Abstract
Human familial/idiopathic-type scoliosis (IS) is a complex genetic disorder for which the cause is unknown. The curve phenotype characteristically demonstrates pronounced morphological and developmental variability that is likely a consequence of biomechanical, environmental, and genetic differences between individuals. In addition, risk factors that affect the propensity for curves to progress to severity are unknown. Progress in understanding the fundamental biology of idiopathic-type scoliosis has been limited by the lack of a genetic/developmental animal model. Prior to consideration of teleosts, developmental idiopathic-type scoliosis has been considered to be exclusive to humans. Consequently, there is the notion that the syndrome is a result of bipedalism, and many studies try to explain the deformity from this anthrocentric viewpoint. This perspective has been reinforced by the choice of animals used for study, in that chickens and bipedal rats and mice demonstrate idiopathic-type curvature when made melatonin deficient, but quadrupedal animals do not. Overlooked is the fact that teleosts also demonstrate similar curvature when made melatonin-deficient. Our characterization of the guppy curveback has demonstrated that non-induced idiopathic-type curvature is not exclusive to humans, nor bipedalism. We hypothesize that unique morphological, developmental and genetic parallels between the human and guppy syndromes are due to common molecular pathways involved in the etiopathogenesis of both phenotypes. We explore established gene conservation between human and teleost genomes that are in pathways hypothesized to be involved in the IS syndrome. We present non-induced vertebral wedging as a unique shared feature in IS and curveback that suggests a similar interaction between a molecular phenotype on the level of the vertebral anatomy, and biomechanics. We propose that rather than bipedalism per se, expression of idiopathic-type scoliosis is dependent on normal spinal loading applied along the cranio-caudal axis that interacts with an unknown factor causing the primary curve. In this regard, a comparative biological approach using a simplified teleost model will promote discovery of basic processes integral to idiopathic-type scoliosis in teleosts and humans, and highlight human-specific aspects of the deformity.
Introduction
Familial/idiopathic-type scoliosis (IS) is a complex genetic disorder that accounts for 80% of all human spinal curvatures (MIM 181800, Online Mendelian Inheritance in Man). It is broadly defined as a three-dimensional curve deformity with no known etiology that manifests after birth and has a propensity to increase in magnitude with growth, until sexual maturity. Curve magnitude, morphology, rate of and propensity for progression are highly variable among individuals, due to biomechanical, environmental, developmental and possibly genetic variability between individuals.
An understanding of fundamental aspects of the deformity has been limited by the lack of a genetic/developmental animal model. Before consideration of teleost fishes, all observed forms of scoliosis in animals have been the result of congenital anomalies, or have been induced in laboratory animals [1]. Hence, it appears that idiopathic-type spinal curvature is exclusive to humans, and therefore is alleged to be a consequence of bipedalism [1-6]. Abandoning this anthrocentric perspective will help advance our comprehension of not only the cause(s) of curve onset, but also risk factors associated with progression. A comparative biological approach using a simplified teleost model will promote discovery of basic processes integral to idiopathic-type scoliosis and highlight human specific aspects of the deformity.
How the choice animal models for IS support the bipedal notion
That idiopathic-type scoliosis has never been observed in any animal other than humans certainly has encouraged the notion that the deformity is contingent on bipedalism. Experimentally, scoliosis has been produced using a variety of animals (i.e., rabbit, lamb, goat, mouse, rat, monkey, dog, pig, chicken). These all have the fundamental goal of producing a model that is comparable to IS in order to elucidate the etiology, and promote new therapeutic methods [7]. Methods for induction of scoliosis include dietary deficiency, immobilization, local procedures (i.e. damage to spinal, neural or surrounding tissues), or pinealectomy. Ultimately, because they are induced, it remains controversial whether conclusions drawn from such experiments relate to primary or secondary influences for curvature [7,8].
Because most of the animals used for study of IS are quadrupedal, they have limitations for research into an etiology that is presumed to be influenced by gravity [7]. The relevance of bipedalism and gravity to IS pathogenesis has been supported by the fact that pinealectomy (or melatonin deficiency) can induce spinal curvature in chickens, but not in quadrupedal mammals unless they are forced to be bipedal [3,9-11]. For example, pinealectomized rats and mice made melatonin-deficient do not demonstrate spinal curvature as quadrupeds, but do if their front legs and tail are amputated in order to force them to be bipedal [9,10, 12].
Importantly, although never reviewed in orthopeadic studies, pinealectomy in the teleosts guppy and salmon induces spinal curvature with a physiological response similar to that in pinealectomized chickens [13-15]. Hence, the conviction that idiopathic-type scoliosis is exclusive to bipedalism and dependent on gravity has been biased by the selection of animals used for study.
Background on the curveback guppy
The curveback guppy is the first model for human IS to demonstrate spinal curvature in otherwise healthy fish that is not induced nor caused by congenital malformation of the vertebrae [16]. Our characterization of the guppy curveback syndrome has revealed unique morphological, developmental, and genetic parallels to human idiopathic-type scoliosis (IS).
