of the motif has no effect on either invasion phenotype or PfRh2b expression and trafficking. Although the PfRh2b gene can be readily disrupted, our results suggest that modification of the PfRh2b cytoplasmic tail results in strong dominant negative activity, highlighting important differences between the PfRh2b and EBA-175 invasion ligands.
Keywords: Plasmodium, Erythrocyte invasion, PfRh2b, Cytoplasmic Tail, Genetic, Motif
1. Introduction
Invasion of erythrocytes by the malaria parasite is a complex, incompletely understood process mediated by engagement of Plasmodium ligands to host cell receptors. Adhesive proteins are stored within the organelles of the apical complex of Plasmodium spp. merozoites, the invasive form of the parasite, and their sequential release from these organelles are integral to erythrocyte invasion (Cowman and Crabb, 2006). The Plasmodium Reticulocyte binding-like (RBL) proteins represent one such class of type-1 transmembrane parasite ligands that are localized to the rhoptries. There are five Plasmodium falciparum RBL homolog (PfRh) genes: PfRh1, PfRh2a, PfRh2b, PfRh3 and PfRh4 (Rayner et al., 2000, 2001; Triglia et al., 2001a; Kaneko et al., 2002; Taylor et al., 2002; Stubbs et al., 2005). Another class of invasion genes are the Duffy binding-like (DBL) genes found in micronemes, encoded by the PfEBA-175, PfEBA-140, PfEBA-181, PfEBA-165 and PfEBL1 that make up the P. falciparum Erythrocyte Binding Antigen (PfEBA) family (Sim et al., 1990; Peterson and Wellems, 2000; Thompson et al., 2001; Triglia et al., 2001b; Gilberger et al., 2003b; Lobo et al., 2003).
Variations in levels of expression in members of the PfEBA and PfRh families have been documented in both laboratory and field isolates (Taylor et al., 2002; Duraisingh et al., 2003b; Triglia et al., 2005; Nery et al., 2006; Bei et al., 2007). Such variant expression may present a mechanism by which P. falciparum parasites can evade human immune responses (Persson et al., 2008). Additionally, the use of different invasion ligands results in the use of alternative erythrocyte receptors, which may allow the parasite to respond to erythrocyte polymorphisms (Reed et al., 2000a; Duraisingh et al., 2003a, 2003b; Maier et al., 2003; Stubbs et al., 2005; Triglia et al., 2005). Members of the PfRh and PfEBA genes can be readily deleted, resulting in the utilization of alternative receptors for invasion, presumably due to the ability of alternative ligands to compensate for these missing ligands. Regions within the ectodomains of both the EBA and PfRh families have been shown to bind directly to erythrocyte receptors (Sim et al., 1994; Rayner et al., 2001; Thompson et al., 2001; Gilberger et al., 2003b; Gaur et al., 2007). Interestingly, both selection on sialic acid-depleted erythrocytes and deletion of EBA-175 lead to the up-regulation of the PfRh4 gene, suggesting a functional compensation of PfRh4 for EBA-175 (Stubbs et al., 2005). This observation supports a ‘space-filling’ model in which the overall expression of different members of the PfRh and PfEBA protein families is reflected in the proportions of these ligands present at the apical end of the merozoite, the location at which the interaction occurs between the parasite and the erythrocyte surface.
The cytoplasmic domains of adhesive microneme proteins are postulated to play important roles in trafficking to the apical complex, forming interactions with the actinomyosin motor, or allowing signal transduction following engagement of host receptors to initiate downstream events such as discharge of the rhoptry and microneme organelles. For the microneme proteins, Thrombospondin related anonymous protein (TRAP) in Plasmodium berghei sporozoites and EBA-175 in P. falciparum merozoites, it has been shown that while the cytoplasmic domains are required for function, they are not required for trafficking (Gilberger et al., 2003a; Treeck et al., 2006). In contrast, the cytoplasmic domain of the rhoptry body protein ROP-1 was essential for trafficking of this protein in Toxoplasma gondii (Hoppe et al., 2000).
