Abstract
We previously reported that the majority of in vitro monocyte/macrophage activation exhibited by extracts of Echinacea and other botanicals depends on bacterial lipopolysaccharides and Braun type bacterial lipoproteins. We determined the contribution made by these bacterial components to the overall immune enhancing activity detected in E. purpurea and E. angustifolia from bulk root and aerial material obtained from six major growers/suppliers in North America. Substantial variation in activity (up to 200-fold) was observed in extracts of these materials when tested in two monocyte/macrophage cell lines. The majority of activity was negated by treatment with agents that target bacterial lipoproteins (lipoprotein lipase) and lipopolysaccharides (polymyxin B). Experiments comparing the activity of freeze dried, freshly harvested Echinacea plants with those harvested and dried using various commercially relevant conditions, suggest that post-harvesting procedures do not substantially contribute to the variation observed in the commercial material.
Keywords: Echinacea, macrophage activation, immune stimulation, Braun type lipoprotein, lipopolysaccharide, bacteria
INTRODUCTION
Echinacea plant material can vary with respect to the species used (E. purpurea, E. angustifolia, E. pallida), the plant part (root, aerial and whole), and the agricultural/harvesting methods. Since these different plant materials all have different chemistries, it is not surprising that Echinacea products manufactured from different sources would exhibit different pharmacological effects. This complexity most likely contributes to the problem of interpreting the inconsistent results of clinical evaluations of Echinacea products. For example, while many studies have demonstrated some benefit in treatment of common colds, others have failed to show any efficacy, or only equivocal efficacy (reviewed in 1). This problem highlights the importance of characterizing the clinically relevant components of Echinacea so that standardized and efficacious products are available for consumers and clinicians.
We reported that the majority of in vitro monocyte/macrophage activation exhibited by extracts of Echinacea, and seven other botanicals traditionally used to enhance immune function, was due to the presence of lipopolysaccharides (LPS) and bacterial lipoproteins of the Braun type (2). Therein, three types of extracts were tested, two that are common practice in the supplement industry to obtain polysaccharide-rich extracts (hot water extract 98° C) and to produce tinctures (50% ethanol) and a hot water extract containing 4% SDS (conditions optimal for both LPS and lipoprotein extraction). In extracts that induced substantial macrophage activation, the majority of the activity was lost when the three types of extracts were treated with lipoprotein lipase (to inactivate lipoprotein) and the LPS inhibitor polymyxin B. Bacterial lipoproteins and LPS were studied since they were determined to be the most potent activators of monocytes/macrophages, as compared to other bacterial components tested. That study also suggested that a significant source of these bacterial components were derived from bacterial endophytes. It also highlighted the importance of monitoring for trace amounts of bacterial lipoproteins, as is presently done for LPS, during the isolation of immunostimulatory fractions or extracts.
The objective of the present study was to evaluate the variation in the immune enhancing activity of Echinacea bulk material obtained from six major growers/commercial suppliers in North America and to determine how much of this activity was due to the presence of bacterial components.
MATERIALS AND METHODS
Materials
Ultra pure E. coli LPS (0111:B4 strain) and synthetic bacterial lipoprotein Pam3CSK4 were obtained from InvivoGen (San Diego, CA). Lipoprotein lipase from Pseudomonas sp., crude E. coli LPS (026:B6 strain) and polymyxin B (7870 units/mg solid) were obtained from Sigma (St. Louis, MO). SDS-out reagent was purchased from Pierce (Rockford, IL).
Bulk root and herb (aerial) material for E. purpurea and E. angustifolia were obtained from the following six commercial suppliers: Frontier Natural Products Co-op, lot numbers 50811.2331, 769.3052, 50964.3034 (Norway, IA), Gaia Herbs, lot numbers 00033874, 00033507, 00034316 (Brevard, NC), Glenbrook Farms Herbs & Such, lot numbers not available (Live Oak, FL), Mountain Rose Herbs, lot numbers 11987, 12066, 12358 (Eugene, OR), Richters, lot numbers 21283, 21580, 21084 (Goodwood, Ontario, Canada), and Trout Lake Farm LLC, lot numbers EPR-K6041-BCP, EPH-S2051-E3P, EAR-BLO51-BCP (Trout Lake, WA). Voucher specimens were deposited in the NCNPR repository at the University of Mississippi.
