Abstract
The mechanisms controlling the differentiation of immature Schwann cells (SCs) into non-myelinating SCs is not known. Laminins are extracellular matrix proteins critical for myelinating SC differentiation, but their roles in non-myelinating SC development have not been established. Here we show that the peripheral nerves of mutant mice with laminin-deficient SCs do not form Remak bundles, which consist of a single non-myelinating SC interacting with multiple unmyelinated axons. These mutant nerves show aberrant L1 and N-CAM expression pattern during development. The homophilic and heterophilic interactions of N-CAM are also impaired in the mutant nerves. Other molecular markers for non-myelinating SCs, including Egr-1, GFAP, and AN2/NG2, are all absent in adult mutant nerves. Analysis of expression of SC lineage markers demonstrates that non-myelinating SCs do not develop in mutant nerves. Additionally, mutant mice are insensitive to heat stimuli and show a decreased number of C-fiber sensory neurons, indicating reduced nociceptive sensory function. These results show that laminin participates in non-myelinating SC development and Remak bundles and suggest a possible role for laminin deficiency in peripheral sensory neuropathies.
Keywords: non-myelinating Schwann cell, laminin, NCAM, L1, sensory function, peripheral nerves
Introduction
Ensheathment and myelination of axons in the peripheral nervous system (PNS) is accomplished by Schwann cells (SCs). SC precursors are derived from neural crest cells and differentiate into immature SCs. Immature SCs proliferate in response to axonal signals and adopt one of two alternative fates according to the size of the axon with which they are associated (Jessen and Mirsky 2005). Large diameter axons produce high levels of neuregulin (NRG1) type III and promote SC differentiation into myelinating SCs. In contrast, small diameter axons have lower levels of NRG1 type III leading to non-myelinating SCs (Michailov et al. 2004; Taveggia et al. 2005). SCs destined to myelinate extend processes to progressively separate axonal bundles to form a 1:1 ratio with individual axons (radial sorting) and to enwrap them with a myelin sheath (Jessen and Mirsky 2005). SCs that enter a non-myelinating lineage ensheath multiple small caliber axons (C-fibers, < 1 mm diameter) and keep individual axons separated by membrane extensions but do not myelinate (Taveggia et al. 2005). This non-myelinating SC/unmyelinated axon structure is called a Remak bundle.
Non-myelinating SCs appear in the PNS at approximately P15-20 (Arroyo et al. 1998; Berti et al. 2006) and ensheath a definite number (5-30) of small caliber axons (Friede and Samorajski 1968). They have a phenotype similar to immature SCs, as both express neural cell adhesion molecule (N-CAM), L1, the low-affinity neurotrophin receptor (p75), growth associated protein 43 (GAP43), and glial fibrillary acidic protein (GFAP) (Arroyo et al. 1998). However, the detailed mechanism of how immature SCs differentiate into non-myelinating SCs remains to be elucidated.
Substantial evidence indicates that SCs require laminins to properly myelinate axons (Yu et al. 2007). Laminins are heterotrimeric glycoproteins composed of an α-, β-, and γ-chain, and 15 isoforms have been observed (Yin et al. 2003). In vitro studies using SC/neuronal co-culture showed that laminin deposition is required for myelination (Fernandez-Valle et al. 1993; Fernandez-Valle et al. 1994; Podratz et al. 2001). Mice with laminin-deficient SCs show reduced SC proliferation and aberrant expression of transcription factors required for myelination (Yang et al. 2005; Yu et al. 2005). These mutant SCs are arrested at the premyelinating stage and fail to separate axons, resulting in impaired radial axonal sorting. All these results indicate that laminins play a pivotal role in the differentiation of myelinating SCs.
Non-myelinating SCs are also surrounded by a basement membrane, in which laminin is a major component. However, it is not known whether laminins are required for non-myelinating SC differentiation. In this study, we examined the role of laminins in non-myelinating SC development using P0/Cre:fLAMγ1 mice (referred to as mutant mice hereafter) (Yu et al. 2005), in which all laminin isoforms are specifically disrupted in SCs at the immature stage. In the absence of laminins, non-myelinating SCs do not exist in the adult PNS of mutant mice. Furthermore, the lack of non-myelinating SCs results in reduced response to heat and a decreased number of C-fiber sensory neurons. These results indicate that laminins are essential for non-myelinating SC development and normal nociceptive sensory function.
Materials and Methods
Mice
P0/Cre:fLAMγ1 mice were generated as described (Chen and Strickland 2003; Feltri et al. 2002; Yu et al. 2005). The use of animals was approved by the Institutional Animal Care and Use Committee of The Rockefeller University.
