Abstract
Context
There is an increased prevalence of obesity and insulin resistance in African-American compared with Caucasian females. Metabolic inflexibility (MI) is the inability to switch the use of lipids and carbohydrates in the peripheral tissue (i.e. muscle) based upon substrate availability.
Objective
We examined whether MI exists in African-American females.
Main Outcome Measures and Design
We measured substrate use differences during eucaloric, macronutrient-manipulated diets [high fat (50% fat, 35% carbohydrate, 15% protein) vs. low fat (30% fat, 55% carbohydrate, 15% protein)] between Caucasian and African-American women. We also compared differences in substrate use in response to insulin infusion during two-step pancreatic-euglycemic clamps and epinephrine infusion during lipolysis studies. In each study, similar groups of Caucasian and African-American women were compared.
Results
Caucasians had significantly higher fat oxidation (FO) (P = 0.01) and lower carbohydrate oxidation (P < 0.01) during the high-fat vs. low-fat diet, whereas no significant differences were observed in African-Americans. The African-American women also failed to significantly suppress FO during the second step of the pancreatic-euglycemic clamp despite a doubling of their fasting plasma insulin and failed to increase their FO or decrease their carbohydrate oxidation in response to epinephrine infusion as much as Caucasian women did. The response of free fatty acid turnover rates to insulin and epinephrine stimulation was similar between races.
Conclusion
The impaired substrate use observed in African-American women during these three studies demonstrates the existence of MI and may contribute to their greater prevalence of obesity and insulin resistance.
With the increased obesity epidemic in the United States, new physiological dysregulations have been observed. One such dysregulation is metabolic inflexibility (MI). MI was identified and extensively studied by Kelley and colleagues (1–5) in obese and type 2 diabetics and is characterized by an absence of increased fat oxidation in skeletal muscle during fasting conditions and the impaired ability to switch from fat to carbohydrate oxidation in response to insulin. Healthy populations (6–8) have been shown to switch their fuel use based upon substrate availability; however, within healthy individuals, the degree of metabolic flexibility varies (9). Myocytes obtained from healthy, lean males were found to be more inflexible in vitro (decreased fat oxidation suppression with glucose and a smaller increase in fat oxidation with palmitate) with increased percent body fat and free fatty acid (FFA) levels and decreased insulin sensitivity (9). Insulin resistance is hypothesized as one mechanism for MI (4–5).
African-American females have a higher obesity prevalence than age-matched Caucasians (10). Nearly half of the African-American females aged 30–44 yr are overweight, whereas only 33% of similarly aged Caucasians are overweight (11). African-Americans are more insulin resistant than Caucasians (12, 13) even when adjusting for degree of obesity (14). There are some data suggesting that MI exists in African-American women. African-American males have higher fasting and 24-h respiratory quotients (RQs) than Caucasian males (15), and African-American postmenopausal females have lower postabsorptive fat oxidation rates than Caucasian counterparts (16). Differences in fat oxidation between African-Americans and Caucasians have been studied under varied conditions (postabsorptive, postprandial, or postexercise), and most (17, 18) but not all studies (19) have reported lower fat oxidation in African-American women.
In this paper, we analyze whether MI is present in premenopausal, nondiabetic, healthy African-American females. We report three studies. First we report substrate use measurements in response to eucaloric, macronutrient-manipulated diets: high fat (HF) vs. low fat (LF). We then report physiological mechanistic studies measuring differences in rates of substrate use in response to insulin infusion during pancreatic clamps and epinephrine infusion during lipolysis studies.
Subjects and Methods
Studies overview
All studies were performed in the General Clinical Research Center at St. Luke’s-Roosevelt Hospital New York Obesity Research Center. Subjects signed consent forms for each experiment that was approved by the St. Luke’s-Roosevelt Institute for Health Sciences Institutional Review Board. Subjects were defined sedentary by physical activity questionnaires and completed an oral glucose tolerance test (75-g glucose load) to ensure absence of diabetes [mean fasting glucose, 5.0 mmol/liter (4.2–5.9 mmol/liter); mean 2-h glucose, 5.9 mmol/liter (4.1–8.3 mmol/liter)]. Subjects completed medical histories and physical exams and were excluded if they self-reported taking any medications including oral contraceptives or if they self-reported smoking within the past 6 months, consuming alcohol in excess (>2 oz. ethanol/d) or having irregular menstrual cycles (skipping more than two monthly cycles per year). Subjects were included if they self-reported all four grandparents were either from Caucasian or African ancestry. There was no overlap of subjects between studies.
Study 1
Twenty-one Caucasian and 21 African-American lean and obese [body mass index (BMI), 18.7–46.5 kg/m2], premenopausal (age 22–44 yr) women participated. Subjects had their resting metabolic rate (RMR) determined by indirect calorimetry as previously described (20) and provided with a 7-d eucaloric diet based upon their RMR adjusted for their activity level. Subjects were randomized to begin with either a LF (30% fat, 55% carbohydrate, 15% protein) or a HF (50% fat, 35% carbohydrate, 15% protein) diet. The fat calories were distributed as one third saturated, one third monounsaturated, and one third polyunsaturated for both diets. There was a minimum 2-wk washout period between the diets.
The subjects were provided with menus and 6-d food supplies to consume at home. Dietary compliance was assured through weight stability measurements, and adjustments were planned for a weight change of ±1 kg. However, because diets were based upon earlier RMR and physical activity measurements, adjustments were not necessary. On the sixth night, subjects were admitted to the General Clinical Research Center. Postabsorptive substrate use measurements were made through indirect calorimetry (Horizon Metabolic Cart or VMax29; Sensor Medics, Yorba Linda, CA) in 20 subjects [nine African-Americans (five obese and four lean) and 11 Caucasians (six obese and five lean)] (21) or by room calorimetry in 26 subjects [13 African-Americans (eight obese and five lean) and 13 Caucasians (seven obese and six lean)] (22). Fasting blood samples were collected after indirect calorimetry measurements.
