Summary
Early life stress (ELS) is expected to increase reactivity of the hypothalamic–pituitary–adrenocortical (HPA) axis; however, several recent studies have shown diminished cortisol reactivity among adults and children with ELS exposure. The goal of this study was to examine cortisol activity in 10–12-year-old internationally adopted children to determine if moderate and severe ELS have different impacts on the HPA axis. Salivary cortisol and two measures of autonomic activity were collected in response to the Trier Social Stress Test for Children (TSST-C). Three groups reflecting moderate, severe, and little ELS were studied: early adopted children who came predominantly from foster care overseas (early adopted/foster care (EA/FC), n = 44), later adopted children cared for predominantly in orphanages overseas (late adopted/post-institutionalized (LA/PI), n = 42) and non-adopted (NA) children reared continuously by their middle- to upper-income parents in the United States (n = 38). Diminished cortisol activity was noted for the EA/FC group (moderate ELS), while the LA/PI group (severe ELS) did not differ from the NA group. Overall, few children showed cortisol elevations to the TSST-C in any group. The presence/absence of severe growth delay at adoption proved to be a critical predictive factor in cortisol activity. Regardless of growth delay, however, LA/PI children exhibited higher sympathetic tone than did NA children. These results suggest that moderate ELS is associated with diminished cortisol activity; however, marked individual differences in cortisol activity among the LA/PI children suggest that child factors modify the impact of severe ELS. Lack of effects of severe ELS even for growth delayed children may reflect the restorative effects of adoption or the generally low responsiveness of this age group to the TSST-C.
Keywords: Cortisol, Group-based trajectory modeling, Growth curve modeling, Linear mixed modeling, Early life stress, Internationally adopted children
For over 50 years, animal studies have yielded evidence that early life stress can produce both stress resilience and stress vulnerability (see Levine, 2005). More attention has been paid to the vulnerability findings as these appear to provide a biological explanation for the heighted risk of psychopathology among maltreated children (Felliti et al., 1998; Heim and Nemeroff, 2001; Sanchez et al., 2001; De Bellis, 2005). Nonetheless, there is a large body of animal data showing that early life stress (ELS) can have stress resilience effects, such as reducing fearfulness and neurobiological reactivity to later stressors (see Parker et al., 2006). The distinction between ELS that produces vulnerability and ELS that produces resilience is presumed to lie in individual difference, contextual factors, and stressor factors that affect whether the ELS is manageable or overwhelming (Southwick et al., 2005).
Animal studies show that ELS impacts the development of both the corticotropin-releasing hormone/hypothalamic–pituitary–adrenocortical (CRH/HPA) and locus ceruleus/norepinephrine (LC–NE) arms of the mammalian stress system (Chrousos and Gold, 1992; Habib et al., 2001). There are a number of ways of measuring activity of these systems in human populations; however, in many studies, including the present one, saliva samples are assayed for cortisol to assess the HPA system, while cardiac measures are obtained to assess effects on the autonomic nervous system (ANS). ANS activity is important in supporting fight/flight responses (Sapolsky et al., 2000). Cortisol, on the other hand, has widespread regulatory influences (Sapolsky et al., 2000). Because of it profound and multiple impacts on neurodevelopment, cortisol has been the primary focus of neurobiological studies of ELS.
In the animal literature, early handling reliably produces stress resilience (e.g., Denenberg, 1999), while maternal separation less reliably produces stress vulnerability (Sanchez et al., 2001). Early handling involves handling and brief periods of maternal separation, while maternal separation involves handling and prolonged periods of maternal separation. When first introduced, handling was viewed as a stressor (Landauer and Whiting, 1964), an interpretation that was enhanced when it was shown that electric shock produced similar effects (Levine et al., 1956). However, the stressor interpretation gave way to the maternal mediation hypothesis (see Smotherman and Bell, 1980; Plotsky and Meaney, 1993; Caldji et al., 2000). That is, it was argued that the effects of ELS were not bidirectional (a little producing resilience, a lot producing vulnerability), but only appeared so because of the bidirectional effect of these manipulations on maternal care.
Recent results have resurrected questions about the potential bi-directionality of ELS effects. In non-human primates there is increasing evidence that early experiences that produce repeated or prolonged elevations in stress hormones can produce stress resilience rather than stress vulnerability (Parker et al., 2006; Lyons and Parker, 2007). Lyons and Parker (2007) argued that their results were due to the fact that, while their separation procedures produced marked and repeated elevations in cortisol, they involved challenges that were within the infants' coping capacity. This they argued was supported by evidenced that these separations did not impair normal physical and/or behavioral development. Studies of adults exposed to ELS have also begun to show that ELS is, at times or for some individuals, associated with decreased HPA axis responses rather than hyper-reactivity of the HPA system (Carpenter et al., 2007; Elzinga et al., 2008).
Known as the stress-inoculation hypothesis, the argument is that stress exposures that are not overwhelming, while still being significant enough to activate emotional and physiological coping processes, may inoculate or steel the individual against later stress exposures (Garmezy, 1991; Rutter, 1993). In the developmental literature, there is evidence that work-related stressors are less likely to induce depression in adults who experienced stress in work environments as adolescents (Mortimer and Staff, 2004). There is also evidence that highly protective parenting increases the odds that a fearful infant will continue to be extremely fearful (Rubin, 2002), and that going to child care, which is known to activate the HPA axis (e.g., Ahnert et al., 2004), reduces fearfulness among these same extremely fearful children (Fox et al., 2001). Likewise, Taylor et al. (2006) found that harsh parenting predicted reduced, rather than enhanced, amygdala responses to threat stimuli among psychiatrically healthy adults. Thus it may be that children exposed to some degree of adversity early in life develop stress systems that are hardened against later stressor exposure, while those exposed to severe ELS become more stress-reactive and vulnerable to later stressor exposure. If so, then impacts of mild/moderate versus severe ELS on later resilience versus vulnerability may be influenced by impacts of these early experiences on stress-mediating neurobiological systems.
When reviewing the current literature on ELS and stress biology, it is unclear whether the severity (intensity/duration) or individual differences determine resilience versus vulnerability. To date, most of the evidence that ELS produces increased activation of the HPA system come from studies of individuals with affective disorders or those with high genetic risk of these disorders. Heim et al. (2001a,b, 2002) have shown that among clinically depressed adults, greater ELS exposure is associated with greater ACTH and cortisol responses to a standard stressor task and more aberrant pituitary and adrenal responses to pharmacological challenges. Maltreated children with PTSD have also been shown to produce higher levels of cortisol and catecholamines (De Bellis et al., 1999; Carrion et al., 2002). Likewise, children whose mothers exhibit high depressive symptoms have been shown to have higher cortisol levels, especially when maternal depression occurred during the child's infancy (Dawson and Ashman, 2000; Halligan et al., 2004) or when the family is experiencing high economic stress (Lupien et al., 2000; Essex et al., 2002; Fernald et al., 2008), as well as when children of depressed mothers also exhibit high internalizing symptoms (Ashman et al., 2002). Finally, maltreated children with depression or high internalizing symptoms show disrupted daytime cortisol patterns (Kaufman, 1991; Hart et al., 1996; Cicchetti et al., submitted for publication).
However, there is growing evidence that when the impact of ELS exposures are examined in psychiatrically healthy, and thus by definition, psychologically resilient individuals, diminished or blunted pattern of HPA activity are often observed, particularly at the adrenal level. Heim et al. (2001a,b, 2002) found that women exposed to ELS who were not concurrently depressed showed no larger cortisol reactivity to the Trier Social Stress Test (TSST) than did non-depressed, non-ELS exposed women, although they did show a larger ACTH response. When exposed to pharmacological challenges, psychiatrically healthy women exposed to ELS showed a blunted cortisol response to ACTH compared to healthy women with no ELS exposure, although they showed a larger ACTH response to CRH (Heim et al., 2001a,b). Thus, both studies suggest a diminished adrenal response to ACTH among psychologically resilient survivors of ELS (however, see Carpenter et al., 2007). Elzinga et al. (2008), using psychologically healthy college students, also observed a diminished cortisol response to the TSST with greater ELS, especially among the men, but no differences in heart rate, blood pressure, or subjective tension. Similarly, Carpenter et al. (2007) noted lower HPA responses to the TSST among currently healthy ELS participants compared to healthy individuals within the non-ELS group. This effect was significant for men for both ACTH and cortisol, while among women, ELS was only associated with lower baseline ACTH. In general, a similar lack of evidence of cortisol hyper-reactivity has been noted in the child literature among maltreated children who do not exhibit significant symptoms of depression or internalizing problems (see Cicchetti and Rogosch, 2001a,b).
