Abstract
Apelin, a novel peptide originally isolated from bovine stomach tissue extracts, is widely but selectively distributed throughout the nervous system. Vasopressin and oxytocin are synthesised in the magnocellular neurons of the hypothalamic supraoptic (SON) and paraventricular nuclei (PVN), which are apelin-rich regions in the central nervous system. We made extracellular electrophysiological recordings from the transpharyngeally exposed SON of urethane-anaesthetised rats to assess the role of apelin in the control of the firing activity of identified magnocellular vasopressin and oxytocin neurons in vivo. Apelin-13 administration onto SON neurons via microdialysis revealed cell-specific responses; apelin-13 increased the firing rates of vasopressin cells, but had no effect on the firing rate of oxytocin neurons. A direct excitatory effect of apelin-13 on vasopressin cell activity is also supported by our in vitro studies showing depolarisation of membrane potential and increase in action potential firing. To assess the effects of apelin-13 on somato/dendritic peptide release we used in vitro release studies from SON explants in combination with highly sensitive and specific radioimmunoassays. Apelin-13 decrease basal (by 78%, p<0.05, n=6) and potassium-stimulated (by 57%, p<0.05, n=6) vasopressin release but had no effect on somato/dendritic oxytocin release.
Taken together, our data suggest a local autocrine feedback action of apelin on magnocellular vasopressin neurons. Furthermore, these data show a marked dissociation between axonal and dendritic vasopressin release with a decrease in somato/dendritic release but an increase in electrical activity at the cell bodies, indicating that release from these two compartments can be regulated wholly independently.
Keywords: hypothalamus, posterior pituitary, dendritic release, APJ
Introduction
Immunohistochemical and in situ hybridisation studies have shown that apelin, a peptide originally isolated from bovine stomach tissue extracts (1), is widely distributed throughout the body, as well the central nervous system (2-8). Apelin is derived from a 77-amino acid precursor, pre-pro-apelin, which is processed to several different forms in different tissues (4;7). Those reported to have the highest in vitro activity on the G-protein-coupled, seven-transmembrane domain apelin receptor (APJ) are apelin-13 and apelin-17 (3;9). The highly conserved structure of apelin and its receptor between species has prompted suggestions of important physiological functions for apelin and, so far, roles in pressure/volume homeostasis, modulating pituitary hormone release, food and water intake, and stress and immune system activation have been described (reviewed by (10-17).
Like APJ, apelin is found in a variety of brain areas including hippocampus, cerebellum, striatum and hypothalamus (4-6;8;11;18;19). Some of the most apelin-rich regions in the brain, with a dense network of apelin-immunoreactive nerve cell bodies, dendrites and axons, are the vasopressin and oxytocin synthesising magnocellular neurons of the supraoptic (SON) and paraventricular nuclei (PVN) of the hypothalamus (9;20). The neuropeptides are released from axon terminals in the neural lobe of the pituitary and from somata and dendrites within the hypothalamus. Vasopressin and oxytocin secretion from the pituitary into the general circulation plays a considerable role in the physiological regulation of fluid homeostasis and reproduction. Oxytocin and vasopressin released from dendrites act as retrograde signals that modulate synaptic transmission, electrical activity and, in some cases, morphology of the cell of origin (reviewed in (21;22)).
Initially a subpopulation of apelin-immunoreactive magnocellular neurons were shown to be oxytocinergic, as indicated by their signal for neurophysin I (9). However, a recent paper has shown that almost all apelin-immunoreactive cells are immunopositive for vasopressin; although the apelin-immunoreactive cells represented only a small subset of vasopressin neurons. Although both were found in the cytoplasm, the apelin and vasopressin signals were not co-localised within the neurons. Moreover, a 48 hour dehydration stimulus applied in vivo depleted the somato-dendritic vasopressin signal while resulting in an increased apelin signal in both the number of apelin positive cells as well as labelling density, suggesting that the two peptides might be stored in, and therefore differentially released from, two distinct vesicular pools within the same cells (23). In addition to apelin, APJ mRNA is expressed in magnocellular neurons (3;18), suggesting a local role of apelin/APJ in regulating vasopressin neurons.
However, contrasting effects of apelin in regulating vasopressin release and drinking behaviour have been reported. Intracereboventricular (i.c.v.) injection of apelin-17 decreased the electrical activity of vasopressin cells in lactating animals (24). I.c.v. injection of apelin-13 or apelin-17 inhibited basal and dehydration-induced release of vasopressin and water intake in mice and rats (18), suggesting an inhibitory role of apelin in the regulation of vasopressin release. Two other studies observed regulatory actions of apelin on fluid homeostasis; apelin-13 administered i.c.v. dose-dependently increased water intake (5;25) or had no effect on water intake in sated or water-deprived rats (26).
Therefore, the aim of this study was to determine the direct effects of apelin-13 on the electrical activity and somato/dendritic peptide release of vasopressin and oxytocin neurons.
Material and Methods
Animals
Sprague-Dawley control (Bantin & Kingman, UK) or Wistar rats expressing a vasopressin-enhanced green fluorescent protein (vasopressin-eGFP (27-29)) were used in accordance with the UK Animals (Scientific Procedures) Act, 1996. Experiments conducted in Japan were in accordance with guidelines on the use and care of laboratory animals as set out by the Physiological Society of Japan and was approved by the Ethics Committee of Animal Care and Experiments of the University of Occupational and Environmental Health, Kitakyushu, Japan. Only the minimum number of rats necessary to produce reliable scientific data was used. All rats were housed under controlled conditions (12 h light: 12 h dark, 21 C) with free access to food and water.
Immunocytochemistry
Adult (250-300 g) random cycling female rats were deeply anaesthetised (Sagatal, 0.6ml, intraperitoneal) then perfused through the ascending aorta first with heparin (5000 U/ml; 300 ml) in 0.9% saline solution followed by 300 ml of a 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The brain and pituitary were carefully removed and immersed overnight in a solution of 0.2% paraformaldehyde and 15% sucrose in 0.1 M PB at 4 C. The tissue was then placed in a solution of 30% sucrose in 0.1 M PB and left at 4 C until the tissue had sunk (usually 48 h). Tissue sections (40 μm) were cut with a freezing microtome and rinsed in 0.1 M PB. Sections were first incubated for 30 min at room temperature in a blocking buffer consisting of 1% BSA + 0.2% Triton X-100 in 0.1 M PB. Sections were incubated for 60 min at room temperature then 48 h at 4 C with primary antibodies against the apelin receptor (APJ, rabbit polyclonal, diluted 1:50, Santa Cruz, UK, (30)), plus glial fibrillary acidic protein (GFAP, mouse monoclonal, IMGENEX; diluted 1:200) or oxytocin (neurophysin-oxytocin PS38, mouse monoclonal, diluted 1:5000) or vasopressin (neurophysin-vasopressin PS41 mouse monoclonal; diluted 1:5000). The vasopressin and oxytocin antibodies were a kind gift of Professor H. Gainer. After rinsing in 0.1 M PB, sections were incubated for 60 min at room temperature in secondary antibodies against rabbit IgG conjugated with Alexa 488 and mouse IgG Alexa 568 (each diluted 1:1000, Molecular Probes, Eugene, OR, USA). Both primary and secondary antibodies were diluted in blocking buffer. The sections were mounted in a Mowiol mounting medium (Calbiochem, San Diego, CA, USA), supplemented with 2.5% DABCO (Sigma) and fluorescence immunoreactivity observed with a Leica TCS-NT microscope equipped with an Argon/Krypton laser. Emissions from each fluorescent secondary antibody were acquired consecutively to ensure no cross-talk from one channel to another. No fluorescent labelling was detected when primary antibodies were omitted. Images were acquired and viewed using Leica Confocal Software (Leica Microsystems, Heidelberg, Germany).