Study 2
Nine Caucasian and nine African-American obese (BMI, 28.4–40.9 kg/m2), premenopausal (ages 26–49 yr) women participated in lipolysis studies at postabsorptive baseline followed by two-step (low- and high-dose insulin) pancreatic euglycemic clamps (21). Briefly, subjects were fed weight-maintaining diets for 3 d and on d 4 were studied. [1-13C]Palmitic acid (99% 13C; Masstrace, Woburn, MA) in an albumin mixture (Bayer Laboratories, Elkhart, IN) prepared as a sterile, pyrogen-free solution was infused [0.07 μmol/kg fat-free mass (FFM) per minute] from −90 min to +180 min. Somatostatin (Bachem, Torrence, CA) was infused (0.14 μg/kg FFM·min) starting at time 0 and lasting throughout the clamp (+180 min) to suppress endogenous insulin production. Exogenous insulin infusion (Humulin; Eli Lilly, Indianapolis, IN) was started at time 0 at 2 mU/m2·min and then at 90 min at 8 mU/m2·min until 180 min. Results from the lipolysis portion of the study have been previously published (21). Substrate use was measured at baseline and during the last 30 min of steady states of the two-step pancreatic euglycemic clamp via indirect calorimetry (Horizon Metabolic Measurement Cart; Sensor Medics).
Study 3
Eight Caucasian and nine African-American obese (BMI, 28.3–40.3 kg/m2), premenopausal (ages 26–48 yr) women participated in epinephrine-induced in vivo lipolysis studies. Palmitate turnover rates were measured using stable isotopically labeled tracers as described for study 2. At −90 min, a continuous infusion of [1-13C]palmitate (0.07 μmol/kg FFM·min in an albumin mixture) was started. At min 0, epinephrine, a mixed α- and β-adrenergic agonist, was infused at a rate of 1.0 μg/min for a total of 90 min to measure its effect on stimulating a lipolytic response and subsequent fat use. The epinephrine was diluted in 0.9% normal saline containing 1 mg/ml ascorbic acid to prevent epinephrine degradation. Substrate use was measured during baseline and the last 30 min of epinephrine infusion via indirect calorimetry (Vmax29; Sensor Medics).
Body composition
Fat mass, FFM, and percent body fat were measured in studies 1 and 2 by dual-energy x-ray absorptiometry (23) and in study 3 by densitometry (24, 25). Adipose tissue areas, sc (SAT) and visceral (VAT) (L2–L3 level), were measured by magnetic resonance imaging in all studies as described (21).
Insulin sensitivity measurements
Baseline insulin sensitivity was measured during the follicular phase of the menstrual cycle in studies 2 and 3 via an iv glucose tolerance test using the Bergman minimal model (26).
Assays
For all studies, blood samples were immediately centrifuged, aliquoted, and frozen at −70 C. Insulin was measured by RIA (Linco Research, St. Charles, MO), glucose was measured by a Beckman glucose analyzer (Beckman, Fullerton, CA), and epinephrine was measured via HPLC (27). The interassay coefficients of variation for insulin, glucose, and epinephrine were 4.5, 3, and 6.2%, respectively.
Indirect calorimetry calculations
Substrate use was calculated for all studies based upon O2 consumption and CO2 production (28) as follows: fat oxidation (FO) = (1.67 × vO2) − (1.67 × vCO2), carbohydrate oxidation (CO) = (4.55 × vCO2) − (3.21 × vO2), and nonprotein respiratory quotient (NPRQ) = (vCO2/vO2).
Determination of [1-13C]palmitate enrichment in the plasma
In studies 2 and 3, plasma palmitate enrichment was measured using gas chromatography/isotope-ratio mass spectrometry as described (21).
Calculations
Steady-state appearance and disappearance rates of palmitate (Ra palmitate) were calculated using the mean enrichment values and tracer infusion rates as described (21). For study 2, FFA turnover rates (Ra FFA) were calculated as Ra of palmitate multiplied by the percentage of palmitate from total plasma FFA (21).
Statistics
Log-transformed values were used for variables that were not normally distributed. Paired t tests and ANOVA with repeated measures were used to determine significant differences between African-American and Caucasian subjects. For the different indirect calorimetry measurements (study 1), a categorical factor was used in all ANOVA. No significant interactions were observed, and results were pooled. Post hoc analysis was performed using Fisher’s least significant difference, with the significance level defined as α = 0.05. Analysis of covariance using a general linear model was used for all studies to adjust for difference of pertinent variables between groups. Statistica version 6.0 (Statsoft Inc., Tulsa, OK) was used for all analyses.
Results
Dietary intervention studies: study 1
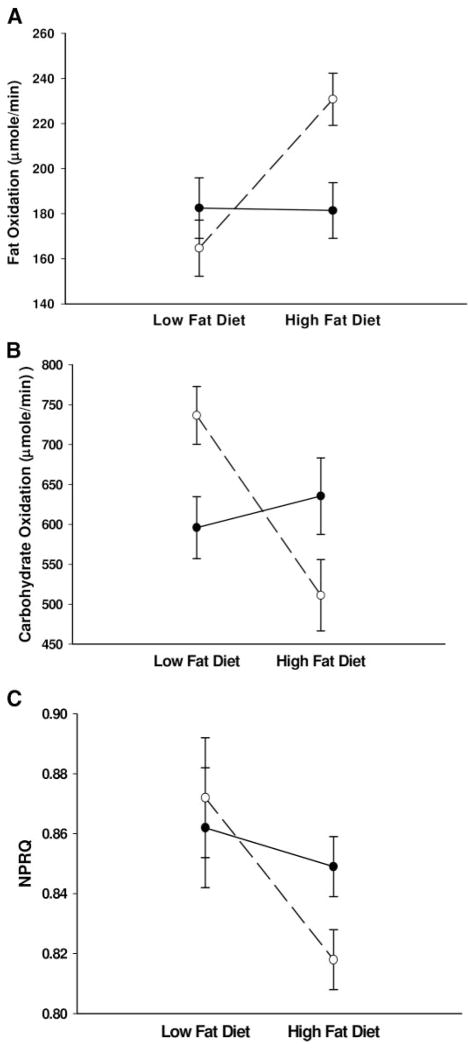
The Caucasian and African-American women’s baseline characteristics were not significantly different (Table 1). There was a significant diet by race interaction for FO (P < 0.01, Fig. 1A) and CO (P < 0.01, Fig. 1B) but not NPRQ (P = 0.10, Fig. 1C).
