Abstract
We investigated the relation between parental education and dementia in the United States. Participants in the Aging, Demographics, and Memory Study were included, with information regarding parental education obtained from the Health and Retirement Study. The odds of dementia in elderly Americans whose mothers had less then 8 years of schooling were twice (95% CI, 1.1–3.8) that of individuals with higher maternal education, when adjusted for paternal education. Of elderly Americans with less educated mothers, 45.4% (95% CI, 37.4–53.4%) were diagnosed with dementia or “cognitive impairment, no dementia” compared to 31.2% (95% CI, 25.0–37.4%) of elderly Americans whose mothers had at least an 8th grade education. The population attributable risk of dementia due to low maternal education was 18.8% (95% CI, 9.4–28.2%). The education of girls in a population may be protective of dementia in the next generation.
Keywords: all cognitive disorders/dementia, MCI (mild cognitive impairment), risk factors in epidemiology, other education
Introduction
Increasing evidence suggests that risk factors from across the entire life-course, including those from early-life, may have an impact on the frequency of dementia in late-life.1,2 These risk factors include germline and somatic genetic mutations, the intrauterine environment, birth-related and postpartum events, as well as environmental factors that affect growth, brain development, and cognition in childhood. 1,2 With the exception of genetic factors, there have been few studies of transgenerational effects on the likelihood of developing clinically-diagnosed dementia. One such transgenerational variable is parental education, which for most historical cohorts occurred prior to the birth of the affected individual. In a study of Finnish men, Kaplan and colleagues assessed the effects of parental education on cognitive ability in adult mid-life and found that maternal education, in particular, was associated with better adult cognition in areas including verbal memory, visual memory, verbal fluency, complex information processing, and global cognitive status.3 Other studies have used maternal education as part of a composite index of socioeconomic status (SES) and found higher levels of this index to be associated with better cognitive function in later life4–6; however, such composite indices did not show significant results for cognitive decline5 or Alzheimer’s disease.6 It is difficult to understand the influence of maternal education on later cognitive function when it is embedded in a composite measure of SES or other early-life influences because its association to cognition may be diluted by other measures (eg, paternal occupation 7) that are less directly related to the outcome of interest. In addition, such composite indices make it difficult to isolate the most influential variables, or those with the most potential for targeted efforts to reduce the prevalence of cognitive decline and dementia.
We report here an investigation of the distinct effects of maternal and paternal education on the frequency of clinically diagnosed cognitive impairment in a nationally-representative sample of the US population. We hypothesized that lower maternal education would be associated with increased risk of both dementia and cognitive impairment in the absence of dementia (“cognitive impairment, not demented” [CIND]). We used a path analysis approach to examine direct and indirect influences of transgenerational factors (ie, parental education, race, and apolipoprotein E genotype) on late-life dementia and CIND.
Methods
Participants
The study design for this investigation was case-control, nested within a prospective longitudinal panel study. The participants were 856 individuals in the Aging, Demographics, and Memory Study (ADAMS). Full details of the ADAMS design and methods have been reported previously.8 Briefly, the sampling frame for the ADAMS was the nationally-representative Health and Retirement Study (HRS) of persons born in 1947 or earlier, which was designed to investigate the health, social, and economic implications of aging in the American population.9 A stratified sample was taken from the HRS of individuals aged 70 years and older to derive the ADAMS participants. Specifically, sampling was stratified by cognition, ranging from “low” to “high normal” function on the HRS cognitive measures.8 The 3 highest cognitive functions were further stratified by age (70–79 years vs. 80 or older) and sex to ensure relatively adequate numbers of participants by age and gender in these subgroups.
Procedures
To determine dementia status, each participant in ADAMS underwent a battery of neuropsychological tests, a videotaped standardized neurological examination, and a 7-minute videotaped segment covering portions of the cognitive status and neurological examinations. In addition, the participant’s clinical history was obtained from a proxy informant, which included (1) chronological history of cognitive symptoms, (2) medical history, (3) current medications, (4) current neuropsychiatric symptoms, (5) measures of severity of cognitive and functional impairment, and (6) family history of memory problems. Medical record releases were obtained to evaluate relevant neuroimaging and laboratory results. A buccal swab DNA sample was collected to determine apolipoprotein E (ApoE) genotype. A consensus expert panel of neuropsychologists, neurologists, geropsychiatrists, and internists reviewed all of this information and then assigned a diagnosis of normal cognition, CIND, or dementia. Dementia diagnosis was based on Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (DSM-III-R; 22) and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; 23) criteria. “Cognitive impairment, not demented” was defined as: (1) mild cognitive or functional impairment reported by the subject or informant that did not meet criteria for dementia or (2) performance on neuropsychological measures that was both below expectation and ≥1.5 standard deviations below published norms on any test. Of the 856 participants, 241 were diagnosed with CIND, 99% of whom met criterion 2, 28% of whom met criterion 1 (using Dementia Severity Rating Score of >5 and <12 as the guideline), and 28% of whom met both criteria. For criterion 2, 95% were impaired on more than one test. Further details of subcategories of dementia and CIND have been described elsewhere.8,10
Information regarding father’s education and mother’s education was based on self- or proxyreported information collected during the baseline HRS interview. Most often, the proxy was the spouse. For purposes of this study, “low education” was defined as attending less than 8 years of schooling and “high education” was defined as attending 8 or more years of schooling. This cut point reflected historical levels of education in the United States at the end of the 1800s and early 1900s, when the parents of the ADAMS participants were schooled.11 Information regarding maternal education was available for 83.5% of the participants. The comparable percentage for paternal education was 81.8%. For 11.3% of the participants, data on both maternal and paternal education were not available.