Here we present a genetic analysis of the cytoplasmic domain and the conserved motif of the PfRh2b protein, which has been shown to localize to the rhoptry neck of P. falciparum merozoites. Our analysis of the PfRh2b protein and its homologs reveals the existence of a conserved acidic motif in the cytoplasmic tail domain of all members of the RBL super-family. In contrast to the EBA-175 protein, the PfRh2b cytoplasmic domain appears to be sensitive to genetic modification, suggesting differences in function between the cytoplasmic tails of these two important invasion ligands.
2. Materials and methods
2.1. Parasites and plasmids
Plasmodium falciparum asexual stages were maintained in vitro in human O+ erythrocytes at 4% hematocrit in RPMI 1640 (Sigma)-25 mM HEPES (EMD Biosciences) media supplemented with 0.5% Albumax II (Gibco). Plasmodium falciparum 3D7 genomic DNA was used as a template in the construction of all plasmids. A linker containing AvrII and ClaI sites was cloned between the BglII and XhoI sites of the plasmid pHH1 (Reed et al., 2000b), which contains the human dihydrofolate reductase (hDHFR) gene positive selectable marker. PCR fragments of the PfRh2b cytoplasmic tail domain were cloned between the AvrII and ClaI sites. Primers used to generate the targeting fragments are described in Supplementary Table S1. 3D7 parasites were obtained from the Walter and Eliza Hall Institute (Melbourne, Australia).
Sorbitol-synchronized ring-stage parasites were transfected with 80 μg of purified plasmid DNA. Selection of stable transfectants by single-crossover recombination events by drug cycling was carried out using WR99210, as described previously (Baldi et al., 2002). One cycle consisted of approximately 4 weeks on drug, followed by 4 weeks off drug.
2.2. Southern blotting
Genomic DNA was harvested from synchronous, late-stage parasite cultures using the QIAamp DNA Mini Isolation Kit (QIAGEN). Chromosomal integration was assessed by Southern blot using standard procedures. The southern probe (PrB), which is specific to the PfRh2b 3′ unique region, was amplified from genomic DNA using the following primers: 5′-GGACCCCTAGGCAACAACAAAGAAATATCCAAGAATTAG – 3′ and 5′-GGACCATCGATTTAAAAATATTTTTCTTCATTTTCATCAAAC – 3′.
2.3. SDS-PAGE and Western blot analysis
Supernatants enriched in proteins required for merozoite invasion were obtained from synchronous parasite cultures. Proteins were resolved on 5% SDS-PAGE gels, transferred to a 0.45 μm nitrocellulose membrane (Schleicher and Schuell) and probed with antibodies specific to PfRh2a (1:500) and PfRh2b (1:500) (Triglia et al., 2005). Protein transfer to nitrocellulose membranes was performed according to standard protocols and blots were visualized with a chemiluminescent detection system (Pierce).
2.4. Invasion assays
The invasion efficiencies of cloned mutant lines were assessed using invasion assays, as described previously (Reed et al., 2000a). Invasion assays were performed on sorbitol-synchronized ring-stage parasites plated at a final parasitemia of 0.7% and a hematocrit of 2%. Parasites treated with enzymes to prevent re-invasion (donor cells) were added to an equal volume of differentially enzyme-treated erythrocytes (acceptor cells) in a 96-well plate. Erythrocytes were treated using the following enzymes: neuraminidase (Calbiochem, 66 mU/ml), trypsin (Sigma, 0.66 mg/ml) and chymotrypsin (Worthington Biochemical, 1 mg/ml)/trypsin (0.66 mg/ml). Donor cells were treated with both neuraminidase (66 mU/ml) and chymotrypsin (1 mg/ml) for 1 h at 37°C, which cleaves the majority of known erythrocyte receptors, preventing reinvasion of these cells. Negative control cells were treated in the same way to prevent reinvasion and positive control cells were incubated in the presence of RPMI alone. Plates were incubated at 37°C for 48 h, at which point tritium-labeled hypoxanthine was added at a final concentration of 1 μCi/well. Parasite maturation was permitted for an additional 24 h, after which assays were harvested on glass filter plates and the level of radiolabel incorporation was measured using a scintillation counter. Percent invasion into enzyme-treated erythrocytes was calculated relative to invasion into untreated erythrocytes. Assays were performed three times in triplicate.
