Abstract
This study aimed to investigate ventilatory correlates of conditioned fear responses. Respiratory, end-tidal carbon dioxide pressure (PetCO2) and heart rate changes were studied in a differential fear conditioning paradigm. Forty-two participants viewed pictures of faces. One picture (CS+) was followed by a human scream (US) during the acquisition phase, but not in a subsequent extinction phase. Conditioning of PetCO2 (decrease), respiratory cycle time (decrease) and inspiratory duty time (increase) was established and subsequently extinguished. When participants were clustered according to their conditioned PetCO2 responses during acquisition, only a group showing a conditioned decrease in PetCO2 showed also a differential cardiac acceleration, a decrease in expiratory duration and an increase in inspiratory duty time in response to the CS+. These results suggest that preparation for defensive action is characterized by a tendency towards hyperventilation and cardiac acceleration.
Keywords: Fear Conditioning, Respiration, Heart rate, Hyperventilation
Introduction
Both clinical phenomenology and theory highlight the centrality of respiratory behavior and respiratory distress in states of fear (e.g., Ley, 1985; Gorman, Kent, Sullivan, & Coplan, 2000; Sinha, Papp, & Gorman, 2000; Wilhelm, Trabert, & Roth, 2001). Despite this, ventilatory correlates of conditioned fear responses in humans are not well documented, perhaps because, compared to other psychophysiological measures of learning (e.g., heart rate), changes in the mechanics of respiration are slower and more variable. A more tractable respiratory parameter, however, may be carbon dioxide pressure, since, in many circumstances, healthy subjects are able to regulate arterial carbon dioxide pressure, maintaining it within relatively narrow limits. As a consequence, normal breathing is characterized by little variability in carbon dioxide pressure (Shea, 1997), compared to the variability in respiratory timing and volume parameters (Bruce & Daubenspeck, 1995). In the current study, we assessed end-tidal carbon dioxide pressure (PetCO2, a valid approximation of arterial CO2 pressure; Gardner, 1996; Pahn, Tremer, Lee, & Barker, 1987) during affective conditioning. Not only is PetCO2 thought to be less variable than timing and volume parameters of breathing, it is also the only relevant outcome measure in the context of hyperventilation, which reflects a breathing pattern in excess of metabolic needs that occurs during emotional arousal, particularly fear (Van Diest et al., 2001a,b; Van Diest, De Peuter, Devriese, Wellens, Van de Woestijne, & Van den Bergh, 2005).
The control of breathing is a complex interplay that relies on many factors, including the bulbopontine respiratory network, central and peripheral chemoreceptor control, modulation of respiratory muscles by mechanoreceptors, and numerous suprapontine networks located in the limbic, cerebellar, and cortical areas (Gallego, Nsegbe, & Durand, 2001; Shea, 1996). It is not currently known how these different networks interact during an emotional event and in which measures they are reflected. Addressing this concern, Boiten (1993; 1998) suggested that, in addition to assessing traditional respiratory parameters of depth (tidal volume) and rate (total cycle time, inspiratory time, expiratory time, post-expiratory pause) of breathing, compound measures should also be pursued. One such parameter is the ratio of inspiratory time to the total breathing cycle time (inspiratory duty cycle), which reflects the cyclical on /off switching of the central inspiratory drive mechanism (Gautier, 1980). Several authors suggest that, in contrast to volume parameters, this timing parameter is weakly controlled by the chemical drive to breathe, allowing for its modulation by non-metabolic factors (Gallego, Denot-Ledunois, Vardon, & Perruchet, 1996; Rafferty & Gardner, 1996).
Thus, to the extent that respiratory changes in anticipation of an aversive event are not driven by changes in metabolism, one would expect to see effects of affective learning on timing, compared to volume parameters. This is exactly what has been observed in studies of humans using a standard aversive conditioning paradigm (e.g., tone CS and shock US; Ley, 1999). Obrist (1968) described important interindividual differences in the direction of the conditioned breathing responses: Whereas most participants showed a slightly increased breathing frequency following conditioning, a subgroup of participants showed a marked decrease in respiratory activity in response to the CS. The latter also showed a more sustained conditioned cardiac deceleration.
Similar to respiration, fear conditioned heart rate responses also show different patterns among individuals (Hamm, & Vaitl, 1996; Hodes, Cook, & Lang, 1985; Moratti & Keil, 2005). Heart rate decreases or increases have been interpreted as distinguishing between defensive attention and fear in the context of the defense cascade model (Lang, Bradley, & Cuthbert, 1997). This model describes an aversive motivational circuit that, with increasing arousal, triggers reactions ranging from orienting to fight/flight. The associated autonomic and somatic responses can be functionally organized into two broad output classes of defensive immobility and attention (i.e., freezing and hypervigilance in which the organism is passive, but primed to respond) and defensive action (contextual variations in fight/flight that are more or less direct responses to nociception or imminent attack).
