Abstract
Objective
Adiponectin influences insulin sensitivity (SI) and fat oxidation. Little is known about changes in adiponectin with changes in the fat content of eucaloric diets. We hypothesized that dietary fat content may influence adiponectin according to an individual’s SI.
Research Methods and Procedures
We measured changes in adiponectin, insulin, glucose, and leptin in response to high-fat (HF) and low-fat (LF) eucaloric diets in lean (n = 10) and obese (n = 11) subjects. Obese subjects were further subdivided in relation to a priori SI.
Results
We found significantly higher insulin, glucose, and leptin and lower adiponectin in obese vs. lean subjects during both HF and LF. The mean group values of these measurements, including adiponectin (lean, HF 21.9 ± 9.8; LF, 20.8 ± 6.6; obese, HF 10.0 ± 3.3; LF, 9.5 ± 2.3 ng/mL; mean ± SD), did not significantly change between HF and LF diets. However, within the obese group, the insulin-sensitive subjects had significantly higher adiponectin during HF than did the insulin-resistant subjects. Additionally, the change in adiponectin from LF to HF diet correlated positively with the obese subjects’ baseline SI.
Discussion
Although in lean and obese women, group mean values for adiponectin did not change significantly with a change in fat content of a eucaloric diet, a priori measured SI in obese subjects predicted an increase in adiponectin during the HF diet; this may be a mechanism that preserves SI in an already obese group.
Keywords: insulin resistance, adiponectin, macronutrient manipulated diets
Introduction
As the obesity epidemic continues to grow in the United States (1), researchers seek potential physiological mechanisms that explain the presence of enlarged adipose tissue depots. Adipose tissue is an endocrine organ, producing many adipocytokines with important physiological functions. One such adipocytokine is adiponectin, first reported as a novel collagen-like secretory protein (2). Adiponectin has been shown in animals to have insulin-sensitizing properties in liver and to increase fat oxidation in muscle (3). Adiponectin levels are decreased in the db/db mouse (4) and in human obesity (5). In addition, lower adiponectin levels are associated with increased insulin resistance in humans, independently of the degree of obesity (6). Adiponectin infusion restores insulin sensitivity (SI)1 in highly insulin-resistant (IR) db/db mice (7) and protects wild-type mice from developing obesity and insulin resistance on a high-fat (HF) diet (8).
There is little known about the changes in adiponectin levels in humans with changes in the fat content of eucaloric diets. Given adiponectin’s role in SI regulation and fat oxidation, we hypothesized that dietary fat content may regulate adiponectin according to an individual’s SI.We measured adiponectin levels in healthy lean and obese, premenopausal, non-diabetic women in response to HF and low-fat (LF) eucaloric diets. We compared the effects of these diets on lean and obese subjects’ adiponectin, glucose, insulin, leptin, and free fatty acid (FFA) levels. We further determined whether, in the obese subjects, the effect of the diet was related to their baseline SI.
Research Methods and Procedures
Subjects
Ten lean and 11 obese premenopausal (22 to 44 years old) women participated in this study. Lean was defined as BMI < 23 and obese as BMI > 28 kg/m2. Subjects were sedentary as defined by physical activity questionnaires. All subjects signed a consent form that was approved by the St. Luke’s-Roosevelt Institute for Health Sciences Institutional Review Board.
Pretest
All subjects completed an oral glucose tolerance test (75-gram glucose load) to ensure that they were not diabetic. Subjects also completed a medical history and physical exam and were excluded if they were taking any medications including oral contraceptives or if they smoked, consumed alcohol in excess, or did not have regular menstrual cycles.
Fasting weight and height were measured with subjects wearing their undergarments. Minimum waist circumference (minimum circumference between the lower rib margin and the iliac crest, usually midpoint or midwaist) and maximum hip circumference (below the iliac crest, with the subject viewed from the front) were measured while the subjects were standing upright and with their heels together.
