Abstract
Background
Cognitive impairment without dementia is associated with increased risk for disability, increased health care costs, and progression to dementia. There are no population-based prevalence estimates of this condition in the United States.
Objective
To estimate the prevalence of cognitive impairment without dementia in the United States and determine longitudinal cognitive and mortality outcomes.
Design
Longitudinal study from July 2001 to March 2005.
Setting
In-home assessment for cognitive impairment.
Participants
Participants in ADAMS (Aging, Demographics, and Memory Study) who were age 71 years or older drawn from the nationally representative HRS (Health and Retirement Study). Of 1770 selected individuals, 856 completed initial assessment, and of 241 selected individuals, 180 completed 16- to 18-month follow-up assessment.
Measurements
Assessments, including neuropsychological testing, neurologic examination, and clinical and medical history, were used to assign a diagnosis of normal cognition, cognitive impairment without dementia, or dementia. National prevalence rates were estimated by using a population-weighted sample.
Results
In 2002, an estimated 5.4 million people (22.2%) in the United States age 71 years or older had cognitive impairment without dementia. Prominent subtypes included prodromal Alzheimer disease (8.2%) and cerebrovascular disease (5.7%). Among participants who completed follow-up assessments, 11.7% with cognitive impairment without dementia progressed to dementia annually, whereas those with subtypes of prodromal Alzheimer disease and stroke progressed at annual rates of 17% to 20%. The annual death rate was 8% among those with cognitive impairment without dementia and almost 15% among those with cognitive impairment due to medical conditions.
Limitations
Only 56% of the nondeceased target sample completed the initial assessment. Population sampling weights were derived to adjust for at least some of the potential bias due to nonresponse and attrition.
Conclusion
Cognitive impairment without dementia is more prevalent in the United States than dementia, and its subtypes vary in prevalence and outcomes.
Cognitive impairment that does not reach the threshold for dementia diagnosis is associated with increased risk for progression to dementia in most studies, with progression rates of 10% to 15% per year compared with 1% to 2.5% among cognitively healthy older adults (1–3). However, even among those without dementia, cognitive impairment contributes to decreased quality of life, increased neuropsychiatric symptoms, and increased disability (4, 5), as well as increased health care costs (6, 7). All of these negative outcomes make accurate national estimates of the prevalence of cognitive impairment without dementia essential for determining the full societal impact of cognitive impairment on patients, families, and health care programs. However, previous estimates of the prevalence of this condition from regional and non-U.S. samples have varied from 3% to 29% (8–10), a range that is most likely due to differences in diagnostic criteria and sample characteristics. Estimates of the total number of people with cognitive impairment without dementia in the United States are not available.
We conducted ADAMS (Aging, Demographics, and Memory Study) to determine the national prevalence of dementia and cognitive impairment without dementia in the United States. We previously (11) reported our estimates of the prevalence of dementia. In this article, we report prevalence rates from what we believe to be the first population-based study of cognitive impairment without dementia to include individuals from all regions of the country, as well as rates of progression from cognitive impairment without dementia to dementia and death.
Methods
Participants
We drew the ADAMS sample from the larger HRS (Health and Retirement Study), an ongoing nationally representative cohort study of individuals born before 1954 that was designed to investigate the health, social, and economic implications of aging in the U.S. population (12–14). The HRS began in 1992, and the current sample includes approximately 22 000 participants.
The ADAMS sample began with a stratified random subsample of 1770 individuals age 70 years or older from 5 cognitive strata based on participants’ scores on a self-reported or proxy-reported cognitive measure (15) from the most recent HRS interview (either 2000 or 2002). We further stratified the 3 highest cognitive strata by age (age 70 to 79 years vs. ≥80 years) and sex to ensure adequate numbers in each subgroup. Full details of the ADAMS sample design and selection procedures are described elsewhere (16, 17). The ADAMS initial assessments occurred between July 2001 and December 2003, on average, 13.3 months (SD, 6.9) after the HRS interview. Thus, participants were 71 years of age or older at the initial assessment.
As part of the ADAMS assessment, proxies (usually a spouse or adult child) provided information about the participant’s cognitive and functional decline, neuropsychiatric symptoms, and medical history. Use of proxies to collect this information is preferred, because self-reporting of this type of information may not be reliable, particularly among cognitively impaired individuals.
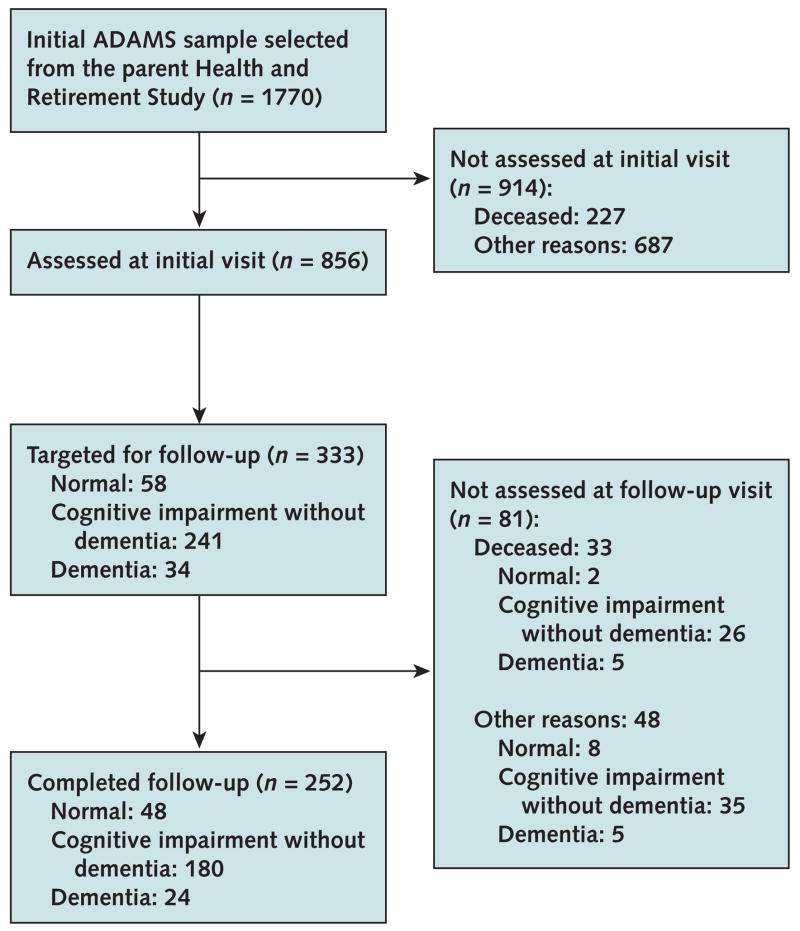
The Figure summarizes the number of participants at each phase of the study; additional details on participation rates are reported elsewhere (17). A total of 856 individuals, 56% of the nondeceased target sample, participated in all phases of the dementia assessment. A major concern in ADAMS, as in similar population-based studies, is the potential for selective nonparticipation to bias prevalence estimates. However, because the ADAMS sample was derived from the HRS sample, a wide range of health and social information was available to assess and correct for potential selection bias due to nonparticipation in our sample. Using logistic regression, we modeled the probability that a sample individual participated in the ADAMS assessment as a function of covariates, such as age, sex, education, marital status, HRS cognition scores, nursing home residency, and indicators of past or existing major health conditions. We used the results of this response propensity analysis to develop nonresponse adjustments to the ADAMS sample selection weights (18). We then constructed population sample weights to take into account the probabilities of selection in the stratified sample design and to adjust for differential nonparticipation in ADAMS (16).
