Abstract
Glycogen storage disease type II (GSD-II; Pompe disease; MIM 232300) stems from the deficiency of acid-α-glucosidase (GAA; acid maltase; EC 3.2.1.20), which primarily involves cardiac and skeletal muscles. We hypothesized that systemic administration of an adeno-associated virus (AAV) vector containing a muscle specific regulatory cassette could drive efficacious transgene expression in GAA-knockout (GAA-KO) mice. AAV2/8 vectors containing the muscle creatine kinase (CK1) or hybrid α-myosin heavy chain enhancer-/muscle creatine kinase enhancer-promoter (MHCK7) cassettes were compared. The CK1 reduced glycogen content by approximately 50% in the heart and quadriceps, in comparison to untreated GAA-KO mice, whereas the MHCK7 containing vector reduced glycogen content even further: >95% in heart and >75% in the diaphragm and quadriceps. Administration of the MHCK7-containing vector significantly increased striated muscle function as assessed by increased Rotarod times at 18 weeks post-injection, whereas the CK1-containing vector did not increase Rotarod performance. Transduction efficiency was evaluated with an AAV2/8 vector in which MHCK7 drives alkaline-phosphatase, revealing that many more myofibers were transduced in the quadriceps than in the gastrocnemius. An AAV2/9 vector containing the MHCK7 cassette corrected GAA deficiency in the skeletal muscles of the distal limb, including the gastrocnemius, extensor digitalis longus, and soleus; furthermore, glycogen accumulations were substantially cleared by hGAA expression therein. Importantly, type IIb myofibers in the extensor digitalis longus were transduced, thereby correcting a myofiber type that is unresponsive to enzyme replacement therapy. In summary, AAV8 and AAV9-pseudotyped vectors containing the MHCK7 regulatory cassette achieved enhanced efficacy in Pompe disease mice.
Keywords: Glycogen storage disease type II, adeno-associated virus, acid alpha-glucosidase, acid maltase, Pompe disease
INTRODUCTION
Glycogen storage disease type II (GSD-II; Pompe disease; MIM 232300) is a classical lysosomal storage disease that causes death in infancy from cardiomyopathy and cardiorespiratory failure. The single gene deficiency in acid α-glucosidase (GAA; acid maltase; EC 3.2.1.20) results in lysosomal accumulation of glycogen in various tissues, primarily in heart and skeletal muscle. Pompe disease could be effectively treated by the correction of GAA deficiency in striated muscle. GAA expression with pseudotyped AAV vectors 1-5 or with a helper-dependent adenovirus vector has achieved prolonged efficacy 6 in Pompe disease mice. None of the aforementioned vectors completely corrected the glycogen content of all skeletal muscles, and none of these studies evaluated the distal hindlimb muscles.
The clinical presentation of Pompe disease resembles that of the muscular dystrophies, featuring weakness of the proximal leg muscles that generalizes to all skeletal muscles.7 Gene therapy in the muscular dystrophies represents a unique challenge, because humoral and cytotoxic immune responses occur frequently in response to introduced proteins.8,9 Cytotoxic T lymphocyte responses against gene therapy vectors have been reduced by substituting muscle-specific regulatory cassettes for ubiquitously active viral promoter/enhancers. An AAV2 vector containing a muscle-specific creatine kinase (MCK) regulatory cassette evoked an attenuated immune response in mdx mice, in comparison to an analogous AAV vector containing the CMV promoter.10 An AAV2/6 vector containing the MCK CK6 cassette11 transduced skeletal muscle with lower efficiency, in comparison to an analogous vector containing a CMV promoter/enhancer; however, the MCK-driven β-galactosidase expression persisted longer than CMV-driven expression.12 An AAV2/6 vector containing another MCK regulatory cassette (CK113,14) produced high-level human GAA expression and glycogen clearance in the injected gastrocnemius muscle;2 and the analogous AAV2/7 vector partially cleared glycogen storage in multiple muscle groups of GAA-KO mice following intravenous administration.2