Regional adipose tissue volumes were measured by whole-body magnetic resonance imaging, as described (9), in 20 of the 21 subjects. The scans were acquired using contiguous axial slices, 10-mm thickness, and at 40-mm intervals, below L4 to L5 to the toes, and above this level to the fingertips (General Electric, 6X Horizon, Milwaukee, WI). SliceOmatic 4.2 image analysis software (Tomovision, Montreal, CA) was used to analyze images on a PC workstation (Gateway, Madison, WI). Visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) volumes were calculated. The technical errors for three repeated readings of the same scan of magnetic resonance imaging-derived SAT and VAT and volumes in our laboratory are 0.96% and 1.97%, respectively.
Only the obese subjects had their baseline SI determined by an intravenous glucose tolerance test according to the Bergman minimal model (10). This test was completed during the follicular phase of the menstrual cycle while subjects consumed their normal diet. Subjects were defined as IR with SI < 2.5 or insulin sensitive (IS) with SI > 2.5, according to a large population database with demographics similar to those of our subjects (11).
Dietary Intervention
All subjects had their resting metabolic rate determined by indirect calorimetry as previously described (12). Subjects were provided with a 7-day eucaloric diet based on their resting metabolic rate and adjusted for their activity level. The subjects were randomized to begin with either an LF (30% fat, 55% carbohydrate) or an HF (50% fat, 35% carbohydrate) diet. The protein content was 15% for both diets. The subjects were provided with menus and a 6-day food supply to consume at home. Dietary compliance was assured through weight stability measurements. On the 6th night, subjects were admitted to the metabolic research unit. Energy balance was maintained by determining total energy expenditure measurements and total food consumed during 24 hours in a room calorimeter (13). On exiting the chamber, fasting blood samples were collected. The subjects then repeated the experiment with the opposite diet with a minimum 2-week washout.
The blood samples were immediately centrifuged, and the plasma or serum portions were collected and frozen at -70 °C. Radioimmunoassays were used to measure serum adiponectin, plasma leptin, and plasma insulin (Linco Research, St. Charles, MO). Serum FFAs were quantified by a commercial kit from Wako Pure Chemical Industries (Richmond, VA), and glucose values were determined by the Beckman Glucose Analyzer (Beckman, Fullerton, CA). The interassay coefficients of variation for serum adiponectin, plasma leptin, plasma insulin, serum FFA, and plasma glucose were 5.6%, 6.8%, 4.5%, 0.8%, and <3% respectively.
Statistics
Log-transformed values were used for variables that were not normally distributed. Paired Student’s t tests and ANOVA with repeated measures were used to determine significant differences between lean and obese subjects and between IS and IR obese subjects. Post hoc analysis was performed using Fisher’s least significant difference, with the significance level defined as α = 0.05. Correlation coefficients were also computed to determine significant associations among the variables. Statistica version 6.0 (Statsoft Inc., Tulsa, OK) was used for all analyses.
Results
The characteristics of the lean and obese groups (age, anthropometrics, and body composition) are shown in Table 1. The metabolic measurements (mean values for the lean and the obese groups) at the LF and HF are shown in Table 2. At initial screening, the obese subjects had significantly higher weight, BMI, VAT, SAT (Table 1) and postabsorptive insulin, and glucose (not shown) compared with the lean subjects. During each of the diets, the obese subjects had significantly lower adiponectin levels and significantly higher postabsorptive levels of insulin, glucose, and leptin than the lean subjects (Table 2). The fasting FFA levels were not different between lean and obese subjects on either diet (Table 2). There were also no significant changes in response to diet within either the lean or the obese groups for any of these measurements (Table 2).
Table 1.
Subjects’ characteristics
| Lean (n = 10) | Obese, all (n = 11) | Obese, IS (n = 6) | Obese, IR (n = 5) | |
|---|---|---|---|---|
| Age | 32.4 ± 8.4 | 37.5 ± 6.2 | 37.0 ± 6.3 | 38.0 ± 6.7 |
| BMI | 21.1 ± 1.9* | 34.3 ± 4.8 | 31.9 ± 2.6 | 37.3 ± 5.3 |
| WHR | 0.83 ± 0.19 | 0.86 ± 0.08 | 0.87 ± 0.07 | 0.85 ± 0.10 |
| Total SAT (liters) | 15.5 ± 5.6* | 40.3 ± 6.7 | 37.2 ± 5.7 | 44.9 ± 5.7 |
| Total VAT (liters) | 0.72 ± 0.42* | 2.51 ± 0.90 | 2.54 ± 0.94 | 2.45 ± 0.96 |
p < 0.01 for lean vs. all obese subjects. Data are expressed as mean ± SD. Total SAT and VAT were measured by whole-body MRI.