Electron microscopy and immuno-EM analyses
Electron microscopic (EM) analyses of ultra-thin sciatic nerve cross sections were as described (Chen and Strickland 2003). For immuno-EM, mice were intracardially perfused with PBS and fixed with 4% paraformaldehyde (PFA). Sciatic nerves were removed, postfixed, and cryoprotected. Thirty micron-thick sections were cut on a cryostat and mounted onto slides. The sections were blocked and incubated in anti-N-CAM antibodies (Chemicon). Biotinylated secondary antibodies were used, and the avidinbiotin-peroxidase complex (ABC reaction, Vector Laboratories) was visualized with diaminobenzidine and hydrogen peroxide. The stained samples were fixed in 2.5% glutaradehyde in 0.1 M sodium cacodylate buffer briefly and postfixed in reduced osmium tetroxide. After staining in 1% uranyl acetate for 1 hr, the samples were dehydrated through a graded series of ethanol and propylene oxide and embedded in Durcapan. Ultrathin sections were cut using an Ultracut E Microtome and analyzed by EM. The differences in staining patterns between control and mutant mice at the same stage were compared.
Immunohistochemistry
Mice were perfused with PBS and 4% PFA, and the vertebra and spinal cords were dissected and postfixed in 4% PFA for two days and then 30% sucrose for 48 hours. The spinal cords and dorsal root ganglia (DRG) were dissected, and sections were prepared. Sciatic nerves were collected without 4% PFA perfusion, and fresh frozen sections were prepared. For immunohistochemistry, sections were blocked in PBS containing 0.3% Triton X-100 and 5% normal donkey serum. The primary antibodies used were rat anti-L1 (Chemicon), rat anti-N-CAM (Chemicon), rabbit anti-Egr1 (Santa Cruz), rabbit anti-GFAP (Dako), rabbit anti-AN2/NG2 (Chemicon), rabbit anti-Oct-6 (a gift from Dr. Meijer, Erasmus University, Rotterdam, The Netherlands), rabbit anti-Krox-20 (Covance Research Products), rat anti-laminin γ1 (Chemicon), goat anti-calcitonin gene-related peptide (AbD serotec), and guinea pig anti-P2X3 (Chemicon). The samples were then incubated with the appropriate secondary antibodies (Jackson ImmunoResearch Laboratories) for 1 hr at room temperature. The sections were mounted in Vectashield® mounting medium with or without DAPI (Vector Laboratories), examined under an Axioskop 2 fluorescent microscope (Carl Zeiss) equipped with appropriate filters, and photographed with the AxioVision System (Carl Zeiss).
Thermal sensory function test
The left hind paws of mice were immersed for in a 52 °C water bath, and the time to withdraw the hind paws was recorded to determine nociceptive function. The left hind paws of a separate set of mice were immersed in the water bath for one minute, and mice were sacrificed and perfused,with PBS and 4% PFA two hours later. Spinal cords were removed, and coronal sections were prepared and stained for c-Fos (Chemicon) and NeuN (Chemicon).
Results
Absence of Remak bundles in sciatic nerves containing laminin-deficient Schwann cells
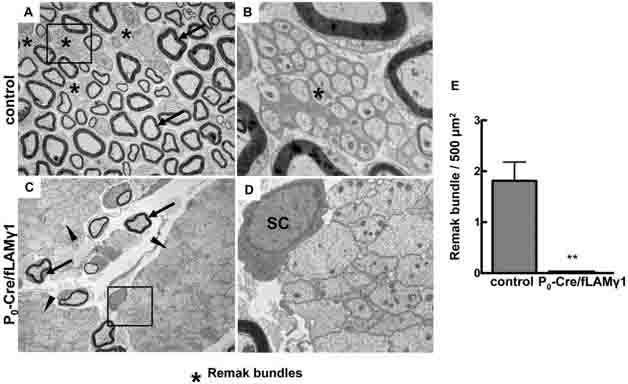
Mutant mice were generated as described previously (Yu et al. 2005). These mice lose expression of all laminin isoforms in immature SCs around embryonic day 14. At adult stages (2 to 3 months-of-age), the ultrastructure of control and mutant sciatic nerves was compared by electron microscopy (EM). In control nerves, large diameter axons were myelinated (arrows in Fig. 1A), whereas small diameter axons were separated by cytoplasmic processes of non-myelinating SCs and formed Remak bundles (stars in Fig. 1A & B). In mutant sciatic nerves, most axons were not sorted (arrowheads in Fig. 1C), very few axons were myelinated (arrows in Fig. 1C), and no Remak bundles were observed. The unsorted axonal bundles contained no SC processes between axons (Fig. 1D), which is the major difference when compared to the Remak bundle (Fig. 1B). Quantitative analysis of sciatic nerves from three control and mutant mice confirmed that Remak bundles did not form in the mutant peripheral nerves (Fig. 1E).
Figure 1. Remak bundles are not present in the peripheral nerves of the mutant mice.
Electron micrographs show that Remak bundles form in sciatic nerves of adult control mice (A and B), but do not form in those of mutant mice (C and D). Quantitative analysis is shown in E. BoxedElectron micrographs show that Remak bundles form in sciatic nerves of adult control mice (A and B), but do not form in those of mutant mice (C and D). Quantitative analysis is shown in areas in A & C are shown in B & D, respectively. Stars, Remak bundles; Arrows, myelinated axons; Arrowheads, unsorted axonal bundles; SC, Schwann cells.