TABLE 1.
Subject characteristics for studies 1 (LF and HF diets), 2 (pancreatic-euglycemic clamp), and 3 (epinephrine-stimulated lipolysis)
| Study 1a |
Study 2b |
Study 3c |
||||
|---|---|---|---|---|---|---|
| African-American (n = 21) | Caucasian (n = 21) | African-American (n = 9) | Caucasian (n = 9) | African-American (n = 9) | Caucasian (n = 8) | |
| Age (yr) | 32.8 (7.4) | 34.9 (6.9) | 38 (6) | 35 (7) | 38 (7) | 36 (8) |
| BMI (kg/m2) | 28.4 (6.3) | 28.0 (8.1) | 35.6 (3.5) | 32.7 (4.4) | 32.7 (4.7) | 32.7 (5.2) |
| % fat | 35.3 (10.6) | 36.5 (11.9) | 45.8 (3.0) | 45.5 (2.8) | 40.7 (6.6) | 42.9 (4.6) |
| Fat mass (kg) | 29.3 (14.2) | 29.1 (15.6) | 46.1 (7.5) | 40.7 (6.4) | 36.4 (10.2) | 37.1 (7.7) |
| FFM (kg) | 49.1 (7.2) | 45.9 (6.6) | 53.5 (7.0) | 49.7 (9.2) | 51.7 (4.6) | 48.8 (2.7) |
| WHR | 0.84 (0.09) | 0.83 (0.12) | 0.86 (0.06) | 0.79 (0.07) | 0.83 (0.07) | 0.86 (0.08) |
| VAT (cm2) | 60 (44) | 68 (36) | 107 (39) | 122 (56) | 74 (53) | 109 (62) |
| SAT (cm2) | 216 (111) | 205 (99) | 421 (90) | 364 (55) | 320 (150) | 339 (142) |
| SI | 3.0 (2.5) | 2.9 (2.7) | 3.2 (2.6) | 3.7 (1.8) | ||
Results are shown as mean (SD). SI, Insulin sensitivity measured by iv glucose tolerance test in studies 2 and 3 only.
African-American vs. Caucasian; all P values > 0.60.
All P values > 0.25 (except WHR, P = 0.09).
All P values > 0.28.
Fig. 1.
A, FO rates; B, CO rates; C, NPRQ in Caucasian (○) and African-American (●) women during HF and LF diets. A, The diet by race interaction was P < 0.01 and the change within Caucasian women was P < 0.01 and within African-American women was P = 0.86 (LF vs. HF); B, the diet by race interaction was P < 0.01 and the change within Caucasian women was P < 0.01 and within African-American women was P = 0.69; C, the diet by race interaction was P = 0.10 and the change within Caucasian women was P < 0.01 and within African-American women was P = 0.42.
Post hoc analyses showed only Caucasian women increased FO (164.7 ± 12.5 vs. 230.8 ± 11.6 μmol/min, P < 0.01; for African-American, 182.5 ± 13.4 vs. 181.4 ± 12.3 μmol/min, P = 0.86), decreased CO (736.5 ± 36.1 vs. 511.2 ± 44.7 μmol/min, P < 0.01; for African-American, 596.0 ± 38.7 vs. 635.5 ± 47.9 μmol/min, P = 0.69) and decreased NPRQ (0.872 ± 0.02 vs. 0.818 ± 0.01, P < 0.01; for African-American, 0.862 ± 0.02 vs. 0.849 ± 0.01, P = 0.42) when switching from LF to HF diet. Additionally, Caucasian women had significantly higher FO (P = 0.05) during HF diet and higher CO (P = 0.01) during LF diet.
The significant differences in substrate use described for Caucasian compared with African-American women retained a P < 0.05 after correcting for age, waist-to-hip ratio (WHR), BMI, percent body fat, FFM, fat mass, and VAT or SAT area.
When switching from a LF to HF diet, the Caucasian women trended toward increased fasting insulin (68.0 ± 10.2 vs. 76.2 ± 9.7 pM, P = 0.08) whereas African-American women significantly decreased fasting insulin (99.8 ± 10.1 vs. 89.6 ± 9.7 pM, P = 0.05). The diet by race interaction for fasting plasma insulin was significant (P = 0.02). There were no significant differences for glucose between diets or races (P > 0.8 for all analyses).