The guppy is a small live-bearing teleost fish, and offspring are born approximately 3 weeks after conception. As with humans, the onset of curvature begins at variable ages after birth (guppy skeleton is completely ossified before birth) and can either stabilize at a moderate magnitude, resolve to normal or nearly normal, or progress to severity [16-18]. The curve phenotype is a primary sagittal lordosis of variable magnitude with most individuals exhibiting a posterior kyphosis, coronal deviation and axial rotation (figure 1). Beyond complex inheritance, the human and curveback idiopathic-type curvature syndromes share: a female bias for severe curve magnitude, despite an equal incidence rate among males and females; similar variability for curve magnitude and morphology; variable age of curve onset and rate/ propensity for progression; curve stabilization at sexual maturity; the incidence of resolving curves; and vertebral shape distortion at the apex of severe curves [16].
Figure 1. Example of curveback phenotype.
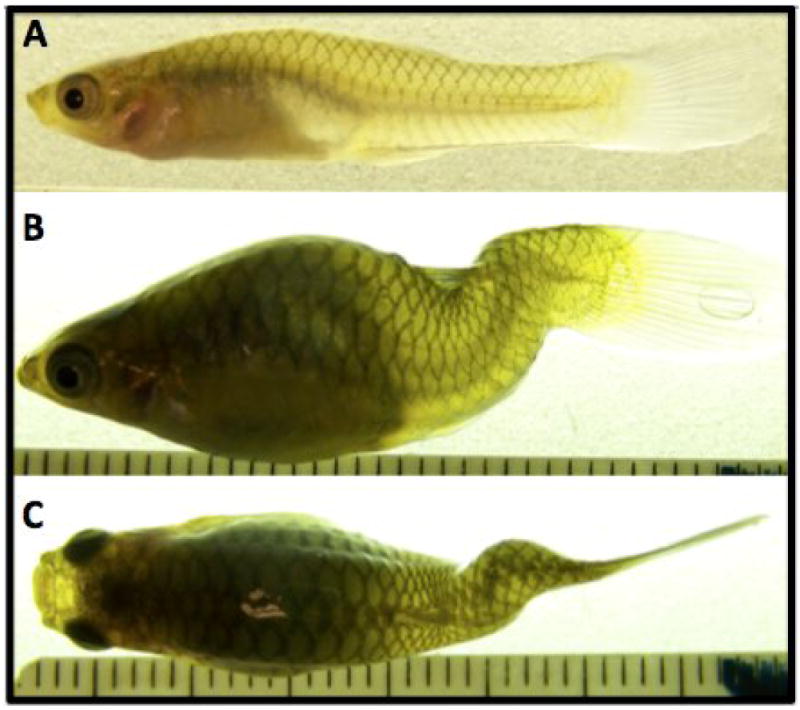
A and B: Sagittal profile of a normal (A), and curved (B) adult female curveback guppy. C: Coronal profile of the same female as shown in B. Photos taken on euthanized fish with digital camera (Kodak Easyshare Z612) under 3X magnification on a light table (scale shown in mm).
Hypothesis
Study of the teleost curveback provides an important insight: that idiopathic-type scoliosis is not a human exclusive deformity. Here we explore the hypothesis that common molecular pathways are involved in the etiopathogenesis of the guppy and human phenotypes. This idea is based on the fact that curveback demonstrates so many phenotypic parallels to IS, and that humans and teleosts share many genes involved in basic biological processes. It is possible that the same genes in human and guppy idiopathic-type scoliosis are mutated, or it is also possible that different sets of genes are mutated in guppy and human systems, but that they affect common molecular pathways. Either way, comparison of the two systems has the potential to illuminate important biological pathways involved in the maintenance of spinal stability throughout growth.
An important corollary of our hypothesis is that rather than a consequence of gravity and bipedalism per-se, the deformity is likely contingent on the interaction of force/loading applied along the cranio-caudal axis with the vertebral anatomy, in the presence of a genetic predisposition. An important question that emerges from our hypothesis is whether genes for idiopathic-type curvature are present in terrestrial animals, but their expression is constrained by quadrupedal biomechanics, or if indeed the primary etiology is exclusive to humans and teleosts.
Is the genetic predisposition exclusive to humans?
It is possible that the genetic predisposition and components related to curve progression for idiopathic-type curvature in guppies and humans are in the same genes or in genes controlling common genetic pathway(s). The observed phenotypic variation and lack of concordant loci identified among human linkage studies has suggested that there may be multiple predisposing genes for IS [19-22]. With complex syndromes such as IS, different polymorphisms in the same gene or in different genes within the same molecular pathway could cause observed phenotypic variability [23-26].
