Abstract
Marine food webs are important links between Hg in the environment and human exposure via consumption of fish. Estuaries contain sediment repositories of Hg and are also critical habitat for marine fish and shellfish species consumed by humans. MeHg biotransfers from sites of production in estuarine sediments to higher trophic levels via both benthic and pelagic pathways. In this study, we investigated the potential for Hg biotransfer to estuarine food webs across a Hg contamination gradient in the Gulf of Maine. Despite the variation in sediment Hg concentrations across sites (>100 fold), Hg concentrations in biota ranged by only 2–4 fold for each species across sites. Sediment contamination alone explained some variation in Hg and MeHg concentrations in biota across sites. However, biogeochemical and ecological factors also explained significant variation in Hg bioaccumulation across species. Contaminated sites had higher total organic carbon concentrations in sediments, which related to a decrease in Hg bioaccumulation (measured as biota-sediment concentration factors, BSCF). Moreover, concentrations of MeHg were higher in pelagic-feeding than benthic-feeding fauna (determined from delta 13C) indicating the importance of pelagic pathways in transferring MeHg. Lastly, the proportion of total Hg as MeHg increased with trophic level (measured as delta 15N). These results reveal the importance of both biogeochemical and ecological factors in determining the bioavailability and trophic transfer of MeHg in estuarine food webs.
Keywords: methylmercury, bioaccumulation, aquatic food web, estuarine
Introduction
Hg is a ubiquitous environmental contaminant and a potent neurotoxin that accumulates in aquatic ecosystems, posing a risk to humans and wildlife. Mercury enters the environment from both natural and anthropogenic sources, but predominantly from coal fired power plant emissions (1), and eventually enters aquatic systems via direct deposition and watershed transport. In freshwater and marine ecosystems, inorganic Hg is methylated in sediments and in the water column and MeHg is biomagnified in the food web. Human exposure is almost entirely due to fish consumption that is comprised mostly of marine fish and shellfish. In fact, marine fisheries make up more than 90% of the global fish harvest (2) and provide an important source of protein for the global population (3). Hg levels in many top predatory marine fish are often above the USEPA recommended criterion of <0.29 μg/g (wet wt.) for safe unrestricted consumption (4).
Based on Hg budgets calculated for the oceans, MeHg produced in coastal sediments may provide an important source of MeHg to offshore pelagic food webs (5,6). Coastal sediments, particularly in estuaries with many industrialized watersheds, are known repositories of contaminants (7). Estuaries are also critical areas of Hg methylation due to geochemical conditions in sediments that are conducive to MeHg production such as anoxia and periodic wetting and drying from tidal flux (8–10). MeHg from estuarine sediments may be transferred to offshore fisheries via physical or ecological transport processes.
Estuaries are critical habitat for many commercially and recreationally harvested fish and shellfish species (11,12) that are consequently exposed to MeHg produced in situ. Estuaries support many species over their entire life cycle and provide nursery grounds for other species that move to deeper portions of the estuary as they develop and grow (13). MeHg produced in estuaries may be transported offshore via the ‘trophic nekton relay’ (13,14) or ‘bioadvection’ (6,15) through feeding by transient subtidal species on resident intertidal species and migration of juveniles to offshore adult habitat (16). This horizontal trophic transfer also results in biomagnification of MeHg to top predator species consumed by humans and wildlife (17).
While MeHg produced in the water column is likely bioaccumulated in marine food webs via direct uptake by phytoplankton and pelagic feeding, MeHg that is produced in sediments can be taken up through either benthic or pelagic pathways (9,18). Chemical flux from sediments or methylation in the water column makes MeHg available to phytoplankton at the base of the pelagic food web whereas feeding in or on sediments links MeHg to the benthic food web. Feeding and burrowing activities by benthic epifauna and infauna influence methylation as well as expose them to MeHg (19). However, despite their direct contact with sediments, benthic food webs in lakes have lower levels of Hg than pelagic food webs even in relatively contaminated systems (20). This counterintuitive relationship may be due to Hg speciation in these different food webs and the influence of organic carbon on the bioavailability of Hg and MeHg. The question remains as to whether benthic fauna are important links between MeHg production in sediments and higher trophic levels in marine food webs.
In this study, we investigate Hg biotransfer from sediments to benthic and pelagic food webs across a gradient of sites, from pristine to highly industrialized. We seek to test the following hypotheses: 1) Hg bioaccumulation in intertidal fauna varies with concentrations in sediments; 2) Hg and MeHg bioaccumulation does not differ between benthic and pelagic fauna; and 3) MeHg biomagnifies in intertidal food webs. These are fundamental transfer processes that need to be addressed in order to determine the pathways of MeHg transfer from sites of production in estuarine sediments to coastal fisheries.
Methods
We studied four sites in the Gulf of Maine (GOM) and measured Hg, MeHg, and percent of total Hg as MeHg (%MeHg) in benthic and pelagic fish and invertebrates and compared them to in situ sediment concentrations and to their stable isotope signatures (13C and 15N). To represent a wide range of functional feeding groups and trophic levels, we sampled major benthic primary consumers, secondary consumers, and tertiary consumers. In 2003, we collected a full range of taxa and in 2004, we focused on four focal taxa: a filter feeder (Mytilus edulis), a vertebrate omnivore (Fundulus heteroclitus), a benthic grazer (Littorina littorea), and a benthic omnivore (Carcinus maenas).
We sampled at four sites in intertidal areas with similar patterns of tidal inundation but differing in degrees of Hg contamination. Sites were located in 1) Adams Point (ADAMS) in southeast Great Bay NH where Hg inputs are low (21) and land use is relatively forested; 2) the Portsmouth Harbor Region of Great Bay (PHGB), which is highly industrialized and adjacent to numerous contaminated sites; 3) the Webhannet Estuary in Wells ME (WELLS), which is undeveloped except for some residential areas (mostly seasonal); and 4) Northeast Cove on Mount Desert Island Maine (MDI) adjacent to Acadia National Park, which is pristine but receives relatively high atmospheric inputs of Hg (22).