Whereas defensive attention is associated with cardiac deceleration, cardiac acceleration prepares the organism to actively escape from an imminent threat, and previous studies have found that different individuals show different cardiac reactions following aversive conditioning (Hamm & Vaitl, 1996; Hodes et al., 1985). For instance, when a picture was paired with a loud noise, participants could be clustered based on their conditioned heart rate responses, which consisted of acceleration or deceleration (Hodes et al., 1985). Compared to individuals showing cardiac deceleration, individuals showing a conditioned cardiac acceleration to the CS+, also reported greater fear to the CS+ than the CS- (Hodes et al., 1985), showed more resistance to extinction in electrodermal responses (Hodes et al., 1985) and displayed potentiated startle responses to the CS+ (Hamm & Vaitl, 1996). This pattern of results is interpreted as indicating that, whereas all participants learned an anticipatory orienting response (late interval heart rate deceleration), only accelerators showed a conditioned preparation for defensive action.
Several findings from other studies suggest that respiration also distinguishes between defensive attention and action. Decreases in breathing frequency and/or a tendency towards expiration (i.e., decreased inspiratory duty cycle time) have been reported as part of the orienting response and during sustained attention in humans (Barry, 1982; Boiten, Wientjes, & Frijda, 1994; Denot-Ledunois, Vardon, Perruchet, & Gallego, 1998; Obrist, Webb, & Sutterer, 1969; Stekelenburgh & Van Boxtel, 2001). This may either be a direct consequence of a decreased somatic activity during defensive immobility (Obrist et al., 1969), or, it may be functional to inhibit breathing under threat for two reasons: (1) prompting an increase in blood flow to the brain, due to the cerebrovascular dilating effect of CO2 (Giardino, Friedman, & Dager, 2007; Kastrup, Dichgans, Niemeier, & Schabet, 1998), or (2) suppressing the noise associated with breathing in the presence of a predator (Fokkema, 1999). Defensive action, on the other hand, seems to prompt an increased cardio-respiratory activation, as well as a tendency towards hyperventilation (decreased PetCO2; Van Diest et al., 2001a,b).
The present study aimed to investigate fear conditioning of breathing behavior. To this end, respiratory and PetCO2, as well as heart rate responses, were studied during a prototypical affective learning paradigm. Pictures of human faces served as the conditioned stimulus (CS), a loud human scream as the unconditioned stimulus (US). We expected that differential conditioning of respiratory timing parameters, PetCO2 and heart rate would be established during acquisition and dissipate during a subsequent extinction phase. In addition, we expected that heart rate responses would closely follow respiratory timing and PetCO2 responses.
Method
Participants
Forty-two students aged 18 – 31 years old (mean age 20 years; 24 women) from the University of Florida volunteered to participate in return for course credit in their General Psychology class.
Design and Materials
A differential delay conditioning paradigm was used. Coloured pictures of neutral or angry male faces (“Karolinska Directed Emotional Faces”; Lundqvist, Flykt, & Öhman, 1998) presented for 8 s served as conditioned stimuli (CSs). A 95 dB human scream (6 sec duration) presented at picture offset was the unconditioned stimulus (US). The inter trial interval (ITI) was 80 s. For half of the participants, the US was preceded by an angry face (CS+), while the neutral face remained unpaired with the US (CS-). This was reversed for the other half. The expression of the CS faces was primarily manipulated for exploratory reasons. The expression of the CS+/CS- had no effect on the establishment of conditioning in any of the cardio-respiratory measures, and thus the rationale and interpretation of this variable are not central to the primary aims of this study.
The experiment consisted of an Acquisition (6 trials of CS+/CS-, with CS+ followed by the US) and an Extinction (4 CS+/4CS-, no US) phase. Trial types (CS+/CS-) were presented in a semi-randomized order. Extinction always started with a CS+ trial.
Measures
VPM software (Cook, Atkinson, & Lang, 1987) was used for physiological recordings. The ECG was obtained using standard Ag/AgCl electrodes placed on each forearm. The signal was sampled at 1000 Hz and transduced, amplified and filtered through a Coulbourn S75-05 Isolated Bioamplifier. A Dual Comparator/Window Discriminator (Coulbourn S21-10) detected the R-waves in the ECG.
Respiration was measured using an aneroid chest bellow (Coulbourn V94-19) strapped around the participant's chest wall and connected to a differential aneroid pressure transducer (Coulbourn V94-05) and a DC coupler with a 7.5V excitation voltage (Coulbourn V72-25).
Carbon dioxide pressure in the inhaled and exhaled air was continuously measured using a nasal CO2-sampling cannula connected to a non-dispersive infrared CO2-monitor (Datek 223 CO2 monitor, Pruiton-Bennet Worp., Wilmington, MA 01887). The CO2 signal was digitized at 25 Hz. A calibration procedure preceded each experimental session: a gas mixture containing 55 mmHg CO2 was sent to the capnograph and the corresponding A/D units were recorded, as well as the A/D corresponding to regular room air. This allowed for an off-line linear transformation yielding calibrated PetCO2 values.
Two types of custom made pen and paper tools were employed. In the first tool, participants rated the CS pictures on pleasantness and arousal. To this end, a bipolar scale was presented below each picture, consisting of a horizontal line of 150 mm with at the extreme points the labels ‘completely happy’ and ‘completely unhappy’ or ‘completely calm’ to ‘completely excited’. Participants could indicate their feeling by putting a vertical bar on the scale. The distance in mm from the left extreme was used as a score of unpleasantness or arousal.
The second tool was a post-experimental questionnaire that asked whether participants at times had voluntary controlled their breathing pattern during the presentation of the CS pictures. Participants could answer this question on a 5-point Likert type scale ranging from ‘never’ to ‘all the time’.