Figure. Study flow diagram.
ADAMS = Aging, Demographics, and Memory Study.
The ADAMS data are publicly available and can be obtained from the HRS Web site (http://hrsonline.isr.umich.edu). The institutional review boards at Duke University Medical Center and the University of Michigan approved all study procedures, and study participants or their surrogates provided informed consent.
Measurements
A nurse and a neuropsychology technician assessed all participants at their residence for cognitive impairment. The full details of the assessment and diagnostic procedures are described elsewhere (17). In brief, the following information about the participant was collected from a knowledgeable informant: chronological history of cognitive symptoms, medical history, current medications, current neuropsychiatric symptoms, measures of severity of cognitive and functional impairment, and family history of memory problems. During the assessment, the participant completed a battery of neuropsychological measures; a self-reported depression measure; a standardized neurologic examination; a blood pressure measurement; collection of buccal DNA samples for apolipoprotein E (APOE) genotyping; and a 7-minute, videotaped segment covering portions of the cognitive status and neurologic examinations. Specific assessment measures reported here are the Mini-Mental State Examination (19) and the Dementia Severity Rating Scale (20). The Dementia Severity Rating Scale is completed by an informant and assesses the presence and severity of impairment in 12 cognitive and functional domains. Scores range from 0 to 54, with higher scores reflecting more impairment. We also sought medical record releases to obtain relevant neuroimaging and laboratory results from participants’ physicians.
A consensus expert panel of neuropsychologists, neurologists, geropsychiatrists, and internists reviewed all information collected during the in-home assessment and assigned final diagnoses. The consensus panel reviewed each case and assigned a diagnosis in 2 stages, first without and then with medical records. For most cases, the consensus panel reached agreement with little discussion; however, diagnostic agreement was more challenging for participants with little or no education or with substantial sensory or physical impairment. The medical records often provided the necessary neuroimaging results to change a diagnosis from possible Alzheimer disease or vascular dementia to probable Alzheimer disease or vascular dementia. Except for these situations, the diagnoses seldom changed after the consensus panel reviewed the additional information in the medical records.
Diagnoses were divided within the 3 general categories: normal cognitive function, cognitive impairment without dementia, and dementia. The consensus panel used clinical judgment to assign the final diagnosis, based on the following criteria. Dementia diagnosis was based on guidelines from the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (21), and the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (22); diagnoses of Alzheimer disease and other types of dementia were based on currently accepted criteria (23–26). The definition of cognitive impairment without dementia and its subtypes has been developed over 17 years, primarily on the basis of the accumulated clinical experience of a group of researchers common to ADAMS and 3 other epidemiologic studies of dementia (27–29). Before ADAMS, we operationalized the definition for cognitive impairment without dementia on the basis of analyses of both neuropsychological data and an objective measure of daily function from participants with this diagnosis in our other studies (27, 28). We defined cognitive impairment without dementia as mild cognitive or functional impairment, reported by the participant or informant, that did not meet criteria for dementia (that is, Dementia Severity Rating Scale score of 6 to 11), or performance on neuropsychological measures that was both below expectation and at least 1.5 SDs below published norms on any test.
To reflect the variation in clinical presentation and potential differences in the cause of the impairment we used 12 diagnostic subcategories for cognitive impairment without dementia, unspecified cognitive impairment without dementia: prodromal Alzheimer disease, amnestic mild cognitive impairment (30, 31), vascular cognitive impairment without dementia, stroke, medical conditions or sensory impairment, neurologic conditions, depression, other psychiatric disorders, low baseline intellect or learning disorder, past alcohol abuse, and current alcohol abuse (Appendix Table 1, available at www.annals.org). In population-based samples, few individuals meet the criteria typically used for amnestic mild cognitive impairment (30, 31). For this reason, we used the additional category of “prodromal Alzheimer disease,” defined as cognitive impairment without dementia with a pattern of clinical symptoms or performance on neuropsychological testing suggestive of prodromal Alzheimer disease and no other medical or neuropsychiatric conditions present to preclude an eventual diagnosis of Alzheimer disease. To show that cognitive impairment is often the consequence of more than 1 pathologic process, we assigned a primary and secondary diagnosis denoting these multiple causes when appropriate.
Appendix Table 1.
