Abstract
Purpose
Angiogenin undergoes nuclear translocation and stimulates ribosomal RNA transcription in both prostate cancer cells and endothelial cells. The purpose of this study is to assess the anti-tumor activity of neamine, a nontoxic degradation product of neomycin that blocks nuclear translocation of angiogenin.
Experimental Design
The anti-prostate cancer activity of neamine was first evaluated in a xenograft animal model. It was then examined in the murine prostate-restricted AKT transgenic mice (MPAKT) that develop prostate intraepithelial neoplasia (PIN) owing to AKT transgene overexpression.
Results
Neamine inhibits xenograft growth of PC-3 human prostate cancer cells in athymic mice. It blocks nuclear translocation of angiogenin, inhibits rRNA transcription, cell proliferation as well as angiogenesis. Neamine also prevents AKT-induced PIN formation as well as reverses fully developed PIN in MPAKT mice, accompanied by a decrease in rRNA synthesis, cell proliferation, and angiogenesis, and an increase in prostate epithelial cell apoptosis.
Conclusion
We confirmed that angiogenin is a molecular target for cancer drug development and that blocking nuclear translocation of angiogenin is an effective means to inhibit its activity. Our results also suggested that neamine is a lead compound for further preclinical evaluation.
Keywords: Angiogenin, prostate cancer, rRNA transcription, neamine, nuclear translocation
Introduction
Increasing evidence points to an important role of angiogenin (ANG), a 14 kDa angiogenic ribonuclease, in the development and progression of prostate cancer (1–5). ANG has been shown to be up-regulated in human prostate cancer (5). The circulating level of ANG in plasma is significantly higher in prostate cancer patients, especially those with hormone refractory diseases, as compared with normal controls (4). Immunohistochemical (IHC) studies indicated that ANG expression in the prostate epithelial cells is increased as prostate cancer progresses from a benign phenotype to invasive adenocarcinoma (5). Mouse ANG is the most significantly up-regulated gene in AKT-induced PIN in MPAKT mice (4).
ANG has been shown to undergo nuclear translocation in proliferating endothelial cells (6) where it stimulates rRNA transcription (7), a rate-limiting step in protein translation and cell proliferation (8). We have therefore proposed that ANG-stimulated rRNA transcription is a general requirement for endothelial cell proliferation and angiogenesis (9). ANG inhibitors abolish the angiogenic activity of ANG as well as that of other angiogenic factors including VEGF and bFGF (9). Moreover, ANG has been found to play a direct role in cancer cell proliferation (10). Nuclear translocation of ANG in endothelial cells is inversely dependent on cell density (11) and is stimulated by growth factors (9). However, ANG is constitutively translocated to the nucleus of cancer cells in a cell density-independent manner (10, 12). It is plausible that constitutive nuclear translocation of ANG is one of the reasons for sustained growth of cancer cells, a hallmark of malignancy (1).
The dual role of ANG in prostate cancer progression suggested that ANG is a molecular target for the development of cancer drugs (1). ANG inhibitors would combine the benefits of both anti-angiogenesis and chemotherapy because both angiogenesis and cancer cell proliferation are targeted. Moreover, since ANG-mediated rRNA transcription is essential for other angiogenic factors to induce angiogenesis (9), ANG antagonists would also be more effective as angiogenesis inhibitors than others that target only one angiogenic factor.
The activity of ANG in both endothelial and cancer cells are related to its capacity to stimulate rRNA transcription; for that to occur ANG needs to be in the nucleus physically (7). ANG has a typical signal peptide and is a secreted protein (13). The mechanism by which it undergoes nuclear translocation is not clear as yet (14), but it obviously is a target for anti-ANG therapy. Targeting nuclear translocation of ANG would be more advantageous than targeting ANG directly because normally ANG circulates in the plasma (15) at a concentration of 250–350 ng/ml (16, 17) and would require a high dose of inhibitors to neutralize them.
Neomycin, an aminoglycoside antibiotic, has been shown to block nuclear translocation of ANG (18) and to inhibit xenograft growth of human prostate cancer cells in athymic mice (1). However, the nephro- and oto-toxicity of neomycin (19) would seem to preclude its prolonged use as an anti-cancer agent. We have now established that neamine (20), a nontoxic degradation product of neomycin, effectively inhibits nuclear translocation of ANG (12). It has also been shown to inhibit angiogenesis induced both by ANG and by bFGF and VEGF (9). Moreover, it inhibits xenograft growth of HT-29 human colon adenocarcinoma and MDA-MB-435 human breast cancer cells in athymic mice (12). Since the toxicity profile of neamine is close to that of streptomycin and kanamycin, which is ~20-fold less toxic than neomycin (21, 22), it may serve as a lead agent for the development of prostate cancer therapeutics. Therefore, we examined its capacity to prevent the establishment and to inhibit the growth of PC-3 human prostate cancer cells in mice, as well as its capacity to prevent and to reverse AKT-induced PIN in MPAKT mice.
