Abstract
Background
Coinfection with hepatitis B virus (HBV) and human immunodeficiency virus (HIV) is common in Africa; however, the impact of HBV infection on the outcomes of antiretroviral therapy programs is unclear. We evaluated the impact of chronic hepatitis B on HIV virologic response, changes in CD4 cell count, hepatotoxicity, and mortality among Africans receiving highly active antiretroviral therapy (HAART).
Methods
We conducted a retrospective cohort study involving a workplace HAART program in South Africa. Participants received HAART according to a protocol and were followed up for up to 72 weeks. On the basis of pre-HAART serum assays, patients were classified as being hepatitis B surface antigen (HBsAg) negative, HBsAg positive with a low HBV DNA level (≤1 × 104 copies/mL), and HBsAg positive with a high HBV DNA level (>1 × 104 copies/mL). The relationships between HBV status and HIV RNA suppression, change in CD4 cell count, mortality, and hepatotoxicity were assessed with use of regression techniques.
Results
Five hundred thirty-seven individuals fulfilled the inclusion criteria; 431 (80.3%) of these patients were HBsAg negative, 60 (11.2%) were HBsAg positive with a low HBV DNA level, and 46 (8.6%) were HBsAg positive with a high HBV DNA level. All groups had similar rates of HIV RNA suppression (P =.61), CD4 cell count increases (P =.75), and mortality (17 total deaths; P =.11) for up to 72 weeks after the initiation of HAART. Baseline transaminase levels were highest in the group with high HBV DNA levels (P =.004). Hepatotoxicity was similar between the HBsAg-negative group and the group with low HBV DNA levels but was higher in the group with high HBV DNA levels (incidence rate ratio, 4.4).
Conclusions
We revealed that HBV status does not affect HIV RNA suppression, CD4 cell count response, or mortality during the first 72 weeks of HAART in an African setting. The risk of HBV-associated hepatotoxicity, however, is associated with the baseline HBV DNA level.
Africa has the largest burden of HIV infection and is a setting where hepatitis B virus (HBV) infection is highly endemic [1]. In Africa, hepatitis B is the leading cause of liver disease and hepatocellular carcinoma. The expansion of antiretroviral therapy (ART) has improved the life expectancy of persons infected with HIV; however, as ART expansion continues, it is important to understand the impact of endemic coinfections, such as HBV infection, on treatment response.
Chronic hepatitis B is associated with liver enzyme level elevations (hepatotoxicity) in HAART recipients [2–8] that are exacerbated by concomitant tuberculosis treatment [7]. However, the impact of chronic hepatitis B on HIV treatment outcomes remains uncertain because of contradictory reports [9–13]. It is unclear whether chronic hepatitis B affects the rate and durability of HIV RNA suppression and increases in CD4 cell count (as has been suggested by a few previous studies [10, 11, 14]) or survival during HAART, especially in Africa. Thus, we evaluated the impact of chronic hepatitis B on the outcomes of HIV virologic response, increases in CD4 cell count, hepatotoxicity, and mortality among patients in a South African ART cohort with a high prevalence of HIV-HBV coinfection.
PATIENTS, MATERIALS, AND METHODS
Patients
Patients included in this study were enrolled in workplace HIV programs at several mining and industrial companies in South Africa and fulfilled the following criteria: (1) initiated HAART from November 2002 through January 2006, (2) received follow-up prospectively until November 2006 or for a maximum of 72 weeks, (3) had pre-HAART serum samples available for testing; and (4) had results of at least 1 follow-up laboratory test available (for CD4 cell count, HIV RNA level, or alanine transaminase [ALT] level). This ART program has been described in detail elsewhere [15]. In brief, HAART eligibility was based on modified World Health Organization criteria, which included a CD4 lymphocyte count <250 cells/mm3, World Health Organization stage 3 HIV infection and a CD4 lymphocyte count <350 cells/mm3, or World Health Organization stage 4 HIV infection. The initial HAART regimen consisted of efavirenz, lamivudine, and zidovudine. Testing for HBV infection was not performed as part of routine care; therefore, individuals with and without hepatitis B did not receive different treatment. Follow-up clinical evaluations were performed every 2 weeks for the first 8 weeks and then monthly. Aspartate aminotransferase (AST) and ALT levels were tested at baseline, at weeks 2 and 6, and then every 3 months. CD4 cell count and HIV RNA level (Amplicor HIV-1 Monitor Test [Roche Diagnostics] or Versant bDNA Test [Bayer Diagnostics]) were determined at baseline, at 6 weeks, and then every 6 months.