Statistical Analyses
Statistical analyses were performed in Stata/SE 9.2 for survey data, taking into account the complex sampling design of the HRS and ADAMS.12 Initially, tables were generated for categorical data, weighted to reflect the elderly population in the United States. Multiple imputation by chained equations was used for missing data (based on 20 cycles of regression switching and 10 imputations). All analyses were conducted with and without multiple imputation and the results were similar (n = 856 and n = 654, respectively).
Maternal and paternal education were regressed on dementia status (normal cognition, CIND, dementia) to generate odds ratios, using survey ordinal logistic regression. Because the time sequence of several transgenerational factors (ie, parental education, race, and ApoE genotype) was known, a path model was developed using probability principles previously described.13,14 All possible associations among variables in the model were specifically tested to determine the direction and size of coefficients. Unidirectional arrows represented possible directed associations while bidirectional arrows represented associations without direct causal inference. The lack of an arrow indicated no association. The presence of ApoE ε4 alleles was coded as a count of ε4 alleles (0, 1, or 2). Estimates of effect were generated in survey-weighted logit models, with all “causal parents” (predictors in the proposed model) included for a specific dependent variable. For the final path model, odds ratios are reported for the 4 logit regression models, with outcome variables identifiable by incoming arrows in the model. That is, father’s education and participant’s race were regressed on mother’s education; mother’s education and participant’s race were regressed on participant’s education; race was regressed on ApoE ε4 alleles; and ApoE ε4 alleles and participant’s education were regressed on cognitive impairment in late-life (categorized as normal cognition, CIND, and dementia). All estimates of effect were adjusted for participant’s age (years) at the time when the dementia status was determined and were weighted to reflect the overall elderly population of the United States.
Population attributable risk (PAR) was calculated using the weighted-sumapproach, with the proportion of cases in each adjustment level as the weight (case-load method).15 Under standard assumptions, this PAR yields an asymptotically unbiased estimator of the attributable risk, which reflects both the strength of the association and the prevalence of the predictors. The PAR for maternal education was adjusted for age and presence of ApoE ε4 alleles. Standard errors for PAR and 95% confidence intervals were calculated using Walter’s formula.16
Three sensitivity analyses were conducted on the final path model. The first was conducted to assess the effect of education on dementia status when restricted to whites only, because non-white race was associated with education and non-whites constituted a small percentage (10.7%) of the population. The second and third sensitivity analyses were done to restrict the analyses to those participants in whom memory of parental education would likely be more accurate. In the second sensitivity analysis, individuals whose age of onset of dementia was less than 5 years since the time of the baseline interview were excluded, because questions regarding parental education were asked at the baseline interview. In the third sensitivity analysis, we excluded (1) participants with normal cognition and a baseline proxy interview and (2) subjects with dementia without a proxy baseline interview.
Results
The prevalence of CIND and dementia increased with age, with 31.0% of individuals aged 80 years or older having CIND and 26.6% diagnosed with dementia (Table 1). Although there was no association between dementia status and gender, there was for race; non-whites exhibited a greater probability of dementia and CIND than whites. Elderly Americans with 1 or 2 ApoE ε4 alleles had a greater prevalence of dementia (20.3%) than those without (11.6%). The frequency of ApoE genotype in this population, all cognitive groups combined, was 24.2% for 1 ApoE ε4 allele and 2.2% for 2 ApoE ε4 alleles. There was variability in the cognitive status by region of birth in the United States, with those born in the south exhibiting a greater prevalence of dementia (18.6%). The birth years for the participants ranged from 1893 through 1932.
Table 1.