2.5. Immunofluorescence microscopy
Smears of mature schizont-stage parasite cultures were made, fixed in ice cold methanol for 2 min at -20°C and allowed to air dry. Samples were stained with anti-PfRh2b (1:100) or anti-RhopH3 (1:1000) antibodies for 1 h at room temperature and incubated with Alexa 488 α-rabbit-IgG (1:750) or Alexa 555 α-mouse-IgG (1:1,000), respectively, for 1 h at room temperature. Nuclei were stained with DAPI. Slides were imaged with the assistance of MetaMorph 7.0 software [Molecular Devices] and a Nikkon Eclipse TE300 microscope.
3. Results
3.1. Bioinformatic analysis of the RBL cytoplasmic tail region
PfRh2b belongs to the large RBL superfamily of proteins that includes the different members of the Plasmodium yoelli 235 and Plasmodium vivax reticulocyte-binding proteins (Preiser et al., 1999; Rayner et al., 2000). Most of the members of the RBL superfamily are type-1 transmembrane proteins, with large ectodomains, followed by a transmembrane domain and a short cytoplasmic tail (Fig. 1). We hypothesized a role for the cytoplasmic domain of PfRh2b in either protein trafficking to the rhoptry neck region, signal transduction or tethering to the parasite cytoskeleton. Amino acid sequence comparison of the cytoplasmic tails of the RBL homologs reveals that the cytoplasmic tails are very acidic, between 29 and 42%. No canonical protein binding or trafficking motifs are identified (Bonifacino and Traub, 2003). In contrast to the rhoptry proteins, such as the rhoptry bulb protein RAP-1, we do not find the canonical YXXΦ motif (Y-tyrosine, X- any amino acid, Φ-hydrophobic amino acid) in any of the expressed PfRh paralogs (Sam-Yellowe et al., 2004).
Fig. 1.
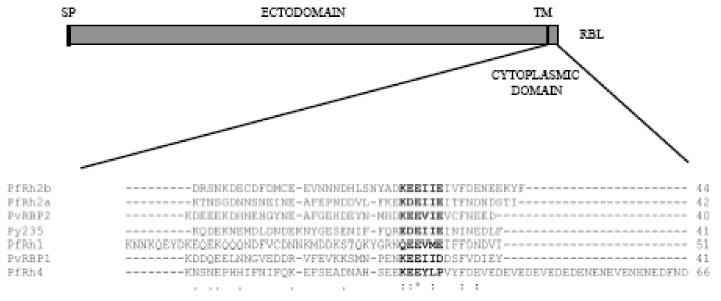
Alignment of the conserved cytoplasmic tail motif found in reticulocyte binding-protein like (RBL) homologs. Schematic of an RBL protein of Plasmodium spp. with a sequence alignment of the cytoplasmic tail region. The asterisk denotes species-wide conserved residues. The motif is found in all RBL homologs with cytoplasmic tails for which sequence is available. A representative subset is shown. ClustalW alignment was performed on sequences obtained from PlasmoDB for the different Plasmodium homologs. SP- signal peptide; TM - transmembrane. PlasmoDB: PfRh2b- PF14_0675; PfRh2a- PF13_0198; PvRB2- Pv090325; Py235- Py01185; PfRh1- PFD0110w: PvRBP1- Pv098585; PfRh4- PFD1150c.
Alignment of the cytoplasmic tail domains of a number of RBL orthologs reveals the presence of a conserved cytoplasmic tail motif consisting of six amino acids (Fig. 1). The motif consists of a charged amino acid (usually lysine) in the first position, a glutamate in the third position and a hydrophobic amino acid (usually isoleucine) amino acid in the fifth position. The lysine residue is followed by two negatively-charged amino acids, in a combination of glutamate and aspartate, generally succeeded by two non-polar amino acids. The composition of the PfRh4 motif differs slightly in that it is contains three non-polar amino acids and only two acidic residues.
The motif may be extended to include two additional amino acids, one hydrophobic and the other charged, three and five amino acids C-terminal from the six amino acid stretch, respectively.