Table 2.
Postabsorptive metabolic measurements in response to LF and HF diets in lean and obese women
| Lean (n = 10) |
Obese (n = 11) |
|||
|---|---|---|---|---|
| LF diet | HF diet | LF diet | HF diet | |
| Adiponectin (ng/mL) | 20.79 ± 6.62* | 21.89 ± 9.79† | 9.51 ± 2.31 | 9.99 ± 3.27 |
| Insulin (pM) | 69 ± 24* | 68 ± 30† | 121 ± 53 | 117 ± 28 |
| Glucose (mM) | 5.1 ± 0.5* | 5.2 ± 0.5† | 5.7 ± 0.7 | 5.7 ± 0.6 |
| Leptin (ng/mL) | 7.0 ± 4.0* | 7.9 ± 5.2† | 31.7 ± 15.5 | 32.3 ± 13.5 |
| FFA (g/L) | 0.15 ± 0.05 | 0.13 ± 0.04 | 0.14 ± 0.05 | 0.13 ± 0.05 |
p < 0.05 for lean vs. obese on LF diet.
p < 0.05 for lean vs. obese on HF diet. Data are expressed as mean ± SD.
We then analyzed only our obese subjects, classifying them as IS (SI > 2.5, n = 6) or IR (SI < 2.5, n = 5) based on their baseline SI. There were no significant differences in age, BMI, waist-to-hip ratio (WHR), or VAT or SAT between these small groups (Table 1). We noticed that adiponectin levels tended to increase in response to HF diet for the IS subjects (+1.54 ± 2.3 ng/mL, P = 0.07) but tended to decrease in response to HF diet for IR subjects (-0.81 ± 0.72 ng/mL, P > 0.1) (Table 3). An interaction between the effect of diet (HF vs. LF) and baseline SI group (IS vs. IR) was computed using an ANOVA with repeated measures. The interaction for adiponectin approached significance (p = 0.0659), whereas no significant interactions were found for the other measurements: insulin (p > 0.3), glucose (p > 0.5), leptin (p > 0.4), and FFA (p > 0.7). Further post hoc analysis showed that there was a significant difference in adiponectin levels of the IS and IR groups while on an HF diet (p = 0.006, Table 3). Other post hoc analyses showed a trend for increased FFA levels on the HF diet for the IR compared with the IS group (p = 0.063). All other p values for both within and between comparisons (insulin, glucose, and leptin) were >0.1.
Table 3.
Postabsorptive metabolic measurements in response to LF and HF diets in IS and IR obese women
| IS (n = 6) |
IR (n = 5) |
|||
|---|---|---|---|---|
| LF diet | HF diet | LF diet | HF diet | |
| Adiponectin (ng/mL) | 9.73 ± 2.77 | 11.27 ± 2.79 | 9.25 ± 1.91 | 8.45 ± 1.50* |
| Insulin (pM) | 113 ± 53 | 119 ± 17 | 131 ± 57 | 113 ± 41 |
| Glucose (mM) | 5.6 ± 0.8 | 5.6 ± 0.8 | 5.7 ± 0.5 | 5.7 ± 0.4 |
| Leptin (ng/mL) | 24.7 ± 7.4 | 26.9 ± 7.2 | 40.1 ± 19.2 | 38.7 ± 17.2 |
| FFA (g/L) | 0.12 ± 0.04 | 0.11 ± 0.02 | 0.16 ± 0.04 | 0.16 ± 0.06† |
p < 0.01 IS vs. IR on HF diet.
p < 0.063 for IS vs. IR on HF diet. Data are expressed as mean ± SD.
