Abstract
Early embryos of some metazoans polarize radially to facilitate critical patterning events such as gastrulation and asymmetric cell division; however, little is known about how radial polarity is established. C. elegans early embryos polarize radially when cell contacts restrict the polarity protein PAR-6 to contact-free cell surfaces, where PAR-6 regulates gastrulation movements. Here, we identify a Rho GTPase activating protein (RhoGAP), PAC-1, which mediates C. elegans radial polarity and gastrulation by excluding PAR-6 from contacted cell surfaces. We show that PAC-1 is recruited to cell contacts, and we suggest that PAC-1 controls radial polarity by restricting active CDC-42 to contact-free surfaces, where CDC-42 binds and recruits PAR-6. Thus, PAC-1 provides a dynamic molecular link between cell contacts and PAR proteins that polarizes embryos radially.
Early embryos can polarize radially when cell contacts differentiate the contacted (inner) and contact-free (outer) surfaces of each cell. Radial polarity, called compaction in mammals, provides a foundation for executing critical patterning events such as cell fate specification and gastrulation (1, 2). For example, radial polarity in C. elegans allows gastrulating cells to enrich myosin at their outer surfaces; myosin constricts these surfaces to help drive gastrulating cells into the embryo (2, 3). The C. elegans embryo polarizes radially when cell contacts restrict the polarity proteins PAR-6 (PSD-95/DLG/ZO-1 (PDZ) and semi-Cdc42/Rac-interactive-binding (semi-CRIB) domain protein), PAR-3 (PDZ domain protein) and PKC-3 (atypical protein kinase C) to the outer surfaces of early embryonic somatic cells (EES cells) (2, 4–6). This ‘inner-outer’ PAR asymmetry begins at the four-cell stage and persists through early embryogenesis (2). The molecular link between cell contacts and the inner-outer PAR asymmetries they induce to polarize embryos is not known.
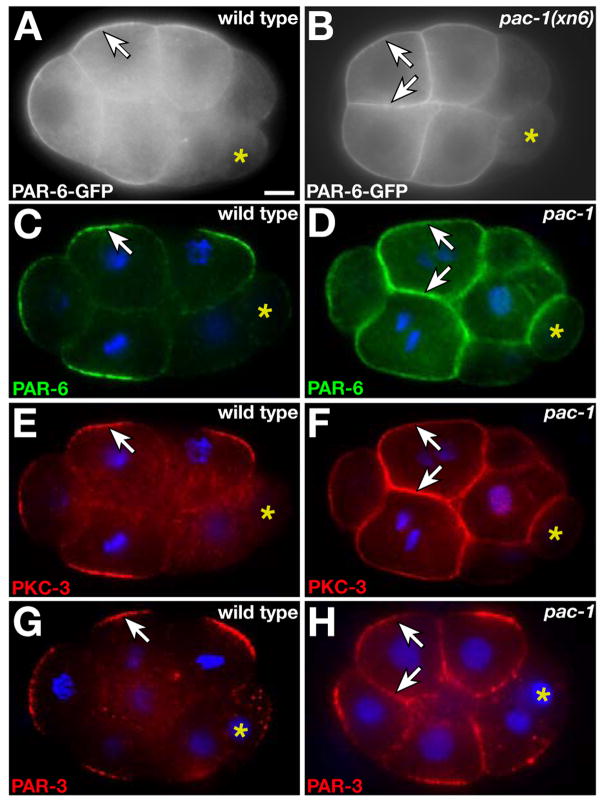
To learn how radial polarity is established, we screened for mutations preventing the inner-outer asymmetry of GFP-tagged PAR-6 (PAR-6-GFP). Two mutations (xn1, xn6) in a gene we named pac-1 (PAR-6-at-contacts) caused PAR-6-GFP to associate with both inner and outer surfaces of EES cells (Fig. 1A,B). pac-1 mutations are maternal-effect, and hereafter we refer to embryos produced by pac-1(xn6) mutant mothers as pac-1 embryos.
Fig. 1.
Inner-outer PAR asymmetry. In all figures anterior is left, nuclei are blue, and asterisk indicates germline precursor cell. Genotypes (italicized) and proteins shown (capitalized) are indicated. Bar in first panel applies to all panels unless indicated. (A, B) Live 8-cell embryos. (C-H) Immunostained 8-cell embryos. Arrows indicate PAR proteins. Bar, 5μm.