Sediment sampling and analysis
Sediment samples were collected in summer 2004 and 2006 at different sites using a 6 cm diameter coring tube. The top 2 cm of nine sediment cores were composited into a single sample. Subsamples of the composite were freeze-dried, homogenized, and analyzed for total Hg (THg as μg/g DW) and percent total organic carbon (% TOC using thermal partitioning at 550°C; EPA 440.0; see Supporting Information (SI)).
SEM-AVS (Simultaneously Extracted Metals – Acid Volatile Sulfides) is a measure of biovailability in which the AVS (largely iron sulfides available for binding divalent cationic metals to form insoluble metal-sulfide complexes) reduces the metal biovailability and toxicity of metals in sediments. Values <1.0 indicate no bioavailability of metals. Separate sediment samples (three replicates per site) were collected for SEM-AVS analysis by taking a plug of sediment under water to prevent contact with air. Samples were frozen immediately and later analyzed (see SI).
Chlorophyll and Zooplankton sampling
We collected chlorophyll, dissolved organic carbon (DOC), and zooplankton samples in summer 2003 at each GOM site at high tide. These samples were not indicative of conditions throughout the tidal cycle but a measure to compare aqueous conditions across sites at a single time point. Zooplankton samples were collected at high tide using a 202 μm non-metallic plankton net for multiple vertical tows. Samples were collected and stored in 30 ml acid-cleaned Teflon vials (23). In the lab, samples were freeze-dried, milled in acid cleaned Teflon vessels with Teflon coated steel balls, and split for Hg and stable isotope analyses. Trace metal clean protocols were used in all field and analytical work (24). Reagents were trace-metal grade wherever possible, and procedural blanks were included in all cases.
Benthic invertebrate sampling
Invertebrates were sampled using plastic trowels, minnow traps, D nets, pitfall traps, or collected by hand. Sediment samples were sieved through a 0.5 mm, nylon coated mesh, returned to the lab where invertebrates were sorted, and identified to the lowest practical taxon. All samples were handled with trace metal clean technique and stored in either acid cleaned plastic bags or acid cleaned teflon vials (for smaller organisms) and frozen. Later, frozen samples were thawed, rinsed with ultra-clean water, weighed, and freeze-dried. Mollusks were removed from their shells prior to freeze-drying.
Fish sampling
Fish sampling was conducted at mid to high tide levels using fish seines, fyke nets, and minnow traps. Fish total lengths and wet weights were measured. Sizes of individuals for each fish and invertebrate species were standardized for Hg tissue samples to reduce the influence of size on feeding habits and trophic position (25). Fish were frozen in acid rinsed plastic bags for storage and prepared as in Chen and Folt (26).
Stable Isotope Analysis
Whole fish tissue, whole invertebrates, and gastropods without shells all sampled in 2004 were analyzed for stable isotopes at the Colorado Plateau Stable Isotope Laboratory. Animal samples were freeze-dried, ground and homogenized. Isotopic signatures (13C/12C, 15N/14N) were measured for each species. 13C was used to identify food sources (27) such as benthic vs. pelagic production (28) or marsh plants versus phytoplankton (29). 15N was used to identify the trophic levels of the organisms studied (27). Stable isotope samples of M. edulis were not collected from Wells in 2004 but samples from 2006 were used after stable isotope values from multiple years were compared to ensure minimal year to year variation.
Hg and MeHg Analysis
All metal samples were analyzed by the Dartmouth Trace Element Core Facility using a magnetic sector inductively coupled plasma-mass spectrometer (ICP-MS ELEMENT2, Thermo-Finnigan, Waltham, MA). Total Hg samples were microwave digested for analysis with a nitric acid digestion (Seastar™). Total Hg in biota and sediments was analyzed using cold vapor-ICP-MS (instrument detection limits of ca. 0.1 ng L−1). External quality control was achieved by digesting and analyzing similar amounts of standard reference materials (SRM’s; NIST SRM 2976 mussel tissue, n=4, and DORM-2, NRC-CNRC Canada). Average Hg recovery rates were 103.5±5% for the mussel SRM and 93% for DORM-2. For total Hg the method detection limit is approximately 2 ng g−1 based on a sample weight of 30 mg and digestion volume of 10 ml. Field blanks for zooplankton were 16 ng L−1, 100 times lower than the average sample concentrations.
Samples from 2004 were analyzed for Hg speciation using isotope dilution gas chromatography-ICPMS. Samples were freeze-dried, spiked with an appropriate amount of enriched inorganic 199Hg (HgI) and enriched methyl201Hg (MeHg) and then extracted in 2–3 mls of KOH/methanol (25% w/v). One of two methods for Hg speciation was employed depending on the expected level of Hg in the original sample, a function of the initial available sample mass. For <20 mg the methodology involved purging with inert gas and trapping on a Tenax trap which was thermally desorbed and Hg species were quantified by isotope dilution GC-ICP-MS using a high sensitivity Element2 ICP-MS in low resolution mode. For >20 mg, samples were analyzed according to Lorenzo et al. (30). The latter methodology is less time-consuming than the purge and trap method but has higher detection limits and is only suitable for larger initial sample masses. Quality control was conducted through the analysis of two SRM’s: NIST 2976, mussel tissue with MeHg certified at 0.0278 ± 1.1 μg g−1 and CRC (Ottawa, Canada) DORM-2, dogfish muscle, MeHg concentration of 4.47 ± 0.32 μg g−1. Average recovery for MeHg in DORM2 was 108% (n=13, r.s.d. = 3.4%) and for NIST 2976 average recovery was 114% (n=12, r.s.d. = 10%). Method detection limits for MeHg analysis by isooctane extraction and capillary GC-ICP-MS (Agilent 7500c, Palo Alto, CA) are 5 ng g−1 assuming an initial sample mass of 200 mg. For the purge and trap GC-ICP-MS (Element 2, Thermo-Fisher, Bremen, Germany) method detection limits are 0.2 ng g−1 based on an initial sample weight of 25 mg.