Materials and Methods
Cells and animals
PC-3 cells were cultured in DMEM + 10% FBS. Outbred male athymic mice (nu/nu) were from Charles River Laboratories. A breeding pair of MPAKT mice was provided by Dr. W. R. Sellers of Dana Farber Cancer Institute. All animal experiments were approved by IACUC of Harvard Medical School.
Xenograft growth of PC-3 cell tumors
Five-week-old male athymic mice were inoculated s. c. with 100 μl of a mixture containing 5 × 105 PC-3 cells and 33 μl of Matrigel. The mice were treated s. c. with PBS or neamine (30 mg/kg) twice weekly for 8 weeks. Tumor sizes were measured every 3 days and recorded in mm3 (length × width2, the longer side of the tumor was designated as the length). Mice were sacrificed at day 56 and the tumors were removed and the wet weights of the PC-3 tumors were recorded.
Treatment of MPAKT mice with neamine
For PIN prevention experiments, 4-week-old MPAKT mice were treated with daily i.p. injection of PBS or neamine at a dose of 10 mg/kg body weight for 4 weeks. To examine the effect of neamine on established PIN, 12-week-old MPAKT mice with fully developed PIN were treated with daily i.p. injection of PBS or neamine at a dose of 10 mg/kg body weight for 4 weeks. The animals were sacrificed and the entire genitourinary tract was removed and fixed with 4% paraformaldehyde and embedded in paraffin.
Immunohistochemistry (IHC)
Tissue sections of 4 μm were hydrated, incubated for 30 min with 3% H2O2 in methanol at RT, washed with H2O and PBS, and microwaved in 10 mM citrate buffer, pH 6.0, for 10 min. Sections were blocked in 5% non-fat dry milk in PBS for 30 min and incubated with antibodies against human ANG (30 μg/ml, 26-2F), mouse ANG (10 μg/ml, R163), PCNA (1:200, Dako), vWF (1:200, Dako), and p-Akt-S473 (1:100; Cell signaling) in 1% BSA in PBS at 4°C for 16 h. For detection of Ki67, the sections were blocked in the M. O. M.™ mouse Ig blocking reagent for 60 min and incubated with anti-Ki-67 antibody (1:100; Vector Laboratories) in the M. O. M.™ diluent at 25°C for 1 h. The slides were washed with PBS, and incubated with HRP-labeled second antibody and visualized with the DakoCytomation EnVision System.
In situ hybridization (ISH) for 47S rRNA
Riboprobes for human and mouse 47S rRNA were prepared and labeled with digoxigenin as described by Qian et al. (23). The templates for the sense riboprobes was prepared by PCR from mouse genomic DNA with sense primer containing a T7 promoter (5′-GGGTAATACGACTCACTATAGGGCGA). The primers for the initiation site of the 47S rRNA precursor were as follows. Human: forward, 5′-GCTGACACGCTGTCCTCTGG-3′; reverse, 5′-GAGAACGCCTGACACGCACG-3′. Mouse: forward, 5′-GCCTGTCACTTTCCTCCCTG; reverse, 5′-GCCGAAATAAGGTGGCCCTC; PCR conditions were: 5 min at 94 °C; 35 cycles (94 °C for 1 min, 60 °C for 1 min, and 72 °C for 1 min) and at 72 °C for 7 min. Digoxigenin-labeled probes were generated by in vitro transcription from the above PCR templates using Digoxigenin RNA labeling Kit (Roche Diagnostics). The control probe was the digoxigenin-labeled “antisense” Neo transcripts of 760 bases in length, which was transcribed by T7 RNA polymerase according to the standard protocol using pSPT18-Neo as the template. Tissue sections were deparaffined with xylene and rehydrated with ethanol. After proteinase K treatment (1.5 μg/ml for 10 min at RT) and acetylation reaction (0.25% acetic anhydride in 0.1 mM Triethanolamine at RT for 20 min), the sections were washed with 4 × SSC, prehybridized at 45 °C for 1 h in 5 × SSC containing 50% formamide, 0.5 mg/ml heparin, and 0.1 mg/ml salmon sperm DNA. Hybridization was carried out in the same buffer as prehybridization but containing 800 ng/ml digoxigenin labeled probe at 45 °C for 16 h. After successive washing in 4 × SSC (1 min at RT), 50% formamide in 2 × SSC/(1 h at 45 °C), 0.1 × SSC (2 h at 45 °C), TTBS (5 min at RT), the hybridization signal was visualized using an alkaline phosphatase-conjugated anti-digoxigenin antibody with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate as the substrate.
Silver staining of nucleolar organizer region (NOR)
Formalin-fixed tissue sections were deparaffinized in xylene, rehydrated in ethanol and H2O, immersed in 10 mM sodium citrate buffer, pH 6.0, and autoclaved in at 120 °C for 20 min. After extensive wash with H2O, the slides were incubated at 37 °C for 13 min in a solution containing 1 vol of 2% gelatin in 1% aqueous formic acid, and 2 vol of 50% silver nitrate.
