Summary
Adult neurogenesis in the hippocampus leads to the incorporation of thousands of new granule cells into the dentate gyrus every month, but its function remains unclear. Here we present computational evidence that indicates that adult neurogenesis may make three separate but related contributions to memory formation. First, immature neurons introduce a degree of similarity to memories learned at the same time, a process we refer to as pattern integration. Second, the extended maturation and change in excitability of these neurons make this added similarity a time-dependent effect, supporting the possibility that temporal information is included in new hippocampal memories. Finally, our model suggests that the experience-dependent addition of neurons results in a dentate gyrus network well suited for encoding new memories in familiar contexts while treating novel contexts differently. Taken together, these results indicate that new granule cells may affect hippocampal function in several unique and previously unpredicted ways.
Introduction
The dentate gyrus (DG) is one of two brain regions with substantial neurogenesis throughout the lifetime of mammals (Altman and Das, 1965; Eriksson et al., 1998). In rats, thousands of new granule cells (GC) are born into the existing circuitry every day (Cameron and McKay, 2001), though only a fraction of these cells survive to become fully functional neurons (Kempermann et al., 2003). Each newborn neuron undergoes a maturation process lasting several months, developing electrical properties that are highly similar to developmentally born GC and forming synaptic contacts with the same afferent and efferent neurons (Esposito et al., 2005; van Praag et al., 2002; Zhao et al., 2006). While these adult-born neurons ultimately appear identical to those born in utero and post-natally, the maturation process progresses through states that make immature neurons distinct from mature GC. The integration of new neurons into the existing circuitry involves complex mechanisms for synaptogenesis (Toni et al., 2008; Toni et al., 2007) and is accompanied by distinct physiological properties, including lower threshold and higher amplitude long-term potentiation (LTP) (Ge et al., 2007; Schmidt-Hieber et al., 2004) and potentially greater excitability (Esposito et al., 2005). Furthermore, there is a pronounced relationship between behavior and neurogenesis. Physical activity, environmental enrichment, and learning increase proliferation and survival of new neurons (Gould et al., 1999; Kempermann et al., 1997; van Praag et al., 1999) whereas age and stress adversely affect the neurogenesis process (Gould et al., 1991; Kuhn et al., 1996). Anti-depressants have been shown to stimulate proliferation and require neurogenesis for their function (Sahay and Hen, 2007). The regulation of survival appears to be particularly dependent on activity, as new neurons pass through a critical period for survival that requires NMDA activation and that benefits strongly from environmental enrichment (Tashiro et al., 2007; Tashiro et al., 2006).
Despite this increasing understanding of how new neurons integrate into the functional DG network, it is still unclear what the function of this process is. Computational studies have demonstrated how neurogenesis may affect memory formation (Aimone and Wiskott, 2008; Becker, 2005; Chambers et al., 2004; Deisseroth et al., 2004; Wiskott et al., 2006). While the functional implementation of neurogenesis differs greatly between models, ultimately most of these computational results suggest that, without this addition of new neurons, new information might be encoded in a manner that disrupts previous memories. Conversely, numerous behavioral studies (using a range of knockdown techniques) investigating the role of new neurons on several different hippocampal memory tasks have reported mixed results (Leuner et al., 2006). For example, at least three separate studies have demonstrated that rodents with reduced neurogenesis showed impaired performance on the Morris water maze (Dupret et al., 2008; Snyder et al., 2005; Zhang et al., 2008), but no differences in water maze performance were seen in several other studies using different (and in one case the same) knockdown techniques (Saxe et al., 2006; Shors et al., 2002).
The difficulty in observing a strong knockdown phenotype on classic hippocampal memory tasks, combined with the observation that the DG may only be required for certain hippocampus-dependent behaviors (McHugh et al., 2007; Nakashiba et al., 2008), suggests that neurogenesis may not be critical to many of the functions that the hippocampus has classically been assigned. Rather than suggesting that neurogenesis has no cognitive relevance, it is important to consider an alternative: that new neurons provide functions that have not previously been described for the hippocampus. For example, in a recent communication, we described a hypothesis for how immature neurons may alter the DG’s function of reducing similarity between information sent to the hippocampus (i.e., pattern separation) by being more active than fully mature GC. Such increased participation over transient periods could be the source of the temporal associations seen in long-term memory (Aimone et al., 2006).
To address this question about neurogenesis function, we have developed a computational model of the DG system that incorporates many of the aforementioned features of the maturation process. The analysis of the model was principally focused on the pattern separation function of the DG, which has been predicted theoretically (McNaughton and Morris, 1987; O’Reilly and McClelland, 1994; Treves and Rolls, 1992) and examined using behavioral and physiological approaches (Bakker et al., 2008; Jung and McNaughton, 1993; Kesner et al., 2004; Leutgeb et al., 2007; McHugh et al., 2007). Because most theories about DG function pre-dated the wide acceptance of adult neurogenesis, they do not account for the role of continuous GC addition to the network. Therefore, a theoretical basis for how neurogenesis may affect this pattern separation function should be developed. Of particular interest is the question of whether a neurogenic DG provides any functional benefit apart from that proposed for the non-neurogenic DG. Any such insights, in turn, could influence the design of new behavioral and physiological tasks that are necessary for fully understanding the role of new neurons in cognition.
In this paper, we will describe several distinct theoretical results from our study. First, we examined how neurogenesis affects the similarity between DG outputs when tested with a simple pattern separation experiment. Second, we looked at how the dynamics of the neurogenesis process affect pattern separation over time. Third, we used the model to show how the addition of new neurons shapes the way that the DG will encode different contexts in the future as well as in the present. Finally, we investigated how changes in neurogenesis rate that are observed in clinical conditions may affect these functions.