Fish share most developmental pathways, physiological mechanisms and organ systems with humans [reviewed in 27-29]. Comparisons between human and fish genomes have identified DNA sequences and entire gene networks that have significant functional activity in humans, many of which are in systems implicated for human IS, suggesting some gene conservation for genetic factors thought to be involved in the deformity (e.g. osteoblast and chondrocyte differentiation [30], bone formation [31,32], muscle formation [33], gene regulation [34], pineal gland (Lhx9) [35], neural development [36], somitogenesis [37], cell proliferation [38-40], pituitary(pitx) function [41], osteoclast function [42]). These include regulation of hormones that might be involved in idiopathic-type curvature (based on suggestions from human studies): e.g. calmodulin and steroidogenesis [43], nutritional regulation of growth hormone and insulin-like growth factor-I [44], IGF-binding proteins (IGFBPs) [45], growth hormone-releasing hormones and receptors [46], a neuromodulatory role for nitric oxide [47], thyroid hormones (TH) in bone remodeling [48], thyroid and muscle growth [49], the midbrain locomotor region (MLR) and descending (reticulospinal) pathways that activate spinal networks for rhythmic movements such as swimming in fishes, and walking and running in humans [50,51].
Physical evidence for shared biomechanical and/or physiological factors
Distortion of vertebrae at the apex of curvature is considered an important component of the human phenotype that has not been observed in other animals unless it is induced. It is broadly suggestive of an unknown physiological dysfunction involving asymmetrical loading (the details of which are a subject of speculation) the spine during growth that directly affects the vertebral body growth plates, so that longitudinal growth of a vertebral body is modified [reviewed in 52]. Because the non-induced phenotype is (without consideration of teleosts) exclusively human, it has generated many hypotheses that are difficult to test [reviewed in 53]. Symptomatic distortion of apical vertebrae may provide valuable perspective regarding the interaction between a molecular phenotype on the level of the vertebrae and biomechanics. Therefore, study of vertebral distortion has involved simulation of the phenotype by bracing in rat and cow tails or tethering in goats or rabbits [54-58]. Importantly, the question of to what extent curvature is due to altered biomechanics and growth and how much is due to a more primary etiology cannot be answered only using physical simulations in induced models.
In both humans and guppies primary loading on the spine is along the cranial-caudal axis. In simplified terms, normal loading on human vertebrae is from the weight of the head and gravity coupled with the loading associated with bipedalism (i.e. standing and walking); in guppies, from swimming through the dense medium of water coupled with the force associated with the tail-beat motion. In guppies, non-induced distortion of apical vertebrae is similar to that observed in human IS, in which vertebral bodies are compressed on the concave side of a curve [16, 59-61]. An important question in both the curveback and human phenotypes is whether the vertebral bodies are compromised so that they are less capable of handling normal cranio-caudal loading (i.e., failure of mechanotransdution), or if the vertebral bodies are normal, but there is excessive/pathological force on the vertebrae sufficient to cause distortion (i.e., dysfunctional growth). There are hypotheses to support the idea that the predisposing defect may involve vertebral bodies [62-65], and also there are hypotheses to support that there may be excessive force on the vertebrae from growth related dysfunctions [66-68].
Consequences of hypothesis
One of the main insights of the curveback model is that idiopathic-type scoliosis is not exclusive to bipedalism. We hope that such a consideration will provoke new ideas regarding which components of the syndrome are primary or initiating, and which are secondary (complicating, or risk factors associated with the propensity for curve progression), and how these factors might interact. Human and guppy biomechanical similarities might elucidate essential components of idiopathic-type curvature, and differences between the two animals may offer the opportunity to specify which aspects of IS are indeed exclusive to humans.
Comparative studies of guppy and human physiology and curve phenotypes might direct hypotheses regarding how biomechanics can interact with intrinsic aspects of curve etiology (i.e. genetic and molecular aspects) and/or progression (i.e. growth related aspects). Hypotheses regarding the relative contribution of factors such as tallness/length [69-73], dorsal shear force [1], pelvic association [74-76], or posture [77-81] can be critically evaluated by comparison to the anatomy of curveback.
With complex human syndromes that involve interactions among genetic, physiological, and environmental forces, a successful experimental approach is to first identify genes and molecular pathways in a model animal with a similar phenotype [82-85], and we have presented one for IS, the guppy curveback. Once genes involved in the etiology of curveback are identified, we can determine whether mutations in these genes are correlated to the human IS phenotype. An important question that then can be answered is whether the genetic predisposition to idiopathic-type curvature is common to all vertebrates but not expressed in quadrupeds because of biomechanical constraints, or if unique mutations in the human lineage have lead to this prevalent syndrome.