Cell adhesion molecules of non-myelinating SCs are not detected in the mutant nerves
Lack of Remak bundles indicates an impairment of differentiation in non-myelinating SCs. L1 and N-CAM are two transmembrane cell adhesion molecules of the immunoglobulin superfamily that mediate cell-cell adhesion (Takeda et al. 2001). They are expressed on the surface of developing axons, growth cones, immature SCs, mature unmyelinated fibers, and non-myelinating SCs (Martini and Schachner 1986). The homophilic and heterophilic interactions of L1 and N-CAM promote axon/SC adhesion (Seilheimer et al. 1989), which is important for non-myelinating SC differentiation (Haney et al. 1999). Both L1 and N-CAM serve as molecular markers of differentiated non-myelinating SCs (Martini and Schachner 1986).
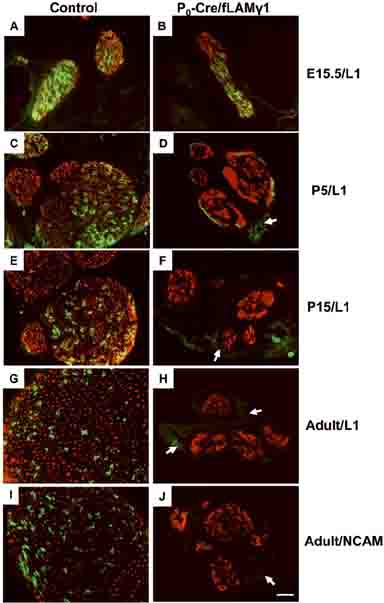
To evaluate differentiation of non-myelinating SCs, sciatic nerve sections were immunostained for L1 and N-CAM to detect their expression levels at different developmental stages. L1 and N-CAM are normally expressed in the growing axons and immature SCs, but are down-regulated in myelinating SCs upon differentiation and are restricted to non-myelinated axons and non-myelinating SCs when nerves mature (Martini and Schachner 1986). In both control and mutant embryonic nerves, L1 staining appeared normal in developing axons and immature SCs (Fig. 2 A and B). During postnatal stages, L1 was gradually confined to non-myelinating SCs and unmyelinated axons in control nerves (Fig. 2C, E, and G). In contrast, mutant nerve L1 expression was progressively down-regulated and was very low at P5 (Fig. 2D) and undetectable in P15 and in adulthood (Fig. 2F and H). N-CAM was present in control nerves (Fig. 2I), but showed changes similar to L1 in developing mutant peripheral nerves (data not shown) and was not detected in adult mutant nerves (Fig. 2J).
Figure 2. L1 and N-CAM show aberrant expression patterns during development in mutant peripheral nerves.
Whole embryo sections at E15.5 (A and B) and transverse sciatic nerve sections at P5 (C and D), P15 (E and F), and adult (G-J) were stained for neurofilament (red) and L1 (green, A-H) or N-CAM (green, I and J) to detect their expression patterns. In mutant nerves, L1 was expressed at early developmental stages (B) but was progressively undetectable at later stages (D, F, and H). N-CAM showed a similar expression pattern as L1 in mutant nerves and was not detected at the adult stage (J). Bar: 25 μm.
Mutant nerves show impaired homophilic and heterophilic interactions of N-CAM in SCs
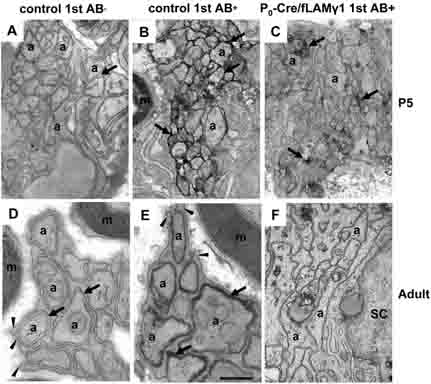
One explanation for the abnormal expression of L1 and N-CAM in mutant nerves was that these cell adhesion molecules do not form homophilic and heterophilic interactions when non-myelinating SCs develop. Since both L1 and N-CAM showed a very similar expression profile during development, we performed immuno-EM for N-CAM to further examine its subcellular expression pattern.
In control nerves, N-CAM appeared at the site of apposed axons in early developmental stages (arrows in Fig. 3B). Its expression was progressively down-regulated in myelinating SCs and was gradually confined to non-myelinating SCs and unmyelinated axons. When nerves matured, the non-myelinating SCs differentiated and extended processes to enwrap individual small diameter axons (arrowheads in Fig. 3D and E), and N-CAM expression was restricted to the regions where SC membranes were apposed to axolemma (arrows in Fig. 3E). In mutant nerves, N-CAM was present in the growing axons but showed a dramatic decrease in most regions of the nerves and was only preserved at a few sites of apposed axons (arrows in Fig. 3C) at early postnatal stages, the time when most SCs enter the myelinating stage. At adult stages, these mutant SCs did not extend processes to ensheath axons, and they failed to establish the homophilic and heterophilic interactions of N-CAM between the surface of axons and SCs (Fig. 3F). N-CAM expression was not detected at any sub-cellular localization of axons and SCs, suggesting that impaired homophilic and heterophilic interactions may lead to the degradation of cell adhesion molecules.
Figure 3. Homophilic and heterophilic interaction of N-CAM was impaired in mutant nerves.