AAV2/8 vectors have efficiently transduced striated muscle following systemic delivery in mice. 15 As few as 3×1011 vector particles transduced the majority of cardiomyocytes.15,16 More relevant to muscular dystrophy, an AAV2/8 vector encoding δ-sarcoglycan corrected the leakage of creatine kinase from striated muscle in the TO-2 hamster model for limb-girdle muscular dystrophy;16 and an AAV2/1 vector encoding GAA reduced glycogen storage following intravenous administration to neonatal GAA-KO mice.4 Thus, current data from Pompe and muscular dystrophy mice endorses the further investigation of AAV pseudotypes with enhanced muscle tropism in these models.2,4,17,18
Salva et al designed a series of highly active muscle specific regulatory cassettes that were evaluated in striated muscle Following systemic delivery via tail vein injections of AAV6 vectors.14 The most active cassette in a variety of anatomical muscles combined a 190-bp enhancer from the murine alpha-myosin heavy chain gene with a 570 bp abbreviated MCK regulatory cassette, termed the MHCK7. The latter expressed human placental alkaline phosphatase at very high levels in murine heart and skeletal muscle, exceeding levels achieved with the CMV promoter/enhancer in the heart, and expressed high levels of microdystrophin in skeletal and cardiac muscles of mdx mice.14
We hypothesized that systemic administration of an AAV vector containing a muscle-specific regulatory cassette could achieve long-term correction of multiple muscles in GAA-KO mice. An AAV vector containing the MHCK7 cassette was pseudotyped with AAV serotypes 7, 8, and 9, and the efficacy of each serotype was evaluated in GAA-KO mice.
RESULTS
The transduction of striated muscle with AAV2/8 vectors containing either an abbreviated MCK regulatory cassette (AAV-CK1hGAApA 2) or a hybrid α-myosin heavy chain enhancer/MCK cassette (AAV-MHCK7hGAApA) were evaluated, following intravenous administration in adult GAA-KO mice (1×1011 or 1×1012 vector particles (vp)/mouse). The former vector contains CK1 promoter13 and the latter contains MHCK7.14 GAA activity and glycogen content in the heart, liver, and skeletal muscles were analyzed 18 weeks following vector administration (Fig. 1). GAA activity and glycogen content were significantly affected by both dosage and vector type for all tissues examined (p<0.05 with a 2-way ANOVA). Bonferroni post-test comparisons of the two vectors showed GAA levels were significantly greater in all tissues treated with the higher dose of the MHCK7-containing vector compared with the equivalent dose of the CK1-containing vector (Fig 1A). At the low dose the difference between the two vectors was not significant for any of the tissues, although the mean GAA levels were greater in each tissue for the MHCK7-vector group. The lower GAA activity in the liver following AAV-CK1hGAApA administration suggested a more stringent muscle-restricted expression with the CK1 cassette relative to the MHCK7 cassette (Fig. 1A).14 Glycogen was significantly lower in all muscles treated with the MHCK7-containing vector at both low and high doses, in comparison to the equivalent dose of the CK1-containing vector (Fig 1B). An exception was the glycogen level in the gastrocnemius, which was significantly lower in the MHCK7-containing vector treated group only at the lower dose, in comparison with the CK1-containing vector treated group; however, this result demonstrated that a low number of MHCK7-containing vector particles achieved maximal efficacy in the gastrocnemius for these two vectors.
Fig. 1. Transduction of striated muscle in adult GAA-KO mice with intravenously administered AAV2/8 vectors containing the CK1 or MHCK7 regulatory cassettes.
(A) GAA activity in the indicated striated muscles, 18 weeks following vector administration. AAV-CK1hGAApA (CK1), either 1×1011 (1E+11; n=3) or 1×1012 (1E+12; n=3) vp/mouse, and AAV-MHCK7hGAApA (MHCK7), either 1×1011 (1E+11; n=4) or 1×1012 (1E+12; n=5) vp/mouse were injected intravenously at 3 months of age. Mock-treated GAA-KO mice were negative controls (PBS; n=4). (B) Glycogen content for GAA-KO mice in A. (C) Vector genome quantitation with realtime PCR for GAA-KO mice in A. (D) Ratio of GAA activity to vector genome quantity for GAA-KO mice in A. Mean +/- s.d. shown. P<0.05 (*) and P<0.001(**) indicated for the values for groups of mice following AAV-MHCK7hGAApA injection, in comparison to the group receiving the equivalent number of AAV-CK1hGAApA vector particles.