We immunostained pac-1 embryos to examine the localization of endogenous PAR proteins. PAR-6, PAR-3, and PKC-3 are restricted to outer surfaces of wild-type EES cells, but each protein showed a symmetric cortical localization in pac-1 EES cells (Fig. 1C-H, Table S2). PAR proteins within the zygote and germline precursor cell of wild-type early embryos develop anterior-posterior (A/P) asymmetries that are not patterned strictly by cell contacts. These PAR asymmetries appeared normal in pac-1 embryos (Fig. S1). pac-1 mutations also did not disrupt PAR-6 asymmetry in epithelial cells, which are born at later stages and localize PAR-6 apically (7). Thus, pac-1 is essential for contact-mediated PAR asymmetries that develop during radial polarization but appears dispensable for other types of PAR asymmetries.
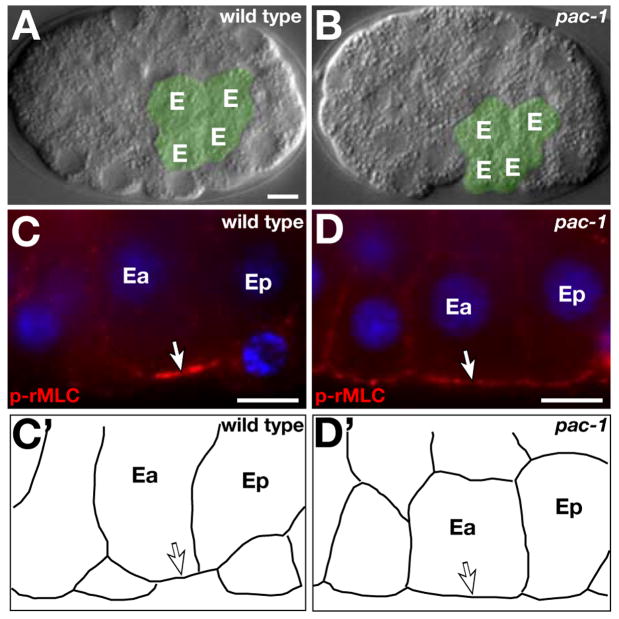
Depleting PAR-6 or PAR-3 specifically from EES cells causes slowed gastrulation (2). In wild-type embryos, gastrulation begins when the two endodermal precursor cells (EPCs) ingress into the interior. We filmed pac-1 embryos to determine if loss of inner-outer PAR asymmetry also disrupts gastrulation. EPCs ingressed significantly more slowly in pac-1 embryos (8), and were often present on the surface at a time that they would be internalized in wild-type (Fig. 2A,B; Movies S1,S2). Similar to embryos lacking PAR-3 or PAR-6 in EES cells (2), the slowed cell ingressions in pac-1 embryos did not prevent EPC descendants from ultimately internalizing and embryos were viable.
Fig. 2.
Gastrulation and myosin localization. (A, B) Live 44-cell embryos. EPC descendants (‘E’, colored green) have ingressed in wild type (A) but not in the pac-1 embryo (B). (C, D) mid-26-cell embryos. EPCs (Ea, Ep) are indicated. Arrows, p-rMLC. (C’,D’) Cell outlines. Bar, 5μm.
PAR-3 is required for non-muscle myosin to concentrate at and constrict the EPC outer surfaces (2, 3). To determine if the gastrulation defects we detected might be explained by altered myosin localization or activity, we immunostained embryos for activated myosin regulatory light chain (p-rMLC) (9). In wild type, p-rMLC concentrated at outer surfaces of ingressing EPCs, similar to published reports (Fig. 2C) (9). By contrast, levels of p-rMLC at outer surfaces of pac-1 EPCs were reduced significantly (Figs. 2D, S2). These data suggest that PAC-1 regulates gastrulation by restricting PAR-3 to the outer cortex, where PAR-3 is needed to concentrate active myosin.