At early developmental stages, immuno-EM shows that N-CAM is located at the site of apposed axons in control nerves (B) but is decreased in mutant nerves (C). When the non-myelinating SC/unmyelinated axon unit becomes mature and forms Remak bundles in the control nerves, N-CAM expression is restricted to the site between SC processes and axons (E), indicating that a homophilic or heterophilic interaction of N-CAM forms when axolemma adhere to the SC membrane. However, neither Remak bundles nor N-CAM staining are observed in the mutant nerves (F). Negative controls (no primary antibody) did not show staining in growing nerves (A) or in the Remak bundle of mature nerves (D). m, myelin sheath; a, axons; arrows, spaces between SC processes and axons or N-CAM-positive staining; arrowheads, SC processes. Bar: 4 mm (A-C); 3 mm (D-F).
Molecular markers of non-myelinating SCs are not expressed in the mutant nerves
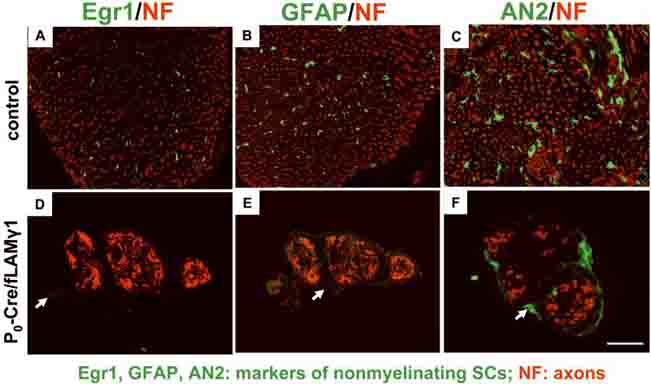
Since the homophilic and heterophilic interactions of L1 and N-CAM are essential for non-myelinating SC differentiation, it was possible that the impaired interaction impeded development of these cells. To address this question, expression levels of three molecular markers of differentiated non-myelinating SCs were examined in mutant nerves: Egr-1 (a transcription factor), GFAP (an intermediate filament protein), and AN2 (a cell-surface glycoprotein) (Jessen and Mirsky 1984; Schneider et al. 2001; Topilko et al. 1997). Egr-1, GFAP, and AN2 were present in adult control nerves (Fig. 4, A-C), and the expression patterns of these proteins were mostly co-localized with L1 or N-CAM (data not shown), indicating they were expressed normally in mature non-myelinating SCs. In contrast, Egr-1, GFAP, and AN2 were not detected in mutant nerves (Fig. 4, D-F). These results indicate that molecular makers for non-myelinating SCs are not expressed in the mutant nerves, suggesting non-myelinating SCs may improperly differentiate.
Figure 4. Molecular markers of non-myelinating SCs are not detected in the mutant sciatic nerves.
Immunohistochemistry shows that Egr1 (A and D), GFAP (B and E), and AN2 (C and F) are expressed in the sciatic nerves of adult control mice (A-C), but are not detected in those of mutant mice (D-F). Arrows: background staining of connective tissue (epineurium). Bar: 25 μm.
Non-myelinating SCs do not develop in the mutant nerves
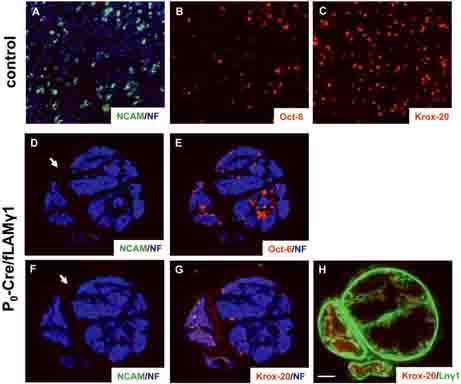
Upon specific disruption of laminins in SCs, mutant nerves show decreased cell number due to reduced cell proliferation and increased cell death (Yu et al. 2005). However, some mutant SCs are still present in the nerve. To determine the developmental status of these mutant SCs, we stained mutant nerve sections with lineage markers for SCs at different developmental stages. Oct-6 is a transcription factor that is transiently expressed in premyelinating and promyelinating SCs, and its function is required in promyelinating SCs for timely differentiation into myelinating SCs (Bermingham et al. 1996; Jaegle et al. 1996). Krox-20 is another transcription factor that is expressed continuously and specifically in myelin-producing Schwann cells (Topilko et al. 1994). As mentioned above, N-CAM is a cell adhesion molecule expressed in immature and non-myelinating SCs. Adjacent transverse sections of control and mutant sciatic nerves at P15 were stained for Oct-6 (detect pre/promyelinating SCs), Krox-20 (detect myelinating SCs) and N-CAM (detect immature SCs or non-myelinating SCs). In control nerves at this stage, even distribution of neurofilament staining indicated that peripheral axons are completely sorted (Fig. 5A). Some SCs in control nerves develop into myelinating lineage and differentiate into the pre/promyelinating stage (expressing Oct-6, Fig. 5B) or myelinating stage (expressing Krox-20, Fig. 5C). It is expected that a few SCs show overlapping expression of Oct-6 and Krox20, as these two transcription factors are co-expressed transiently at the early promyelinating stage (Zorick et al. 1996). Other SCs in control nerves show N-CAM expression in confined regions, indicating that they develop into non-myelinating SCs (Fig. 5A). In contrast, in mutant nerves, axons are presented as large unsorted bundles (Figs. 5D and F) and mutant SCs are located outside these axonal bundles (Figs. 5E and G). Most mutant SCs are arrested at premyelinating stage as they express very high level of Oct-6 (Yu et al. 2005); Fig. 5E). Some mutant SCs show Krox-20 expression, indicating that they obtain laminins from nearby connective tissues and further differentiate into myelinating stage (Yu et al. 2005) and Fig. 5G and H). However, no N-CAM staining was detected inside mutant nerves (Figs. 5D or F), indicating that mutant SCs do not arrest at the immature SC stage and they do not develop into non-myelinating SCs. We were unable to detect apoptosis for N-CAM or L1-positive cells in postnatal mutant sciatic nerves (data not shown), which further suggests that the lack of non-myelinating SCs results from a lack of development and was not due to cell death.