Results
Computational Model of DG Neurogenesis
We modeled adult neurogenesis by designing a complex neural network that included many of the specific details of adult neurogenesis and the DG (described in Supplemental Methods and Supplemental Model Description). While there are many approaches to modeling neural systems, we hoped that this “bottom-up” approach would reveal possible functions that would otherwise go unnoticed in a simpler model. The DG networks we used contained six separate populations (layers) of cells. These included two input regions - the lateral and medial entorhinal cortex (lEC and mEC, respectively) - the neurogenic GC layer and three local interneuron populations: excitatory mossy cells (MC), inhibitory basket cells (BC) and hilar interneurons (HI) (Figure 1A). Before experimentation, newly generated networks were “grown” to full size using a paradigm designed to reflect the developmental growth of the DG. The non-neurogenic cell layers (all but GC) and the connections between them were initialized fully when the network was generated. The GC layer was initialized with a large number of immature neurons, and these, as well as all later newborn neurons, matured and developed connections according to the maturation process (Figure 1B; Supplementary text). Initially the GC layer had twice the number of input EC neurons, 800 GC compared to 400 EC neurons (including both the mEC and lEC layers), but after full growth the GC layer had approximately five times the total number of EC neurons (Figure 1C). This ratio corresponds to the ratio observed in the developed rat DG (200,000 EC neurons to 1 million GC; (Amaral et al., 2007). New neurons were born at a rate of 10 per day -though not all survived (Figure S2A). At the time of testing, the model GC layer grew at roughly 10% per month, similar to what has been estimated in young rats (~6%; (Cameron and McKay, 2001).
Figure 1. Overview of neural network model.
(A) Simplified block diagram of network architecture. (B) Sketch of newborn granule cell (GC) maturation process implemented in model. (C) Growth of the GC layer and cell death. (D) Timeline of model growth initialization and growth. (E) Sample input neuron activity in different environments (Env). Medial entorhinal cortex (mEC) neurons (top) have spatial response, lateral EC (lEC) neurons (bottom) fire at equal rates at all spatial locations. (F) Illustration of how the network is trained and tested. During training (top), model “explores” random paths within an environment. During testing (bottom), network activity is measured in a series of spatial locations that tile the environment
After initialization, the input layers provided highly structured inputs representing different “environments” for the equivalent of 120 days, during which time each network grew by generating new neurons and integrating them in the circuit in an activity-dependent manner (Figure 1D). On each training trial the model’s inputs were determined by a random path through the environment that activated spatial grid cells (mEC) (Solstad et al., 2006) and context-specific neurons (lEC)(Figure 1E–F; Figure S1), which in turn activated the GC population (Figure S2B). At 120 days, the network was duplicated, with one network continuing to grow with neurogenesis (“NG” network) while the other network ceased to have new neurons born (“No NG” network). These two networks were presented with a fourth environment for 40 days before experimentation.
The afferent and efferent connections of new neurons were not formed immediately but rather gradually as the neuron matured within the network according to the rate of connectivity seen biologically (Toni et al., 2008; Zhao et al., 2006) (Figure S2C). New GC competed with existing GC for many of their excitatory inputs (Figure S2D)(Toni et al., 2007), and these synapses were the only plastic synapses in the model, experiencing LTP and long-term depression (LTD) at a rate determined by the age of the synapse (Figure S2E). Due to having only young synapses, immature neurons exhibited the highest levels of LTP in the model, though mature neurons were capable of learning at lower levels. This finding is consistent with several studies of LTP and adult neurogenesis (Ge et al., 2007; Schmidt-Hieber et al., 2004). The connections formed within the network mimicked the general topography of the observed connectivity in vivo (Amaral et al., 2007)(Figure S3). The maturation of the immature neuron physiology, including membrane resistance, resting potential, and firing rates, proceeded according to what has been observed biologically (Esposito et al., 2005; Ge et al., 2006)(Figure S4).
Immature neurons contribute “pattern integration” to DG pattern separation
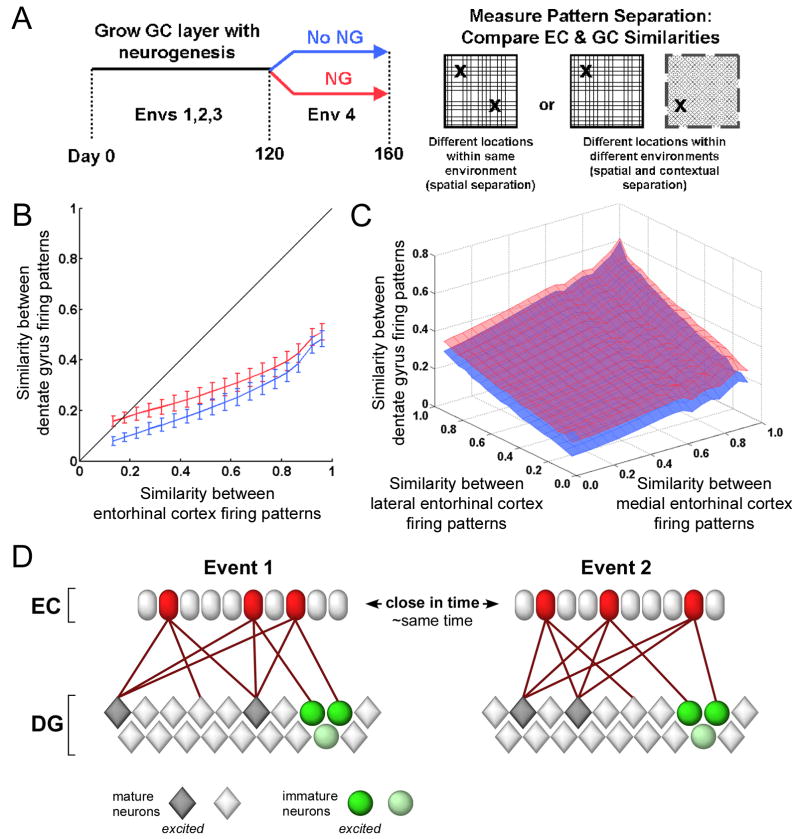
The first behavior we directly examined in the DG networks was pattern separation. Pattern separation is a computational process by which similar information entering a network results in distinct outputs. This process is believed to be critical in the formation of memories in the CA3’s auto-associative network (Treves and Rolls, 1992) and has long been considered a natural function for the DG, due to its high density of sparsely active GC. Following the growth of the NG and No NG networks (Figure 2A), we tested the output of the DG layer using different EC inputs that varied by changing the context (lEC input) and spatial location (mEC input), expecting that the DG’s outputs from these events would be considerably more distinct from one another than the inputs were (O’Reilly and McClelland, 1994). For highly similar EC inputs (similar spatial and contextual information), the two networks performed comparably at pattern separation. However, when the inputs become more dissimilar, the orthogonalization of outputs was inversely related to the degree of neurogenesis, with the No NG network outputting the most separate signals whereas the NG networks actually appeared to blur together outputs for very dissimilar inputs (NG vs. No NG; p<0.01; Figures 2B–C).