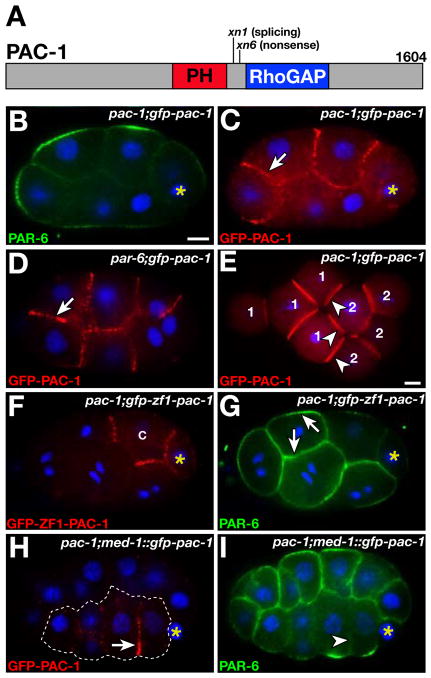
We cloned the pac-1 gene (8), which encodes a protein containing a pleckstrin homology (PH) and a RhoGAP domain (Figs. 3A, S3A). RhoGAP domains inhibit Rho GTPase signaling by converting active GTP-bound Rho proteins to inactive GDP-bound forms (10). xn6 contains a nonsense mutation predicted to truncate PAC-1 before the RhoGAP domain and causes a phenotype indistinguishable from pac-1(RNAi) (Fig. S3D-F), suggesting that xn6 eliminates pac-1 function. xn1 alters an invariant splice donor base. BLAST searches identified two related human proteins, ARHGAP10 and ARHGAP23, as PAC-1 homologues (Fig. S3B). In cultured cells, ARHGAP10 can regulate Golgi morphology, endocytosis, and α-catenin localization at epithelial junctions. ARHGAP10 is thought to function by inhibiting the Rho GTPase Cdc42 (11–13).
Fig. 3.
PAC-1 localization and time of function. (A) Predicted PAC-1 protein showing domains and mutations. (B–C) Coimmunostained 7-cell embryo; arrow, GFP-PAC-1. (D) 8-cell embryo; arrow, GFP-PAC-1. (E) Double embryo with ectopic contacts (arrowheads); cells from each embryo are numbered differently. GFP-PAC-1 is present at endogenous and ectopic contacts. (F,G) Coimmunostained 8-cell embryo. GFP-ZF1-PAC-1 has degraded from somatic cells except C cell (labeled). PAR-6 (arrows) localizes symmetrically in cells lacking GFP-ZF1-PAC-1. (H,I) Coimmunostained 26-cell embryo; cells expressing GFP-PAC-1 (arrow) are outlined (H). PAR-6 is absent from inner surfaces (arrowhead) of cells expressing GFP-PAC-1 (I). Bar, 5μm.
To learn where PAC-1 localizes within EES cells, we expressed a GFP-PAC-1 fusion protein in early embryos. GFP-PAC-1 rescued PAR-6 localization in pac-1 embryos (Fig. 3B, Table S2) and localized to inner but not outer surfaces of EES cells (Fig. 3C). GFP-PAC-1 localized identically in par-6 mutant embryos (22/23 embryos; Fig. 3D), indicating that PAC-1 functions upstream of PAR-6 to polarize EES cells. We combined cultured embryos to create new contacts; GFP-PAC-1 localized to endogenous and induced contacts (7/7 embryos; Fig. 3E), indicating that cell contacts recruit PAC-1 to inner surfaces.
To define when PAC-1 is needed for inner-outer PAR asymmetry, we first removed PAC-1 specifically from EES cells and asked if PAR-6 asymmetry was lost. To remove PAC-1, we fused it to the PIE-1 ZF1 domain, which targets proteins for degradation in EES cells but not in the germline precursor cell (2, 14). Because EES cells arise sequentially from asymmetric divisions of the germline precursor cell, ZF1-fusion proteins degrade in a reproducible mosaic pattern. GFP-ZF1-PAC-1 was functional (Fig. S4A,B), and showed the same localization as GFP-PAC-1 before degrading from EES cells after the four-cell stage (Fig. 3F). In pac-1(ZF1) embryos (pac-1 embryos expressing GFP-ZF1-PAC-1), PAR-6 localized symmetrically in cells lacking GFP-ZF1-PAC-1 (69/69 embryos; Fig. 3G). We next asked if introducing PAC-1 into EES cells of pac-1 embryos could induce PAR-6 asymmetry. We expressed GFP-PAC-1 using the med-1 promoter, which is active zygotically after the four-cell stage in a subset of EES cells (15). Cells expressing GFP-PAC-1 showed normal inner-outer PAR-6 asymmetry (21/22 embryos), whereas PAR-6 remained symmetric in cells not expressing GFP-PAC-1 (19/22 embryos; Fig. 3H,I). Together, these experiments suggest that PAC-1 functions continuously within EES cells to exclude PAR-6 from the inner cortex.
