Abstract
We have generated a humanized double-reporter transgenic rat for whole-body in vivo imaging of endocrine gene expression, using the human prolactin (PRL) gene locus as a physiologically important endocrine model system. The approach combines the advantages of bacterial artificial chromosome recombineering to report appropriate regulation of gene expression by distant elements, with double reporter activity for the study of highly dynamic promoter regulation in vivo and ex vivo. We show first that this rat transgenic model allows quantitative in vivo imaging of gene expression in the pituitary gland, allowing the study of pulsatile dynamic activity of the PRL promoter in normal endocrine cells in different physiological states. Using the dual reporters in combination, dramatic and unexpected changes in PRL expression were observed after inflammatory challenge. Expression of PRL was shown by RT-PCR to be driven by activation of the alternative upstream extrapituitary promoter and flow cytometry analysis pointed at diverse immune cells expressing the reporter gene. These studies demonstrate the effective use of this type of model for molecular physiology and illustrate the potential for providing novel insight into human gene expression using a heterologous system.
Reporter-gene transgenic rats have been generated that provide the first in vivo model to accurately study the physiological regulation of the human prolactin gene.
A major challenge in physiology is the understanding and analysis of dynamic temporal control of gene expression in living intact tissues in real time in different physiological conditions. In this study we developed transgenic rat lines using large reporter transgenes in bacterial artificial chromosomes (BACs), with the purpose of studying dynamic regulation of the important hormone prolactin (PRL), assessing gene expression in the intact animal in vivo and in living cells ex vivo. Surprisingly little is known about the timing of gene expression in living tissues and whole organisms, although previous work has indicated that in cell lines, gene transcription in individual cells behaves in a highly dynamic and heterogeneous manner (1, 2).
PRL is a polypeptide hormone mainly produced by the lactotrope cells of the anterior pituitary gland, and its major function in mammals is related to lactation (3). The PRL gene is also expressed in humans and primates at extrapituitary sites, such as brain, decidualized endometrium, myometrium, and circulating lymphocytes (4), and more than 300 biological functions have been attributed to this hormone, including reproduction, immunomodulation, and behavior. The human PRL gene maps on the short arm of chromosome 6, and it is organized in five exons and four introns (4). In human decidua and in lymphocytes the PRL mRNA is longer including an extra exon (exon 1a), produced by the activity of an alternative promoter located 5.8 kb upstream of the pituitary transcription start site (4, 5, 6). A number of comparisons suggest that the human and rodent prolactin (Prl) loci are significantly different. Where the human PRL locus lies in a gene desert (7) surrounded by around one megabase of noncoding DNA (http://genome.ucsc.edu), rodent Prl genes exist as a large family of 26 closely related paralogous genes arising from gene duplication (8). The PRL gene families in the mouse and rat are clustered on chromosomes 13 and 17, respectively (4). Differential expression of the alternative human promoter has been suggested to compensate for the lack of expansion of the PRL gene in primates (9). No mutations have been described in the human PRL gene, suggesting that such mutations might be lethal. This differs from studies in mouse where neither vitality nor immune competency is compromised in Prl (10) and Prl receptor knockout mice (10, 11), despite many indications for a role of PRL in the immune system (12). This is surprising given the high conservation of the PRL gene sequence in humans. Therefore, the mouse knockout models available may not be ideal for studying the human PRL gene, as a result of redundancy of the rodent system.
Here we describe the generation of a humanized double-transgenic rat that expresses luciferase and destabilized enhanced green fluorescent protein (d2eGFP) under the control of the entire human PRL gene locus. Our strategy is based on a BAC recombineering approach to permit the inclusion of long-distance regulatory elements and to minimize site of transgene integration effects. We have previously used luciferase to image the dynamic pattern of gene expression, which is characteristic of the human PRL promoter (2, 13, 14). We show that the resulting rat double-transgenic model allows in vivo imaging and ex vivo analysis of human PRL gene expression driven by the pituitary and also the extrapituitary promoter, making this an ideal tool for the study of human PRL gene expression in different physiological and pathological conditions.
Results
Generation of a BAC-reporter transgene
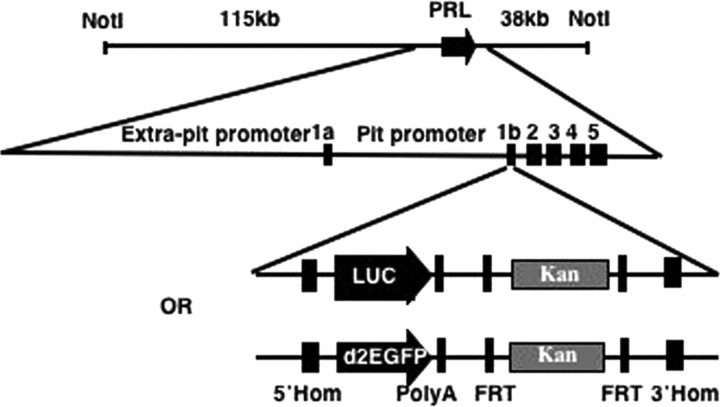
We have generated a BAC-luciferase and a BAC-destabilized eGFP (d2eGFP) construct by BAC recombineering (15) using BAC RP11-237G3, which spans 163 kb of the human PRL genomic locus including 115 kb upstream and 38 kb downstream of the PRL gene (Fig. 1A). Both Photinus pyralis luciferase and d2eGFP were selected as reporter genes due to their short half-life, which allows for the imaging of highly dynamic gene expression patterns (2) and for their suitability for in vivo imaging (16, 17).
Fig. 1.
Diagram showing the BAC targeting strategy using two (luciferase and d2eGFP) double-strand linear cassettes. 5′ Hom, 5′-homology arm; Luc, luciferase; 3′Hom, 3′-homology arm; Pit, pituitary.
The BAC was targeted with a linear double-strand DNA cassette containing either the luciferase or the d2eGFP gene and a Kan selectable marker flanked by FRT sites. Homologous recombination arms were designed to span the PRL gene 5′-untranslated region (UTR) and the first intron to substitute exon 1b with the targeting cassette (Fig. 1) (verified using Southern blot hybridization; see supplemental Figs. 1b and 2b published as supplemental data on the Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Exon 1b contains the translation ATG initiator, and its removal prevents the production of PRL from the targeted transgene.