One difficulty in interpreting any of these data is that ongoing adversity in the individual's life and the presence or absence of coping resources during and following the period of ELS exposure may influence both the impact on the HPA axis and on the individual's psychological resilience (e.g., Kaufman et al., 1997, 2004; Gunnar and Donzella, 2002). Furthermore, the only way to address whether exposure to ELS during early childhood produces a long-term impact on stress reactivity and regulation is to find natural experiments in which, once removed from their ELS conditions, children live in relatively low-stress conditions, and ELS exposure groups clearly differ in the nature and duration of early exposure.
These concerns led us to examine children adopted internationally from ELS conditions. Adoption into a supportive home can provide a profound natural intervention in the life of a child exposed to significant ELS (van Ijzendoorn and Juffer, 2005). International adoption (IA) has increased dramatically in the United States and other industrialized countries over the last few decades (Gunnar et al., 2000). Prior to adoption, IA children have experienced multiple forms of ELS that differ depending on their country of origin. All IA children have experienced parental loss/abandonment and multiple changes in care-giving arrangements. However, some have experienced orphanage or institutional care while others have experienced foster care. Whether they are placed in an institution or foster home largely depends on the type of care used for wards of the state in their countries of origin (Gunnar et al., 2000). Country-of-origin policies also strongly influence when the child is available for adoption. Generally speaking, countries using foster care get children into permanent adoptive placements sooner than countries that rely on institutional care. Once adopted, currently available data suggests that post-adoption family life stressors tend to be very low (Kertes et al., 2008), parenting on the whole tends to be adequate to good (Croft et al., 2001), and there are very few adoption disruptions (e.g., Brumble, 2007). As a result, IA children provide a natural experiment on the impact of different degrees and durations of care disruption on subsequent stress reactivity and regulation.
This study was designed to examine the impact of two levels of ELS on later stress reactivity and regulation: severe and moderate. Children adopted from orphanages or other institutions when they were a year or older constituted the severe ELS group (late adopted/post-institutionalized or LA/PI). The types of adversity to which these children were exposed likely varied, but nearly all institutions included the lack of a consistent, responsive, adult caregiver. Exposure to pathogens is also greater in group care settings such as orphanages. While many institutions make adequate nutrition available, problems in delivery (propped bottles) and health issues (parasites) may reduce the nutrition the child actually receives (Zeanah et al., 2006). The longer a child is in institutional care, the more delayed the child becomes physically, cognitively, and socially. Studies of HPA activity in institutional settings suggest that many infants and toddlers exhibit cortisol patterns consistent with chronic stress (Carlson and Earls, 1997), such as low early morning levels and slightly higher late afternoon levels, resulting in relatively flat diurnal rhythm over the daytime hours (Miller et al., 2007).
The second group consisted of children adopted early, before 8 months, predominantly from foster care overseas (early adopted/foster care or EA/FC). We considered this the moderate ELS group. Like the LA/PI children, they experienced being abandoned or removed from their natal family. However, unlike the LA/PI children, they spent either no or very little time in institutional care and thus most of their early life was spent in the care of one or a few consistent caregivers. They then experienced the loss of this foster family when they were adopted. Thus the EA/FC group is clearly an ELS group, but as a group their early life adversity was briefer as they were adopted at a younger age. And, because they were cared for in families, it is likely that they experienced less neglect of their emotional, cognitive and physical needs than did the LA/PI children. Indeed, studies of children adopted early from foster care settings overseas show that as a group they tend to be physically healthy and display behavioral development that is age appropriate (van Londen et al., 2007), consistent with our argument that the EA/FC children meet the criteria for moderate ELS.
Finally, our non-adopted (NA) comparison children were born and raised in their birth families. To roughly match adoptive parental education and income, they were recruited from a registry of families who had agreed at the time of their child's birth to be contacted for child development studies. Previous evidence indicates that these families tend to be highly educated and to have high incomes, similar to families who adopt internationally. Children in this group were expected to have a minimal history of major life events and thus to represent a group who, relative to the two other groups, experienced the least amount of early life adversity.
All studies of children adopted or fostered from institutions, even one using random assignment, have shown that once out of the orphanage, children begin to show remarkable rebounds in physical and cognitive development (Maclean, 2003; Kreppner et al., 2007; Nelson et al., 2007). However, these studies also provide evidence of remarkable variability in resilience (Rutter et al., 2001). This is also the case for the two studies of basal cortisol levels examined 6 or more years after adoption (Gunnar et al., 2001; Kertes et al., 2008). Indeed, the second of these studies found that only those children who were more severely affected at adoption, as measured by their physical growth delay, were the ones with higher cortisol levels years after adoption (Kertes et al., 2008). As with studies of psychiatrically healthy versus disordered adults, this may indicate that it is not the merely the severity of ELS exposure but also factors in the individual that determine how ELS impacts the developing stress system. Thus, in the following study, we not only examined cortisol and autonomic responses to a stressor task as a function of level of ELS exposure, but also as a function of how adversely affected the child was by that exposure as indexed by stunted physical growth at adoption.
1. Method
1.1. Participants
The participants were 124 children ages 10.02–12.21 years (M = 11.25, S.D. = 0.68). Children in the LA/PI group (23 girls, 19 boys) were adopted when they were 12 months or older (range 12–64 months, M = 26.8 months, S.D. = 13.4 months) having spent at least 75% of their pre-adoption lives in institutional care. Nearly 70% of this group had lived only in an institution before adoption (range 0–64 months, M = 25.5 months of institutional care, S.D. = 13.5 months). They came from Asia (10 girls, 5 boys), Latin America (3 girls, 2 boys) and Russia/Eastern Europe (10 girls, 12 boys). Children in the EA/FC group (20 girls, 24 boys) had been adopted before 8 months (range 0.5–8 months, M = 4.2 months, SD = 1.7 months) and had spent less than 2 months in institutional care. Fifty percent of this group had never been institutionalized, while over half had spent 80% or more of their pre-adoption lives in foster care. They came from Asia (12 girls, 16 boys) and Latin America (8 girls, 8 boys). Children in the NA group (17 girls, 21 boys) were raised continuously by their birth parents in the United States. Descriptive data are shown in Table 1.
Table 1.
Family socioeconomic measures and child growth, puberty, and psychiatric diagnoses
| LA/PI | EA/FC | NA | |
|---|---|---|---|
| Median family income (thousands) | 75–100 | 75–100 | 75–100 |
| Median paternal education | College graduate | College graduate | College graduate |
| Median maternal education | College graduate | College graduate | College graduate |
| Standardized weight | |||
| At birth | −1.68 (1.1) | −1.07 (1.1) | −0.14 (1.0) |
| At adoption | −2.13 (2.0) | −0.57 (1.6) | NA |
| At testing | −.39 (1.0) | 0.24 (1.2) | 0.26 (1.0) |
| Standardized height | |||
| At adoption | −2.1 (1.9) | −0.49 (1.3) | NA |
| At testing | −0.27 (1.3) | −0.03 (0.9) | .59 (1.0) |
| Mean puberty score (range 1–4) | 2.1 (0.9) | 2.1 (0.9) | 1.9 (0.6) |
| %Psychiatric diagnosis | 24 | 14 | 8 |
| %ADD/ADHD | 19 | 14 | 5 |
Note. LA/PI: late adopted/post-institutionalized; EA/FC: early adopted/foster care; NA: non-adopted. Numbers in parentheses are standard deviations.