Procedure
After providing informed consent, participants rated the two pictures on pleasantness and arousal. Following this, the sensors were attached, as well as the respiratory belt and nose cannula. Participants were then instructed that a series of pictures would be presented and that they occasionally would hear an unpleasant loud noise that could be ignored. After this, the lights were dimmed and the experimenter left the room. Following the experiment, participants completed the post-experimental questionnaire and rated both CSs again on pleasantness and arousal.
Data reduction and Analyses
Heart rate
Recorded R-R intervals were edited with an off-line program (VPM; Cook et al., 1987) and transformed to average beats/minute for each half second. Heart rates per half second bins were averaged for each CS type (CS+, CS-) within each block of 2 trials. Heart rate in the 1 s before picture onset was subtracted from the average heart rate for every 0.5 s after picture onset. Initial and secondary deceleration (D1, D2) and midinterval acceleration (A) were scored according to Hodes et al. (1985):
D1 = slowest heart rate during the first 2 s following CS onset
A = fastest heart rate subsequent to D1 and within the first 5 s after CS onset
D2 = slowest heart rate subsequent to A, but before the US onset
Respiration
Both the CO2 and the respiratory signal were treated off-line using PSPHA (De Clerck, Verschuere, Crombez, & De Vlieger, 2006), a modular script-based program which we extended to extract the following parameters for each breath: end-tidal CO2-pressure (PetCO2, in mmHg), inspiratory time (Ti, in s), expiratory time (Te, in s), end-expiratory pause (Pexpin, in s), total cycle time (Ttot, in s), inspiratory duty cycle time (Ti/Ttot, in %) and amplitudo (A/D units standardized within subject). Respiratory parameters for each breath were averaged across the 15 s preceding picture onset (baseline) and across 8 s picture presentation. Only breaths falling entirely within a time window (baseline, picture presentation) were included. Next, averages for each CS type (CS+, CS-) and for each block of two consecutive trials were calculated. Change scores were calculated subtracting the mean base of each parameter from the mean during picture presentation.
Analyses
In a first set of repeated measure ANOVAs, data from each experimental phase (Acquisition, Extinction) were tested separately in a CS (+/-) × Block (1/2/3) design. Pre- and post-experimental subjective ratings of the CSs on pleasantness and arousal were analyzed in a CS (+/-) × Time (Pre/Post) design.
Data from five participants were excluded from respiratory analyses (final N=37), because of too many movement artefacts (n = 3), crying (n = 1) and self-reported voluntary breath holding upon CS presentation (n = 1). Data for four additional participants had to be excluded from the PetCO analysis (final N=33), one for an occluded sample line and three because of mouth breathing. For the heart rate data, four participants were excluded (final N=38) for a bad ECG signal in which R peaks were hard to detect.
A second set of analyses aimed to explore individual differences in conditioned response patterns. A k-means cluster analysis was performed to group participants according to their conditioned responses in PetCO2 during the second and third acquisition block. Subsequently, these groups were introduced as a between subject variable in ANOVAs with Cluster × CS (+/-) × Block (1/2/3) design. The Block variable had 3 levels for acquisition and 2 for extinction. Data for the latter are only presented if differential conditioning was present during acquisition. Only effects involving the Cluster factor will be reported. Pre- and post-experimental subjective ratings of the CSs on pleasantness and arousal were analyzed in a Cluster × Time (Pre/Post) × CS(+/-) design.
α was set at .05. Only significant effects are reported. Significant interactions were further tested on the simple main level. Greenhousse – Geisser correction were applied where appropriate. Effect sizes are reported as partial ηp2 scores.
Results
Respiration
During acquisition, participants breathed faster (had a shorter total respiratory cycle time) in response to the CS+ compared to the CS-, F(1, 36) = 6.66, p < .01, ηp2 = .16; see Table 1.
Table 1. Mean (SD) Respiratory Responses during each Block of Acquisition and Extinction.