TUNEL assay
Formalin-fixed tissue sections were deparaffinized in xylene, rehydrated in ethanol and incubated with proteinase K (0.02mg/ml) for 20 min at RT. TUNEL staining was carried out using the Fluorescein-FragEL DNA Fragmentation Detection kit (Calbiochem) per the manufacturer’s instructions. TUNEL-positive luminal epithelial cells were counted in all ducts of the ventral prostate.
Results
Neamine inhibits xenograft growth of PC-3 human prostate cancer cells in athymic mice
The anti-prostate cancer activity of neamine was examined first in a xenograft tumor model in which PC-3 human prostate cancer cells were injected into athymic mice. Fig. 1 shows that subcutaneous (s. c.) treatment with neamine at 30 mg/kg, a nontoxic dose that is 2.4% of the reported LD50 of 1250 mg/kg (19), prevented tumor establishment in 50% of the athymic mice. At day 20, all of the untreated mice (n=12) had tumors, while only 5 of the 12 neamine-treated animals had palpable tumors. Fifty percent of the animals never developed ectopic PC-3 tumors as a consequence of neamine treatment (Fig. 1A). In the animals that did develop tumors, their growth rate was decreased significantly (Fig. 1B). At day 56, when all animals were sacrificed, the average tumor weight in the control and neamine-treated groups was 620 ± 310 and 170 ± 50 mg, respectively (Fig. 1C and D), representing a 72.5% inhibition of tumor growth by neamine.
Fig 1.
Neamine inhibited xenograft growth of PC-3 human prostate cancer cells in athymic mice. Male athymic mice were inoculated with 5 × 105 of PC-3 cells, and treated s. c. with PBS or neamine at a dose of 30 mg/kg body weight twice weekly for 8 weeks. Twelve mice were used per group. A, Mice were examined by palpation for tumor appearance. B, Tumor sizes were measured with a caliper and were expressed as length × width2. At day 56, mice were sacrificed and tumor tissues were removed, photographed (C) and weighed (D).
Neamine blocks nuclear translocation of ANG, suppresses rRNA transcription, and inhibits cell proliferation and angiogenesis
In efforts to understand how neamine inhibits PC-3 cell tumor growth in athymic mice, we next examined the status of nuclear human ANG in the tumor tissues grown in untreated and neamine-treated mice. IHC staining with a human ANG-specific monoclonal antibody (26-2F) shows that ANG is stained predominantly in the nucleus of tumor cells grown in untreated animals (Fig. 2A, left panel), whereas in neamine treated tumors most of the ANG is extracellular (Fig. 2A, right panel). These results indicate that in PC-3 cells neamine blocks nuclear translocation of ANG. Since the function of nuclear ANG is known to be related to rRNA transcription, we used in situ hybridization (ISH) with a probe specific for the initiation site of the 47S rRNA precursor to clarify the effect of neamine on rRNA transcription. Fig. 2B shows that in neamine-treated tumor tissues the 47S rRNA level is decreased significantly when compared with that of control tumor tissues. IHC with antibodies against PCNA (Fig. 2C) and von Willebrand factor (vWF) (Fig. 2D) were used to determine cell proliferation and angiogenesis status, respectively. Neamine treatment decreased PCNA positive cells from 75.4 ± 6 to 25.6 ± 6.4 % (Fig. 2C), representing a 66% decrease in cell proliferation. Vessel density decreased from 82 ± 3.2 to 22.3 ± 9.6 vessels per mm2, representing a 72.8% decrease in tumor angiogenesis (Fig. 2D). Jointly, all data suggest that neamine decreases nuclear accumulation of ANG thereby suppressing rRNA transcription, cell proliferation and angiogenesis, consistent with the previous report that ANG plays a dual role in prostate cancer progression by stimulating both angiogenesis and cancer cell proliferation (1). They also concur with the reports that nuclear function of ANG is related to rRNA transcription (7) and that the neomycin family of aminoglycoside antibiotics blocks nuclear translocation of ANG (18).
Fig. 2.
Effect of neamine treatment on cell proliferation, angiogenesis and 47S rRNA synthesis. PBS- and neamine-treated tumor tissues were fixed in formalin, embedded in paraffin, and sections of 5 μM were cut. A, Localization of ANG was determined by IHC with anti-human ANG monoclonal antibody 26-2F. B, ISH with a probe specific for the initiation site of the 47S rRNA. C, Proliferating cells were stained with an anti-PCNA mAb. PCNA positive and total numbers of cells were counted in 5 randomly selected areas at 200 × magnification. D, Blood vessels were stained with an anti-vWF antibody and counted in five most vascularized areas at 200 × magnification.