It was shown previously that the PAC-1 RhoGAP domain inhibits the Rho GTPases RHO-1/RHOA, CED-10/RAC, and CDC-42 in vitro (16). To determine if the RhoGAP domain is required for inner-outer PAR asymmetry, we mutated a conserved catalytic arginine (R984) essential for the activity of other RhoGAPs, including ARHGAP10 (Fig. S3C) (11, 12). GFP-PAC-1(R984A) was expressed comparably to GFP-PAC-1, localized to inner surfaces of EES cells (Fig. 4A), but could not rescue PAR-6 localization in pac-1 embryos (Fig. 4B; Table S2). Thus, PAC-1 likely excludes PAR-6 from the inner cortex by inhibiting Rho GTPases.
Fig. 4.
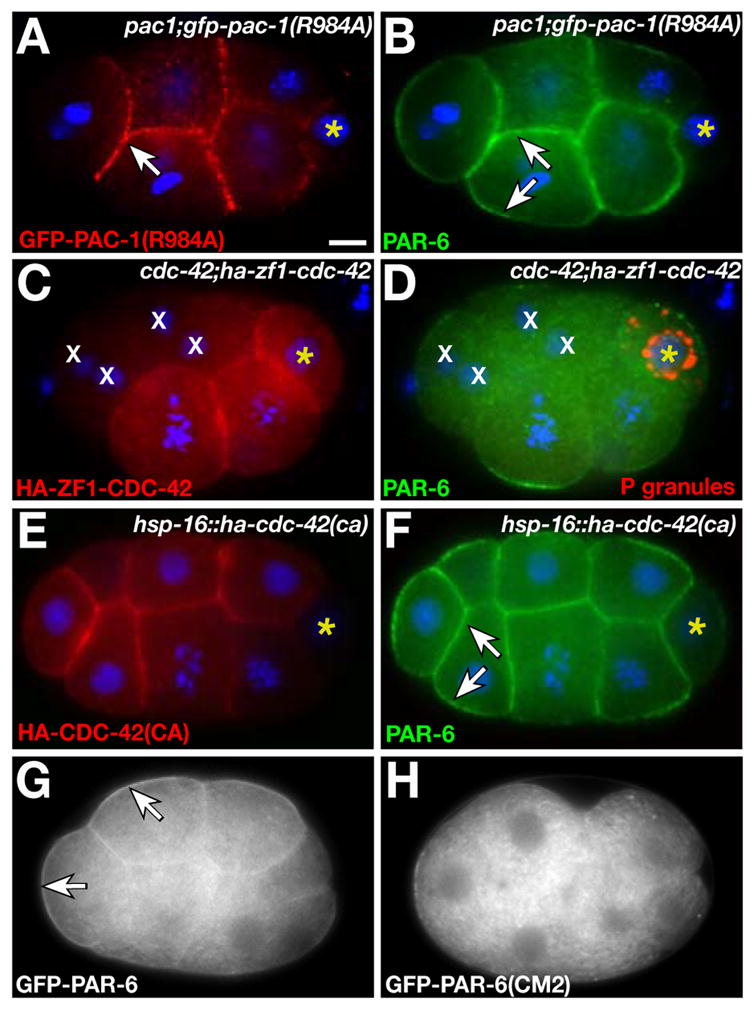
PAR-6 localization by the PAC-1 RhoGAP domain and CDC-42. (A,B) Coimmunostained 8-cell embryo. GFP-PAC-1(R984A) localizes to inner surfaces (arrow in A) but PAR-6 localizes symmetrically (arrows in B). (C,D) Coimmunostained 8-cell embryo; restriction of P granules to germline precursor cell indicates normal A/P polarity. HA-ZF1-CDC-42 has mostly degraded from cells marked ‘x’ and PAR-6 is cytoplasmic in these cells. (E,F) Coimmunostained 12-cell embryo expressing HA-CDC-42(CA). PAR-6 localizes to inner and outer surfaces (arrows). (G,H) Live 8-cell embryos. GFP-PAR-6 is enriched at the outer cortex (arrows); GFP-PAR-6(CM2) localizes to the cytoplasm. Bar, 5μm.