Pancreatic euglycemic clamp studies: study 2
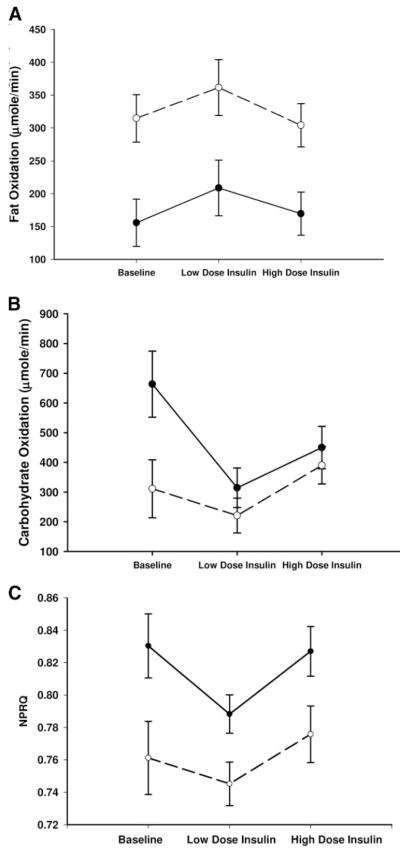
The Caucasian and African-American women’s baseline physical characteristics were not significantly different (Table 1). The overall time point (baseline, low-dose insulin, high-dose insulin) by race interaction was significant for CO (P = 0.05) but not for FO (P = 0.40) or NPRQ (P = 0.25) (Fig. 2, A–C).
Fig. 2.
A, FO rates; B, CO rates; C, NPRQ in Caucasian (○) and African-American (●) women during pancreatic euglycemic clamp. A, The overall time point by race interaction was P = 0.40, and the overall race effect was P = 0.02; B, the overall time point by race interaction was P = 0.05; C, the overall time point by race interaction was P = 0.25, and the overall race effect was P = 0.01.
Caucasian women had higher FO (baseline 314.6 ± 36.0, low dose 361.4 ± 42.4, and high dose 304.0 ± 32.9 μmol/min vs. African-American baseline 155.8 ± 36.1, low dose 208.7 ± 42.3, and high dose 169.6 ± 32.9 μmol/min, P = 0.02) and lower NPRQ (baseline 0.743 ± 0.02, low dose 0.728 ± 0.03, and high dose 0.764 ± 0.02 vs. African-American baseline 0.828 ±0.02, low dose 0.780 ±0.01, and high dose 0.815 ±0.02, P = 0.01) during the clamp. CO was significantly lower in the Caucasian women at baseline (311.1 ± 97.9 vs. African-American 663.5 ± 111.0 μmol/min, P = 0.04) but not different at low-dose insulin (220.9 ± 58.5 vs. African-American 314.5 ± 66.4 μmol/min, P = 0.43) or high-dose insulin (390.0 ± 62.9 μmol/min vs. African-Americans 449.8 ± 71.3 μmol/min, P = 0.38).
During low-dose insulin infusion, the Caucasian women did not change their FO (P = 0.07), CO (P = 0.29), or NPRQ (P = 0.37), whereas the African-American women had significantly increased FO (P = 0.02) and decreased CO (P < 0.01) and NPRQ (P < 0.01). During the high-dose insulin infusion, Caucasian women significantly suppressed FO (P < 0.01) and increased CO (P = 0.04) and NPRQ (P = 0.05), whereas the African-American women did not suppress FO (P = 0.11), although they increased CO (P = 0.05) and NPRQ (P = 0.05).
The significant differences in substrate use described for Caucasian compared with African-American women retained a P < 0.05 after correcting for age, WHR, BMI, percent body fat, FFM, fat mass, and VAT or SAT area.
There was no significant race effect or time point by race interaction for FFA turnover rate (μmol/min) (P = 0.73 and P = 0.64, respectively; Table 2). Both African-Americans and Caucasians significantly increased FFA turnover rate (~20%) from baseline (P < 0.01 for both races) to low-dose insulin and significantly decreased FFA turnover rate (~37%) when switched to high-dose insulin (P < 0.01 for both races).
TABLE 2.
Substrate and hormone levels during study 2 (pancreatic euglycemic clamp)
| African-American (n = 9) | Caucasian (n = 9) | P valuea | |
|---|---|---|---|
| Ra FFA (μmol/min)b | |||
| Baseline | 549.8 (38.0) | 558.3 (37.9) | 0.92 |
| Low dose | 671.3 (50.0) | 670.3 (51.1) | 0.93 |
| High dose | 389.9 (46.0) | 443.7 (45.8) | 0.77 |
| Insulin (pM)b | |||
| Baseline | 81.4 (16.2) | 99.0 (16.2) | 0.37 |
| Low dose | 44.0 (5.1) | 37.6 (5.2) | 0.77 |
| High dose | 136.7 (11.2) | 112.5 (11.1) | 0.24 |
| Glucose (mM)b | |||
| Baseline | 4.9 (0.1) | 5.2 (0.1) | 0.31 |
| Low dose | 5.0 (0.1) | 5.2 (0.1) | 0.49 |
| High dose | 5.2 (0.2) | 5.5 (0.2) | 0.36 |
Results are shown as mean (SEM).
African-American vs. Caucasian.
Overall interaction for time point by race for Ra FFA (P = 0.60), insulin (P = 0.02), and glucose (P = 0.77).
There was an overall time point by race interaction for plasma insulin (P = 0.03) but not for plasma glucose (P = 0.70) (Table 2). African-American women had significantly higher plasma insulin levels during the high-dose insulin compared with baseline levels (P < 0.01), whereas the levels in Caucasian women did not differ (P = 0.29).