3.2. Strategy used to generate plasmid constructs to assess PfRh2b functional domains
The PfRh2a and PfRh2b genes encode for type-I transmembrane rhoptry proteins that can be readily deleted by either single or double crossover recombination (Duraisingh et al., 2003b). They are identical for approximately three quarters of the proteins, and diverge only in their C-terminal regions that correspond to a portion of the ectodomain, a transmembrane domain and a short cytoplasmic tail (Fig. 2A). We conducted analyses of the unique C-terminal domain of PfRh2b using a series of mutant and deletion constructs that serve to create PfRh2b 3′ allelic replacements. These constructs were transfected into P. falciparum 3D7 parasites, where loss of function of PfRh2b results in a measurable change in invasion pathway. Two classes of constructs were made for i) targeted deletion of the conserved motif and transmembrane domain and ii) mutagenesis of the conserved cytoplasmic domain. The control consists of parasites transfected with a plasmid encoding the wild-type PfRh2b gene for reconstitution (Fig. 2B). PfRh2bΔMotif encodes 27 of the 44 amino acids that comprise the cytoplasmic tail but the conserved cytoplasmic tail motif is deleted. PfRh2bΔTM lacks the transmembrane domain in addition to the cytoplasmic tail (Fig. 2B).
Fig. 2.

Schematic of merozoite invasion protein PfRh2b and plasmids used in the functional analysis of PfRh2b. A) PfRh2b consists of a signal peptide, a large ectodomain, a single transmembrane and a short cytoplasmic tail. PfRh2b diverges from PfRh2a at a complex polymorphic repeat region. The variable repeat region, which differs both in sequence and number for PfRh2a and PfRh2b, lies upstream of the unique region, which consists of (i) a portion of the ectodomain, (ii) a transmembrane domain, and (iii) a short cytoplasmic tail, with a sequence of six amino acids that are conserved across all reticulocyte binding-protein like (RBL) orthologs. B) Plasmids used in functional analysis. The unique region of the wild-type PfRh2b gene is shown on top. Letters below the conserved motif represent the single letter amino acid designations of the residues comprising the conserved region. Underlined residues indicate introduced sequence changes. The plasmid sequence shown at the bottom represents PfRh2b/WT, a control plasmid whose sequence is identical to that of wild-type PfRh2b (TM - transmembrane domain).
In PfRh2b/Alanine and PfRh2b/Shuffle, the six amino acids that comprise the conserved cytoplasmic tail motif have been altered (Fig. 2B). In PfRh2b/Alanine (KEEIIE → KAAIIA) the charged amino acids have been converted to alanine. In PfRh2b/Shuffle (KEEIIE → KIEIEI), the overall charge is maintained but the sequence has been reordered.
3.3. The cytoplasmic tail of PfRh2b is indispensable for erythrocyte invasion
After drug selection, all transfected parasites acquired the plasmid episomally. Single-crossover integrants were selected for using the positive selectable marker WR99210 (Fig. 3A). After two cycles 3D7Rh2b/WT, reconstituting wildtype Rh2b, was integrated into the P. falciparum 3D7 genome. After 2 years of drug cycling (five to eight cycles), neither PfRh2bΔMotif nor PfRh2bΔTM integrated into the genome, but rather, these plasmids persisted as episomes (Fig. 3B). We have previously reported the targeted disruption of PfRh2b in 3D7 by both double crossover and single crossover recombination (Duraisingh et al., 2003b). Our results suggest that an integration event for either of these plasmids which results in either partial and whole deletions of the cytoplasmic tail domain is deleterious to the parasite, and is supportive evidence for a dominant negative phenotype.
Fig. 3.

Targeted integration at the PfRh2b locus. A) The pHH1 plasmid contains the positive selectable marker human dihydrofolate reductase (hDHFR), which is flanked on one side by sequence homologous to the 3D7 PfRh2b unique region for targeted integration by single crossover recombination resulting in modification of the 3′ region encoding the PfRh2b cytoplasmic tail. B) Southern blot assessing integration of PfRh2b deletion constructs into positive transfectants of the Plasmodium falciparum 3D7 genetic background. Of the original transfectants, only two—PfRh2b/WT and PfRh2b/Shuffle (as indicated by arrows)—yielded the diagnostic 3.3 kb fragment. C) Southern blot of PfRh2b/WT and PfRh2b/Shuffle clones obtained by limiting dilution.