Characteristics of Elderly Americans by Cognitive Status (Percentage, 95% Confidence Intervals)
| Characteristic | Normal | CIND | Dementia | P Value |
|---|---|---|---|---|
| Age, years | ||||
| 71–79 | 79.0 (74.1–83.9) | 16.0 (11.5–20.5) | 5.0 (2.6–7.3) | |
| ≥80 | 42.4 (37.2–47.5) | 31.0 (26.4–35.6) | 26.6 (21.8–31.5) | P < .001 |
| Sex | ||||
| Male | 63.5 (59.1–67.8) | 25.4 (21.8–29.0) | 11.1 (7.8–14.5) | |
| Female | 64.1 (58.4–69.7) | 20.2 (14.8–25.6) | 15.7 (12.4–19.1) | P = .766 |
| Race | ||||
| White | 66.2 (63.0–69.5) | 21.3 (17.7–24.8) | 12.5 (10.3–14.7) | |
| Non-white | 43.9 (30.6–57.2) | 30.2 (21.0–39.4) | 25.9 (14.4–37.4) | P =.001 |
| APOE-e4 | ||||
| 0 alleles | 66.4 (62.2–70.6) | 22.0 (18.5–25.5) | 11.6 (9.0–14.1) | |
| 1 or 2 alleles | 57.0 (48.1–65.8) | 22.7 (14.2–31.2) | 20.3 (14.2–26.4) | P = .026 |
| Region at birth | ||||
| Northeast US | 73.2 (63.9–82.5) | 18.7 (12.3–25.2) | 8.1 (2.9–13.3) | |
| Midwest US | 65.2 (57.8–72.5) | 22.1 (15.4–28.7) | 12.8 (8.0–17.5) | |
| South US | 57.2 (50.9–63.4) | 24.2 (17.5–30.9) | 18.6 (12.9–24.3) | |
| West US | 63.3 (50.3–76.2) | 22.3 (7.5–37.2) | 14.4 (1.5–27.3) | |
| Foreign | 60.6 (45.4–75.7) | 24.5 (13.3–35.8) | 14.9 (7.5–22.3) | P = .026 |
Note: CIND = cognitively impaired but no dementia.
Maternal and paternal education were strongly related (P < .001); there was concordance in parental educational status (< 8 years vs. 8+ years of schooling) in 82.4% of participants. To evaluate the individual contributions of maternal versus paternal education on dementia status of their children, each combination of parental education was considered (Table 2). Of the elderly Americans 52% had parents who both received schooling for at least 8 years, while 30.4% had parents who both had less than an eighth grade education. Of the elderly Americans with more highly educated mothers, 68.8% had normal cognition, (95% CI, 62.6–75.0%), while 54.6% of those with less educated mothers had normal cognition (95% CI, 46.6–62.6%), irrespective of the level of paternal education. In contrast, 45.4% of elderly Americans whose mothers did not have an eighth grade education were diagnosed with either CIND or dementia (95% CI, 37.4–53.4%), regardless of paternal education; this compared with 31.2% (95% CI, 25.0–37.4%) of elderly Americans with higher maternal education.
Table 2.
Percentages of Elderly Americans by Parental Education and Cognitive Status
| Normal |
CIND |
Dementia |
All Cognitive Groups |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | Percentage | 95% CI | Percentage | 95% CI | Percentage | 95% CI | Percentage | 95% CI |
| High maternal and high paternal education | 343 | 68.9 | 62.8–75.0 | 21.0 | 15.3–26.8 | 10.1 | 6.7–13.5 | 52.0 | 46.0–58.0 |
| High maternal and low paternal education | 100 | 68.6 | 54.4–82.8 | 19.1 | 7.6–30.6 | 12.3 | 4.0–20.6 | 12.9 | 9.0–16.8 |
| Low maternal and high paternal education | 59 | 42.2 | 17.5–66.9 | 38.1 | 15.5–60.7 | 19.7 | 8.1–31.3 | 4.7 | 2.7 –6.6 |
| Low maternal and low paternal education | 354 | 56.5 | 47.2–65.9 | 23.1 | 14.8–31.5 | 20.3 | 14.7–25.9 | 30.4 | 24.5–36.3 |
| All educational groups | 856 | 63.8 | 60.3–67.4 | 22.2 | 18.7–25.7 | 13.9 | 11.4–16.4 | 100.0 | |
Note: CIND = cognitively impaired but no dementia.