Vector genomes were quantified to allow a comparison of the transduction efficiency following administration of each of the AAV2/8 vectors (Fig. 1C). Long-term hGAA expression in heart and skeletal muscle was associated with persistence of vector DNA. Quantification of vector DNA confirmed the presence of 0.3 to 1.1 vector genomes (vg) per nuclear genome in the heart, gastrocnemius, and quadriceps, whereas up to 100 vg per nuclear genome were present in the liver (Fig. 1C). Vector DNA was significantly higher in the liver following AAV-CK1hGAApA administration (p < 0.05), whereas it was significantly higher in the heart and diaphragm following AAV-MHCK7hGAApA administration (p < 0.05). Vector DNA was also more elevated in the gastrocnemius from the AAV-MHCK7hGAApA treated group, but the difference was not significant (p = 0.06). The normalized expression of GAA activity from the MHCK7 cassette was significantly increased (p < 0.05) for all tissues, except quadriceps, when compared with normalized expression from the CK1 cassette (Fig. 1D).
Western blot analysis revealed the ∼76 kD processed form of GAA in the heart, quadriceps, gastrocnemius and liver of GAA-KO mice following administration of the higher number of particles of both vectors, whereas a very low signal was detected in the diaphragm following transduction with AAV-CK1hGAApA (Fig. 2, Liver GAA not shown). GAA activity and glycogen content were not significantly changed in diaphragm following administration of AAV-CK1hGAApA (Fig. 1), consistent with the low activity for the CK1 cassette in the diaphragm.12,14
Fig. 2. Western blot detection of hGAA in striated muscle 18 weeks following intravenous administration of AAV2/8 vectors in GAA-KO mice.
Lanes labeled as follows: P, control mouse injected with PBS; C, AAV-CK1hGAApA (1×1012 vp); and M, AAV-MHCK7hGAApA (1×1012 vp).
Glycogen staining revealed the basis for incomplete correction of skeletal gastrocnemius with AAV-MHCK7hGAApA (Fig. 1B). Multiple glycogen laden myofibers were detected in the gastrocnemius, consistent with the lack of correction of GAA deficiency within individual myofibers (Fig. 3A). The quadriceps contained a higher number of normal-appearing myofibers (Fig. 3A, arrows), consistent with the lower glycogen content in the quadriceps (Fig. 1B). Thus, the relatively higher residual glycogen in the gastrocnemius could be attributed to the greater prevalence of untranduced myofibers. Lymphocytic infiltrates were absent in skeletal muscle, indicating a lack of cytotoxic T cell responses in transduced muscle (not shown). To evaluate the distribution of functionally transduced myofibers, mice were injected with 1×1012 vp of an AAV2/8 vector carrying a human placental alkaline phosphatase reporter cDNA driven by the MHCK7 regulatory cassette. The highest level of functional transduction was detected in the heart, followed by the quadriceps, whereas fewer transduced myofibers were present in the gastrocnemius (Fig. 3B). The soleus and extensor digitalis longus were transduced at even lower frequency than the gastrocnemius (<3 positive myofibers/field; not shown). The low transduction of the soleus and extensor digitalis longus demonstrated a clear limitation for the AAV2/8 pseudotype.
Fig. 3. Transduction of striated muscle with an AAV2/8 vector.
(A) Periodic acid/Schiff staining following intravenous AAV-MHCK7hGAApA vector administration to GAA-KO mice. Control mice were sham-treated, age-matched GAA-KO mice (PBS). Original magnification: 200. (B) Alkaline phosphatase staining following intravenous AAV-αMHCKChAP administration to GAA-KO mice. Sham-treated GAA-KO mice stained negatively for alkaline phosphatase in striated muscle (not shown; see ref. 14, Figs 3 and 5). Original magnification: 400.