Categories and Definitions of Cognitive Impairment without Dementia*
| Category | Definitions |
|---|---|
| Unspecified cognitive impairment without dementia | Meets criteria for cognitive impairment without dementia, but pattern of clinical symptoms and medical history do not fit criteria for other subtypes. |
| Prodromal Alzheimer disease | Pattern of clinical symptoms or performance on neuropsychological testing suggestive of prodromal Alzheimer disease (typically gradual onset of impairment in the areas of memory and executive function); no other medical or neuropsychiatric conditions present to preclude an eventual Alzheimer disease diagnosis. |
| Amnestic mild cognitive impairment | Memory symptom as noted by a Dementia Severity Rating Scale Memory item score greater than 2; at least 1.5 SD below mean on Wechsler Memory Scale Revised Logical Memory II or Delayed Recall on CERAD Word List Delayed Recall; MMSE score of at least 24; memory domain score on CDR of 0.5 and overall CDR score less than 1.0; and symptoms not due to major depression (adapted from reference 31). |
| Vascular cognitive impairment without dementia | Cognitive or functional impairment that had a gradual onset, seems to progress, and includes inconsistent memory performance or slowed processing speed. These problems occur in the presence of clinically significant cardiovascular or cerebrovascular conditions, but are not temporally linked to a single stroke or transient ischemic attack. |
| Stroke | Cognitive or functional deficits and a history of stroke or focal neurologic signs consistent with a stroke. |
| Medical conditions or sensory impairment | Cognitive or functional impairment due to medical conditions or sensory impairment, such as heart disease, lung disease, renal disease, sleep apnea, and other primarily chronic illnesses. Sensory impairments include clinically significant loss of vision or hearing. |
| Neurologic conditions | Cognitive or functional impairment in the presence of a neurologic condition, such as Parkinson disease, traumatic brain injury, or other conditions affecting the central nervous system. Pattern of cognitive impairment is consistent with that expected for the specific condition, and impairment is not due solely to motor impairment from the neurologic conditions. |
| Depression | Cognitive or functional impairment in the presence of reported symptoms consistent with major depression. |
| Other psychiatric disorders | Cognitive or functional impairment in the presence of a neuropsychiatric disorder, such as bipolar disorder, schizophrenia, or characteristics of personality disorder. |
| Past alcohol abuse | Cognitive or functional impairment in the presence of a history of alcohol abuse at least 6 months before. |
| Current alcohol abuse | Cognitive or functional impairment in the presence of ongoing alcohol abuse less than 6 months before. |
| Low baseline intellect or presence of a learning disorder | Cognitive or functional impairment that is thought to be consistent with the individual’s adult baseline function level. Performance on neuropsychological measures is impaired because of lifelong low intellect or a learning disorder. |
Cognitive decline from a previous higher level of function is implied for all other subtypes except the low baseline intellect or presence of learning disorder group. CDR = Clinical Dementia Rating scale; CERAD = Consortium to Establish a Registry for Alzheimer Disease; MMSE = Mini-Mental State Examination.
We selected a subset of ADAMS participants (Figure) to undergo a follow-up assessment approximately 16 to 18 months after the initial assessment. Participants were chosen if they received an initial diagnosis of cognitive impairment without dementia or if they received an initial diagnosis of normal cognition or dementia and the consensus panel thought the findings from the initial assessment were ambiguous and that longitudinal follow-up would help clarify the diagnosis. We used the same assessment protocol for both the initial and follow-up assessments.
Statistical Analysis
Using the ADAMS sample weights described previously, we computed estimates for the national prevalence of cognitive impairment without dementia and its most common subtypes in 2002. We then estimated the number of individuals age 71 years or older with these conditions in the United States in 2002 by using the ADAMS sample weights. We compared characteristics of the major subtypes by using analysis of variance and chi-square tests adjusted for the HRS complex sample design. Initially, we used Rao chi-square tests adjusted for the HRS complex sample design to compare rates of survival and follow-up cognitive outcomes among the 4 most frequent subtypes of cognitive impairment without dementia.
To examine the characteristics associated with cognitive impairment without dementia and its progression to dementia, we used logistic regression to assess the association of age, years of education, sex (male = 1; female = 0), race (black = 1; white = 0; other ethnic and racial groups were excluded because of small sample sizes), and APOE genotype (presence of ε4 allele = 1; absence = 0).
We conducted all analyses by using SAS software, version 9.1.3 (SAS Institute, Cary, North Carolina), and the special survey procedures, which account for the influence of weighting and the other features of the complex sample design on the SEs and CIs of sample estimates, as well as the values of test statistics.
Role of the Funding Source
The National Institute on Aging had no role in the collection, management, analysis, and interpretation of the data or the preparation, review, or approval of the manuscript.
Results
Table 1 shows characteristics of the 856 ADAMS participants, stratified by cognitive status. The cognitive impairment without dementia group comprised individuals who were well distributed across education levels and age ranges and included 36 participants age 90 years or older. Mean age increased from the normal cognition group to the cognitive impairment without dementia and dementia groups, whereas the mean level of education progressively decreased across these groups. The Mini-Mental State Examination score (19) progressively declined from the normal cognition group to the cognitive impairment without dementia group to the dementia group, whereas the Dementia Severity Rating Scale (20) score progressively increased across these groups.
Table 1.
Characteristics of Baseline ADAMS Sample*
| Characteristic | All Dementia | All Cognitive Impairment without Dementia | All Normal Cognition |
|---|---|---|---|
| Total participants, n (%) | 308 (100) | 241 (100) | 307 (100) |
| Mean Mini-Mental State Examination score (SD) | 15.94 (4.27) | 24.75 (3.27) | 27.84 (2.99) |
| Mean Dementia Severity Rating Scale score (SD) | 22.13 (8.50) | 6.17 (4.78) | 1.59 (2.86) |
| Mean age, y | 84.45 (4.06) | 80.92 (5.90) | 77.37 (6.69) |
| Men, n (%) | 95 (31.5) | 119 (44.9) | 141 (39.1) |
| Mean education (SD), y | 10.33 (2.46) | 10.81 (3.46) | 12.36 (4.16) |
| Race/ethnicity, n (%) | |||
| Non-Hispanic white | 218 (83.4) | 157 (84.2) | 238 (89.0) |
| Non-Hispanic black | 67 (12.4) | 52 (10.4) | 40 (5.7) |
| Hispanic | 23 (4.2) | 32 (5.4) | 29 (5.4) |
Values for n are unweighted. Means and percentages are weighted and calculated within columns. Because of missing data, the total number for the Mini-Mental State Examination is 814 and the total number for Dementia Severity Rating Scale is 808. Totals do not all add up to 100% because of rounding. ADAMS = Aging, Demographics, and Memory Study.
Table 2 shows the frequency and characteristics of the subtypes of cognitive impairment without dementia. Patients with the 4 most frequent subtypes (prodromal Alzheimer disease, medical conditions, stroke, and vascular cognitive impairment without dementia) were similar in age, years of education, Mini-Mental State Examination score, and Dementia Severity Rating Scale score. However, women were more frequent in the medical conditions group than in the 3 other most common subtypes (chi-square, 12.17; P = 0.004).
Table 2.