| Acquisition | Extinction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Block 1 | Block 2 | Block 3 | Block 1 | Block 2 | ||||||
| CS+ | CS- | CS+ | CS- | CS+ | CS- | CS+ | CS- | CS+ | CS- | |
| Ttot | -0.1 (0.6) |
0.1 (0.6) |
-0.1 (0.7) |
-0.0 (0.6) |
-0.2 (0.9) |
0.2 (0.5) |
-0.2 (0.9) |
-0.0 (0.8) |
-0.1 (1.0) |
-0.0 (0.6) |
| Ti | -0.0 (0.3) |
0.0 (0.3) |
0.1 (0.3) |
0.0 (0.2) |
0.1 (0.2) |
0.1 (0.3) |
0.1 (0.3) |
0.1 (0.4) |
0.1 (0.4) |
0.0 (0.6) |
| Te | 0.0 (0.5) |
0.1 (0.4) |
0.1 (0.4) |
0.1 (0.3) |
0.1 (0.4) |
0.1 (0.3) |
-0.1 (0.5) |
0.0 (0.6) |
0.1 (0.5) |
-0.1 (0.4) |
| Pexpin | 0.0 (0.7) |
0.1 (0.7) |
-0.2 (0.5) |
-0.1 (0.4) |
-0.2 (0.5) |
-0.1 (0.3) |
-0.2 (0.6) |
-0.0 (0.7) |
-0.1 (0.7) |
0.1 (0.8) |
| Ti/Ttot | .01 (.06) |
.00 (.05) |
.03 (.07) |
.00 (.05) |
.03 (.09) |
.02 (.05) |
.01 (.07) |
.01 (.06) |
.00 (.08) |
-.00 (.06) |
| Amp | -0.3 (0.7) |
-0.2 (0.7) |
-0.1 (0.4) |
-0.1 (0.5) |
-0.0 (0.6) |
0.0 (0.6) |
-0.2 (0.6) |
0.0 (0.8) |
-0.3 (0.9) |
-0.0 (1.4) |
| PetCO2 | 0.4 (0.9) |
0.1 (0.9) |
-0.1 (1.5) |
0.4 (0.8) |
-0.1 (1.3) |
0.3 (0.8) |
0.2 (1.4) |
-0.0 (1.2) |
0.2 (1.3) |
-0.0 (1.5) |
Note. Ttot = respiratory cylce time (s); Ti= inspiratory time (s); Te = expiratory time (s); Pexpin = post-expiratory pause duration (s); Amp = respiratory amplitude (standardized within subjects); Ti/Ttot = inspiratory duty time (%); PetCO2 = end-tidal carbon dioxide pressure (mmHg)
A significant conditioning effect was also found for inspiratory duty cycle time: the relative inspiratory time increased more in response to the CS+ than to the CS-, F(1, 33) = 4.77, p < .05, ηp2 = .13, see Table 1. No significant conditioning effects were found for the subcomponents of the respiratory cycle time (inspiratory and expiratory time, and end-expiratory pause), or for amplitude measures.
For PetCO2, a significant CS × Block interaction was present during acquisition (F(2, 66) = 4.06, p < .05, ε = .99, ηp2 = .11; see Table 1). Compared to the CS-, a significant decrease in response to the CS+ was present in the second and third (F(1, 33) = 4.70, p < .05), but not in the first (F(1, 33) = 2.37, n.s.) acquisition block.
No significant effects were found for the extinction data.
Heart rate
No significant effects on any of the heart rate components (initial and secondary deceleration, midinterval acceleration) were observed during acquisition. During extinction, heart rate increased more in response to the CS+ than to the CS- in the first (F(1, 37) = 6.23, p < .05), but not in the second block of extinction (F(1, 37) = 0.01, n.s.; CS × Block interaction: F(1, 37) = 4.21, p < .05, ηp2 = .10; see Table 2). No significant effects were found for the initial or secondary deceleration during extinction.
Table 2. Mean (SD) Heart Rate Responses during each Block of Acquisition and Extinction.
| Acquisition | Extinction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Block 1 | Block 2 | Block 3 | Block 1 | Block 2 | ||||||
| CS+ | CS- | CS+ | CS- | CS+ | CS- | CS+ | CS- | CS+ | CS- | |
| D1 | -4.6 (6.0) |
-3.4 (3.2) |
-3.8 (5.5) |
-2.0 (3.5) |
-3.6 (5.0) |
-3.2 (4.2) |
-2.7 (4.5) |
-4.7 (6.0) |
-3.3 (4.6) |
-3.6 (4.9) |
| A | 1.1 (7.0) |
2.5 (7.0) |
3.5 (7.7) |
4.3 (5.9) |
5.6 (6.0) |
3.1 (5.6) |
4.8 (5.9) |
1.4 (7.9) |
2.9 (7.7) |
2.9 (5.3) |
| D2 | -5.9 (5.7) |
-5.5 (6.0) |
-4.0 (7.3) |
-3.9 (5.2) |
-4.0 (7.4) |
-5.9 (6.0) |
-6.5 (6.5) |
-8.8 (7.6) |
-7.4 (7.6) |
-6.5 (8.0) |
Note. D1 = initial cardiac deceleration (bpm); A = midinterval cardiac acceleration (bpm); D2 = late cardiac deceleration (bpm)
Subjective Ratings
Compared to the pre-experimental ratings, participants rated the CS+, but not the CS- as more unpleasant following conditioning, compared to these ratings prior to conditioning (Time × CS, F(1, 40) = 23.09, p < .01, ηp2 = .37). Furthermore, participants rated the CS+ picture higher in arousal than the CS- picture following, but not prior to the experiment (Time × CS, F(1, 40) = 23.07, p < .01, ηp2 = .37).
Clustering by PetCO2 response
Cluster analysis
Two clusters were identified based on PetCO2 responses to both CSs in the two last acquistion blocks. Table 3 displays the descriptive statistics of the PetCO2 responses averaged over the last 2 acquistion blocks for each cluster. In the first cluster (n=14), participants showed minor non-differential increases in PetCO2 to both CSs; this group will be further called hypoventilators. In the second cluster (n=19), participants showed a marked decrease in PetCO2 in response to the CS+, but not to the CS-; this cluster will be further labeled hyperventilators. The clusters' labeling refers to a response tendency and does not reflect a clinically significant state of hypo- or hyperventilation, which would imply more pronounced and sustained PetCO2 responses.