Data Analysis
We used log10-transformed metal concentrations in all analyses as this equalized variance and normalized residuals. All analyses were conducted using the statistical software program, JMP 5.01a. Biota-Sediment Concentration Factors (BSCF) were calculated for the four focal taxa collected in 2004 (BSCF = THg concentration in biota/THg concentration in sediment). The relationship between BSCF values and % TOC or SEM-AVS across sites were determined using analysis of covariance (ANCOVA) with species as an additive classification term. We used ANCOVA to test for a relationship between sediment THg vs. Hg and MeHg concentrations in the focal species across sites. The ANCOVA model included species as a classification term and sediment Hg as a covariate, with mean Hg or MeHg for each species at each site as the response. In ANCOVA analyses, we initially tested for interaction terms, but none were significant and were dropped from the models. We only conducted this analysis for the 2004 data as not all species were collected at all sites in 2003. We used factorial analysis of variance (ANOVA) to test for differences in Hg and MeHg concentrations in focal species across sites. The ANOVA model included classification terms for both species and site, as well as their interaction.
We analyzed stable isotopes of N and C to identify organisms that feed more on pelagic resources (more depleted 13C) and feed at higher trophic levels (more enriched 15N). These patterns in stable isotopes reflect position within a food web, but there was confounding spatial variation in isotopic signatures across sites. Therefore, we included site as an additive blocking factor in an ANOVA to test for differences among the focal species. Thus, stable isotope values were compared between taxa within sites not across sites.
Results
The four sites investigated in this study captured a range of Hg concentrations in sediments from 0.008–1.135 μg THg g−1 DW (Table 1). As expected, the more industrialized Great Bay NH sites had higher sediment concentrations of total Hg and %TOC. Although grain size was not measured, there was variation across sites with the WELLS site (the most pristine site) having the greatest contribution of sand vs. silt. The WELLS site also had the lowest sediment Hg concentrations by more than 2 orders of magnitude and the lowest %TOC. All sites had excess AVS relative to total SEM, but the WELLS site had the least negative SEM-AVS levels (−0.77, all others <−10.0) suggesting it had the smallest amount of sulfide available to bind free metal ions. DOC concentrations in the water column were similar across sites but chlorophyll concentrations differed between sites being highest at MDI followed by ADAMS, PHGB, and WELLS (Table 1; p= < 0.0001).
Table 1.
Sediment and water attributes of four sites (ADAMS, PHGB, WELLS, MDI) in the Gulf of Maine. Sediment samples (%TOC, SEM-AVS, Total Hg) from composites of 9 sediment subsamples collected in 2006. Chl a (value ± SD) and DOC from water column samples collected in 2003.
| System | Site | Sediment TOC (%) | SEM-AVS | Sediment Total Hg (μg/g DW) | Chl a μg/L | DOC mg/L |
|---|---|---|---|---|---|---|
| Webhannet ME | Harbor Road (WELLS) | 0.071 | −0.77 | 0.008 | 1.4±0.3 | 0.95 |
| Mount Desert ME | Northeast Creek (MDI) | 1.39 | −14.08* | 0.069 | 5.2±0.8 | 0.59 |
| Great Bay NH | Adams Point (ADAMS) | 2.17 | −11.66 | 0.424 | 3.1±0.3 | 0.65 |
| Great Bay NH | Portsmouth Harbor (PHGB) | 2.76 | −27.99 | 1.135 | 2.0±0.4 | 0.71 |
Sample taken from Somes Sound also on Mount Desert Island.
There was a significant positive relationship between Hg in sediments and %TOC across our sites (Figure S1, SI, P=0.003). Consistent with higher Hg in sediments at sites with high %TOC, Hg concentrations in the four focal taxa were also positively related to %TOC (Table 2, P=0.05). However, BSCF’s calculated for the four focal taxa across the four sites were negatively related to %TOC (Table 2; P<0.001, Figure 1a). Calculated BSCF’s were also positively related to SEM-AVS (Table 2, P<0.001, Figure 1b) indicating that WELLS, the most pristine site with the least negative SEM-AVS had the highest BSCF. The negative AVS-SEM values across all the sites would suggest that inorganic Hg is not bioavailable at any of the sites but the bioaccumulation data indicate otherwise.
Table 2.
Summary of statistical analyses for relationships between Hg and MeHg in biota, BSCF’s, %TOC, sediment concentrations, and sites.