Figure 2. Pattern separation by dentate gyrus (DG) model.
(A) Schematic showing pattern separation experiment. Once grown for 160 days, NG and No NG networks were tested at different locations and environments, each providing a different entorhinal cortex (EC) input to the model. (B) Effect of EC similarity (x-axis) on the similarity between DG outputs (y-axis). In networks with neurogenesis (NG, red), very low input similarity results in relatively higher DG similarity, an effect we refer to as pattern integration. Pattern integration does not occur in non-neurogenic networks (No NG, blue). Similarity is measured by the normalized dot product (NDP). The difference between NG and No NG networks was significant (p<0.01). (C) The decrease in pattern separation with neurogenesis occurs with both spatial (medial EC) and contextual (lateral EC) inputs. (D) Cartoon schematic of pattern integration effect. Two events encoded by similar EC populations activate distinct mature DG neurons, yet activate the same immature neurons.
The observation that pattern separation is affected by the presence of new neurons is consistent with our previous theory that the new neurons respond too broadly to be effective separators (Aimone et al., 2006). Furthermore, we had also predicted that the mature neurons in the neurogenic DG would still be separating the EC inputs. When the immature neurons were removed from the analysis, we indeed saw that it was the new neurons that were affecting this response, as fully mature neurons were still sparsely active and effective at separating the cortical inputs (Figure S5A). Therefore, while new neurons appeared to affect the global pattern separation capability of the DG, the mature cells continued to perform as expected. The degree to which the outputs from NG networks were more similar than those from the No NG networks was dependent on the rate of neurogenesis: as expected, the greater the neurogenesis rate, the larger the pattern integration effect (Figure S5B).
Based on these results, we speculate that the pattern separation function of the DG is more complex than previously considered: while mature GC effectively separate information arriving from EC, the immature GC provide associations between events (Figure 2D). This latter role, which we refer to here as pattern integration, is most prominent when events are highly dissimilar and may help to form associations in the CA3 during memory formation. This pattern integration effect is different from the pattern completion function that has been proposed for downstream hippocampal areas. Pattern completion produces the same output from related but different inputs, allowing the reconstruction of a memory from a partial cue, whereas pattern integration, as described here, limits the amount of separation of very distinct inputs.
Dynamics of adult neurogenesis result in “temporal separation” of memories
The observation that immature neurons increase the similarity between DG outputs suggests that young GC are contributing information while the DG pattern separates. One possibility that we discussed in our previous report is that this added association relates to time (Aimone et al., 2006). Namely, the pattern integration effect observed with neurogenesis may represent information about the temporal relationship between two events. Whereas events close in time will encounter the same immature neurons, thus adding similarity to their DG representations, events encoded far apart in time will utilize distinct sets of immature neurons, making their representations more distinct.
To examine whether the time between events presented to the network affects the decrease in pattern separation that is observed for events presented to the same network, we tested the network daily in one environment while continuing to grow it in a separate environment (Figure 3A). Each day, after the growth phase, the network was tested in each of the previous test environments at 400 distinct positions (with plasticity disabled), and the outputs of the network were compared across time. The NG network’s ability to separate two inputs strongly depended on the amount of time that elapsed between their presentations. If the two events occurred within a short time of one another (within 1–2 days), the resulting DG output demonstrated the same added similarity (when compared to the No NG networks) that was observed between events occurring at the same time (Figure 3B). However, for events presented further apart in time, the influence of immature neurons reversed, and the separation of temporally distant events in NG networks was better than that of the No NG networks (interaction of neurogenesis and time; p<0.01). This improved separation was not a result of the network learning, as there was no plasticity in response to the test environments, but rather was a result of temporally separated events being encoded with distinct populations of immature neurons.
Figure 3. Effect of time between events on pattern separation and pattern integration.
(A) Schematic showing the pattern separation experiment extended over time. The model continued to grow with maturation, neurogenesis and cell death between testing sessions, at which time the response of the model was measured at different environments and spatial locations. (B) Effect of time between events on pattern separation of inputs that are 80% (top), 50% (middle), and 10% (bottom) similar. Note how DG similarities between events separated in time are lower than those tested on the same day. Both the decrease in similarity over time and the interaction between time and NG/No NG groups were significant for each of the input similarity groups (p<0.01). (C) Cartoon schematic of temporal separation. Two similar events, when separated by time, will activate distinct mature DG neurons, but also a different population of immature neurons, increasing the separation of the two events.