1.2. Recruitment and screening
The children were drawn from two sources. The internationally adopted children came from the Minnesota International Adoption Project (MnIAP) registry of families interested in participating in research (Hellerstedt et al., 2008). The non-adopted children came from the Institute of Child Development Participant Pool, a similar registry of children in their birth families. Within 6 months of the present study, the children were all screened for normal IQ (≥78), use of steroidal medications, congenital defects (downs/cerebral palsy), and fetal alcohol syndrome (FAS). Methods of this screening are available upon request. Based on an analysis of 332 children screened for a number of studies of internationally adopted children over a 3-year period, 87.2% of LA/PI, 96.7% of EA/FC, and 96.9% of NA children met screening criteria and were available for subsequent studies. Once in the pool of screened participants, children were assigned to be contacted for the present study based on being between 10 and 12 years of age and not having a sibling already in the study. We attempted to roughly balance for sex of child while also obtaining a distribution of adoptions from Asia, Latin America and Russia/Eastern Europe.
1.3. Procedures
The child and at least one parent attended a laboratory session that started roughly at 3:30 p.m. (M = 3:49 p.m., S.D. = 22 min). The children were asked not to eat for 1 h prior to coming to the laboratory. After consent/assent, four spot electrodes were applied for autonomic recording while parents were present. The parent was then escorted to a nearby room and the electrodes were allowed to acclimate for 20 min while the child worked on a puzzle. Baseline autonomic measures (6 min) were then obtained while the child relaxed and watched nature videos (Baseline Period). The TSST-C was then administered (Buske-Kirschbaum et al., 1997). This task involved providing the child with a story scenario and then giving him/her 6 min to develop a story (Speech Preparation Period). The child was then taken to another room where s/he was confronted with two unfamiliar adult judges to whom the child delivered the speech for 5 min, followed by 5 min of mental arithmetic (Speech/Math Period). The children were told their performance was being videotaped and they delivered their speeches facing not only the judges but also a highly visible video camera. The story stem used was one in which the child was erroneously accused of stealing and had to describe to a “principal and teacher” why they were innocent (for details, see Gunnar et al., in press).
After the speech/math period, the child was congratulated and reminded that the adults knew it was all pretend. The child then returned to the original room where autonomic recording resumed while s/he watched another 12 min of the nature videos (Post and Recovery Periods). The child then completed a rating of the stressfulness of each test segment and a puberty scale (see below), and was compensated with a $10 gift certificate for attending the lab session and a second $10 gift certificate for completing the home portion of the study.
1.4. Measures
1.4.1. Salivary cortisol
Saliva was collected by having the child chew a piece of original flavor Trident™ gum for 1 min and then spit through a 3-in. straw into a cryos vial (Eppendorf, Westbury, NY; Schwartz et al., 1998). Samples collected at home were stored in a zip-locked bag in the family's refrigerator and then mailed to the laboratory (Clements and Parker, 1998). In the laboratory, all samples were stored at −20 °C until assayed in duplicate using a time-resolved fluorescence immunoassay (DELFIA) with intra- and inter-assay coefficients of variation were 5.8% and 8.7%, respectively.
Eight samples were taken in the laboratory. Three were collected over the initial baseline period [after consent/assent (0 min), after electrode placement (+15 min) and after completion of baseline autonomic assessment (+30 min)]. Five were collected during the stress and recovery periods [immediately after the speech preparation (+40 min), after the speech/math period (+55 min), and then at 10 min intervals until +75 min]. To provide a home baseline, participants took saliva samples on 2 regular school days at 4 p.m. Diary reported times of home sampling averaged 4:09 p.m., S.D. = 30 min. Of the 124 participants, 112 provided home samples (LA/PI = 98%, EA/FC = 92%, NA = 82%).
Cortisol data were log10 transformed prior to analysis (Gunnar and Talge, 2007). The home cortisol data were used to determine which of the pre-TSST-C samples could serve as a valid baseline; paired t-tests showed that none of the first three laboratory values were elevated over home-baseline levels. Given this, the 3rd baseline sample was used as the index of baseline.
1.4.2. Autonomic measures
Pre-ejection period (PEP) and respiratory sinus arrhythmia or vagal tone (VT) were obtained simultaneously during four 6-min collection periods: Baseline, Speech Preparation, Post and Recovery. The latter two periods reflected the first and second 6 min following the Speech/Math stressor. All autonomic measures were obtained when participants were seated and not speaking to control for effects of posture and speaking (Berntson et al., 1997). PEP was analyzed from electrocardiogram (ECG) readings obtained using a SORBA Medical Systems Inc. CIC-1000 (Critchley, 1998). VT was obtained using raw ECG input from the electrodes into a Polar heart rate monitor to detect R-spikes and a Mini-Logger (Mini-Mitter Co. Inc., Bend, OR) recorded inter-beat intervals (IBI) throughout each collection period. MXedit software was used to calculate the heart period and Vagal Tone Index using methods described by Porges (1985). Complete data were available for 95% of the participants for PEP and 82% for VT.
1.4.3. Perceived stress
Children were shown the self-assessment manikin (SAM) developed by Bradley and Lang (1994). The first figure was characterized to the child as being “completely relaxed” while the fifth figure was said to be “freaking out”. Children were asked to use this scale to describe their reactions at five points in the testing: arrival at the laboratory, during speech preparation, while giving the speech, during the math segment, and during the recovery period.
1.4.4. Current height, weight, and body mass index (BMI)
Researchers obtained height and weight during the session. BMI was calculated using the equation for U.S. units (BMI = 703 × −weight (lbs)/height2 (in.2)). The Center for Disease Control (CDC) standard of a BMI for age above the 95th percentile was used to define obesity. EA/FC children were more likely to be obese (16%) than were either LA/PI (2%) or NA (5%), χ2(2) = 5.8, p = 0.05. Thus, BMI was included in the analyses.
1.4.5. Birth and adoption height and weight
Parents reported on the participant's birth weight and length, if the information was available, and for the adopted children, on these measures from the child's first doctor's visit in the U.S. If the parent did not know this information, permission was obtained to contact the child's pediatrician. For the birth measures, the most commonly available measure was birth weight (LA/PI 38%, EA/FC 80%, NA 90%). For measures at adoption, either height or weight measures or both were available on 92% of the IA children. Percentiles and standard scores for height and weight at adoption were calculated using the CDC 2000 growth norms (C.D.C., 2004), and children scoring two or more standard deviations below the mean on either measure were classified as severely growth delayed at adoption.
1.4.6. Parent income, education, and child life stress
Parents completed a questionnaire that included questions about family income (25 K units) and each parent's education (<High School, High School or GED, Some College or Associates Degrees, Bachelor's Degree, Masters, Professional Degree or Doctorate). No group difference were noted in maternal, χ2(8) = 10.94, ns., or paternal education, χ2(10) = 10.38, ns., or family income, F(2,116) = 0.17, ns. However, as socioeconomic factors have been shown to influence stress reactivity (e.g., Evans and Kim, 2007), education and income were included in the analyses. Parents also completed the Child Life Events Scale for the last 3 months and for the time since the child entered the family (Boyce et al., 1995). This measure includes normative stressors (i.e., birth/adoption of sibling, move to a new house) as well as non-normative stressors (i.e., death of family member, incarceration of a parent, parental divorce/separation). Neither of these time periods yielded group differences (F = .009 and F = .82, ns.) and are not considered further. In general, since birth (NA) or since adoption (EA/FC and LA/PI), child life events were low in all three groups (Grand Mean = 7.9 out of 37, S.D. = 3.4) and generally normative.