Neamine prevents AKT-induced PIN in MPAKT mice
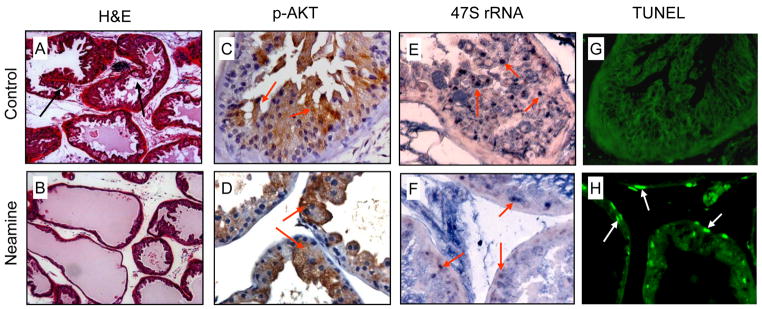
The anti-prostate cancer activity of neamine was examined further in AKT transgenic mice known to develop PIN spontaneously, the precursor of prostate cancer. ANG is the highest up-regulated gene in the PIN lesion of MPAKT mice (4). However, the role of ANG in AKT-induced proliferation of prostate epithelial cells has been uncertain. To understand whether ANG is involved in AKT-induced prostate epithelial cell proliferation and PIN formation, we have treated 4-week-old MPAKT mice with neamine at a daily i.p. dose of 10 mg/kg for 4 weeks. The mice were sacrificed at week 8 and the ventral prostates were examined histologically for PIN formation. H and E staining shows that neamine inhibited PIN formation (Fig. 3A and B). The percentage of PIN in the ventral prostate decreased from 55.1 ± 4.3 to 9.6 ± 1.4 % after neamine treatment, as determined by the use of established criteria for PIN such as intraglandular cell expansion and lumen formation, nuclear atypia, and loss of cell polarity (4). IHC with an anti-mouse ANG antibody shows strong nuclear staining of ANG in the prostate epithelial cells from the ventral prostate of untreated animals (Fig. 3C). In neamine-treated ones, ANG was predominantly cytoplasmic and extracellular (Fig. 3D), indicating blockage of nuclear translocation of ANG in the prostate epithelial cells. To exclude the possibility that neamine might have affected AKT transgene expression or phosphorylation, we performed IHC with an anti-pAKT antibody and showed that AKT phosphorylation in neamine-treated samples (Fig. 3F) did not differ from those of controls (Fig. 3E), demonstrating that nuclear ANG is not involved in the AKT phosphorylation pathway and that neamine does not affect AKT transgene expression and phosphorylation. ISH with a probe specific for the initiation site of the mouse 47S rRNA shows that the level of 47S rRNA in the ventral prostate epithelial cells decreased dramatically after neamine treatment (Fig. 3G and H), thereby confirming the activity of nuclear ANG in rRNA transcription. We did not notice any adverse reactions of the animals after neamine treatment. There were no difference in body weight, grooming behaviors and food and fluid intakes between PBS and neamine treated groups.
Fig. 3.
Neamine prevented AKT-induced PIN formation in MPAKT mice. Four-week-old MPAKT mice were treated with daily i.p. injection of PBS control or neamine at a dose of 10 mg/kg body weight, respectively, for 4 weeks. The mice were sacrificed at week 8 and the ventral prostates were processed for histological examinations. A and B, H&E staining of the ventral prostates. PIN lesions are indicated by arrows. C and D, IHC examinations of nuclear translocation of ANG. Staining of nuclear ANG is indicated by arrows. E and F, IHC examinations of phosphorylation status of AKT. Positive signals are indicated with arrows. G and H, ISH analysis for rRNA transcription. Positive signals are indicated by arrows. I and J, Silver stained NOR of the ventral prostate epithelial cells. Data shown are the average numbers of NOR of 60 cells.
To obtain more quantitative assessment of the changes in rRNA transcription, we examined the effect of ANG siRNA on nucleolar organizer region (NOR) of the prostate epithelial cells. NOR are loops of rDNA that are actively being transcribed (24). NOR are associated with argyrophilic proteins and can be visualized by silver staining. Both the numbers and size of the NOR reflect the degree of ribosome biogenesis (25). Treatment of neamine decreased the average number of NOR per cell from 2.9 ± 0.8 (Fig. 3I) to 1.9 ± 0.9 (Fig. 3J), indicating a significant decrease in ribosome biogenesis (p<10−8).
ANG plays a dual role in prostate cancer progression by stimulating rRNA transcription in both endothelial and cancer cells (1). It undergoes nuclear translocation in both cell types and can be inhibited by neomycin (1, 18). We therefore examined the effect of neamine treatment on both angiogenesis and AKT-induced prostate luminal cell proliferation. IHC with an anti-CD31 antibody shows that neamine treatment decreased interluminal angiogenesis (Fig. 4A). Vessel density in the control and treated ventral prostate was 11.5 ± 4.5 and 4 ± 0.5 per mm2, respectively. Neamine treatment also decreases cell proliferation in the ventral prostate (Fig. 4B). Ki-67 positive cells decreased from 61.1 ± 9.3 % in untreated PIN to 24.9 ± 8.4 in neamine-treated samples. Thus, neamine inhibits both angiogenesis and cell proliferation.
Fig. 4.