Table 3. Mean, SD and Range of PetCO2 responses to CS+ and CS- during the last two Blocks of Acquisition for Hypoventilators (N = 14) and Hyperventilators (N = 19).
| Acquisition Block 2 | Acquisition Block 3 | ||||
|---|---|---|---|---|---|
| CS+ | CS- | CS+ | CS- | ||
| Hypoventilators | Mean | 0.5 | 0.6 | 0.8 | 0.3 |
| SD | 0.7 | 0.7 | 1.1 | 0.7 | |
| Range | -0.7 - 1.5 | -0.6 – 1.5 | -0.4 – 4.2 | -0.9 – 1.8 | |
| Hyperventilators | Mean | -0.8 | 0.2 | -0.8 | 0.3 |
| SD | 0.9 | 0.8 | 0.9 | 0.9 | |
| Range | -2.6 – 0.7 | -1.1 – 1.9 | -3.4 – 0.5 | -0.9 – 2.5 | |
Respiration
During acquisition, a significant Cluster × CS × Block interaction: F(2, 62) = 4.00, p < .05, ε = .96, ηp2 = .11) was present for PetCO2, reflecting the clustering procedure. No differences were observed for PetCO2 responses during extinction.
During acquisition, hyperventilators had a shorter expiration in response to the CS+ compared to the CS- (F(1, 29) = 8.37, p < .01; means (SD) were -0.08 (0.25) and -0.22 (0.28) respectively), whereas expiratory time during CS+ versus CS- did not differ for the hypoventilators (F(1, 29) = 1.17, n.s.; means (SD) were 0.15 (0.18) and 0.13 (0.23) respectively). The overall Cluster × CS × Block interaction for expiratory time was significant, F(1, 29) = 7.34, p < .05, ε = .96, ηp2 = .11.
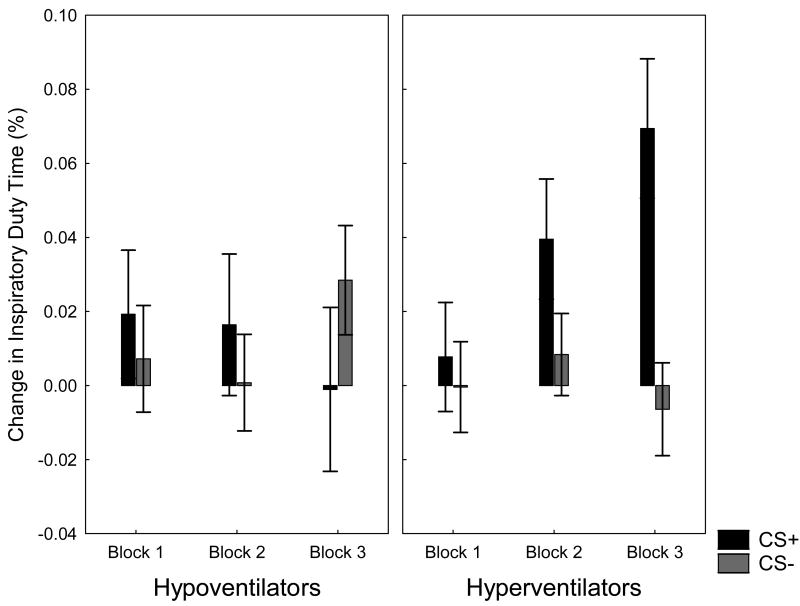
Also during acquisition, hyperventilators learned to respond with an increased inspiratory duty time in response to the CS+, but not to the CS- (linear trends were F(1, 31) = 5.47, p < .05 and F(1, 31) = 0.11, n.s., respectively). No such increases were observed for the hypoventilators (CS+: F(1, 31) = 0.31, n.s: CS-: F(1, 31) = 1.01, n.s.). Figure 1 displays the Cluster × CS × Block interaction for responses in inspiratory duty time during acquisition, F(2, 58) = 3.73, p < .05, ε = .81, ηp2 = .11.
Figure 1.
Changes in Inspiratory Duty Time (means and standard errors) in response to CS+ and CS- during three Acquisition Blocks for each cluster (Hypoventilators/Hyperventilators)
During extincition, a differential increase in inspiratory duty time in response to the CS+ tended to be present for the hyperventilators (F(1, 31) = 3.64, p < .07), but not for the hypoventilators (F(1, 31) = 0.58, n.s.). The overall Cluster × CS interaction was only marginally significant (F(1, 31) = 3.32, p < .08).
Heart rate
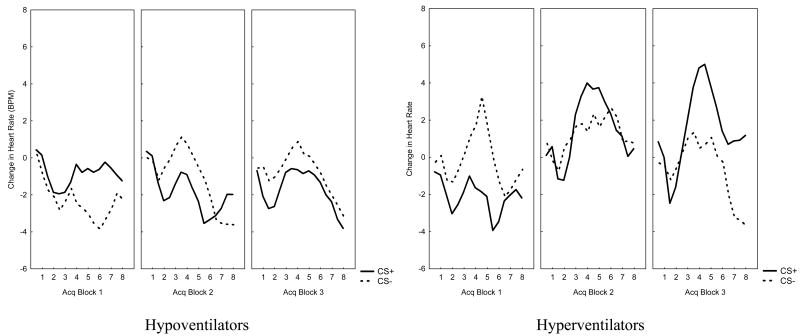
Participants in the two clusters differed in their conditioned midinterval cardiac acceleration during the acquisition phase (Cluster × CS × Block interaction, F(2, 62) = 4.20, p < .05, ηp2 = .12; see Figure 2). Only hyperventilators showed a progressive increase across acquisition blocks in midinterval cardiac acceleration in response to the CS+ (linear trend: F(1, 31) = 11.89, p < .01). This did not happen for the CS- (F(1, 31) = 0.05, n.s.). No significant effects were found for the initial or secondary deceleration components.