Interestingly, in addition to the time that elapsed between events, the degree that neurogenesis affected separation of these events was also a function of how similar the inputs were: inputs that were already well separated were more affected by the immature neuron population and appeared to retain a neurogenesis-dependent similarity for a longer time (Figure 3B; Figure S6). In contrast, immature neurons were not as effective at contributing similarity to events that were initially very similar, as these inputs were strongly separated by mature cells in the network. Although the pattern integration effect was strongest during the encoding of events with already separate EC representations, neurogenesis improved the separation of two events most profoundly at high levels of input similarity when the events were separated by several days.
The temporal dynamics observed in the pattern integration effect emerge from the continuously changing immature GC population, with attrition of older immature neurons through maturation and cell death and replenishment by the birth of new neurons (Figure 3C). This dependence on time suggests that the pattern integration effect does not simply reduce pattern separation, but rather fundamentally changes the DG’s separation function. Because of the changing immature neuron population, the DG not only separates events based on their contextual and spatial similarities, but also by their temporal relationship. This temporal separation is accomplished even though the “when” part of memory may not be explicitly part of the inputs.
New neuron maturation allows specialization in encoding familiar environments
While our modeling results concerning pattern separation show that young GC have unique properties that may affect DG function, the long-term survival of these neurons suggests that adult-born GC continue to affect hippocampal function after they pass through this immature phase. The influence of environment on the survival of adult-born GC indicates that the ultimate function of these neurons is determined by their experience. To investigate this long-term function of neurogenesis, we examined the response of the network to the four environments that it was exposed to during the development of the network (familiar environments: “FEs”) as well as a novel environment (“NE”; Figure 4).
Figure 4. Response of DG network to familiar and novel environments.
Environments were examined in each of the environments used during network growth. Within each environment, firing rates in response to 400 spatial locations were determined. (A) A sample response of a NG network’s granule cell (GC) population upon presentation to 400 spatial locations within each familiar environment (FE) and one novel environment (NE) on Day 160 (gray:>2Hz; green:>4Hz; blue:>6Hz; firing 2Hz or below not shown). Neurons are sorted on the x-axis by age - oldest on the left, youngest on the right. Note how neurons of similar ages respond to the same environments. (B) Response of the same NG network to the same environments on Day 200. Note the increase of the preferring group to Env 4 and the development of a preferring group to Env 5. The young population of GC that respond to all inputs are labeled with an asterisk (‘*’) in (A) and (B). (C–D) The response of a sample No NG network to the four FEs and one NE on Day 160 (C) and Day 200 (D). Note the failure of the No NG to develop a population of neurons that preferred Env 5. (E) Cartoon schematic of DG specialization. Adult-born neurons are involved in the encoding of events during their maturation. Those same adult-born neurons, once mature, are utilized when that event is remembered or experienced again.
An inspection of one network’s response to testing at different locations in each of the four different FEs and an NE after training in all four FEs revealed that each environment activated different groups of GC neurons (Figure 4A). Exposure to the first three FEs resulted in the activation of large, separate fractions of the GC population, whereas the most recently experienced FE, environment 4, had a smaller, yet still grouped, response. Testing within the NE (environment 5), however, activated only a disperse set of neurons, with few GC showing a preference for the NE. As suggested by the results showing the temporal dependence of pattern integration, the population of immature neurons changed as the network passed through time. We allowed the network to grow and mature within the NE, and as a result, a population of GC that preferred the NE emerged (Figure 4B). In addition, the response to the fourth FE (environment 4) was stronger, even though the network did not experience that environment again.
When considered by date of neuron birth, it is apparent that the GC that responded the most to an FE were those neurons that matured within that environment (Figure S7A–B). Neurons did not begin to acquire specificity to an environment until they were about 3 weeks old; when the environment changed, the existing population of immature neurons was the first to specialize, followed by the neurons being born. This population of immature neurons that has yet to specialize (labeled with an ‘*’) responded to all environments, and it was this non-discriminating response that led to the pattern integration function observed only in the NG networks described earlier.
We performed a similar analysis on networks where neurogenesis was halted after the third FE. While the No NG networks had specialized neurons that responded preferentially to the four FEs because new neurons continued to enter the network until day 120, the No NG networks did not have a group of neurons that responded preferentially to the NE on day 160 (Figure 4C), and they failed to develop one even after extended exposure within that environment on day 200 (Figure 4D). In addition, the No NG networks lacked the population of immature neurons observed in the NG networks (Figure S7C–D), explaining the lack of pattern integration by No NG networks (Figure 2).
The development of these dedicated populations suggests that the continual growth of the DG is not simply the random addition of new dimensions, but rather a process by which young GC form dimensions specialized to environmental features experienced during maturation (Figure 4E). Starting with the large population of GC maturing at birth, the DG appears to be growing into a structure designed to process information in the context of what the network has experienced in the past. In such a network, new events will be encoded using the dimensions defined by previous events. Importantly, because there may be aspects of new events that are fundamentally novel (thus cannot be accounted for by existing GC), neurogenesis allows the DG to adapt by adding new dimensions.
Aging and stress affect adult neurogenesis function
One of the most pronounced features of adult neurogenesis is that it is heavily regulated by experience. We measured the role of neurogenesis modulation by approximating two conditions that decrease neurogenesis levels: aging, which results in a chronic decrease in the number of new neurons (Kuhn et al., 1996), and stress, which can induce a rapid decrease in neurogenesis rates (Gould et al., 1991). Both aging and stress are complex physiological states that affect many neural systems, and their interactions with other modulators of neurogenesis are likely complex. In this study, we have used simple decreases in neurogenesis rates to investigate what the general effects of changing neurogenesis rates are on memory formation.