Frequencies and Estimated Population Characteristics for Cognitive Impairment without Dementia Subtypes*
| Cognitive Impairment without Dementia Subtype after Initial Assessment | Frequency, n (%) | Mean Age (SD), y | Mean Education (SD), y | Mean Mini-Mental State Examination Score (SD) | Men, % | Mean Dementia Severity Rating Scale Score (SD) |
|---|---|---|---|---|---|---|
| Prodromal Alzheimer disease | 94 (34.2) | 81.88 (5.94) | 10.97 (3.58) | 24.13 (3.44) | 53.8 | 4.86 (3.94) |
| Medical conditions | 55 (23.9) | 80.28 (5.96) | 10.51 (2.81) | 24.63 (2.77) | 26.5† | 7.08 (4.97) |
| Stroke | 34 (15.5) | 81.04 (5.41) | 11.78 (3.07) | 25.70 (3.45) | 55.5 | 6.25 (5.82) |
| Vascular cognitive impairment without dementia | 20 (10.1) | 78.63 (6.25) | 11.10 (3.39) | 25.80 (2.98) | 36.2 | 5.20 (4.61) |
| Depression and other psychiatric disorders | 10 (5.1) | 80.21 (3.59) | 11.21 (3.60) | 23.67 (1.25) | 14.6 | 7.98 (5.92) |
| Neurologic conditions | 10 (5.2) | 82.71 (6.71) | 8.59 (5.29) | 27.00 (2.89) | 72.2 | 8.08 (5.19) |
| Amnestic mild cognitive impairment | 4 (2.8) | 82.45 (12.52) | 12.59 (3.56) | 25.72 (0.93) | 45.8 | 12.08 (5.93) |
| Past or current alcohol abuse | 6 (1.7) | 76.89 (1.34) | 10.58 (2.78) | 25.37 (1.92) | 91.8 | 8.20 (2.89) |
| Low baseline intellect or presence of learning disorders | 8 (1.4) | 81.12 (4.66) | 3.06 (1.91) | 16.51 (2.64) | 37.2 | 2.52 (1.64) |
| Total | 241 (100) | 80.92 (5.90) | 10.81 (3.46) | 24.75 (3.27) | 44.9 | 6.17 (4.78) |
Values for n are unweighted. Means and percentages are weighted and are calculated within columns. Some subtypes are grouped together because of small sample sizes. Means or percentages are compared among the 4 most frequent cognitive impairment without dementia subtypes.
Persons in the medical conditions subgroup were more likely to be female (P = 0.004).
Table 3 shows the national prevalence estimates for cognitive impairment without dementia and its more frequent subtypes, stratified by 9- or 10-year age ranges. It also reports the estimated number of individuals with cognitive impairment without dementia in the United States for the same groups. We estimated that 5.4 million people age 71 years or older had cognitive impairment without dementia in 2002, whereas an estimated 2.0 million had prodromal Alzheimer disease. Because of the uncertainty surrounding the diagnostic criteria for cognitive impairment without dementia, we explored how the prevalence estimates for dementia would change if we considered some of those with a diagnosis of cognitive impairment without dementia at the initial assessment to have had dementia at baseline. For these analyses, we considered all individuals who progressed to dementia at the follow-up visit to have had dementia at baseline, whereas all those who progressed to Alzheimer disease were considered to have Alzheimer disease at baseline. Because estimates of the prevalence of cognitive impairment without dementia for persons younger than age 71 years are not available, we used rates of dementia for persons age 60 to 71 years from other studies (32, 33) and combined them with estimates from this study and our previous research (11). This resulted in an estimate of almost 4.7 million individuals age 60 years or older with dementia in the United States, up from our previous estimate of 3.8 million, and a total of 3.3 million with Alzheimer disease, up from 2.5 million (11).
Table 3.
Estimated National Prevalence of Cognitive Impairment without Dementia, by Age Categories*
| Age | All Cognitive Impairment without Dementia (n = 241) |
Prodromal Alzheimer Disease (n = 98)† |
Vascular Cognitive Impairment without Dementia and Stroke (n = 54)† |
Medical Conditions (n = 55)† |
||||
|---|---|---|---|---|---|---|---|---|
| Prevalence (95% CI), % | Population Estimate (95% CI) | Prevalence (95% CI), % | Population Estimate (95% CI) | Prevalence (95% CI), % | Population Estimate (95% CI) | Prevalence (95% CI), % | Population Estimate (95% CI) | |
| 71–79 y | 16.0 (11.5–20.5) | 2.29 (1.65–2.94) | 5.5 (2.6–8.4) | 0.79 (0.37–1.20) | 3.5 (1.4–5.6) | 0.50 (0.20–0.80) | 4.7 (1.2–8.5) | 0.674 (0.17–1.22) |
| 80–89 y | 29.2 (24.3–34.1) | 2.41 (2.00–2.81) | 9.7 (6.4–13.1) | 0.80 (0.528–1.081) | 10.1 (6.4–13.9) | 0.83 (0.53–1.15) | 5.4 (2.1–8.7) | 0.45 (0.17–0.72) |
| ≥90 y | 39.0 (25.7–52.2) | 0.73 (0.48–0.98) | 22.4 (11.9–32.9) | 0.42 (0.22–0.62) | 2.9 (0.0–6.3) | 0.05 (0.0–0.12) | 9.2 (0.2–18.2) | 0.17 (0.00–0.34) |
| Total | 22.2 (18.7–25.7) | 5.43 (4.57–6.29) | 8.2 (6.5–10.0) | 2.01 (1.59–2.45) | 5.7 (3.8–7.6) | 1.39 (0.93–1.86) | 5.3 (2.6–8.0) | 1.30 (0.64–1.96) |
Proportions are weighted. Population estimates in millions (and their CIs) were calculated by using the Aging, Demographics, and Memory Study population sampling weights.
“Prodromal Alzheimer Disease” includes amnestic mild cognitive impairment subtype. The 3 right columns are a subset of the column “All Cognitive Impairment without Dementia.” The percentages across rows for these 3 subgroups do not equal the percentage for the “All Cognitive Impairment without Dementia” group because the latter group includes additional subgroups not presented in the table.
In a logistic regression model, cognitive impairment without dementia was more likely in older persons (odds ratio [OR], 1.13 [95% CI, 1.09 to 1.17] per year) and men (OR, 1.62 [CI, 1.09 to 2.41]) and less likely in those with more years of education (OR, 0.89 [CI, 0.84 to 0.94] per year). Among those with prodromal Alzheimer disease, 48.3% had at least 1 APOE ε4 allele, a rate higher than that in the 3 other most common subtypes (chi-square, 12.23; P = 0.007). However, the presence of an APOE ε4 allele was not significantly related in the model to the odds of cognitive impairment, including all subtypes (OR, 1.56 [CI, 0.92 to 2.67]). Race was not significantly associated with cognitive impairment without dementia (OR, 1.29 [CI, 0.66 to 2.54]).
Appendix Tables 2 to 4 (available at www.annals.org) present unweighted values from Tables 1 to 3, respectively.