Acknowledgments
Support from Natural Sciences and Engineering Research Council of Canada; US National Institutes of Health, 1R21AR053730-01A2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Castelein RM, van Dieen JH, Smit TH. The role of shear forces in the pathogenesis of idiopathic scoliosis- A hypothesis. Medical Hypotheses. 2005;65:501–508. doi: 10.1016/j.mehy.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Burwell RG, Cole AA, Cook TA, Grivas TB, Kiel AW, Moulton A, Thirlwall AS, et al. Pathogenesis off idiopathic scoliosis: The Nottingham concept. Acta Orthopaedica Belgica. 1992;58(Suppl 1):33–58. [PubMed] [Google Scholar]
- 3.Machida M, Murai I, Miyashita Y, Dubousset J, Yamada T, Kimura J. Pathogenesis of idiopathic scoliosis: experimental study in rats. Spine. 1999;24(19):1985–9. doi: 10.1097/00007632-199910010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Xiao J, Wu ZH, Qiu GX, Yang XY, Li JY, Weng XS. Upright posture impact on spine susceptibility in scoliosis and progression patterns of scoliotic curve. Zhonghua Yi Xue Za Zhi. 2007 Jan 2;87(1):48–52. [PubMed] [Google Scholar]
- 5.Bagnall KM. Using a synthesis of the research literature related to the aetiologiy of adolescent idiopathic scoliosis to provide ideas on future directions for success. Scoliosis. 2008;3(5) doi: 10.1186/1748-7161-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burwell RG, Dangerfield PH, Freeman BJ. Concepts on the pathogenesis of adolescent idiopathic scoliosis. Bone growth and mass, vertebral column, spinal cord, brain, skull, extra-spinal left-right skeletal length asymmetries, disproportions and molecular pathogenesis. Stud Health Technol Inform. 2008;135:3–52. [PubMed] [Google Scholar]
- 7.Kawakami N, Deguchi M, Kanemura T. Animal models of scoliosis. In: An YH, Friedman RJ, editors. Animal Models in Orthopaedic Research. CRC Press; 1999. pp. 549–564. [Google Scholar]
- 8.Braun JT, Ogilvie JW, Akyuz E, Brodke DS, Bachus KN, Stefko RM. Experimental scoliosis in an immature goat model: A method that creates idiopathic-type deformity with minimal violation of the spinal elements along the curve. Spine. 2006;28(19):2198–2203. doi: 10.1097/01.BRS.0000085095.37311.46. [DOI] [PubMed] [Google Scholar]
- 9.O'Kelly C, Wang X, Raso J. The production of scoliosis after pinealectomy in young chickens, rats, and hamsters. Spine. 1999;24(1):35–43. doi: 10.1097/00007632-199901010-00009. [DOI] [PubMed] [Google Scholar]
- 10.Machida M, Saito M, Dubousset J, Yamada T, Kimura J, Shibasaki K. Pathological mechanism of idiopathic scoliosis: experimental scoliosis in pinealectomized rats. Eur Spine J. 2005;14:843–848. doi: 10.1007/s00586-004-0806-1. [DOI] [PubMed] [Google Scholar]
- 11.Cheung KM, Wang T, Poon AM, Carl A, Tranmer B, Hu Y, Luk KD, Leong JC. The effect of pinealectomy on scoliosis development in young nonhuman primates. Spine. 2005;30(18):2009–2013. doi: 10.1097/01.brs.0000179087.38730.5d. [DOI] [PubMed] [Google Scholar]
- 12.Oyama J, Murai I, Kanazawa K, Machida M. Bipedal ambulation induces experimental scoliosis in C57BL/6J mice with reduced plasma and pineal melatonin levels. J Pineal Res. 2006 Apr;40(3):219–24. doi: 10.1111/j.1600-079X.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 13.Plugfelder O. Wirkung der epiphysektomie auf die postembryonalentwicklung von Lebistes reticulatua (Peters) Wilhelm Roux Arch Entwiklungsmech Der Org. 1953;146:115–136. doi: 10.1007/BF00575348. [DOI] [PubMed] [Google Scholar]
- 14.Mayer I. Effect of long-term pinealectomy on growth and precocious maturation in Atlantic salmon, Salmo salar parr. Aquat Living Resour. 2000;13:139–144. [Google Scholar]
- 15.Fjelldal PG, Grotmol S, Kryvi H, Gjerdet NR, Taranger GL, Hansen T, Porter MJ, Totland GK. Pinealectomy induces malformation of the spine and reduces the mechanical strength of the vertebrae in Atlantic salmon, Salmo salar. Journal of Pineal Research. 2004;36:132–139. doi: 10.1046/j.1600-079x.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 16.Gorman KF, Tredwell SJ, Breden F. The mutant guppy syndrome curveback as a model for human heritable spinal curvature. Spine. 2007;32(7):735–741. doi: 10.1097/01.brs.0000259081.40354.e2. [DOI] [PubMed] [Google Scholar]
- 17.Mookerjee HK, Mitra GN, Mazumdar SR. The development of the vertebral column of a viviparous teleosts, Lebistes reticulates. Journal of Morphology. 1940;67(2):241–269. [Google Scholar]