| Analysis | Response | Term | DF | SS | F Ratio | Prob > F | r2 |
|---|---|---|---|---|---|---|---|
| ANCOVA | Tissue log10THg | Species | 3 | 0.72 | 5.32 | 0.02 | 66% |
| Sediment log10THg | 1 | 0.24 | 5.23 | 0.04 | |||
| Error | 11 | 0.5 | |||||
|
| |||||||
| Tissue log10MeHg | Species | 3 | 0.63 | 4.25 | 0.03 | 61% | |
| Sediment log10THg | 1 | 0.21 | 4.27 | 0.06 | |||
| Error | 11 | 0.54 | |||||
|
| |||||||
| ANCOVA | Tissue log10THg | Species | 3 | 0.72 | 5.23 | 0.02 | 65% |
| Sediment %TOC | 1 | 0.23 | 4.96 | 0.05 | |||
| Error | 11 | 0.5 | |||||
|
| |||||||
| Tissue log10MeHg | Species | 3 | 0.63 | 4.25 | 0.03 | 61% | |
| Sediment %TOC | 1 | 0.21 | 4.29 | 0.06 | |||
| Error | 11 | 0.54 | |||||
|
| |||||||
| ANCOVA | log10BSCF | Species | 3 | 0.72 | 5.23 | 0.02 | 94% |
| Sediment %TOC | 1 | 7.81 | 172.78 | <0.0001 | |||
| Error | 11 | 0.5 | |||||
|
| |||||||
| log10BSCF | Species | 3 | 0.72 | 1.69 | 0.22 | 83% | |
| AVS-SEM | 1 | 6.75 | 47.5 | <0.0001 | |||
| Error | 11 | 1.56 | |||||
|
| |||||||
| ANOVA | Tissue log10THg | Site | 3 | 1.17 | 18.1 | <.0001 | 86% |
| Species | 3 | 2.21 | 34.14 | <.0001 | |||
| Site*Species | 9 | 1.04 | 5.34 | 0.0002 | |||
| Error | 32 | 0.69 | |||||
|
| |||||||
| Tissue log10MeHg | Site | 3 | 0.73 | 10.94 | <.0001 | 84% | |
| Species | 3 | 1.7 | 25.44 | <.0001 | |||
| Site*Species | 9 | 1.38 | 6.86 | <.0001 | |||
| Error | 32 | 0.71 | |||||
|
| |||||||
| Tissue %MeHg | Site | 3 | 0.06 | 5.82 | 0.0027 | 95% | |
| Species | 3 | 1.78 | 160.48 | <.0001 | |||
| Site*Species | 9 | 0.6 | 18.1 | <.0001 | |||
| Error | 32 | 0.12 | |||||
|
| |||||||
| ANOVA | Tissue d13C | Site | 3 | 55 | 18.38 | <.0001 | 62% |
| Species | 3 | 12.99 | 4.34 | 0.0095 | |||
| Error | 41 | 40.89 | |||||
|
| |||||||
| Tissue d15N | Site | 3 | 30.51 | 9.13 | <.0001 | 70% | |
| Species | 3 | 74.3 | 22.24 | <.0001 | |||
| Error | 41 | 45.66 | |||||
Figure 1.
Relationship of biotic-sediment concentration factors (BSCF) across sites (ADAMS, PHGB, WELLS, MDI) and four focal taxa to: a) TOC (2006) and b) SEM-AVS (2006). Symbols: (open square) Mytilus, (solid square) Carcinus, (open circle) Littorina, (solid circle) Fundulus. Equation for %TOC ANCOVA: Log10 BSCF= X − 0.69 · (% TOC), where X=0.87 for Carcinus, 1.23 for Fundulus, 1.19 for Littorina, and 1.46 for Fundulus; see Table 2 for statistics. Equation for SEM-AVS ANCOVA: Log10 BSCF= X − 0.067 · (SEM-AVS), where X=0.68 for Carcinus, 1.03 for Fundulus, 1.01 for Littorina, and 1.27 for Fundulus; see Table 2 for statistics.
Both total Hg and MeHg concentrations in organisms collected in 2004 tended to be higher at sites where sediment total Hg concentrations were higher (Table 2, THg, P=0.04; MeHg, P=0.06). However, sediment Hg concentrations alone were a poor predictor of concentrations in organisms (partial r2<0.3) and the 2–4 fold range in organism Hg and MeHg concentrations for each taxa across sites did not reflect the more than one hundred-fold range in sediment Hg concentrations (Figure 2a–c). Furthermore, PHGB sediments were by far the most contaminated, but organisms at PHGB did not consistently have the highest total Hg or MeHg concentrations.
Figure 2.
Concentrations (μg/g DW) of THg across sites (ADAMS, PHGB, WELLS, MDI) in: a) sediments (collected in 2006) and b) four focal species collected in 2003 (not all four species collected at all sites) and c) 2004 (percent moisture for each taxa: Carcinus 76±9%, Fundulus 71±3%, Littorina 78±2%, and Mytilus 84±5%). Note difference in vertical scale for panel b. Bars = SE.
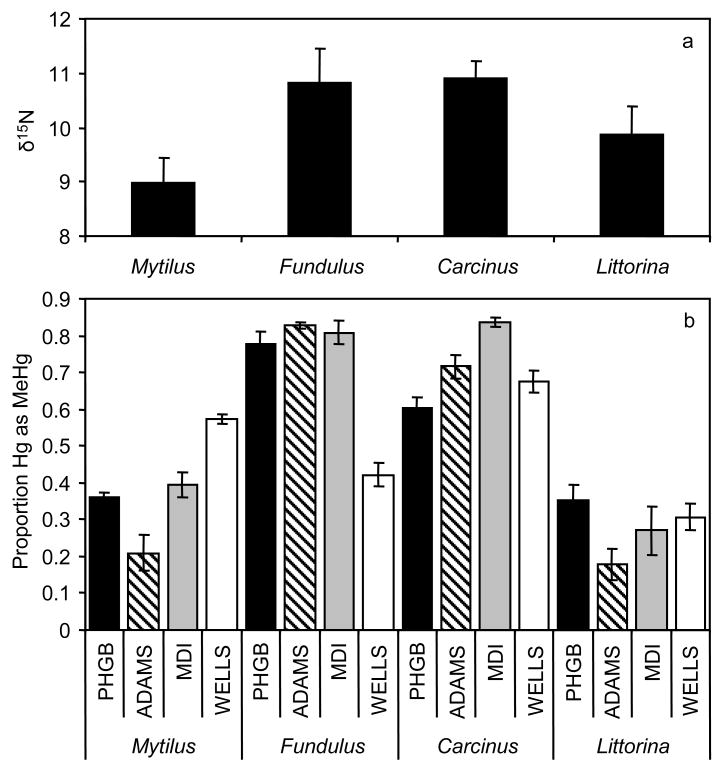
Hg concentrations in 2004 biota varied significantly across both sites and species (Table 2, Figure 2, site: P<0.0001; species: P<0.0001). However, there was a significant interaction (Table 2, P=0.0002); species with the highest Hg concentrations were not the same across sites. MeHg concentrations also varied significantly across both sites and species (Table 2, site: P<0.0001; species: P<0.0001), again with a significant interaction (Table 2, P<0.0001). Despite the interaction, Fundulus and Mytilus clearly tend to have higher MeHg concentrations than Carcinus and Littorina (Figure 3). The % MeHg varied significantly across species (Table 2, P<0.0001) with a relatively small but statistically significant difference across sites (Table 2, P=0.003) and an interaction (Table 2, P<0.0001). Despite the interaction, predatory species, Fundulus and Carcinus, clearly tend to have higher %MeHg than Littorina and Mytilus (Figure 4).