Figure 5. SCs in the mutant sciatic nerves develop into pre/promyelinating or myelinating SCs but not into non-myelinating SCs.
Transverse sections (A and B) and an adjacent section (C) of control sciatic nerves at P15 were stained for N-CAM (green, A), neurofilament (blue, A), Oct-6 (red, B), or Krox-20 (red, C) to detect pre/promyelinating, myelinating, and non-myelinating SCs. Adjacent transverse sections (D and E, F and G, and H) of mutant sciatic nerves at P15 were stained for N-CAM (green, D and F), neurofilament (blue, D-G), Oct-6 (red, E), Krox-20 (red, G and H) and laminin γ1 to detect pre/promyelinating, myelinating, and non-myelinating SCs and laminin expression. In mutant sciatic nerves, only pre/promyelinating.or myelinating SCs are present but no non-myelinating SCs are detected. Arrows: background staining of connective tissue (epineurium). Bar: 25 μm.
Mutant mice show impaired sensory response
Non-myelinating SCs ensheath C-fibers to form Remak bundles. C-fibers are the axons of postganglionic sympathetic efferents and primary sensory neurons (Julius and Basbaum 2001). Most of these nerve fibers regulate the noxious thermal sensory function. Therefore, possible thermal sensory deficiencies due to the lack of development of non-myelinating SCs to ensheath C-fibers were investigated by a hot stimulus assay (Chen et al. 2003). When the hind paw of control mice was immersed in hot water, it was quickly withdrawn (2-4 sec). However, when the hind paw of mutant mice was immersed, the mice slightly twisted their paws 20-30 sec after the immersion and did not withdraw their paws (data not shown).
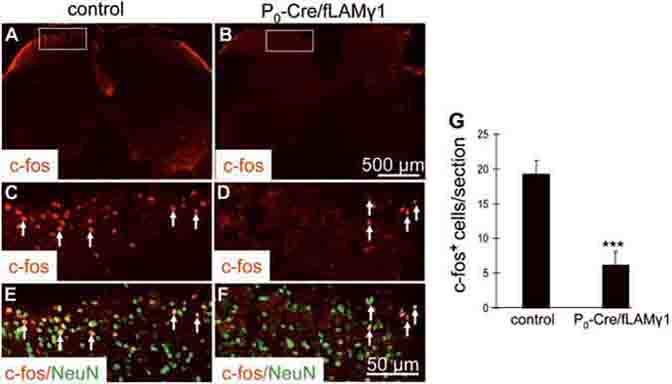
Since motor function of the mutant mice was impaired, it was difficult to attribute the delayed reaction to hot stimuli to sensory function impairment. Noxious heat stimuli activate small-diameter cutaneous sensory afferents and result in the rapid induction of c-Fos expression in the superficial layers of the dorsal horn (Hunt et al. 1987). Therefore, we analyzed c-Fos induction in the lumbar dorsal horn to determine if the delayed response of the reflex withdrawal to hot stimuli was due to the sensory function deficit. The left hind paws of control or mutant mice were immersed in 52 °C water for one minute. After two hours, the lumbar 4/5 regions of the spinal cord were collected for c-Fos and NeuN immunostaining. c-Fos was induced in the lumbar dorsal horn of control mice (Figs. 6A, C, and E) and c-Fos-positive nuclei colocalized with NeuN immunostaining (Fig. 6E), indicating that c-Fos expression was induced in neurons. There were significantly fewer c-Fos-positive cells in the ipsilateral dorsal horn of the lumbar 4/5 spinal cords of mutant mice (Figs. 6B, D, and F) when compared to control. These results indicate that the thermal sensation of the mutant mice is impaired. One possible explanation for the reduced thermal sensation is that C-fibers were lost in mutant nerves. To determine whether C-fibers were still present in unsorted axonal bundles, mutant nerves were stained for the calcitonin gene-related peptide (CGRP) to detect C-fiber axons originating from NGF-dependent small DRG neurons (Murinson et al. 2005). CGRP-positive axons were detected in unsorted axonal bundles of mutant nerves (arrows in Fig. S1), indicating that the reduced thermal sensation in mutant mice results from the lack of ensheathment but not the loss of C-fibers.