HBV laboratory testing
Presence of hepatitis B surface antigen (HBsAg) was determined in specimens that were obtained up to 6 months prior to HAART initiation and stored at −70°C with use of the ADVIA Centaur automated immunoassay system (Bayer Diagnostics). Patients who were positive for HBsAg had a pre-HAART sample tested for quantitative HBV DNA with use of the Versant HBV DNA Quantitative Assay, version 3.0 (Bayer Diagnostics); the lower limit of detection for this assay was 2000 copies/mL. Previous testing for hepatitis C virus antibody provided no positive results for a random sample of 50 patients; thus, no further hepatitis C virus testing was performed.
Definitions
We classified participants according to their HBV status as (1) HBsAg negative, (2) HBsAg positive with a low HBV DNA level (≤1 × 104 copies/mL), and (3) HBsAg positive with a high HBV DNA level (>1 × 104 copies/mL). The cutoff for HBV DNA level was chosen on the basis of reported increased risk for hepatocellular carcinoma and cirrhosis in individuals with HBV DNA levels >1 × 104 copies/mL [16, 17]. Hepatotoxicity was classified on the basis of the AIDS Clinical Trials Network definition of grade 3 or 4 hepatotoxicity, defined as an ALT or AST level >5 times the upper limit of normal. The upper limit of normal was 40 IU/L for both ALT and AST levels. This classification allowed inclusion of patients with missing baseline ALT and AST values but differs in method from a previous study involving this cohort [7]. Because the definition for hepatotoxicity uses either ALT or AST level, we reported the maximum of either value at a visit and termed this the “liver enzyme” level. Death was classified as traumatic or nontraumatic on the basis of company personnel records; details on the cause of death were not available. We limited analysis to deaths classified as nontraumatic. HIV RNA suppression was defined as an HIV RNA level <400 copies/mL.
Analysis
Baseline factors were compared among the 3 groups with use of the Kruskal-Wallis test for continuous variables and the χ2 test for categorical variables. Odds ratios for the association of HBV status with HIV RNA suppression (at follow-up visits at 6, 12, 24, 48, and 72 weeks after HAART initiation) were determined with use of logistic regression with robust variance estimates and generalized estimating equations. Because increases in CD4 cell count during HAART are biphasic [18], we modeled these 2 phases separately. For the first phase, we used linear regression to evaluate the effect of HBV status on the weekly change in CD4 cell count from baseline to 6 weeks after the initiation of HAART. For the second phase, with use of a summary measures approach, linear regression of differences between the CD4 cell count at 6 weeks and CD4 cell counts at subsequent weeks was used to determine the slope for each patient. We then modeled the slopes of individuals with analysis of variance and linear regression to assess differences among the groups.
Mortality rates (number of deaths per 100 person-years) were calculated with the follow-up time starting on the date of HAART initiation and ending on the date of death, November 2006, 72 weeks after HAART initiation, or the date when the patient left the workforce. Poisson regression was used to calculate rates and incidence risk ratios for mortality. Grade 3 or 4 hepatotoxicity rates (number of events per 100 person-years) were calculated from the date of HAART initiation to the date that a grade 3 or 4 event occurred, November 2006, the date of death, the date that a patient left the workforce, or 72 weeks after HAART initiation, whichever came first. Kaplan-Meier plots were used to show cumulative survival without hepatotoxicity by HBV infection group, and Poisson regression was used to assess incidence risk ratios for hepatotoxicity by HBV infection group.