In vivo electrophysiology
Female Sprague-Dawley rats were anaesthetised with intraperitoneal injection of urethane (1.25 g/kg); a femoral vein and the trachea were cannulated and the pituitary stalk and right SON were exposed transpharyngeally as described previously (31). In some animals an additional i.c.v. cannulae was implanted. A home-made U-shaped dialysis probe (molecular cut-off 6000Da, Spectra/Por RC Hollow Fibers®, Spectrum Med. Inc.) was bent to position the loop of the membrane flat onto the exposed ventral glial lamina of the SON after removal of the meninges (32). A glass micropipette (filled with 0.15 M NaCl, 20-40 MΩ) was introduced into the centre of the loop of the dialysis probe to record the extracellular activity of single neurons in the SON. A bipolar stimulating electrode (SNEX-200X, Clarke Clarke Electromedical Instruments, Reading, UK) was placed on the pituitary stalk and set to deliver single matched biphasic pulses (1 ms, <1 mA peak to peak) for antidromic identification of SON neurons. Oxytocin neurons were distinguished from vasopressin neurons by their firing pattern and by their opposite response to i.v. cholecystokinin (CCK, 20 μg/kg, cholecystokinin-(26-33)-sulphated, Bachem Ltd., Saffron Walden, Essex, UK), i.e. transient excitation of oxytocin neurons and no effect or short-term inhibition of vasopressin neurons (33). Artificial cerebrospinal fluid (aCSF, pH 7.2, composition in mM: NaCl 138, KCl 3.36, NaHCO3 9.52, Na2HPO4 0.49, urea 2.16, CaCl2 1.26, MgCl2 1.18) was dialysed at 3 μl/min throughout the experiment. During recording, dialysis fluid was changed to aCSF containing apelin-13 fragment (1 μg/μl) for 30 min. Drugs administered by microdialysis in this way penetrate only a short distance into the brain - the concentrations achieved 0.5-1 mm below the surface of the brain are about 3 to 4 orders of magnitude below the dialysate concentration over this duration of infusion (32). In some animals apelin-13 was given i.c.v. at a dose of 0.1 μg/2 μl. At the end of each experiment the rats were killed by overdose of pentobarbitone anaesthetic (60 mg/kg, i.v.). The firing rates of cells were recorded using Spike2 software (Cambridge Electronic Design, Cambridge, UK) and interfaced to a personal computer. The mean firing rate (spikes/s) and the activity quotient (proportion of time in which a cell is active) were analysed for 10 min intervals before, during and after drug treatment.
In vitro electrophysiology
Recordings of apelin-induced currents were made in magnocellular neurons from isolated neuron and brain slice preparations. Neurons were isolated as previously described (34) with the following minor modifications. In brief, 600 μm thick coronal hypothalamic slices, including the SON, were prepared in constantly O2 bubbled ice-cold Locke solution (in mM: NaCl 140, KCl 5, Hepes 10, Glucose 10, KH2PO4 1.2, MgSO4 1.2 and CaCl2 1.8, adjusted to pH 7.4 with NaOH and osmolarity 295-300 mOsm) from brains removed from 60-80 g vasopressin-eGFP or wild type rats. Punches (1.8mm in diameter) were made of the SON, transferred to a conical container and incubated in O2-bubbled Locke solution containing DNAse (0.5 mg/ml) and Protease X (1 mg/ml) for 20 min at room temperature. This solution was replaced with one containing DNAse (0.5 mg/ml) and Protease XIV (1 mg/ml) and incubated for a further 20 min at room temperature. The tissue punches were washed with normal Locke buffer for at least 2 h before gentle manual dispersal of cells with fire-polished Pasteur pipettes. The use of tissue punches was observed to result in a far greater number of healthy cells with less cellular debris and non-magnocellular neurons than using SONs dissected from the brain. The cell suspensions were plated onto glass-bottom culture dishes (HBSt or GWSt-3522 series; 22 mm diameter, 0.17 mm thickness; WillCo Wells BV, Amsterdam, The Netherlands) with custom-made polycarbonate inserts to allow rapid perfusion. The eGFP-expressing isolated magnocellular neurons were identified by their green fluorescence and cells were patched under visible light at room temperature.
Recordings were also made in 250μm hypothalamic slices containing SONs. The slices were prepared as above, without enzyme treatment, from wild-type Sprague Dawley rats as well as vasopressin-eGFP rats. Slices were allowed to rest for at least 60min in oxygenated Locke solution at room temperature before being transferred to a heated perfusion chamber mounted on a Zeiss Axioskop epifluorescence microscope. Again, eGFP cells were identified using their green fluorescence and SON magnocellular neuron cell bodies were patched using infrared-DIC optics. Images were acquired using a Hamamatsu Orca-ER camera controlled by Simple PCI software (Digipixel). Patch electrodes were fabricated from borosilicate capillary tubing of 1.5 μm diameter (Garner Glass Co., Claremont, CA). The pipette solution contained (in mM) 134 potassium methanesulfonate, 5 KCl, 0.1 K-EGTA, 5 Na-HEPES, 3 Mg-ATP, and 0.4 Na-GTP (pH 7.4) (4–6 MΩ). Membrane potentials were recorded in current-clamp mode, with a patch-clamp amplifier (Axopatch 200B; Axon Instruments, Union City, CA). Recorded signals were low-pass-filtered at 1–2 kHz, digitized at 2–5 kHz with an A/D converter (Digidata 1322A; Axon Instruments), and analyzed with the pClamp software (Axon Instruments).
Calcium imaging from isolated magnocellular neurons
Magnocellular neurons from wild type rats were enzymatically treated as described above. The tissue pieces were then rinsed with normal Locke solution and maintained in oxygenated normal Locke solution at room temperature until dissociated mechanically by gentle titration. The resulting cell suspension was gently mixed with a solution containing Fura-2/AM and pluoronic acid in normal Locke buffer to give a final concentration of 1 μM and 0.01% respectively. The cell suspension was then plated onto clean poly-L-lysine treated coverslips (22 mm diameter No. 0: Merk) which formed the base of heated perfusion chambers for [Ca2+]i measurements. After 30 min at room temperature, the coverslips were gently rinsed to remove unattached cells and unincorporated dye. After a further 30 min incubation at room temperature to allow de-esterification of the Fura-2/AM, perfusion chambers were placed on the temperature-controlled stage of an inverted microscope (Nikon Diaphot TMD) and cells were perfused with oxygenated normal Locke solution or test solutions as described below at 1 ml/min at 35 C. Cells were viewed with a 40x CF fluorescence objective (numerical aperture 1.3), and putative magnocellular neurons were chosen on the basis of size (soma diameter of ~15 μm) and morphology. The Fura-2 was excited at 340 and 380 nm and emission (520 nm) detected and displayed (ImageMaster Pro software; Photon Technology International, New Jersey). Images were collected at 3 Hz, alternating between the two excitation wavelengths. Data were collected from defined regions of interest within the neurons. Fluorescence values were corrected for background and dark current and [Ca2+]i was calculated from the ratio between 340 and 380 nm recordings using a standard curve derived from a Fura-2 Imaging Calibration Kit (Molecular Probes).
In vitro release experiments
For the in vitro release experiments female Sprague-Dawley rats (100-150 g) were killed by decapitation and the neurohypophysis, devoid of the pars intermedia, and both SON, were quickly removed and placed in normal Locke solution as previously described (35). Release was measured from either individual neurohypophyses, or from four SONs kept in chambers containing normal Locke media at 37 C. This media was removed and replaced every 5 min for 30-45 min before collecting any samples, to achieve stable basal levels. Following this equilibration period, the media samples were retained and stored at -20 C. At time periods described below, the normal Locke solution was replaced with either a high potassium (K+; in mM 50 KCl, NaCl 90, Hepes 10, Glucose 10, KH2PO4 1.2, MgSO4 1.2 and CaCl2 1.8, adjusted to pH 7.4 with NaOH and osmolarity 295-300 mOsm) solution, apelin-13 (50 nM), or a combination of apelin-13 and high K+. The evoked vasopressin and oxytocin release during each period was calculated by subtracting the release under basal conditions (five times the mean of the three samples before the stimulus was applied) from that observed during, and directly after, the stimulus (a total of five fractions).