The odds ratio for the association between low maternal education and dementia status (normal, CIND, dementia) was 2.0 (95% CI, 1.1–3.8; P = .033) when adjusted for paternal education. The odds ratio for the relation between paternal education and dementia status was 0.9 (95% CI, 0.5–1.5; P = .642) when adjusted for maternal education. Because the results reflect ordinal logistic regression, this odds ratio indicates that the odds of dementia (versus normal cognition or CIND) were twice as great in elderly Americans who had less educated versus more educated mothers. Similarly, the odds of cognitive impairment in late-life (CIND or dementia, versus normal cognition) were 100% greater (or twice as great) in elderly Americans whose mothers did not have an eighth grade education compared to those whose mothers had at least an eighth grade education. When stratified by gender, the odds ratio for the association between low maternal education and late-life dementia in men was 2.2 (95% CI, 1.0–4.8) and in women was 1.9 (95% CI, 0.9–4.2); effect modification by gender was not significant (P = .490). Likewise, gender was not a confounding variable in these data; adjustment for gender did not appreciably change the odds ratios, as gender was not significantly related to the outcome, cognitive status (P = .766). The population-attributable risk of dementia due to low maternal education was 18.8% (95% CI, 9.4–28.2%).
Table 3 lists the results for combinations of maternal and paternal education. The odds ratio for low maternal and low paternal education was 1.9, which indicates that the odds ratio of cognitive impairment (either CIND or dementia) were 90% greater in those with low parental education compared to participants with high parental education. When ApoE ε4 allele status was considered, the odds ratios tended to be greater in those groups with low maternal education and in those groups with at least one allele. The odds ratio for low maternal and high paternal education was significant (OR = 2.9) in those without ApoE ε4 alleles, as was the odds ratio (OR = 1.9) for those with both low maternal and low paternal education. For individuals with at least one ApoE ε4 allele, the odds ratio for low parental education was significant at 3.7.
Table 3.
Results of Ordered Logistic Regression: Parental Education Regressed on Cognitive Statusa
| Characteristic | Odds Ratio | P Value | 95% Confidence Interval | |
|---|---|---|---|---|
| Regardless of ApoE status: | ||||
| High maternal and high paternal education | 1.0 | (reference) | ||
| High maternal and low paternal education | 1.1 | .707 | 0.6–2.4 | |
| Low maternal and high paternal education | 2.2 | .108 | 0.8–5.8 | |
| Low maternal and low paternal education | 1.9 | .031 | 1.1–3.2 | |
| With ApoE status: | ||||
| High maternal and high paternal education | No ApoE ε4 alleles | 1.0 | (reference) | |
| High maternal and low paternal education | No ApoE ε4 alleles | 1.2 | .669 | 0.5–3.0 |
| Low maternal and high paternal education | No ApoE ε4 alleles | 2.9 | .030 | 1.1–7.3 |
| Low maternal and low paternal education | No ApoE ε4 alleles | 1.9 | .025 | 1.1–3.5 |
| High maternal and high paternal education | 1–2 ApoE ε4 alleles | 2.0 | .041 | 1.0–3.9 |
| High maternal and low paternal education | 1–2 ApoE ε4 alleles | 1.6 | .378 | 0.5–5.1 |
| Low maternal and high paternal education | 1–2 ApoE ε4 alleles | 2.7 | .255 | 0.5–15.2 |
| Low maternal and low paternal education | 1–2 ApoE ε4 alleles | 3.7 | .004 | 1.6–8.7 |
Cognitive status was modeled in 3 categories: normal, cognitively impaired but no dementia, and dementia. All odds ratios were adjusted for age.
The relation between maternal education, participant’s education, and dementia status in late-life is shown in Table 4. Only 8.7% of elderly Americans had less than an eighth grade education, while 35.1% had a mother with this educational background. Normal cognition was most prevalent (at 70.0%) in families in which both the mother and child had a higher level of education. The highest prevalence of dementia was in participants who did not have an eighth grade education (24.4% in those with high maternal education and 32.3% in those with low maternal education). Because an educated mother was strongly associated with higher education in her child, with an odds ratio of 6.6 (95% CI, 2.9–15.3), most families with an educated mother also had an educated child (63.3%). This relation was reflected by the low percentage (1.6%) of families in which the mother had a high education level and the child had a low education level.
Table 4.
Percentages of Elderly Americans by Education and Cognitive Status
| Normal |
CIND |
Dementia |
All Cognitive Groups |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | Percentage | 95% CI | Percentage | 95% CI | Percentage | 95% CI | Percentage | 95% CI |
| High maternal and high subject education | 403 | 70.0 | 63.8–76.2 | 19.4 | 13.9–24.9 | 10.6 | 7.7–13.6 | 63.3 | 57.4–69.1% |
| Low maternal and high subject education | 258 | 61.1 | 51.7–70.4 | 22.9 | 15.5–30.3 | 16.1 | 11.0–21.1 | 28.0 | 23.0–32.9% |
| High maternal and low subject education | 40 | 18.9 | 0.0–44.6 | 56.7 | 26.7–86.7 | 24.4 | 0.0–49.9 | 1.6 | 0.6–2.7% |
| Low maternal and low subject education | 155 | 30.8 | 16.7–44.9 | 36.9 | 20.6–53.2 | 32.3 | 18.5–46.1 | 7.1 | 5.2–9.1 |
| All educational groups | 856 | 63.8 | 60.3–67.4 | 22.2 | 18.7–25.7% | 13.9 | 11.4–16.4 | 100.0% | |
Note: CIND = cognitively impaired but no dementia.