We evaluated univariate associations of the following baseline covariates with each outcome: CD4 cell count, hemoglobin level, log10 HIV RNA level, age, and weight. When we evaluated increases in CD4 cell count, we included the proportion of visits up to and including the visit date when HIV RNA suppression was observed. Data on isoniazid use or combination antituberculosis therapy were not available for many patients and were not included. Multivariate models were constructed with use of a backward stepwise method; HBV status was included in all models, and any other covariates with P <.1 were included in univariate analysis. The sample size was determined by the available population with stored serum samples in the workplace ART program. Stata, version 10.0 (StataCorp), was used for all calculations.
Ethical approval for this study was obtained from the Research Ethics Committee of the Anglo-Gold Health Service, the University of KwaZulu-Natal, the Research Ethics Board of the London School for Hygiene and Tropical Medicine, and the Institutional Review Board at Johns Hopkins University School of Medicine.
RESULTS
Study participants
Five hundred thirty-seven individuals met our inclusion criteria; 431 (80%) of these patients were HBsAg negative, 60 (11%) were HBsAg positive with a low HBV DNA level (≤1 × 104 copies/mL), and 46 (9%) were HBsAg positive with a high HBV DNA level (>1 × 104 copies/mL). Of the 60 patients with a low HBV DNA level, 45 (75%) had an HBV DNA level ≤2000 copies/mL (the level of detection), and 15 (25%) had an HBV DNA level >2000 copies/mL. The median age was 41 years (interquartile range [IQR], 36–46 years), and 393 (94%) of the 417 patients were men. Baseline CD4 cell counts and log10 HIV RNA levels did not differ among the 3 groups; however, baseline liver enzyme levels were higher in the group with high HBV DNA levels than in the other groups (table 1). Follow-up data on any laboratory measurement (CD4 cell count, HIV RNA level, or liver enzyme level) were available for 400 patients (74%) at week 2, 456 (85%) at week 6, 419 (78%) at week 12, 392 (73%) at week 24, 331 (62%) at week 36, 313 (58%) at week 48, and 251 (47%) at week 72. The proportion of patients for whom data were available through week 72 was not significantly different among the groups by baseline CD4 cell count category or by occurrence of grade 3 or 4 hepatotoxicity. However, the group of patients with the lowest percentage of visits during which HIV RNA levels were <400 copies/mL had the fewest week-72 follow-up data. The median number of visits during which liver enzyme levels were available was 7 (mean, 6.2 visits) and did not vary among the groups.
Table 1.
Baseline characteristics of the study population.
| HBsAg-positive patients |
||||
|---|---|---|---|---|
| Characteristic | HBsAg-negative patients (n = 431) | HBV DNA level ≤1 × 104 copies/mL (n = 60) | HBV DNA level >1 × 104 copies/mL (n = 46) | P |
| Sex, proportion (%) of patients | ||||
| Men | 393/417 (94) | 57/59 (97) | 40/42 (95) | .60 |
| Women | 24/417 (6) | 2/59 (3) | 2/42 (5) | |
| Age, years | 41 (36–46) | 42 (36–47) | 40 (36–46) | .51 |
| Weight, kg | 65 (58–70) | 64 (58–73) | 62 (60–67) | .56 |
| CD4 cell count, cells/mm3 | 146 (79–224) | 174 (114–246) | 148 (85–232) | .23 |
| HIV RNA level, log10copies/mL | 4.9 (4.5–5.3) | 4.8 (4.5–5.2) | 5.0 (4.6–5.3) | .45 |
| Maximum ALT or AST level, IU/L | 38 (31–58) | 40 (31–56) | 56 (36–89) | .004 |
| ALT or AST level >ULNa % | 45 | 46 | 70 | .006 |
| Hemoglobin level, g/dL | 12.4 (11–14) | 12.6 (11–13) | 12.5 (11–14) | .92 |
NOTE. Data are median value (interquartile range [IQR]), unless otherwise indicated. ALT, alanine aminotransaminase; AST, aspartate aminotransaminase; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; ULN, upper limit of normal.
The ULN for ALT and AST was 40 IU/L.