1.4.7. Puberty measure
The Pubertal Development Scale (Petersen et al., 1988) was administered to both the parent and the child, independently. Parent and child puberty reports were significantly correlated, r = .65, d.f. = 122, p < .001, and were averaged for analysis. Puberty scores did not differ by ELS group F(2, 116) = 0.13, ns., nor did ELS group interact with sex, F(2, 116) = 0.60, ns. Thus, this measure was not considered further.
1.4.8. Clinical diagnoses and medications
The parent completed the computerized version of the Diagnostic Interview for Children and Adolescents, Revised (DICA-R; Reich and Welner, 1988; Reich et al., 1995) and also reported on children's psychotropic medications. Children were scored as having a psychiatric diagnosis if either report so indicated. Only 19 children had a psychiatric diagnosis, predominantly ADD or ADHD (84% of the 19 children). LA/PI children were more likely than NA children to have a psychiatric diagnosis χ2(2) = 3.71, p = .05, while EA/FC and NA children did not differ, χ2(1) = 0.68, ns. There were no group difference in medication use on the day of testing, χ2(2) = 4.03, ns. Because recent studies indicate no impact of stimulant medications on cortisol activity (Hibel et al., 2007; Lee et al., 2007), neither medication nor diagnosis was considered further.
1.5. Analysis plan
Covariates with missing data were used, so the sample size varied slightly by analysis. N's are reported for each analysis. The statistical tests we used enable analyses with unbalanced designs. In all analyses, covariates were examined and, if non-significant, were removed from the models. Thus, only significant covariates appear in the results. Because ELS was expected to impact not only the level of stress response but also its organization, we approached each physiological measure first by examining whether the population included latent groups that differed in their response patterns, not just in their level or baseline intercept. To examine the data for latent groups, we used a form of finite mixture modeling known as group-based trajectory modeling (Nagin, 2005) implemented as SAS Proc Traj (Jones et al., 2001). Mixture modeling takes into account potential population heterogeneity, in which a single sample is actually representative of two or more sub-populations with qualitatively different response patterns (for use of similar methods in small samples, see Davis et al., 2004; Mareschal and Tan, 2007). When we noted latent groups that varied in response pattern, we then examined whether ELS was related to the probability of exhibiting each of the patterns observed. If the group-based procedure yielded only evidence of latent groups that differed in level but not in pattern of response, we reanalyzed the data using Linear Mixed Modeling (LMM), which enabled the specification of a mean growth curve for the entire sample according to a polynomial function (Fitzmaurice et al., 2004). Our interest was in examining the ability of ELS to predict variation from the mean growth curve (e.g., EA/FC vs. NA and LA/PI vs. NA). Finally, for physiological variables that yielded evidence of differences in terms of ELS groups, we examined whether severe growth delay at adoption, as an index of the individual's sensitivity to ELS, explained the ELS effects. These analyses involved contrasting the NA children with internationally adopted children (combining both groups) who were and were not severely growth delayed at adoption.
2. Results
2.1. Perceived stressfulness of the TSST-C
Application of RM-ANOVA to the cortisol data yielded neither a main effect of ELS group, F(2, 120) = 1.35, ns., nor an interaction of group with trials, F(8, 480) = 1.13, ns. The trials effect was highly significant, F(4, 480) = 181.5, p < .001, yielding evidence that children perceived the preparation, speech and math segments of the test as more stressful than the baseline and recovery periods.
2.2. Differences in cortisol response patterns
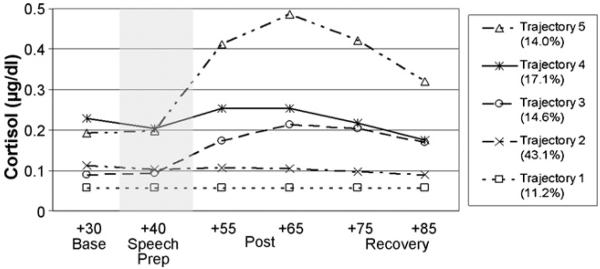
Means and standard deviations for all physiological data are shown in Table 2. To identify unique or non-normative response trajectories, group-based trajectory modeling (Nagin, 2005) was performed employing the Bayesian Information Criteria (BIC) to determine the appropriate number of latent groups (Jones et al., 2001; Nagin, 2005). Model fit improved up to five latent groups, but not at six groups; thus, the 5-group model was preferred (see Fig. 1). All model adequacy measures indicated a very well-fitting model (Nagin, 2005); these data are available upon request. As shown in Fig. 1, most children did not show a cortisol response to the TSST-C. Trajectories 1 (11.2% of children), 2 (43%), and 4 (17.2%) were all non-responsive, while Trajectories 3 (14.6%) and 5 (14%) differed in both cortisol level and magnitude of response. As shown in Table 3, relative to Trajectory 5, being younger predicted membership in Trajectories 2–4, since negative coefficients indicate that greater age predicts reduced likelihood of membership in the lower trajectories as compared to Trajectory 5; thus, the older children were more likely to be more reactive to the TSST procedure, controlling for family income and adoption status. Relative to Trajectory 5, children from wealthier families tended to be in Trajectory 2, which was the most common trajectory (the positive coefficient indicates increased likelihood of wealthier families in Trajectory 2); thus, income seems to predict reduced reactivity when controlling for age and adoption status.
Table 2.
Physiological measures
| Measure | LA/PI |
EA/FC |
NA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| M | S.D. | N | M | S.D. | N | M | S.D. | N | |
| Cortisol (μg/dl) | |||||||||
| Baseline 1 | .17 | .11 | 42 | .15 | .08 | 44 | .18 | .12 | 37 |
| Baseline 2 | .16 | .10 | 42 | .13 | .05 | 44 | .15 | .09 | 38 |
| Baseline 3 | .16 | .12 | 42 | .12 | .05 | 44 | .16 | .09 | 38 |
| Speech prep | .15 | .12 | 42 | .11 | .04 | 44 | .16 | .10 | 38 |
| Post 1 | .20 | .19 | 42 | .16 | .10 | 44 | .20 | .11 | 38 |
| Post 2 | .21 | .17 | 42 | .19 | .16 | 44 | .24 | .16 | 38 |
| Recovery 1 | .18 | .15 | 42 | .17 | .14 | 44 | .19 | .14 | 38 |
| Recovery 2 | .15 | .12 | 42 | .14 | .09 | 44 | .16 | .11 | 38 |
| Pre-ejection period (ms) | |||||||||
| Baseline | 10.40 | .97 | 41 | 10.53 | 1.33 | 42 | 10.90 | 1.49 | 36 |
| Speech prep | 10.39 | 1.22 | 41 | 10.43 | 1.21 | 42 | 10.68 | 1.55 | 36 |
| Post | 10.43 | 1.16 | 41 | 10.60 | 1.14 | 42 | 11.03 | 1.47 | 36 |
| Recovery | 10.33 | .87 | 41 | 10.50 | 1.30 | 42 | 11.03 | 1.44 | 36 |
| Vagal tone (ms2) | |||||||||
| Baseline | 6.90 | 1.01 | 35 | 6.55 | .98 | 40 | 6.81 | 1.04 | 34 |
| Speech prep | 6.74 | .78 | 36 | 6.12 | 1.16 | 41 | 6.46 | 1.12 | 36 |
| Post | 6.86 | .98 | 37 | 6.53 | 1.02 | 41 | 6.88 | 1.00 | 34 |
| Recovery | 6.97 | .92 | 36 | 6.65 | 1.04 | 41 | 6.97 | 1.08 | 34 |
Note. LA/PI: late adopted/post-institutionalized; EA/FC: early adopted/foster care; NA: non-adopted.
Figure 1.
Cortisol response trajectories using group-based trajectory modeling. Percentage of trajectory membership indicated. Shaded area indicates the TSST-C stressor task period.
Table 3.