Simultaneous entry of maternal education, paternal education, and child’s education into an ordinal logistic regression model with cognitive status (normal, CIND, dementia) as the dependent variable yielded an odds ratio of 1.6 (95% CI, 0.8–3.1) for maternal education, an odds ratio of 0.9 (95% CI, 0.5–1.6) for paternal education, and an odds ratio of 3.5 (95% CI, 2.1–5.9) for child’s education, when adjusted for age. When missing data were excluded from these analyses (ie, imputed data were excluded), simultaneous adjustment yielded an odds ratio for maternal education of 1.6 (95% CI, 0.8–3.4), an odds ratio for paternal education of 0.9 (95% CI, 0.5–1.6), and an odds ratio for child’s education of 2.6 (95% CI, 1.2–5.3), when adjusted for age.
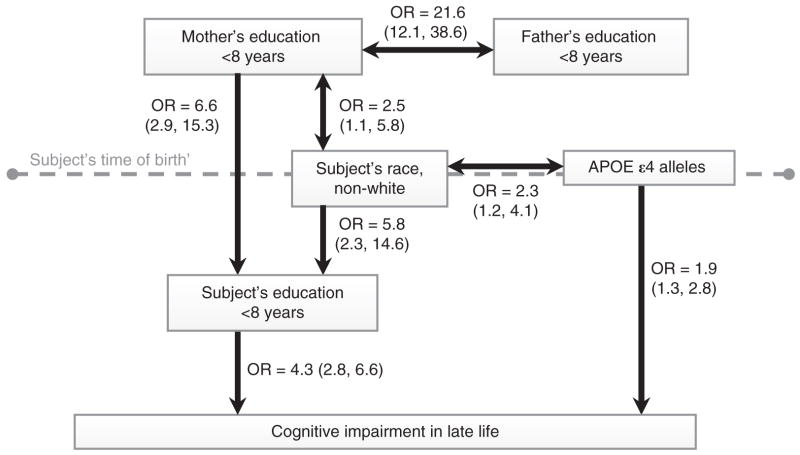
The path model, generated from these data, is shown in Figure 1. Odds ratios are displayed in relation to time, with cognitive status represented as an ordered variable (normal cognition, CIND, dementia). The odds ratio for the association between maternal and paternal education was 21.6 (95% CI, 12.1–38.6). The association between race and maternal education was also significant (OR = 2.5), as was the relation between race and ApoE ε4 alleles (OR = 2.3 for non-white versus white). Both maternal education and race significantly predicted the participant’s education; the odds ratio for the relation between maternal education and participant’s education was 6.6 and for the relation between race and participant’s education was 5.8. Participant’s education was associated with cognitive impairment in later life, adjusted for ApoE ε4 alleles and age. The odds of dementia were 4.3 times greater in participants with less than an eighth grade education compared to those without dementia (normal or CIND). Because these results were generated from ordinal logistic regression, the odds ratio remains constant across comparison groups; that is, the odds of cognitive impairment (CIND or dementia) were 4.3 times greater in participants with less than an eighth grade education compared to those without cognitive impairment (normal).
Figure 1.
Time-related odds ratios (95% Confidence Intervals) for associations among education variables and cognitive impairment (CIND or dementia). ApoE, apolipoprotein E; OR, odds ratio.
To assess fit, all independent variables in Figure 1 were regressed simultaneously on cognitive impairment in late-life (normal/CIND/dementia). Only paternal education yielded a negative Bayesian Information Criterion difference between the full and reduced models, suggesting that paternal education was not a predictor of cognitive impairment while the other variables (maternal education, race, APOE e4 alleles, subject’s education and age) were.
In the sensitivity analysis using whites only, the association between maternal and child education yielded an odds ratio of 9.6 (95% CI, 3.4–26.8) and the association between child education and dementia yielded an odds ratio of 3.6 (95% CI, 2.0–6.4). In the sensitivity analysis including only those cases with dementia whose onset was 5 or more years after the baseline interview, the odds ratio for the relation between maternal and child education was 9.2 (95% CI, 3.9–21.7) and the odds ratio for the relation between child education and dementia was 4.1 (95% CI, 2.6–6.4). In the sensitivity analysis excluding proxy interviews for participants with normal cognition and self interviews for participants with dementia, the odds ratio for the association between maternal and child education in the path model was 12.6 (95% CI, 4.2–37.5); the odds ratio for the relation between participant’s education and dementia was 7.3 (95% CI, 3.7–14.1).