HIV RNA suppression
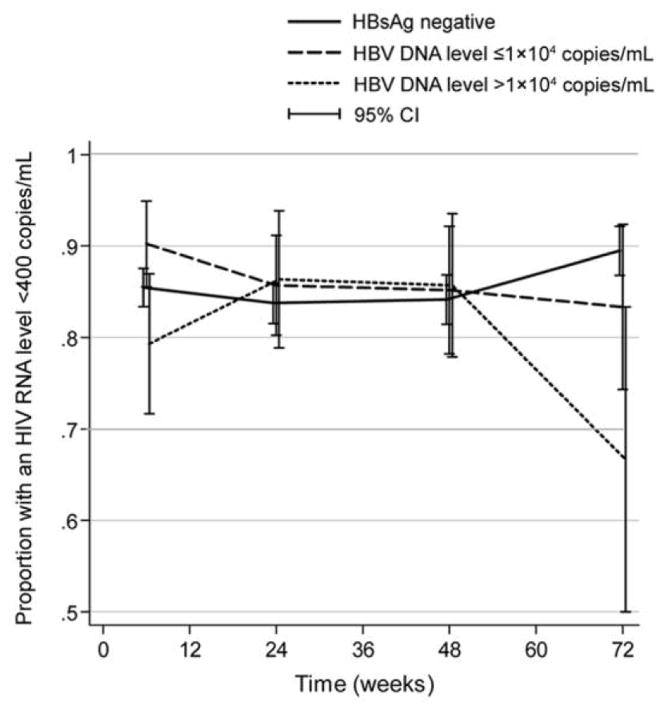
At 6, 24, 48, and 72 weeks, a similar proportion of patients in all of the groups achieved HIV RNA suppression (HIV RNA level, <400 copies/mL) (figure 1). At 24 and 48 weeks, the percentages of patients who had experienced HIV RNA suppression were 84% in the HBsAg-negative group, 85% in the group with low HBV DNA levels, and 86% in the group with high HBV DNA levels (P =.91). At week 72, these percentages were 89%, 83%, and 67%, respectively (P =.11).
Figure 1.
Proportion of patients who achieved HIV RNA suppression (HIV RNA level, <400 copies/mL) at 6, 24, 48, and 72 weeks after initiation of HAART, by hepatitis B virus (HBV) infection group. HBsAg, hepatitis B surface antigen.
With use of repeated measures analysis, the effect of HBV status on HIV RNA suppression over time was similar among the 3 groups; the unadjusted ORs were 1.2 (95% CI, 0.59–2.3) in the group with low HBV DNA levels and 0.81 (95% CI, 0.38–1.7) in the group with high HBV DNA levels (P =.75, overall). Evaluation of associated covariates identified an inverse association between low baseline CD4 cell count and HIV RNA suppression but no other associations. When baseline CD4 cell count was included, the adjusted ORs in the groups were similar to the unadjusted ORs, and HBV status remained unassociated with HIV suppression (P =.61).
CD4 cell count recovery
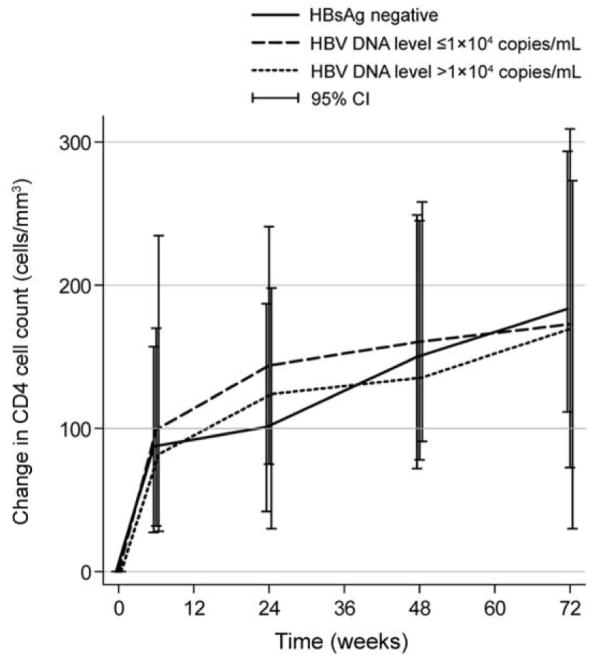
In univariate analysis, the increase in CD4 cell count from HAART initiation to 6 weeks was similar among the groups (figure 2). The estimated increase in CD4 cell count per week was 15.7 cells/mm3 (95% CI, 14–18 cells/mm3) in the group of HBsAg-negative patients, 16.3 cells/mm3 (95% CI, 11–22 cells/mm3) in the group with low HBV DNA levels, and 21 cells/mm3 (95% CI, 15–28 cells/mm3) in the group with high HBV DNA levels (P =.28). Increases were inversely associated with baseline CD4 cell count (P =.02) and baseline hemoglobin level (P =.05); increases were directly associated with baseline log10 HIV RNA level (P < .001). In a multivariate model that included these covariates, the adjusted increases in CD4 cell count (per week) in each group were similar to the unadjusted increases (P =.14, for comparison among the groups).