ELS group and covariate predictors of membership relative to Trajectory 5 (N = 119)
| Predictor | Trajectory 1 |
Trajectory 2 |
Trajectory 3 |
Trajectory 4 |
||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. B | B | S.E. B | B | S.E. B | B | S.E. B | |
| EA/FC (vs. NA) | 2.76 * | 1.36 | 1.21 | .86 | 2.42 * | 1.07 | −.24 | 1.06 |
| LA/PI (vs. NA) | 1.33 | 1.32 | .26 | .76 | −.28 | 1.21 | −.87 | .93 |
| Child test age | −1.25 | .84 | −1.71 * | .70 | −1.95* | .79 | −1.82 * | .82 |
| Family income | .18 | .23 | .37 * | .18 | .27 | .21 | −.03 | .23 |
Note. LA/PI: late adopted/post-institutionalized; EA/FC: early adopted/foster care; NA: non-adopted.
p < .05.
With age and family income in the model and using Trajectory 5 as the comparison, we found no evidence for LA/PI and NA children to differ in their cortisol response patterns. In contrast, relative to Trajectory 5, EA/FC were more likely than NA children to be in Trajectories 1 (lowest) and 3 (low cortisol, modest response), with a similar but not significant bias for Trajectory 2. Because the lowest 3 and highest 2 trajectories appeared to differ in cortisol intercept, we examined the percentages of children who were assigned to these levels. Of the EA/FC children, 85% were on the lowest three trajectories, compared to 53% of the NA children, χ2(1) = 9.53, p < .001. Of the LA/PI children, 69% were on the lowest three trajectories, which did not differ from the NA children, χ2(1) = 2.3, ns. To assist the reader in visualizing these group differences, a chi-square table for the ELS and trajectory groups is presented in Table 4.
Table 4.
Latent group by ELS group (N = 124)
| ELS groups | Latent groups |
||||
|---|---|---|---|---|---|
| Trajectory 1 | Trajectory 2 | Trajectory 3 | Trajectory 4 | Trajectory 5 | |
| LA/PI | |||||
| Actual count | 5.0 | 21.0 | 3.0 | 6.0 | 7.0 |
| Expected count | 4.7 | 18.3 | 6.1 | 7.1 | 5.8 |
| EA/FC | |||||
| Actual count | 7.0 | 18.0 | 12.0 | 3.0 | 4.0 |
| Expected count | 5.0 | 19.2 | 6.4 | 7.5 | 6.0 |
| NA | |||||
| Actual count | 2.0 | 15.0 | 3.0 | 12.0 | 6.0 |
| Expected count | 4.3 | 16.5 | 5.5 | 6.4 | 5.2 |
Note. LA/PI: late adopted/post-institutionalized; EA/FC: early adopted/foster care; NA: non-adopted.
2.3. Differences in PEP/VT response patterns
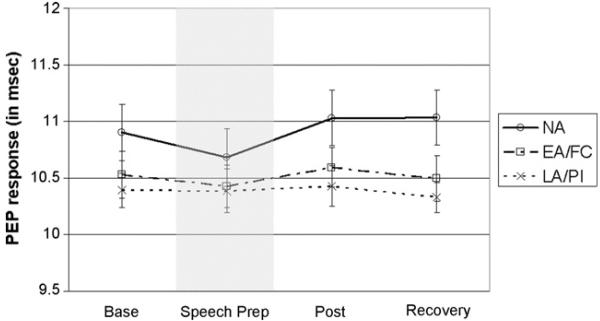
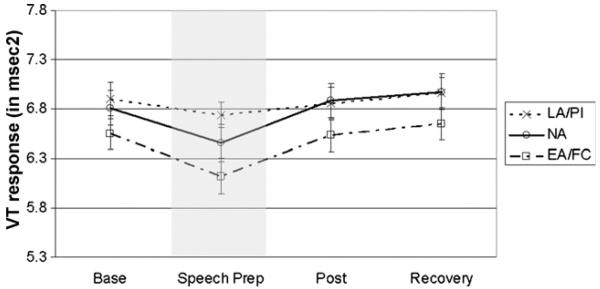
Group-based trajectory modeling for the PEP and VT data yielded evidence of three and four latent groups, respectively, that differed only in intercept and not in pattern of change (these analyses are available upon request). Therefore, we used growth curve modeling via LMM to analyze these data. In the PEP model (see Fig. 2), neither the linear nor quadratic terms were significant, indicating that no mean change in PEP occurred during the procedure (we retained the linear term because the associated random effect was significant). The effect of LA/PI on the intercept was negative and significant (see Table 5), indicating that the LA/PI but not the EA/FC children had lower overall PEP when compared to NA children. In the VT model (Fig. 3), the linear term was negative and significant and the quadratic term was positive and significant (see Table 5), but no significant ELS group effects were noted.
Figure 2.
PEP response (in ms) by ELS group. Bars reflect standard error of the mean (S.E.M.s). Shaded area indicates the TSST-C stressor task period.
Table 5.
Linear mixed model for PEP and VT (N = 124)
| Variable | B | S.E. B |
|---|---|---|
| Pre-ejection period | ||
| Intercept | 10.88 *** | .21 |
| EA/FC vs. NA | −.41+ | .24 |
| LA/PI vs. NA | −.55 * | .25 |
| Linear | .02 | .04 |
| Vagal tone | ||
| Intercept | 6.70 *** | .17 |
| EA/FC vs. NA | −.26 | .23 |
| LA/PI vs. NA | .22 | .24 |
| Linear | −.22 *** | .06 |
| EA/FC × linear | −.02 | .04 |
| LA/PI × linear | −.08+ | .04 |
| Quadratic | .11 *** | .02 |
Note. LA/PI: late adopted/post-institutionalized; EA/FC: early adopted/foster care; NA: non-adopted.
p < .10
p < .05
p < .001.
Figure 3.
VT response (in ms2) by ELS group. Bars reflect standard error of the mean (S.E.M.s). Shaded area indicates the TSST-C stressor task period.
2.4. Severe growth delay at adoption
Severe growth delay at adoption was defined as having height-for-age or weight-for-age at adoption of equal to or greater than 2 standard deviations below CDC norms. Of the LA/PI children, 54% of the 37 with adoption growth data were severely growth delayed, while 19% of the EA/FC children were this severely affected, χ2(1) = 10.53, p < .01. We computed the same kind of growth delay variable using the height and weight measures at testing and obtained no group differences; thus, at testing the children in the IA groups were no longer severely growth delayed. Among the LA/PI children, t-tests were conducted to determine if the severely growth delayed children differed from the other LA/PI children in age at adoption or duration of institutional care. No differences were found (t's < 1.0, ns). Using chi-square analyses, the severely growth delayed LA/PI children also were not more likely to come from Russia/Eastern Europe, Asia or Latin America χ2(2) = 1.8, ns, nor did they differ by sex χ2(1) = 0.8, ns. Only 16 of these children had birth weight information; however, for these children, birth weight for gestational age did not differentiate those who became severely growth delayed by the time of adoption from those who did not, t = −1.4, d.f. = 14, ns. Thus, these data suggest that severe growth delay at adoption was not due to our measures of duration of ELS or genetic or prenatal impacts on growth.
To examine whether individual differences in the severity of response to ELS predicted levels or patterns of cortisol response to the TSST-C, we used the group-based trajectory modeling results to examine the impact of growth delay on trajectory membership. We compared the NA children to the IA children (combined EA/FC and LA/PI groups) with and without severe growth delay at adoption (see Table 6). One-third of the IA children without severe growth delay were LA/PI children, while 29% of the IA children with severe growth delay at adoption were EA/FC children. Despite this, as shown in Table 6, the results followed the same pattern as the ELS group analysis. IA children without severe growth delay at adoption were more likely than the NA children to be in the lowest three trajectories relative to Trajectory 5 (with some differences only marginally significant), while growth delayed and NA children did not differ. Relative to the 53% of the NA children who were on the lowest three trajectories, among the IA children without severe growth delay these percentages were 83% for LA/PI and 88% for EA/FC children. Examining the IA children who were severely growth delayed at adoption, 60% of LA/PI and 63% of EA/FC were in these three low cortisol trajectories. Thus, sustaining relatively normal growth prior to adoption appeared to be as relevant as ELS group in predicting lower cortisol activity in the years following adoption.