We simulated aging by gradually decreasing the rates at which new neurons are introduced to the model over time (Figure 5A). Although the neurogenesis rates slowed, the network continued to grow in size throughout the experiment. We repeated the three studies described above at different points in the network’s growth. Pattern integration is significantly lower in networks with decreasing neurogenesis than networks with a constant neurogenesis rate (p<0.01; Figure 5B), though pattern separation is not as strong in aged networks with constant neurogenesis (Figure S8). In contrast to young networks, the time between events did not affect pattern integration in networks with decreasing neurogenesis, suggesting that temporal associations in aged networks will be impaired (Figure 5C). Temporal associations remained in networks that aged with full neurogenesis. Re-exposure of the aging networks to their FEs revealed that the groups of specialized GC are smaller in the FEs experienced later (Figure 5D). This finding is in contrast to networks without decreasing neurogenesis rates, where the size of the specialized GC populations does not decrease substantially with aging (Figure S9).
Figure 5. Effect of neurogenesis modulation on function.
(A) Time-course of neurogenesis in aging study. After day 120, networks were grown for 400 days with either decreasing neurogenesis (red) or constant neurogenesis (blue). (B) Pattern integration in aging networks, measured by ability of network to separate already dissimilar inputs (input similarity of 10%; p<0.01). (C) Temporal dynamics of pattern integration (input similarity of 10%) in young (solid line) and old (dashed line) networks. Pattern integration depends on time in young networks, but time between events has limited effect on old networks. (D) Response of aged network to familiar environments (FEs) after full growth (gray:>2Hz; green:>4Hz; blue:>6Hz; firing 2Hz or below not shown). (E) Time-course of neurogenesis in stress study. After day 120, networks had 60 days of decreased neurogenesis followed by full recovery (red), or no change in neurogenesis (blue). (F) Pattern integration in stressed networks, measured by ability of network to separate already dissimilar inputs (input similarity of 10%; p<0.01). (G) Temporal dynamics of pattern integration (input similarity of 10%) before (solid line), during (dashed line), and after (dotted line) stressful experience. (H) Response of stressed network to familiar environments (FEs) after full growth.
While aging is a chronic condition that results in a gradual decrease of proliferation, stress is one of several conditions that can result in a sharply decreased level of neurogenesis (Gould et al., 1991; Mirescu and Gould, 2006). We modeled stress by immediately decreasing the neurogenesis rate to 75% of its baseline amount, followed by a subsequent recovery 60 days later (Figure 5E). While the rate changes were acute, the effects on DG function were gradual. The depletion of immature neurons shifted the DG pattern integration response (measured at 10% EC similarity) to pattern separation, and the response shifted back to pattern integration following recovery of neurogenesis (Figure 5F). Importantly, both transitions took between 10 and 15 days to reach their steady state due to time required for new neuron maturation. Although the pattern integration was diminished in the stress condition, the dependence on time remained, albeit at a lower level (Figure 5G). Similar to the aging results, re-exposure of the stress networks to their FEs revealed that the FEs experienced at the time of low neurogenesis have a diminished representation (Figure 5H), though environments after recovery are represented well. This finding suggests that a transient lack of neurogenesis may affect the way memories are later encoded within environments associated with a stressful period, but that this effect can be reversed for future memories in subsequent environments.
Discussion
Adult-born neurons have multiple functions
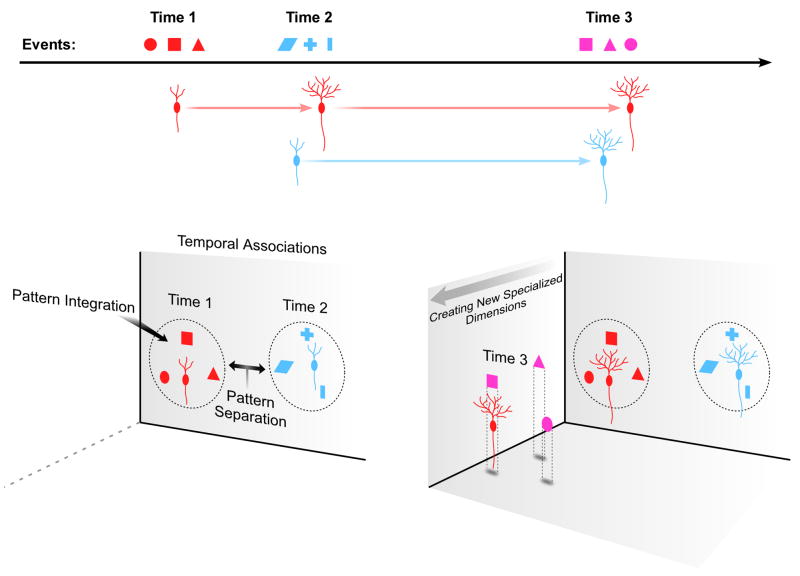
Our study suggests three possible functions for adult-born GC: 1) new GC provide a level of similarity to events that would otherwise be separated, a process we refer to as pattern integration; 2) this integration effect is temporally dependent, possibly leading to associations between contemporaneous events while increasing the separation of events further apart in time; and 3) the neurons involved in this integration effect mature into unique dimensions that may be used to improve the encoding of future memories. These functions are not independent; rather, they each emerge from the experience-dependent maturation process that new neurons undergo (Figure 6). During maturation, new GC transition from progenitor cells to fully functional neurons. For the first few weeks of this process, the electrical properties of immature neurons are quite different from those of mature GC (Esposito et al., 2005). We observed that the population of immature neurons with increased excitability might actually decrease the separation function performed by the DG. Furthermore, because the maturation of immature GC is continuous, this pattern integration effect is dependent on the amount of time between two events, providing the mechanism for encoding the temporal relationship between events that we had proposed before (Aimone et al., 2006). Our data suggest that memories formed within a few days will utilize the same immature GC, allowing for associations between memories that occur at the same time.