Hormonal responses of stably transfected BAC cell lines
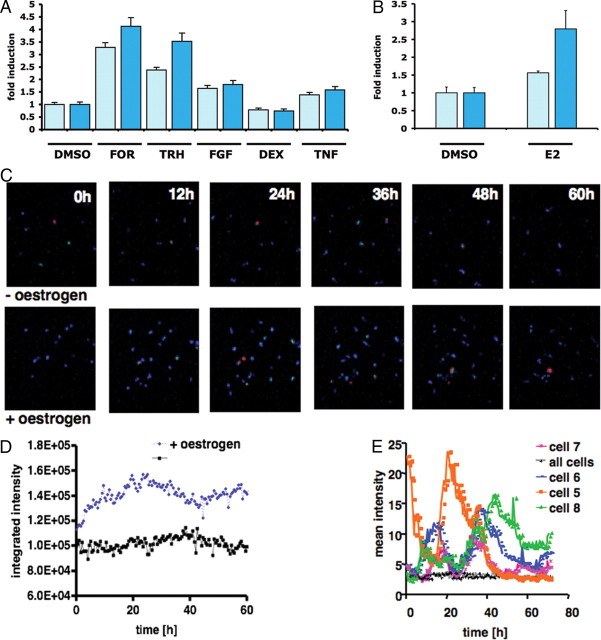
PRL-Luc BAC construct validation was performed by generating stably transfected pituitary GH3 cell lines. Eighteen recombinant clones were analyzed for basal luciferase activity (see supplemental Fig. 3), and a subset of nine were challenged with a variety of well-characterized PRL-regulating stimuli. A comparison with GH3 cells expressing luciferase under the control of 5 kb of human PRL promoter [D44 cell line (2)] is presented in Fig. 2A. A 2.8-fold induction of luciferase activity was observed in the PRL-Luc BAC cell lines after stimulation with estrogen compared with the 1.6-fold induction in D44 (P < 0.05) (Ref. 18 and Fig. 2B).
Fig. 2.
Analysis of cell lines stably transfected with the PRL-Luc BAC construct. A, Stimulation of BAC cell lines and D44 cells (FOR, 1 μm forskolin; TRH 300 nm; FGF-2, 10 ng/ml; Dex 10 nm; and TNF-α, 10 ng/ml) for 8 h. Results are shown as fold induction ± sd. B, Estrogen (E2) stimulation of BAC cell lines and D44 (1 nm) for 24 h. Results are shown as fold induction ± sd. C, Cell imaging. Estrogen was added at time 0, and images for untreated (upper panels) and treated cells (lower panels) are shown. Scale bar, 125 μm. D, Total photon counts of a whole field of cells (∼20 cells) with (blue) or without (black) addition of estrogen. E, Quantification of the transcriptional activity in single cells under resting conditions. The data are the mean intensity of individual cell areas and the mean intensity of the whole field of cells (∼20 cells). Dex, Dexamethasone; DMSO, dimethylsulfoxide; FOR, forskolin.
Real-time luminescence imaging showed significantly greater estrogen induction in the PRL-Luc BAC-transfected GH3 cells than that observed using the 5-kb PRL promoter (Fig. 2C). Single cells revealed heterogeneous, fluctuating transcriptional activity under resting conditions (Fig. 2, D and E), as seen previously in clonal cell lines (2), adenovirus infected (14), or microinjected primary pituitary cells (19).
Generation of PRL-Luc and PRL-d2eGFP transgenic rats
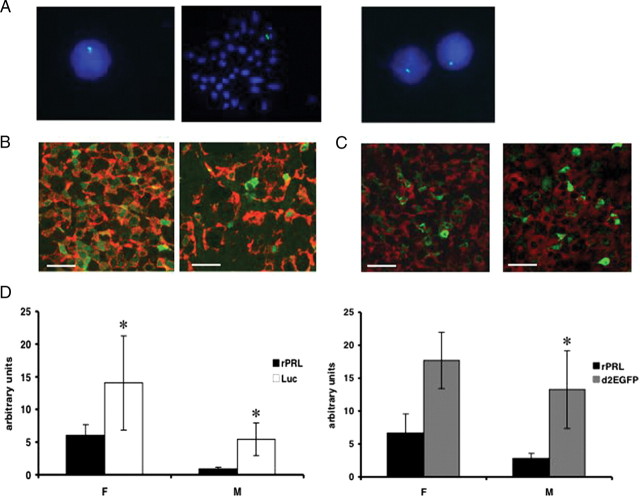
The targeted PRL-Luc and PRL-d2eGFP BAC constructs were injected into the pronucleus of Fisher 344 fertilized rat oocytes. Of 64 potential founder rats for PRL-Luc construct, five transgenic rats were identified (PRL-Luc25, PRL-Luc34, PRL-Luc37, PRL-Luc47, PRL-Luc49), and of 26 potential founders for PRL-d2eGFP construct, two transgenic rats were identified by PCR and confirmed by Southern blot hybridization (data not shown). All the lines except PRL-Luc25 and PRL-Luc34 transmitted the transgene to their progeny and showed normal growth and viability. Fluorescence in situ hybridization (FISH) analysis of interphase and metaphase nuclei showed multiple insertion sites of the transgene in lines PRL-Luc34, PRL-Luc37, and PRL-Luc47 (see supplemental Fig. 4), but a single insertion site in line PRL-Luc49, PRL-d2eGFP455 (Fig. 3A) and PRL-d2eGFP485. Southern blot analysis showed that more than 155 kb of the transgene was integrated into the genome of line PRL-Luc 49 in high copy number and in PRL-d2eGFP455 in low copy number. Thus, these two lines were selected for further characterization and study. Double immunocytochemistry analysis shows that a good colocalization of PRL/reporter gene signal can be observed in male and female pituitaries. A higher density of lactotropes was observed using immunocytochemistry throughout the female pituitary, compared with male pituitary (Fig. 3B; P = 0.0005) in PRL-Luc rats. In these animals, expression of luciferase was higher in females than males (Fig. 3B), and the two genes were coexpressed in 93.9% ± 5.9 of female lactotropes and in 59% ± 13.9 of male lactotropes (P < 0.0001). In PRL-d2eGFP transgenic rats the two genes were coexpressed in 18.4% ± 8% of female lactotropes and in 21.6% ± 8% of male lactotropes (Fig. 3C).
Fig. 3.