Neamine treatment decreased angiogenesis and cell proliferation in the ventral prostate of MPAKT mice. Four-week-old MPAKT mice were treated by daily injection of PBS or neamine at a dose of 10 mg/kg body weight for 4 weeks. A, Formalin-fixed, paraffin-embedded ventral prostate sections were stained with anti-CD31 antibody and interductal neovessels were counted in five microscopic areas at 200 × magnification. The numbers shown are means ± SD of the numbers of neovessels per mm2 from one representative mouse. B, IHC with an anti-Ki67 antibody was used to show proliferative cells. Ki67 positive cells were counted from a total of 500 cells in each sample.
Neamine treatment reverses established PIN in MPAKT mice
We next examined the effect of neamine on established PIN. For this purpose, 12-week-old MPAKT mice were treated by daily i.p. injection of neamine (10 mg/kg) for a period of 4 weeks. The animals were sacrificed at week 16 and the ventral prostates subjected to histological, IHC and ISH examinations. Neamine treatment shrank established PIN and restored normal luminal architectures of the ventral prostate in AKT over-expressing mice (Fig. 5A and B). Again, AKT expression and phosphorylation were not affected (Fig. 5C and D), but rRNA transcription was inhibited by neamine (Fig. 5E and F). For established PIN to reverse its phenotype, cell death at the prostate lumens would have to occur. TUNEL staining shows that neamine treatment does induce apoptosis of the prostate luminal epithelial cells of MPAKT mice in contrast with the untreated samples (Fig. 5G and H). The apoptotic index in the untreated and neamine-treated samples were 0.89 ± 0.12 and 2.15 ± 0.17 per duct, respectively. Jointly, these data demonstrate that neamine blocks nuclear translocation of ANG thereby inhibiting rRNA transcription and inducing cell apoptosis, leading to a phenotypic reversal of established PIN.
Fig. 5.
Neamine treatment reversed established PIN in MPAKT mice. Twelve-week-old MPAKT mice with fully developed PIN were treated with daily i.p. injection of PBS control or neamine at a dose of 10 mg/kg body weight, respectively, for 4 weeks. The mice were sacrificed at week 16 and the ventral prostates were examined. A and B, H&E staining, PIN lesions are indicated with arrows. C and D, IHC detection of p-AKT. Positive signals are indicated by arrows. E and F, ISH for rRNA transcription. Positive signals are indicated by arrows. G and H, Apoptosis of luminal epithelial cells were examined by TUNEL staining. Apoptotic cells are indicated by arrows.
Discussion
ANG is a proven target for prostate cancer therapy owing to its dual role in prostate cancer progression (1). ANG-stimulated rRNA transcription in endothelial cells is a general requirement for angiogenesis (9). Earlier work has shown that ANG is essential for angiogenesis induced by a variety of other angiogenic factors including aFGF, bFGF, EGF, and VEGF (9). Targeting ANG would therefore be more effective than targeting other individual angiogenic factors. Moreover recent work has shown ANG to play a direct role in prostate cancer proliferation (1, 10), making inhibition of ANG an even more attractive target for cancer drug development. It is conceivable that ANG inhibitors would provide the benefits of both anti-angiogenic and traditional chemotherapy.
To develop anti-ANG therapy, both ANG and its receptor could serve as targets. The cell surface receptor of ANG has not been identified as yet and the signal transduction pathways are not yet fully characterized. Past efforts have focused on targeting ANG itself. A variety of approaches have been explored and proofs-of-principle have been established that ANG inhibitors are possible anti-cancer agents. Thus, ANG inhibitors including specific antisense (3) and siRNA (1), monoclonal antibodies (26) or soluble binding protein (27), as well as a small-molecule enzymatic inhibitors (28) have all been shown to inhibit xenograft growth of human cancer. The relatively high concentration of ANG (~250–350 ng/ml) that circulates in plasma (16, 17) is a caveat of these strategies. The majority of the circulating ANG is produced by the liver (29). Moreover, with a seeming fast turnover rate and a half-life of 2 h (30), a large quantity of ANG inhibitors would be needed to neutralize the circulating ANG.
An alternative approach to inhibit the function of ANG would be blockage of its nuclear translocation. The biological function of ANG is related to rRNA transcription (31), which requires ANG to be in the nucleus physically (7). Nuclear translocation of ANG seems to be essential for its biological function (6). Targeting nuclear translocation of ANG would avoid potential problems caused by its high plasma concentration. A distinct advantage of targeting nuclear translocation of ANG would be that it might not have serious side effects since nuclear translocation of ANG occurs only in proliferating endothelial and cancer cells.