3.4. Sequence, but not charge, of conserved motif can be altered in the PfRh2b cytoplasmic tail
The PfRh2b/Alanine plasmid (KEEIIE → KAAIIA) did not integrate after five cycles on and off drug (2 years). This suggests that the charged amino acids in the cytoplasmic tail are indispensable for erythrocyte invasion. In contrast, the PfRh2b/Shuffle (KEEIIE → KIEIEI) successfully integrated into the P. falciparum 3D7 genome after two cycles on and off drug (4 months) (Fig. 3A and B). The bulk population was serially diluted to obtain clonal lines and integration was confirmed by Southern blot (Fig. 3C). These results suggest that shuffling the six amino acids in the conserved motif of the cytoplasmic tail domain is tolerated.
3.5. PfRh2b expression, trafficking and function are independent of sequence of conserved cytoplamsic motif
Polyclonal antibodies specific to the PfRh2b unique region confirmed similar levels of expression of PfRh2b in 3D7Rh2b/WT and 3D7Rh2b/Shuffle (Fig. 4A). To test whether the shuffled sequence in the cytoplasmic tail of PfRh2b has an effect on trafficking, we employed immunofluorescence to determine PfRh2b localization in 3D7Rh2b/Shuffle parasites. PfRh2b localizes to the apical end of the merozoite in all three lines in late-stage schizonts of 3D7, 3D7Rh2b/WT and 3D7Rh2b/Shuffle (Fig. 4B). We then performed invasion assays on 3D7Rh2b/WT and 3D7Rh2b/Shuffle clonal lines and found no significant difference in invasion pathway utilization (Fig. 4C). The function of the PfRh2b protein with shuffled sequence remains unchanged.
Fig. 4.

Shuffled cytoplasmic tail does not impair merozoite invasion protein PfRh2b expression or rhoptry trafficking. A) PfRh2a and PfRh2b expression remains intact in clonal parasite populations transfected with wild-type (PfRh2b/WT) and shuffled mutant (PfRh2b/Shuffle) PfRh2b constructs. The PfRh2a and PfRh2b polyclonal antibodies used are specific to the unique region of the ectodomain (Rayner et al., 2000a). B) Immunofluorescence imaging of schizont-stage Plasmodium falciparum wild-type and transfected parasites reveals an apical localization of PfRh2b in 3D7Rh2b/Shuffle, which encodes a reordering of the cytoplasmic tail motif residues. Parasites were stained with PfRh2b (green) and DAPI (blue). C) Invasion pathway utilization is unchanged in parasites wih the shuffled cytoplasmic tail motif of PfRh2b. Values represent the percent invasion relative to untreated controls. No statistically significant differences emerge between wild-type 3D7 and the PfRh2b transfectants in any of the enzyme treatments. Assays were performed three times in triplicate. Error bars represent 95% confidence intervals.
4. Discussion
Here we report a genetic analysis of the C-terminal region of the PfRh2b protein. It has been shown that PfRh2b is not essential for P. falciparum invasion and growth in vitro through targeted gene disruption in parasites of the 3D7 genetic background (Duraisingh et al., 2003a). Here, we infer an important role for the cytoplasmic domain as we find that we are unable to obtain tail-less PfRh2b. This is most likely due to a dominant negative effect of the attempted tail mutations. This is not without precedent, as deletion of the cytoplasmic domain in integrins results in dominant negative phenotypes (eg. Spinardi et al., 1995). In our model, mutant PfRh2b is translated and trafficked to the merozoite apical complex. There, the ectodomain of PfRh2b engages its cognate receptor, but no signal is transduced and events downstream of apical orientation and ligand-receptor interaction are unlikely to occur. Merozoite invasion will be unsuccessful due to the predominance of unproductive ligand-receptor interactions. In contrast, when PfRh2b is knocked out completely, alternative parasite ligands will take the place of the missing ligands, leading to productive interactions via alternative ligand-receptor interactions. As long as PfRh2b mutant protein persists at the apical interface, parasites are unable to reorganize their invasion ligand repertoire to mediate invasion via an alternative pathway (Baum et al., 2005). Importantly, this model requires trafficking of the PfRh2b mutant proteins to the apical end. If this occurs in PfRh2b without the trasnsmembrane cytoplasmic domains, this implies that these regions are not required for trafficking. Unfortunately, P. falciparum parasites are not easily tractable to transient or inducible expression. As an alternative model, misfolding of the mutant proteins in the endoplasmic reticulum may lead to a generalized defect in the secretory system.