Figure 6. Heat stimulus-induced c-Fos expression in the dorsal horn of the spinal cord is impaired in mutant mice.
Immunostaining with anti-c-Fos antibody after immersion of the hind paw in a 52 °C water bath shows significantly fewer c-Fos-positive cells in the ipsilateral dorsal horn of lumbar 4/5 regions of the spinal cords of mutant mice (B, D, and F) than in those of control mice (A, C, and E). High magnification of boxed areas in A and B are shown in C and D, respectively. Merged images of double immunostainings for c-Fos and NeuN in boxed areas of A and B are shown in E and F, respectively. c-Fos-positive nuclei colocalized with NeuN immunostaining (E and F). Arrows: c-Fos- positive cells. (G) Quantitative analysis of c-Fos positive cells in sections of lumbar 4/5 spinal cord of control and mutant mice (4 representative serial sections from each animal; n=4 mice per genotype).
Mutant mice have a decreased number of C-fiber sensory neurons
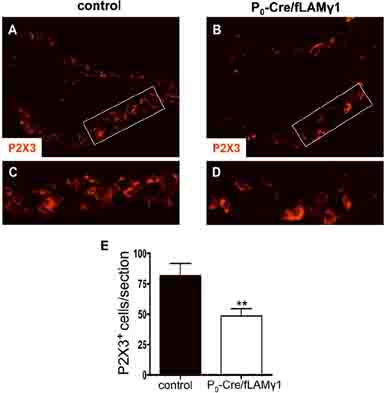
Since non-myelinating SCs play an important role in the maintenance of C-fiber sensory neuron survival (Chen et al. 2003) and since non-myelinating SCs are absent in the peripheral nerves of the mutant mice, we analyzed the number of C-fiber sensory neurons in the DRG of the mutant mice. In order to analyze C-fiber sensory neurons, we used antibodies against the P2X3 sensory-specific ATP-gated channel (Averill et al. 1995; Chen et al. 2003; Snider and McMahon 1998). We stained DRG sections from control and mutant mice and compared the number of labeled neurons between control and mutant mice. Many P2X3-positive cells were detected in control DRGs (Figs. 7A and C), whereas there were significantly fewer P2X3-positive cells in the mutant DRGs (Figs. 7B, D, and E). This result indicates that the survival of C-fiber sensory neurons is affected in the absence of non-myelinating SCs.
Figure 7. P2X3-positive DRG neurons is decreased in mutant mice.
DRG sections from control (A and C) and mutant mice (B and D) were stained with anti-P2X3 antibodies. High magnification of boxed areas in A and B are shown in C and D, respectively. (E) P2X3-positive cells were counted, and differences between control and mutant mice were analyzed by student's t test (4 representative serial sections from each animal; n=4 mice per genotype).
Discussion
Non-myelinating SCs do not develop in peripheral nerves of mice containing SCs lacking laminins. There are several possibilities as to how the disruption of laminins may influence the development of non-myelinating SCs. The P0Cre transgene in P0/Cre:fLAMγ1 mice activates Cre-mediated disruption of laminins around E13.5 and 14.5 (Yu et al. 2005), a time when SCs enter the immature stage. At this stage, SCs have not yet differentiated to myelinating and non-myelinating SCs and disruption of laminins could affect steps required for development of both cell types, including proliferation and ensheathment of SCs. One contributing factor could therefore be the reduced SC number in the mutant nerves (Yang et al. 2005; Yu et al. 2005). However, there are some SCs in the nerve, and the complete lack of non-myelinating cells indicates that laminin affects this differentiation step. Second, laminin signals are required for SC process extension, which may be essential for ensheathment and differentiation of non-myelinating SCs. Third, the impaired radial sorting of axons could impede non-myelinating SC development. Non-myelinating SCs appear late in the PNS, at approximately P15-20 (Arroyo et al. 1998; Berti et al. 2006). Development of myelinating SCs precedes that of non-myelinating SCs, and non-myelinating SCs ensheath multiple small caliber axons only after the myelinating SCs reach a 1:1 ratio with large individual axons (Eccleston et al. 1987). The impaired radial sorting of axons may inhibit the “sorting out” of small caliber axons or the “presorting” of larger bundles to smaller bundles, and may therefore prevent the development of non-myelinating SCs. Fourth, the failure of SCs to be exposed to axon-derived signals might inhibit non-myelinating SC differentiation. The amount of NRG1 type III in axons determines the ensheathment fate of axons (Michailov et al. 2004; Taveggia et al. 2005). Low levels of NRG1 type III are required for non-myelinating SCs to ensheath multiple small axons, whereas high levels of NRG1 type III are required for myelinating SCs to myelinate large axons. Lack of exposure of axon-derived NRG1 in laminin-deficient SCs (Yu et al. 2005) may result in inappropriate development of both myelinating and non-myelinating SCs. Finally, laminins may affect non-myelinating SC development directly by mediating the clustering of L1 and N-CAM in the cell membrane during postnatal PNS development, which is an essential step for non-myelinating SC differentiation.