We then looked at the relationship of how much each obese subject changed the levels of these measurements in response to dietary fat from LF to HF and correlated this change with the subjects’ baseline SI. There were significant positive correlations between the subjects’ change in adiponectin level and baseline SI (r = 0.78, p = 0.005; Figure 1) and the subjects’ change in fasting insulin levels and baseline SI (r = 0.63, p = 0.041). There were no significant correlations for changes in glucose, leptin, and FFA with baseline SI (data not shown). There were also no significant correlations for change in adiponectin levels with BMI (r = -0.31, p = 0.36), WHR, (r = 0.43, p = 0.21), SAT (r = -0.42, p = 0.23), and VAT (r = 0.25, p = 0.49).
Figure 1.
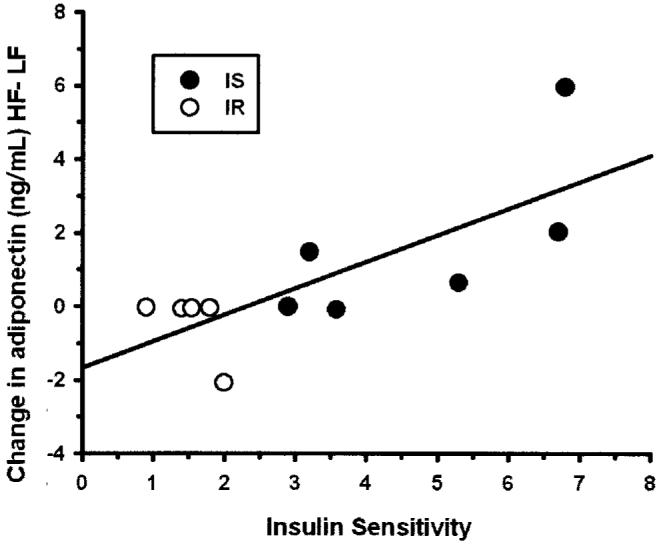
The change in adiponectin (nanograms per milliliter) levels between HF and LF diets correlated with subjects’ baseline SI in obese women (r = 0.78, p = 0.005). (Black circles) IS, SI < 2.5. (White circles) IR, SI > 2.5.
Discussion
We studied non-diabetic lean and obese subjects with controlled eucaloric diets. We show large differences in circulating glucose, insulin, and leptin levels (increased) and in circulating adiponectin levels (decreased) between lean and obese individuals, and these differences were independent of the dietary fat composition of eucaloric diets. Overall, the short-term influence of changing the fat content of eucaloric diets on these measurements (adiponectin, glucose, insulin, and leptin) was minimal because there were no significant changes in response to the diets, when examining the effect within each of the lean or obese groups.
However, when we further examined our obese group based on their baseline SI, with two well-matched groups, IS and IR, we noticed that, during the HF diet only, the IR obese subjects had significantly lower adiponectin levels compared with the more IS obese subjects. We then found a significant correlation between the changes in adiponectin levels from an LF to HF diet with baseline SI.
Previous reports in the literature suggest that adiponectin plays a role in mediating SI and fat oxidation (8,14-17); however, whether dietary fat content influences adiponectin levels in humans is not entirely clear. Yannakoulia et al. (18) did not find any correlations between dietary macronutrient composition and fasting adiponectin levels in lean subjects in a 3-day, cross-sectional, dietary recall study. A single HF meal has been shown to either decrease postprandial adiponectin levels in healthy lean subjects, patients with type 2 diabetes, and their relatives (19) or to have no effect on postprandial adiponectin levels in healthy lean or non-diabetic, IR subjects (20). In contrast, we studied lean and obese subjects maintained on a eucaloric diet with manipulations of carbohydrates and fat content for 1 week. We detected differences in the effect of diet manipulations between the obese IR and obese IS subjects but not between the lean and obese groups. The differences between our results and those of previous studies may have been due to different durations of diet, with some of the previous experiments occurring over too short a time period for any compensatory changes in adiponectin levels, or may have been due to differences in the populations studied. In obese IR and in patients with type 2 diabetes, who may already show severely impaired insulin action and fat oxidation, an increase in adiponectin may be defective or it may provide no further benefit for these functions when challenged with an increased fat load. In lean subjects, there may be no need to improve fat oxidation and SI through an increase in adiponectin levels. Alternatively, the number of lean women we studied may have been too small to detect a significant change in adiponectin. A low baseline adiponectin level predicted a decrease in SI during a 3-day dietary fat challenge in 27 lean males. However, this study did not report changes in adiponectin levels during the dietary intervention (21).