Bacterial endotoxin levels were determined using a Limulus Amebocyte Lysate (LAL) assay (Pyrochrome Chromogenic Test Kit with diazo coupling) from Associates of Cape Cod Inc. (East Falmouth, MA). Human monocytic THP-1 cells and RAW 264.7 mouse macrophages were obtained from American Type Culture Collection (Manassas, VA).
Preparation of extracts
All crude extracts were prepared using the following extraction procedure. For each sample 0.5 g of finely ground Echinacea raw material was extracted with 5 mls of 4% SDS at 98°C for 1 hour. After removal of SDS using SDS-out reagent, extracted material was precipitated by addition of 4 volumes of 95% ethanol. Precipitate was then washed twice with 95% ethanol, air dried at 50–55°C and dissolved in 2% octylglucoside for analysis.
Monocyte and macrophage activation assays
The THP-1 human monocyte cell line was transfected with a luciferase reporter gene construct containing two copies of NF-kappa B motif from HIV/IgK and test samples were evaluated as described previously (3). RAW 264.7 cells were cultured in RPMI 1640 medium supplemented with fetal bovine serum (10% v/v) and amikacin (60 mg/L) at 37°C, under 5% CO2. Actively growing cells were transiently transfected with the same reporter plasmid as used for THP-1 cells using electroporation at 150V and one 70 ms pulse (4). Following electroporation, cells were resuspended in culture medium at 0.5 million cells/ml and plated at a density of 1 × 105 cells/well in 96-well plates. After 24 hours, test samples were added. After a six hour incubation, the medium was removed by aspiration and cells lysed by addition of 40 µl of a 1:1 mixture of luciferase assay reagent (Promega) and phosphate buffered saline containing 1 mM calcium and magnesium. Light output was measured using a Packard microplate scintillation counter in single photon mode.
Cultivation and post-harvest drying conditions for Echinacea plants
Seeds of E. purpurea (lot. 27912) and E. angustifolia (lot. 27353) were purchased from Johnny’s Selected Seeds (Winslow, Maine) and sown in plastic trays on March 3, 2006.
For E. purpurea, two-month old seedlings were transplanted to raised beds at the University of Mississippi Field Station on May 3, 2006. Seedlings were spaced 8 inches apart within each row and rows were separated by 1.6 to 2.2 ft. Mulch was laid on each bed as soil coverage and two foliar applications of Miracle-Gro® were made in 2006, followed by one application in 2007. During June, July and August of 2006 and 2007 plants were manually watered twice/week (25L/bed on each day).
For E. angustifolia, seedlings were transplanted to pots (6″x 6″x15″) on April 12, 2006. The substrate in the pots was a mixture of sand and Pro-Mix BRK with mycorrhiza soil of Premier Horticultures (Hummert International Co., Earth City, MO). After seedlings were planted, pot soil was covered with wood mulch (Delta Select, Louisiana Soil Product of Ruston LLC, Ruston, LA) and pots were arranged into completely randomized blocks and maintained under full sun. Water was supplied using a drip system, with each pot receiving 200mls/day. Foliar fertilizer (Miracle-Gro®) was applied twice in 2006 and once in 2007, at emergence.
Echinacea plants were harvested during second year growth (last week of July, 2007). Roots (E. purpurea and E. angustifolia) and aerial parts (E. purpurea) were washed extensively and then subjected to 4 different post-harvest drying treatments. For treatment 1, plant parts were placed in brown paper bags and arranged on tables inside a greenhouse with adequate air circulation for 2 weeks (daily ambient temperature ranged between 35 and 43°C). For treatment 2, plant parts were placed outside, under a shaded area, near the growing field for 24 hours and then transferred to an air forced drier for 10 days, maintained at 40°C. For treatment 3, plant parts were immediately transferred to an air forced drier for 10 days maintained at 40°C. For treatment 4, plant parts were immediately frozen and then freeze-dried for 5 days.