It has been suggested that members of the PfRh and PfEBA families are functionally equivalent, as deletion of EBA-175 leads to increased reliance on PfRh4 (Stubbs et al., 2005). However, our results reveal important differences between the functional contributions of the cytoplasmic domains of EBA-175 and PfRh2b. Truncated mutants of EBA-175 which lack both the cytoplasmic tail and transmembrane domain are easily obtained (Duraisingh et al., 2003a; Gilberger et al., 2003a); however, the cytoplasmic tail is found to be required for function but nor for trafficking to the micronemes (Gilberger et al., 2003a). This ability to delete the cytoplasmic tail domain of EBA-175 suggests that tail-less EBA-175 does not produce a dominant negative effect. Alternatively, the EBA-175 tail may function in trafficking, but is redundant because it is trafficked to the micronemes in complexes with other proteins with intact trafficking signals. Recent evidence suggests that this function localizes to the cysteine-rich C-terminal region of the ectodomain (Treeck et al., 2006) and a similar region of PfRh2b may be involved in protein trafficking. Interestingly, deletion of the cytoplasmic domain of the P. berghei sporozoite microneme protein TRAP also has no effect on trafficking but results in a loss of function (Kappe et al., 1999). This protein does possess a YXXΦ motif in its cytoplasmic domain which is required for trafficking (Bhanot et al., 2003).
We have identified a conserved motif in the cytoplasmic tail that is found in nearly all members of the RBL family in different Plasmodia spp. (Fig. 1). This species-wide conservation argues for an important role for this motif in RBL function, either in signal transduction, protein trafficking or tethering to the actinomyosin motor. However, the PfRh2b tails do not possess any of the previously described evolutionarily conserved trafficking motifs. Nevertheless, we found through our genetic analysis that we were able to dramatically shuffle the order of motif, without affecting the charge, with no affect on protein trafficking of PfRh2b or erythrocyte invasion of P. falciparum. However, this motif may play a role in trafficking that is masked due to the presence of other redundant sequences in the PfRh2b cytoplasmic. We were unable to obtain parasites where the charged residues of the motif were substituted by alanines, suggesting that the charge of the tail is important for its function. These residues may be required for binding to downstream proteins relaying signals following ligand-receptor engagement. In the Apicomplexan parasite T. gondii, the cytoplasmic tail of the micronemal MIC2 proteins was found to be essential (Starnes et al., 2006). Interestingly, acidic amino acid stretches were found to be critical for MIC2 function, but were not required for proper trafficking.
In conclusion, our genetic analysis highlights the importance of the PfRh2b cytoplasmic domain region and reveals important differences with the EBA-175 tail. Future studies aimed at identifying the proteins that interact directly with the cytoplasmic domains of the PfRh and PfEBA will further elucidate the essential functions of these domains.
Acknowledgments
This work was supported by NIH grant R01AI057919 (Duraisingh). We thank Jeff Dvorin for critical reading of the manuscript.