Radioimmunoassay
Vasopressin and oxytocin released from the explants was assayed as described before (oxytocin: (34); vasopressin: (36)), with antibodies kindly supplied by Dr. R. J. Bicknell, (Babraham Institute, Cambridge, UK). The final antibody dilution for the vasopressin and oxytocin antibodies was 1:140000 and 1:30000 respectively. The oxytocin and vasopressin radioimmunoassays had sensitivities of 0.5 pg and 1.0 pg, respectively. The inter- and intra-assay coefficients of variation were 5-7 %.
Drugs and Reagents
Apelin-13 was bought from Peptide Institute Inc. (Osaka, Japan) and Phoenix Pharmaceuticals (CA, U.S.A.). Apelin-13(F13A) was also obtained from Phoenix Pharmaceuticals. All other reagents were purchased from Sigma unless otherwise stated.
Statistical analysis
Statistical analyses were performed using SigmaStat® software package (SigmaStat software, Systat Software Inc., Richmond, CA, USA). Data were analysed by t-tests (paired or unpaired as appropriate after passed normality tests) or, when appropriate, by ANOVA followed by Student-Newman-Keuls post-hoc tests. All values are expressed as mean ± S.E.M., and differences were considered significant at P ≤ 0.05.
Results
APJ receptor distribution in the SON and pituitary
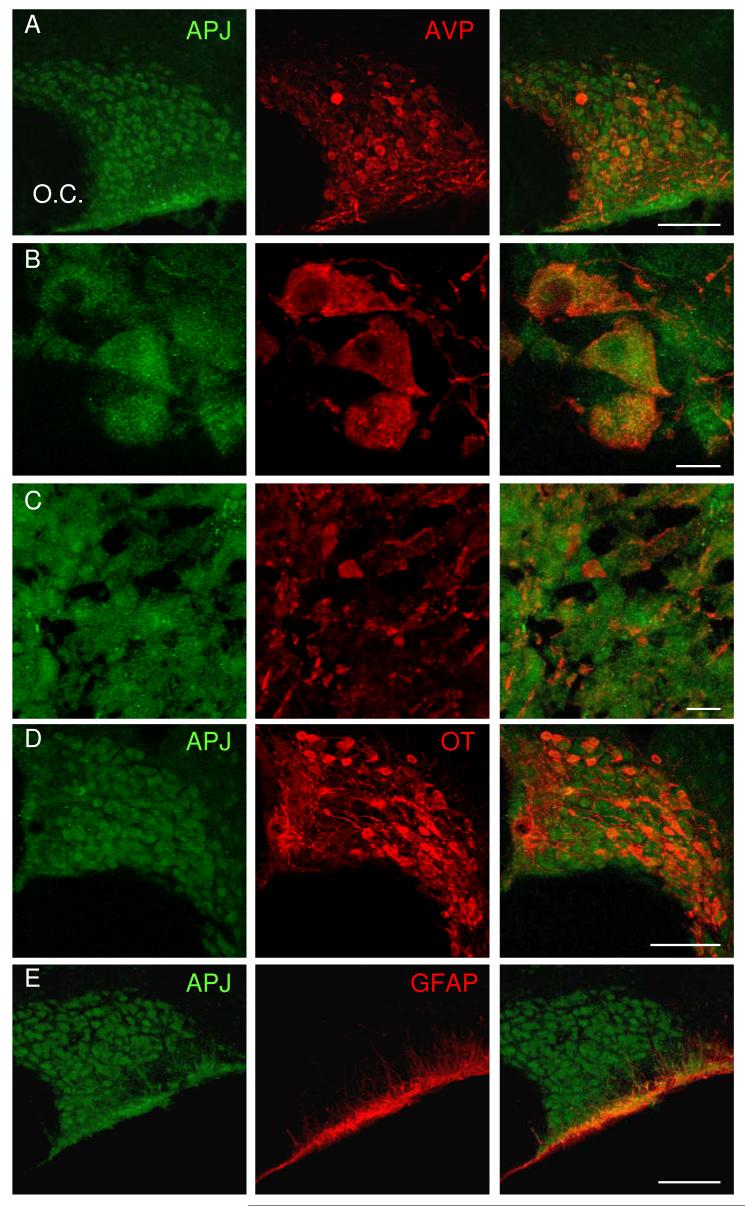
Hypothalamic and pituitary sections were double-labelled with immunofluorescence using antibodies against vasopressin, oxytocin or glial fibrillary acidic protein (VP, OT and GFAP respectively, red) and the apelin receptor (APJ, green). Confocal images showed APJ immunoreactivity in the somata, dendrites, axon fibres and axon terminals of magnocellular vasopressin and oxytocin neurons (Fig 1A-D). APJ immunoreactivity was also seen in the ventral glial lamina (Fig. 1E) and in some cells of the anterior pituitary (not shown).
Fig. 1. Immuno-visualisation of APJ in the SON and pituitary.
A) Confocal images from hypothalamic and pituitary sections double-labelled with immunofluorescence antibodies against vasopressin, oxytocin or glial fibrillary acidic protein (VP, OT and GFAP respectively, red) and the apelin receptor (APJ, green). APJ immunoreactivity was found in somata/dendrites of vasopressin A, B) and oxytocin neurons in the SON (D) and axon terminals (C) in the neural lobe of magnocellular neurons. Immunoreactivity was also seen in the ventral glial lamina of the SON (E). Scale bars in A, D and E are 100μm and B-C are 10μm.
In vivo electrophysiology
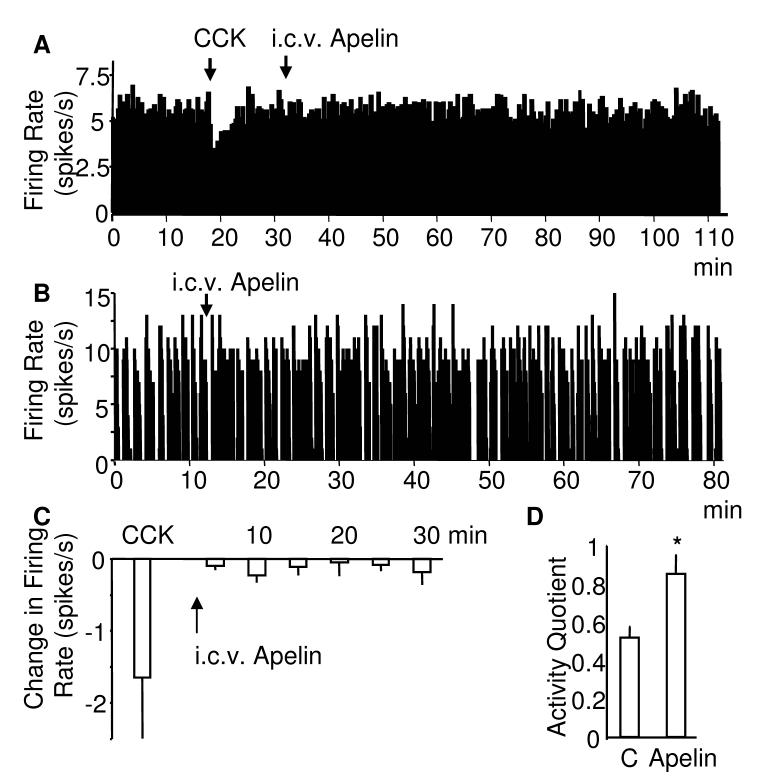
Experiments to determine the direct effects of apelin-13 on magnocellular neurons were carried out on 8 identified vasopressin and 7 oxytocin neurons. Microdialysis administration of apelin-13 onto the supraoptic nucleus (one neuron/rat) excited all vasopressin neurons; characterised by their frequency (spikes/s) and activity quotient (calculated for the 5 min periods before and during apelin treatment). All continuously active cells were identified by their response to i.v. CCK (33) showing an increase in the firing rate in oxytocin (to 173% ± 16.7% of control) and a decrease in the firing rate in vasopressin cells (to 76.2% ±9.17% of control, p<0.01).