Figure 6. Schematic summarizing possible functions for adult-born neurons.
(A) Distinct events (different shapes) occurring at different times (labeled Time 1, Time 2, and Time 3). The events are colored by the time that they are presented. The different events that are experienced will tune the maturing neurons to eventually fire specifically to those events. (B) While immature, the new neurons associate events that occur around the same time (pattern integration). Events encoded at distinct times (Time 1 and Time 2) activate different neurons (temporal separation). (C) The young neurons that matured at time 1 (colored red) will later specify new dimensions specifically tuned to the same events they experienced when young. If the events that occurred during their maturation are re-experienced, the red neurons will be utilized to increase the dimensionality of the memory that is formed.
Our earlier hypothesis did not address the role of the neurons that survive beyond this pattern integration stage. While a significant fraction of immature neurons dies before they are fully mature, a non-trivial proportion of them remains alive indefinitely (Kempermann et al., 2003; Kempermann et al., 1997; Tashiro et al., 2006). Our results show that the activity-dependent maturation of these surviving neurons results in the generation of specialized groups of GC that may improve the encoding of that environment in the future, consistent with biological studies using immediate-early genes that showed that neurons responded preferentially to events that occurred during their maturation (Kee et al., 2007; Tashiro et al., 2007). These populations of neurons represent new dimensions that the DG can use to encode new memories – dimensions that are “custom-built” for the information contained in those memories. Indeed, the same neurons that perform pattern integration between events when they are young ultimately comprise the new dimensions to better encode those events when they are older. While pattern integration is adding similarity to the encoding of current events, the new neurons are gaining specificity that will lead them to improve the encoding of future events.
Relationship of current hypotheses to previous theories of hippocampal function and neurogenesis function
The idea that DG sparse coding leads to pattern separation has been developed over recent decades (Kesner et al., 2004; Leutgeb et al., 2007; McNaughton and Morris, 1987; O’Reilly and McClelland, 1994; Treves and Rolls, 1992). Our results support a pattern separation function of mature neurons in the DG, although the presence of neurogenesis in our model suggests that pattern separation is not as straightforward as previously considered. Instead, we propose that the separation effect of the DG is dependent on both the structure of inputs and when the inputs are presented. If two stimuli are very similar, the separation provided by the mature neurons outweighs the integration effect of immature neurons, but when inputs are already encoded separately, the pattern integration effect contributed by immature neurons is more evident. Pattern integration essentially acts as a lower bound to the pattern separation process for temporally proximal events, but for events occurring at different times, pattern separation dominates. Furthermore, while the hippocampus has long been considered critical for the encoding of temporal information, these studies have focused mostly on recurrent network dynamics in the CA3 and spike-timing dependent plasticity (Dan and Poo, 2004; Dragoi and Buzsaki, 2006). Both these effects operate at time scales considerably shorter (seconds and milliseconds) than the temporal associations proposed here (hours and days). These different temporal dynamics would not be redundant but rather complement one another in the addition of temporal context to new memories.
Several hippocampal studies have suggested that the DG’s pattern separation function is only required during initial memory formation, with memory retrieval bypassing the DG via the direct EC-CA3 projection (Kesner et al., 2004; Treves and Rolls, 1992). Limiting the DG’s involvement in this way would also confine the effects of neurogenesis to the encoding-phase of memory formation. This conclusion suggests that any temporal information contributed by immature neurons would be stored and ultimately recalled elsewhere in downstream hippocampal areas (CA subfields) or cortex. Accordingly, we have focused our study on the role of neurogenesis in the encoding of memories, though future work may reveal that the presence of neurogenesis affects the retrieval of memories.
Our approach to modeling adult neurogenesis differs considerably from that of previous models of adult neurogenesis (for review, see Aimone and Wiskott, 2008). These distinctions likely underlie the differences between our results and previous theoretical results. The model presented here has at least three major features that distinguish it from previous modeling studies: (1) the inclusion of details about the maturation process; (2) simulation over long time scales, allowing successive generations of new neurons to populate the DG; and (3) assaying DG function by measuring pattern separation while using biologically derived inputs. The extent of biological detail included in this model is in contrast to previous computational studies of neurogenesis that investigated the effect of either neuron addition or turnover on specific network functions in less complicated models. While those models have revealed several possible functions for the addition of new neurons in simple network architectures, we believe that the inclusion of biological details was important for our observation of several network behaviors heretofore not described.
In some cases, our results were similar to those of other models. For example, our results showing that FEs attain dimensional independence due to the maturation process are similar to the hypotheses put forth by two previous computational studies that suggested that new neurons protect old memories by increasing the capacity available for encoding new memories (Becker, 2005; Wiskott et al., 2006). However, these studies suggest that the acute effects of stopping neurogenesis would be substantial, potentially leading to the collapse of previously encoded memories, whereas our model predicts that the cessation of neurogenesis would result in a more subtle deficit: new environments would continue to be encoded using a combination of previous environments, but their transition to being familiar would be impaired.
Limitations of our computational approach
While the complexity of this model was important for the generation of novel, behaviorally testable predictions, both the accuracy and completeness of the model are issues that remain to be addressed by both biological studies and future modeling work. Adult neurogenesis is a dynamic area of research and, as is the case with all computational models, future results may make it necessary to revisit certain assumptions made in the model. This caveat does not negate the validity of the results proposed here, but it underscores the importance of future biological investigation of these hypotheses, as described in the next section.