Footnotes
Note: Supplementary data associated with this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldi DL, Good R, Duraisingh MT, Crabb BS, Cowman AF. Identification and disruption of the gene encoding the third member of the low-molecular-mass rhoptry complex in Plasmodium falciparum. Infect Immun. 2002;70:5236–5245. doi: 10.1128/IAI.70.9.5236-5245.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J, Maier AG, Good RT, Simpson KM, Cowman AF. Invasion by P. falciparum Merozoites Suggests a Hierarchy of Molecular Interactions. PLoS Pathog. 2005;1:e37. doi: 10.1371/journal.ppat.0010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei AK, Membi CD, Rayner JC, Mubi M, Ngasala B, Sultan AA, Premji Z, Duraisingh MT. Variant merozoite protein expression is associated with erythrocyte invasion phenotypes in Plasmodium falciparum isolates from Tanzania. Mol Biochem Parasitol. 2007;153:66–71. doi: 10.1016/j.molbiopara.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Frevert U, Nussenzweig V, Persson C. Defective sorting of the thrombospondin-related anonymous protein (TRAP) inhibits Plasmodium infectivity. Mol Biochem Parasitol. 2003;126:263–273. doi: 10.1016/s0166-6851(02)00295-5. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Duraisingh MT, Maier AG, Triglia T, Cowman AF. Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2003a;100:4796–4801. doi: 10.1073/pnas.0730883100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisingh MT, Triglia T, Ralph SA, Rayner JC, Barnwell JW, McFadden GI, Cowman AF. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. Embo J. 2003b;22:1047–1057. doi: 10.1093/emboj/cdg096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur D, Singh S, Jiang L, Diouf A, Miller LH. Recombinant Plasmodium falciparum reticulocyte homology protein 4 binds to erythrocytes and blocks invasion. Proc Natl Acad Sci U S A. 2007;104:17789–17794. doi: 10.1073/pnas.0708772104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilberger TW, Thompson JK, Reed MB, Good RT, Cowman AF. The cytoplasmic domain of the Plasmodium falciparum ligand EBA-175 is essential for invasion but not protein trafficking. J Cell Biol. 2003a;162:317–327. doi: 10.1083/jcb.200301046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilberger TW, Thompson JK, Triglia T, Good RT, Duraisingh MT, Cowman AF. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J Biol Chem. 2003b;278:14480–14486. doi: 10.1074/jbc.M211446200. [DOI] [PubMed] [Google Scholar]
- Hoppe HC, Ngo HM, Yang M, Joiner KA. Targeting to rhoptry organelles of Toxoplasma gondii involves evolutionarily conserved mechanisms. Nat Cell Biol. 2000;2:449–456. doi: 10.1038/35017090. [DOI] [PubMed] [Google Scholar]
- Kaneko O, Mu J, Tsuboi T, Su X, Torii M. Gene structure and expression of a Plasmodium falciparum 220-kDa protein homologous to the Plasmodium vivax reticulocyte binding proteins. Mol Biochem Parasitol. 2002;121:275–278. doi: 10.1016/s0166-6851(02)00042-7. [DOI] [PubMed] [Google Scholar]
- Kappe S, Bruderer T, Gantt S, Fujioka H, Nussenzweig V, Menard R. Conservation of a gliding motility and cell invasion machinery in apicomplexan parasites. J Cell Biol. 1999;147:937–943. doi: 10.1083/jcb.147.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo CA, Rodriguez M, Reid M, Lustigman S. Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl) Blood. 2003;101:4628–4631. doi: 10.1182/blood-2002-10-3076. [DOI] [PubMed] [Google Scholar]
- Maier AG, Duraisingh MT, Reeder JC, Patel SS, Kazura JW, Zimmerman PA, Cowman AF. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat Med. 2003;9:87–92. doi: 10.1038/nm807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Deans AM, Mosobo M, Marsh K, Rowe JA, Conway DJ. Expression of Plasmodium falciparum genes involved in erythrocyte invasion varies among isolates cultured directly from patients. Mol Biochem Parasitol. 2006;149:208–215. doi: 10.1016/j.molbiopara.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson KE, McCallum FJ, Reiling L, Lister NA, Stubbs J, Cowman AF, Marsh K, Beeson JG. Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J Clin Invest. 2008;118:342–351. doi: 10.1172/JCI32138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DS, Wellems TE. EBL-1, a putative erythrocyte binding protein of Plasmodium falciparum, maps within a favored linkage group in two genetic crosses. Mol Biochem Parasitol. 2000;105:105–113. doi: 10.1016/s0166-6851(99)00173-5. [DOI] [PubMed] [Google Scholar]