Epinephrine-induced lipolysis studies: study 3
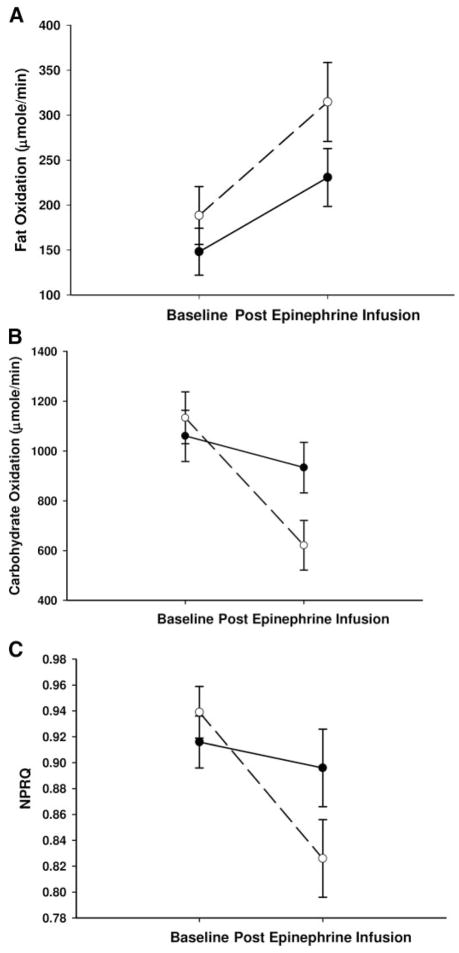
The Caucasian and African-American women’s baseline physical characteristics were not significantly different (Table 1). The time point (baseline vs. final 30 min of epinephrine infusion) by race interaction was not significant for FO (P = 0.30, Fig. 3A) but was significant for CO (P < 0.01, Fig. 3B) and approached significance for NPRQ (P = 0.06, Fig. 3C).
Fig. 3.
A, FO rates; B, CO rates; C, NPRQ in Caucasian (○) and African-American (●) women during an epinephrine-induced lipolysis study. A, The time point by race interaction was P = 0.30, and the change within Caucasian women was P < 0.01 and within African-American women was P = 0.07; B, the time point by race interaction was P = 0.01, and the change within Caucasian women was P < 0.01 and within African-American was P = 0.20; C, the time point by race interaction was P = 0.06 and the change within Caucasian was P < 0.01 and within African-American women was P = 0.53.
Post hoc analysis found that only Caucasian women significantly increased FO (baseline 188.3 ± 35.5 vs. final 30 min 314.7 ± 44.0 μmol/min, P < 0.01; for African-American, baseline 148.0 ± 26.1 vs. final 30 min 230.7 ± 32.2 μmol/min, P = 0.07), decreased CO (baseline 1133.2 ± 104.3 vs. final 30 min 621.2 ± 99.6 μmol/min, P < 0.01; for African-American, baseline 1060.4 ± 102.7 vs. final 30 min 933.3 ± 101.1 μmol/min, P = 0.20) and decreased NPRQ (baseline 0.939 ± 0.02 vs. final 30 min 0.826 ± 0.03, P < 0.01; for African-American, baseline 0.916 ± 0.02 vs. final 30 min 0.896 ± 0.03, P = 0.53).
Caucasians’ CO and NPRQ trended toward being significantly lower after epinephrine infusion compared with African-American women (P = 0.09 and P = 0.06, respectively).
The significant differences in substrate use described for Caucasian compared with African-American women retained a P < 0.05 after correcting for age, WHR, BMI, percent body fat, FFM, fat mass, and VAT or SAT area.
The rate of palmitate appearance (Ra palmitate) was equal between races at baseline. Both groups increased their Ra palmitate in response to epinephrine (P = 0.01), and there was no difference in this increase between groups (P = 0.40) (Table 3).
TABLE 3.
Substrate and hormone profile during study 3 (epinephrine-stimulated lipolysis)
| African-Americana (n = 9) | Caucasiana (n = 8) | P valueb | |
|---|---|---|---|
| Ra palmitate (μmol/min)c | |||
| Baseline | 135.2 (20.7) | 125.9 (17.4) | 0.42 |
| Final 30 min | 191.3 (18.3) | 169.3 (17.9) | 0.46 |
| Epinephrine (pM)c | |||
| Baseline | 142.2 (55.8) | 195.9 (59.1) | 0.81 |
| Final 30 min | 1677.7 (170.4) | 1451.7 (180.7) | 0.32 |
| Insulin (pM)c | |||
| Baseline | 115.0 (19.8) | 79.6 (21.0) | 0.36 |
| Final 30 min | 108.7 (17.2) | 88.9 (18.2) | 0.60 |
| Glucose (mM)c | |||
| Baseline | 5.3 (0.14) | 4.9 (0.15) | 0.19 |
| Final 30 min | 5.9 (0.14) | 5.8 (0.15) | 0.71 |
Results are shown as mean (SEM).
Within differences for Ra palmitate (P < 0.01), epinephrine (P < 0.01), insulin (P > 0.3), and glucose (P < 0.01) for Caucasians and African-Americans.
African-American vs. Caucasian.
Overall interaction for time point by race for Ra palmitate (P = 0.4), epinephrine (P = 0.2), insulin (P = 0.2), and glucose (P = 0.3).
There were no racial differences or interactions for plasma epinephrine, glucose, and insulin levels. Both African-Americans and Caucasians significantly increased their plasma epinephrine (P < 0.01) and glucose levels (P < 0.01) but did not significantly change their plasma insulin in response to epinephrine infusion (P > 0.1; Table 3).
Discussion
We performed multiple studies to examine whether MI exists in nondiabetic African-American females. We studied this topic because African-Americans have been reported to be more obese and insulin resistant than Caucasians (12–14). In each of our studies, our African-American subjects were similar in age and BMI compared with Caucasian subjects. These studies examined lean (study 1) and obese (studies 1, 2, and 3) premenopausal women who were nondiabetic and healthy.
We found increased postabsorptive FO and decreased CO and NPRQ in Caucasian women when switched from a LF to HF eucaloric diet; this was not observed in African-American women. Macronutrient manipulation of isocaloric diet studies have been shown to affect nutrient use through 24-h energy expenditure measurements in lean and obese populations (29). Using our paradigm of eucaloric macronutrient-manipulated diets, we found that African-American women were unable to switch their substrate use, and we believe that this phenomenon is equivalent to what has been described as MI (1–5).