Figure 2.
Median change in CD4 cell count at 6, 24, 48, and 72 weeks after the initiation of HAART, by hepatitis B virus (HBV) infection group. HBsAg, hepatitis B surface antigen; IQR, interquartile range.
In modeling of the second phase of increases in CD4 cell count, HBV status was not associated with increases in CD4 cell count (figure 2). The estimated increase in CD4 cell count per week was 0.74 cells/mm3 (95% CI, 0.17–1.3 cells/mm3) in the group of HBsAg-negative patients, 2.2 cells/mm3 (95% CI, 1.2–3.2 cells/mm3) in the group with low HBV DNA levels, and 1.4 cells/mm3 (95% CI, −1.9 to 4.9 cells/mm3) in the group with high HBV DNA levels (P =.18). The mean proportion of visits when HIV RNA suppression was observed was strongly associated with changes in CD4 cell count (P < .001). However, including this proportion in a regression model did not change the lack of association between HBV status and changes in CD4 cell count (P =.14).
Hepatotoxicity
At baseline, the median liver enzyme level in the group with high HBV DNA levels was 56 IU/L (P = .004) (table 1). By 48 weeks after the initiation of HAART, the median liver enzyme level decreased in the group with high HBV DNA levels but remained higher than the levels in the other groups (HBsAg-negative group, 33 IU/L [IQR, 27–44 IU/L]; group with low HBV DNA levels, 29 IU/L [IQR, 25–38 IU/L]; group with high HBV DNA levels, 46 IU/L [IQR, 33–56 IU/L]; P =.004).
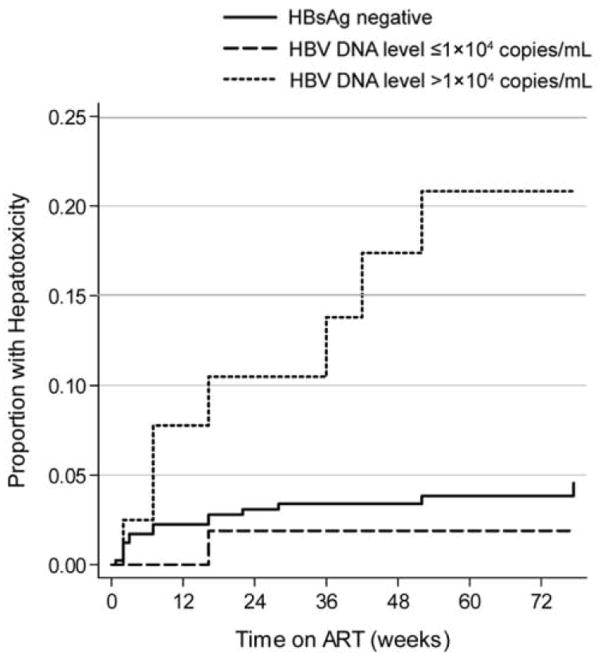
A total of 23 episodes of grade 3 or 4 hepatotoxicity were detected in 23 patients during the observation period. The risk of hepatotoxicity was strongly associated with a high baseline serum HBV DNA level. The rate of hepatotoxicity events was 3.8 events per 100 person-years (95% CI, 2.3–6.2 events per 100 person-years; 15 events) among HBsAg-negative patients, 1.7 events per 100 person-years (95% CI, 0.23–12 events per 100 person-years; 1 event) in the group with low HBV DNA levels, and 17.0 events per 100 person-years (95% CI, 8.3–37 events per 100 person-years; 7 events) in the group with high HBV DNA levels (figure 3). The unadjusted incidence rate ratio was 4.4 (95% CI, 1.9–10) in the group with high HBV DNA levels and 0.42 (95% CI, 0.06–3.1) in the group with low HBV DNA levels, compared with the HBsAg-negative reference group (P < .001).