Molecular characterization of BAC PRL-Luc and PRL-d2eGFP transgenic rats. A, FISH analysis of metaphase and interphase nuclei of transgenic rat spleenocytes. Left panels, PRL-Luciferase line 49; right panel, PRL-d2eGFP line 455. B and C, Fluorescent immunocytochemistry of female (left) and male (right) pituitary sections. Green fluorescence: luciferase (panel B) or d2eGFP (panel C); red fluorescence indicates PRL. Scale bar, 20 μm. D, Real-time qPCR of luciferase, d2eGFP, and rat Prl in the pituitary gland of female and male transgenic rats. (*, P < 0.05 rat Prl vs. luciferase in males and females; n = 5 female; n = 4 male; *, P < 0.05 rat Prl vs. d2eGFP in males; n = 3 female; n = 4 male). F, Female; M, male.
Endogenous rat Prl and luciferase or d2eGFP gene expression analysis by quantitative PCR (qPCR) shows a significantly higher expression of the reporter genes compared with the endogenous Prl (Fig. 3D) in both male and females. In male luciferase transgenic rats, expression of the Prl gene is significantly decreased, suggesting competition for transcription factors (transcriptional squelching; Fig. 3D). Human PRL activity was undetectable by RIA in the transgenic rat plasma and pituitary homogenates.
In vivo imaging of pituitary-specific expression
In vivo bioluminescence imaging detected strong signals, 1.33 × 106 ± 6.7 × 105 p/sec/cm2/steradian (sr) in females and 5.6 × 105 ± 4.2 × 105 in males (Fig. 4A), after 30 sec signal integration time in luciferase transgenic rats lying supine. No strong signal was detected in this position with the animal lying prone, most likely due to the optical properties of the brain and the skull (20). Specificity of the signal for the pituitary gland was assessed after the rat was killed (Fig. 4B).
Fig. 4.
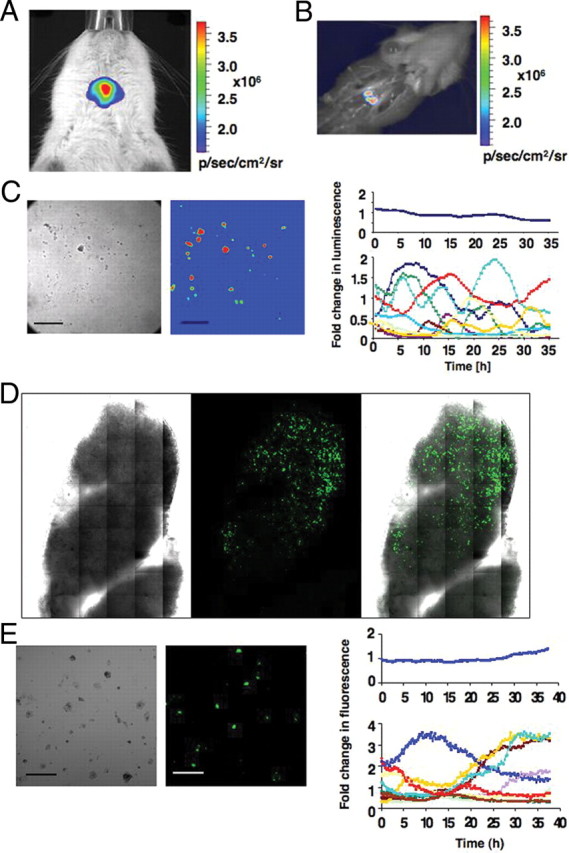
In vivo imaging and ex vivo analysis of BAC PRL-Luc and PRL-d2eGFP transgenic rats. A, In vivo imaging of a female BAC PRL-Luc transgenic rat. The image was acquired with an IVIS Spectrum (Caliper Life Sciences): 60 sec integration time, Bin 4, FOV 6.6, f1. B, Image taken after the rat has been euthanized. C, Ex vivo imaging of single-cell preparations derived from PRL-Luc transgenic rat pituitary. Left, Bright-field image of the pituitary cells. Middle, Pseudocolor image of the same cells. Right, Quantification of the dynamic changes in luminescence from individual cells. Scale bar, 200 μm. D, Tile scan imaging, with a ×40 1.3 fluar objective, of a pituitary thick section (400 μm) from PRL-d2eGFP rat. E, Ex vivo imaging of single-cell preparations derived from PRL-d2eGFP rat pituitary. Left, Bright-field image of the pituitary cells. Middle, Color image of the same cells. Right, Quantification of the dynamic changes in fluorescence from individual cells. Scale bar, 200 μm.
Ex vivo study of pituitary single-cell gene expression
Pituitaries from transgenic positive animals were harvested and the cells dispersed. Light emission could be detected in approximately one third of cells from each dispersed pituitary gland as assessed by bright-field imaging. Continuous imaging and photon emission were recorded over 35 h. The cells showed a dynamic expression pattern, which was not synchronized (Fig. 4C), but similar to the responses in the BAC cell lines (Fig. 2E). A movie showing the dynamic and oscillatory pattern of expression is in supplemental movie 1. Heterogeneity in PRL expression was also observed in d2eGFP-expressing cells dispersed from transgenic positive pituitaries (Fig. 4, D and E).
Extrapituitary luciferase expression
PRL expression in man has been demonstrated in a number of extrapituitary sites including cells of the immune system especially in T lymphocytes and lymphoid tissues (12). Detectable levels of luciferase and d2eGFP gene expression and luciferase activity were found to be present in the thymus and spleen of transgenic rats, whereas endogenous rat Prl gene expression was absent (see supplemental Fig. 5). Luminescence signals were also detected in the paws and ears of male and female transgenic rats, suggesting human PRL promoter-driven luciferase gene expression in cartilage (Semprini, S., J. R. McNeilly, D. G. Brownstein, K. Featherstone, L. Ramage, D. M. Salter, J. R. E. Davis, and J. J. Mullins, manuscript in preparation).
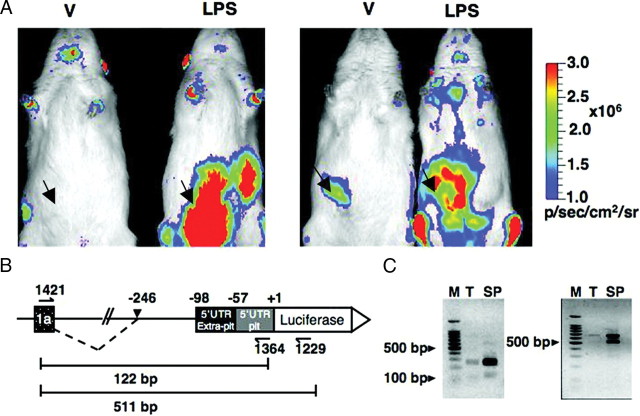
Fig. 5.