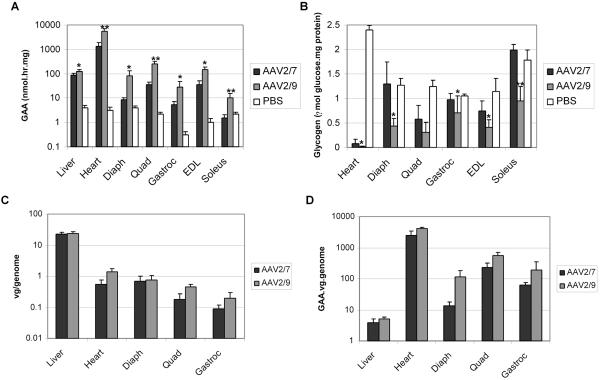
The presence of residual glycogen accumulation in hindlimb muscles following AAV2/8 vector administration prompted pseudotyping of AAV-MHCK7hGAApA as AAV2/7 and AAV2/9. The GAA activities of the heart and skeletal muscles were significantly increased following AAV2/9 vector administration (p < 0.05), in comparison to AAV2/7 (Fig. 4A). The glycogen contents of the diaphragm, extensor digitalis longus (EDL) and soleus were significantly decreased following AAV2/9 vector administration, in comparison to AAV2/7 (Fig. 4B) (p < 0.05). The enhanced correction with the AAV2/9 vector correlated with slightly increased numbers of vector genomes in the heart, quadriceps, and gastrocnemius (Fig. 4C); moreover, normalized GAA expression was also signifcantly increased in striated muscle and heart for the AAV2/9 vector (Fig. 4D) (p < 0.05). Taken together, these data indicated that AAV2/9 transduced striated muscle more efficiently and produced higher GAA activity in transduced myofibers, in comparison to the AAV2/7 vector.
Fig. 4. Transduction of striated muscle in adult GAA-KO mice with intravenously administered AAV2/7 or AAV2/9 vector containing the MHCK7 regulatory cassette.
(A) GAA activity in the indicated striated muscles, 18 weeks following administration of AAV-MHCK7hGAApA (5×1011 vp) pseudotyped as AAV2/7 (n=5) and AAV2/9 (n=5). Mock-treated GAA-KO mice were negative controls (PBS; n=4). (B) Glycogen content for GAA-KO mice in A. (C) Vector genome quantitation with realtime PCR for GAA-KO mice in A. (D) Ratio of GAA activity to vector genome quantity for GAA-KO mice in A. Mean +/- s.d. shown. P<0.05 (*) and P<0.001 (**) indicated for the values for each group of mice following AAV2/9 injection, in comparison to the group receiving the AAV2/7 vector.
Antibodies against hGAA have prevented efficient cross-correction of GAA deficiency through receptor-mediated uptake, despite the presence of secreted hGAA in the blood.2,3,19 Anti-hGAA antibodies were significantly elevated by 6 weeks following both low and high doses of AAV2/8 vector (p<0.05), as compared with PBS-treated controls, regardless of the cassette type (Fig. 5A). Despite the presence of anti-GAA antibodies, Rotarod times were significantly increased (p < 0.05) at 12 and 18 weeks following administration of AAV-MHCK7hGAApA-injected mice for the AAV2/7, AAV2/8 and AAV2/9 serotypes (Fig. 5B). However, Rotarod times were not significantly increased at 12 or 18 weeks for AAV-CK1hGAApA treated mice. A glucotetrasaccharide biomarker for increased glycogen storage, Glcα1-6Glcα1-4Glcα1-4Glc, (Glc4) was significantly reduced (p < 0.05) at 18 weeks for the high-dose group of all vector-injected GAA-KO mice, regardless of the regulatory cassette or serotype in comparison to PBS-injected mice (Fig 5C). There was no difference in mean Glc4 levels between the different vector-treated groups. Hence, a significant increase in Rotarod time and reduction of Glc4 biomarker were achieved, despite the presence of an antibody response against hGAA.
Fig. 5. Humoral response, biomarker reduction, and Rotarod testing following systemic AAV2/8 vector administration in GAA-KO mice.