RESULTS
In vitro monocyte/macrophage activation potential varies substantially in bulk Echinacea plant material obtained from different sources
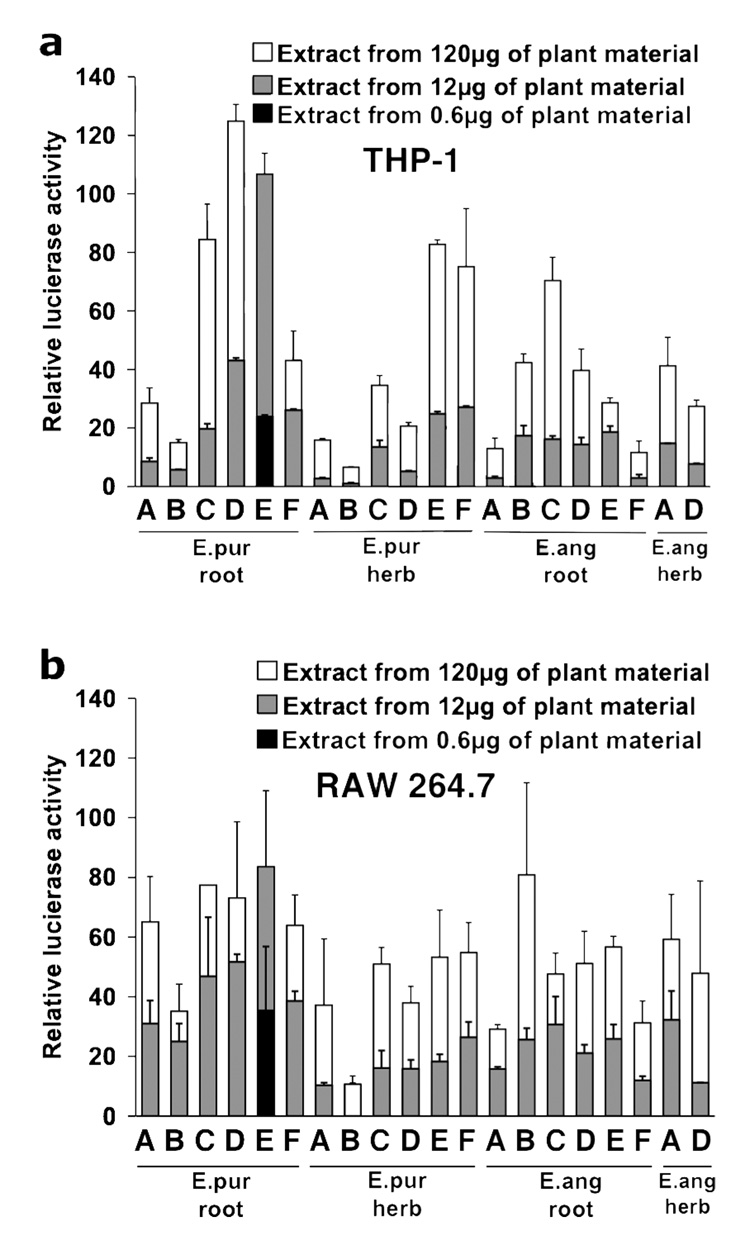
Plant material was first extracted with hot water (98° C for 1 hr) containing 4% SDS. Immune enhancing components were then precipitated by the addition of ethanol and then washed twice with 95% ethanol. The ethanol precipitation and washing steps are necessary to remove anti-inflammatory (inhibitors of macrophage activation) plant components such as alkylamides so as to allow accurate detection of macrophage activating components within the precipitated material. Two cell lines were used to assess in vitro activity. The THP-1 human monocyte cell line responds more robustly with respect to activation of NF-kappa B to the TLR2 agonist Braun type bacterial lipoproteins than to the TLR4 agonist LPS, while RAW 264.7 macrophages exhibit the opposite response (2). Therefore, THP-1 and RAW 264.7 cells were used to detect the contribution of bacterial lipoproteins and LPS, respectively, to the overall activity found within these extracts. Figure 1a and b shows that despite the difference in response of these two cell lines to bacterial lipoproteins and LPS, there was a significant correlation between the activities exhibited by the Echinacea extracts in the two assays (r=0.896, p< 0.0001). Extracts from E. purpurea exhibited the largest variation in activity in both cell lines between the different sources (approximately 200 and 100-fold difference in activity between company B (lowest) and E (highest) for root and herb, respectively). In contrast, a smaller variation in activity was observed between extracts from E. angustifolia from different sources (approximately 10-fold difference in activity between lowest and highest values).