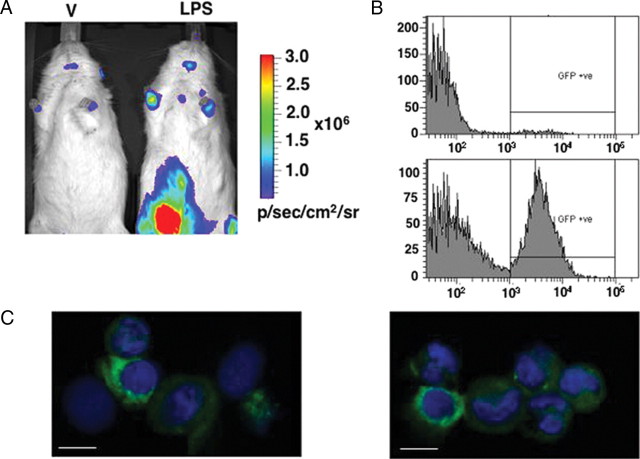
Extrapituitary expression of luciferase in BAC PRL-Luc transgenic rat. A, In vivo imaging of vehicle (V) and LPS-treated rats. The rats were injected ip (arrows indicate site of injection) either with physiological saline or with 3 mg/ml LPS and imaged 16 h after the treatment. The images were acquired with an IVIS Spectrum (Caliper Life Sciences): 30 sec integration time, Bin (HR) 8, FOV 19.6, f1. B, RT-PCR strategy for the analysis of luciferase expression driven by the extrapituitary promoter. Dotted line, Observed alternative splicing. C, RT-PCR result. M, 100-bp ladder; T, thymus; SP, spleen.
To address possible regulation of PRL promoter activity in immune-related cells, male rats were injected ip with either physiological saline (vehicle) or 3 mg/kg of body weight of lipopolysaccharide (LPS). After 16 h the rats were anesthetized, given an ip injection of luciferin, and imaged. One vehicle-treated and one LPS-treated rat were imaged simultaneously. Figure 5A shows two representative experiments in which strong multiple signals were seen in LPS-treated rats in lower and upper left abdomen, but also overlying the mediastinum (thymus and parathymic lymph nodes), the axillae (axillary/brachial lymph nodes), and posterior intermandibular space (mandibular lymph nodes). This pattern of light emission in LPS-treated rats suggests luciferase expression in cells resident in lymphoid tissues, but also in peritoneal exudate cells. Fainter signals could also be seen in the abdomen of vehicle-injected rats, and they may be due to local and superficial LPS-independent inflammation at the site of ip injection of the vehicle and luciferin substrate. The vehicle-treated animal in the right panel showed very low expression of pituitary luciferase (see supplemental Fig. 6).
To assess whether this in vivo expression was reflecting alternative promoter usage in immune-related organs, an RT-PCR approach was designed to amplify luciferase cDNA in thymus and spleen of transgenic rats, using two sets of primers. The first set of primers amplified a portion of cDNA including exon 1a, the extrapituitary 5′-UTR (21), and the pituitary 5′-UTR. The second set of primers amplified a portion of the luciferase gene as well (Fig. 5B). Amplification was successful in both tissues with the two sets of primers, demonstrating luciferase expression driven by the extrapituitary promoter, but a band was obtained that was 148 bp larger than the expected size and corresponded to the most abundant amplicon obtained in addition to the expected fragment (Fig. 5C). Sequence analysis allowed for the precise mapping of the alternative splice acceptor site to position −246 relative to the luciferase translation start site (equivalent to the human PRL ATG position). These data suggest activation of the human alternative PRL promoter upon immune challenge.
In vivo imaging and ex vivo analysis of PRL-mediated LPS response in a double-transgenic rat
Double-transgenic PRL-Luc/d2eGFP rats were generated by mating from single transgenics. Male double-transgenic rats were injected ip with either physiological saline (vehicle) or 3 mg LPS/kg of body weight. After 16 h, the rats were anesthetized, given an ip injection of luciferin, and imaged using the in vivo imaging system (IVIS) Spectrum. One vehicle-treated and one LPS-treated rat were imaged simultaneously. Figure 6A shows bioluminescence imaging of the PRL-mediated immune response as was also observed in the PRL-Luc transgenic animals (Fig. 5A). Furthermore, this model offers the option for fluorescence-based analysis of the cellular response to the LPS challenge. Fluorescent positive cells were observed in the peritoneal exudate of vehicle and LPS-treated rats in dramatically different proportions (Fig. 6B) by flow cytometry. Confocal imaging of peritoneal exudate cells suggested the expression of the d2eGFP protein by different populations of immune cells, including monocytes/macrophages and granulocytes (Fig. 6C).
Fig. 6.
In vivo and ex vivo imaging of reporter genes in LPS-treated PRL-Luc/d2eGFP double-transgenic rats. A, In vivo imaging of vehicle (V) and LPS-treated rats. The rats were injected i.p either with physiological saline or with 3 mg/ml LPS and imaged 16 h after the treatment. The images were acquired with an IVIS Spectrum (Caliper Life Sciences): 30 sec integration time, Bin (HR) 8, FOV 19.6, f1. B, Flow cytometric analysis of d2eGFP-positive cells from the peritoneal exudate of a vehicle and a LPS-treated double-transgenic rat. C, Confocal imaging of cytocentrifuge preparation of cells from the peritoneal exudate of LPS-treated double-transgenic rats. Scale bar, 10 μm.
Discussion
We have used a BAC recombineering approach to generate reporter transgenic rats that express the luciferase and the d2eGFP gene under the control of both the pituitary- and extrapituitary-specific human PRL gene promoters. Whole-body in vivo imaging of human PRL promoter activity in these models readily reveals physiological pituitary expression and striking evidence of alternative promoter activation after immune challenge.
The human PRL gene is surrounded by highly conserved noncoding sequences that span over 1 megabase of genomic DNA. These sequences are likely to contain functional regulatory elements and long-distance enhancers (22, 23). We selected a BAC clone spanning 163 kb of the human PRL locus and engineered it to insert either the luciferase or the destabilized eGFP genes into the human PRL exon 1b, thus removing expression of human PRL. BAC transgenesis offers several advantages over the use of small transgenes. These constructs encompass large stretches of flanking DNA and behave very similarly to the endogenous gene because effects due to the position of insertion can be practically nullified (24). They are also readily modified by BAC recombineering (25) and can be used for generating transgenic animals and for transfecting mammalian cell lines (26). To validate the PRL-Luc BAC construct, stably transfected pituitary GH3-based cell lines were generated. They showed dramatic heterogeneity in PRL transcription at single-cell level even under resting conditions, confirming a dynamic pattern of gene regulation (2, 13, 14, 19).