- 18.Weisel GF. Early ossification in the skeleton of the sucker (Catosomus macrocheilus) and the guppy (Poecilia reticulata) Journal of Morphology. 1967;121:1–18. doi: 10.1002/jmor.1051210102. [DOI] [PubMed] [Google Scholar]
- 19.Miller NH, Marosy B, Justice CM, Novak SM, Tang EY, Boyce P, Pettengil J, et al. Linkage analysis of genetic loci for kyphoscoliosis on chromosomes 5p13, 13q13.3, and 13q32. American Journal of Medical Genetics Part A. 2006;140A:1059–1068. doi: 10.1002/ajmg.a.31211. [DOI] [PubMed] [Google Scholar]
- 20.Miller NH. Genetics of Familial Idiopathic Scoliosis. Clinical Orthopaedics and Related Research. 2007;462:6–10. doi: 10.1097/BLO.0b013e318126c062. [DOI] [PubMed] [Google Scholar]
- 21.Ogilvie JW, Braun J, Argyle V, Nelson L, Meade M, Ward K. The search for idiopathic scoliosis genes. Spine. 2006;31(6):679–681. doi: 10.1097/01.brs.0000202527.25356.90. [DOI] [PubMed] [Google Scholar]
- 22.Cheng JCY, Tang NLS, Yeung HY, Miller N. Genetic Association of Complex Traits Using Idiopathic Scoliosis as an Example. Clinical Orthopeadics and Related Research. 2007;462:38–44. doi: 10.1097/BLO.0b013e3180d09dcc. [DOI] [PubMed] [Google Scholar]
- 23.Marchini J, Donnelly P, Cardon LR. Genome-wide strategies for detecting multiple loci that influence complex diseases. Nat Genet. 2005;37(4):413–7. doi: 10.1038/ng1537. [DOI] [PubMed] [Google Scholar]
- 24.Evans DM, Marchini J, Morris AP, Cardon LR. Two-stage two-locus models in genome-wide association. PLoS Genet. 2006 Sep 22;2(9):e157. doi: 10.1371/journal.pgen.0020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franke L, van Bakel H, Fokkens L, de Jong ED, Egmont Petersen M, Wijmenga C. Reconstruction of a functional human gene network, with an application for prioritizing positional candidate genes. Am J Hum Genet. 2006 Jun;78(6):1011–25. doi: 10.1086/504300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan W. Network-based model weighting to detect multiple loci influencing complex diseases. Hum Genet. 2008 Aug 22; doi: 10.1007/s00439-008-0545-1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cossins AR, Crawford DL. Fish as models for environmental genomics. Nature. 2005;6:324–333. doi: 10.1038/nrg1590. [DOI] [PubMed] [Google Scholar]
- 28.Crollius HR, Weissenbach J. Fish Genomics and biology. Genome Research. 2005;15:1675–1682. doi: 10.1101/gr.3735805. [DOI] [PubMed] [Google Scholar]
- 29.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 30.Inohaya K, Kudo A. Temporal and spatial patterns of cbfal expression during embryonic development in the teleosts, Oryzias latipes. Developmental Genes and Evolution. 2000;210:570–574. doi: 10.1007/s004270000094. [DOI] [PubMed] [Google Scholar]
- 31.Wagner TU, Renn J, Riemensperger T, Volff JN, Köster RW, Goerlich R, Schartl M, Winkler C. The teleost fish medaka (Oryzias latipes) as genetic model to study gravity dependent bone homeostasis in vivo. Adv Space Res. 2003;32:1459–1465. doi: 10.1016/S0273-1177(03)90381-4. [DOI] [PubMed] [Google Scholar]
- 32.Renn J, Winkler C, Schartl M, Fischer R, Goerlich R. Zebrafish and medaka as models for bone research including implications regarding space-related issues. Protoplasma. 2006;229:209–214. doi: 10.1007/s00709-006-0215-x. [DOI] [PubMed] [Google Scholar]
- 33.Hollway GE, Currie PD. Myotome meanderings. EMBO reports. 2003;4(2):855–860. doi: 10.1038/sj.embor.embor920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin JT, Priest JR, Ovcharenko I, Ronco A, Moore RK, Burns CG, MacRae CA. Human-zebrafish non-coding conserved elements act in vivo to regulate transcription. Nucleic Acids Research. 2005;33(17):5437–5445. doi: 10.1093/nar/gki853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alunni A, Blin M, Deschet K, Bourrat F, Vernier P, Rétaux S. Cloning and developmental expression patterns of Dlx2, Lhx7 and Lhx9 in the medaka fish (Oryzias latipes) Mechanisms of Development. 2004;121:977–983. doi: 10.1016/j.mod.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 36.Deyts C, Candal E, Joly JS, Bourrat F. An automated in-situ hybridization screen in the medaka to identify unknown neural genes. Developmental Dynamics. 2005;234:698–708. doi: 10.1002/dvdy.20465. [DOI] [PubMed] [Google Scholar]
- 37.Gajewski M, Elmasri H, Girschick M, Sieger D, Winkler C. Comparative analysis of her genes during fish somitogenesis suggests a mouse/chick-like mode of oscillation in medaka. Developmental Genes and Evolution. 2006;216:315–332. doi: 10.1007/s00427-006-0059-6. [DOI] [PubMed] [Google Scholar]