An attractive candidate target for PAC-1 is CDC-42, which regulates PAR-6 localization in the zygote (17–21). A functional hemagglutinin (HA)-tagged CDC-42 (Fig. S4C,D) was uniformly cortical in wild-type and pac-1 EES cells (Fig. S5), strongly suggesting that PAC-1 and CDC-42 overlap at inner surfaces. In cdc-42(RNAi) embryos, GFP-PAR-6 localized to the cytoplasm of EES cells (16/16 embryos) instead of the outer cortex as in wild type (Fig. S6A,B). We removed CDC-42 specifically from EES cells by fusing HA-CDC-42 to the ZF1 domain and expressing HA-ZF1-CDC-42 in cdc-42 embryos (hereafter cdc-42(ZF1) embryos). PAR-6 remained in the cytoplasm of cells where HA-ZF1-CDC-42 was degraded (31/31 embryos; Fig. 4C,D). By contrast, GFP-PAC-1 localized normally in cdc-42(ZF1) embryos (49/49 embryos; Fig. S6C,D). Thus, CDC-42 is required within EES cells to asymmetrically position PAR-6, but not PAC-1, at the cortex.
The opposite distribution of PAR-6 in pac-1 and cdc-42(ZF1) embryos suggests that PAC-1 controls PAR-6 localization by inhibiting CDC-42. PAR-6 showed the same cytoplasmic localization in pac-1(RNAi) cdc-42(ZF1) embryos as in cdc-42(ZF1) embryos (39/39 embryos; Fig. S6E,F), consistent with CDC-42 functioning downstream of PAC-1. We asked whether expressing constitutively active (CA) CDC-42 (17), which should not be inactivated by PAC-1, caused PAR-6 to bind both inner and outer surfaces of EES cells. In cells expressing HA-CDC-42(CA) from a heat shock promoter (30/30 embryos), but not in control heat-shocked embryos (1/35 embryos; Fig. S7A) or embryos expressing HA-CED-10/RAC(CA) or HA-RHO-1(CA) (Fig. S7C-F), PAR-6 showed a uniform cortical localization indistinguishable from that in pac-1 embryos (Fig. 4E,F) (8). Altogether, these data suggest that PAC-1 controls inner-outer PAR-6 asymmetry by inactivating CDC-42 at the inner surfaces of EES cells, although it is possible that other Rho GTPases also participate in radial polarization.
Active CDC-42 can bind the PAR-6 semi-CRIB domain, and this interaction is required for GFP-PAR-6 to associate stably with the zygote cortex (17). Therefore, we considered the possibility that active CDC-42 at the outer cortex of EES cells recruits PAR-6 directly. PAR-6 containing mutations in the semi-CRIB domain, PAR-6(CM2), cannot bind CDC-42 but still interacts with PAR-3 and PKC-3 (17). When expressed at equivalent levels in EES cells (8), GFP-PAR-6 and GFP-PAR-6(CM2) showed different localizations: both fusion proteins were present within the cytoplasm, but only GFP-PAR-6 was enriched significantly at the outer cortex (Fig. 4G,H; Fig. S8). HA-CDC-42(CA) forced GFP-PAR-6 (34/34 embryos), but not GFP-PAR-6(CM2) (0/26 embryos), to localize symmetrically at the cortex (Fig. S7B,G,H). These results support the hypothesis that active CDC-42 positions PAR-6 at the outer cortex through interactions with the PAR-6 semi-CRIB domain.
Our findings provide a model for how cell contacts polarize embryos radially. We propose that PAC-1, recruited to inner surfaces by cell contacts, locally inactivates CDC-42 and therefore restricts active CDC-42 to outer surfaces. Active CDC-42 would interact with the PAR-6 semi-CRIB domain, allowing PAR-6 to associate stably with the outer cortex and thereby creating an inner-outer asymmetry in PAR-6 localization. Thus, PAC-1 provides a continuous molecular link between cell contacts and cell polarity, allowing embryos to control polarity dynamically as cell divisions and movements remodel patterns of contact. This control may be especially important for rapid cytoskeletal changes during gastrulation, when pac-1 regulates myosin localization in gastrulating cells. In the C. elegans zygote, the RhoGAP CYK-4 provides an analogous link between transient sperm polarity cues and cytoskeletal reorganization that induces A/P PAR asymmetry (22).
It will be interesting to learn if PAC-1 homologues mediate radial polarity in other animal embryos. Radial polarization/compaction of early mammalian embryos shares many molecular similarities with C. elegans radial polarization (1). During mouse compaction, cell contacts restrict PAR-6, PAR-3, and aPKC/PKC-3 to outer surfaces (23, 24); these asymmetries are thought to help parcel cells into separate inner (embryonic) and outer (extra-embryonic) lineages (23). Rho GTPases are required for compaction (25), but how Rho activity is controlled has not been established.