Testing done at the indicated times following AAV vector administration. Mean and standard deviation shown. (A) ELISA of GAA-KO mouse plasma (1:200 dilution) following AAV vector administration. The absorbance for anti-hGAA antibodies at different times following AAV vector administration are shown. The number of mice was as follows: control (PBS, n=4); AAV-CK1hGAApA (CK1), low dose (1×1011, n=3), high dose (1×1012, n=3); and AAV-MHCK7hGAApA (MHCK7), low dose (1×1011, n=4), high dose (1×1012, n=5). (B) Rotarod testing of GAA-KO mice in A, and additional groups following administration of AAV-MHCK7hGAApA (5×1011 vp) pseudotyped as AAV2/7 (n=5) and AAV2/9 (n=5). (C) Urinary Glc4 concentrations for the groups of GAA-KO mice in B, 18 weeks following vector administration, and for age-matched, untreated GAA-KO controls (n=4).
Wide-spread clearance of glycogen in individual myofibers was demonstrated following AAV2/7 and AAV2/9 vector administration (Fig. 6A). Fiber typing confirmed the high prevalence of type I, slow-twitch fibers in the soleus of GAA-KO mice; whereas the EDL was comprised mainly of type IIb myofibers (Fig. 6B). The correction of multiple myofibers in the EDL and soleus indicated that both type I and IIb myofibers were transduced by the AAV2/7 and AAV2/9 vectors.
Fig. 6. Transduction of striated muscle with AAV2/7 and AAV2/9 vectors.
(A) Periodic acid/Schiff staining following intravenous administration of AAV-MHCK7hGAApA, pseudotyped as AAV2/7 or AAV2/9, to GAA-KO mice. Controls were sham-treated, age-matched GAA-KO mice (PBS). (B) Fiber-typing: co-staining of GAA-KO mouse myofibers with Rho-MHC I and FITC-MHC IIb. Original magnification: 200.
DISCUSSION
Gene therapy in the muscular dystrophies will likely require therapeutic gene expression in striated muscle generally, especially for lethal muscular dystrophies such as Duchenne muscular dystrophy. Pompe disease uniquely responds to infused or secreted therapeutic protein, because unlike other muscular dystrophies Pompe disease is a lysosomal storage disorder amenable to enzyme replacement therapy. Due to the high enzyme level requirements in enzyme replacement therapy and complicating antibody responses to GAA, muscle-targeted gene therapy is under development in Pompe disease.2,20,21 Systemic delivery of an AAV2/8 vector encoding muscle-restricted hGAA achieved significant efficacy; however, the transduction of myofibers was partial, preventing the complete correction of glycogen storage in the distal muscles of the hindlimb. The analogous AAV2/9 vector demonstrated increased transduction of the heart and small hindlimb muscles, in comparison to either the AAV2/8 or AAV2/7 vectors.
Type I fibers were more easily cleared of glycogen during enzyme replacement therapy, indicating the need to focus upon the transduction of Type II fibers in Pompe disease.22,23 Type I myofibers were transduced less efficiently with an AAV2 vector than with an AAV2/6 vector, reflecting the potential advantage of newer serotypes over AAV2.24,25 Currently, the AAV2/9 serotype efficiently transduced both type I and IIb myofibers, as well as cardiomyocytes, following intravenous administration. This study further endorses the role of AAV2/9 vectors for gene therapy in Pompe disease, demonstrating significant correction of not only the heart but all skeletal muscles examined. Previously AAV2/9 vectors have transduced striated muscle more efficiently than either an AAV2/1 vector in neonatal mice26 or an AAV2/8 vector in adult mice.27 Neither of these earlier studies demonstrated significant correction of multiple skeletal muscles in adult mice26,27. The improved efficacy of this new AAV2/9 vector further strengthens preclinical data in favor of clinical trials of gene therapy in Pompe disease.
The successful development of gene therapy in GAA-KO mice indicates that curative therapy for Pompe disease may become available in the foreseeable future; however, the efficacy of gene therapy in these experiments was inversely related to the presence of immune responses. Immunocompetent GAA-KO mice produced high titer anti-hGAA IgG in response to an AAV vector containing the ubiquitously active CMV promoter/chicken β-actin (CB) promoter, packaged as either AAV2/6 or AAV2/8.2,3 The CB-containing vector failed to secrete detectable hGAA in the plasma for >2 weeks or to reduce glycogen storage in the muscle of GAA-KO mice.3 Immune responses to ubiquitously expressed hGAA included lymphocytic infiltrates and activation of CD4+ and CD8+ lymphocytes in injected skeletal muscle, and in the liver following intravenous injection.2,2,3 Substitution of the CK1 regulatory cassette in place of the CB promoter prevented CD8+ lymphocytic responses in the injected muscle. Therefore, both antibody production and CTL were elicited in response to hGAA production from a ubiquitously active CB promoter; however, vectors containing muscle specific cassettes did not provoke lymphocytic infiltrates in transduced muscles and expressed hGAA for greater than 18 weeks.2 The latter experiment also demonstrated the imperviousness of muscle-specific hGAA expression to circulating anti-GAA antibodies. The presence of anti-GAA antibodies has prevented cross-correction of untransduced muscle cells2,3,19, implicating the transduction of individual muscle cells as the source of glycogen clearance in the current study.