Discussion
The current study found that maternal education was an important correlate of an individual’s cognitive function in later life. Among elderly Americans who received a clinical assessment of their cognitive function, 45% of those whose mothers had less than 8 years of formal schooling were diagnosed with either cognitive impairment (CIND) or dementia, compared to 31% of individuals whose mothers had at least 8 years of education. Twenty percent of elderly Americans whose mothers did not complete 8 years of education were diagnosed with dementia. Low maternal education level resulted in twice the risk of cognitive impairment or dementia, even after adjusting for paternal education. Approximately 19% of late-life dementia in elderly Americans could be attributed to the lack of an eighth grade education in their mothers.
Previous investigations support these findings.3,4 Kaplan and colleagues found specific effects of maternal education on several areas of cognitive performance,3 while other studies found beneficial effects of maternal education as part of a composite of other factors.4–6 In contrast to previous studies,5,6 we found clinically diagnosed cognitive impairment (ie, CIND and dementia) to be more prevalent among individuals with lower levels of maternal education. One reason for our significant results may be that we examined maternal education individually, rather than as a composite to represent SES or other early-life influences.
There are several potential explanations for these findings. An educated mother may provide the type of stimulation necessary for better cognitive skills during the period of rapid synaptogenesis in the child. Dollaghan found that children of educated mothers use a greater number of different words, a greater total number of words, and longer lengths of utterances in morphemes than children of less educated mothers. 17 Quality ofmother–infant interactions have been found to predict later cognitive functioning in children,18 and higher levels of maternal education are associated with better cognitive functioning from ages 3 to 8 years in high-risk populations.19,20 In addition, research from developmental enrichment interventions shows greater cognitive improvements in children whose mothers have less education than in children with educated mothers, implying that the consequences of an educational deficit may be partially mitigated by external intervention after birth.19,21,22 However, these studies also showed that the absolute cognitive level never reached that of children whose mothers were more educated.21,22 Thus, maternal education may provide the foundation for a developmentally-rich environment during the early childhood period.
Another possible mechanism for the positive effect of maternal education is through the physiologic advantages an educated mother brings to a child. Maternal education is a known predictor of birth weight, and fetal brain development is related to birth weight.23 Low birth weight is associated with reduced volumes of parietal, occipital horn, and frontal gray matter.24 A portion of the birth weight effect could be associated with undernutrition during fetal growth, mediated through poverty.25 In the 1946 British cohort, however, birth weight was predictive of cognitive function at 8 years of age but not at 43 years, although shortness in height, often used as a proxy measure for deficiencies in childhood nutrition, was associated with dementia.26,27 In addition, there are some physiological advantages of an educated mother which are more apparent after the birth of her child. For instance, postnatal head growth was significantly greater in children who had educated mothers, and brain growth after birth was more predictive of cognitive function at 9 years of age than brain growth in utero, suggesting that factors after birth were more important than those during pregnancy.28
The results of our study indicated that there were few instances in which the mother was educated (to eighth grade) but the child was not; thus, we could not adequately assess these effects independently. Without evidence from trials, it is not possible to determine the extent to which maternal education, child education, or correlates thereof have a causal effect on cognitive impairment and dementia in late-life. Indeed, these factors may well be part of a larger causal pathway, with child education serving as a marker for the cognitive milieu present during synaptic proliferation in early childhood. There is considerable evidence that the cognitive environment prior to formal schooling strongly determines cognitive abilities of the school-aged child.29–32 In an in-depth analysis of a wide spectrum of possible mechanisms mediating the effects of poverty in children, Guo and colleagues found that cognitive stimulation during preschool (eg, how often the mother read to the child and the number of children’s books in the home) was the most critical factor determining intellectual development in the school-aged child.29 Mother’s education and the cognitive ability of the mother were both, in turn, significantly associated with this most important factor—preschool cognitive stimulation.
This study has several limitations that merit discussion. One of these is the lack of records to verify the educational status of the parents. Although the sensitivity analyses conducted to address this issue demonstrated that the effect of maternal education remained after restriction to those groups in which accuracy of reporting educational status was likely to be better, verifiable data on educational level and performance is preferable. Krieger and colleagues found that father’s education was accurately recalled from adulthood (more so than social class) and was not differentially recalled based on adult socioeconomic position.33 Another limitation was the inability to assess change in cognitive status (normal, CIND, dementia) over time, to discriminate baseline cognitive performance from change in performance. An additional limitation is the possibility of survivor bias; that is, those who survived to older ages (and thus, were assessed in the HRS) would be different with respect to the variables of interest than the entire cohort born during the early 20th century. Because individuals with lower childhood mental ability tend to have shorter lifespans,34 we anticipate that the inclusion of nonsurvivors could potentially strengthen the association between maternal education and dementia.