Studies in humans where FFA levels were experimentally decreased have provided more consistent results, showing a decrease in adiponectin levels. This would exclude studies of caloric restriction where despite a predicted decrease in FFA, no changes in adiponectin levels were reported (22). Acipimox administration in healthy men resulted in a decrease in both FFA and adiponectin levels (23). The authors speculate that because there was less substrate available for fat oxidation to occur, adiponectin was not needed to deliver FFA to the skeletal muscle and, therefore, decreased. Adiponectin levels have also been reported to decrease during hyperinsulinemic-euglycemic clamp (24). Again, it was speculated that the antilipolytic effects of insulin decreased FFA levels, thus diminishing the need for adiponectin. In contrast, our study’s paradigm included an HF condition with an increased fat exposure. Although we did not find any differences in fasting FFA levels between the diets, FFA turnover rates may have been increased. However, we did find that, on the HF diet only, the FFA levels tended to be lower for obese IS compared with obese IR subjects (p = 0.063). At the same time, the obese IS subjects had higher adiponectin levels than the obese IR subjects during HF diet, and the increase in adiponectin levels from LF to HF diet was predicted by baseline SI status. Preserved action of adipose tissue lipoprotein lipase in the IS obese patients could also provide an explanation for these observations (25,26). We could speculate that in these obese, IS subjects, lipoprotein lipase would facilitate exposure of adipocytes to higher local FFA levels, resulting in turn in increased adiponectin levels during the HF diet.
A limitation of our study is the small number of subjects (n = 21), which may explain why we failed to find significant differences in response to dietary fat within the lean and obese subjects. Other limitations are the lack of direct SI measurements in the lean subjects and the short nature of the dietary intervention. However, despite the small sample size, we observed an interesting trend with regards to adiponectin levels and SI in the obese group. Larger studies are necessary to further validate these observations.
In summary, we found that lean subjects have significantly higher adiponectin levels on either an LF or HF diet compared with obese subjects and that, within our obese subjects only, SI status determines the adiponectin response to a eucaloric dietary fat challenge. It remains to be determined whether increasing adiponectin levels in response to dietary fat challenge, in an already established obese state, is a protective mechanism against the development of insulin resistance and, ultimately, type 2 diabetes.
Acknowledgment
This study was supported by grants R01 DK40414, M01RR00645, and P30DK26687.
Footnotes
- SI
- insulin sensitivity
- IR
- insulin resistant
- HF
- high fat
- LF
- low fat
- FFA
- free fatty acid
- MRI
- magnetic resonance imaging
- VAT
- visceral adipose tissue
- SAT
- subcutaneous adipose tissue
- WHR
- waist-to-hip ratio
References
- 1.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Kaplan JP. The continuing epidemic of obesity and diabetes in the United States. JAMA. 2001;286:1195–200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 2.Maeda K, Okubo K, Shimomuta I, Funahashi T, Matsuzava Y, Matsubuza K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (Adipose most abundant gene transcript 1) Biochem Biophys Res Comm. 1996;221:286–9. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 3.Diez J, Iglesias M. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endo. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 4.Yamauachi T, Koman J, Waki H, et al. The fat derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 5.Arita Y, Kihora S, Ouchi N, et al. Paradoxical decrease of adipose specific protein, adiponectin in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 6.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 7.Berg AH, Combs TP, Du X, Brownlee M, Scherer P. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 8.Maeda N, Shimomura I, Kishida K, et al. Diet induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;7:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. Magnetic resonance imaging provides new insights into the characterization of adipose and lean tissue distribution. Can J Clin Pharmacol. 1996;74:778–85. [PubMed] [Google Scholar]