The MHCK7 regulatory cassette has driven highly efficacious hGAA expression in the Pompe disease mouse model in this study. A comparison of AAV2/6 vectors containing either the MHCK7 or the CK1 cassette demonstrated high transgene expression within cardiac muscle and skeletal muscles14, which has now been replicated in Pompe disease mice for AAV2/7, AAV2/8, and AAV2/9 vectors. Virtually all myofibers were transduced in the muscles examined with the aforementioned AAV2/6 vector, although transgene expression was reduced in the diaphragm; moreover, transduction was enhanced by the co-administration of the vascular permeabilizing agent VEGF.14,28 The current vector-regulatory cassette combinations with GAA achieved remarkable clearance of glycogen from the diaphragm in Pompe mice without the use of VEGF, increasing the likelihood of translation to clinical applications in Pompe disease. The activity of the MCHK7 regulatory cassette was higher than that for the CK1 cassette in heart, quadriceps, gastrocnemius, and diaphragm, all critical targets for gene therapy in Pompe disease and other forms of muscular dystrophy. The combination of highly active muscle-specific regulatory cassettes and novel AAV serotypes promises to advance gene therapy for muscular dystrophy by providing curative therapy for these devastating disorders.
METHODS
Preparation of AAV 2/8 vector
AAV-MHCK7hGAApA contains the MHCK7 regulatory cassette14, the human GAA cDNA, and a human growth hormone polyadenylation sequence. The vector plasmid, pAAV-MHCK7hGAApA, was derived from pAAV-CBhGAApA.29 The pAAV-MHCK7hGAApA was digested with KpnI, blunt-ended with the Klenow fragment of DNA polymerase, then digested with XbaI; subsequently, the 5.7 kb blunt/XbaI fragment from pAAV-MHCK7hGAApA was ligated with a 0.8 kb XbaI/blunt Sal I fragment containing MHCK7 cassette from αMHCKChAP14 (provided by Dr. Stephen Hauschka, University of Washington, Seattle, WA). AAV-CK1hGAApA has been described (formerly AAV-MCKhGAApA).2 Briefly, 293 cells were transfected with an AAV vector, the AAV packaging plasmid30 (courtesy of Dr. James M. Wilson, University of Pennsylvania, Philadelphia, PA), and pAdHelper (Stratagene, La Jolla, CA). Cell lysate was harvested 48 hours following infection and freeze-thawed three times, and isolated by sucrose cushion pelleting followed by 2 cesium chloride gradient centrifugation steps. AAV stocks were dialyzed against three changes of Hanks buffer, and aliquots were stored at -80°C. The number of vector DNA containing-particles in viral stocks was determined by DNase I digestion, DNA extraction, and Southern blot analysis. The Southern blot signal for vector genomes was quantified by comparison to the signals from standards consisting of AhdI-digested vector plasmid. All viral vector stocks were handled according to Biohazard Safety Level 2 guidelines published by the NIH.
In vivo analysis of AAV vector
The AAV type 8 pseudotyped (AAV2/8) vector stocks were administered intravenously (via the retroorbital sinus) in 3 month-old GAA-KO mice.31 At the indicated time points post-injection, plasma or tissue samples were obtained and processed as described below. All animal procedures were done in accordance with Duke University Institutional Animal Care and Use Committee-approved guidelines.
