Abstract
Objective
Higher post-absorptive post-heparin plasma lipoprotein lipase (LPL) activity has been reported in African Americans as compared to non-Hispanic whites but differences in tissue-specific LPL activity are unclear.
Methods and Procedures
Post-absorptive skeletal muscle (SM)-LPL (vastus lateralis) and subcutaneous abdominal adipose tissue (AT)-LPL activity was measured in overweight, sedentary African American females (n = 11) as well as in their non-Hispanic white counterparts (n = 6) during a period of controlled low fat (30%) diet (for 10 days) combined with physical activity (for days 8–10). Post-absorptive substrate utilization was measured on day 10; fasting blood levels and SM and AT biopsies were obtained on day 11.
Results
African Americans had significantly greater post-absorptive SM-LPL activity (P = 0.04) when compared to non-Hispanic whites. There were no significant differences in post-absorptive AT-LPL activity, free fatty acids, and systemic fat oxidation or respiratory quotient between African American and white non-Hispanic women in this study (P > 0.2 for all).
Discussion
During a controlled low fat (30%) diet post-absorptive vastus lateralis SM-LPL activity is higher in sedentary pre-menopausal African American as compared to non-Hispanic white women.
We have shown that, healthy pre-menopausal African American women have higher amounts of intermuscular adipose tissue (AT) (1) and are more insulin resistant and metabolically inflexible than their non-Hispanic White white counterparts (2,3). In addition, we (and others) have reported lower circulating triglyceride (TG) levels in African Americans compared to non-Hispanic whites, despite higher insulin resistance (4–6). Therefore differences in substrate partitioning may exist in African Americans compared to non-Hispanic whites (3,5,6). Lipoprotein lipase (LPL) is an enzyme that hydrolyzes circulating TG, delivering free fatty acid (FFA) to peripheral tissues for utilization and storage (7). It is highly expressed in skeletal muscle (SM) and AT, main TG clearance sites, and plays a key role in substrate partitioning regulation (7). Higher post-absorptive post-heparin plasma LPL (PH-LPL) and higher fasting subcutaneous AT-LPL mRNA and AT-LPL activity were reported in African Americans compared to their non-Hispanic white counterparts (5,6,8–10). No studies have so far examined if race differences exist in SM-LPL activity. We therefore compared heparin-releasable AT- and SM-LPL activity in overweight, healthy pre-menopausal, African American and non-Hispanic white women during a period of controlled low fat (30%) diet and physical activity since these factors highly influence LPL activity (7). Body composition and in vivo systemic fat oxidation were also measured concomitantly.
METHODS AND PROCEDURES
Subjects
Eleven African American and six non-Hispanic white overweight (BMI >25 kg/m2), pre-menopausal (age 25–45 years) women were studied. All subjects signed an approved consent form (from the St. Luke’s-Roosevelt Institute for Health Sciences Institutional Review Board).
Study protocol
Study inclusion/exclusion criteria and protocol have been previously published (3). Briefly, subjects were provided with a 7-day eucaloric low fat diet (30% fat, 55% carbohydrate, 15% protein); dietary compliance was assured through weight measurements. From days 8 to 10 physical activity (supervised scheduled activities; i.e., riding a stationary ergometer, resting, sitting, reading, and sleeping) and the eucaloric diet were controlled at the General Clinical Research Center. On day 10, post-absorptive substrate utilization and fasting plasma glucose, insulin and FFAs were measured as described (3).
SM and AT biopsies
Percutaneous biopsies, of vastus lateralis muscles and subcutaneous abdominal AT, were performed in the fasting state on day 11 under 2% lidocaine anesthetic (Elkins-Sinn, Cherry Hill, NJ). Muscle biopsies were done using a #5 or #6 Bergstrom needle. All visible fat was quickly and carefully removed, the tissue was minced, weighed and frozen within 30 min. AT biopsies were done using a blunt-ended needle designed for liposuction (3-mm Spirotri cannula; Unitech Instruments, Fountain Valley, CA). An aliquot of AT was rinsed with saline, weighed and immediately frozen.
AT- and SM-LPL assay
AT- and SM-LPL activities were measured as reported (11) in duplicate samples weighing between 30 and 35 mg each. One LPL activity unit was defined as the release of 1 μmol FFA in 1 h. Two assays, each including African American and non-Hispanic white samples were performed at different times for SM-LPL activity with different concentrated substrates prepared as described (11) (assay 1, n = 10; assay 2, n= 7). Adipose cell size was measured as described (12).