The homophilic and heterophilic interactions of L1 and N-CAM between unmyelinated axons and non-myelinating SCs are important for non-myelinating SC differentiation (Haney et al. 1999; Macica et al. 2006; Martini and Schachner 1986; Martini and Schachner 1988). L1 interacts with integrins, components of laminin receptors, through the sixth Ig domain (L1-6D), and mice lacking L1-6D lose L1-integrin interactions and L1-L1 homophilic adhesion (Itoh et al. 2005). Disruption of laminins in SCs may destabilize the homophilic and heterophilic interactions of L1 and N-CAM and shorten the lifetime of these adhesion molecules on the cell membrane (Figs. 2 and 3).
In mutant nerves, a few mutant SCs located outside unsorted axonal bundles obtained laminins from nearby perineurial cells and were myelinated (Yu et al. 2005) and Fig. 5G and H) but why do these SCs not form Remak bundles? One possibility is that there may not be sufficient small axons “sorting out” to the peripheral for the Remak bundle formation.
A recent study identified the transcription factor, c-Jun, as a negative regulator of myelination (Parkinson et al. 2008). c-Jun can drive myelinating SCs back to the immature stage in injured nerves and acts as a potent suppressor of the myelinating phenotype during SC differentiation. It is not clear how some SCs maintain a non-myelinating status in adult peripheral nerves. Whether c-Jun plays a role in this process would be interesting to study.
The nociceptive sensory function of the mutant mice is reduced, which may partly be due to a decreased number of C-fiber sensory neurons in the DRG (Fig. 7). Non-myelinating SCs play an important role in the maintenance of C-fiber sensory neuron survival possibly by providing trophic factors (Chen et al. 2003). Disruption of ErbB receptor signaling in adult non-myelinating SCs leads to death of this cell type and also loss of C-fiber sensory neurons (Chen et al. 2003). In the sciatic nerves of these mice, the level of glial cell line-derived neurotrophic factor (GDNF) is decreased, which may be responsible for C-fiber sensory neuron loss. In the peripheral nerves of the laminin γ1 knockout mice, non-myelinating SCs are absent and GDNF is probably also decreased, which may cause C-fiber sensory neuron apoptosis. Our results support the hypothesis that non-myelinating SCs play an important role in the maintenance of a subpopulation of sensory neurons.
In summary, our results suggest that laminins are essential for non-myelinating SC development, which provides novel insight into how these cells ensheath small axons to form Remak bundles. These data may help researchers better understand the pathophysiology of peripheral sensory neuropathies and develop new therapeutic approaches for these disorders.
Supplementary Material
Acknowledgements
We thank Dr. Laura Feltri for providing materials and helpful suggestions, Dr. Erin H. Norris for comments on the manuscript, and Mr. Prabhjot Dhadialla and Dr. Karen Carlson for useful discussions. This work was supported by grants from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, NIH (NS035704, NS038472), and Muscular Dystrophy Association (MDA4066).
References
- Arroyo EJ, Bermingham JR, Jr., Rosenfeld MG, Scherer SS. Promyelinating Schwann cells express Tst-1/SCIP/Oct-6. J Neurosci. 1998;18(19):7891–902. doi: 10.1523/JNEUROSCI.18-19-07891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7(7):1484–94. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham JR, Jr., Scherer SS, O'Connell S, Arroyo E, Kalla KA, Powell FL, Rosenfeld MG. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10(14):1751–62. doi: 10.1101/gad.10.14.1751. [DOI] [PubMed] [Google Scholar]
- Berti C, Nodari A, Wrabetz L, Feltri ML. Role of integrins in peripheral nerves and hereditary neuropathies. Neuromolecular Med. 2006;8(1-2):191–204. doi: 10.1385/nmm:8:1-2:191. [DOI] [PubMed] [Google Scholar]
- Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci. 2003;6(11):1186–93. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol. 2003;163(4):889–99. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston PA, Mirsky R, Jessen KR, Sommer I, Schachner M. Postnatal development of rat peripheral nerves: an immunohistochemical study of membrane lipids common to non-myelin forming Schwann cells, myelin forming Schwann cells and oligodendrocytes. Brain Res. 1987;432(2):249–56. doi: 10.1016/0165-3806(87)90049-6. [DOI] [PubMed] [Google Scholar]
- Feltri ML, Graus Porta D, Previtali SC, Nodari A, Migliavacca B, Cassetti A, Littlewood-Evans A, Reichardt LF, Messing A, Quattrini A, et al. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156(1):199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Valle C, Fregien N, Wood PM, Bunge MB. Expression of the protein zero myelin gene in axon-related Schwann cells is linked to basal lamina formation. Development. 1993;119(3):867–80. doi: 10.1242/dev.119.3.867. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valle C, Gwynn L, Wood PM, Carbonetto S, Bunge MB. Anti-beta 1 integrin antibody inhibits Schwann cell myelination. J Neurobiol. 1994;25(10):1207–26. doi: 10.1002/neu.480251004. [DOI] [PubMed] [Google Scholar]