- 38.Cadal E, Thermes V, Joly JS, Bourrat F. Medaka as a model system for the characterization of cell cycle regulators: a functional analysis of Ol-Gadd45γ during early embryogenesis. Mechanisms of Development. 2004;121:945–958. doi: 10.1016/j.mod.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Yoder JA. Investigating the morphology, function and genetics of cytotoxic cells in bony fish. Comparative Biochemistry and Physiology Part C. 2004;138:271–280. doi: 10.1016/j.cca.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Thermes V, Candal E, Alunni A, Serin G, Bourrat F, Joly JS. Medaka simplet (FAM53B) belongs to a family of novel vertebrate genes controlling cell proliferation. Development. 2006;133:1881–1890. doi: 10.1242/dev.02350. [DOI] [PubMed] [Google Scholar]
- 41.Jaszczyszyn Y, Haeussler M, Heuzé A, Debiais-Thibaud M, Casane D, Bourrat F, Joly JS. Comparison of the expression of medaka (Oryzias latipes) pitx genes with other vertebrates shows high conservation and a case of functional shuffling in the pituitary. Gene. 2007;406(12):42–50. doi: 10.1016/j.gene.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 42.Nemoto Y, Higuchi K, Baba O, Kudo A, Takano Y. Multinucleate osteoclasts in medaka as evidence of active bone remodeling. Bone. 2007;40:399–408. doi: 10.1016/j.bone.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Benninghoff AD, Thomas P. Involvement of calcium and calmodulin in the regulation of ovarian steroidogenesis in Atlantic croaker (Micropogonias undulates) and modulation by Aroclor 1254. General and Comparative Endocrinology. 2005;144:211–223. doi: 10.1016/j.ygcen.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Pedroso FL, de Jesus-Ayson EG, Cortado HH, Hyodo S, Ayson FG. Changes in mRNA expression of grouper (Epinephelus coioides) growth hormone and insulin-like growth factor I in response to nutritional status. General and Comparative Endocrinology. 2006;145:237–246. doi: 10.1016/j.ygcen.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Shepherd BS, Johnson JK, Silverstein JT, Parhar IS, Vijayan MM, McGuire A, Weber GM. Endocrine and orexigenic actions of growth hormone secretagogues in rainbow trout (Oncorhynchus mykiss) Comp Biochem Physiol A. 2007;146(3):390–399. doi: 10.1016/j.cbpa.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Lee LT, Siu FK, Tam JK, Lau IT, Wong AO, Lin MC, Vaudry H, Chow BK. Discovery of growth hormone-releasing hormones and receptors in nonmammalian vertebrates. PNAS. 2007;104(7):2133–2138. doi: 10.1073/pnas.0611008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marley R, Lu W, Balment RJ, McCrohan CR. Evidence for nitric oxide role in the caudal neurosecretory system of the European flounder, Platichthys flesus. General and Comparative Endocrinology. 2007;153:251–261. doi: 10.1016/j.ygcen.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 48.Sbaihi M, Kacem A, Aroua S, Baloche S, Rousseau K, Lopez E, Meunier F, Dufour S. Thyroid hormone-induced demineralization of the vertebral skeleton of the eel, Anguilla anguilla. General and Comparative Endocrinology. 2007;151:98–107. doi: 10.1016/j.ygcen.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Yang X, Xie J, Wu T, Yue G, Chen J, Zhao R. Hepatic and muscle expression of thyroid hormone receptors in association with body and muscle growth in large yellow croaker Pseudosciaena crocea (Richardson) General and Comparative Endocrinology. 2007;151:163–171. doi: 10.1016/j.ygcen.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Fetcho JR. The spinal motor system in early vertebrates and some of its evolutionary changes. Brain Behav Evol. 1992;40:82–97. doi: 10.1159/000113905. [DOI] [PubMed] [Google Scholar]
- 51.Jahn K, Deutschländer A, Stephan T, Kalla R, Wiesmann M, Strupp M, Brandt T. Imaging human supraspinal locomotor centers in brainstem and cerebellum. NeuroImage. 2008;39:786–792. doi: 10.1016/j.neuroimage.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 52.Stokes IA. Mechanical modulation of spinal growth and progression of adolescent scoliosis. Stud Health Technol Inform. 2008;135:75–83. [PubMed] [Google Scholar]
