Abstract
Stem cell transplantation has emerged as a potential modality in cardiovascular therapeutics due to their inherent characteristics of self-renewal, unlimited capacity for proliferation and ability to cross lineage restrictions and adopt different phenotypes. Constrained by extensive death in the unfriendly milieu of ischemic myocardium, the results of heart cell therapy in experimental animal models as well as clinical studies have been less than optimal. Several factors which play a role in early cell death after engraftment in the ischemic myocardium include; absence of survival factors in the transplanted heart, disruption of cell-cell interaction coupled with loss of survival signals from matrix attachments, insufficient vascular supply and elaboration of inflammatory cytokines resulting from ischemia and/or cell death. This article reviews various signaling pathways involved in triggering highly complex forms of cell death and provides critical appreciation of different novel anti-death strategies developed from the knowledge gained from using an ischemic preconditioning approach. The use of pharmacological preconditioning for up-regulation of pro-survival proteins and cardiogenic markers in the transplanted stem cells will be discussed.
1. An overview of heart cell therapy
During the last decade, widespread experimental studies in animal models and clinical studies have shown the safety, feasibility and efficacy of cell based therapies for myocardial regeneration. Starting with the use of unipotent myogenic cells including skeletal muscle derived stem cells and cardiomyocytes, heart cell therapy has progressed to assess the feasibility of multipotent and pluripotent stem cells for cardiac repair 1,2. Despite recent reports for their successful cardiomyogenic differentiation in vitro 3, failure of skeletal myoblasts to electromechanically integrate and synchronously contract with the host myocytes post engraftment have restricted their use as the choice donor cells in clinical settings 4. Similarly, cardiomyocytes are an excellent option for use as donor cells but their limited availability remains an issue of concern in clinical application 5,6. Engraftment of non-myogenic fibroblasts and smooth muscle cells with contractility characteristics different from cardiomyocytes have also been shown to improve cardiac function 7,8. Nevertheless, a repertoire of stem and progenitor cells in the bone marrow with differing plasticity and differentiation potentials have emerged as the most extensively studied cells for their myocardial regenerative potential in experimental animals and clinical studies 9,10. In case of bone marrow derived stem cell engraftment, the mainstay of mechanisms for cardiac repair is their differentiation to adopt cardiac phenotype together with angiogenesis 11–13. More recently, a paracrine hypothesis has been proposed which conceives that grafted bone marrow cells produce cytokines, growth factors and other signaling moieties to promote infarct healing process with an as yet undefined mechanism 14,15. Despite encouraging results in experimental animals and clinical studies, there is a lingering controversy regarding the ability of bone marrow cells to transdifferentiate into cardiomyocytes 16. Additionally, some safety concerns have been raised regarding bone marrow cells multipotentiality which might lead to their uncontrolled differentiation into undesired lineage 17,18.
Embryonic stem cells have emerged as promising candidates for cardiac repair and may adopt different phenotypes 19. However, their use is afflicted with several difficulties including technical limitations of undifferentiated propagation in vitro and purification of spontaneously differentiated cardiomyocytes from cell culture. Despite these difficulties, recent progresses in stem cell biology have paved the way to exploit embryonic stem cells as a clinically relevant and renewable source to generate cardiomyocytes and endothelial cells for engraftment 20,21. The cardiomyocytes and endothelial cells thus generated formed stable grafts in animal hearts without tumor formation. Nevertheless, availability and immune status as cell graft, and teratogenic nature remain debatable issues for their progress in clinical applications 22,23. More recently, the existence of resident cardiac stem cells has added a new dimension to the approach of stem cell therapy and implies a paradigm shift in the long standing dogma about the heart being unable to repair itself in the event of injury 24. The myocardial progenitor populations include cells expressing the c-kit, stem cell antigen-1 (Sca-1), side population (SP cells), MDR1, and cells expressing the transcription factor islet-1 (isl-1) 25,26. The roles of these cells in tissue maintenance, repair, and possible therapeutic applications will be an exciting area of investigation in future.