Table 6.
Growth delay and covariate predictors of membership relative to Trajectory 5 (N = 112)
| Predictor | Trajectory 1 |
Trajectory 2 |
Trajectory 3 |
Trajectory 4 |
||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. B | B | S.E. B | B | S.E. B | B | S.E. B | |
| No growth delay (vs. NA) | 2.58+ | 1.34 | 1.54+ | .85 | 2.33 * | 1.06 | −.31 | 1.03 |
| Growth delay (vs. NA) | 1.39 | 1.38 | −.08 | .83 | −.88 | 2.14 | −.01 | .86 |
| Child test age | −.44 | .82 | −1.24* | .61 | −1.80* | .81 | −1.10 | .67 |
| Family income | .33 | .26 | .55 ** | .21 | .26 | .27 | .17 | .23 |
p < .07
p < .05
p < .01.
To examine whether growth delay at adoption influenced the group difference in PEP activity, we recomputed the LMM analysis, now contrasting NA children with IA children with and without severe adoption growth delay. Along with the significant effect of intercept reported above (B = 10.87, p < .001), the results revealed that children with severe growth delay at adoption had lower PEP (B = −.59, p < .01), while for those without severe adoption growth delay this effect was a trend (B = −.46, p < .10). Overall, however, the pattern for the growth delayed and non-growth delayed IA children was similar.
3. Discussion
The results provide little support for the argument that severe ELS permanently increases the reactivity of the HPA axis in children, although as will be discussed, this conclusion should be tempered by evidence that children of the age tested may not be highly reactive to the TSST-C in general. In contrast, the results do yield evidence that moderate ELS may lower activity of the HPA axis, and that children who manage to continue relatively normal growth in response to conditions of severe ELS may also emerge from their early experiences with a similarly down-regulated axis. Notably, results for the sympathetic nervous system revealed somewhat opposite effects of ELS, with severe ELS being associated with heightened sympathetic tone that was, if anything, more marked among children whose growth was severely delayed at the time of adoption.
Because the results need to be interpreted within the context of the non-adopted children's reactions to the TSST-C, we turn first to those data. The TSST-C has been used with children as young as 7 years and is argued to be the most effective laboratory stressor task if the goal is to activate the HPA axis (Dickerson and Kemeny, 2004). However, there is now evidence that many typically developing, low-risk peri-adolescent children are relatively cortisol hypo-responsive to a variety of laboratory stressor tasks, including the TSST-C. As in the present study, children in this age group report being stressed during the TSST-C and showed marked parasympathetic reactions; however, in two recent studies neither cortisol nor PEP reactions have been observed (Gunnar et al., in press; Stroud et al., in press). Our results were consistent with these findings. Of the NA children, 76% were assigned to three trajectories (Trajectories 1, 2 and 4) showing either no change or decreasing levels of cortisol in response to the stressor test. This was true despite evidence these children were at home-baseline cortisol concentrations when the TSST-C task was introduced. Consistent with both recent normative development studies, age was a significant predictor of responsiveness to this task. Specifically, being older at testing predicted membership in the only trajectory (Trajectory 5) that revealed a robust cortisol response. In previous work (Gunnar et al., in press; Stroud et al., in press), pubertal development has been shown to predict increased cortisol responses to this and another psychosocial stressor task. Notably, we found no group difference in pubertal status and few children in any of the groups who were past the mid-point in pubertal staging. Thus, despite the extant literature at the time we began this study (Kudielka et al., 2004), we may have chosen to assess the impact of severe ELS on hyper-stress responsivity at a developmental period of enhanced HPA hypo-responsiveness.
If it is true that this age period is characterized by relative cortisol hypo-responsiveness, then the present findings indicate that severe ELS did not break through this pattern. There was no evidence that the LA/PI children differed from NA children in trajectory membership. Even when we considered the LA/PI children who were extremely growth delayed at adoption and thus appeared to have either been exposed to or responded more severely to their prolonged period of adverse early care, we did not find that these children differed in cortisol levels or response from NA children. We did find evidence that these children had higher sympathetic tone than did NA children; however, we found a similar trend among the EA/FC children and, ignoring ELS group membership, among IA children who did not present with severe growth delay at adoption. None of our covariates predicted sympathetic, or for that matter parasympathetic, activity during the TSST-C. Thus, it might be that factors other than postnatal care experiences were associated with this higher sympathetic tone among internationally adopted children.
The present results are inconsistent with an earlier study of post-institutionalized Romanian children (Gunnar et al., 2001). In that study, post-institutionalized children exhibited higher overall levels than did children born and raised in their families of origin. There are a number of differences in these two studies, however, that make it difficult to directly compare the results. First, the earlier study involved cortisol assessments at wakeup, noon, and bedtime. No measures were obtained in the late afternoon. Second, the earlier study involved home basal cortisol levels, while that of the present study involved laboratory assessments. Third, because prenatal alcohol exposure impacts the development of the HPA axis (for review, see Zhang et al., 2005), we excluded children with frank facial features of fetal alcohol syndrome and those whose low IQ might indicate significant exposure. This was not done in the earlier study (Gunnar et al., 2001), which may be problematic because of the high expectation of maternal drinking for children placed in institutional care in Russia and Eastern Europe (Gunnar et al., 2000).
Of course, it is also possible that the effects of severe ELS were ameliorated by the care that the LA/PI children experienced in their adoptive families. Although the animal models argue for permanent impacts of ELS on stress reactivity and regulation, in fact the plasticity of stress-sensitive neurobiological systems has not received much empirical attention in the animal literature (Kaffman and Meaney, 2007). There is evidence that rats who have experienced lower levels of licking and grooming as infants will displayed physiological and behavioral reactions that are comparable to animals who experienced high levels of licking and grooming if, at weaning, they are exposed for several weeks to an enriched environment (Francis et al., 2002). There is also evidence that the hyper-fearfulness of isolation-reared juvenile monkeys can be reduced if they are exposed to monkeys who are too young to be aggressive and, instead, can act as therapists for the juvenile isolation-reared monkeys (Suomi and Harlow, 1972). Thus, it is possible that the window for shaping the reactivity of stress neurobiology extends well beyond the infancy period. If so, then this would have significant implications for intervention.
While it is hopeful to consider that the impact of severe ELS on stress neurobiology can be ameliorated with time in a supportive family, it is also possible that by assessing these children prior to the pubertal transition we have not observed the full impact of their early life experiences. Recent studies of children adopted from Romania have shown that while they did not differ in internalizing problems from comparison children prior to adolescence, with the pubertal transition the percentage of children with clinically significant emotional problems increased markedly (Colvert et al., 2008). Thus it may be important to qualify these findings by the developmental time point in which they were obtained.
In contrast to the LA/PI group, the EA/FC group did differ significantly from the NA children. They were more likely to be in the lower cortisol trajectories, reflecting a lower set-point, reduced responsiveness, or both. Notably, although we did not directly compare them (because the LA/PI and NA children did not differ), the EA/FC children also appeared to have lower cortisol activity than the LA/PI children. The present findings replicated previous findings associating patterns of physical growth at adoption and later cortisol levels (Kertes et al., 2008), although they suggest quite a different interpretation of those findings. The Kertes et al. (2008) study did not include a non-adopted comparison group. In that study we noted that IA children who exhibited greater growth delay at adoption had higher home-baseline cortisol levels an average of 6–7 years post-adoption. More adverse pre-adoption care conditions predicted growth delay and growth delay predicted cortisol levels. We interpreted these data as evidence that pre-adoption care that was severe enough to impair normal patterns of physical growth was also severe enough to produce an increase in the set point of the HPA axis. However, the results of the present study clearly argue that our interpretation was in error. Rather than predicting elevated cortisol levels, it seems that the capacity to maintain relatively normal growth despite ELS exposure predicts lower levels of cortisol activity.