- 53.Stokes IA, Burwell RG, Dangerfield PH IBSE. Biomechanical spinal growth modulation and progressive adolescent scoliosis--a test of the ‘vicious cycle’ pathogenetic hypothesis: summary of an electronic focus group debate of the IBSE. Scoliosis. 2006 Oct 18;1:16. doi: 10.1186/1748-7161-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mente PL, Stokes IA, Spence H, Aronsson DD. Progression of vertebral wedging in an asymmetrically loaded rat tail model. Spine. 1997 Jun 15;22(12):1292–6. doi: 10.1097/00007632-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 55.Aronsson DD, Stokes IA, Rosovsky J, Spence H. Mechanical modulation of calf tail vertebral growth: implications for scoliosis progression. J Spinal Disord. 1999 Apr;12(2):141–6. [PubMed] [Google Scholar]
- 56.Stokes IA, Aronsson DD, Clark KC, Roemhildt ML. Intervertebral disc adaptation to wedging deformation. Stud Health Technol Inform. 2006;123:182–7. [PubMed] [Google Scholar]
- 57.Kallemeier PM, Buttermann GR, Beaubien BP, Chen X, Polga DJ, Lew WD, Wood KB. Validation, reliability, and complications of a tethering scoliosis model in the rabbit. Eur Spine J. 2006 Apr;15(4):449–56. doi: 10.1007/s00586-005-1032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Braun JT, Ogilvie JW, Akyuz E, Brodke DS, Bachus KN. Creation of an experimental idiopathic-type scoliosis in an immature goat model using a flexible posterior asymmetric tether. Spine. 2006 Jun 1;31(13):1410–4. doi: 10.1097/01.brs.0000219869.01599.6b. [DOI] [PubMed] [Google Scholar]
- 59.Wever DJ, Veldhuizen AG, Klein JP, Webb PJ, Nijenbanning G, Cool JC, v Horn JR. A biomechanical analysis of the vertebral and rib deformities in structural scoliosis. European Spine Journal. 1999;8(4):252–260. doi: 10.1007/s005860050169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheung J, Veldhuizen AG, Halberts JP, Sluiter WJ, Van Horn JR. Geometric and electromyographic assessments in the evaluation of curve progression in idiopathic scoliosis. Spine. 2006;31(3):322–329. doi: 10.1097/01.brs.0000197155.68983.d8. [DOI] [PubMed] [Google Scholar]
- 61.Modi HN, Suh SW, Song HR, Yang JH, Kim HJ, Modi CH. Differential wedging of vertebral body and intervertebral disc in thoracic and lumbar spine in adolescent idiopathic scoliosis- A cross sectional study in 150 patients. Scoliosis. 2008 Aug 13;3(1):11. doi: 10.1186/1748-7161-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreau A, Wang DS, Forget S, Azeddine B, Angeloni D, Fraschini F, Labelle H, Poitras B, Rivard CH, Grimard G. Melatonin signaling dysfunction in adolescent idiopathic scoliosis. Spine. 2004 Aug 15;29(16):1772–81. doi: 10.1097/01.brs.0000134567.52303.1a. [DOI] [PubMed] [Google Scholar]
- 63.Azeddine B, Letellier K, Wang da S, Moldovan F, Moreau A. Molecular determinants of melatonin signaling dysfunction in adolescent idiopathic scoliosis. Clin Orthop Relat Res. 2007 Sep;462:45–52. doi: 10.1097/BLO.0b013e31811f39fa. [DOI] [PubMed] [Google Scholar]
- 64.Letellier K, Azeddine B, Blain S, Turgeon I, Wang da S, Boiro MS, Moldovan F, Labelle H, Poitras B, Rivard CH, Grimard G, Parent S, Ouellet J, Lacroix G, Moreau A. Etiopathogenesis of adolescent idiopathic scoliosis and new molecular concepts. Med Sci (Paris) 2007 Nov;23(11):910–6. doi: 10.1051/medsci/20072311910. [DOI] [PubMed] [Google Scholar]
- 65.Letellier K, Azeddine B, Parent S, Labelle H, Rompré PH, Moreau A, Moldovan F. Estrogen cross-talk with the melatonin signaling pathway in human osteoblasts derived from adolescent idiopathic scoliosis patients. J Pineal Res. 2008 May 26; doi: 10.1111/j.1600-079X.2008.00603.x. [DOI] [PubMed] [Google Scholar]
- 66.Chu WC, Lam WM, Ng BK, Tze-Ping L, Lee KM, Guo X, Cheng JC, Burwell RG, Dangerfield PH, Jaspan T. Relative shortening and functional tethering of spinal cord in adolescent scoliosis - Result of asynchronous neuro-osseous growth, summary of an electronic focus group debate of the IBSE. Scoliosis. 2008 Jun 27;3:8. doi: 10.1186/1748-7161-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chu WC, Man GC, Lam WW, Yeung BH, Chau WW, Ng BK, Lam TP, Lee KM, Cheng JC. Morphological and functional electrophysiological evidence of relative spinal cord tethering in adolescent idiopathic scoliosis. Spine. 2008 Mar 15;33(6):673–80. doi: 10.1097/BRS.0b013e318166aa58. [DOI] [PubMed] [Google Scholar]
- 68.Kawabata H, Ono K, Seguchi Y, Tanaka M. Idiopathic scoliosis and growth—A biomechanical consideration. Nippon Seikeigeka Gakkai Zasshi. 1998;62(3):161–170. [PubMed] [Google Scholar]