It is important to note that the parents in this study were likely schooled during the early 1900s, a time during which primary education was not mandatory in all states and secondary education was only attained by a minority of the general US population. In 1910, for example, only 3.8% of school-aged children were enrolled in high schools and 70.4% were enrolled in grade schools.11 Moreover, only 72.1% of enrolled students attended school on a daily basis.11 The increasing education of the US population could partially account for the decreasing prevalence of cognitive impairment in this country.35 Furthermore, the cognitive milieu for the HRS participants during childhood may have been quite different from conditions today. Not only were the HRS participants born at a time when fewer mothers worked outside the home, they were also born prior to the advent of television, personal computers, and other methods of communication routinely used today. It is possible that the proportional effect of maternal education on childhood development (in relation to total input) may have been greater in the early 20th century compared to today. In this context, our results may have particular relevance to children in developing countries, where educational levels are still low. Worldwide, there are 60 million girls aged 6 to 11 years who do not attend school and 100 million who are in primary school but will drop out before completion.36 In addition, it is estimated that only 10% to 41% of parents in developing countries provide cognitively stimulating materials to their children.37
The issue of maternal education highlighted in this study is important because the results suggest an actionable intervention. Although education of girls to the primary school level has been achieved in many developed countries, it deserves additional emphasis in developing countries. Whereas the education of girls has been shown to reduce infant mortality, maternal mortality, family size, and poverty,36 it may have an additional effect of fostering late-life cognitive health in the next generation. Moreover, incentives to educate girls may rest, not solely on evidence of the benefits to these particular girls, but also on that to the men and women in the next generation.
Acknowledgments
The National Institute on Aging (NIA) provided funding for the Health and Retirement Study (HRS) and the Aging, Demographics and Memory Study (U01 AG09740), data from which were used for this analysis. The HRS is performed at the Survey Research Center, Institute for Social Research, University of Michigan. Additional support was provided by grants from the NIA (K08 AG019180 and R01 AG027010). Dr Langa was supported by a Paul Beeson Physician Faculty Scholars in Aging Research award.
Footnotes
The authors report no conflicts of interest.
References
- 1.Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:63–72. doi: 10.1097/01.wad.0000201854.62116.d7. [DOI] [PubMed] [Google Scholar]
- 2.Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurol. 2006;5:87–96. doi: 10.1016/S1474-4422(05)70286-6. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol. 2001;30:256–263. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]
- 4.Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. J Gerontol. 2005;60B:S93–S101. doi: 10.1093/geronb/60.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everson-Rose SA, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Early life conditions and cognitive functioning in later life. Am J Epidemiol. 2003;158:1083–1089. doi: 10.1093/aje/kwg263. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RS, Scheer PA, Hoganson G, et al. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiol. 2005;25:8–14. doi: 10.1159/000085307. [DOI] [PubMed] [Google Scholar]
- 7.Richards M, Sacker A. Lifetime antecedents of cognitive reserve. J Clin Exp Neuropsychol. 2003;25:614–624. doi: 10.1076/jcen.25.5.614.14581. [DOI] [PubMed] [Google Scholar]
- 8.Langa KM, Plassman BL, Wallace RB, et al. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology. 2005;25:181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 9.Juster FT, Suzman R. An overview of the Health and Retirement Study. J Human Res. 1995;30(suppl):S7–S56. [Google Scholar]
- 10.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder TD, Dillow SA, Hoffman CM. National Center for Education Statistics, Institute of Education Sciences, U.S. Department of Education. Washington, DC: U.S. Government Printing Office; 2007. Digest of Education Statistics 2006 (NCES 2007-017) [Google Scholar]
- 12.Heeringa SG, Fisher GG, Ofstedal MB, Rodgers WL, Santos K. Sample design, weights, and analysis for ADAMS. Ann Arbor, MI: Survey Research Center, Institute for Social Research, University of Michigan; 2006. [Google Scholar]