Figure 3.
Kaplan-Meier graph showing the proportion of patients who experienced episodes of hepatotoxicity, by hepatitis B virus (HBV) infection group. ART, antiretroviral therapy; HBsAg, hepatitis B surface antigen.
Most hepatotoxic events occurred within the first 12 weeks after HAART initiation. However, among patients with high HBV DNA levels, 3 of the 7 episodes occurred 24 weeks after the initiation of HAART. Of the 23 patients who experienced a hepatotoxicity event, 4 had a change in treatment regimen coincident with the event; 2 had single agent substitutions, 1 had a brief interruption of HAART, and 1 discontinued HAART and was subsequently lost to follow-up.
Mortality
A total of 17 nontraumatic deaths (mortality rate, 3.2 deaths per 100 person-years; 95% CI, 2.0–5.2 deaths per 100 person-years) and 6 traumatic deaths occurred during the first 72 weeks of HAART. There was no difference in non-traumatic mortality rates among the groups (3.4 deaths per 100 person-years [95% CI, 2.0–5.8 deaths per 100 person-years] in the HBsAg-negative group, 0 deaths per 100 person-years [95% CI, 0–5.8 deaths per 100 person-years] in the group with low HBV DNA levels, and 4.2 deaths per 100 person-years [95% CI, 0.54–16.1 deaths per 100 person-years] in the group with high HBV DNA levels). The incidence rate ratios for death were 0.24 (95% CI, 0–1.8) in the group with low HBV DNA levels and 1.3 (95% CI, 0.15–5.7) in the group with high HBV DNA levels, compared with the HBsAg-negative group (P = .1). The number of deaths was insufficient for multivariate modeling, and data on the specific cause of death were generally not available.
DISCUSSION
In this South African cohort of HIV-infected workers from several African countries, HBV coinfection did not affect early mortality or response to HAART that included agents active against HBV infection. Even the group with the highest pre-HAART HBV DNA levels achieved increases in CD4 cell count and HIV RNA suppression at rates similar to those among patients who were HBsAg negative. Furthermore, we demonstrated that the risk of HAART-related hepatotoxicity can be stratified by HBV DNA level, expanding the current knowledge in this area.
Other studies have assessed the impact of hepatitis B on response to HAART and had similar findings [10–12]. However, studies from Taiwan found that individuals with chronic hepatitis B were less likely to achieve HIV RNA suppression at 4 [13] and 24 months after the initiation of HAART [14].
CD4 cell recovery during the first 4 and 8 weeks of HAART was slower in patients with chronic hepatitis B than in patients without chronic hepatitis B in a Thai study; however, there was no difference by 48 weeks after the initiation of HAART [10]. Futhermore, increases in CD4 cell count were lower in Italian patients with chronic hepatitis B than in such patients without chronic hepatitis B, both early and up to 36 months after the initiation of HAART [11]. Other studies have reported no difference in CD4 cell recovery by HBV status during the first 12 months of HAART [12]. Our study adds to the understanding of increases in CD4 cell count, because we did not find a difference in increases in CD4 cell count during the first or second phase, regardless of HBV DNA level. If any difference does exist, we believe that stratification by HBV DNA level increased our ability to detect a difference related to HBV status.
Because of the high early mortality reported from African HAART programs, it is important to identify factors associated with death [19, 20]. It is encouraging that we did not find an association between mortality and HBV status during the first 72 weeks of HAART.