In efforts to understand the mechanism by which ANG is translocated to the nucleus of endothelial cells, neomycin was discovered to block nuclear translocation of ANG and to inhibit ANG-induced cell proliferation and angiogenesis (18). Moreover, neomycin has been shown to inhibit xenograft growth of PC-3 cells in athymic mice (1). Neomycin is an aminoglycoside antibiotic isolated originally from Streptomyces fradiae (32). Similar to other aminoglycosides, neomycin has high activity against Gram-negative bacteria, and has partial activity against Gram-positive bacteria. However, neomycin is nephro- and oto-toxic to humans and its clinical use has been restricted to topical preparation and oral administration as a preventive measure for hepatic encephalopathy and hypercholesterolemia by killing bacteria in the small intestinal tract and keeping ammonia levels low (19). The nephrotoxicity of neomycin is associated with selective accumulation in the kidney where the cortical levels may reach as high as 20 times those of circulating levels in serum. The mechanism underlying selective renal accumulation has been shown to be tubular reabsorption, extraction from the circulation at the basolateral surface, as well as brush border uptake (21). The antibiotic activity and the renal toxicity of neomycin seem to be separable from its capacity to inhibit nuclear translocation of ANG. This led our search for less toxic derivatives and analogues of neomycin and led to the finding that neamine (33), a virtually nontoxic derivative of neomycin, has comparable activity in blocking nuclear translocation of ANG (12). Neamine is equally effective in inhibiting angiogenesis induced by ANG as well as by other angiogenic factors (9). Other aminoglycoside antibiotics including streptomycin, gentamicin, kanamycin, amikacin, and paromomycin do not block nuclear translocation of ANG and are not anti-angiogenic (18).
Neamine is a degradation product of neomycin although there is some evidence that it is also produced in small amounts by Streptomyces fradiae (33). Cell and organ culture experiments have shown that the nephro- and oto-toxicity of neamine is ~5 and 6%, respectively, of that of neomycin (21, 22). Thus, the toxicity of neamine is similar to that of streptomycin, an antibiotic that is in clinical use. Neamine is also less neuromuscularly toxic than neomycin. The acute LD50 (subcutaneous) in mice for neamine, neomycin, and streptomycin is 1.25, 0.22, and 0.60 g/kg, respectively (19). The recommended dosage for intramuscular injection of streptomycin in humans is 25–30 mg/kg twice weekly (34). Since neamine appears to be less toxic than streptomycin, the dose we used in this study (30 mg/kg s. c., and 10 mg/kg i.p.) might be tolerated well. Indeed we did not see any acute or chronic adverse side effects in experiments with these mice.
Neamine is effective in inhibiting prostate cancer growth in both the xenograft and spontaneous mouse models. With the xenograft animal model, neamine prevented the establishment of PC-3 cell tumors in 50% of the animals with an overall inhibition of 72.5% in the growth rate (Fig. 1). Histology and IHC evaluation demonstrated that neamine inhibited both angiogenesis and cancer cell proliferation (Fig. 2A and B). These results closely resemble those which we observed with neomycin, confirming the dual role of ANG and suggesting a similar mechanism of inhibition mediated by neamine and neomycin. Indeed, neamine treatment blocked nuclear translocation of ANG and suppressed rRNA transcription in cancer cells (Fig. 2C and D).
Neamine is effective in preventing AKT-induced PIN in MPAKT mice (Fig. 3), providing a strong rationale for its further development as an anti-prostate cancer agent. AKT kinase activity is frequently elevated in prostate cancers (35). Activated AKT promotes both cell growth and survival. Mouse ANG is the most significantly up-regulated gene in the prostate during PIN development in AKT transgenic mice (4). In these mice, expression of AKT in the ventral prostate results in activation of the p70S6K pathway and induction of PIN similar in character to that observed in PTEN+/− mice (36). PTEN has been shown to regulate cell size in association with its ability to regulate ribosome biogenesis (37). Inactivating somatic mutation of PTEN or loss of the PTEN protein are common in prostate cancer cell lines and in primary and metastatic tumor specimens (38). Mutation of PTEN leads to deregulated PI3K signaling, resulting in constitutive activation of downstream targets including the AKT kinase family. Transformation by PI3K or AKT correlates directly with activation of mTOR and its downstream target S6K (39). S6 phosphorylation has been associated with translation of a specific class of mRNA termed TOP (a terminal oligopyrimidine track in the 5′ untranslated region) mRNA (40). This class of mRNAs includes ribosomal proteins, elongation factors 1A1 and 1A2, and several other proteins involved in ribosome biogenesis or in translation control (41). Thus, AKT activation will enhance ribosomal protein production. However, it is unknown how transcription of rRNA, which needs to be incorporated in an equimolar ratio, is elevated proportionally. It could very well be that ANG is upregulated by AKT so that rRNA transcription can be increased to fulfill the enhanced growth requirement resulting from AKT activation. The findings that neamine treatment decreases rRNA transcription in the luminal epithelial cells of the ventral prostate of the mice support such a hypothesis.
The capacity of neamine to reverse established PIN is a clinically more relevant finding (Fig. 5). Neamine treatment of the MPAKT mice that have fully developed PIN inhibited rRNA transcription, induced cell proliferation, resulting in a reversal of PIN phenotype and normalization of the luminal architecture. These results indicate that ANG is important not only for the initial cell expansion during PIN formation but also for cell survival and maintenance of established PIN. Given the nontoxic nature of neamine and its potent activity against ANG-mediated rRNA transcription that is essential for prostate cancer progression, neamine is a promising candidate for further development as a therapeutic agent in prostate cancer.
Acknowledgments
Grant support: NIH grant R01CA105241 and DOD grant W81XWH-06-1-0031 (G.-f. Hu).