Appendix Table 2.
Characteristics of Baseline ADAMS Sample*
| Characteristic | All Dementia | All Cognitive Impairment without Dementia | All Normal Cognition |
|---|---|---|---|
| Total participants, n (%) | 308 (100) | 241 (100) | 307 (100) |
| Mean Mini-Mental State Examination (SD) score | 14.34 (6.37) | 22.94 (4.24) | 26.99 (2.91) |
| Mean Dementia Severity Rating Scale (SD) score | 23.14 (13.19) | 6.40 (5.41) | 1.83 (2.68) |
| Mean age (SD), y | 85.35 (6.87) | 81.42 (6.91) | 77.93 (5.35) |
| Men, n (%) | 95 (30.8) | 119 (49.4) | 141 (45.9) |
| Mean education (SD), y | 9.47 (4.25) | 8.95 (4.59) | 11.53 (3.77) |
| Race/ethnicity, n (%) | |||
| Non-Hispanic white | 218 (70.8) | 157 (65.1) | 238 (77.5) |
| Non-Hispanic black | 67 (21.8) | 52 (21.6) | 40 (13.0) |
| Hispanic | 23 (7.5) | 32 (13.3) | 29 (9.5) |
Values for n are unweighted. Percentages are unweighted. Unweighted percentages do not represent the population. Because of missing data, the total number for the Mini-Mental State Examination is 814 and the total number for Dementia Severity Rating Scale is 808. Totals do not all add up to 100% because of rounding. ADAMS = Aging, Demographics, and Memory Study.
Appendix Table 4.
Subtypes of Cognitive Impairment without Dementia, by Age Categories (Unweighted)*
| Age | All Cognitive Impairment without Dementia, n | Prodromal Alzheimer Disease, n† | Vascular Cognitive Impairment without Dementia and Stroke, n† | Medical Conditions, n† |
|---|---|---|---|---|
| 71–79 y | 94 | 30 | 21 | 26 |
| 80–89 y | 111 | 46 | 29 | 21 |
| ≥90 y | 36 | 22 | 4 | 8 |
| Total | 241 | 98 | 54 | 55 |
Values for n are unweighted.
“Prodromal Alzheimer Disease” includes mild cognitive impairment subtype. The 3 right columns are subsets of “All Cognitive Impairment without Dementia.” The sums of these 3 subgroups do not equal the total for the “All Cognitive Impairment without Dementia” group because the latter group includes additional subgroups not presented in the table.
We reassessed participants, on average, 17.04 months (SD, 1.62) after their initial assessment. In a logistic regression model, progression from cognitive impairment without dementia to dementia at follow-up was more probable among older individuals (OR, 1.08 [CI, 1.02 to 1.14] per year) and less probable among those with more years of education (OR, 0.88 [CI, 0.81 to 0.96] per year). Men tended to be less likely to progress to dementia (OR, 0.37 [CI, 0.13 to 1.05]). Neither race (OR, 0.46 [CI, 0.16 to 1.27]) nor presence of an APOE ε4 allele (OR, 1.33 [CI, 0.55 to 3.23]) was significantly associated with progression to dementia.
Table 4 summarizes the outcomes of the follow-up assessments for the most frequent subtypes of cognitive impairment without dementia. On follow-up, 80.4% of participants were again classified as having cognitive impairment without dementia or had progressed to dementia. The annualized rate of progression to dementia was about 12%, whereas the rate of progression was 17% to 20% per year in the prodromal Alzheimer disease and stroke groups. Given the small sample sizes, differences in cognitive outcomes (chi-square, 14.84; P = 0.062) and mortality outcomes (chi-square, 4.19; P = 0.24) among the 4 most frequent subtypes did not reach standard significance levels. The 4 participants with amnestic mild cognitive impairment at baseline remained cognitively impaired without dementia at follow-up. Among those who had progressed to dementia at follow-up, 83% had Alzheimer disease, 16.7% had possible vascular dementia, and 0.4% had dementia of undetermined cause.
Table 4.
Outcomes of Cognitive Impairment without Dementia at Follow-up Assessment*
| Follow-up Outcome | Diagnosis at Initial Assessment |
|||||
|---|---|---|---|---|---|---|
| All Cognitive Impairment without Dementia | Prodromal Alzheimer Disease | Medical Conditions | Vascular Cognitive Impairment without Dementia | Stroke | Other | |
| Total participants, n | 241 | 94 | 55 | 20 | 34 | 38 |
| Follow-up completed, n | 180 | 71 | 36 | 15 | 23 | 35 |
| Dementia | ||||||
| Participants, n | 39 | 21 | 7 | 2 | 5 | 4 |
| Rate (±SE), % | 16.7 ± 3.5 | 27.9 ± 7.4 | 8.6 ± 3.4 | 9.3 ± 7.3 | 24.9 ± 5.0 | 2.6 ± 1.3 |
| Annualized rate (±SE), % | 11.7 ± 2.5 | 20.1 ± 5.5 | 6.0 ± 2.4 | 6.1 ± 4.9 | 17.1 ± 3.5 | 1.9 ± 0.9 |
| Cognitive impairment without dementia | ||||||
| Participants, n | 116 | 43 | 19 | 12 | 14 | 28 |
| Rate (±SE), % | 63.8 ± 6.1 | 53.7 ± 8.0 | 54.4 ± 6.5 | 83.8 ± 7.0 | 61.5 ± 11.2 | 85.3 ± 4.1 |
| Normal | ||||||
| Participants, n | 25 | 7 | 10 | 1 | 4 | 3 |
| Rate (±SE), % | 19.6 ± 4.2 | 18.4 ± 4.0 | 37.0 ± 6.8 | 7.0 ± 0.6 | 13.6 ± 7.9 | 12.1 ± 4.0 |
| Follow-up not completed, n | 61 | 23 | 19 | 5 | 11 | 3 |
| No visit | ||||||
| Participants, n | 35 | 14 | 7 | 4 | 7 | 3 |
| Rate (±SE), % | 11.7 ± 2.7 | 10.0 ± 2.8 | 7.4 ± 1.4 | 30.4 ± 11.0 | 16.7 ± 8.2 | 4.9 ± 0.6 |
| Death | ||||||
| Participants, n | 26 | 9 | 12 | 1 | 4 | 0 |
| Rate (±SE), % | 10.2 ± 2.2 | 8.9 ± 4.0 | 20.6 ± 1.8 | 4.4 ± 0.7 | 11.6 ± 1.4 | 0 |
| Annualized rate (±SE), % | 8.0 ± 2.2 | 6.4 ± 3.2 | 14.9 ± 2.3 | 4.4 ± 0.7 | 11.6 ± 1.4 | 0 |
Values for n are unweighted. Percentages are weighted sample-based estimates of the change that occurred in the population that the sample represented. Percentages for “Death” and “No visit” were calculated by column using the total number of participants. Percentages for “Dementia,” “Cognitive Impairment without Dementia,” and “Normal” were calculated by column using the number of patients who completed follow-up assessment. Rate (%) is based on outcome at 16 to 18 months of follow-up. Annualized rate for dementia was based on prorating to a 12-month interval the percentage that progressed to dementia. The annualized rate for the deceased is the percentage of those who died within 12 months of initial assessment. All remaining “Cognitive Impairment without Dementia” subtypes are included in “Other.”