Four of the vasopressin cells were continuously active (5.54 ± 1.29 spikes/s) and four showed phasic firing patterns (activity quotient: 0.68 ± 0.10; intraburst firing rate 7.0 ± 0.63 spikes/s). The activity of the continuous cells rose to 7.0 ± 1.15 spikes/s (p<0.05, Fig 2B) after administration of apelin-13. The activity quotient in the phasic cells increased to 0.87 ± 0.07 (p<0.05, Fig 2C) and the intraburst firing rate increased to 7.93 ± 0.96 spike/s (p<0.05) within 20-30 min of apelin-13 administration. In contrast, retrodialysis of apelin-13 tended to decrease the electrical activity of oxytocin neurons which, however, did not reach statistical significance (4.47 ± 1.16 spikes/s before and 3.13 ± 1.1 spikes/s after apelin, Fig 2D, E).
Fig. 2. Effects of retrodialysis of apelin on the electrical activity on supraoptic neurons in vivo.
Examples of extracellular recordings of the electrical activity of A) vasopressin and C) oxytocin cells. Apelin-13 was administered by microdialysis administration (retrodialysis) over 30 min. Phasic vasopressin cells B) show an increase in their activity quotient (n=4) and continuously active vasopressin cells D) show an overall increase in firing rate (n=4) E) Oxytocin neurons (n=7) were not significantly affected by the apelin-13 treatment. All continuously active cells were identified by their response to i.v. CCK showing a decrease in the firing rate in vasopressin D) and an increase in the firing rate in oxytocin cells C,E). O.C. optic chiasm, Mean ± S.E.M, *p<0.05.
Since the effects of apelin-13 on vasopressin cells observed here were in contrast with a previous report using i.c.v. injection of apelin-17 in lactating rats (24), in nine experiments we administered apelin-13 i.c.v. and recorded from five continuously active and four phasic active vasopressin cells. There was no change in the activity of the continuous active vasopressin cells within the time of recording (Fig 3A, C) but a significant increase in the activity quotient of the phasic cells (from 0.52 ± 0.06 to max 0.86 ± 0.09, p<0.05, Fig 3B, D). The intraburst firing rate did not change significantly in the phasic cells (from 9.29 ± 1.45 spikes/s to 9.86 ± 1.91 spikes/s) within 30 min after apelin-13 administration.
Fig. 3. Effects of i.c.v. apelin on the electrical activity on supraoptic vasopressin neurons in vivo.
Examples of extracellular recordings of the electrical activity of A) continuous and B) phasic active vasopressin cells after intracebroventricular (i.c.v.) injection of apelin-13. Whereas continuous active vasopressin cells C) show no change in firing rate (n=4), phasic vasopressin cells D) showed a significant increase in their activity quotient (n=4). Mean ± S.E.M., *p<0.05.
Effect of apelin on membrane potential and currents in isolated neurons and hypothalamic brain slices
The effects of apelin-13 on membrane potentials and ionic currents of SON neurons were studied by the whole cell patch-clamp technique. Initially studies were made using dissociated SON neurons from heterozygous GFP-vasopressin rats (27). In voltage-clamp mode, cells were held at -40 mV and stepped from -120 to -40 mV in 10 mV increments, each step over 200 ms. All GFP-positive neurons were observed to have a linear current-voltage relationship ie. no hyperpolarisation-induced inward rectification at potentials more negative than -90 mV or a sustained outward rectification at potentials more positive than -70 mV (data not shown). This was consistent with previous studies identifying SON magnocellular neurons with a linear current-voltage relationship to express vasopressin by post-hoc immunocytochemistry (37). Nearly all of the GFP negative cells showed an outward rectification, indicating them to be oxytocin cells. Thus, later studies using non-GFP vasopressin rats utilised the presence or absence of an outward rectification in response to this protocol to identify oxytocin and vasopressin neurons respectively.
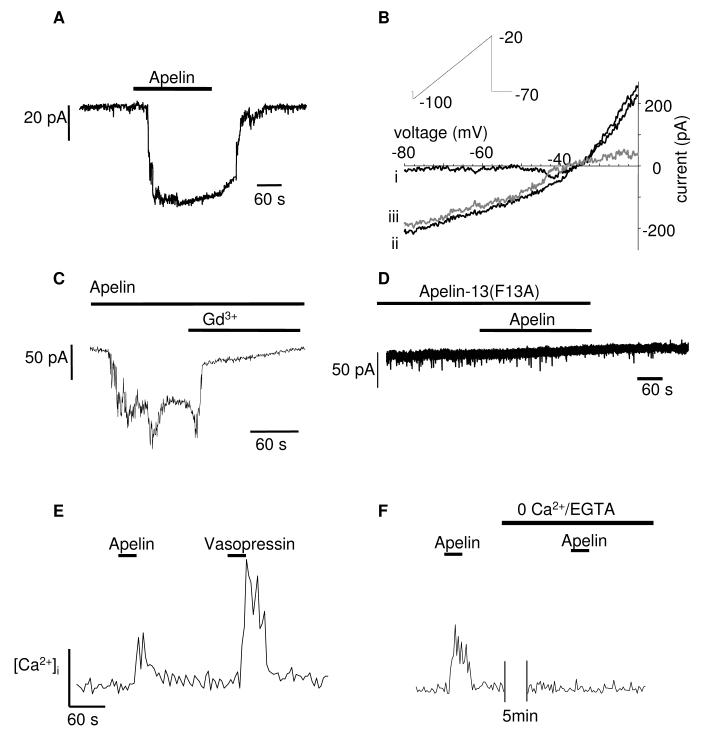
In voltage-clamp mode, all isolated vasopressin neurons (n=10, a typical example is shown in Fig. 4A) showed inward currents, on average 55 ± 16.5 pA in amplitude in response to apelin-13 (50 nM, 3 min), when cells were clamped at -70 mV. The response to apelin-13 was rapid, lasted at least the duration of time that apelin-13 was applied, lasting on average 5.5±1.5 min and was readily reversed upon washing out (Fig. 4A). The identity of the channel(s) carrying the apelin-evoked inward current was investigated using ramp voltage protocols, stepping from -70 mV to -100 mV, and then ramping up to -20 mV over 4 s. These were applied three times before and during apelin-13 treatment. The currents used to calculate the reversal potential were those collected during the maximum evoked current. The reversal potential of the apelin-induced current was -32 ± 2 (n=5, Fig. 4B). The apelin-induced current was also completely blocked by the cation channel blocker gadolinium (Gd3+, 0.1 mM, n=5, Fig. 4C). The peptide apelin-13(F13A) has been described as an APJ-specific antagonist from its effects in vivo in blocking the hypotensive action of apelin-13 (39). In our in vitro experiments, apelin-13(F13A) (0.1 mM) applied before (10 min) and during exposure to apelin-13 blocked the apelin-induced inward current, indicating it was also effective in vitro (n=5, Fig. 5B).
Fig. 4.
Effects of apelin on vasopressin neurons in vitro: A-D) Examples showing effect of applying apelin-13 (50 nM) to isolated vasopressin neurons. A) In voltage-clamp mode VP neurons were held at -70mV and apelin-13 evoked a reversible inward current which lasted at least as long as apelin-13 was applied. B) Using a ramp protocol described in the inset, the whole cell current recorded before apelin was applied (i), was subtracted from that recorded after (ii) and thus the apelin-induced current was derived (iii, grey line). The average apelin-13-induced current had a reversal potential of -32 ± 2 mV (n=5). C) The apelin-13-induced current was also shown to be Gd3+ (0.1 mM, n=5) sensitive, suggesting a non-specific cation current. D) Apelin-13 did not evoke any current when applied after cells were pre-treated with 0.1mM apelin-13(F13A) (n=5). E) Apelin-13 evoked a small increase in [Ca2+]i. in vasopressin neurons (n=12). F) Apelin-13 did not evoke an increase in [Ca2+]i in the absence of extracellular calcium (n=5).