Supplementary Material
Footnotes
SUPPORTING ONLINE MATERIAL
www.sciencemag.org, Materials and Methods, Figs. S1, S2, S3, S4, S5, S6, S7, S8, Tables S1, S2, Movies S1, S2
REFERENCES AND NOTES
- 1.Johnson MH, McConnell JM. Semin Cell Dev Biol. 2004;15:583. doi: 10.1016/j.semcdb.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Nance J, Munro EM, Priess JR. Development. 2003;130:5339. doi: 10.1242/dev.00735. [DOI] [PubMed] [Google Scholar]
- 3.Lee JY, Goldstein B. Development. 2003;130:307. doi: 10.1242/dev.00211. [DOI] [PubMed] [Google Scholar]
- 4.Etemad-Moghadam B, Guo S, Kemphues KJ. Cell. 1995;83:743. doi: 10.1016/0092-8674(95)90187-6. [DOI] [PubMed] [Google Scholar]
- 5.Hung TJ, Kemphues KJ. Development. 1999;126:127. doi: 10.1242/dev.126.1.127. [DOI] [PubMed] [Google Scholar]
- 6.Nance J, Priess JR. Development. 2002;129:387. doi: 10.1242/dev.129.2.387. [DOI] [PubMed] [Google Scholar]
- 7.Totong R, Achilleos A, Nance J. Development. 2007;134:1259. doi: 10.1242/dev.02833. [DOI] [PubMed] [Google Scholar]
- 8.Materials and Methods are available as Supporting Online Material
- 9.Lee JY, et al. Curr Biol. 2006;16:1986. doi: 10.1016/j.cub.2006.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etienne-Manneville S, Hall A. Nature. 2002;420:629. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 11.Dubois T, et al. Nat Cell Biol. 2005;7:353. doi: 10.1038/ncb1244. [DOI] [PubMed] [Google Scholar]
- 12.Sousa S, et al. Nat Cell Biol. 2005;7:954. doi: 10.1038/ncb1308. [DOI] [PubMed] [Google Scholar]
- 13.Kumari S, Mayor S. Nat Cell Biol. 2008;10:30. doi: 10.1038/ncb1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reese KJ, Dunn MA, Waddle JA, Seydoux G. Mol Cell. 2000;6:445. doi: 10.1016/s1097-2765(00)00043-5. [DOI] [PubMed] [Google Scholar]
- 15.Maduro MF, Meneghini MD, Bowerman B, Broitman-Maduro G, Rothman JH. Mol Cell. 2001;7:475. doi: 10.1016/s1097-2765(01)00195-2. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Blanc J, Lim L. J Biol Chem. 1994;269:820. [PubMed] [Google Scholar]
- 17.Aceto D, Beers M, Kemphues KJ. Dev Biol. 2006;299:386. doi: 10.1016/j.ydbio.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotta M, Abraham MC, Ahringer J. Curr Biol. 2001;11:482. doi: 10.1016/s0960-9822(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 19.Kay AJ, Hunter CP. Curr Biol. 2001;11:474. doi: 10.1016/s0960-9822(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 20.Motegi F, Sugimoto A. Nat Cell Biol. 2006;8:978. doi: 10.1038/ncb1459. [DOI] [PubMed] [Google Scholar]
- 21.Schonegg S, Hyman AA. Development. 2006;133:3507. doi: 10.1242/dev.02527. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins N, Saam JR, Mango SE. Science. 2006;313:1298. doi: 10.1126/science.1130291. [DOI] [PubMed] [Google Scholar]
- 23.Plusa B, et al. J Cell Sci. 2005;118:505. doi: 10.1242/jcs.01666. [DOI] [PubMed] [Google Scholar]
- 24.Vinot S, et al. Dev Biol. 2005;282:307. doi: 10.1016/j.ydbio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Clayton L, Hall A, Johnson MH. Dev Biol. 1999;205:322. doi: 10.1006/dbio.1998.9117. [DOI] [PubMed] [Google Scholar]
- 26.We thank A. Hyman, K. Kemphues, and G. Seydoux for reagents. Some antibodies and strains were obtained from the Developmental Studies Hybridoma Bank and the Caenorhabditis Genetics Center. We thank G. Fishell, M. Rosenberg, and Nance lab members for comments. Funding was provided by the NIH (T32HD07520 (D.C.A.) and R01GM078341 (J.N)) and the Edward Mallinckrodt, Jr. Foundation (J.N.). pac-1 Genbank accession: 1098033.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.