Figure 1.
Variation in monocyte/macrophage stimulatory activity in extracts from bulk Echinacea plant material. Root and aerial (herb) raw material from E. purpurea (E.pur) and E. angustifolia (E.ang) was obtained from six commercial growers (companies “A” – “F”). The indicated plant material was extracted with hot water containing 4% SDS, followed by precipitation and washing of the precipitate with ethanol. Twenty-four hours following transfection with the NF-kappa B luciferase reporter plasmid, THP-1 (a) and RAW 264.7 (b) cells were treated with extracts. Average medium concentrations (µg/ml) for the 12 µg group (gray bars) were 15.9 (E.pur root), 16.8 (E.pur herb), 20.1 (E.ang root), and 20.2 (E.ang herb) and there was no correlation between extract yield and activity (data not shown). Four hours (THP-1) or six hours (RAW 264.7) later cells were harvested for luciferase assay. Extract from E. purpurea root (company E) was also evaluated at a concentration 20 times lower (black bar) since maximal activation was observed at the lower concentration tested for the other extracts (gray bar). Control value for untreated THP-1 cells was 0.76±0.13 and for untreated RAW 264.7 cells was 8.4±3.3. Results represent the average of duplicate samples ± range from a representative experiment that was repeated twice.
Differences in activity exhibited by Echinacea bulk plant material from different sources is mainly due to bacterial lipoproteins and lipopolysaccharides
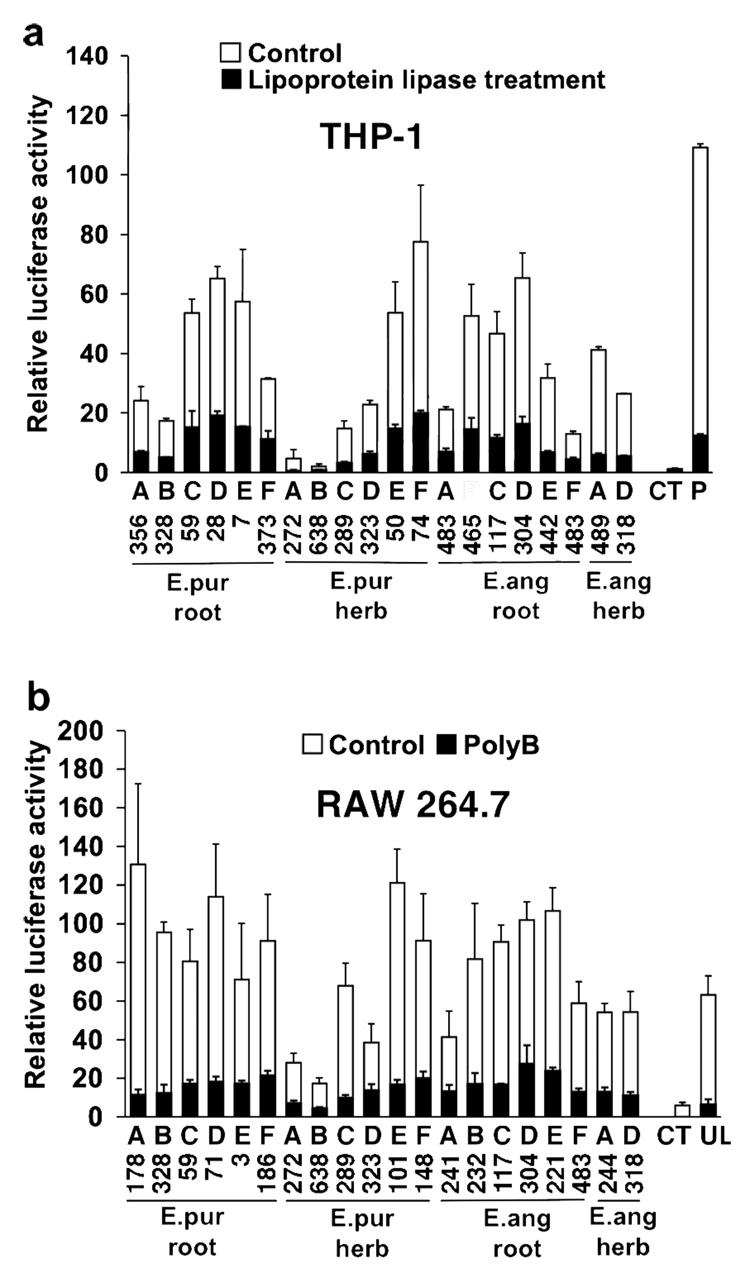
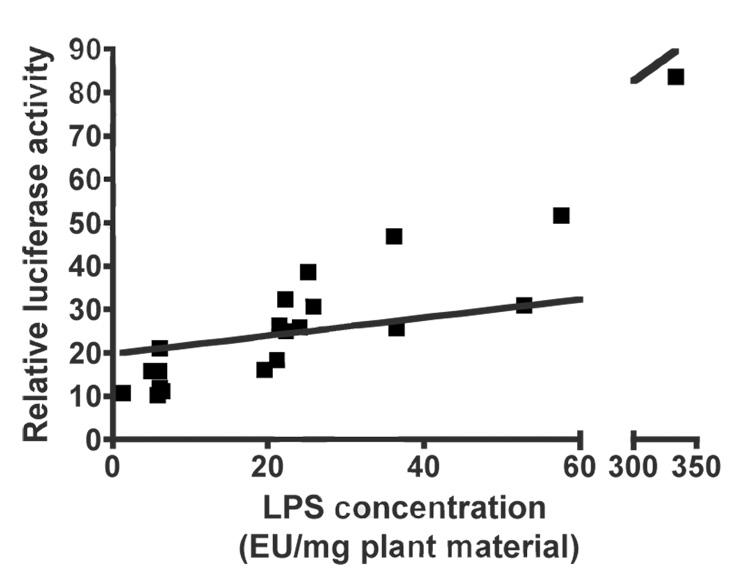