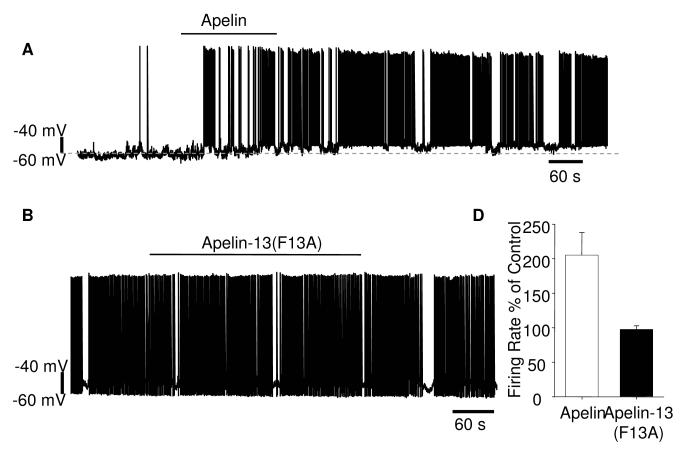
Fig. 5.
The effect of applying apelin-13 (50 nM) and the APJ antagonist apelin-13(F13A) (0.1 mM) on vasopressin neurons in hypothalamic slices. A) Shows an example of apelin-13-induced increase in action potential firing in a phasic vasopressin neuron. Apelin-13 also depolarised the neuron (the original membrane potential is indicated by the dotted line, increasing from -58 to -54 mV). B) An example showing that apelin-13(F13A) had no effect on the spontaneous action potential firing rate or pattern in a vasopressin neuron. C) The average firing rate as a percentage of a control period prior to exposure to apelin-13 or apelin-13(F13A) showed that apelin-13 produced a significant increase in firing rate (*p<0.05, n=10) while apelin-13(F13A) was without effect (n=10).
Apelin-13 (50 nM, 3 min) stimulated a small but reproducible increase in [Ca2+]i in 12 out of 12 vasopressin neurons (Fig. 4D). The identity of the isolated vasopressin neurons was established by their response to vasopressin (Fig. 4D) as oxytocin neurons do not respond to vasopressin with an increase in [Ca2+]i (38).This increased [Ca2+]i was absent when extracellular calcium was removed in 5 of 5 vasopressin neurons (Fig. 4F). As the inward current was evoked at potentials more negative than -32 mV, it is likely that the apelin-induced calcium influx occurs via the non-specific cation channel rather than a voltage operated calcium channel. We also observed apelin-induced increases in [Ca2+]i in oxytocin neurons (data not shown) but did not pursue this further.
The effects of apelin-13 on membrane potential and action potential firing were examined using brain slices, as vasopressin neurons in this preparation show patterns of spontaneous action potentials similar to those recorded in vivo. Cells were identified as vasopressin neurons either from a fluorescence signal or using the voltage-clamp protocol described above. Results from wild-type and vasopressin-eGFP rats and both genders were not different and have been pooled. In current-clamp mode, apelin-13 (50 nM) had a modest effect, depolarising the membrane potential on average by 5.6±1 mV from an average resting membrane potential of -58.5±8.5 mV (n=10, Fig 5A). In both non-phasic and phasically firing neurons, apelin-13 increased the action potential firing rate (Fig 5C). In general, vasopressin cells did not return to pre-apelin membrane potential and firing rates until at least 10 min after perfusion of the drug was stopped. Nevertheless, cells were able to re-respond to a second apelin-13 exposure. Treatment of hypothalamic brain slices by bath perfusion with apelin-13(F13A) (0.1 mM, 15 min) was without any significant effect on spontaneous action potential firing rate or membrane potential of vasopressin neurons (n=10 neurons, Fig. 5B, C).
In contrast to the vasopressin neurons, all isolated neurons or cells in the hypothalamic brain slices identified as oxytocin neurons showed no increase in inward current upon exposure to apelin-13 (0.05 or 0.1 mM) or change in membrane potential or action potential firing rate.
In vitro release experiments
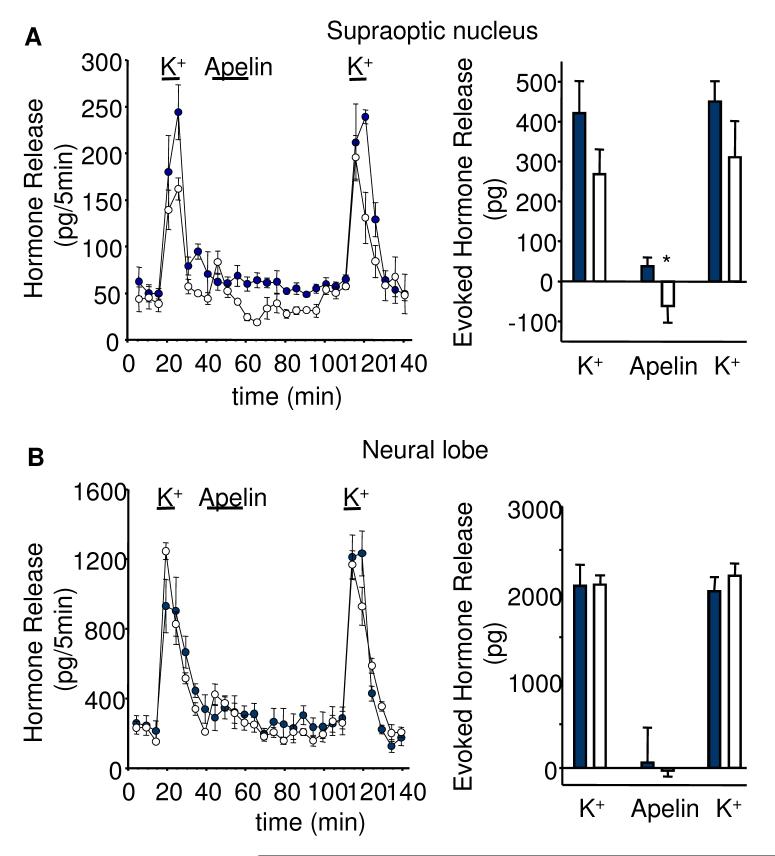
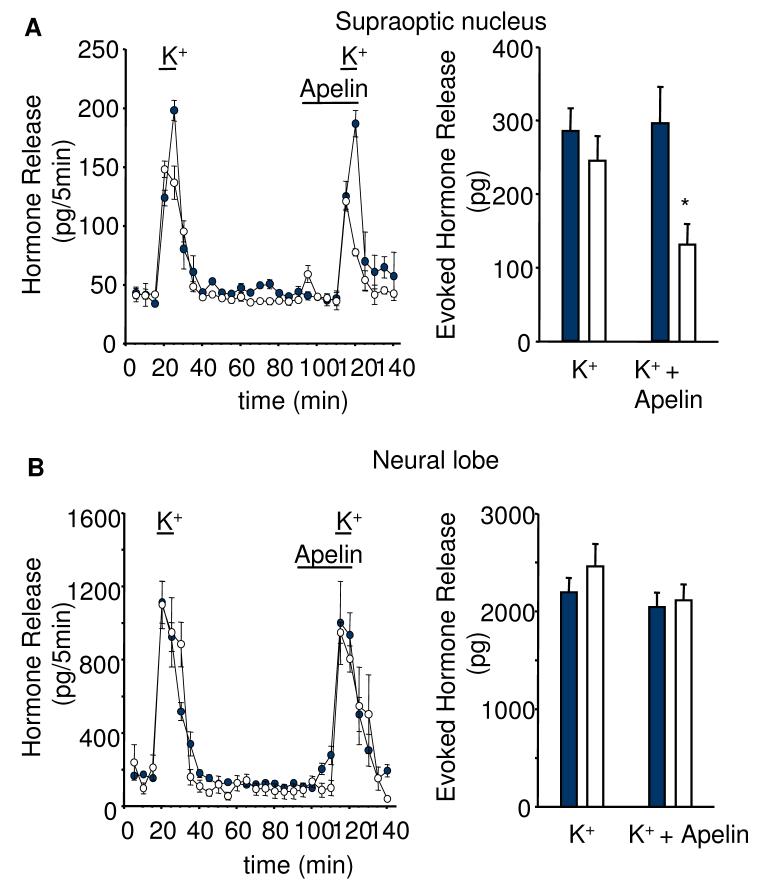
To test whether the effects of apelin-13 are mediated through somato/dendritic or axon terminal release of vasopressin or oxytocin, we studied hormone release from isolated neural lobes and SON in vitro. The responses of vasopressin and oxytocin release from both compartments were similar after repeated stimulation with 50 mM K+ solution (Fig. 6). Apelin-13 (50 nM) given for 20 min between the two potassium stimuli significantly decreased basal vasopressin (by 78%, p<0.05, n=6), but not oxytocin release, from the SON preparations. Release of both hormones from the neural lobe was not affected by the apelin treatment (Fig 6). Apelin-13 (50 nM) also significantly inhibited 50 mM K+-evoked vasopressin release from the SON explants (by 57%, p<0.05, n=6, Fig 7).