Body composition
Fat mass and fat-free mass were determined using dual-energy X-ray absorptiometry and SM volume, total and compartmental AT volumes (as liters and percents) were measured using whole body magnetic resonance imaging (2).
Indirect calorimetry calculations
Post-absorptive fat oxidation and non-protein respiratory quotient were calculated as described (3).
Statistics
t-Tests were used to determine significant differences between African Americans and non-Hispanic whites. Since there was a large range of values for the two different SM-LPL activity assays (assay 1, n = 10; assay 2, n = 7) a nested analysis of variance was used. No significant interactions were observed and results were pooled. The significance level was defined as α = 0.05 for all analyses. Statistica v.6.0 (Statsoft, Tulsa, OK) was used for all analyses.
RESULTS
Subjects’ characteristics and body composition are shown in Table 1. African Americans had significantly greater SM volume compared to non-Hispanic whites (P < 0.01), even after adjusting for weight and height and higher inter-muscular adipose tissue as a percent of total AT (%IMAT) (P < 0.05), consistent with previous reports from our laboratory; none of the other dual-energy X-ray absorptiometry or magnetic resonance imaging measurements were statistically significantly different in African Americans vs. non-Hispanic whites in this study.
Table 1.
Subject characteristics
| Mean ± s.d. | African American (n = 11) | Non-Hispanic white (n = 6) |
|---|---|---|
| Age (years) | 37 ± 7 | 37 ± 7 |
| BMI (kg/m2) | 33.9 ± 3.9 | 35.8 ± 9.5 |
| Fat mass (kg) | 41.2 ± 8.7 | 44.3 ± 12.9 |
| Fat-free mass (kg) | 51.3 ± 7.3 | 49.8 ± 6.5 |
| Skeletal muscle (l) | 25.2 ± 2.6* | 21.1 ± 1.7 |
| Subcutaneous adipose tissue (l) | 41.0 ± 6.5 | 45.8 ± 15.9 |
| Visceral adipose tissue (l) | 2.3 ± 0.9 | 3.5 ± 2.1 |
| Intermuscular adipose tissue (l) | 1.8 ± 0.3 | 1.7 ± 0.7 |
| Subcutaneous adipose tissue (%)a | 91.0 ± 3.6 | 90.5 ± 2.0 |
| Visceral adipose tissue (%)a | 5.3 ± 2.1 | 6.5 ± 1.6 |
| Intermuscular adipose tissue (%)*,a | 4.2 ± 0.7* | 3.4 ± 0.3 |
P < 0.05 for African American vs. non-Hispanic white.
Percentage was calculated for each depot from total adipose tissue.
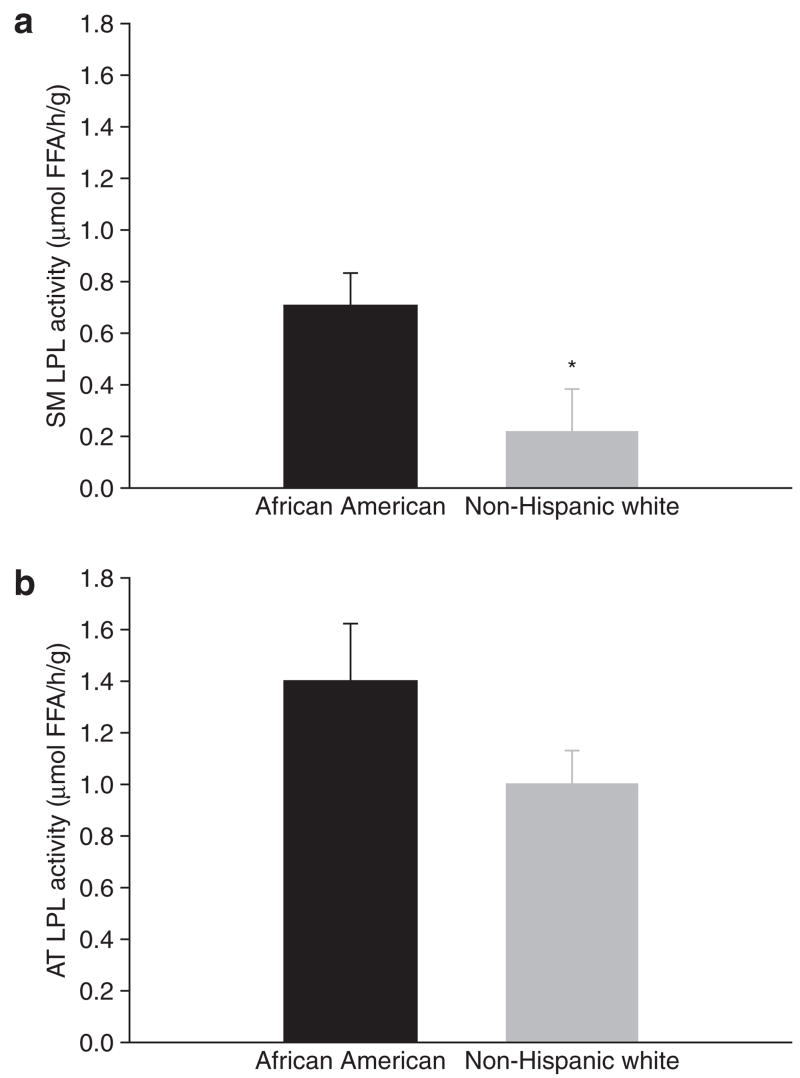
SM-LPL activity was significantly higher in African Americans compared to non-Hispanic whites (0.71 ± 0.12 vs. 0.22 ± 0.16 μmol FFA/h/g, P = 0.04, Figure 1a). The differences in AT-LPL activity (1.37 ± 0.21 vs. 0.98 ± 0.12 μmol FFA/h/g, P = 0.2, Figure 1b) or AT cell size (0.63 ± 0.15 vs. 0.66 ± 0.21 μg P= 0.7) were not significant.
Figure 1.
Differences in (a) SM-LPL activity and (b) AT-LPL activity between African Americans and non-Hispanic whites following a 10-day 30%-fat diet (P = 0.04 and P = 0.20, respectively). AT, adipose tissue; LPL, lipoprotein lipase; SM, skeletal muscle.