The majority of in vitro macrophage activation exhibited by extracts from various botanicals traditionally used to enhance immune function, such as Echinacea, is due to bacterial lipoproteins of the Braun type and LPS (2). The results presented in Figure 2a and b address the question as to how much variation in activity exhibited by bulk Echinacea plant material presented in Figure 1 can be accounted for by these two bacterial components. Concentrations of extracts were used that either gave between 50 and 100 units of relative luciferase activity or, for low activity samples, a concentration of extract from 240µg of plant material was used. Figure 2a shows that in the lipoprotein-sensitive monocyte cell line THP-1, the bulk of the activity exhibited by these extracts was negated by treatment with lipoprotein lipase. Although lipoprotein lipase inactivates both bacterial lipoproteins and lipoteichoic acids (but not LPS) through the removal of fatty acids ester-linked to glycerol, our previous studies demonstrated that most of the activity detected is due to lipoproteins (2). Similarly, in the LPS-sensitive mouse cell line RAW 264.7 most of the activity exhibited by these extracts was blocked by treatment with the LPS inhibitor polymyxin B (Figure 2b). Further evidence that the activity detected by RAW 264.7 cells was due to LPS is demonstrated in Figure 3 which shows a significant correlation between this activity and the content of LPS in the extracts as determined by the LAL assay (r = 0.851, p < 0.0001).
Figure 2.