Fig. 6. Effects of apelin on basal peptide release in vitro.
Apelin-13 (50 nM) reduced A) basal vasopressin (empty circles/bars), but not oxytocin release (filled circles/bars) from SON explants with B) no effect of apelin-13 on either vasopressin or oxytocin release from isolated axon terminals of the neural lobe. Mean ± S.E.M., n=6, *p<0.05.
Fig. 7. Effects of apelin on high potassium stimulated peptide release in vitro.
Apelin-13 (50 nM) reduced A) high potassium stimulated vasopressin (empty circles/bars), but not oxytocin release (filled circles/bars) from SON explants with B) no effect of apelin-13 on either vasopressin or oxytocin release from isolated axon terminals of the neural lobe. Mean ± S.E.M., n=6, *p<0.05.
In both the SON and neural lobe preparations apelin-13 often appeared to stimulate vasopressin release within the first five min. However, this small increase was not statistically significant. The K+-evoked release of oxytocin from the SON and vasopressin and oxytocin from the neural lobes was also not affected by apelin-13 (Fig. 7).
Discussion
The present study demonstrated that both oxytocin and vasopressin neurons are immunoreactive for apelin receptors. Apelin-13 administration to isolated vasopressin and putative oxytocin neurons provoked an increased [Ca2+]i in both cell types. However, apelin-13 increased the electrical activity in vivo and in vitro of vasopressin cells only. Finally, apelin-13 decreased basal and potassium stimulated somato-dendritic vasopressin release without affecting oxytocin release.
These data again show a marked dissociation between axonal and dendritic vasopressin release with a decrease in somato/dendritic release but an increase in electrical activity at the cell bodies, which we anticipate would result in increased terminal secretion. However, we did not measure the contemporaneous vasopressin release from the neural lobes after apelin-13 was applied in vivo to the somata and dendrites via the microdialysis probe. This is because the apelin-13 was applied to only one of the four nuclei that project to the neural lobe and a change in the firing rate of vasopressin neurons in one treated nucleus may not necessarily be reflected in a change in output at the neural lobe.
Generally, increased action potential firing is accompanied by increased secretion. Here we show that apelin-13 increases the electrical activity of vasopressin neurons, while decreasing somato/dendritic release. However, this is not the first observation that a change in action potential firing rate does not alter peptide release from magnocellular neuron somata and dendrites (35).
That release from these two compartments can be regulated wholly independently has been previously shown (35;40). For example, oxytocin cells express MC4 receptors, through which α–MSH acts and MC4 agonists trigger intracellular calcium release in oxytocin cells, evoke dendritic release of oxytocin, and induce expression of the immediate-early gene c-fos. However, α-MSH inhibits the electrical activity of oxytocin neurones and so reduces the secretion of oxytocin into the blood (40).
APJ was distributed throughout magnocellular vasopressin neurons with intensive labelling of cell bodies, dendrites and axon terminals. APJ immunoreactivity was also seen on oxytocin cells and cells of the anterior pituitary. Labelling of vasopressin neurons is in accordance with previous in situ hybridisation studies showing APJ mRNA expression in vasopressin cells which is unregulated during osmotic stimuli (41). Using in situ hybridisation combined with immunohistochemistry, high expression of apelin mRNA in corticotrophs of the anterior pituitary has been shown, suggesting a local interaction between apelin and the release of adrenocorticotropin (42). The physiological significance of APJ expression on oxytocin neurons is currently unknown; in our experiments, apelin administration had no effect on the electrical activity of oxytocin neurones activity in vivo and in vitro or somato/dendritic oxytocin release at doses that affected vasopressin cell activity and vasopressin release. Likewise, despite a strong immunocytochemical APJ signal, application of apelin-13 to neural lobe preparations had no effect on vasopressin release from axon terminals in vitro.
There was also intensive APJ staining in the ventral glia lamina. Co-localisation of APJ with the glial cell marker GFAP has been reported previously (8;43). However, in the former paper, glial cell expression of APJ was thought to be in oligodendrocytes with limited expression in astrocytes where oligodendrocytes and astrocytes were identified by staining against galactosylceramide and GFAP respectively. Choe and colleagues (43) reported that, despite the presence of APJ immunoreactivity, astrocytes stimulated with apelin did not show an increase in [Ca2+]i. The functional significance of APJ expression on astrocytes remains unknown.
The literature regarding the effects of apelin in regulating vasopressin release and drinking behaviour is controversial. I.c.v. injection of apelin-13 and apelin-17 inhibited basal and dehydration-induced release of vasopressin and water intake in mice and rats (18), suggesting an inhibitory role of apelin in the regulation of vasopressin release. However, in other studies apelin-13 administered i.c.v. dose-dependently increased water intake in rats (5;25) or had no effect (26).
In our in vivo preparation, apelin-13 was applied directly onto supraoptic neurons by microdialysis administration (32), resulting in an increase in the activity quotient (phasic cells) and mean spike activity (continuous active cells) of all vasopressin cells tested. This finding is in direct contrast to the results on lactating animals recently published by others (24). I.c.v injection of the apelin agonist K17F induced a gradual and sustained inhibition of phasic vasopressin cells for up to 60 min after injection (24). In our study, in virgin female rats, we did not find any changes in the activity of continuous active vasopressin or oxytocin cells within 15-30 min after i.c.v. apelin-13 injection, but again found a significant increase in the activity quotient of phasic active cells. These differences may be due to the physiological conditions of the animals. In the lactating rat model, vasopressin neurons are hyperactive (44) in order to preserve body water content and to optimise milk production. Alternatively, apelin, as vasopressin, might affect the firing pattern of vasopressin cells dependent on their ongoing electrical activity so that fast-firing neurones are slowed, and slow-firing neurones are excited (45).
A direct excitatory effect of apelin on the electrical activity is supported by our in vitro findings. Apelin-induced inward currents recorded under voltage-clamp suggest the activation of a non-voltage dependent current and the recorded reversal potential was close to the reversal potential of non-selective cation currents of SON neurons, and resembles the currents obtained with other peptides such as cholecystokinin, angiotensin II, activin A, neurotensin and PACAP (46-49). However, further investigation is required to determine the different ion permeability, in other words whether apelin induces an inward calcium current via a non-specific cationic channel.
The second messenger signalling system utilised by APJ upon binding apelin appears to be dependent on cell type. In studies using APJ transfected Chinese hamster ovary cells, apelin inhibits forskolin-induced cAMP production (8;18) suggesting APJ is functionally linked to Gαi. Apelin can also activate kinases involved in cellular survival such as ERK-1/2 (50;51) or AKT (52). However, while the neuronal cell lines 293 (8;53) and NT2 (43) respond to apelin with an increase in [Ca2+]i, APJ expressing Chinese hamster ovary cells do not (2;50;54). Apelin evoked an increase in [Ca2+]i in vasopressin neurons. Furthermore, apelin did not increase [Ca2+]i in cells perfused with Ca2+-free solution, indicating that apelin induces calcium influx, rather than release from intracellular stores. The consequences of apelin-induced changes in intracellular calcium concentration in magnocellular neurons are currently unknown.