Figure 3.
a) Delta 13C signatures and b) MeHg concentrations (μg/g DW) in four focal taxa collected in 2004 across four GOM sites (ADAMS, PHGB, WELLS, MDI). Bars = SE.
Figure 4.
a) Delta 15N signatures and b) % of THg as MeHg in four focal taxa collected in 2004 across four GOM sites (ADAMS, PHGB, WELLS, MDI). Bars = SE.
Differences in food source and trophic levels between the four focal taxa across sites were related to MeHg concentrations and %MeHg. Delta13C signatures differed significantly across species (Table 2, d13C: P<0.0001; Figure 3a) and were more depleted for the nominal pelagic feeding species (Mytilus and Fundulus), confirming that the Mytilus and Fundulus species utilize more pelagic food sources than the benthic-feeding Carcinus and Littorina. As indicated above, these pelagic-feeding species also tended to have elevated MeHg concentrations. Delta 15N values differed significantly across species (Table 2, d15N: P<0.0001; Figure 4a) and were higher in the predator/omnivore species (Carcinus and Fundulus), confirming that Carcinus and Fundulus feed at higher trophic position than Littorina and Mytilus. Although MeHg concentration was not elevated in the predators/omnivores, as indicated above, the %MeHg was elevated indicating that MeHg biomagnifies in these intertidal food webs.
Discussion
Hg deposition in eastern North America is elevated (1) and Hg levels in fish from New England coastal basins are among the highest in the country (31–33). In general, estuaries with major anthropogenic inputs receive higher concentrations of organic and inorganic contaminants as well as nutrients and organic matter which influence the redox status at the sediment-water interface and control metal flux (34). Both the supply of organic matter and the supply of Hg to the sediments influence Hg bioavailability to methylating bacteria and bioaccumulation by benthic and pelagic organisms (10,35,36).
Biogeochemical Factors
Studies of Hg biogeochemistry and bioaccumulation in estuarine systems (5,9,10,37–39) show methylation increasing in sediments with increasing inorganic Hg but decreasing methylation with high levels of carbon and sulfides. However, the presence of organic rich carbon pockets or bulk sulfides indicating the presence of organic carbon can also provide a substrate for enhanced MeHg production (40,41). In this study, total Hg and %TOC in sediments were positively related suggesting, as others have found (33,38,40–42), that a significant portion of the variance in total Hg in sediments is due to retention by %TOC. In other studies, this relationship is also related to grain size where finer sediments are associated with higher %TOC concentrations (41,43).
%TOC and AVS are also biogeochemically linked to one another since TOC in sediments produces the anaerobic conditions under which AVS are created. The extent to which divalent metals bind to AVS is determined by the solubility product of their respective sulfides. Among the heavy metals, HgS is the least soluble and Hg is therefore bound most tightly (i.e., it is the least soluble of metal sulfides). Consequently, the presence of excess AVS at all of our sites implies that all inorganic Hg should be bound (and not bioavailable).
Elevated organic carbon in sediments reduces bioaccumulation of MeHg by benthic fauna (44). In a range of sites in Chesapeake Bay where sediment concentrations of Hg vary by 25 times, concentrations in benthic invertebrates vary by only 2–4 times (42,45,46). Data in the present study show similar trends where total concentrations of Hg in sediments vary by more than two orders of magnitude across sites but the concentrations in biota vary by only 2–4 times. Thus, despite the higher supply of Hg in sediments and the slightly higher bioaccumulation in more contaminated sites, the bioavailability of Hg and MeHg to benthic fauna appears to be strongly influenced by organic carbon.
The effect of organic carbon on Hg bioaccumulation becomes evident when examining the relationship between BSCF, a measure of bioaccumulation relative to in situ sediment Hg concentrations, and %TOC or SEM-AVS. As in previous metal studies, there is a negative relationship between BSCF and TOC and a positive relationship with SEM-AVS (42,45,46). The significant positive relationship between BSCF and SEM-AVS indicates that when normalized to sediment concentration the greater binding capacity in the sediments with more excess AVS lowers bioaccumulation in benthic fauna. However, all of the sites in this study had excess AVS which did not prevent Hg bioaccumulation in benthic fauna. In fact, the higher biotic THg and MeHg concentrations in sites with the most excess AVS suggests that the SEM-AVS is not a good predictor of bioavailability. Thus, TOC may play a greater role in controlling Hg bioavailability in these sediments.
Ecological Factors
MeHg transfer from sites of methylation in sediments to higher trophic levels involves both benthic and pelagic pathways. Studies of food web structure in estuarine systems using stable isotope reveal that primary and secondary consumers utilize a mixture of detrital and algal based food sources (13,47). Numerous studies have shown that more pelagic consumers feeding on phytoplankton are more depleted in 13C than benthic consumers feeding on benthic microalgae and saltmarsh vegetation (47–49). In the food webs studied here, our four focal species represent different functional feeding groups and trophic levels. Delta 13C signatures confirm that Mytilus is the most pelagic in its feeding on phytoplankton and particulate organic matter. Fundulus, an omnivorous resident species is slightly less depleted in 13C reflecting its pelagic-benthic diet of zooplankton, benthic microalgae and Spartina detritus (27,29,47,50,51). The two benthic epifauna, Littorina and Carcinus, have more enriched 13C signatures reflective of their diets on benthic algae and benthic invertebrates, respectively.