Our modeling and theoretical work has focused principally on the DG, and it is possible that neurogenesis has unknown implications on other hippocampal regions. For example, the relationship between GC and CA3 neurons is complex, as it appears that CA3 pyramidal neurons and interneurons respond differentially to bursting of GC (Henze et al., 2002; Lawrence and McBain, 2003). If new neurons do not fire in the same manner as mature cells, it is possible that the CA3 will not respond as predicted. Until the mossy fiber projection is fully investigated in vivo, the precise effect new neurons have on CA3 is not entirely clear, though recent work by our lab shows that they make functional connections (Toni et al., 2008).
In addition to mechanistic details, it is not yet clear how changing the pattern separation function in the DG will affect information processing in the rest of the hippocampus. While generally considered an associative network, the CA3 has been shown to also contribute to pattern separation, though this is believed to be fundamentally different from the separation function of the DG (Leutgeb et al., 2007). Neurogenesis would appear to be one source of this difference, as we are proposing that DG is separating inputs according to time as well as specific features of the events. In addition, further modeling work may reveal how neurogenesis affects the network dynamics of the DG. A more sophisticated understanding of the network dynamics associated with pattern separation in the DG network may clarify how this separation function affects the attractor dynamics in the CA3.
In addition to more complex analyses of the network dynamics, continued examination of the model’s behavior considering other perspectives on hippocampal function will be revealing, particularly with regard to how neurogenesis affects the hippocampal representation of space and neurogenesis’ relationship to depression. The DG is believed to be important in the formation of hippocampal place representations, and GC have distinct spatial behaviors, though how they affect hippocampal spatial processing is still unclear (Leutgeb et al., 2007). Similarly, the role of DG in affective conditions, such as depression, is unknown, though a strong relationship between neurogenesis and certain anti-depressant drugs suggests that adult-born neurons play a role in affective state (Sahay and Hen, 2007). The functional role of neurogenesis in encoding space and affect is unknown, and further work is required to relate the results of the model to these hippocampal functions.
Finally, as with other computational models, our study is limited by details of the system that have not yet been fully described. For instance, although the spatial properties of mEC neurons have been well characterized (Hafting et al., 2005), the structure of the lEC input to the DG remains unclear (Hargreaves et al., 2005). For instance, GC in our model have a spatial structure that is obviously influenced by the grid structure of the mEC neurons (Figure S1C). While in vivo studies have shown spatial structure to GC responses, it has not been reported as significantly grid-like (Jung and McNaughton, 1993; Leutgeb et al., 2007). This difference in model behavior emerges from the grid cells being the only input population with a spatial structure. Furthermore, more examination is required to determine how immature neurons influence in vivo measurements of DG neurons during behaviors and exploration.
Comparison of hypotheses to biological studies and future biological predictions
Because multiple assumptions were required in this model to arrive at the hypotheses presented here, testing the predictions of the model with biological studies is essential. While there have been many behavioral tests of neurogenesis knockdowns, the interpretation of these results has been difficult and the relevance to these specific hypotheses is unclear. Ultimately, since we are proposing that neurogenesis contributes to memory formation in manners not widely considered elsewhere, new behavioral tasks must be designed to directly test these new hypotheses.
Of the previous behavioral results using knockdown models of neurogenesis, perhaps the most relevant to our model is the observation that irradiated animals have improved performance on a working memory (Saxe et al., 2007). One prediction of the pattern integration hypothesis is that reducing neurogenesis might result in an increase of pattern separation during memory encoding. As a result, behaviors that benefit from greater separation may show an improvement after the elimination of new neurons. One interpretation of the working memory results is that normal mice have difficulty distinguishing between the current trial and recent trials, whereas irradiated mice have a better ability to segregate their current actions from those of the past. While pattern integration may make pattern separation more difficult, it may be necessary for other behaviors that require the animal to integrate information across several learning trials. Explicit testing of these hypotheses will require the design of new behavioral tasks. While the design of new tasks is a considerable undertaking, we can anticipate the types of tasks that may be effective for studying each of these ideas. One possibility for testing the model is to simultaneously examine both pattern integration and temporal associations. The hypotheses suggest that events occurring close in time will be associated with one another, whereas events occurring several days apart will be encoded separately. An example behavioral paradigm using fear conditioning would be to present multiple contexts to an animal over time with one context coupled to an aversive stimulus (i.e., shock). The model would predict that animals would fear both the context where the shock occurred and those contexts that were proximal in time. One drawback to this specific example is that context fear conditioning is affected in neurogenesis knockdowns in certain conditions, so care must be taken to ensure the underlying fear memory is present.
The final hypothesis - that adult-born neurons mature to encode new dimensions - can also be examined behaviorally. One implication of developing specialized groups of GC may be an increased ability to acquire new memories that can utilize those new dimensions. Animals that live extensively within an enriched environment have an increased survival of new neurons that may specialize to features of that environment (Kempermann et al., 1997; Tashiro et al., 2007). Given the DG’s presumed role in memory encoding, we would predict that that these animals may have a greater ability to learn within that environment than animals for which the environment is novel. One possible behavioral task would be to pre-expose an animal to several contexts over several weeks, which should induce populations of specialized GC. Later, the animal would be trained to fear one of these contexts, but not the others. We would anticipate that neurogenesis would improve the discrimination of the feared context from the other pre-exposed environments.