- 13.Shipley B. Cause and Correlation in Biology: A User’s Guide to Path Analysis, Structural Equations and Causal Inference. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 14.Pearl J. Causality: Models, Reasoning, and Inference. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 15.Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res. 2001;10:195–216. doi: 10.1177/096228020101000303. [DOI] [PubMed] [Google Scholar]
- 16.Walter SD. Calculation of attributable risks from epidemiological data. Int J Epidemiol. 1978;7:175–182. doi: 10.1093/ije/7.2.175. [DOI] [PubMed] [Google Scholar]
- 17.Dollaghan CA, Campbell TF, Paradise JL, et al. Maternal education and measures of early speech and language. J Speech Lang Hearing Res. 1999;42:1432–1443. doi: 10.1044/jslhr.4206.1432. [DOI] [PubMed] [Google Scholar]
- 18.Tzuriel D. Parent-child mediated learning interactions as determinants of cognitive modifiability: recent research and future directions. Genet Soc Gen Psychol Monogr. 1999;125:109–156. [PubMed] [Google Scholar]
- 19.Ment LR, Vohr B, Allan W, et al. Change in cognitive function over time in very low-birth-weight infants. JAMA. 2003;289:705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
- 20.Strathearn L. Long-term cognitive function in very low-birth-weight infants. JAMA. 2003;289:2209. doi: 10.1001/jama.289.17.2209-a. [DOI] [PubMed] [Google Scholar]
- 21.Berlin LJ, Brooks-Gunn J, McCarton C, McCormick MC. The effectiveness of early intervention: examining risk factors and pathways to enhanced development. Prev Med. 1998;27:238–245. doi: 10.1006/pmed.1998.0282. [DOI] [PubMed] [Google Scholar]
- 22.Ramey CT, Ramey SL. Prevention of intellectual disabilities: early interventions to improve cognitive development. Prev Med. 1998;27:224–232. doi: 10.1006/pmed.1998.0279. [DOI] [PubMed] [Google Scholar]
- 23.Shmueli A, Cullen MR. Birth weight, maternal age, and education: new observations from Connecticut and Virginia. Yale J Biol Med. 1999;72:245–258. [PMC free article] [PubMed] [Google Scholar]
- 24.Kesler SR, Ment LR, Vohr B, et al. Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol. 2004;31:318–325. doi: 10.1016/j.pediatrneurol.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dauncey MJ, Bicknell RJ. Nutrition and neurodevelopment: mechanisms of developmental dysfunction and disease in later life. Nutr Res Rev. 1999;12:231–253. doi: 10.1079/095442299108728947. [DOI] [PubMed] [Google Scholar]
- 26.Richards M, Hardy R, Kuh D, Wadsworth MEJ. Birth-weight and cognitive function in the British 1946 birth cohort: longitudinal population based study. BMJ. 2001;322:199–203. doi: 10.1136/bmj.322.7280.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beeri MS, Davidson M, Silverman JM, Noy S, Schmeidler J, Goldbourt U. Relationship between body height and dementia. Am J Geriatr Psychiatry. 2005;13:116–123. doi: 10.1176/appi.ajgp.13.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gale CR, O’Callaghan FJ, Godfrey KM, Law CM, Martyn CN. Critical periods of brain growth and cognitive function in children. Brain. 2004;127:321–329. doi: 10.1093/brain/awh034. [DOI] [PubMed] [Google Scholar]
- 29.Guo G, Harris KM. The mechanisms mediating the effects of poverty on children’s intellectual development. Demography. 2000;37:431–447. doi: 10.1353/dem.2000.0005. [DOI] [PubMed] [Google Scholar]
- 30.Richards M, Wadsworth MEJ. Long term effects of early adversity on cognitive function. Arch Dis Child. 2004;89:922–927. doi: 10.1136/adc.2003.032490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: specific associations with neurocognitive development. Bran Res. 2006;1110:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 32.Beckett C, Maughan B, Rutter M, et al. Do the effects of early severe deprivation on cognition persist into early adolescence? Findings from the English and Romanian adoptees study. Child Dev. 2006;77:696–711. doi: 10.1111/j.1467-8624.2006.00898.x. [DOI] [PubMed] [Google Scholar]
- 33.Krieger N, Okamoto A, Selby JV. Adult female twins’ recall of childhood social class and father’s education: a validation study for public health research. Am J Epidemiol. 1998;147:704–708. doi: 10.1093/oxfordjournals.aje.a009512. [DOI] [PubMed] [Google Scholar]
- 34.Whalley LJ, Deary IJ. Longitudinal cohort study of childhood IQ and survival up to age 76. BMJ. 2001;322:1–5. doi: 10.1136/bmj.322.7290.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freedman VA, Aykan H, Martin LG. Aggregate changes in severe cognitive impairment among older Americans: 1993 and 1998. J Gerontol Soc Sci. 2001;56B:S100–S111. doi: 10.1093/geronb/56.2.s100. [DOI] [PubMed] [Google Scholar]
- 36.Herz B, Sperling GB. Evidence and Policies from the Developing World. New York: Council on Foreign Relations, Inc; 2004. What Works in Girls’ Education. [Google Scholar]
- 37.Walker SP, Wachs TD, Gardner JM, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]