The BAC cell lines showed a response to a variety of stimuli, such as forskolin, TRH, fibroblast growth factor 2 (FGF-2), and TNF-α. We have recently demonstrated that TNF-α is a stimulator of the human PRL promoter, and its effect is mediated by nuclear factor-κB signaling pathway (27). Interestingly, the stimulation of several BAC cell lines with estrogen elicited larger luciferase emission (18) than D44 cells, which express luciferase under the control of a shorter human PRL promoter (2). These data suggest the presence of further estrogen-responsive regulatory elements in the BAC sequence, in addition to the one identified at −1189 bp relative to the transcription start site.
Although cell studies are very valuable for looking at molecular mechanisms of gene regulation, in vivo imaging strategies are very important tools to understand biological processes as they occur in living animals. We have generated four lines of BAC PRL-Luc and two lines of BAC PRL-d2eGFP transgenics but selected luciferase line 49 and eGFP line 455 for further studies due to the integrity of the BAC construct at a single insertion point in the rat genome. Immunocytochemistry results and real-time qPCR taken together show that endogenous rat Prl and the reporter genes (luciferase and d2eGFP) are expressed in lactotropes cells at good levels. Given the high transgene copy number in the PRL-Luc49 line, male transgenic rats showed a marked reduction in endogenous rat Prl mRNA compared with the luciferase mRNA. Both pituitaries and plasma of transgenic rats were tested for production of human PRL, and results were negative. Having excluded negative feedback by human prolactin on endogenous rat Prl production, one could speculate that given the sexual dimorphism in the expression of the pituitary-specific transcription factor Pit-1 (28), transcriptional squelching could occur in male pituitaries, but to at a lesser extent in female pituitaries. Indeed, silencing of gene expression has been reported for large transgenes when present in higher copy numbers (eight to 14 copies) (29). The reduced production of endogenous rat PRL in male animals could account for the highly significant reduced number of lactotropes in luciferase transgenic male pituitaries. In PRL-d2eGFP animals the low copy number of the transgene is consistent with a normal expression of the endogenous rat PRL as shown by the qPCR. A similar, but low proportion of male and female lactotropes coexpress the two genes; this could be possibly due to different half-lives of the proteins, differential expression of the human and rat Prl promoters, or stochastic expression of individual PRL loci under condition of low stimulation and in different lactotropes subtypes in the pituitary.
The bioluminescent transgenic rats are valuable tools for studying gene expression in vivo, because despite the intracranial location of the pituitary gland, high quality images were obtained with short integration times (30–60 sec) in both males and females. Although the signal resolution was low when imaged through intact tissue (and a precise localization of the light output could not be determined), the removal of the skull and the brain revealed pituitary-specific localization of luciferase expression. Studies on cultured transgenic pituitary cells revealed striking fluctuations in promoter activity, and more detailed ex vivo studies will be important to further characterize these transcription profiles.
Extrapituitary expression of PRL promoter activity was dramatically visualized in BAC transgenic animals when the animals were challenged with an ip injection of LPS, and we confirmed that this arose from alternate promoter usage in vivo. LPS is the major virulence factor of Gram-negative bacteria, and great progress has been made in the elucidation of LPS recognition and signaling in mammalian cells (30). Recognition of LPS by macrophages leads to the rapid activation of an intracellular signaling pathway, which results in the release of proinflammatory mediators and the recruitment of humoral and cellular components of the immune system. In vivo imaging of male luciferase transgenic and luciferase/d2eGFP double-transgenic rats, 16 h after the injection of LPS, showed luciferase signal in the abdominal area and also in the mediastinum and mandibular and axillary lymph nodes. The double-transgenic rat poses a real advantage in the study of the involvement of PRL in the immune response by combining in vivo imaging with ex vivo analysis. Flow cytometry analysis has revealed a population of eGFP-expressing cells in the peritoneal exudate of LPS-treated rats, which has been confirmed by confocal imaging with the suggestion that diverse cells types express d2eGFP. These data suggest that these rat models will therefore allow the in vivo and ex vivo study of the role of human PRL in immune-related processes, clarifying its function in autocrine and paracrine immunomodulation. Moreover, we have developed a unique approach for monitoring inflammation processes in vivo in real time, and this could facilitate the screening of drugs with antiinflammatory potential. Despite the recognition of PRL as an immune cytokine, its synthesis by the immune system is very low, and resting cells normally require activation to express PRL (12). Expression of luciferase and d2eGFP was observed by qPCR in thymus and spleen of unstimulated male and female transgenic rats, but endogenous rat Prl gene expression was undetectable. This observation confirms the conflicting evidence regarding PRL gene expression in the rodent immune system, where it could be both weak and transient. Conversely, stronger evidence is available for human PRL gene expression in T lymphocytes and peripheral blood lymphocytes (12) as confirmed here by the expression of luciferase in thymus and spleen of unstimulated rats. We have analyzed gene expression in vivo and ex vivo in two rat models with independent insertion sites of the BAC transgene and with different copy numbers and appreciated luciferase activity and fluorescence emission in cells of the immune system in both models; therefore, these observations suggest that the transgenic rat models we have generated are humanized model organisms that could be very valuable for studying human PRL gene regulation in vivo under physiological and pathological conditions; further studies are now required to characterize the reporter gene expression in leukocytes.
Interestingly, the extrapituitary mRNA transcribed in thymus and spleen is 148 bp longer than the previously described and most common extrapituitary mRNAs (5, 6). However, it is of note that a less abundant human decidual PRL cDNA has been reported with the same structure (21).
In conclusion, we have generated humanized reporter-gene transgenic rats for whole-body imaging and ex vivo study of human PRL gene expression. These models combine the advantages of BAC recombineering for appropriate regulation of gene expression and of reporter gene activity for the study of highly dynamic promoter regulation in vivo and ex vivo in different tissues. Moreover, it offers the benefit of a heterologous system for the characterization of human-specific PRL gene expression in a rat. To our knowledge, these are the first BAC transgenic rats for in vivo imaging of gene expression. Given the limitations of the Prl and Prl receptor knockout mouse for the understanding of human PRL gene function, these transgenic rats provide the first in vivo model to accurately study the physiological regulation of the human PRL gene.