The local excitatory actions of apelin on the electrical activity of vasopressin neurons may be mediated through somato/dendritic vasopressin release. Vasopressin neurons discharge in a characteristic phasic pattern that optimises the efficiency of stimulus-secretion coupling at the nerve terminals. Magnocellular neurons release large amounts of oxytocin and vasopressin from their dendrites (55) and dendritically-released vasopressin itself modulates this phasic activity by a predominantly inhibitory action (32;45). In vivo retrodialysis of vasopressin onto vasopressin neurons inhibited vasopressin neurons by reducing their firing rate, whereas, retrodialysis of a V1-receptor antagonist partially reversed the effects of vasopressin (32). Functional evidence suggests that the effects of vasopressin on electrical activity are likely to be inhibitory. I.c.v. injection of vasopressin decreases plasma vasopressin concentration (56), while V1/V2 antagonists enhance peripheral vasopressin release in response to osmotic stimulation (57).
To assess the effects of apelin on somato/dendritic peptide vasopressin release we used in vitro release studies from SON explants (35) demonstrating an inhibitory action of apelin on basal and stimulated somato/dendritic vasopressin release. Our data imply that vasopressin and apelin are released differentially from vasopressin neurones in which they are co-localised, but found in neurosecretory granules differing in size and distribution (23). While the APJ antagonist apelin-13(F13A) blocked the exogenous apelin-13 evoked inward current, we did not observe an effect of the antagonist on membrane potential or action potential firing rate, which suggests that apelin is not being tonically released. In addition, we have not been successful in measuring potassium- stimulated apelin release from our SON explant preparation, which may be due to the low amount of apelin released and/or the detection limited of the enzyme immunoassay used (minimum sensitivity: 0.07ng/ml; Apelin-12 Enzyme ImmunoAssay, Phoenix Pharmaceuticals, Inc. Ca, USA).
It is tempting to speculate that inhibition of dendritic vasopressin release by apelin may remove an inhibitory tone leading to excitation of vasopressin cell activity. Osmotic challenges such as dehydration increase both the firing rate of vasopressin cells and vasopressin release (58;59) and in a dual immuno-labelling confocal microscopy study, Reaux-Le Goazigo and colleagues (23), have shown that the number and labelling intensity of magnocellular apelin-immunoreactive cells increased significantly after dehydration, whereas the number and labelling density of vasopressin-immunoreactive neurons significantly decreased. The authors speculate that an inhibition of somato/dendritic apelin release is partly responsible for the observed effect. However, this hypothesis requires further evaluation.
Acknowledgements
Work was supported by grants from the BBSRC (Ludwig) and the Wellcome Trust (O’Carroll) and a BBSRC Japan Partnering Award (Ludwig). We thank Professor Gareth Leng (Edinburgh) for critical reading of the manuscript.
Footnotes
Disclosure summary: The authors have nothing to disclose
Reference List
- 1.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 2.Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, Onda H, Tatemoto K, Fujino M. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta. 1999;1452:25–35. doi: 10.1016/s0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 3.De Mota N, Lenkei Z, Llorens-Cortes C. Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology. 2000;72:400–407. doi: 10.1159/000054609. [DOI] [PubMed] [Google Scholar]
- 4.Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, Kitada C, Honda S, Kurokawa T, Onda H, Nishimura O, Fujino M. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem. 2000;275:21061–21067. doi: 10.1074/jbc.M908417199. [DOI] [PubMed] [Google Scholar]
- 5.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O’Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 6.O’Carroll AM, Selby TL, Palkovits M, Lolait SJ. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim Biophys Acta. 2000;1492:72–80. doi: 10.1016/s0167-4781(00)00072-5. [DOI] [PubMed] [Google Scholar]
- 7.Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, Nishizawa N, Kitada C, Onda H, Nishimura O, Fujino M. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta. 2001;1538:162–171. doi: 10.1016/s0167-4889(00)00143-9. [DOI] [PubMed] [Google Scholar]
- 8.Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G, Bolaky JE, Herrity NC, Murdock P, Darker JG. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem. 2003;84:1162–1172. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 9.Brailoiu GC, Dun SL, Yang J, Ohsawa M, Chang JK, Dun NJ. Apelin-immunoreactivity in the rat hypothalamus and pituitary. Neurosci Lett. 2002;327:193–197. doi: 10.1016/s0304-3940(02)00411-1. [DOI] [PubMed] [Google Scholar]
- 10.Masri B, Knibiehler B, Audigier Y. Apelin signalling: a promising pathway from cloning to pharmacology. Cell Signal. 2005;17:415–426. doi: 10.1016/j.cellsig.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107:198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Lee DK, George SR, O’Dowd BF. Unravelling the roles of the apelin system: prospective therapeutic applications in heart failure and obesity. Trends Pharmacol Sci. 2006;27:190–194. doi: 10.1016/j.tips.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Charles CJ. Putative role for apelin in pressure/volume homeostasis and cardiovascular disease. Cardiovasc Hematol Agents Med Chem. 2007;5:1–10. doi: 10.2174/187152507779315804. [DOI] [PubMed] [Google Scholar]
- 14.Carpene C, Dray C, Attane C, Valet P, Portillo MP, Churruca I, Milagro FI, Castan-Laurell I. Expanding role for the apelin/APJ system in physiopathology. J Physiol Biochem. 2007;63:359–373. [PubMed] [Google Scholar]
- 15.Japp AG, Newby DE. The apelin-APJ system in heart failure: pathophysiologic relevance and therapeutic potential. Biochem Pharmacol. 2008;75:1882–1892. doi: 10.1016/j.bcp.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Llorens-Cortes C, Kordon C. Jacques Benoit lecture: the neuroendocrine view of the angiotensin and apelin systems. J Neuroendocrinol. 2008;20:279–289. doi: 10.1111/j.1365-2826.2007.01642.x. [DOI] [PubMed] [Google Scholar]
- 17.Rayalam S, Della-Fera MA, Krieg PA, Cox CM, Robins A, Baile CA. A putative role for apelin in the etiology of obesity. Biochem Biophys Res Commun. 2008;368:815–819. doi: 10.1016/j.bbrc.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Reaux A, De Mota N, Skultetyova I, Lenkei Z, El Messari S, Gallatz K, Corvol P, Palkovits M, Llorens-Cortes C. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 2001;77:1085–1096. doi: 10.1046/j.1471-4159.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Donnell LA, Agrawal A, Sabnekar P, Dichter MA, Lynch DR, Kolson DL. Apelin, an endogenous neuronal peptide, protects hippocampal neurons against excitotoxic injury. J Neurochem. 2007;102:1905–1917. doi: 10.1111/j.1471-4159.2007.04645.x. [DOI] [PubMed] [Google Scholar]
- 20.Reaux A, Gallatz K, Palkovits M, Llorens-Cortes C. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience. 2002;113:653–662. doi: 10.1016/s0306-4522(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 23.Reaux-Le Goazigo A, Morinville A, Burlet A, Llorens-Cortes C, Beaudet A. Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology. 2004;145:4392–4400. doi: 10.1210/en.2004-0384. [DOI] [PubMed] [Google Scholar]
- 24.De Mota N, Reaux-Le Goazigo A, El Messari S, Chartrel N, Roesch D, Dujardin C, Kordon C, Vaudry H, Moos F, Llorens-Cortes C. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci U S A. 2004;101:10464–10469. doi: 10.1073/pnas.0403518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taheri S, Murphy K, Cohen M, Sujkovic E, Kennedy A, Dhillo W, Dakin C, Sajedi A, Ghatei M, Bloom S. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochem Biophys Res Commun. 2002;291:1208–1212. doi: 10.1006/bbrc.2002.6575. [DOI] [PubMed] [Google Scholar]