Discussion
The ADAMS has produced the first prevalence estimates of cognitive impairment without dementia and its major subtypes in a nationally representative sample in the United States. We estimate that 22.2% (about 5.4 million) of individuals in the United States age 71 years or older have cognitive impairment without dementia. These results suggest that the number of individuals with cognitive impairment without dementia in the United States is about 70% higher than that with dementia, based on our previous estimate (11) of 3.4 million individuals in this age group in the United States with dementia. In the 71- to 79-year age group, 16% had cognitive impairment without dementia, whereas an additional 5% had dementia (11), suggesting that more than 1 of 5 individuals in this age group has cognitive impairment. Overall, individuals with cognitive impairment without dementia progressed to dementia at a rate of about 12% per year, but the annual rate of progression ranged from 2% to 20% across the various subtypes. The overall annual mortality rate was 8%, but it ranged from 0% to almost 15% across the various subtypes of cognitive impairment without dementia.
To date, no other estimates of the number of individuals with cognitive impairment without dementia in the United States are available to compare with these ADAMS estimates (MEDLINE search for English-language articles to December 2007). Reviews often report the prevalence of cognitive impairment without dementia as ranging from 5% to 29% (9, 10). Even so, estimates from the few available U.S. regional and Canadian samples report prevalence rates for cognitive impairment without dementia of 17% to 23% (34–36), closely bracketing the ADAMS estimate of 22.2%. Selected European population studies using different criteria for cognitive impairment without dementia report prevalence rates ranging from 21% to 27% (37, 38).
Our findings suggest that cognitive impairment without dementia due to chronic medical conditions accounts for about 24% of all cognitive impairment without dementia in the United States. Previous research suggests that individuals with this condition are less likely to be seen at university clinics for memory disorders (39) and may be excluded from clinical trials (31) even though numerous epidemiologic studies have reported an association between some of these medical conditions (for example, diabetes and heart failure) and cognitive impairment (40). This large group may be the most underdiagnosed subtype of cognitive impairment without dementia, and their cognitive impairment may get relatively less attention from medical providers as the treatment of their primary health issues takes priority.
Previous epidemiologic research (41) has noted variation across subtypes of cognitive impairment without dementia in rates of prevalence, progression to dementia, and mortality, which shows that cognitive impairment without dementia is a heterogeneous condition with multiple causes. Accurately distinguishing among subtypes of cognitive impairment without dementia will become more important as effective treatments are developed.
No single set of consensus criteria for cognitive impairment without dementia is currently available. This has led to debate about the appropriate classification of individuals in the zone between normal cognition and dementia, which has implications for the interpretation of our results. Whereas some research groups have pointed to clinical, neuropathologic, and functional neuroimaging evidence to suggest that individuals with mild impairment constitute a distinct group in transition from normal cognition to dementia (42), others have suggested that those with mild impairment actually have early dementia (43). When we assessed the effect on the prevalence rate of dementia after classifying some participants with cognitive impairment without dementia to have had dementia at baseline, the prevalence rate for Alzheimer disease increased but was still substantially less than the higher estimates of 4.5 million (44, 45) and 5 million with Alzheimer disease (46) reported by other studies.
Limitations of ADAMS include possible response bias from a participation rate that was lower than we hoped for. To minimize response bias, we used archived information from past interviews to develop response propensity models and associated weighting adjustments. The range of available measures used in these models probably captured most of the major factors that could statistically significantly contribute to selection bias in our population estimates. As proof of this, our calculations showed that the total size of the population age 71 years or older in 2002 using the ADAMS sample weights closely matched the population estimates from the U.S. Census Bureau (47). Diagnostic errors may have occurred because of inaccuracies in the diagnostic criteria and in the assignment of the diagnosis by the consensus panel. The criteria for both cognitive impairment without dementia and its subtypes are in the developmental stages and require further validation (48, 49). The diagnostic subtypes are loosely defined and rely substantially on clinical judgment. They may not truly reflect different causes or predict prognosis. However, just as the criteria for several other neuropsychiatric conditions have evolved over time, we expect that these criteria will be refined after additional investigation to further characterize clinical phenotypes, identification of specific biological markers, and longer follow-up periods to determine outcomes. We attempted to minimize variability in the assignment of the clinical diagnosis within this study by using assessment teams based at a single site that used methods established in our previous epidemiologic studies and by using 1 expert case review panel.
Our findings show that cognitive impairment without dementia affects a very large segment of the elderly population. The heterogeneous presentation of symptoms and outcomes in these individuals implies varied underlying causes that may provide opportunities for prevention strategies on several fronts. Prevention strategies may include programs targeting stroke prevention, cardiovascular risk factor reduction (for example, exercise and nutrition), and similar proactive approaches to education and management of chronic medical conditions. Positive gains from these strategies may reduce the prevalence of cognitive impairment without dementia and may have marked benefits for public health. Future longitudinal research using data from HRS and ADAMS, as well as from other studies of aging, may identify interventions that reduce the prevalence of cognitive impairment without dementia and benefit patients, families, and our aging society.
Appendix Table 3.