- Friede RL, Samorajski T. Myelin formation in the sciatic nerve of the rat. A quantitative electron microscopic, histochemical and radioautographic study. J Neuropathol Exp Neurol. 1968;27(4):546–70. [PubMed] [Google Scholar]
- Haney CA, Sahenk Z, Li C, Lemmon VP, Roder J, Trapp BD. Heterophilic binding of L1 on unmyelinated sensory axons mediates Schwann cell adhesion and is required for axonal survival. J Cell Biol. 1999;146(5):1173–84. doi: 10.1083/jcb.146.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328(6131):632–4. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Itoh K, Fushiki S, Kamiguchi H, Arnold B, Altevogt P, Lemmon V. Disrupted Schwann cell-axon interactions in peripheral nerves of mice with altered L1-integrin interactions. Mol Cell Neurosci. 2005;30(4):624–9. doi: 10.1016/j.mcn.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaegle M, Mandemakers W, Broos L, Zwart R, Karis A, Visser P, Grosveld F, Meijer D. The POU factor Oct-6 and Schwann cell differentiation. Science. 1996;273(5274):507–10. doi: 10.1126/science.273.5274.507. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Nonmyelin-forming Schwann cells coexpress surface proteins and intermediate filaments not found in myelin-forming cells: a study of Ran-2, A5E3 antigen and glial fibrillary acidic protein. J Neurocytol. 1984;13(6):923–34. doi: 10.1007/BF01148594. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6(9):671–82. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Macica CM, Liang G, Lankford KL, Broadus AE. Induction of parathyroid hormone-related peptide following peripheral nerve injury: role as a modulator of Schwann cell phenotype. Glia. 2006;53(6):637–48. doi: 10.1002/glia.20319. [DOI] [PubMed] [Google Scholar]
- Martini R, Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve. J Cell Biol. 1986;103(6 Pt 1):2439–48. doi: 10.1083/jcb.103.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and myelin-associated glycoprotein) in regenerating adult mouse sciatic nerve. J Cell Biol. 1988;106(5):1735–46. doi: 10.1083/jcb.106.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304(5671):700–3. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Murinson BB, Hoffman PN, Banihashemi MR, Meyer RA, Griffin JW. C-fiber (Remak) bundles contain both isolectin B4-binding and calcitonin gene-related peptide-positive axons. J Comp Neurol. 2005;484(4):392–402. doi: 10.1002/cne.20506. [DOI] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, et al. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181(4):625–37. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podratz JL, Rodriguez E, Windebank AJ. Role of the extracellular matrix in myelination of peripheral nerve. Glia. 2001;35(1):35–40. doi: 10.1002/glia.1068. [DOI] [PubMed] [Google Scholar]
- Schneider S, Bosse F, D'Urso D, Muller H, Sereda MW, Nave K, Niehaus A, Kempf T, Schnolzer M, Trotter J. The AN2 protein is a novel marker for the Schwann cell lineage expressed by immature and nonmyelinating Schwann cells. J Neurosci. 2001;21(3):920–33. doi: 10.1523/JNEUROSCI.21-03-00920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilheimer B, Persohn E, Schachner M. Neural cell adhesion molecule expression is regulated by Schwann cell-neuron interactions in culture. J Cell Biol. 1989;108(5):1909–15. doi: 10.1083/jcb.108.5.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20(4):629–32. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Murakami Y, Asou H, Uyemura K. The roles of cell adhesion molecules on the formation of peripheral myelin. Keio J Med. 2001;50(4):240–8. doi: 10.2302/kjm.50.240. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47(5):681–94. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topilko P, Levi G, Merlo G, Mantero S, Desmarquet C, Mancardi G, Charnay P. Differential regulation of the zinc finger genes Krox-20 and Krox-24 (Egr-1) suggests antagonistic roles in Schwann cells. J Neurosci Res. 1997;50(5):702–12. doi: 10.1002/(SICI)1097-4547(19971201)50:5<702::AID-JNR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371(6500):796–9. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Yang D, Bierman J, Tarumi YS, Zhong YP, Rangwala R, Proctor TM, Miyagoe-Suzuki Y, Takeda S, Miner JH, Sherman LS, et al. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J Cell Biol. 2005;168(4):655–66. doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Kikkawa Y, Mudd JL, Skarnes WC, Sanes JR, Miner JH. Expression of laminin chains by central neurons: analysis with gene and protein trapping techniques. Genesis. 2003;36(2):114–27. doi: 10.1002/gene.10206. [DOI] [PubMed] [Google Scholar]
- Yu WM, Feltri ML, Wrabetz L, Strickland S, Chen ZL. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J Neurosci. 2005;25(18):4463–72. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WM, Yu H, Chen ZL. Laminins in peripheral nerve development and muscular dystrophy. Mol Neurobiol. 2007;35(3):288–97. doi: 10.1007/s12035-007-0026-x. [DOI] [PubMed] [Google Scholar]
- Zorick TS, Syroid DE, Arroyo E, Scherer SS, Lemke G. The transcription factors SCIP and Krox-20 mark distinct stages and cell fates in Schwann cell differentiation. Mol Cell Neurosci. 1996;8(2-3):129–45. doi: 10.1006/mcne.1996.0052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.