Figure 2.
Changes in Heart Rate per second during the 8 s presentation of the CS-pictures for each cluster (Hypoventilators/Hyperventilators)
For extinction, no significant effects involving the Cluster variable were observed.
Subjective ratings
No differences in ratings of pleasantness and arousal were found as a function of cluster membership.
Discussion
The present study documents aversive conditioning of respiratory and subjective responses in a human fear conditioning paradigm using human faces as the CS and a loud human scream as the US. Following the experiment, participants rated the CS+, but not the CS- higher in arousal and in unpleasantness as compared to prior to the conditioning procedure. The primary finding, however, was that participants learned to breathe faster with a higher inspiratory duty time in response to the CS+ compared to the CS- picture. Whereas the finding that such fear-related changes in respiration can be established using a prototypical fear conditioning procedure is new, the nature of the observed fear-related respiratory responses is consistent with other findings on fear-related changes in breathing patterns. The observed effects on respiratory timing parameters confirm the general finding that fear-related influences on respiration are primarily reflected in respiratory timing parameters, and less in parameters reflecting respiratory depth (Gallego et al., 1996; Rafferty & Gardner, 1996; Van Diest et al., 2001a). In addition, they are consistent with the major projections of the central nucleus of the amygdala to the brainstem respiratory areas implicated in respiratory timing (Harper, Frysinger, Trelease, & Marks, 1984; Zhang, Harper, & Ni, 1986). The relative shift towards inspiration, as reflected in an increased inspiratory duty time, has been observed during fearful imagery (Van Diest et al., 2001a,b), unpleasant picture viewing (Gomez, Stahel, & Danuser, 2004; Van Diest, Janssens, Bogaerts, Fannes, Davenport, & Van den Bergh, in press) and aversive film viewing (Boiten, 1998).
Participants from the present study also developed a lower PetCO2 in response to CS+ compared to CS- during the acquisition phase, which can be interpreted as a conditioned tendency towards hyperventilation. Although statistically significant, the observed responses in PetCO2 were nonetheless small. The relatively brief (8 s) exposure to the CS is not sufficient to prompt the development of a clinically relevant hypocapnic state and its associated symptoms, as might be encountered in a subgroup of panic disorder patients. Nonetheless, the conditioned decrease in PetCO2 observed here is theoretically relevant, particularly from the perspective of a continuum in defensive responding proposed by Lang et al. (1997). Hyperventilation has been conceptualized as a feedforward-response that compensates for an anticipated increase in the production of CO2 associated with defensive action (Van Diest et al., 2001a,b; 2005). Together with the observations of conditioned faster breathing and a relative shift towards inspiration, the conditioned changes in PetCO2 are consistent with an interpretation that they reflect preparation for defensive action (fight/flight behavior).
These data are consistent with the animal model, in which electrical stimulation of the dorsal periaqueductal gray (dPAG) in rats, a midbrain region known to play a crucial role in fight/flight behavior and its autonomic response, evokes a set of respiratory changes that remarkably resembles the responses observed in the present study (Zhang, Hayward & Davenport, 2005). In addition to increases in heart rate and blood pressure, electrical stimulation of the rat's dPAG, also evokes a decrease in PetCO2 and a shortening of both the inspiratory and expiratory phases. Interestingly, the shortening of expiratory duration is more pronounced than that of inspiratory duration, leading to an increase in inspiratory duty time.
An interpretation in terms of the respiratory differences in the current study as an index of defensive activation is further supported by secondary analyses that differentiated between participants who showed a clear conditioned tendency towards hyperventilation or not. When participants were clustered using their conditioned PetCO2 responses during the last two blocks of acquisition, only the subgroup showing a conditioned decrease in PetCO2 in response to the CS+ (‘hyperventilators’) also displayed conditioned cardiac midinterval acceleration. Cardiac acceleration is considered to be a valid discriminator between states characterized by defensive attention and states implying action-oriented defensive strategies (Lang et al., 1997; Bradley & Lang, 2007). Furthermore, only this subgroup also showed a conditioned decrease in expiratory time and a conditioned increased inspiratory duty time during acquisition. A higher inspiratory duty cycle time would reduce vagal tone (Eckberg, 2003; Obrist, Wood, & Perez-Reyes, 1965), which is consistent with an interpretation in terms of preparation for defensive action. In this respect, future research could try to sort out to what extent a pattern of fear conditioned cardiac acceleration is secondary to changes in inspiratory duty time.
The present results are both theoretically and clinically relevant. First, respiratory distress is a cardinal symptom in many anxiety disorder patients. Fear conditioned alterations in breathing behavior may constitute a potential mechanism for such symptoms. Future research could explore whether psychological vulnerability factors (e.g., anxiety sensitivity) affect the conditioning process. Second, the present results highlight the relevance of respiratory responses in the defense cascade model of human fear (Lang et al., 1997). They suggest that an increase in inspiratory duty time and a decrease in expiratory time and PetCO2 may be indicators of preparation for defensive action as is cardiac acceleration.