We thank Drs. W. R. Sellers for providing a breeding pair of MPAKT mice, and Bert L. Vallee for his critical reading of the manuscript and for his continuous support and advice.
Footnotes
Translational Relevance
Angiogenin plays a dual role in prostate cancer progression by stimulating rRNA transcription in both endothelial and cancer cells. Angiogenin inhibitors would have the benefit combining chemotherapy and anti-angiogenesis therapy. Neomycin, a FDA-approved aminoglycoside antibiotic, has been shown to block nuclear translocation of angiogenin and to inhibit xenograft growth of PC-3 cells in athymic mice. Because of the nephro-toxicity, neomycin itself cannot be directly applied as a prostate cancer therapeutic. We have now established that neamine, a non-toxic derivative of neomycin, effectively blocks nuclear translocation of angiogenin, inhibits xenograft growth of human prostate cancer cells in athymic mice, as well as prevents and reverses prostate intraepithelial neoplasia in AKT transgenic mice. Therefore, the current work identified neamine as a lead compound for further development of prostate cancer therapeutics.
References
- 1.Yoshioka N, Wang L, Kishimoto K, Tsuji T, Hu GF. A therapeutic target for prostate cancer based on angiogenin-stimulated angiogenesis and cancer cell proliferation. Proc Natl Acad Sci USA. 2006;103:14519–24. doi: 10.1073/pnas.0606708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olson KA, Byers HR, Key ME, Fett JW. Inhibition of prostate carcinoma establishment and metastatic growth in mice by an antiangiogenin monoclonal antibody. Int J Cancer. 2002;98:923–9. doi: 10.1002/ijc.10282. [DOI] [PubMed] [Google Scholar]
- 3.Olson KA, Byers HR, Key ME, Fett JW. Prevention of human prostate tumor metastasis in athymic mice by antisense targeting of human angiogenin. Clin Cancer Res. 2001;7:3598–605. [PubMed] [Google Scholar]
- 4.Majumder PK, Yeh JJ, George DJ, et al. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci U S A. 2003;100:7841–6. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katona TM, Neubauer BL, Iversen PW, Zhang S, Baldridge LA, Cheng L. Elevated expression of angiogenin in prostate cancer and its precursors. Clin Cancer Res. 2005;11:8358–63. doi: 10.1158/1078-0432.CCR-05-0962. [DOI] [PubMed] [Google Scholar]
- 6.Moroianu J, Riordan JF. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proc Natl Acad Sci U S A. 1994;91:1677–81. doi: 10.1073/pnas.91.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu ZP, Tsuji T, Riordan JF, Hu GF. Identification and characterization of an angiogenin-binding DNA sequence that stimulates luciferase reporter gene expression. Biochemistry. 2003;42:121–8. doi: 10.1021/bi020465x. [DOI] [PubMed] [Google Scholar]
- 8.Ruggero D, Pandolfi PP. Does the ribosome translate cancer? Nat Rev Cancer. 2003;3:179–92. doi: 10.1038/nrc1015. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene. 2005;24:445–56. doi: 10.1038/sj.onc.1208223. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji TYS, Kishimoto K, et al. Angiogenin is translocated to the nucleus of HeLa cells and is involved in rRNA transcription and cell proliferation. Cancer Research. 2005;65:1352–60. doi: 10.1158/0008-5472.CAN-04-2058. [DOI] [PubMed] [Google Scholar]
- 11.Hu G, Xu C, Riordan JF. Human angiogenin is rapidly translocated to the nucleus of human umbilical vein endothelial cells and binds to DNA. J Cell Biochem. 2000;76:452–62. doi: 10.1002/(sici)1097-4644(20000301)76:3<452::aid-jcb12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Hirukawa S, Olson KA, Tsuji T, Hu GF. Neamine inhibits xenografic human tumor growth and angiogenesis in athymic mice. Clin Cancer Res. 2005;11:8745–52. doi: 10.1158/1078-0432.CCR-05-1495. [DOI] [PubMed] [Google Scholar]
- 13.Kurachi K, Davie EW, Strydom DJ, Riordan JF, Vallee BL. Sequence of the cDNA and gene for angiogenin, a human angiogenesis factor. Biochemistry. 1985;24:5494–9. doi: 10.1021/bi00341a032. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Riordan JF, Hu G. Nuclear translocation of human angiogenin in cultured human umbilical artery endothelial cells is microtubule and lysosome independent. Biochem Biophys Res Commun. 1997;238:305–12. doi: 10.1006/bbrc.1997.7290. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro R, Strydom DJ, Olson KA, Vallee BL. Isolation of angiogenin from normal human plasma. Biochemistry. 1987;26:5141–6. doi: 10.1021/bi00390a037. [DOI] [PubMed] [Google Scholar]
- 16.Miyake H, Hara I, Yamanaka K, Gohji K, Arakawa S, Kamidono S. Increased angiogenin expression in the tumor tissue and serum of urothelial carcinoma patients is related to disease progression and recurrence. Cancer. 1999;86:316–24. [PubMed] [Google Scholar]