As in previous studies, there were increased rates of hepatotoxicity among HBsAg-positive individuals, compared with HBsAg-negative individuals [6, 7, 21–24]. Of interest, the risk of hepatoxicity was increased only among those with the highest serum HBV DNA levels. This finding suggests that hepatotoxicity may be related to the level of HBV replication rather than to only the presence of hepatitis B surface antigenemia. This is important because it indicates that assaying HBsAg alone is insufficient to predict hepatotoxicity or to attribute a liver enzyme level increase to HBV infection. Another import finding is that a large proportion of episodes of hepatotoxicity in the group with high HBV DNA levels occurred after the first 12 weeks of HAART, whereas in HBV-negative patients, hepatotoxicity most commonly occurred during the first few months after HAART initiation. The later hepatotoxic events may be attributable to the development of lamivudine-resistant HBV [25, 26]. An alternative explanation may be that these events were associated with immune recovery inflammatory syndrome. Additional evaluation is needed to understand hepatotoxicity events that occur late after the initiation of HAART and to evaluate the long-term clinical consequences.
Our findings should be considered in the context of several limitations. First, the cohort is made up of industrial and mine workers; thus, the level of general illness and malnutrition in our setting may be less than that in other low-income settings. However, the fact that the participants were part of a work program is a strength because of the excellent data collection and routine laboratory testing. Furthermore, accurate records were maintained on patient mortality, because death of an employee is reported to the personnel office for reasons of both salary to workers and compensation to family members. Second, because this cohort primarily consisted of men, the results do not necessarily extend to women. Third, although 20% of the study cohort was positive for HBsAg, the small number of deaths and hepatotoxic events limited power to assess multiple factors associated with HBV status. Fourth, patients were classified as having hepatitis B on the basis of a single HBsAg test; the standard definition is based on 2 HBsAg tests performed ≥6 months apart [27]. We believe that little misclassification occurred, because HBV transmission usually occurs during childhood in southern Africa; thus, it is unlikely that the individuals identified as HBsAg positive had acute HBV infection. Fifth, although tuberculosis therapy has been associated with hepatotoxicity in 1 population [7], we were unable to determine the impact of tuberculosis therapy in this study, because such data were not available for the majority of patients. Because it is likely that tuberculosis therapy did not differ by group, we do not believe that having these data would alter our results. Sixth, even with a sample size sufficiently large to find clinically meaningful differences, it is possible that small differences between groups might not be detected with precision; thus, we cannot exclude a small difference in viral load suppression by group.
In conclusion, in an African setting with a high prevalence of chronic hepatitis B (20%), chronic HBV coinfection does not alter response to HAART or increase mortality during the first 18 months of therapy and, thus, should not deter clinicians from initiating HAART. Of note, the risk of hepatotoxicity was highest among the HBV-infected patients with high HBV DNA levels and occurred late after the initiation of HAART. Thus, monitoring of liver enzyme levels may be important even after the first few months of HAART for individuals with high HBV DNA levels (if known). However, studies of the cost effectiveness of HBV screening and long-term liver enzyme monitoring for those with high HBV DNA levels in low-income settings is an area that requires further evaluation. In addition, additional follow-up is needed to assess the long-term impact of treatment (including HBV-suppressive and nonsuppressive HAART regimens), outcome, and mortality among individuals coinfected with HIV and chronic HBV.
Acknowledgments
Financial support. National Institutes of Health (AI5535901, DK074348 to C.J.H., AI60449 and AI71820 to C.L.T., and AI5535901 and AI016137 to R.E.C.), the Aurum Institute, Bristol Myers Squibb Virology Fellows Research Program 2005–2006 (to C.J.H.), UK Department of Health Public Health Career Scientist Award (to A.D.G.), and Johns Hopkins University Center for AIDS Research.