Frequencies and Percentages for Cognitive Impairment without Dementia Subtypes*
| Cognitive Impairment without Dementia Diagnosis after Initial Assessment | Frequency, n (%) | Mean Age (SD), y | Mean Education (SD), y | Mean Mini-Mental State Examination Score (SD) | Male Sex, % | Mean Dementia Severity Rating Scale Score (SD) |
|---|---|---|---|---|---|---|
| Prodromal Alzheimer disease | 94 (39.0) | 83.35 (6.99) | 8.90 (5.06) | 22.12 (4.41) | 47.9 | 5.70 (5.31) |
| Medical conditions | 55 (22.8) | 80.62 (7.05) | 8.95 (4.03) | 23.39 (3.79) | 34.5 | 7.11 (5.53) |
| Stroke | 34 (14.1) | 80.50 (6.72) | 9.94 (4.07) | 23.53 (4.52) | 64.7 | 5.91 (5.25) |
| Vascular cognitive impairment without dementia | 20 (8.3) | 79.45 (6.53) | 9.90 (3.95) | 24.50 (2.89) | 50.0 | 6.09 (5.04) |
| Depression and other psychiatric disorders | 10 (4.1) | 79.00 (3.83) | 8.70 (4.24) | 23.20 (1.93) | 60.0 | 8.33 (6.24) |
| Neurologic conditions | 10 (4.1) | 82.20 (6.18) | 9.60 (3.81) | 26.00 (3.46) | 70.0 | 7.38 (5.63) |
| Amnestic mild cognitive impairment | 4 (1.7) | 79.75 (9.22) | 11.50 (3.32) | 25.50 (1.00) | 50.0 | 11.00 (4.24) |
| Past or current alcohol abuse | 6 (2.5) | 77.33 (2.88) | 6.33 (5.72) | 23.60 (3.85) | 66.7 | 8.50 (7.40) |
| Low baseline intellect or presence of learning disorders | 8 (3.3) | 79.00 (8.05) | 3.00 (3.21) | 17.25 (4.59) | 37.5 | 2.86 (2.97) |
| Total | 241 (100.0) | 81.42 (6.91) | 8.95 (4.59) | 22.94 (4.24) | 49.4 | 6.40 (5.41) |
Values for n are unweighted. Percentages are unweighted and are calculated within columns. Unweighted percentages do not represent the population. Some subtypes are grouped together because of small sample sizes.
Acknowledgments
The authors gratefully acknowledge the scientific and clinical input of the other members of the consensus conference panel: Kathleen A. Welsh-Bohmer, PhD; John C.S. Breitner, MD; Norman L. Foster, MD; Bruno Giordani, PhD; Hugh Hendrie, MB, ChB, DSc; and Frederick W. Unverzagt, PhD. Drs. Breitner and Welsh-Bohmer were involved in the early planning and development of ADAMS and in obtaining the project funding. The authors also acknowledge the invaluable contributions of the ADAMS participants and the research staff at Duke University Medical Center and the University of Michigan.
Grant Support: By the National Institute on Aging (U01 AG09740). Dr. Langa was supported by National Institute on Aging grants K08 AG019180 and R01 AG027010 and a Paul Beeson Physician Faculty Scholars award.
Footnotes
Author Contributions: Conception and design: B.L. Plassman, K.M. Langa, G.G. Fisher, S.G. Heeringa, D.R. Weir, M.B. Ofstedal, J.R. Burke, M.D. Hurd, G.G. Potter, W.L. Rodgers, D.C. Steffens, R.J. Willis, R.B. Wallace.
Analysis and interpretation of the data: B.L. Plassman, K.M. Langa, G.G. Fisher, S.G. Heeringa, D.R. Weir, M.B. Ofstedal, J.R. Burke, M.D. Hurd, G.G. Potter, W.L. Rodgers, D.C. Steffens, J.J. McArdle, R.B. Wallace.
Drafting of the article: B.L. Plassman, K.M. Langa, G.G. Fisher.
Critical revision of the article for important intellectual content: B.L. Plassman, K.M. Langa, G.G. Fisher, S.G. Heeringa, D.R. Weir, M.B. Ofstedal, J.R. Burke, M.D. Hurd, G.G. Potter, W.L. Rodgers, D.C. Steffens, J.J. McArdle, R.J. Willis, R.B. Wallace.
Final approval of the article: B.L. Plassman, K.M. Langa, G.G. Fisher, S.G. Heeringa, D.R. Weir, M.B. Ofstedal, J.R. Burke, M.D. Hurd, G.G. Potter, W.L. Rodgers, D.C. Steffens, J.J. McArdle, R.J. Willis, R.B. Wallace.
Statistical expertise: B.L. Plassman, K.M. Langa, G.G. Fisher, S.G. Heeringa, D.R. Weir, M.D. Hurd, J.J. McArdle, W.L. Rodgers. Obtaining of funding: B.L. Plassman, K.M. Langa, S.G. Heeringa, D.R. Weir, M.B. Ofstedal, M.D. Hurd, W.L. Rodgers, R.J. Willis, R.B. Wallace.
Administrative, technical, or logistic support: B.L. Plassman, G.G. Fisher, S.G. Heeringa, J.R. Burke, G.G. Potter, D.C. Steffens.
Collection and assembly of data: B.L. Plassman, K.M. Langa, G.G. Fisher, M.B. Ofstedal, J.R. Burke, G.G. Potter, D.C. Steffens, R.B. Wallace.
Potential Financial Conflicts of Interest: None disclosed.
Reproducible Research Statement: The study protocol and data are available on the HRS Web site (http://hrsonline.isr.umich.edu/adams/overview/summary_2.htm). The statistical code is available to interested readers by contacting Dr. Heeringa (e-mail, sheering@isr.umich.edu) or Dr. Fisher (e-mail, gwenithf@isr.umich.edu).
Current author addresses and author contributions are available at www.annals.org.
Current Author Addresses: Drs. Plassman, Potter, and Steffens: 905 West Main Street, Box 41, Suite 25-D, Durham, NC 27701.
Dr. Langa: 300 North Ingalls Building, Room 7E01, Ann Arbor, MI 48109-0429.
Drs. Fisher, Heeringa, Weir, Ofstedal, and Willis: 426 Thompson Street, Ann Arbor, MI 48104.
Dr. Burke: Box 2900, Duke University Medical Center, Durham, NC 27710.
Dr. Hurd: 1776 Main Street, PO Box 2138, Santa Monica, CA 90407-2138.
Dr. Rodgers: 2050 West 22nd Avenue, Eugene, OR 97405.
Dr. McArdle: 3620 South McClintock Avenue, SGM 501, Los Angeles, CA 90089-1061.
Dr. Wallace: 200 Hawkins Drive, C21-N GH, Iowa City, IA 52242.