- 10.Bergman R, Beard J, Chen M. The minimal modeling method: assessment of insulin sensitivity and B-Cell function in vivo. In: Larner J, Pohl S, editors. Methods in Clinical Diabetes Research. Wiley International; New York: 1986. pp. 13–20. [Google Scholar]
- 11.Haffner SM, D’Agostino R, Jr., Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. Diabetes. 1996;45:742–8. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 12.Albu J, Shur M, Curi M, Murphy L, Heymsfield, Pi-Sunyer FX. Resting metabolic rate in obese, pre-menopausal black women. Am J Clin Nutr. 1997;66:531–8. doi: 10.1093/ajcn/66.3.531. [DOI] [PubMed] [Google Scholar]
- 13.Zhang K, Sun M, Werner P, et al. Sleeping metabolic rate in relation to body mass index and body composition. Intl J Obesity. 2002;26:376–83. doi: 10.1038/sj.ijo.0801922. [DOI] [PubMed] [Google Scholar]
- 14.Shand B, Scott R, Elder P, George P. Plasma adiponectin in overweight, nondiabetic individuals with or without insulin resistance. Diabetes Obes Metab. 2003;5:349–53. doi: 10.1046/j.1463-1326.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 15.Abbasi F, Chu J, Lamendola C, et al. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes. 2004;53:585–90. doi: 10.2337/diabetes.53.3.585. [DOI] [PubMed] [Google Scholar]
- 16.Fruebis J, Tsao T, Javorsci S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shklyaev S, Aslanidi G, Tennant M, et al. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci USA. 2003;100:14217–22. doi: 10.1073/pnas.2333912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yannakoulia M, Yiannakouris N, Bluher S, Matalas A, Klumis Z, Montzeris C. Body fat mass and macronutrient intake in relation to circulation soluble leptin receptor, free leptin index, adiponectin and resistin concentrations in healthy humans. J Clin Endo & Metab. 2003;88:1730–6. doi: 10.1210/jc.2002-021604. [DOI] [PubMed] [Google Scholar]
- 19.Esposito K, Nappo F, Giugliano F, Masella M, Mafella K, Guigliano D. Meal modulation of circulating interleukin 18 and adiponectin concentrations in healthy subjects and in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2003;78:1135–40. doi: 10.1093/ajcn/78.6.1135. [DOI] [PubMed] [Google Scholar]
- 20.Peake P, Krikeots A, Denyer G, Campbell L, Charles-worth J. The postprandial response of adiponectin to a high-fat meal in normal and insulin-resistant subjects. Intl J Obesity. 2003;27:657–62. doi: 10.1038/sj.ijo.0802289. [DOI] [PubMed] [Google Scholar]
- 21.Thamer C, Haap M, Bachmann O, et al. Serum adiponectin levels predict the effect of short-term dietary interventions on insulin sensitivity in humans. Diabetologia. 2004;47:1303–5. doi: 10.1007/s00125-004-1430-7. [DOI] [PubMed] [Google Scholar]
- 22.Boden G, Sargard K, Homko C, Mozzoli M, Stein P. Effect of a low-carbohydrate diet on appetite, blood glucose levels and insulin resistance in obese patients with type 2 diabetes. Ann Int Med. 2005;142:403–11. doi: 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein E, Koutikia P, Ljungquist K, Brev J, Canavan B, Grinspoon S. Acute regulation of adiponectin by free fatty acids. Metabolism. 2004;53:790–3. doi: 10.1016/j.metabol.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 24.Möhlig M, Wegewitz U, Osterhoff M, et al. Insulin decreases human adiponectin plasma levels. Horm Metab Res. 2002;34:665–8. doi: 10.1055/s-2002-38248. [DOI] [PubMed] [Google Scholar]
- 25.Mead J, Irvine S, Ramji D. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med. 2002;80:753–9. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- 26.von Eynattien M, Schneider J, Humpert P, et al. Decreased plasma lipoprotein lipase in hypoadiponectinemia. Diabetes Care. 2004;12:2925–9. doi: 10.2337/diacare.27.12.2925. [DOI] [PubMed] [Google Scholar]