The majority of the in vitro monocyte/macrophage stimulatory activity of extracts from bulk Echinacea plant material is abrogated by treatment with lipoprotein lipase and polymyxin B. Root and aerial (herb) raw material from E. purpurea (E.pur) and E. angustifolia (E.ang) was obtained from six commercial growers (companies “A” – “F”). Preparation of extracts and assay protocols are the same as described in Fig. 1. In the lipoprotein-sensitive THP-1 cells (a), black bars represent extracts added to cell culture after they were incubated at 37°C for 16 hours with lipoprotein lipase (1 mg/ml), 10 μM AEBSF protease inhibitor cocktail solution (Sigma), 1% octylglucoside and 0.2% BSA. Control extracts (without lipoprotein lipase, white bars) were run under identical conditions prior to adding to culture wells. In the LPS-sensitive RAW 264.7 cells (b), extracts were added to cell culture in the presence (black bars) or absence (white bars) of polymyxin B (100µg/ml). Polymyxin B was added 30 minutes prior to sample addition. Ultra pure E. coli LPS (UL) was run at 100ng/ml and synthetic lipoprotein Pam3CSK4 (P) at 0.5ng/ml. (CT) refers to untreated cells. Numbers below bars indicate extract medium concentrations in µg/ml. Values are the average of duplicate determinations ± range from a representative experiment that was repeated twice.
Figure 3.
Correlation between RAW 264.7 macrophage stimulatory activity and endotoxin levels in extracts from Echinacea. The twenty extracts were prepared from bulk Echinacea plant material obtained from commercial growers (refer to Fig. 1b). The y-axis represents the total activity extracted from 12µg of dried ground plant material that was determined in Fig. 1b using the LPS-sensitive RAW 264.7 cells. The x-axis represents the number of endotoxin units (EU) in each extract, expressed per mg of dried ground plant material, determined by the LAL assay. Linear correlation analysis was performed using GraphPad Prism version 4 (p <0.0001; r = 0.8512).
Differences in activity exhibited by bulk Echinacea plant material probably do not originate from different post-harvest drying conditions
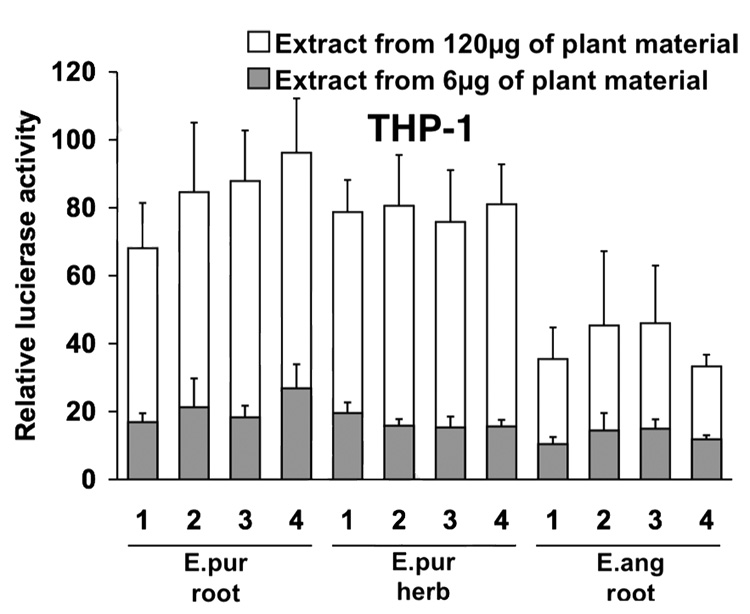
The results presented in Figure 2 raise the question as to what factors contribute to the substantial variation in activity exhibited by this Echinacea material. Since the vast majority of the activity detected was due to the presence of bacterial components, potential bacterial sources could include endophytic bacteria, naturally occurring surface bacteria, post-harvest contamination, or a combination of these sources. Since the bulk Echinacea plant material studied was obtained from various commercial growers, it is possible that different post-harvest drying procedures were used and this could contribute to varying amounts of bacterial growth during the drying process. The experiment presented in Figure 4 evaluated the variation in monocyte activation potential of extracts from Echinacea plant material subjected to different drying treatments that are typically used during commercial harvesting. Although there are a few statistically significant differences between some of the treatment groups, clearly these differences are minor compared to the variation in activity observed between extracts of bulk Echinacea plant material from the different growers reported in Figure 1. This result suggests that different post-harvest drying conditions do not substantially contribute to the variation observed in the level/activity of bacterial components within diversely sourced Echinacea plant material.