The findings for the EA/FC or moderate ELS group are highly consistent with the rodent early handling work and work by Lyons and colleagues on repeated separation in infant squirrel monkeys (Parker et al., 2006). Furthermore, the fact that the impact of severe ELS appears to depend on the child's ability or inability to sustain relatively typical growth patterns is consistent with recent studies of healthy (and thus by definition resilient) adults with ELS histories (e.g., Elzinga et al., 2008). What is not clear is whether diminished HPA activity is a consequence or a cause of resilience. We can find evidence for both hypotheses in our data. EA/FC children were less impaired in growth at adoption and also showed diminished cortisol activity in response to the TSST-C. Because ending up in foster care with early adoption or an institution with later adoption was likely due in large part to where the children were born and not to any other personal characteristics, this suggests that assignment to moderate (EA/FC) ELS conditions caused a reduction in cortisol activity. Of course, we cannot determine whether it was the shorter duration or the nature of the care conditions (foster vs. institution) that predicted this outcome. On the other hand, among the LA/PI children, relatively normal physical growth prior to adoption was a strong predictor of low cortisol activity. While it is possible that LA/PI children who grew relatively normally were subjected to less adversity, we found no evidence of less adversity in terms of briefer exposure, as LA/PI children with and without severe growth failure were comparable in duration of institutionalization, age at adoption, and the region of the world from which they were adopted. This finding, then, suggests the importance of personal characteristics, among which we cannot rule out the possibility of an inherently less active HPA axis.
Others with data similar to that reported here have interpreted diminished cortisol activity for individuals who self-reported more ELS as evidence of impaired HPA axis functioning (Carpenter et al., 2007). That is, they interpreted their findings as evidence of hypo-cortisolism. We cannot rule out that interpretation. However, if the reduced HPA axis activity associated with the EA/FC group and the LA/PI children without growth delay does reflect impairment in the axis and thus heightened vulnerability to stressors, it will be important to determine why we observed this for moderate and not severe ELS, and for the severe ELS children with less growth impairment than for the ones with severe growth impairment. Indeed, the present data would argue for diminished cortisol levels as a reflection of resilience rather than vulnerability.
3.1. Limitations
There are a number of limitations to this study, most of which derive from not being able to randomly assign children to groups. Indeed, this is the challenge of translating the animal ELS studies into human development. Experiments of nature are not true experiments. Accordingly, even though we argue that country-of-origin rather than child factors account for group membership, this was only the case when considering one IA group versus the other. Such an argument does not address the many factors that vary uncontrollably between the adopted children and the non-adopted comparison children. Notably, it is very likely that prenatal conditions differed for the NA compared to the two internationally adopted groups. Indeed, for the children with birth weight data, birth weight was much higher in the NA group than in the EA/FC or LA/PI groups.
Second, while we can have a general idea of the conditions to which the children in each group were exposed prior to adoption, we lack specific information that would allow us to determine the unique conditions for each child. Certainly, foster families differ and some may have provided severely adverse care, while institutions differ and some may have provided relatively supportive care. Unfortunately, it is very difficult to obtain reliable information on pre-adoption care in the type of heterogeneous sample we studied. Prospective studies examining children in their care conditions prior to adoption and following them afterwards are required to address this issue.
Finally, in many ways it would have been preferable to have two groups, either foster care or institutional care, who differed in terms of early versus late adoption. With such a sample, moderate and severe ELS could have been defined by duration of exposure, holding type of care relatively constant. The problem, as noted earlier, is that most children adopted from countries that use foster care are released for adoption at younger ages than are children adopted from countries using institutional care. Foster care and institutional care differ in many ways beyond those that might be ranked on scales of stress severity. We cannot rule out the possibility these differences rather than stressor severity affected our results.
3.2. Implications
The implications of this study are largely hopeful. Despite the large body of animal literature indicating that early exposure to depriving, neglectful conditions produces a hyper-responsive stress and fear system that should increase the risk of affective psychopathology and drug addiction, our results yield no evidence of this for children who are removed from such condition in infancy and early childhood. They raise the possibility that the ELS hypothesis of risk for later psychopathology mediated by relatively permanent impacts on stress neurobiology should be revisited (Heim et al., 2004). If the stress system is more plastic for longer periods of time, then early intervention may have significant impacts on whether early stress exposure is carried forward into heightened risk for adult morbidity and mortality (Gunnar and Fisher, 2006). The present results also should lead us to revisit questions about early stress exposure and resilience. The animal literature provides strong support for the possibility that early life experiences that are highly protective and/or extremely low in stressor exposure may not yield the most stress resilient animal (e.g., Lyons and Parker, 2007). In the human literature, there is also evidence that being extremely protected may increase the likelihood that behavioral inhibition in infancy is carried forward to extreme shyness in preschool and older children (Rubin, 2002). Thus, it may be that children exposed to some degree of adversity early in life develop stress systems that are somewhat hardened against later stressor exposure. Because we have been so focused on the negative impacts of early stressor exposure, we know too little about when, whether, for whom, and for which systems ELS exposure might increase resilience. Nor do we have much understanding of whether there are costs to such resilience. Broadening our examination of the impact of early adversity to include these questions should provide a fuller understanding of the sequelae of early life stress.
Acknowledgements
The authors wish to thank the parents and children who took part in this study. We also owe our thanks to the staff of the Center for Neurobehavioral Development for their help in conducting this study.
Role of the funding source
This research was supported by the National Institute of Mental Health through a research grant, MH068857 and Senior Research Scientist Award, MH066208, to the first author and by grant M01-RR00400 from the National Institutes of Health to the University of Minnesota's General Clinical Research Center. The funding source had no influence, beyond the initial review and award of the grant, into the design, analysis, or decision to submit this manuscript.
Footnotes
Conflict of interest
None declared.