Of all these available options, choice of donor cell is a matter of the required outcome of the procedure 27. Whereas the engrafted skeletal myoblasts form neofibers and provide a tenacious support for the weakened myocardium 28, improve oxygenation in the ischemic myocardium 29 and enhance diastolic cardiac function 30, bone marrow cell transplantation mainly improves systolic function and induces angiogenesis. A more relevant approach would be co-transplantation of the two cell types in order to achieve benefits associated with the use of each cell type 31. Moreover, their transplantation needs to be combined with other strategies in order to generate donor cells which have been “pre-formatted” to survive and engraft in the ischemic heart to support the inadequate intrinsic repair mechanisms.
2. Massive cell death as an impediment of successful heart cell therapy
Besides choice of donor cells, their engraftment is confronted with the problem of poor survival in the host myocardium. A significantly high percentage of the donor cells die within hours after transplantation, thus compromising the optimal outcome of the procedure 32. In some studies, more than 70–80% of the cells die within 3 days whilst others have shown that 93% of skeletal myoblasts were lost in 2 days after transplantation 33. Toma and colleagues have reported less than 0.44% mesenchymal stem cell survival by day 4 after engraftment in an immunodeficient mouse heart model 34. As meager as 1% survival of the transplanted cells have been reported in patient hearts 35. Although the underlying cause of unusually high and accelerated cell mortality generally remains undefined, these disconcerting reports related with the problematic high attrition of donor cells have implicated multiple factors. Besides other mechanisms, the dynamics of early donor cell death incriminates pathological processes including local immune and inflammatory responses, loss of trophic factors and local tissue ischemia as the prime factors responsible for massive cell death 36. Hence, it is imperative to reinforce the donor cells to withstand the rigors of the microenvironment of the infarcted heart incurred from ischemia, inflammatory response, and pro-apoptotic factors in order to develop heart cell therapy into an effective therapeutic modality.
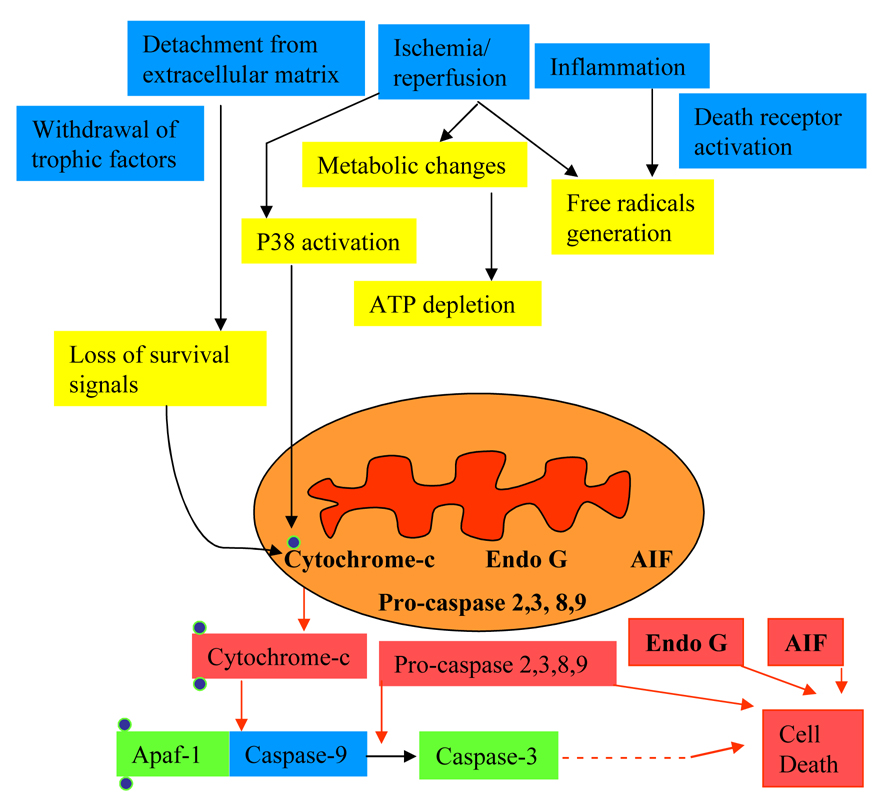
Apoptosis is a highly orchestrated physiological process for programmed killing of the damaged cells. This has been implicated as one of the key pathologic features in acute myocardial infarction and heart failure 37,38. Depending upon the nature of both intrinsic as well as extrinsic death signals, separate self-regulated signaling pathways get activated to coordinately execute apoptosis by means of common death effector machinery. A schematic representation of the mechanism of apoptotic cell death is given in Figure 1. Initiated by extrinsic or intrinsic stimuli such as the loss of trophic factors, detachment from extracellular matrix, ischemia reperfusion injury or stimulation of death receptors, apoptosis is characterized by morphological alterations including membrane blebbing, chromatin clumping, nuclear condensation and cell shrinkage. These characteristic features of apoptosis are a direct consequence of caspase activation 39. During extrinsic apoptosis