Additionally, there were no significant differences in fasting insulin (144.9 ± 59.6 vs. 118.6 ± 42.7 pmol/l, P = 0.4), fasting FFA (0.57 ± 0.26 vs. 0.63 ± 0.15 mmol/l, P = 0.7), or fasting glucose (5.4 ± 0.9 vs. 5.2 ± 0.6 mmol/l, P = 0.5) for African Americans vs. non-Hispanic whites nor were there significant differences in post-absorptive substrate utilization (fat oxidation, 176.5 ± 105.8 vs. 207.1 ± 96.8 μmol/min, P = 0.6; non-protein respiratory quotient, 0.84 ± 0.08 vs. 0.82 ± 0.07, P = 0.6).
DISCUSSION
We found significantly greater SM-LPL activity in overweight, sedentary, healthy, pre-menopausal African American women as compared to their counterparts among non-Hispanic white women, during a period of controlled, weight maintainance, low fat (30%) diet and controlled physical activity conditions. Simultaneously, the subcutaneous abdominal AT-LPL activity was not significantly different, nor were there significant differences in systemic substrate utilization (systemic RQ) between groups. The African American women in this study had higher SM volume and a higher distribution of AT within the intermuscular space rather than in subcutaneous or visceral depots, similar to previous cohorts about whom we have reported (1–3).
Circulating fatty acids provided to SM can be oxidized or stored as TGs; in general, increased SM-LPL activity has been related to increased systemic utilization of fat in humans (7,13) but some animal model studies have demonstrated that SM-LPL overexpression results also in increased deposition of lipids inside the myocytes (14,15). Our current finding suggests that under the conditions studied, African American women may take up more fatty acids into their muscle, but not utilize them by means of oxidation, thus favoring intramuscular storage.
Our finding is novel; however, since this is the first study to report on a higher SM-LPL in African American women compared to non-Hispanic whites, the limitations of this study include (i) a lack of measurements of intra vs. extra-myocellular lipids, thus being unable to determine whether the increased SM-LPL may be contributing to intra-myocellular lipid accumulation and thus directly to increased insulin resistance; (ii) measurements of only systemic substrate utilization, thus being unable to dismiss the possibility that differences exist in tissue-specific fat oxidation (i.e., less in liver and more in SM); (iii) a small sample size, which may have precluded the ability to detect significant differences in AT-LPL activity. Indeed since the overweight state is associated with higher AT-LPL activity, differences due to race may have been blunted. Power analysis showed that while our sample size was adequate to find differences in SM-LPL given the effect size (β = 0.8 for a difference of 0.5 with a s.d. of 0.14), the ability to detect observed differences in AT-LPL with the same number of subjects was only 50%. The strengths of our study included carefully selected subjects comparable to those in our previously published studies and very carefully controlled diet and exercise conditions, factors that are known to highly influence the SM-LPL activity.