In the intertidal food webs studied here, MeHg concentrations are higher in more pelagic feeding species (more depleted delta 13C signature) suggesting that the more important pathway from MeHg production in sediments to marine fish may be chemical flux into the water column and absorption by and ingestion of particulates rather than direct ingestion of sediments. The more pelagic taxa, Mytilus and Fundulus, represent different trophic levels but both derive a major portion of their diet from particulates or organisms feeding on particulates and both contain higher concentrations of MeHg. Total Hg concentrations for these two taxa are within the ranges found in other studies (Fundulus: 250±160 ppb DW (52); mussels: 250–1200 ppb DW (53)). The two benthic taxa (Littorina, Carcinus) also representing different trophic levels have lower MeHg concentrations potentially due to their sediment-based diets. The bioconcentration of MeHg by particulates that can range up to six orders of magnitude may drive the higher exposures to MeHg in pelagic fauna than benthic fauna. The importance of the pelagic pathway in transferring MeHg also suggests that methylation in the water column could have an important role in supplying MeHg to pelagic food webs and marine fish species that humans consume.
In this study of the lower trophic levels of benthic and pelagic food webs, the %MeHg increases with trophic level while MeHg concentrations do not. The biomagnification of MeHg in most aquatic food webs results in increasing concentration or %MeHg with trophic level (1,54). However, these generally involve food webs extending from lower trophic levels to apex predators (1,5,17). For most piscivorous fish species, total Hg is comprised almost entirely of MeHg (>95%) making these species particularly important vectors of human exposure. However, lower trophic level organisms where MeHg enters marine food webs vary greatly in their %MeHg ranging from 5%–80% (1,35). Similar to these studies, we find that %MeHg ranges from 18–35% in Littorina, 20–57% in Mytilus, 60–84% in Carcinus, and 42–82% in Fundulus.
These estuarine food webs are potential sources of MeHg biotransfer from sediments to coastal food webs by virtue of their proximity to MeHg production and flux from the sediments and their ecological connection to offshore fisheries via a “trophic nekton relay” (13,14,47). This ecological transport mechanism of both energy and contaminants may be an important source of MeHg to offshore fish that reside in environments far from MeHg sources (3,5). Thus, the biogeochemical and ecological processes mediating MeHg bioaccumulation in lower trophic levels in estuarine sediments can have far reaching effects on the ultimate fate of MeHg in marine food webs. Although more contaminated sites have much greater supply of Hg for methylation and uptake, Hg bioavailability to benthic fauna is substantially reduced by sediment organic matter. In addition, across a range of estuarine sites, benthic feeding in or on sediments may provide a less efficient pathway for MeHg biotransfer than pelagic feeding on suspended particulate organic matter.
Supplementary Material
The following methods descriptions, figure (Figure S1), and table (Table S1) are included as part of this manuscript. The supplementary materials comprise a total of three pages. This information is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We gratefully acknowledge the assistance of Chip Glaholt, Amy Wallace, Heather Hudenko, Richard Mackenzie, Ata Bilgili, Jim Dochtermann, and Jeremy Miller in conducting fieldwork. We thank Lorenzo Perna and Angela Lacroix-Fralish for their analytical assistance and David Shay at the UNH Jackson Estuarine Laboratory and the Wells National Estuarine Research Reserve for making facilities available for our use. The paper also was greatly improved by the comments of four anonymous reviewers. This research was supported by NIH Grant Number P42 ESO7373 (to C.L.F. and C.Y.C.) from the National Institute of Environmental Health Sciences, SERDP Project ER-1503 funds, UNH Sea Grant development funds, Dartmouth CEHS pilot funds, and a New Investigator Award from the Mount Desert Biological Laboratory.
References
- 1.Driscoll CT, Han YJ, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK. Mercury contamination in forest and freshwater ecosystems in the Northeastern United States. BioScience. 2007;57(1):17–28. [Google Scholar]
- 2.UNEP, Global Mercury Assessment; United Nations Environment Programme: 2003.
- 3.Sunderland EM. Mercury Exposure from Domestic and Imported Estuarine and Marine Fish in the U.S. Seafood Market. Environ Health Persp. 2007;115(2):235–242. doi: 10.1289/ehp.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.USEPA, Origin of the 1 meal/week noncommercial fish consumption rate in national advisory for mercury. Office of Water, National Fish and Wildlife Contamination Program 2004.