The increased prevalence of insulin resistance in African-Americans (12–14) may be an underlying mechanism to explain the manifestation of MI. We did not directly measure peripheral insulin sensitivity during dietary intervention, limiting the interpretation of our data. However, insulin regulates many cellular pathways (30), and changes in its levels during dietary intervention may provide some insight into our findings.
The African-American women we studied had greater fasting plasma insulin during the LF diet compared with the HF diet, whereas the opposite response occurred in the Caucasian women. A similar finding has previously been reported with changes in insulin sensitivity between African-Americans and Caucasians during a comparable dietary intervention (31). Normally, elevated insulin levels suppress FFA levels and FO. We did not find differences in plasma FFA between races or diets (data not shown); thus the observed hyperinsulinemia in African-American women during LF diet failed to suppress FO. Obese adolescents were shown to increase their insulin secretion to maintain nor-moglycemia and metabolic flexibility under high-carbohydrate, isocaloric diets similar to our study (32). The higher insulin levels during the LF diet in the African-American women could have preserved their metabolic flexibility but failed.
During the HF diet, we expected the African-American women to increase their FO because of increased fat availability. Their response was contrary to our hypothesis and the Randle cycle, which assumes that in insulin-resistant subjects, increased FFA availability results in increased fatty acid use subsequently leading to impaired glucose oxidation (33). Our African-American women did not increase systemic FO or decrease CO during HF compared with LF diet. This finding supports hypotheses by Sidossis and Wolfe (34) and Mandarino et al. (35), which stated that carbohydrates remain the preferential substrate for muscle even during high FFA levels.
Although we did not find differences in plasma FFA levels between races or diets, differences in FFA turnover rates or FFA availability may have been present. Therefore, we turned to additional mechanistic studies to further identify differences in fat use and availability between African-American and Caucasian women.
During euglycemic clamp studies, the African-American women did not suppress systemic FO in response to the high-dose insulin infusion similarly to the Caucasian women despite the African-American women doubling their baseline plasma insulin. In fact, the African-American women increased FO and decreased CO only when their insulin levels were below basal levels (low-dose infusion). When plasma epinephrine levels were in physiological ranges favoring fat use (36), the African-American women did not significantly increase their FO but rather maintained significantly greater CO compared with Caucasian women. Epinephrine could be promoting glycogenolysis and gluconeogenesis (37) in addition to lipolysis.
Despite differences in substrate use in both of our mechanistic studies, FFA availability, measured as FFA turnover rate or Ra palmitate, at baseline and their response to insulin or epinephrine did not differ between African-American and Caucasian women. However, although systemic FFA turnover rates during the two diets may not have differed in African-Americans compared with Caucasians, lipid distribution in the muscle could have differed. African-American women tend to have greater intermuscular adipose tissue compared with Caucasian women (14) and may store fat ectopically as intramyocellular lipids (IMCL) (13). Myocytes rapidly increase IMCL in response to dietary fat (38). In African-American women, the lack of increase in FO during HF diet creates a metabolic profile favoring fat storage. Elevated IMCL is associated with insulin resistance (39); therefore, the relationship between this ectopic fat deposition, increased prevalence of insulin resistance, and MI in African-American women needs to be further explored.
Our design did not include a direct measure of circulating FFA oxidation. Therefore, we can only speculate whether the FFA oxidation we measured by indirect calorimetry represented oxidation of circulating vs. stored fat. Sumner et al. (40) demonstrated lower triglyceride levels in African-American women compared with African-American men and hypothesized that decreased adipose tissue FFA release explained this observation. However, we have previously reported in the Caucasians and African-Americans from study 2 no significant differences in basal or insulin-suppressed FFA release in relation to their regional adipose tissue distribution (VAT and SAT) (21), nor did we find any differences in study 3. Additional analysis of FFA kinetics in studies 2 and 3 showed that FFA clearance (calculated as the Ra FFA or Ra palmitate divided by the plasma FFA or palmitate concentration, respectively) was slightly lower but not significantly different in Caucasian vs. African-American women (0.72 ± 0.10 vs. 0.84 ± 0.09 liter/min, P = 0.37, for study 2 and 0.90 ± 0.12 liter/min vs. 1.16 ± 0.09, P = 0.11, for study 3). We can therefore only speculate that the defect in substrate use in the African-American group may be occurring on an intracellular level.
Other factors may have affected substrate use during our dietary intervention study. We examined whether leptin and adiponectin, adipokines that affect insulin sensitivity and substrate use, were altered. We measured levels of these adipokines in a subset of women but found no differences between races or diets (data not shown).
Our study has some limitations. Each of the three studies examined different sets of Caucasian and African-American women, which may limit the overall interpretation of our data. However, within each study, our subjects had similar characteristics, and we repeatedly observed impaired substrate use after correcting for measurable differences in body composition, further substantiating our finding of MI in African-American women. In addition, the Caucasian and African-American women in studies 2 and 3 had similar baseline insulin sensitivity, suggesting that MI may exist before the development of insulin resistance. Moreover, we did not measure intermuscular adipose tissue or IMCL, and how these adipose tissue depots contribute toward MI remains unknown. Finally, in study 1, we did not control for follicular phase of the menstrual cycle in all women. However, we did control for follicular phase in a subset of women (six African-Americans and seven Caucasians), and similar substrate use results were observed.
In summary, we found that healthy, premenopausal, non-diabetic Caucasian women are more systemically, metabolically flexible than their African-American counterparts. African-American women failed to switch fat and carbohydrate use based upon dietary macronutrient composition. Disordered substrate use may explain these findings; African-Americans favored fat storage, whereas Caucasians favored fat use. These studies may help explain why African-American women are at increased risk for the development of obesity and type 2 diabetes compared with Caucasian women.