Rotarod testing was performed as described 1. GAA activity and glycogen content were analyzed as described.32 Western blotting of hGAA was performed as described 1 using the hGAA monoclonal antibody (courtesy of Genzyme Corp., Framingham, MA). Alkaline phosphatase staining was performed as described.14 The ELISA was performed as described.3 All samples yielded absorbance values that were within the linear range of the assay at this dilution. Urinary Glc4 concentrations were determined relative to creatinine by stable isotope-dilution electrospray tandem mass spectrometry as previously described.33
Statistical analyses
2-way analyses of variance (ANOVA) were performed using the independent variables of vector type and dose of vector, with Bonferroni post-tests for specific comparison between groups. (Prism 3.0, San Diego, CA). Comparison of two groups were assessed by a homoscedastic Student T-test. A P value of <0.05 indicated a significant difference between the observed values for each group.
ACKNOWLEDGEMENTS
This work was supported by NIH Grant R01 HL081122-01A1 from the National Heart, Lung, and Blood Institute. DDK was supported by the Muscular Dystrophy Association and Genzyme Corporation. BS was supported by a Development Grant from the Muscular Dystrophy Association. Development and evaluation of the MCK regulatory cassettes was supported by NIH grants RO1 AR18860, 1 U54 HD047175, and R24 HL64387, and the Muscular Dystrophy Association to SDH. GAA-KO mice were provided courtesy of Dr. Nina Raben at the National Institutes of Health (Bethesda, MD). The AAV8 packaging plasmid, p5E18-VD 2/8, was provided courtesy of Dr. James M. Wilson at the University of Pennsylvania (Philadelphia, PA).
REFERENCES
- 1.Sun B, Zhang H, Franco LM, Young SP, Schneider A, Bird A, et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther. 2005;11:57–65. doi: 10.1016/j.ymthe.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Sun B, Zhang H, Franco LM, Brown T, Bird A, Schneider A, et al. Correction of glycogen storage disease type II by an adeno-associated virus vector containing a muscle-specific promoter. Mol Ther. 2005;11:889–898. doi: 10.1016/j.ymthe.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Franco LM, Sun B, Yang X, Bird A, Zhang H, Schneider A, et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther. 2005;12:876–884. doi: 10.1016/j.ymthe.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Mah C, Cresawn KO, Fraites TJ, Jr., Pacak CA, Lewis MA, Zolotukhin I, et al. Sustained correction of glycogen storage disease type II using adeno-associated virus serotype 1 vectors. Gene Ther. 2005;12:1405–1409. doi: 10.1038/sj.gt.3302550. [DOI] [PubMed] [Google Scholar]
- 5.Cresawn KO, Fraites TJ, Wasserfall C, Atkinson M, Lewis M, Porvasnik S, et al. Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid alpha-glucosidase in a model of glycogen storage disease type II. Hum Gene Ther. 2005;16:68–80. doi: 10.1089/hum.2005.16.68. [DOI] [PubMed] [Google Scholar]
- 6.Kiang A, Hartman ZC, Liao SX, Xu F, Serra D, Palmer DJ, et al. Fully deleted adenovirus persistently expressing GAA accomplishes long-term skeletal muscle glycogen correction in tolerant and nontolerant GSD-II mice. Mol Ther. 2006;13:127–134. doi: 10.1016/j.ymthe.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Hirschhorn R, Reuser AJJ. Glycogen Storage Disease Type II: Acid α-Glucosidase (Acid Maltase) Deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis for Inherited Disease. 8th ed. McGraw-Hill; New York: 2001. [Google Scholar]
- 8.Ferrer A, Wells KE, Wells DJ. Immune responses to dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Gene Therapy. 2000;7:1439–1446. doi: 10.1038/sj.gt.3301259. [DOI] [PubMed] [Google Scholar]
- 9.Cordier L, Gao GP, Hack AA, McNally EM, Wilson JM, Chirmule N, et al. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum Gene Ther. 2001;12:205–215. doi: 10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- 10.Yuasa K, Sakamoto M, Miyagoe-Suzuki Y, Tanouchi A, Yamamoto H, Li J, et al. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Ther. 2002;9:1576–1588. doi: 10.1038/sj.gt.3301829. [DOI] [PubMed] [Google Scholar]