- 5.Hammerschmidt CR, Fitzgerald WF. Bioaccumulation and trophic transfer of methylmercury in Long Island Sound. Arch Environ Con Tox. 2006;51:416–424. doi: 10.1007/s00244-005-0265-7. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald WF, Lamborg CH, Hammerschmidt CR. Marine biogeochemical cycling of mercury. Chem Rev. 2007;107(2):641–662. doi: 10.1021/cr050353m. [DOI] [PubMed] [Google Scholar]
- 7.Long ER. Spatial extent of sediment toxicity in U.S. estuaries and marine bays. Environ Monit Assess. 2000;64:391–407. [Google Scholar]
- 8.Marvin-DiPasquale MC, Agee JL, Bouse RM, Jaffe BE. Microbial cycling of mercury in contaminated pelagic and wetland sediments of San Pablo Bay, California. Environ Geol. 2003;43:260–267. [Google Scholar]
- 9.Mason RP, Lawrence AL. Concentration, distribution, and bioavailability of mercury and methylmercury in sediments of Baltimore Harbor and Chesapeake Bay, Maryland, USA. Environ Toxicol Chem. 1999;18:2438–2447. [Google Scholar]
- 10.Sunderland EM, Gobas FAPC, Branfireun BA, Heyes A. Environmental controls on the speciation and distribution of mercury in coastal sediment. Mar Chem. 2006;102:111–123. [Google Scholar]
- 11.Jury SH, Field JD, Stone SL, Nelson DM, Monaco ME. Distribution and abundance of fishes and invertebrates in North Atlantic estuaries. 1994:221. [Google Scholar]
- 12.Nelson DM, Monaco ME. National overview and evolution of NOAA's Estuarine Living Marine Resources (ELMR) Program. 2000:60. [Google Scholar]
- 13.Deegan LA, Hughes JE, Rountree RA. Salt marsh ecosystem support of marine transient species. In: Weinstein MP, Kreeger DA, Kreeger DA, editors. Concepts and Controversies in Tidal Marsh Ecology. Kluwer Academic Publishers; Boston: 2000. pp. 333–365. [Google Scholar]
- 14.Kneib RT. Salt marsh ecoscapes and production transfers by estuarine nekton in the southeastern United States. In: Weinstein MP, Kreeger DA, editors. Concepts and Contoversies in Tidal Marsh Ecology. Kluwer Academic Publishers; Boston: 2000. pp. 267–291. [Google Scholar]
- 15.Mason RP, Lawson NM, Lawrence AL, Leaner JJ, Lee JG, Sheu GR. Mercury in the Chesapeake Bay. Mar Chem. 1999;65:77–96. [Google Scholar]
- 16.Chen C, Amirbahman A, Fisher N, Harding G, Lamborg C, Nacci D, Taylor D. Methylmercury in Marine Ecosystems: Spatial Patterns and Processes of Production, Bioaccumulation, and Biomagnification. Ecohealth. doi: 10.1007/s10393-008-0201-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bank MS, Chesney EJPS, Maage A, Senn DB. Mercury bioaccumulation trophic transfer in sympatric snapper species from the Gulf of Mexico. Ecol Appl. 2007;17:2100–2110. doi: 10.1890/06-1422.1. [DOI] [PubMed] [Google Scholar]
- 18.Monperrus M, Tessier E, Point D, Vidimova K, Amouroux D, Guyoneaud R, Leynaert A, Grall J, Chauvaud L, Thouzeau G, Donard OFX. The biogeochemistry of mercury at the sediment-water interface in the Thau Lagoon. 2. Evaluation of mercury methylation potential in both surface sediment and the water column. Estuar Coast Shelf S. 2007;72:485–496. [Google Scholar]
- 19.Benoit JM, Shull DH, Robinson P, Ucran LR. Infaunal burrow densities and sediment monomethyl mercury distributions in Boston Harbor, Massachusetts. Mar Chem. 2006;102:124–133. [Google Scholar]
- 20.Power M, Klein GM, Guiguer K, Kwan MKH. Mercury accumulation in the fish community of a sub-Arctic lake in relation to trophic position and carbon sources. J Appl Ecol. 2002;39:819–830. [Google Scholar]
- 21.Jones SH. A Technical Characterization of Estuarine and Coastal New Hampshire. New Hampshire Estuaries Project: Portsmouth, NH. 2000 [Google Scholar]
- 22.Kahl JS, Nelson SJ, Fernandez IHT, Norton S, Wiersma GB, Jacobson G, Amirbahman A, Johnson K, Schauffler M, Rustad L, Tonnessen K, Lent R, Bank MEJ, Eckhoff J, Caron H, Ruck P, Parker J, Campbell J, Manski D, Breen R, Sheehan K, Grygo A. Watershed nitrogen and mercury geochemical fluxes integrate landscape factors in long-term research watersheds at Acadia National Park, Maine, USA. Environ Monit Assess. 2007;126:9–25. doi: 10.1007/s10661-006-9328-0. [DOI] [PubMed] [Google Scholar]
- 23.Chen CY, Stemberger RS, Klaue B, Blum JD, Pickhardt PC, Folt CL. Accumulation of heavy metals in food web components across a gradient of lakes. Limnol Oceanogr. 2000;45:1525–1536. [Google Scholar]
- 24.USEPA Method 1669: Sampling ambient water for Trace Metals at EPA Water Quality Criteria Levels., EPA 821-R-95-034; U.S. Environmental Protection Agency, Office of Water: 1995.
- 25.Bennett WA, Ostarch DJ, Hinton DE. Larval striped bass condition in a drought-stricken estuary: evaluating pelagic food-web limitation. Ecol Appl. 1995;5:680–692. [Google Scholar]
- 26.Chen CY, Folt CL. Bioaccumulation and diminution of arsenic and lead in a freshwater food web. Environ Sci Technol. 2000;34:3878–3884. [Google Scholar]
- 27.Peterson BJ, Fry B. Stable Isotopes in Ecosystem Studies. Annu Rev Ecol Syst. 1987;18:293–320. [Google Scholar]
- 28.Stribling JM, Cornwell JC. Identification of important primary producers in a Chesapeake Bay tidal creek system using stable isotopes of carbon and sulfur. Estuaries. 1997;20:77–85. [Google Scholar]
- 29.Sullivan MJ, Moncreiff CA. Edaphic Algae Are an Important Component of Salt-Marsh Food-Webs - Evidence from Multiple Stable Isotope Analyses. Mar Ecol- Prog Ser. 1990;62:149–159. [Google Scholar]
- 30.Lorenzo P, LaCroix-Fralish A, Stürup S. Determination of inorganic mercury and methylmercury in zooplankton and fish samples by speciated isotopic dilution GC-ICP-MS after alkaline digestion. J Anal At Spectrom. 2005;20:236–238. [Google Scholar]
- 31.Brumbaugh WG, Krabbenhoft DP, Helsel DR, Weiner JG, Echols KR. A national pilot study of mercury contamination in aquatic ecosystems along multiple gradients - Bioaccumulation in fish. U.S. Geological Survey Water Resources Investigations; 2001. [Google Scholar]
- 32.Robinson KW, Flanagan SM, Ayotte JD, Campo KW, Chalmers A, Coles JF, Cuffney TF. Water quality in New England coastal basins, Maine, New Hampshire, Massachusetts, and Rhode Island, 1999–2001. U. S. Department of the Interior and U. S. Geological Survey; 2004. [Google Scholar]
- 33.USEPA. Environmental Monitoring and Assessment Program, National Coastal Assessment. 2007 http://www.epa.gov/emap/index.html.