References
- 1.Edland SD, Rocca WA, Petersen RC, Cha RH, Kokmen E. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch Neurol. 2002;59:1589–93. doi: 10.1001/archneur.59.10.1589. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–42. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 4.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–83. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 5.Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58:758–64. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 6.Albert SM, Glied S, Andrews H, Stern Y, Mayeux R. Primary care expenditures before the onset of Alzheimer’s disease. Neurology. 2002;59:573–8. doi: 10.1212/wnl.59.4.573. [DOI] [PubMed] [Google Scholar]
- 7.Ernst RL, Hay JW. Economic research on Alzheimer disease: a review of the literature. Alzheimer Dis Assoc Disord. 1997;11 (Suppl 6):135–45. [PubMed] [Google Scholar]
- 8.Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–9. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie K. Mild cognitive impairment: an epidemiological perspective. Dialogues Clin Neurosci. 2004;6:401–8. doi: 10.31887/DCNS.2004.6.4/kritchie. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. International Psychogeriatric Association Expert Conference on Mild Cognitive Impairment. Mild cognitive impairment. Lancet. 2006;367:1262–70. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 11.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–32. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour. 1995;30 (Suppl):135–45. [Google Scholar]
- 13.Willis RJ. Theory confronts data: how the HRS is shaped by the economics of aging and how the economics of aging will be shaped by the HRS. Labour Economics. 1999;6:119–45. [Google Scholar]
- 14.Soldo BJ, Hurd MD, Rodgers WL, Wallace RB. Asset and health dynamics among the oldest old: an overview of the AHEAD Study. J Gerontol B Psychol Sci Soc Sci. 1997;52(Spec No):1–20. doi: 10.1093/geronb/52b.special_issue.1. [DOI] [PubMed] [Google Scholar]
- 15.Ofstedal MB, Fisher G, Herzog AR. Documentation of cognitive functioning measures in the Health and Retirement Study. Ann Arbor, MI: University of Michigan; 2005. Accessed at http://hrsonline.isr.umich.edu/docs/userg/dr-006.pdf on 22 January 2008. [Google Scholar]
- 16.Heeringa SG, Fisher GG, Hurd MD, Langa KM, Ofstedal MB, Plassman BL, et al. Aging, Demographics and Memory Study (ADAMS) Sample design, weights, and analysis for ADAMS. 2006 Accessed at http://hrsonline.isr.umich.edu/meta/adams/desc/ADAMSSampleWeights_Nov2007.pdf on 22 January 2008.
- 17.Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, et al. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology. 2005;25:181–91. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 18.Little RJ, Rubin DB. Statistical Analysis with Missing Data. 2. New York: J Wiley; 2002. [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Clark CM, Ewbank DC. Performance of the dementia severity rating scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10:31–9. [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Assoc; 1987. [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Assoc; 1994. [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 24.Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 25.The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1994;57:416–8. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 27.Plassman BL, Steffens DC, Burke JR, Welsh-Bohmer KA, Newman TN, Drosdick D, et al. Duke Twins Study of Memory in Aging in the NAS-NRC Twin Registry. Twin Res Hum Genet. 2006;9:950–7. doi: 10.1375/183242706779462381. [DOI] [PubMed] [Google Scholar]
- 28.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–66. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 29.Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–31. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 30.Petersen RC, Morris JC. Clinical features. In: Petersen RC, editor. Mild Cognitive Impairment. New York: Oxford Univ Pr; 2003. [Google Scholar]
- 31.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 32.Hofman A, Rocca WA, Brayne C, Breteler MM, Clarke M, Cooper B, et al. The prevalence of dementia in Europe: a collaborative study of 1980–1990 findings. Eurodem Prevalence Research Group. Int J Epidemiol. 1991;20:736–48. doi: 10.1093/ije/20.3.736. [DOI] [PubMed] [Google Scholar]
- 33.U.S. General Accounting Office. Alzheimer’s disease: estimates of prevalence in the United States. Washington, DC: U.S. General Accounting Office; 1998. [Google Scholar]
- 34.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–9. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 35.Unverzagt FW, Gao S, Baiyewu O, Ogunniyi AO, Gureje O, Perkins A, et al. Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging. Neurology. 2001;57:1655–62. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- 36.Graham JE, Rockwood K, Beattie BL, Eastwood R, Gauthier S, Tuokko H, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–6. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 37.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 38.Hanninen T, Koivisto K, Reinikainen KJ, Helkala EL, Soininen H, Mykkänen L, et al. Prevalence of ageing-associated cognitive decline in an elderly population. Age Ageing. 1996;25:201–5. doi: 10.1093/ageing/25.3.201. [DOI] [PubMed] [Google Scholar]
- 39.Barnhart RL, van Belle G, Edland SD, Kukull W, Borson S, Raskind M, et al. Geographically overlapping Alzheimer’s disease registries: comparisons and implications. J Geriatr Psychiatry Neurol. 1995;8:203–8. doi: 10.1177/089198879500800401. [DOI] [PubMed] [Google Scholar]
- 40.Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 41.Fisk JD, Merry HR, Rockwood K. Variations in case definition affect prevalence but not outcomes of mild cognitive impairment. Neurology. 2003;61:1179–84. doi: 10.1212/01.wnl.0000089238.07771.c7. [DOI] [PubMed] [Google Scholar]
- 42.Petersen RC, Bennett D. Mild cognitive impairment: is it Alzheimer’s disease or not? J Alzheimers Dis. 2005;7:241–5. doi: 10.3233/jad-2005-7307. discussion 255–62. [DOI] [PubMed] [Google Scholar]
- 43.Morris JC. Mild cognitive impairment is early-stage Alzheimer disease: time to revise diagnostic criteria [Editorial] Arch Neurol. 2006;63:15–6. doi: 10.1001/archneur.63.1.15. [DOI] [PubMed] [Google Scholar]
- 44.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 45.Evans DA. Estimated prevalence of Alzheimer’s disease in the United States. Milbank Q. 1990;68:267–89. [PubMed] [Google Scholar]
- 46.Alzheimer’s Association. Alzheimer’s disease facts and figures. Chicago: Alzheimer’s Association; 2007. Accessed at www.alz.org/alzheimers_disease_facts_figures.asp on 22 January 2008. [Google Scholar]
- 47.U.S. Census Bureau. Table 1. Annual estimates of the population by five-year age groups and sex for the United States: April 1, 2000 to July 1, 2006. Washington, DC: U.S. Census Bureau; 2007. Accessed at www.census.gov/popest/national/asrh/NC-EST2006-sa.html on 22 January 2008. [Google Scholar]
- 48.Matthews FE, Stephan BC, Bond J, McKeith I, Brayne C Medical Research Council Cognitive Function and Ageing Study. Operationalisation of mild cognitive impairment: a graphical approach. PLoS Med. 2007;4:1615–9. doi: 10.1371/journal.pmed.0040304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–6. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]