Figure 4.
Variation in THP-1 monocyte stimulatory activity in extracts from Echinacea plant material exposed to different post-harvest drying conditions. Seeds were germinated in trays and E. purpurea (E.pur) seedlings transferred to field beds and E. angustifolia (E.ang) seedlings transferred to outdoor pots. Roots and aerial (herb) plant parts were harvested during second year growth and subjected to the following drying conditions: (1) 35–43°C in greenhouse; (2) 24-hours under shade outside and then placed in an air forced drier at 40°C; (3) placed in an air forced drier at 40°C; and, (4) freeze-dried (control treatment). Dried plant material was extracted with hot water containing 4% SDS, followed by precipitation and washing of the precipitate with ethanol. Activity of extracts was determined using the THP-1 monocyte activation assay described in Fig. 1a. Each bar represents the average activity ± s.d. of extracts from six (E. pur root and herb) or three (E. ang root) individual plants, with each extract tested in duplicate. Control value for untreated cells was 0.9±0.24. Statistical differences between treatment groups (determined using two-tailed, Student’s t-tests) are as follows: E.pur root white bars (1 vs 3, p=0.035; 1 vs 4, p=0.007), E.pur root gray bars (1 vs 4, p=0.009; 3 vs 4, p=0.024), and, E.pur herb gray bars (1 vs 2, p=0.034; 1 vs 3, p=0.045; 1 vs 4, p=0.027).
DISCUSSION
In the present study we assessed the variation in the immune enhancing activity of diverse Echinacea bulk material. We show that the in vitro monocyte/macrophage activation potential of this material varies substantially, and that the majority of this activity is due to bacterial lipoproteins and LPS. In addition, we demonstrate that different post-harvest drying conditions do not significantly influence the content/activity of the bacterial components within Echinacea plant material.
Several approaches were used to determine that the majority of the activity detected in this bulk Echinacea material was derived from bacterial components. Investigators that study the immune enhancing properties of botanical extracts, or high molecular weight polysaccharide preparations routinely monitor for the presence of bacterial LPS. The LAL assay is useful for estimating the amount of LPS in a botanical sample, while treatment with polymyxin B, within an in vitro immune assay system, is useful for determining how much of the overall activity exhibited is due to LPS. We used both methods in concert and found that the activity exhibited by this material correlated strongly (Figure 3, r = 0.851, p < 0.0001) with the LAL-determined LPS content and that the majority of this activity was inhibited by polymyxin B treatment. Since these experiments suggest that bacterial LPS is responsible for the detected activity, additional support for this conclusion would come from the detection of other bacterial components. We tested for the presence of bacterial lipoproteins since we previously showed, in several monocyte/macrophage cell systems, that both synthetic bacterial lipoproteins of the Braun/murein type and LPS were much more potent than other tested bacterial components (2). Herein, activity detected by the bacterial lipoprotein-sensitive THP-1 cell line was abolished by lipoprotein lipase treatment. We previously showed that this lipase-sensitive activity detected in Echinacea was due to the presence of bacterial lipoproteins of the Braun/murein type (2). Since the activity detected in the THP-1 assay system was highly correlated (r = 0.896, p < 0.0001) with LPS content/activity, it further indicates that the activities detected in these samples are of bacterial origin. Based on this data, it is speculated that either differences in bacterial amount, or bacterial type, are responsible for the substantial variation seen in the activity exhibited by this plant material (up to 200-fold).
It is our hypothesis that a major source for the bacterial components detected in this Echinacea plant material originates from endophytic bacteria. Our previous research showed that ampicillin and other antibiotics suppressed the appearance of activity (NF-kappa B activation of THP-1 cells by the lipoprotein fraction) during the aseptic germination of surface sterilized alfalfa seeds (2). This indicated that endophytic bacteria, carried within the seeds, proliferated during the seven day germination period and gave rise to the detected activity. The activity that appeared during the germination of these alfalfa sprouts was substantial and is equivalent to that detected in the most active Echinacea plant sample tested in Figure 1. This indicates that endophytic bacteria carried within the alfalfa seeds are capable of increasing to levels during germination similar to those detected within the Echinacea plant material.