Materials and Methods
Transgene construction
BAC RP11-237G3 (CHORI, http://www.chori.org/) was selected. For the generation of the luciferase recombination cassette, a 5′-homology arm (234 bp) was amplified using primers 1 and 2 from human BAC RP11-237G3 and using primers 3 and 4 (see supplemental Table 1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org) for the 3′-homology arm (227 bp). The homology arms were designed into the human PRL 5′-UTR and the first intron, in order to replace the starting ATG of the human PRL gene (in exon 1b) with the starting codon of the reporter gene. The FRT-Neo-FRT cassette was PCR amplified from the pIGCN21 (15) plasmid (kindly provided by Neal Copeland). The PCR fragments were subcloned into pGL3-promoter plasmid (see supplemental Fig. 1a). The luciferase/kanamycin targeting cassette was released from the pGL3-promoter plasmid by a HindIII/SalI digestion, gel purified and used for targeting RP11-273G3 BAC by recombineering (15) in EL250 bacterial strain according to the protocol, available at http://recombineering.ncifcrf.gov/Protocol.asp. In a second step of recombination the kanamycin gene was removed after arabinose induction of the cells.
Recombinant PRL-Luc BAC clones were screened by Southern blot hybridization after EcoRI digestion (see supplemental Fig. 1b).
For the generation of the d2eGFP recombination cassette, the pGL3-promoter plasmid containing the luciferase/kanamycin cassette was targeted with a double-strand linear cassette containing d2eGFP together with a tetracycline gene flanked by lox-P sites. The luciferase gene was substituted by the d2eGFP/tetracycline cassette by homologous recombination in EL350 cells (15). In the second step of recombination, the tetracycline gene was removed after arabinose induction (see supplemental Fig. 2).
The new d2eGFP/kanamycin cassette was then released from the pGL3-promoter plasmid by a HindIII/SalI digestion, gel purified, and used for targeting RP11-273G3 BAC by recombineering (15) as described above.
Cell culture and generation of stable transfected BAC cell lines
The construction of the GH3/hPRL-luc (D44) cell line was described previously (2). Cells were maintained in DMEM with pyruvate /glutamax (Life Technologies Inc., Gaithersburg, MD) and 10% fetal calf serum (Harlan Sera-lab, Belton, UK).
For the generation of the PRL-Luc BAC cell line, GH3 cells (107 cells/ml) were mixed with 30 μg of BAC-DNA (after Kanamycin being substituted by neomycin in the BAC construct) and transfected using an Easyjet Plus (Equibio (York, UK)) electroporation system (250 V, 600 μF). Stable transfectant clones were selected and recloned using G418 (500 μg/ml). The clones were screened for luciferase expression and responses to stimuli (10 nm 17β-estradiol; 1 μm forskolin; FGF-2, 10 ng/ml; 300 nm TRH; 10 nm dexamethasone; TNF-α, 10 ng/ml).
Production of transgenic rats
Recombined RP11-237G3 BAC inserts were freed by a NotI digestion and purified by preparative pulse field gel electrophoresis, β-agarase treatment (New England Biolabs, Beverly, MA), and dialysis against injection buffer (10 mm Tris-HCl, pH 7.5; and 0.1 mm EDTA with 100 mm NaCl) (31). BAC DNA (1 μg/ml) was microinjected into the pronucleus of Fisher 344 rats (Harlan Sera-lab) zygotes. Five transgenic PRL-Luc-positive founders were identified by PCR using primers 5 and 6 (supplemental Table 1) and Southern blot hybridization. Primers 14 and 15 (see supplemental Table 1) were used instead to identify PRL-d2eGFP-positive founders by PCR. Animal studies were undertaken under UK Home Office License, following review by local ethics committee. All rats were housed individually, given free access to water and standard commercial rat chow (Special Diet Service, Witham Health Services, Essex, UK), and maintained under controlled conditions of temperature (21 ± 1 C) and humidity (50 ± 10%), under a 12-h light, 12-h dark cycle. Female rats were treated with 0.04 mg of LHRH (Sigma-Aldrich) at d −3 to synchronize their estrous cycle before real-time qPCR.
Cell imaging
Either collagenase type I-dispersed pituitaries or GH3 BAC-transfected cells (105) were cultured in 10% fetal calf serum and then serum starved for 24 h before imaging. Luciferin (1 mm; BioSynth AG, Staad, Switzerland) was added at least 10 h before the start of the experiment, and the cells were transferred to a Zeiss (Welwyn Garden City, Hertfordshire, UK) Axiovert 100M in a dark room. The cells were maintained at 37 C with 5% CO2–95% air. Bright-field images were taken before and after luminescence imaging. Luminescence images were obtained using either a Fluar ×20, 0.75 NA objective and captured using a photon-counting charge-coupled device camera (Orca II; Hamamatsu Photonics, Bridgewater, NJ; Fig. 2) or a Fluar ×10, 0.5 numerical aperture objective using a photon-counting Hamamatsu two-stage video intensification microscope intensified camera (Fig. 4). Sequential images, each integrated over 30 min, were taken using 4 × 4 binning and analyzed using Kinetic Imaging software AQM6 (Andor, Belfast, Ireland). Regions of interest were drawn around each single cell, and total photon counts for individual cell areas were obtained from each image.
Mean intensity data were collected after the average instrument noise (corrected for the number of pixels being used) was subtracted from the luminescence signal.
FISH
Rat spleen cells were cultured in RPMI 1640 medium/10% fetal calf serum with LPS (O111:B4, Sigma-Aldrich), 25 μg/ml; Concanavalin A (Sigma-Aldrich), 5 μg/ml; and β-mercaptoethanol, 0.5%. After 48 h, colcemid (0.25 μg/ml) was added for 60 min. Standard techniques for hypotonic treatment, methanol/acetic acid fixation, and slide preparation were used (32).
FISH was performed as previously described (33) using biotin 16-dUTP (Roche, Indianapolis, IN)-labeled PRL-Luc BAC DNA, a 1:500 dilution of an avidin-fluorescein isothiocyanate (Vector Laboratories) antibody, followed by 30-min incubation with an antiavidin biotin antibody (1:100) (Vector Laboratories), and a second incubation with the avidin-fluorescein isothiocyanate antibody. Slides were finally mounted in Vectashield (Vector Laboratories) containing 4′,6-diamidino-2-phenylindole counterstain.