Finally, our data suggests that, at least partly, the higher PH-LPL activity reported by others among African Americans may also be due to higher SM-LPL activity. In our study the TG measured during the subjects’ normal diet (not during the intervention) was not significantly different in African Americans compared to non-Hispanic whites (P = 0.20); therefore, the contribution of higher SM-LPL activity towards lowering the TG in African Americans needs to be confirmed in a future study.
In summary, during a controlled 30%-fat diet and physical activity, healthy pre-menopausal African American women with higher percentage of intermuscular adipose tissue had higher post-absorptive SM-LPL activity than did their non-Hispanic white counterparts. The relationship of this finding to the insulin resistance and lower TG in African Americans needs further study.
Acknowledgments
This study was supported by these grants: R01 DK40414, M01RR00645, DK52398 and P30DK26687.
Footnotes
The authors declared no conflict of interest.
References
- 1.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with increased inter-muscular adipose tissue and larger acute insulin response to glucose in African American vs. Caucasian non-diabetic women. Am J Clin Nutr. 2005;82:1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berk ES, Kovera AJ, Boozer CN, Pi-Sunyer FX, Albu J. Metabolic inflexibility in substrate utilization is present in African-American but not Caucasian healthy, pre-menopausal, non-diabetic women. J Clin Endocrinol Metab. 2006;91:4099–4106. doi: 10.1210/jc.2005-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes. 1997;46:456–462. doi: 10.2337/diab.46.3.456. [DOI] [PubMed] [Google Scholar]
- 5.Després JP, Couillard C, Gagnon J, et al. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) family study. Arteroscler Thromb Vasc Biol. 2000;20:1932–1938. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 6.Bower JF, Deshais Y, Pfeifer M, Tananberg R, Barakat H. Ethnic differences in the postprandial triglyceride response to a fatty meal and lipoprotein lipase in lean and obese African American and Caucasian women. Metabolism. 2002;51:211–217. doi: 10.1053/meta.2002.29991. [DOI] [PubMed] [Google Scholar]
- 7.Eckel R. Lipoprotein lipase. A multifunctional enzyme relavent to common metabolic diseases. N Engl J Med. 1989;320:1060–1068. doi: 10.1056/NEJM198904203201607. [DOI] [PubMed] [Google Scholar]
- 8.Friday K, Srinivasan S, Elkasabany A, et al. Black-White differences in the postprandial triglyceride response and postheparin lipoprotein lipase and hepatic triglyceride lipase among young men. Metabolism. 1999;48:749–754. doi: 10.1016/s0026-0495(99)90175-0. [DOI] [PubMed] [Google Scholar]
- 9.Bergeron J, Couillard C, Despres JP, et al. Race differences in the response of postheparin plasma lipoprotein lipase and hepatic lipase activities to endurance training in men: results from the HERITAGE family study. Arthrosclerosis. 2001;159:399–406. doi: 10.1016/s0021-9150(01)00515-9. [DOI] [PubMed] [Google Scholar]
- 10.Ama PF, Poehlman ET, Simoneau JA, et al. Fat distribution and adipose tissue metabolism in non-obese male black African and Caucasian subjects. Int J Obes. 1986;10:503–510. [PubMed] [Google Scholar]
- 11.Dourmashkin JT, Chang GO, Gayles EC, et al. Different forms of obesity as a function of diet composition. Int J Obes. 2005;29:1368–1378. doi: 10.1038/sj.ijo.0803017. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JA, Fried SK, Pi-Sunyer FX, Albu JB. Impaired insulin action in subcutaneous adipocytes from women with visceral obesity. Am J Physiol Endocrinol Metab. 2001;280:E40–E49. doi: 10.1152/ajpendo.2001.280.1.E40. [DOI] [PubMed] [Google Scholar]
- 13.Ferraro RT, Eckel RH, Larson DE, et al. Relationship between skeletal muscle lipoprotein lipase activity and 24-hour macronutrient oxidation. J Clin Invest. 1993;92:441–445. doi: 10.1172/JCI116586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JK, Fillmore JJ, Chen Y, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira LD, Pulawa LK, Jensen DR, Eckel R. Overexpressing human lipoprotein lipase in mouse skeletal muscle is associated with insulin resistance. Diabetes. 2001;50:1064–1068. doi: 10.2337/diabetes.50.5.1064. [DOI] [PubMed] [Google Scholar]