- 17.Shimoyama S, Gansauge F, Gansauge S, Negri G, Oohara T, Beger HG. Increased angiogenin expression in pancreatic cancer is related to cancer aggressiveness. Cancer Res. 1996;56:2703–6. [PubMed] [Google Scholar]
- 18.Hu GF. Neomycin inhibits angiogenin-induced angiogenesis. Proc Natl Acad Sci U S A. 1998;95:9791–5. doi: 10.1073/pnas.95.17.9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glasby JS. Encyclopedia of antibiotics. 3. New York, NY: John Wiley &Sons Ltd; 1993. pp. 363–7. [Google Scholar]
- 20.Ford JH, Bergy ME, Brooks AA, et al. Further characterization of neomycin B and neomycin C. J Am Chem Soc. 1955;77:5311–4. [Google Scholar]
- 21.Williams PD, Bennett DB, Gleason CR, Hottendorf GH. Correlation between renal membrane binding and nephrotoxicity of aminoglycosides. Antimicrob Agents Chemother. 1987;31:570–4. doi: 10.1128/aac.31.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Au S, Weiner N, Schacht J. Membrane perturbation by aminoglycosides as a simple screen of their toxicity. Antimicrob Agents Chemother. 1986;30:395–7. doi: 10.1128/aac.30.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian J, Lavker RM, Tseng H. Mapping ribosomal RNA transcription activity in the mouse eye. Dev Dyn. 2006;235:1984–93. doi: 10.1002/dvdy.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruschoff J, Plate K, Bittinger A, Thomas C. Nucleolar organizer regions (NORs). Basic concepts and practical application in tumor pathology. Pathol Res Pract. 1989;185:878–85. doi: 10.1016/S0344-0338(89)80290-0. [DOI] [PubMed] [Google Scholar]
- 25.Trere D, Pession A, Derenzini M. The silver-stained proteins of interphasic nucleolar organizer regions as a parameter of cell duplication rate. Exp Cell Res. 1989;184:131–7. doi: 10.1016/0014-4827(89)90371-6. [DOI] [PubMed] [Google Scholar]
- 26.Olson KA, French TC, Vallee BL, Fett JW. A monoclonal antibody to human angiogenin suppresses tumor growth in athymic mice. Cancer Res. 1994;54:4576–9. [PubMed] [Google Scholar]
- 27.Olson KA, Fett JW, French TC, Key ME, Vallee BL. Angiogenin antagonists prevent tumor growth in vivo. Proc Natl Acad Sci U S A. 1995;92:442–6. doi: 10.1073/pnas.92.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kao RY, Jenkins JL, Olson KA, Key ME, Fett JW, Shapiro R. A small-molecule inhibitor of the ribonucleolytic activity of human angiogenin that possesses antitumor activity. Proc Natl Acad Sci U S A. 2002;99:10066–71. doi: 10.1073/pnas.152342999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiner HL, Weiner LH, Swain JL. Tissue distribution and developmental expression of the messenger RNA encoding angiogenin. Science. 1987;237:280–2. doi: 10.1126/science.2440105. [DOI] [PubMed] [Google Scholar]
- 30.Hatzi E, Bassaglia Y, Badet J. Internalization and processing of human angiogenin by cultured aortic smooth muscle cells. Biochem Biophys Res Commun. 2000;267:719–25. doi: 10.1006/bbrc.1999.2015. [DOI] [PubMed] [Google Scholar]
- 31.Xu ZP, Tsuji T, Riordan JF, Hu GF. The nuclear function of angiogenin in endothelial cells is related to rRNA production. Biochem Biophys Res Commun. 2002;294:287–92. doi: 10.1016/S0006-291X(02)00479-5. [DOI] [PubMed] [Google Scholar]
- 32.Waksman SA, Lechevalier HA. Neomycin, a new antibiotic active against streptomycin-resistant bacteria, including tuberculosis organisms. Science. 1949;109:305–7. doi: 10.1126/science.109.2830.305. [DOI] [PubMed] [Google Scholar]
- 33.Leach BE, Teeters CM. Neamine, an antibacterial degradation product of neomycin. J Am Chem Soc. 1951;73:2794–7. [Google Scholar]
- 34.Wintrobe M, Thorn G, Adams R, et al. Harrison’s Principles of Internal Medicine. 6. New York: McGRAW-HILL; 1971. p. 749. [Google Scholar]
- 35.Sun M, Wang G, Paciga JE, et al. AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol. 2001;159:431–7. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–55. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 37.Vogt PK. PI 3-kinase, mTOR, protein synthesis and cancer. Trends Mol Med. 2001;7:482–4. doi: 10.1016/s1471-4914(01)02161-x. [DOI] [PubMed] [Google Scholar]
- 38.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–90. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 39.Aoki M, Blazek E, Vogt PK. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc Natl Acad Sci U S A. 2001;98:136–41. doi: 10.1073/pnas.011528498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terada N, Patel HR, Takase K, Kohno K, Nairn AC, Gelfand EW. Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc Natl Acad Sci U S A. 1994;91:11477–81. doi: 10.1073/pnas.91.24.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]