- 26.Mitra A, Katovich MJ, Mecca A, Rowland NE. Effects of central and peripheral injections of apelin on fluid intake and cardiovascular parameters in rats. Physiol Behav. 2006;89:221–225. doi: 10.1016/j.physbeh.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Ueta Y, Fujihara H, Serino R, Dayanithi G, Ozawa H, Matsuda K, Kawata M, Yamada J, Ueno S, Fukuda A, Murphy D. Transgenic expression of enhanced green fluorescent protein enables direct visualization for physiological studies of vasopressin neurons and isolated nerve terminals of the rat. Endocrinology. 2005;146:406–413. doi: 10.1210/en.2004-0830. [DOI] [PubMed] [Google Scholar]
- 28.Fujio T, Fujihara H, Shibata M, Yamada S, Onaka T, Tanaka K, Morita H, Dayanithi G, Kawata M, Murphy D, Ueta Y. Exaggerated response of arginine vasopressin-enhanced green fluorescent protein fusion gene to salt loading without disturbance of body fluid homeostasis in rats. J Neuroendocrinol. 2006;18:776–785. doi: 10.1111/j.1365-2826.2006.01476.x. [DOI] [PubMed] [Google Scholar]
- 29.Shibata M, Fujihara H, Suzuki H, Ozawa H, Kawata M, Dayanithi G, Murphy D, Ueta Y. Physiological studies of stress responses in the hypothalamus of vasopressin-enhanced green fluorescent protein transgenic rat. J Neuroendocrinol. 2007;19:285–292. doi: 10.1111/j.1365-2826.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhong JC, Yu XY, Huang Y, Yung LM, Lau CW, Lin SG. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc Res. 2007;74:388–395. doi: 10.1016/j.cardiores.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Leng G, Dyball REJ. Functional identification of magnocellular neuroendocrine neurones. In: Greenstein B, editor. Neuroendocrine Research Methods. vol 2. Harwood Academic Publishers GmbH; Switzerland, Chur: 1991. pp. 769–791. [Google Scholar]
- 32.Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 33.Sabatier N, Brown CH, Ludwig M, Leng G. Phasic spike patterning in rat supraoptic neurones in vivo and in vitro. J Physiol. 2004;558:161–180. doi: 10.1113/jphysiol.2004.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chevaleyre V, Dayanithi G, Moos FC, Desarmenien MG. Developmental regulation of a local positive autocontrol of supraoptic neurons. J Neurosci. 2000;20:5813–5819. doi: 10.1523/JNEUROSCI.20-15-05813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- 36.Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong WE, Stern JE. Phenotypic and state-dependent expression of the electrical and morphological properties of oxytocin and vasopressin neurones. Prog Brain Res. 1998;119:101–113. doi: 10.1016/s0079-6123(08)61564-2. [DOI] [PubMed] [Google Scholar]
- 38.Gouzenes L, Sabatier N, Richard P, Moos FC, Dayanithi G. V1a- and V2-type vasopressin receptors mediate vasopressin-induced Ca2+ responses in isolated rat supraoptic neurones. J Physiol. 1999;517:771–779. doi: 10.1111/j.1469-7793.1999.0771s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee DK, Saldivia VR, Nguyen T, Cheng R, George SR, O’Dowd BF. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology. 2005;146:231–236. doi: 10.1210/en.2004-0359. [DOI] [PubMed] [Google Scholar]
- 40.Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XMM, Jiang M, Van der PL, Leng G. alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23:10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Carroll AM, Lolait SJ. Regulation of rat APJ receptor messenger ribonucleic acid expression in magnocellular neurones of the paraventricular and supraopric nuclei by osmotic stimuli. J Neuroendocrinol. 2003;15:661–666. doi: 10.1046/j.1365-2826.2003.01044.x. [DOI] [PubMed] [Google Scholar]
- 42.Reaux-Le Goazigo A, Alvear-Perez R, Zizzari P, Epelbaum J, Bluet-Pajot MT, Llorens-Cortes C. Cellular localization of apelin and its receptor in the anterior pituitary: evidence for a direct stimulatory action of apelin on ACTH release. Am J Physiol Endocrinol Metab. 2007;292:E7–15. doi: 10.1152/ajpendo.00521.2005. [DOI] [PubMed] [Google Scholar]
- 43.Choe W, Albright A, Sulcove J, Jaffer S, Hesselgesser J, Lavi E, Crino P, Kolson DL. Functional expression of the seven-transmembrane HIV-1 co-receptor APJ in neural cells. J Neurovirol. 2000;6(Suppl 1):S61–S69. [PubMed] [Google Scholar]
- 44.Poulain DA, Wakerley JB, Dyball RE. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc Royal Soc London - Series B: Biol Sci. 1977;196:367–384. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- 45.Gouzenes L, Desarmenien MG, Hussy N, Richard P, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular neurons. J Neurosci. 1998;18:1879–1885. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang CR, Phillips MI, Renaud LP. Angiotensin II receptor activation depolarizes rat supraoptic neurons in vitro. Am J Physiol. 1992;263:R1333–R1338. doi: 10.1152/ajpregu.1992.263.6.R1333. [DOI] [PubMed] [Google Scholar]
- 47.Oliet SH, Plotsky PM, Bourque CW. Effects of activin-A on neurons acutely isolated from the rat supraoptic nucleus. J Neuroendocrinol. 1995;7:661–663. doi: 10.1111/j.1365-2826.1995.tb00806.x. [DOI] [PubMed] [Google Scholar]
- 48.Chakfe Y, Bourque CW. Excitatory peptides and osmotic pressure modulate mechanosensitive cation channels in concert. Nat Neurosci. 2000;3:572–579. doi: 10.1038/75744. [DOI] [PubMed] [Google Scholar]
- 49.Shibuya I, Kabashima N, Tanaka K, Setiadji VS, Noguchi J, Harayama N, Ueta Y, Yamashita H. Patch-clamp analysis of the mechanism of PACAP-induced excitation in rat supraoptic neurones. J Neuroendocrinol. 1998;10:759–768. doi: 10.1046/j.1365-2826.1998.00260.x. [DOI] [PubMed] [Google Scholar]
- 50.Masri B, Lahlou H, Mazarguil H, Knibiehler B, Audigier Y. Apelin (65-77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem Biophys Res Commun. 2002;290:539–545. doi: 10.1006/bbrc.2001.6230. [DOI] [PubMed] [Google Scholar]
- 51.Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y. Apelin (65-77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. FASEB J. 2004;18:1909–1911. doi: 10.1096/fj.04-1930fje. [DOI] [PubMed] [Google Scholar]
- 52.Hashimoto Y, Ishida J, Yamamoto R, Fujiwara K, Asada S, Kasuya Y, Mochizuki N, Fukamizu A. G protein-coupled APJ receptor signaling induces focal adhesion formation and cell motility. Int J Mol Med. 2005;16:787–792. [PubMed] [Google Scholar]
- 53.Zhou N, Zhang X, Fan X, Argyris E, Fang J, Acheampong E, DuBois GC, Pomerantz RJ. The N-terminal domain of APJ, a CNS-based coreceptor for HIV-1, is essential for its receptor function and coreceptor activity. Virology. 2003;317:84–94. doi: 10.1016/j.virol.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 54.Wang G, Anini Y, Wei W, Qi X, OCarroll AM, Mochizuki T, Wang HQ, Hellmich MR, Englander EW, Greeley GH., Jr. Apelin, a new enteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology. 2004;145:1342–1348. doi: 10.1210/en.2003-1116. [DOI] [PubMed] [Google Scholar]
- 55.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 56.Wang BC, Share L, Crofton JT. Central infusion of vasopressin decreased plasma vasopressin concentration in dogs. Am J Physiol. 1982;243:E365–E368. doi: 10.1152/ajpendo.1982.243.5.E365. [DOI] [PubMed] [Google Scholar]
- 57.Liu H-W, Wang Y-X, Crofton JT, Funyu T, Share L. Central vasopressin blockade enhances its peripheral release in response to peripheral osmotic stimulation in conscious rats. Brain Res. 1996;719:14–22. doi: 10.1016/0006-8993(96)00054-6. [DOI] [PubMed] [Google Scholar]
- 58.Wakerley JB, Poulain DA, Brown D. Comparison of firing patterns in oxytocin- and vasopressin- releasing neurones during progressive dehydration. Brain Res. 1978;148:425–440. doi: 10.1016/0006-8993(78)90730-8. [DOI] [PubMed] [Google Scholar]
- 59.Kadekaro M, Summy-Long JY, Harris JS, Freeman S, Eisenberg HM. Cerebral metabolic and vasopressin and oxytocin response during osmotic stimulation in conscious rats. J Neuroendocrinol. 1992;4:217–222. doi: 10.1111/j.1365-2826.1992.tb00162.x. [DOI] [PubMed] [Google Scholar]