Immunocytochemistry
Immunofluorescent staining was performed as described previously (34) using the following antibodies: rabbit antiovine PRL (MCNR51) (35), mouse antiluciferase (P. pyralis) (Invitrogen, Carlsbad, CA/Zymed Laboratories (South San Francisco, CA) and a monoclonal antibody against GFP (Aequorea victoria) (CLONTECH) were used at 1:4000 and 1:50 and 1:100, respectively. All tissues were blocked using goat serum (NGS; Diagnostics Scotland) diluted 1:5 in PBS (pH 7.4) containing 5% BSA before the addition of the primary antibody. All primary antibodies were incubated overnight at 4 C. For the direct detection of PRL in PRL-Luc rats, goat antirabbit Alexa fluor 488 (1:200; Molecular Probes, Inc., Eugene, OR) was used. However, indirect detection using biotinylated goat antimouse IgG (1:500; Vector Laboratories) followed by Streptavidin Alexa 546 (1:200; Molecular Probes) was used to reveal luciferase. For the detection of PRL in PRL-d2eGFP rats a goat antirabbit peroxidase (1:200; DAKO Corp., Carpinteria, CA) was used followed by tyramide Cy3 (1:50; PerkinElmer, Wellesley, MA). However, antigen retrieval and indirect detection with goat antimouse biotinylated (1:200; DAKO) followed by avidin Alexa 488 (1:200; Invitrogen) was performed for detecting eGFP. Finally, sections were mounted using Permafluor fluorescent mounting medium and then examined using a Zeiss LSM510 confocal (Zeiss). For each section, four fields were examined at random, and the number of bihormonal PRL/luciferase and PRL-only cells were determined using Image-Pro Plus analysis software (Media Cybernetics, Bethesda, MD) (n = 8, total number of fields counted for each sex).
RT-PCR and qPCR
Total mRNA was extracted from rat pituitaries, thymus (parathymic lymph nodes), and spleen using the TRIzol reagent (Invitrogen). First-strand cDNA was synthesized using random octamers (8-mers) and Superscript II (Invitrogen) on 2 μg total RNA.
PRL-specific primers and probe were nos. 7, 8, and 9; luciferase-specific primers and probe were nos. 10, 11, and 12; d2EGFP-specific primers and probes were nos. 16, 17, and 18 (see supplemental Table 1). RT-PCR for the detection of extrapituitary mRNA was performed using forward primer 98 (6) in PRL exon 1a and reverse primers no. 13 in PRL 5′-UTR and no. 11 in the luciferase gene (supplemental Table 1).
Real-time quantitative PCR (qPCR) was performed as described previously (36) on a LightCycler 480 System (Roche). Each sample was analyzed in triplicate along with no template controls. The data were normalized to 18S gene expression.
In vivo studies
Adult male and female rats from line PRL-Luc49 and from double-transgenics line PRL-Luc/d2EGFP were analyzed. PRL-Luc transgene expression was monitored in vivo using the approach of biophotonic imaging (16).
Photons were detected noninvasively using a sensitive IVIS Spectrum (Caliper Life Sciences, Hopkinton, MA). Rats were anesthetized with isoflurane and injected with luciferin (Biosynth AG) (150 mg/kg ip injection) 10 min before imaging. Images were taken using the following parameters: exposure time 30–60 sec, binning 4–8, no filter, f-stop 1, variable field of view (FOV) 6.6–26. Images were analyzed by using Living Image Software (Caliper Life Sciences).
Adult PRL-Luc transgenic rats were treated with either 3 mg/kg LPS O127:B8 (Sigma-Aldrich) or physiological saline by ip injection and imaged 16 h later.
Flow cytometry analysis
Peritoneal exudate was recovered from double-transgenic rats in PBS/heparin (5 U/ml) solution. Red blood cells were lysed with a 1 mm NH4HCO3 and 114 mm NH4Cl solution. The cells were then fixed in 2% formaldehyde in PBS solution and analyzed with a FACSVantage SE (Becton Dickinson and Co., Clifton Lakes, NJ) apparatus.
Statistical analysis
Results for real-time PCR are shown as arbitrary units ± sd and for the immunocytochemistry as mean percentage ± sd. Statistical significance for both of these assays was determined by nonparametric two-tailed Mann-Whitney test. Results were considered significant at P < 0.05.
Acknowledgments
We thank M. Sharp, I. Dransfield, A. Rossi, and J. Horn for useful discussion; D. Salter, L. Ramage for access to human samples; K. Featherstone for preparation of thick pituitary sections; S. Boyle for performing the FISH analysis; and D. Dunbar for the statistical analysis.
Footnotes
This work was supported by Wellcome Trust Programme Grant 067252. S.S. is the recipient of a Wellcome Trust Intermediate Fellowship (Cardiovascular Research Initiative); J.J.M. is the recipient of a Wellcome Trust Principal Research Fellowship; for part of this work, C.H. was the recipient of the Professor John Glover Memorial Fellowship; A.D.A. held an Medical Research Council Ph.D. studentship. A.S.M. and J.R.M. are funded by the Medical Research Council UK. Cell imaging instrumentation was funded by BBSRC, Carl Zeiss, and Hamamatsu Photonics. We also acknowledge the support of the Wellcome Trust Functional Genomics Initiative and the British Heart Foundation Centre for Research Excellence (CORE).
First Published Online January 15, 2009
* S.F. and C.V.H. contributed equally to this work.
Abbreviations: BAC, Bacterial artificial chromosome; eGFP, enhanced green fluorescent protein; d2eGFP, destabilized eGFP; FGF-2, fibroblast growth factor 2; FISH, fluorescence in situ hybridization; FOV, field of view; IVIS, in vivo imaging system; LPS, lipopolysaccharide; PRL, prolactin; qPCR, quantitative PCR; sr, steradian; UTR, untranslated region.