Acknowledgments
We thank all of the women who participated in these studies.
This work was supported by National Institutes of Health Grants R01 DK40414, M01RR00645, and P30DK26687 and the American Diabetes Association Grant 1-05-RA-03.
Abbreviations
- BMI
Body mass index
- CO
carbohydrate oxidation
- FFA
free fatty acids
- FFM
fat-free mass
- FO
fat oxidation
- HF
high-fat
- IMCL
intramyocellular lipids
- LF
low-fat
- MI
metabolic inflexibility
- NPRQ
nonprotein respiratory quotient
- Ra
appearance rate
- RMR
resting metabolic rate
- RQ
respiratory quotient
- SAT
sc adipose tissue
- VAT
visceral adipose tissue
- WHR
waist-to-hip ratio
References
- 1.Kelley DE, Goodpaster BH, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity and weight loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 2.Kelley DE, Goodpaster BH, Storlien LH. Muscle triglycerides and insulin resistance. Annu Rev Nutr. 2002;22:325–346. doi: 10.1146/annurev.nutr.22.010402.102912. [DOI] [PubMed] [Google Scholar]
- 3.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 4.Storlien L, Oakes ND, Kelley DE. Metabolic inflexibility. Proc Nutr Soc. 2004;63:363–368. doi: 10.1079/PNS2004349. [DOI] [PubMed] [Google Scholar]
- 5.Kelley DE. Skeletal muscle triglycerides: an aspect of regional adiposity and insulin resistance. Ann NY Acad Sci. 2002;967:135–145. [PubMed] [Google Scholar]
- 6.Treuth M, Sunehag A, Trautwein L, Bier D, Haymond M, Butte N. Metabolic adaptation to high-fat and high carbohydrate diets in children and adolescents. Am J Clin Nutr. 2003;77:479–489. doi: 10.1093/ajcn/77.2.479. [DOI] [PubMed] [Google Scholar]
- 7.Roy H, Lovejoy J, Keenan Bray G, Windhauser M, Wilson J. Substrate oxidation and energy expenditure in athletes and non-athletes consuming isoenergetic high- and low-fat diets. Am J Clin Nutr. 1998;67:405–411. doi: 10.1093/ajcn/67.3.405. [DOI] [PubMed] [Google Scholar]
- 8.Smith S, de Jonge L, Zachweiga JJ, Roy H, Nguyen T, Rood J, Windhauser M, Bray G. Fat and carbohydrate balances during adaptation to a high-fat diet. Am J Clin Nutr. 2000;71:450–457. doi: 10.1093/ajcn/71.2.450. [DOI] [PubMed] [Google Scholar]
- 9.Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray G, Smith S. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest. 2005;115:1934–1941. doi: 10.1172/JCI24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics 1981 Plan and operation of the Health and Nutrition Examination Survey, United States, 1976–1980: vital and health statistics. DHHS Pub. No. (PHS)81-1317, Series 1, No. 15. Washington, DC: Public Health Service, U.S. Government Printing Office
- 11.Kuczmarski R, Flegal K, Campbell S, Johnson C. Increasing prevalence of overweight among US adults: the National Health and Nutrition Examination Surveys: 1969 to 1991. JAMA. 1995;272:205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Haffner S, D’Agostino R, Saad M, Rewers M, Mykkannen L, Selby J, Howard G, Savage P, Hamman R, Wagenknecht LE, Bergman R. Increased insulin resistance and insulin secretion in non-diabetic African Americans and Hispanics compared to non-Hispanic whites. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 13.Ryan A, Nicklas B, Berman D. Racial differences in insulin resistance and mid thigh fat deposition in post menopausal women. Obes Res. 2002;10:336–344. doi: 10.1038/oby.2002.47. [DOI] [PubMed] [Google Scholar]
- 14.Albu J, Kovera A, Allen L, Wainwright M, Berk E, Raja-Khan N, Janumala I, Burkey B, Heshka S, Gallagher D. Independent association of insulin resistance with increased inter-muscular adipose tissue and larger acute insulin response to glucose in African American vs. Caucasian non-diabetic women. Am J Clin Nutr. 2005;82:1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weyer C, Snitker S, Bogardus C, Ravussin E. Energy metabolism in African Americans: potential risk factors for obesity. Am J Clin Nutr. 1999;70:13–20. doi: 10.1093/ajcn/70.1.13. [DOI] [PubMed] [Google Scholar]
- 16.Nicklas BJ, Berman DM, Davis DC, Dobrovolny CL, Dennis KE. Racial differences in metabolic predictors of obesity among postmenopausal women. Obes Res. 1999;7:463–468. doi: 10.1002/j.1550-8528.1999.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 17.Hickner R, Privette J, McIver K, Barakat H. Fatty acid oxidation in African-American and Caucasian women during physical activity. J Appl Physiol. 2001;90:2319–2324. doi: 10.1152/jappl.2001.90.6.2319. [DOI] [PubMed] [Google Scholar]
- 18.Chitwood L, Brown S, Lundy M, Dupper M. Metabolic propensity towards obesity in black vs. white females: response during rest, exercise and recovery. Int J Obes. 1996;20:455–462. [PubMed] [Google Scholar]
- 19.Melby C, Ho Jeckel K, Goran M, Donahoo W. Comparison of risk factors for obesity in young, nonobese African American and Caucasian women. Int J Obes. 2000;24:1514–1522. doi: 10.1038/sj.ijo.0801413. [DOI] [PubMed] [Google Scholar]