Acknowledgments
This research was supported in part by grants from the National Institute of Dental Research (DE 13956) to Margaret M. Bradley and the National Institute of Mental Health (P50 MH 72850) to Peter J. Lang. The research was conducted while Ilse Van Diest was at the NIMH Center for the Study of Emotion and Attention at the University of Florida, funded as a post-doctoral research associate by the Fund of Scientific Research – Flanders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barry RJ. Novelty and significance effects in the fractionation of phasic OR measures: a synthesis with traditional OR theory. Psychophysiology. 1982;19(1):28–35. doi: 10.1111/j.1469-8986.1982.tb02595.x. [DOI] [PubMed] [Google Scholar]
- Boiten F. Component analysis of task-related respiratory patterns. International Journal of Psychophysiology. 1993;15:91–104. doi: 10.1016/0167-8760(93)90067-y. [DOI] [PubMed] [Google Scholar]
- Boiten F, Frijda NH, Wientjes CJ. Emotions and respiratory patterns: Review and critical analysis. International Journal of Psychophysiology. 1994;17:103–128. doi: 10.1016/0167-8760(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Boiten FA. The effects of emotional behaviour on components of the respiratory cycle. Biological Psychology. 1998;49:29–51. doi: 10.1016/s0301-0511(98)00025-8. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson G, editors. Handbook of Psychophysiology. 2nd. New York: Cambridge University Press; 2007. pp. 581–607. [Google Scholar]
- Bruce EN, Daubenspeck AJ. Mechanisms and analysis of ventilatory stability. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. 2nd. Vol. 79. Hong Kong: Marcel Dekker, Inc.; 1995. pp. 285–313. [Google Scholar]
- Cook BW, Atkinson L, Lang KG. Stimulus control and data acquisition for IBM PCs and compatibles. Psychophysiology. 1987;24:626–627. [Google Scholar]
- De Clerck A, Verschuere B, Crombez G, De Vlieger P. Psychophysiological analysis (PSPHA): A modular script based program for analyzing psychophysiological data. Behavior Research Methods. 2006;38(3):504–510. doi: 10.3758/bf03192805. [DOI] [PubMed] [Google Scholar]
- Denot-Ledunois S, Vardon G, Perruchet P, Gallego J. The effect of attentional load on the breathing pattern in children. International Journal of Psychophysiology. 1998;29:13–21. doi: 10.1016/s0167-8760(97)00086-x. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. The human respiratory gate. Journal of Physiology. 2003;548(2):339–352. doi: 10.1113/jphysiol.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokkema DS. The psychobiology of strained breathing and its cardiovascular implications: A functional system review. Psychophysiology. 1999;36:164–175. [PubMed] [Google Scholar]
- Gallego J, Denot-Ledunois S, Vardon G, Perruchet P. Ventilatory responses to imagined exercise. Psychophysiology. 1996;33:711–719. doi: 10.1111/j.1469-8986.1996.tb02367.x. [DOI] [PubMed] [Google Scholar]
- Gallego J, Nsegbe E, Durand E. Learning in respiratory control. Behavior Modification. 2001;25(4):495–512. doi: 10.1177/0145445501254002. [DOI] [PubMed] [Google Scholar]
- Gardner WN. The pathophysiology of hyperventilation disorders. Chest. 1996;109(2):516–534. doi: 10.1378/chest.109.2.516. [DOI] [PubMed] [Google Scholar]
- Gautier H. Control of pattern of breathing. Clinical Science. 1980;58:343–348. doi: 10.1042/cs0580343. [DOI] [PubMed] [Google Scholar]
- Giardino ND, Friedman SD, Dager SR. Anxiety, respiration and cerebral blood flow: implication for functional brain imaging. Comprehensive Psychiatry. 2007;48:103–112. doi: 10.1016/j.comppsych.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez P, Stahel WA, Danuser B. Respiratory responses during affective picture viewing. Biological Psychology. 2004;67:359–373. doi: 10.1016/j.biopsycho.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. American Journal of Psychiatry. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Vaitl D. Affective learning: awareness and aversion. Psychophysiology. 1996;33:698–710. doi: 10.1111/j.1469-8986.1996.tb02366.x. [DOI] [PubMed] [Google Scholar]
- Harper RM, Frysinger RC, Trelease RB, Marks D. State-dependent alteration of respiratory cycle timing by stimulation of the central nucleus of the amygdala. Brain Research. 1984;306:1–8. doi: 10.1016/0006-8993(84)90350-0. [DOI] [PubMed] [Google Scholar]
- Hodes RL, Cook EW, Lang PJ. Individual differences in autonomic response: conditioned association or conditioned fear? Psychophysiology. 1985;22(5):545–560. doi: 10.1111/j.1469-8986.1985.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Dichgans J, Niemeier M, Schabet M. Changes of cerebrovascular CO2 reactivity during normal aging. Stroke. 1998;(29):1311–1314. doi: 10.1161/01.str.29.7.1311. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation and action. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. first. Mahwah, New Jersey: Lawrence Erlbaum Associates; 1997. pp. 97–136. [Google Scholar]