It is possible that high levels of bacterial components in some of the plant material could originate from post-harvest events. However, our data indicate that it is possible to consistently obtain Echinacea plant material containing high levels of these bacterial components under controlled conditions. Echinacea samples that were harvested, washed extensively and then immediately freeze dried, ground and extracted (Figure 4, sample 4) exhibited activities comparable to the higher activity bulk Echinacea samples from growers/suppliers analyzed in Figure 1. This processing procedure would have prevented the post-harvest growth or introduction of bacteria. Likewise, it is unlikely that the bulk Echinacea samples that exhibited very low levels of activity in Figure 1 resulted from the degradation of bacterial components. We find that the activity exhibited by LPS and bacterial lipoproteins within ground Echinacea material, stored at room temperature and analyzed over a period of several years, is extremely stable (data not shown).
Recent research indicates that consumption of foods containing probiotic bacteria can have a beneficial effect on the immune system (reviewed in 5). For example, a double blind, three-stage before-and-after intervention trial showed that consumption of Bifidobacterium lactis HN019 for three weeks by healthy volunteers significantly enhanced polymorphonuclear cell phagocytosis and natural killer cell tumor cell killing (6). Many of these immune enhancing effects do not require viable bacterial cells, since they can be mimicked by consumption of heat killed organisms (7). More research is required to determine if the bacterial components detected in Echinacea contribute to the therapeutic properties of this botanical.
Acknowledgments
This research was partially funded by grants from the National Institutes of Health RO1 AT002360 (NCAAM) to DSP and the USDA, Agricultural Research Service Specific Cooperative Agreement No. 58-6408-7-012.
ABBREVIATIONS USED
- E.
Echinacea
- LPS
lipopolysaccharides
- SDS
sodium dodecyl sulfate
- LAL
Limulus Amebocyte Lysate
- NF-kappa B
nuclear factor-kappa beta
- TLR
toll-like receptor
LITERATURE CITED
- 1.Woelkart K, Linde K, Bauer R. Echinacea for preventing and treating the common cold. Planta Med. 2008;74:633–637. doi: 10.1055/s-2007-993766. [DOI] [PubMed] [Google Scholar]
- 2.Pugh ND, Tamta H, Balachandran P, Wu X, Howell J, Dayan FE, Pasco DS. The majority of in vitro macrophage activation exhibited by extracts of some immune enhancing botanicals is due to bacterial lipoproteins and lipopolysaccharides. Int. Immunopharmacol. 2008;8:1023–1032. doi: 10.1016/j.intimp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pugh N, Ross SA, ElSohly MA, Pasco DS. Characterization of Aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J. Agric. Food Chem. 2001;49:1030–1034. doi: 10.1021/jf001036d. [DOI] [PubMed] [Google Scholar]
- 4.Subbaramaiah K, Bulic P, Lin Y, Dannenberg AJ, Pasco DS. Development and use of a gene promoter-based screen to identify novel inhibitors of cyclooxygenase-2 transcription. J. Biomol. Screen. 2001;6:101–110. doi: 10.1177/108705710100600206. [DOI] [PubMed] [Google Scholar]
- 5.Ezendam J, van Loveren H. Probiotics: Immunomodulation and evaluation of safety and efficacy. Nutr. Rev. 2006;64:1–14. doi: 10.1111/j.1753-4887.2006.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 6.Chiang BL, Sheih YH, Wang LH, Liao CK, Gill HS. Enhancing immunity by dietary consumption of a probiotic lactic acid bacterium (Bifidobacterium lactis HN019): optimization and definition of cellular immune responses. Eur. J. Clin. Nutr. 2000;54:849–855. doi: 10.1038/sj.ejcn.1601093. [DOI] [PubMed] [Google Scholar]
- 7.Hirose Y, Murosaki S, Yamamoto Y, Yoshikai Y, Tsuru T. Daily intake of heat-killed Lactobacillus plantarum L-137 augments acquired immunity in healthy adults. J. Nutr. 2006;136:3069–3073. doi: 10.1093/jn/136.12.3069. [DOI] [PubMed] [Google Scholar]