- 11.Hauser MA, Robinson A, Hartigan-O’Connor D, Williams-Gregory D, Buskin JN, Apone S, et al. Analysis of muscle creatine kinase regulatory elements in recombinant adenoviral vectors. Mol Ther. 2000;2:16–25. doi: 10.1006/mthe.2000.0089. [DOI] [PubMed] [Google Scholar]
- 12.Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shield MA, Haugen HS, Clegg CH, Hauschka SD. E-box sites and a proximal regulatory region of the muscle creatine kinase gene differentially regulate expression in diverse skeletal muscles and cardiac muscle of transgenic mice. Mol Cell Biol. 1996;16:5058–5068. doi: 10.1128/mcb.16.9.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salva MZ, Himeda CL, Tai PW, Nishiuchi E, Gregorevic P, Allen JM, et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol Ther. 2007;15:320–329. doi: 10.1038/sj.mt.6300027. [DOI] [PubMed] [Google Scholar]
- 15.Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y, Kay MA. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005;79:214–224. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Zhu T, Qiao CP, Zhou LQ, Wang B, Zhang J, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 17.Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bostick B, Ghosh A, Yue Y, Long C, Duan D. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther. 2007;14:1605–1609. doi: 10.1038/sj.gt.3303029. [DOI] [PubMed] [Google Scholar]
- 19.Ding EY, Hodges BL, Hu H, McVie-Wylie AJ, Serra D, Migone FK, et al. Long-term efficacy after [E1-, polymerase-] adenovirus-mediated transfer of human acid-alpha-glucosidase gene into glycogen storage disease type II knockout mice. Hum Gene Ther. 2001;12:955–965. doi: 10.1089/104303401750195917. [DOI] [PubMed] [Google Scholar]
- 20.Raben N, Plotz P, Byrne BJ. Acid alpha-glucosidase deficiency (glycogenosis type II, Pompe disease) Curr Mol Med. 2002;2:145–166. doi: 10.2174/1566524024605789. [DOI] [PubMed] [Google Scholar]
- 21.Fraites TJ, Jr., Schleissing MR, Shanely RA, Walter GA, Cloutier DA, Zolotukhin I, et al. Correction of the enzymatic and functional deficits in a model of Pompe disease using adeno-associated virus vectors. Mol Ther. 2002;5:571–578. doi: 10.1006/mthe.2002.0580. [DOI] [PubMed] [Google Scholar]
- 22.Raben N, Danon M, Gilbert AL, Dwivedi S, Collins B, Thurberg BL, et al. Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab. 2003;80:159–169. doi: 10.1016/j.ymgme.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda T, Ewan L, Bauer M, Mattaliano RJ, Zaal K, Ralston E, et al. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann Neurol. 2006;59:700–708. doi: 10.1002/ana.20807. [DOI] [PubMed] [Google Scholar]
- 24.Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Pruchnic R, Cao B, Peterson ZQ, Xiao X, Li J, Samulski RJ, et al. The use of adeno-associated virus to circumvent the maturation-dependent viral transduction of muscle fibers. Hum Gene Ther. 2000;11:521–536. doi: 10.1089/10430340050015716. [DOI] [PubMed] [Google Scholar]
- 26.Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- 27.Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun B, Chen YT, Bird A, Xu F, Hou YX, Amalfitano A, et al. Packaging of an AAV vector encoding human acid alpha-glucosidase for gene therapy in glycogen storage disease type II with a modified hybrid adenovirus-AAV vector. Mol Ther. 2003;7:467–477. doi: 10.1016/s1525-0016(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 30.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raben N, Nagaraju K, Lee E, Kessler P, Byrne B, Lee L, et al. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem. 1998;273:19086–19092. doi: 10.1074/jbc.273.30.19086. [DOI] [PubMed] [Google Scholar]
- 32.Amalfitano A, McVie-Wylie AJ, Hu H, Dawson TL, Raben N, Plotz P, et al. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc Natl Acad Sci U S A. 1999;96:8861–8866. doi: 10.1073/pnas.96.16.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young SP, Stevens RD, An Y, Chen YT, Millington DS. Analysis of a glucose tetrasaccharide elevated in Pompe disease by stable isotope dilution-electrospray ionization tandem mass spectrometry. Anal Biochem. 2003;316:175–180. doi: 10.1016/s0003-2697(03)00056-3. [DOI] [PubMed] [Google Scholar]