- Ley R. The modification of breathing behavior: Pavlovian and operant control in emotion and cognition. Behavior Modification. 1999;23(3):441–479. doi: 10.1177/0145445599233006. [DOI] [PubMed] [Google Scholar]
- Ley R. Blood, breath, and fears: A hyperventilation theory of panic attacks and agoraphobia. Clinical Psychology Review. 1985;5:271–285. [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. The Karolinska Directed Emotional Faces (KDEF) [CD-ROM] Stockholm: Karolinska Institute, Department of Clinical Neuroscience, Section Psychology; 1998. [Google Scholar]
- Moratti S, Keil A. Cortical activation during Pavlovian fear conditioning depends on heart rate response patterns: An MEG study. Cognitive Brain Research. 2005;25:459–471. doi: 10.1016/j.cogbrainres.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Obrist PA. Heart rate and somatic-motor coupling during classical aversive conditioning in humans. Journal of Experimental Psychology. 1968;77(2):180–193. doi: 10.1037/h0025814. [DOI] [PubMed] [Google Scholar]
- Obrist PA, Webb RA, Sutterer JR. Heart rate and somatic changes during aversive conditioning and a simple reaction time task. Psychophysiology. 1969;5(6):696–723. doi: 10.1111/j.1469-8986.1969.tb02872.x. [DOI] [PubMed] [Google Scholar]
- Obrist PA, Wood DM, Perez-Reyes M. Heart rate during conditioning in humans: Effects of UCS intensity, vagal blockade, and adrenergic block of vasomotor activity. Journal of Experimental Psychology. 1965;70:32–42. doi: 10.1037/h0022033. [DOI] [PubMed] [Google Scholar]
- Pahn CQ, Tremer KK, Lee SE, Barker SJ. Nonivasive monitoring of carbon dioxide: a comparison of the partial pressure of transcutaneous and end-tidal carbon dioxide with the partial pressure of arterial carbon dioxide. Journal of Clinical Monitoring. 1987;3:149–154. doi: 10.1007/BF01695936. [DOI] [PubMed] [Google Scholar]
- Rafferty GF, Gardner WN. Control of the respiratory cycle in conscious humans. Journal of Applied Physiology. 1996;81(4):1744–1753. doi: 10.1152/jappl.1996.81.4.1744. [DOI] [PubMed] [Google Scholar]
- Shea SA. Behavioural and arousal-related influences on breathing in humans. Experimental Physiology. 1996;81:1–26. doi: 10.1113/expphysiol.1996.sp003911. [DOI] [PubMed] [Google Scholar]
- Shea SA. Life without ventilatory chemosensitivity. Respiration Physiology. 1997;110:199–210. doi: 10.1016/s0034-5687(97)00084-4. [DOI] [PubMed] [Google Scholar]
- Sinha S, Papp LA, Gorman JM. How study of respiratory physiology aided our understanding of abnormal brain function in panic disorder. Journal of Affective Disorders. 2000;61:191–200. doi: 10.1016/s0165-0327(00)00337-2. [DOI] [PubMed] [Google Scholar]
- Stekelenburg JJ, Van Boxtel A. Inhibition of pericranial muscle activity, respiration, and heart rate enhances auditory sensitivity. Psychophysiology. 2001;38:629–641. [PubMed] [Google Scholar]
- Van Diest I, De Peuter S, Devriese S, Wellens E, Van de Woestijne KP, Van den Bergh O. Imagined risk of suffocation as a trigger for hyperventilation. Psychosomatic Medicine. 2005;67:813–819. doi: 10.1097/01.psy.0000181275.78903.64. [DOI] [PubMed] [Google Scholar]
- Van Diest I, Janssens T, Bogaerts K, Fannes S, Davenport PW, Van den Bergh O. Affective modulation of inspiratory motor drive. Psychophysiology. 2008 doi: 10.1111/j.1469-8986.2008.00715.x. in press. [DOI] [PubMed] [Google Scholar]
- Van Diest I, Winters W, Devriese S, Vercamst E, Han JN, Van de Woestijne KP, Van den Bergh O. Hyperventilation beyond fight/flight: Respiratory responses during emotional imagery. Psychophysiology. 2001a;(38):961–968. doi: 10.1111/1469-8986.3860961. [DOI] [PubMed] [Google Scholar]
- Van Diest I, Proot P, Van de Woestijne KP, Han JN, Devriese S, Winters W, Van den Bergh O. Critical conditions for hyperventilation responses: The role of autonomic response propositions during emotional imagery. Behavior Modification. 2001b;25(4):621–639. doi: 10.1177/0145445501254008. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Trabert W, Roth WT. Characteristics of sighing in panic disorder. Biological Psychiatry. 2001;49:606–614. doi: 10.1016/s0006-3223(00)01014-3. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Harper RM, Ni H. Cryogenic blockade of the central nucleus of the amygdala attenuates aversily conditioned blood pressure and respiratory responses. Brain Research. 1986;386:136–145. doi: 10.1016/0006-8993(86)90150-2. [DOI] [PubMed] [Google Scholar]
- Zhang W, Hayward LF, Davenport PW. Respiratory muscle responses elicited by dorsal periaqueductal gray stimulation in rats. American Journal of Physiology: Integrative, Regulatory and Comparative Physiology. 2005;289(5):R1338–47. doi: 10.1152/ajpregu.00828.2004. [DOI] [PubMed] [Google Scholar]