References
- 1.Elowitz MB, Levine AJ, Siggia ED, Swain PS2002. Stochastic gene expression in a single cell. Science 297:1183–1186 [DOI] [PubMed] [Google Scholar]
- 2.Takasuka N, White MRH, Wood CD, Robertson WR, Davis JRE1998. Dynamic changes in prolactin promoter activation in individual living lactotrophic cells. Endocrinology 139:1361–1368 [DOI] [PubMed] [Google Scholar]
- 3.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA1998. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19:225–268 [DOI] [PubMed] [Google Scholar]
- 4.Ben-Jonathan N, Lapensee CR, Lapensee EW2008. What can we learn from rodents about prolactin in humans? Endocr Rev 29:1–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berwaer M, Martial JA, Davis JRE1994. Characterization of an up-stream promoter directing extrapituitary expression of the human prolactin gene. Mol Endocrinol 8:635–642 [DOI] [PubMed] [Google Scholar]
- 6.Gellersen B, Kempf R, Telgmann R, DiMattia GE1994. Nonpituitary human prolactin gene transcription is independent of Pit-1 and differentially controlled in lymphocytes and in endometrial stroma. Mol Endocrinol 8:356–373 [DOI] [PubMed] [Google Scholar]
- 7.Venter JC2003. A part of the human genome sequence. Science 299:1183–1184 [DOI] [PubMed] [Google Scholar]
- 8.Soares MJ2004. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol 2:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerlo S, Davis JRE, Mager DL, Kooijman R2006. Prolactin in man: a tale of two promoters. Bioessays 28:1051–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K1997. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 16:6926–6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchard B, Ormandy CJ, Di Santo JP, Kelly PA1999. Immune system development and function in prolactin receptor-deficient mice. J Immunol 163:576–582 [PubMed] [Google Scholar]
- 12.Matera L1996. Endocrine, paracrine and autocrine actions of prolactin on immune cells. Life Sci 59:599–614 [DOI] [PubMed] [Google Scholar]
- 13.McFerran DW, Stirland JA, Norris AJ, Khan RA, Takasuka N, Seymour ZC, Gill MS, Robertson WR, Loudon AS, Davis JRE, White MRH2001. Persistent synchronized oscillations in prolactin gene promoter activity in living pituitary cells. Endocrinology 142:3255–3260 [DOI] [PubMed] [Google Scholar]
- 14.Stirland JA, Seymour ZC, Windeatt S, Norris AJ, Stanley P, Castro MG, Loudon AS, White MRH, Davis JRE2003. Real-time imaging of gene promoter activity using an adenoviral reporter construct demonstrates transcriptional dynamics in normal anterior pituitary cells. J Endocrinol 178:61–69 [DOI] [PubMed] [Google Scholar]
- 15.Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG2001. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73:56–65 [DOI] [PubMed] [Google Scholar]
- 16.Contag CH, Bachmann MH2002. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng 4:235–260 [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Qu R, Ogura M, Shibata T, Harada H, Hiraoka M2005. Real-time imaging of hypoxia-inducible factor-1 activity in tumor xenografts. J Radiat Res 46:93–102 [DOI] [PubMed] [Google Scholar]
- 18.Adamson AD, Friedrichsen S, Semprini S, Harper CV, Mullins JJ, White MRH, Davis JRE2008. Human prolactin gene promoter regulation by estrogen convergence with TNFα signaling. Endocrinology 149:687–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shorte SL, Leclerc GM, Vazquez-Martinez R, Leaumont DC, Faught WJ, Frawley LS, Boockfor FR2002. PRL gene expression in individual living mammotropes displays distinct functional pulses that oscillate in a noncircadian temporal pattern. Endocrinology 143:1126–1133 [DOI] [PubMed] [Google Scholar]
- 20.Cheong WF, Prahl SA, Welch AJ1990. A review of the optical properties of biological tissues. IEEE J Quantum Electron 26:2166–2185 [Google Scholar]
- 21.DiMattia GE, Gellersen B, Duckworth ML, Friesen HG1990. Human prolactin gene expression. The use of an alternative noncoding exon in decidua and the IM-9-P3 lymphoblast cell line. J Biol Chem 265:16412–16421 [PubMed] [Google Scholar]
- 22.Dubchak I, Brudno M, Loots GG, Pachter L, Mayor C, Rubin EM, Frazer KA2000. Active conservation of noncoding sequences revealed by three-way species comparisons. Genome Res 10:1304–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tautz D2000. Evolution of transcriptional regulation. Curr Opin Genet Dev 10:575–579 [DOI] [PubMed] [Google Scholar]
- 24.Giraldo P, Montoliu L2001. Size matters: use of YACs, BACs and PACs in transgenic animals. Transgenic Res 10:83–103 [DOI] [PubMed] [Google Scholar]
- 25.Copeland NG, Jenkins NA, Court DL2001. Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet 2:769–779 [DOI] [PubMed] [Google Scholar]
- 26.Illenye S, Heintz NH2004. Functional analysis of bacterial artificial chromosomes in mammalian cells: mouse Cdc6 is associated with the mitotic spindle apparatus. Genomics 83:66–75 [DOI] [PubMed] [Google Scholar]
- 27.Friedrichsen S, Harper CV, Semprini S, Wilding M, Adamson AD, Spiller DG, Nelson G, Mullins JJ, White MRH, Davis JRE2006. Tumor necrosis factor-α activates the human prolactin gene promoter via nuclear factor-κB signaling. Endocrinology 147:773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Parra S, Chowen JA, Garcia Segura LM, Argente J1996. Ontogeny of pituitary transcription factor-1 (Pit-1), growth hormone (GH) and prolactin (PRL) mRNA levels in male and female rats and the differential expression of Pit-1 in lactotrophs and somatotrophs. J Neuroendocrinol 8:211–225 [DOI] [PubMed] [Google Scholar]
- 29.Li S, Hammer RE, George-Raizen JB, Meyers KC, Garrard WT2000. High-level rearrangement and transcription of yeast artificial chromosome-based mouse Ig κ transgenes containing distal regions of the contig. J Immunol 164:812–824 [DOI] [PubMed] [Google Scholar]
- 30.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zahringer U, Seydel U, Di Padova F, Schreier M, Brade H1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J 8:217–225 [DOI] [PubMed] [Google Scholar]
- 31.Mullins LJ, Kotelevtseva N, Boyd AC, Mullins JJ1997. Efficient Cre-lox linearisation of BACs: applications to physical mapping and generation of transgenic animals. Nucleic Acids Res 25:2539–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rooney DE, Czepulkowski BH1986. Human cytogenetics: a practical approach. Oxford: IRL
- 33.Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, Rae F, Forrester L, Turner ML, Hayes PC, Harrison DJ, Bickmore WA, Plevris JN2003. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology 124:1891–1900 [DOI] [PubMed] [Google Scholar]
- 34.Pope C, McNeilly JR, Coutts S, Millar M, Anderson RA, McNeilly AS2006. Gonadotrope and thyrotrope development in the human and mouse anterior pituitary gland. Dev Biol 297:172–181 [DOI] [PubMed] [Google Scholar]
- 35.Davis JRE, McVerry J, Windeatt S, Castro MG, Lowenstein PR, Lincoln GA, McNeilly AS2001. Cell-type specific adenoviral transgene expression in the intact ovine pituitary gland after steriotaxic delivery: an in vivo system for long-term multiple parameter evaluation of human pituitary gene therapy. Endocrinology 142:795–801 [DOI] [PubMed] [Google Scholar]
- 36.Gibson UE, Heid CA, Williams PM1996. A novel method for real time quantitative RT-PCR. Genome Res 6:995–1001 [DOI] [PubMed] [Google Scholar]