- 34.Covelli S, Faganeli J, Horvat M, Brambati A. Porewater distribution and benthic flux measurements of mercury and methylmercury in the Gulf of Trieste (Northern Adriatic Sea) Est Coast Shelf Sci. 1999;48:415–428. [Google Scholar]
- 35.Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL. Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. In: Cai Y, Braids OC, editors. Biogeochemistry of Environmentally Important Trace Elements. Vol. 835. American Chemical Society; Washington: 2003. pp. 262–297. [Google Scholar]
- 36.Lambertsson L, Nilsson M. Organic material: The primary control on mercury methylation and ambient methyl mercury concentrations in estuarine sediments. Environ Sci Technol. 2006;40:1822–1829. doi: 10.1021/es051785h. [DOI] [PubMed] [Google Scholar]
- 37.Baeyens W, Meuleman C, Muhaya B, Leermakers M. Behaviour and speciation of mercury in the Scheldt estuary (water, sediments and benthic organisms) Hydrobiologia. 1998;366:63–79. [Google Scholar]
- 38.Hammerschmidt CR, Fitzgerald WF. Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ Sci Technol. 2004;38:1487–1495. doi: 10.1021/es034528q. [DOI] [PubMed] [Google Scholar]
- 39.Heyes A, Miller C, Mason RP. Mercury and methylmercury in Hudson River sediment: Impact of tidal resuspension on partitioning and methylation. Mar Chem. 2004;90:75–89. [Google Scholar]
- 40.Lawrence AL, Mason RP. Factors controlling the bioaccumulation of mercury and methylmercury by the estuarine amphipod Leptocheirus plumulosus. Environ Pollut. 2001;111:217–231. doi: 10.1016/s0269-7491(00)00072-5. [DOI] [PubMed] [Google Scholar]
- 41.Sunderland EM, Gobas FAPC, Heyes A, Branfireun BA, Bayer AK, Cranston RE, Parsons MB. Speciation and bioavailability of mercury in well-mixed estuarine sediments. Mar Chem. 2004;90:91–105. [Google Scholar]
- 42.Sunderland EM, Gobas FAPC, Branfireun BA, Heyes A. Environmental controls on the speciation and distribution of mercury in coastal sediments. Mar Chem. 2006;102:111–123. [Google Scholar]
- 43.Loring D, Asmund G. Geochemical factors controlling accumulation of major and trace elements in Greenland coastal and fjord sediments. Environ Geol. 1996;28:2–11. [Google Scholar]
- 44.Muhaya B, Leermakers M, Baeyens W. Total mercury and methylmercury in sediments and in the polychaete Nereis diversicolor at Groot Buitenschoor (Scheldt estuary, Belgium) Water Air Soil Poll. 1997;94:109–123. [Google Scholar]
- 45.Wright DA, Mason RP. Biological and chemical influences on trace metal toxicity and bioaccumulation in the marine and estuarine environment. Int J Environ Pollut. 2000;13:226–248. [Google Scholar]
- 46.Mason RP. Chapter 6: The Bioaccumulation of Mercury, Methylmercury, and other toxic elements into pelagic and benthic organisms. In: Newman MC, Roberts MHJ, Hale RC, editors. Coastal and Estuarine Risk Assessment. Lewis Publishers; Boca Raton (NY): 2002. pp. 127–149. [Google Scholar]
- 47.Deegan LA, Garritt RH. Evidence for spatial variability in estuarine food webs. Mar Ecol- Prog Ser. 1997;147:31–47. [Google Scholar]
- 48.Michener RH, Kaufman L. Chapter 9: Stable isotope ratios as tracers in marine food webs: An update. In: Lajtha K, Michener RH, editors. Stable Isotopes in Ecology and Environmental Science. Blackwell Scientific; Boston: 2006. pp. 238–282. [Google Scholar]
- 49.Winemiller KO, Senol Akin, Zeug SC. Production sources and food web structure of a temperate tidal estuary: integration of dietary and stable isotope data. Mar Ecol- Prog Ser. 2007;343:63–76. [Google Scholar]
- 50.Hughes JE, Deegan LA, Peterson BJ, Holmes RM, Fry B. Nitrogen flow through the food web in the oligohaline zone of a New England estuary. Ecology. 2000;81:433–452. [Google Scholar]
- 51.Kneib RT. Bioenergetic and landscape considerations for scaling expectations of nekton production from intertidal marshes. Mar Ecol- Prog Ser. 2003;264:279–296. [Google Scholar]
- 52.Weis P, Ashley JTF. Contaminants in fish of the Hackensack Meadowlands, New Jersey: Seasonal relationships as related to health risks. Arch Environ Con Tox. 2007;52:80–89. doi: 10.1007/s00244-006-0093-4. [DOI] [PubMed] [Google Scholar]
- 53.Chase ME, Jones SH, Hennigar P, Sowles J, Harding GCH, Freeman K, Wells PG, Krahforst C, Cooms K, Crawford R, Pederson J, Taylor D. Gulfwatch: Monitoring spatial and temporal patterns of trace metal and organic contaminants in the Gulf of Maine (1991–1997) with the blue mussel, Mytilus edulis L. Mar Pollut Bull. 2001;42:491–505. doi: 10.1016/s0025-326x(00)00193-4. [DOI] [PubMed] [Google Scholar]
- 54.Mason RP, Laporte JM, Andres S. Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish. Arch Environ Con Tox. 2000;38:283–297. doi: 10.1007/s002449910038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following methods descriptions, figure (Figure S1), and table (Table S1) are included as part of this manuscript. The supplementary materials comprise a total of three pages. This information is available free of charge via the Internet at http://pubs.acs.org.