- 20.Albu J, Shur M, Curi M, Murphy L, Heymsfield, Pi-Sunyer FX. Resting metabolic rate in obese, pre-menopausal black women. Am J Clin Nutr. 1997;66:531–538. doi: 10.1093/ajcn/66.3.531. [DOI] [PubMed] [Google Scholar]
- 21.Albu J, Curi M, Shur M, Murphy L, Matthews D, Pi-Sunyer FX. Systemic resistance to the antilipolytic effect of insulin in black and white women with visceral obesity. Am J Physiol. 1999;277:E551–E560. doi: 10.1152/ajpendo.1999.277.3.E551. [DOI] [PubMed] [Google Scholar]
- 22.Zhang K, Sun M, Werner P, Kovera A, Albu J, Pi-Sunyer FX, Boozer C. Sleeping metabolic rate in relation to body mass index and body composition. Int J Obes. 2002;26:376–383. doi: 10.1038/sj.ijo.0801922. [DOI] [PubMed] [Google Scholar]
- 23.Siri W. Body composition from fluids and density: analysis of methods. In: Brozek J, Henschel A, editors. Techniques for measuring body composition. Washington, DC: National Academy of Science; 1961. [Google Scholar]
- 24.Sopher A, Thorton J, Wang J, Pierson R, Heymsfield S, Horlick M. Measurements of percentage of body fat in 411 children and adolescents: a comparison of dual energy X-ray absorptiometry with a four compartment model. Pediatrics. 2004;113:1285–1290. doi: 10.1542/peds.113.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunez C, Kovera AJ, Pietrobelli A, Heshka S, Horlick M, Kehayais JJ, Wang Z, Heymsfield S. Body composition in children and adults by air displacement plethysmography. Eur J Clin Nutr. 1999;53:382–387. doi: 10.1038/sj.ejcn.1600735. [DOI] [PubMed] [Google Scholar]
- 26.Bergman R, Beard J, Chen M. The minimal modeling method: assessment of insulin sensitivity and β-cell function in vivo. In: Larner J, Pohl S, editors. Methods in clinical diabetes research. New York: Wiley International; 1986. pp. 13–20. [Google Scholar]
- 27.Neilsen S, Guo Z, Albu J, Klien S, O’Brien P, Jensen M. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest. 2003;11:981–988. doi: 10.1172/JCI16253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White M, Bouchard G, Buemann B, Almeras N, Després J, Bouchard C, Tremblay A. Reproducibility of 24-hr energy expenditure and macronutrient oxidation rates in an indirect calorimeter. J Appl Physiol. 1996;80:133–139. doi: 10.1152/jappl.1996.80.1.133. [DOI] [PubMed] [Google Scholar]
- 29.Shah M, Garg A. High-fat and high-carbohydrate diets and energy balance. Diabetes Care. 1996;19:1142–1152. doi: 10.2337/diacare.19.10.1142. [DOI] [PubMed] [Google Scholar]
- 30.Coppack S, Frayn K, Humphreys S, Dhar H, Hockaday T. Effects of insulin on human adipose tissue metabolism in vivo. Clin Sci. 1989;77:663–670. doi: 10.1042/cs0770663. [DOI] [PubMed] [Google Scholar]
- 31.Lovejoy J, Windhauser M, Rood J, de la Bretonne J. Effect of a controlled high-fat versus low-fat diet on insulin sensitivity and leptin levels in African-American and Caucasian women. Metabolism. 1998;47:1520–1524. doi: 10.1016/s0026-0495(98)90080-4. [DOI] [PubMed] [Google Scholar]
- 32.Sunehag A, Toffolo G, Campioni M, Bier D, Haymond M. Effects of dietary macronutrient intake on insulin sensitivity and secretion and glucose and lipid metabolism in healthy, obese adolescents. J Clin Endocrinol Metab. 2005;90:4496–4502. doi: 10.1210/jc.2005-0626. [DOI] [PubMed] [Google Scholar]
- 33.Randle P, Garland P, Hales C, Newsholme E. The glucose fatty-acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 34.Sidossis L, Wolfe R. Glucose and insulin induced inhibition of fatty acid oxidation: the glucose-fatty acid cycle reversed. Am J Physiol. 1996;270:E733–E738. doi: 10.1152/ajpendo.1996.270.4.E733. [DOI] [PubMed] [Google Scholar]
- 35.Mandarino L, Consoli A, Jain A, Kelley D. Interaction of carbohydrate and fat fuels in human skeletal muscle: impact of obesity and NIDDM. Am J Physiol. 1996;270:E463–E470. doi: 10.1152/ajpendo.1996.270.3.E463. [DOI] [PubMed] [Google Scholar]
- 36.Samra J, Simpson E, Clark M, Forster C, Humphreys S, Macdonald I, Frayn K. Effects of epinephrine infusion on adipose tissue: interactions between blood flow and lipid metabolism. Am J Physiol. 1996;271:E834–E839. doi: 10.1152/ajpendo.1996.271.5.E834. [DOI] [PubMed] [Google Scholar]
- 37.Sherwin R, Sacca L. Effect of epinephrine on glucose metabolism in humans: contribution of the liver. Am J Physiol. 1984;247:E157–E165. doi: 10.1152/ajpendo.1984.247.2.E157. [DOI] [PubMed] [Google Scholar]
- 38.Bachmann O, Dahl D, Bretchel K, Machann J, Haap M, Maier T, Loviscach M, Stumvoll M, Claussen C, Schick F, Haring H, Jacob S. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 2001;50:2579–2584. doi: 10.2337/diabetes.50.11.2579. [DOI] [PubMed] [Google Scholar]
- 39.Krssak M, Petersen K, Dresner A, Dipietro L, Vogel S, Rothman D, Shulman G, Roden M. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 40.Sumner A, Kushner H, Tulenko T, Falkner B, Marsh J. The relationship in African Americans of sex differences in insulin-mediated suppression of non-esterified acids to sex differences in fasting triglyceride levels. Metabolism. 1997;46:400–405. doi: 10.1016/s0026-0495(97)90055-x. [DOI] [PubMed] [Google Scholar]