- Preiser PR, Jarra W, Capiod T, Snounou G. A rhoptry-protein-associated mechanism of clonal phenotypic variation in rodent malaria. Nature. 1999;398:618–622. doi: 10.1038/19309. [DOI] [PubMed] [Google Scholar]
- Rayner JC, Galinski MR, Ingravallo P, Barnwell JW. Two Plasmodium falciparum genes express merozoite proteins that are related to Plasmodium vivax and Plasmodium yoelii adhesive proteins involved in host cell selection and invasion. Proc Natl Acad Sci USA. 2000;97:9648–9653. doi: 10.1073/pnas.160469097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner JC, Vargas-Serrato E, Huber CS, Galinski MR, Barnwell JW. A Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1) defines a trypsin-resistant erythrocyte invasion pathway. J Exp Med. 2001;194:1571–1581. doi: 10.1084/jem.194.11.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MB, Caruana SR, Batchelor AH, Thompson JK, Crabb BS, Cowman AF. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid independent pathway of invasion. Proc Natl Acad Sci USA. 2000a;97:7509–7514. doi: 10.1073/pnas.97.13.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000b;403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- Sam-Yellowe TY, Florens L, Wang T, Raine JD, Carucci DJ, Sinden R, Yates JR., 3rd Proteome analysis of rhoptry-enriched fractions isolated from Plasmodium merozoites. J Proteome Res. 2004;3:995–1001. doi: 10.1021/pr049926m. [DOI] [PubMed] [Google Scholar]
- Sim B, Orlandi PA, Haynes JD, Klotz FW, Carter JM, Camus D, Zegans ME, Chulay JD. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol. 1990;111:1877–1884. doi: 10.1083/jcb.111.5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim BKL, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- Spinardi L, Einheber S, Cullen T, Milner TA, Giancotti FG. A recombinant tail-less integrin beta 4 subunit disrupts hemidesmosomes, but does not suppress alpha 6 beta 4-mediated cell adhesion to laminins. J Cell Biol. 1995;129:473–487. doi: 10.1083/jcb.129.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnes GL, Jewett TJ, Carruthers VB, Sibley LD. Two separate, conserved acidic amino acid domains within the Toxoplasma gondii MIC2 cytoplasmic tail are required for parasite survival. J Biol Chem. 2006;281:30745–30754. doi: 10.1074/jbc.M606523200. [DOI] [PubMed] [Google Scholar]
- Stubbs J, Simpson KM, Triglia T, Plouffe D, Tonkin CJ, Duraisingh MT, Maier AG, Winzeler EA, Cowman AF. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science. 2005;309:1384–1387. doi: 10.1126/science.1115257. [DOI] [PubMed] [Google Scholar]
- Taylor HM, Grainger M, Holder AA. Variation in the expression of a Plasmodium falciparum protein family implicated in erythrocyte invasion. Infect Immun. 2002;70:5779–5789. doi: 10.1128/IAI.70.10.5779-5789.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JK, Triglia T, Reed MB, Cowman AF. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol Microbiol. 2001;41:47–58. doi: 10.1046/j.1365-2958.2001.02484.x. [DOI] [PubMed] [Google Scholar]
- Treeck M, Struck NS, Haase S, Langer C, Herrmann S, Healer J, Cowman AF, Gilberger TW. A conserved region in the EBL proteins is implicated in microneme targeting of the malaria parasite Plasmodium falciparum. J Biol Chem. 2006;281:31995–32003. doi: 10.1074/jbc.M606717200. [DOI] [PubMed] [Google Scholar]
- Triglia T, Thompson J, Caruana SR, Delorenzi M, Speed T, Cowman AF. Identification of proteins from Plasmodium falciparum that are homologous to reticulocyte binding proteins in Plasmodium vivax. Infect Immun. 2001a;69:1084–1092. doi: 10.1128/IAI.69.2.1084-1092.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglia T, Thompson JK, Cowman AF. An EBA175 homologue which is transcribed but not translated in erythrocytic stages of Plasmodium falciparum. Mol Biochem Parasitol. 2001b;116:55–63. doi: 10.1016/s0166-6851(01)00303-6. [DOI] [PubMed] [Google Scholar]
- Triglia T, Duraisingh MT, Good RT, Cowman AF. Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum. Mol Microbiol. 2005;55:162–174. doi: 10.1111/j.1365-2958.2004.04388.x. [DOI] [PubMed] [Google Scholar]