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7:402–9. doi: 10.1016/S1473-3099(07)70135-4. [DOI] [PubMed] [Google Scholar]
- 2.Wit FWM, Weverling GJ, Weel J, Jurriaans S, Lange JMA. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis. 2002;186:23–31. doi: 10.1086/341084. [DOI] [PubMed] [Google Scholar]
- 3.Thio CL, Seaberg EC, Skolasky RL, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter AIDS Cohort Study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 4.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 5.Nunez M, Soriano V. Hepatotoxicity of antiretrovirals. Drug Safety. 2005;28:53–66. doi: 10.2165/00002018-200528010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996–2001. AIDS. 2003;17:2191–9. doi: 10.1097/00002030-200310170-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS. 2007;21:1301–8. doi: 10.1097/QAD.0b013e32814e6b08. [DOI] [PubMed] [Google Scholar]
- 8.Puoti M, Torti C, Ripamonti D, et al. Severe hepatotoxicity during combination antiretroviral treatment: incidence, liver histology, and outcome. J Acquir Immune Defic Syndr. 2003;32:259–67. doi: 10.1097/00126334-200303010-00004. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Torres M, Gonzalez-Garcia J, Brau N, et al. Occult hepatitis B virus infection in the setting of hepatitis C virus (HCV) and human immunodeficiency virus (HIV) co-infection: clinically relevant or a diagnostic problem? J Med Virol. 2007;79:694–700. doi: 10.1002/jmv.20836. [DOI] [PubMed] [Google Scholar]
- 10.Law WP, Duncombe CJ, Mahanontharit A, et al. Impact of viral hepatitis co-infection on response to antiretroviral therapy and HIV disease progression in the HIV-NAT cohort. AIDS. 2004;18:1169–77. doi: 10.1097/00002030-200405210-00010. [DOI] [PubMed] [Google Scholar]
- 11.de Luca A, Bugarini R, Lepri AC, et al. Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med. 2002;162:2125–32. doi: 10.1001/archinte.162.18.2125. [DOI] [PubMed] [Google Scholar]
- 12.Konopnicki D, Mocroft A, De WS, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593–601. doi: 10.1097/01.aids.0000163936.99401.fe. [DOI] [PubMed] [Google Scholar]
- 13.Sheng WH, Chen MY, Hsieh SM, et al. Impact of chronic hepatitis B virus (HBV) infection on outcomes of patients infected with HIV in an area where HBV infection is hyperendemic. Clin Infect Dis. 2004;38:1471–7. doi: 10.1086/420744. [DOI] [PubMed] [Google Scholar]
- 14.Sheng WH, Kao JH, Chen PJ, et al. Evolution of hepatitis B serological markers in HIV-infected patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;45:1221–9. doi: 10.1086/522173. [DOI] [PubMed] [Google Scholar]
- 15.Charalambous S, Grant AD, Day JH, et al. Establishing a workplace antiretroviral therapy programme in South Africa. AIDS Care. 2007;19:34–41. doi: 10.1080/09500340600677872. [DOI] [PubMed] [Google Scholar]
- 16.Chen CJ, Yang H-I, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 17.Iloeje UH, Yang H-I, Su J, Jen C-L, You S-L, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–86. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Goicoechea M, Smith DM, Liu L, et al. Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: association of immune activation, T cell maturation markers, and cellular HIV-1 DNA. J Infect Dis. 2006;194:29–37. doi: 10.1086/504718. [DOI] [PubMed] [Google Scholar]
- 19.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368:1254–9. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 20.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1–infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 21.den Brinker M, Wit FW, Wertheimvan Dillen PM, et al. Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS. 2000;14:2895–902. doi: 10.1097/00002030-200012220-00011. [DOI] [PubMed] [Google Scholar]
- 22.Nunez M. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol. 2006;44:S132–9. doi: 10.1016/j.jhep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology. 2002;35:182–9. doi: 10.1053/jhep.2002.30319. [DOI] [PubMed] [Google Scholar]
- 24.Wit FW, Weverling GJ, Weel J, Jurriaans S, Lange JM. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis. 2002;186:23–31. doi: 10.1086/341084. [DOI] [PubMed] [Google Scholar]
- 25.Benhamou Y, Bochet M, Thibault V, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus–infected patients. Hepatology. 1999;30:1302–6. doi: 10.1002/hep.510300525. [DOI] [PubMed] [Google Scholar]
- 26.Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30:567–72. doi: 10.1002/hep.510300221. [DOI] [PubMed] [Google Scholar]
- 27.EASL. Consensus statement (short version). J Hepatol; International Consensus Conference on Hepatitis B; 13–14 September, 2002; Geneva, Switzerland. 2003. pp. 533–40. [DOI] [PubMed] [Google Scholar]