References
- Ahnert L, Gunnar MR, Lamb ME, Barthel M. Transition to child care: associations with infant–mother attachment, infant negative emotion, and cortisol elevations. Child Development. 2004;75:639–650. doi: 10.1111/j.1467-8624.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Development and Psychopathology. 2002;14:333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, JR., Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Chesney M, Alkon A, Tschann JM, Adams S, Chesterman B, et al. Psychobiologic reactivity to stress and childhood respiratory illnesses: results of two prospective studies. Psychosomatic Medicine. 1995;57:411–422. doi: 10.1097/00006842-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brumble K. Intercountry transracial special needs adoptees: today's teenagers and young adults. How have they fared? The parents' perspective. Vol. 68. 2007. p. 1404. Dissertation Abstracts International. [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W, et al. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- C.D.C. EPI Info Version 3.3 (Computer Software) 2004 [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001a;13:783–804. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001b;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth L. The differential Impacts of early abuse on internalizing problems and diurnal cortisol activity in school-aged children. doi: 10.1111/j.1467-8624.2009.01393.x. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AD, Parker RC. The relationship between salivary cortisol concentrations in forzen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Beckett C, Castle J, Groothues C, Hawkins A, et al. Emotional difficulties in early adolescence following severe early deprivation: findings from the English and Romanian Adoptees study. Development & Psychopathology. 2008;20:547–567. doi: 10.1017/S0954579408000278. [DOI] [PubMed] [Google Scholar]
- Critchley LAH. Impedance cardiography: the impact of new technology. Anaesthesia. 1998;53:677–684. doi: 10.1046/j.1365-2044.1998.437-az0550.x. [DOI] [PubMed] [Google Scholar]
- Croft C, O'Connor TG, Keavene L, Groothues C, Rutter M. Longitudinal change in parenting associated with developmental delay and catch-up. Journal of Child Psychology & Psychiatry. 2001;42:649–659. [PubMed] [Google Scholar]
- Davis M, Banks S, Fisher W, Grudzinskas A. Longitudinal patterns of offending during the transition to adulthood in youth from the mental health system. Journal of Behavior Health Services & Research. 2004;31:351–366. doi: 10.1007/BF02287689. [DOI] [PubMed] [Google Scholar]
- Dawson G, Ashman S. On the origins of a vulnerability to depression: the influence of early social environment on the development of psychobiological systems related to risk for affective disorder. In: Nelson CA, editor. The Effects of Adversity on Neurobehavioral Development. Minnesota Symposia on Child Psychology. Vol. 31. Erlbaum; New York: 2000. pp. 245–278. [Google Scholar]
- De Bellis M. The psychobiology of neglect. Child Maltreatment. 2005;10:150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- De Bellis M, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, et al. Developmental traumatology. Part 1. Biological stress systems. Biological Psychiatry. 1999;9:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Commentary: is maternal stimulation the mediator of the handling effects in infancy? Developmental Psychobiology. 1999;34:1–3. doi: 10.1002/(sici)1098-2302(199901)34:1<1::aid-dev2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: a study among healthy young subjects. Psychneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein M, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health: cumulative risk exposure and stress dysregulation. Psychological Science. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Felliti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. The relationship of adult health status to childhood abuse and household dysfunction. American Journal of Preventative Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fernald LC, Burke HM, Gunnar MR. Salivary cortisol levels in children of low-income women with high depressive symptoms. Development and Psychopathology. 2008;20:423–436. doi: 10.1017/S0954579408000205. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Wiley; New York: 2004. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. Journal of Neuroscience. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmezy N. Resilience in children's adaptation to negative life events and stressed environments. Pediatric Annals. 1991;20(459–460):463–466. doi: 10.3928/0090-4481-19910901-05. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: research and policy. Development and Psychopathology. 2000;12:677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher P. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology. 2006;18:651–677. [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM. Neuroendocrine measures in developmental research. In: Schmidt LA, Segalowitz SJ, editors. Developmental Psychophysiology: Theory, Systems, and Methods. Cambridge University Press; Cambridge: 2007. pp. 343–366. [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in HPA activity over the transition to adolescence: normative changes and associations with puberty. Development & Psychopathology. doi: 10.1017/S0954579409000054. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinological and Metabolic Clinics of North America. 2001;30:695–728. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry. 2004;55:376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hart J, Gunnar MR, Cicchetti D. Altered neuroendocrine activity in maltreated children related to symptoms of depression. Development and Psychopathology. 1996;8:201–214. [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary–adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001a;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall RW, Miller AH, Nemroff CB. Altered pituitary–adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001b;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport J, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depression and Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky P, Nemeroff CB. The importance of studying the contributions of early adverse experiences to the neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, Johnson DE. The international adoption project: population-based surveillance of Minnesota parents who adopted children internationally. Maternal and Child Health Journal. 2008;12:162–171. doi: 10.1007/s10995-007-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Cicchetti D, Rogosch FA. Salivary biomarker levels and diurnal variation: associations with medications prescribed to control children's problem behavior. Child Development. 2007;78:927–937. doi: 10.1111/j.1467-8624.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based upon mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29:3740393. [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. Journal of Child Psychology & Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Depressive disorders in maltreated children. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:257–265. doi: 10.1097/00004583-199103000-00014. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, et al. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biological Psychiatry. 1997;42:669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR, Madsen NJ, Long J. Early deprivation and home basal cortisol levels: a study of internationally-adopted children. Development & Psychopathology. 2008;20:473–491. doi: 10.1017/S0954579408000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreppner JM, Rutter M, Beckett C, Castle J, Colvert E, Groothues C, et al. Normality and impairment following profound early institutional deprivation: a longitudinal follow-up into early adolescence. Developmental Psychology. 2007;43:931–946. doi: 10.1037/0012-1649.43.4.93. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuoendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Landauer TK, Whiting JWM. Infantile stimulation and adult stature of human males. American Anthropologist. 1964;66:1007–1028. [Google Scholar]
- Lee MS, Yang JW, Ko YH, Han C, Kim SH, Lee MS. Effects of methylphenidate and bupropion on DHEA-S and cortisol plasma levels in attention-deficit hyperactivity disorder. Child Psychiatry and Human Development. 2007 Aug 31; doi: 10.1007/s10578-007-0081-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuoendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Levine S, Chevalier JA, Korchin SJ. The effects of early handling and shock on later avoidance behavior. Journal of Personality. 1956;24:475–493. doi: 10.1111/j.1467-6494.1956.tb01283.x. [DOI] [PubMed] [Google Scholar]
- Lupien S, King S, Meaney MJ, McEwen B. Child's stress hormone levels correlate with mother's socioeconomic status and depressive state. Biological Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. Journal of Traumatic Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- Maclean K. The impact of institutionalization on child development. Development and Psychopathology. 2003;15:853–884. doi: 10.1017/s0954579403000415. [DOI] [PubMed] [Google Scholar]
- Mareschal D, Tan SH. Flexible and content-dependent categorization by eighteen-month-olds. Child Development. 2007;78:19–37. doi: 10.1111/j.1467-8624.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mortimer JT, Staff J. Early work as a source of developmental discontinuity during the transition to adulthood. Development & Psychopathology. 2004;16:1047–1070. doi: 10.1017/s0954579404040131. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Group-based Modeling of Development. Harvard University Press; Cambridge, MA: 2005. [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proceedings of the National Academy of Sciences. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Research. Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Porges SW. U.S. Patent No. 4,510,944 Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. 1985
- Reich W, Cottler L, McCallum K, Corwin D, VanEerdewegh M. Computerized interviews as a method of assessing psychopathology in children. Comprehensive Psychiatry. 1995;36:4045. doi: 10.1016/0010-440x(95)90097-f. [DOI] [PubMed] [Google Scholar]
- Reich W, Welner Z. Revised Version of the Diagnostic Interview for Children and Adolescents (DICA-R) Department of Psychiatry, Washington University School of Medicine; St. Louis, MO.: 1988. [Google Scholar]
- Rubin KH. “Brokering” emotion dysregulation: the moderating role of parenting in the relation between child temperament and children's peer interactions. In: Zuckerman BS, Lieberman AF, Fox NA, editors. Emotional Regulation and Developmental Health: Infancy and Early Childhood. 2002. pp. 81–99. [Google Scholar]
- Rutter M. Resilience: Some conceptual considerations. Journal of Adolescent Health. 1993;14(626–631):690–696. doi: 10.1016/1054-139x(93)90196-v. [DOI] [PubMed] [Google Scholar]
- Rutter ML, Kreppner JM, O'Connor TG. Specificity and heterogeneity in children's responses to profound institutional privation. British Journal of Psychiatry. 2001;179:97–103. doi: 10.1192/bjp.179.2.97. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development & Psychopathology. 2001;13:419–450. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69(6):1503–1513. [PubMed] [Google Scholar]
- Smotherman WP, Bell RW. Maternal mediation of early experience. In: Bell RW, Smotherman WP, editors. Maternal Influences and Early Behavior. Spectrum Puublications; New York: 1980. pp. 201–210. [Google Scholar]
- Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annual Review of Clinical Psychology. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Stroud L, Foster E, Handwerger K, Papandonatos GD, Granger D, Kivlighan KT, et al. Stress response and the adolescent transition: performance versus peer rejection stress. Development & Psychopathology. doi: 10.1017/S0954579409000042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ, Harlow HF. Social rehabilitation of isolate-reared monkeys. Developmental Psychology. 1972;6:487–496. [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with chcildhood family stress. Bioilogical Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn M, Juffer F. Adoption is a successful natural intervention enhancing children's IQ and school performance. Current Directions in Phychological Science. 2005;14:326–330. [Google Scholar]
- van Londen WM, Juffer F, van Ijzendoorn MH. Attachment, cognitive, and motor development in adopted children: short-term outcomes after international adoption. Journal of Pediatric Psychology. 2007;32:1249–1258. doi: 10.1093/jpepsy/jsm062. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Smyke AT, Settles L. Children in orphanages. In: McCartney K, Phillips DA, editors. Handbook of Early Childhood Development. Blackwell Publishing; Malden, MA: 2006. pp. 224–254. [Google Scholar]
- Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Experimental Biology and Medicine. 2005;230:376–388. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]