Relationship to human memory
While these behavioral studies may be effective at testing simple predictions that emerge from the model, the more complex aspects of the effect of neurogenesis on memory may prove too difficult to test in animal models. Examination of types of memory in humans predicted to be affected by neurogenesis may help reveal the role of new neurons in memory. Aging and stress are two conditions prevalent in the human population that have been correlated with low neurogenesis rates in rodent models. Our results indicate that the chronic and acute decreases in neurogenesis due to aging and stress, respectively, may affect memory formation significantly.
The discovery of functional imaging measures that correlate with human neurogenesis (Pereira et al., 2007) may permit the examination of the effect of neurogenesis on performance on psychological tasks that investigate the structure of human memories (Bakker et al., 2008; Schacter and Slotnick, 2004; Shohamy and Wagner, 2008). Although most hippocampal network theories assume the DG’s role is limited to pattern separation, some more general ideas for the structure of human memories suggest a use for the added similarity that pattern integration provides. One example is the “constructive memory hypothesis,” which postulates that memories are composed of distinct elements that are stored separately and reconstructed at the time of retrieval, as opposed to a pure reproduction of a past event (Schacter and Addis, 2007). If memories are indeed stored in a distributed form, there is probably a requirement for some additional information that binds the distributed pieces together. While the pattern completion circuitry in the hippocampus would be effective at forming and recapitulating associations between items that occur at the same time or in sequence (Rolls and Kesner, 2006), complex memories might require a different mechanism to bind distributed components together. Although the classical view of the DG is that it would separate context from this information, immature neurons may limit the amount of separation performed at the time of encoding. Memories encoded by the network would still be adequately separated to the extent that effective attractors could be formed, but the attractor states of these memories would remain related to one another. Additionally, such associations would only be meaningful if the added similarity was temporally constrained, as there would be little benefit if all memories were linked to one another.
We find that the acute drop in neurogenesis due to stress greatly eliminates the pattern integration provided during memory encoding. In the aforementioned constructive memory framework, we would anticipate that this lack of pattern integration may result in a decreased ability to combine distinct memory components into uniform memories and may be revealed by an improved performance on tasks designed to confuse information with contextual clues. The effect of aging on pattern integration is less dramatic in our model; however, the increased similarity occurring in older networks is not temporally dependent, suggesting that, while the ability to bind memories together remains with aging, this process loses its temporal precision.
In addition to a role for pattern integration, the possibility that novel environments are encoded using a combination of neurons previously used to encode familiar environments also fits nicely into the constructive memory framework. Consistent with the idea that memories are encoded in a distributed manner, we observed that the DG’s representation of an FE included not only those neurons that matured within that environment but also neurons that showed a preference for other, previously experienced, environments (Figure 4). One possibility is that those neurons that are used in multiple environments encode features that are invariant between the two contexts. Furthermore, in our study, NEs were initially encoded entirely by using “familiar” dimensions. Without having developed a set of neurons customized to the current inputs, it appears that the network approximated the entire context by utilizing other neurons that matured in previous environments. Such a process is similar to recent proposals about the process of imagination: that thinking about the future consists of constructing a new combination of old memories into a new package (Schacter and Addis, 2007). Our results suggest that recently experienced environments will not transition to being familiar after aging, as there are few new neurons to commit to those contexts. A failure of environments to transition to familiar may affect how memories are formed in aged or chronically stressed individuals; even environments that should be familiar may be considered novel if there is little neurogenesis available when previously experienced.
Conclusion
In conclusion, the results of our study suggest that neurogenesis may be acting on several different aspects of memory formation. The computational effects of immature neurons integrating into the network in this model were consistent with the hypothesis we outlined earlier regarding the inclusion of temporal context in new memories. In addition, we propose a new hypothesis that fully mature, adult-born neurons are important for the system’s response to new environments to progress from novel to familiar. These hypotheses emerge from the features of the neurogenesis process as the anatomy and function is currently understood. While these hypotheses will be modified as more is learned about this system, they provide a new direction for future behavioral studies in both animal and human models seeking the function of adult neurogenesis.
Supplementary Material
Acknowledgments
We thank W. Deng, L. Rangel, M. Saxe, and A. Tashiro for useful discussion and comments, M.L. Gage for editorial comments, and J. Simon for assistance with figures. This work was funded in part by the James S. McDonnell Foundation, Kavli Institute for Brain and Mind, the NSF Temporal Dynamics of Learning Center, and the U.S. National Institutes of Health (NS-050217).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nature Neuroscience. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiskott L. Computational Modeling of Adult Neurogenesis. In: Gage FH, Kempermann G, Song H, editors. Adult Neurogenesis. Woodbury, NY: Cold Spring Harbor Laboratory Press; 2008. pp. 463–481. [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S. A computational principle for hippocampal learning and neurogenesis. Hippocampus. 2005;15:722–738. doi: 10.1002/hipo.20095. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN, Hoffman RE, Miranker W. Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology. 2004;29:747–758. doi: 10.1038/sj.npp.1300358. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Buzsaki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal Differentiation in the Adult Hippocampus Recapitulates Embryonic Development. Journal of Neuroscience. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol. 1991;313:479–485. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Henze DA, Wittner L, Buzsaki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation--feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26:631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Morris RG. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends in Neurosciences. 1987;10:408–415. [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic Inhibition of Synaptic Transmission Reveals Role of CA3 Output in Hippocampal Learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A. 2007;104:4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Slotnick SD. The cognitive neuroscience of memory distortion. Neuron. 2004;44:149–160. doi: 10.1016/j.neuron.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Solstad T, Moser EI, Einevoll GT. From grid cells to place cells: a mathematical model. Hippocampus. 2006;16:1026–1031. doi: 10.1002/hipo.20244. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008 doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskott L, Rasch MJ, Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A Role for Adult TLX-positive Neural Stem Cells in Learning and Behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.