signaling, ligand binding with cell surface death receptors such as TNF-α or Fas/CD95 stimulates recruitment of adaptor proteins to form death inducing signaling complex (DISC) and activation of caspase-8 which ultimately triggers the downstream caspase cascade 40. Alternatively, intrinsic initiation of apoptosis has been well documented and involves a connection between destabilization of mitochondria by reduction of the mitochondrial transmembrane potential and concomitant release of apoptogenic factors cytochrome-c, endonuclease G and AIF, and caspase-3 activation 41. In summary, the released cytochrome c thus initiates the formation of the poptosome, a complex comprising Apaf-1, cytochrome c, dATP and pro-caspase-9 to activate caspase-9 which in turn activates pro-caspase-3. Although, cell death receptor and mitochondrial pathways appear separate, a cross-talk between the two is possible and has been reported as the responsible mechanism for the massive host myocyte and donor cell death in the infarcted heart.
Figure 1.
Schematic representation of the key players in signaling pathways responsible for apoptotic cell death.
(AIF= apoptosis inducing factor; Apaf= apoptosis protease activating factor; EndoG=endonuclease G).
3. Strategies to enhance donor cell survival
Cell survival may greatly enhance the effectiveness of transplantation therapy. Therefore, development of a strategy to alleviate apoptotic cell death may be of prime consequence in the treatment of infarcted heart 42. Concisely, the survival of transplanted cells requires adoption to unfriendly milieu in ischemic myocardium. Several remedial approaches have been recommended to overcome the menace of apoptotic cell death albeit with less than the desired results (Table-1). Exposure to brief hypoxia or anoxia may precondition the cells and make them resistant to subsequent lethal ischemic injury 43. The approach of therapeutic transgene delivery to the heart either by direct injection of the transgene encoding vector or by engraftment of the genetically modified donor stem cells has given encouraging results 11,44,45. Growth factor gene delivery to the heart combined with cell transplantation has given encouraging results in terms of donor cell survival 46. Ischemic myocardium primed with recombinant growth factor protein treatment prior to transplantation of the donor cells allows better survival of the cell graft 47. Loss of adherence to the matrix is one of the main initiator of apoptotic cascade. Suspension of donor cells in fibrin glue for intramyocardial delivery significantly improves their retention in the infarcted heart. Moreover, subsequent to intramyocardial injection, fibrin also contributes towards increased survival of the cell graft by providing temporary extracellular matrix for the transplanted cells 48. Alternatively, injection of fibrin glue into ischemic myocardium increases regional blood supply via neovascularization and provides better oxygenation for the cells. In the same context, collagen as a natural extracellular matrix component has been shown to support cell survival and growth both in cell culture and in vivo after transplantation into ischemic hearts and leads to improved left ventricle contractile function 49. These strategies will be discussed in length in the later part of the review.
Table 1.