- 69.Siu King Cheung C, Tak Keung Lee W, Kit Tse Y, Ping Tang S, Man Lee K, Guo X, Qin L, Chun Yiu Cheng J. Abnormal peri-pubertal anthropometric measurements and growth pattern in adolescent idiopathic scoliosis: A study of 598 patients. Spine. 2003;28(18):2152–2157. doi: 10.1097/01.BRS.0000084265.15201.D5. [DOI] [PubMed] [Google Scholar]
- 70.Dickson RA, Sevitt EA. Growth and idiopathic scoliosis: A longitudinal cohort study. J Bone Joint Surg. 1982;64B:385. [Google Scholar]
- 71.Leong JC, Low WD, Mok CK, Kung LS, Yau AC. Linear growth in southern Chinese female patients with adolescent idiopathic scoliosis. Spine. 1982;7(5):471–475. doi: 10.1097/00007632-198209000-00011. [DOI] [PubMed] [Google Scholar]
- 72.Nicolopoulos KS, Burwell RG, Webb JK. Stature and its components in adolescent idiopathic scoliosis. Journal of Bone and Joint Surgery [Br] 1985;67:594–601. doi: 10.1302/0301-620X.67B4.4030857. [DOI] [PubMed] [Google Scholar]
- 73.Escalada F, Marco E, Duarte E, Muniesa JM, Belmonte R, Tejero M, Cáceres E. Growth and curve stabilization in girls with adolescent idiopathic scoliosis. Spine. 2005;30(4):411–417. doi: 10.1097/01.brs.0000153397.81853.6a. [DOI] [PubMed] [Google Scholar]
- 74.Gum JL, Asher MA, Burton DC, Lai SM, Lambart LM. Transverse plane pelvic rotation in adolescent idiopathic scoliosis: primary or compensatory? Eur Spine J. 2007 Oct;16(10):1579–86. doi: 10.1007/s00586-007-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burwell RG, Dangerfield PH, Freeman BJ. Concepts on the pathogenesis of adolescent idiopathic scoliosis. Bone growth and mass, vertebral column, spinal cord, brain, skull, extra-spinal left-right skeletal length asymmetries, disproportions and molecular pathogenesis. Stud Health Technol Inform. 2008;135:3–52. [PubMed] [Google Scholar]
- 76.Kotwicki T, Walczak A, Szulc A. Trunk rotation and hip joint range of rotation in adolescent girls with idiopathic scoliosis: does the “dinner plate” turn asymmetrically? Scoliosis. 2008 Jan 19;3:1. doi: 10.1186/1748-7161-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Champain N, Dupuis R, Pomero V, Mouilleseaux B, Dubousset J, Skalli W. Geometric and postural analysis of mild idiopathic scoliotic patients. Stud Health Technol Inform. 2002;91:267–71. [PubMed] [Google Scholar]
- 78.Nault ML, Allard P, Hinse S, Le Blanc R, Caron O, Labelle H, Sadeghi H. Relations between standing stability and body posture parameters in adolescent idiopathic scoliosis. Spine. 2002 Sep 1;27(17):1911–7. doi: 10.1097/00007632-200209010-00018. [DOI] [PubMed] [Google Scholar]
- 79.Chow DH, Kwok ML, Cheng JC, Lao ML, Holmes AD, Au-Yang A, Yao FY, Wong MS. The effect of backpack weight on the standing posture and balance of schoolgirls with adolescent idiopathic scoliosis and normal controls. Gait Posture. 2006 Oct;24(2):173–81. doi: 10.1016/j.gaitpost.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 80.Karski T. Recent observations in the biomedical etiology of so-called idiopathic scoliosis. New classification of spinal deformity- Ist, II-nd and III-rd etiological groups. Studies in Health Technology and Informatics. 2006;123:473–482. [PubMed] [Google Scholar]
- 81.Zabjek KF, Leroux MA, Coillard C, Prince F, Rivard CH. Postural characteristics of adolescents with idiopathic scoliosis. J Pediatr Orthop. 2008 Mar;28(2):218–24. doi: 10.1097/BPO.0b013e3181651bdc. [DOI] [PubMed] [Google Scholar]
- 82.Masciotra S, Picard, Deschepper CF. Cosegregation Analysis in Genetic Crosses Suggests a Protective Role for Atrial Natriuretic Factor Against Ventricular Hypertrophy. Circ Res. 1999;84:1453–1458. doi: 10.1161/01.res.84.12.1453. [DOI] [PubMed] [Google Scholar]
- 83.Duong C, Charron S, Deng Y, Xiao C, Ménard A, Roy J, Deng AY. Individual QTLs controlling quantitative variation in blood pressure inherited in a Mendelian mode. Heredity. 2007;98:165–171. doi: 10.1038/sj.hdy.6800920. [DOI] [PubMed] [Google Scholar]
- 84.Siebenhaar F, Sharov AA, Peters EM, Sharova TY, Syska W, Mardaryev AN, Freyschmidt-Paul P, Sundberg JP, Maurer M, Botchkarev VA. Substance P as an Immunomodulatory Neuropeptide in a Mouse Model for Autoimmune Hair Loss (Alopecia Areata) Journal of Investigative Dermatology. 2007;127:1489–1497. doi: 10.1038/sj.jid.5700704. [DOI] [PubMed] [Google Scholar]
- 85.Ibrahim J, Miyashiro JK, Berk BC. Shear Stress Is Differentially Regulated Among Inbred Rat Strains. Circ Res. 2003;92:1001–1009. doi: 10.1161/01.RES.0000069687.54486.B1. [DOI] [PubMed] [Google Scholar]