Strategies to improve cell survival
| Pro-survival Strategy | References |
|---|---|
| Anti-inflmmatory or immune treatment | |
| Anti-LFA-1 | 135,136 137 |
| Interleukin-1 receptor antagonist | 33,138,139 |
| Depletion of C3 complement | 140 |
| Treatment of anti-CD154 antibody | 141 |
| CD4 and CD8 depletion | 36 |
| Natural killer cells depletion | 36 |
| Treatment of immunosuppression | 142,143 |
| Growth factors | |
| Fibroblast growth factor | 144,145 |
| Transforming growth factor-1 | 146,147 |
| Erythropoietin | 148 |
| Insulin-like growth factor-I | 149 |
| Vascular endothelial growth factor | 150,151 |
| Others | |
| Purity of myoblasts (Desmin) | 33 |
| Donor and host myosin heavy chain | 152 |
| Heat shock treatment | 55,153 |
| Optimization of injection procedure | 142 |
| Fibrin glue | 48 |
3.1 Combining preconditioning approach with stem cell engraftment
A more effective approach in this regard, however, would be to combine the powerful cytoprotective effects of preconditioning with stem cell engraftment 50,51. Since the pioneering work of Murry and colleagues which showed that cyclic episodes of brief ischemia and reperfusion, referred to as ischemic preconditioning, rendered the heart more tolerant to subsequent lethal ischemia, preconditioning is considered as the most potent and effective mean of cytoprotection 52. These authors showed that four 5-minute cycles of ischemia with intermittent reperfusion protected the heart from a subsequent episode of sustained ischemia and infarct size was reduced up to 75%. The cytoprotective effects of ischemic preconditioning can be mimicked in multiple ways such as pharmacological treatment, heat shock intervention, genetic modification of cells for overexpression of growth factors and survival pathway molecules, and ultrasound treatment of cells etc. 53–55.
3.1.1 Ischemic preconditioning for cytoprotection
Ischemic preconditioning improves ischemic tolerance in various body tissues including skeletal and cardiac muscles 56,57 58. In the experimental settings for cardioprotection, ischemic preconditioning significantly attenuated myocardial infarct size by reducing both necrotic and apoptotic myocyte death 59,60. Nevertheless, the protective mechanism of ischemic preconditioning remains contentious despite more than two decades of research. To date, the reported signal transduction pathways initiated by ischemic preconditioning involve the activation of a diverse array of survival proteins including protein kinases A, C, and G, members of the MAPK family (Erk1/2, p38, JNK and BMK1), the phosphatidylinositol`3 -kinase (PI3K) PI3K-Akt cascade, and the JAK-STAT pathway 56,61–63. In addition to initiation of survival signaling, induction of iNOS in host cardiomyocytes in response to ischemic preconditioning has been implied as a possible alternative mechanism underlying cardioprotection. Subsequent studies have shown that endothelial progenitor cells which act as reservoirs of cytokines get mobilized to the heart in response to ischemic preconditioning and express an array of potentially cardioprotective cytokines including nitric oxide synthase to impart cardioprotection 64. Further studies confirmed the role of growth factors which are upregulated in response to ischemic preconditioning. In the mice subjected to ischemic preconditioning, myocardial SDF-1α mRNA increased within hours after preconditioning and attenuated the infarct size 65. Similar observations were made in vitro in cardiomyocytes and resulted in phosphorylation of both ERK1/2 and Akt, decreased phosphorylation of JNK and p38 thus increasing their resistance to hypoxia/ reoxygenation damage. Ischemic preconditioning also enhances vascular endothelial growth factor (VEGF) in the heart and the cardioprotective effects of ischemic preconditioning were abolished in heterozygous Flt-1 knockout mice 66. These studies show a clear relationship between cardioprotection and growth factor expression.
3.1.2 Pharmacological preconditioning
Activation of mitochondrial pathways which promote cell survival is endogenously occurring process and constitutes an integral part of the homeostatic mechanism 52. These effects have been mimicked via pharmacological intervention using mitochondrial potassium channel openers 67,68. A chemically diverse group of compounds such as nicorandil, bimakalim, minoxidil and diazoxide share the property of promoting K+ current through ATP sensitive K+-channels 69–72. Amongst these, the prototype mitoKATP channel opener diazoxide has been widely demonstrated to suppress cell apoptosis and promote cell survival 50,73. Although their exact mechanism of cytoprotection remains undefined, these pharmacological agents act via multiple mechanisms. This may include succinate dehydrogenase inhibition, mitochondrial depolarization, protein kinase-C (PKC) activation and involvement of a potassium conductance-independent pathway for cellular protection 74–77. Our group has shown that cardiac protection from mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria 78.
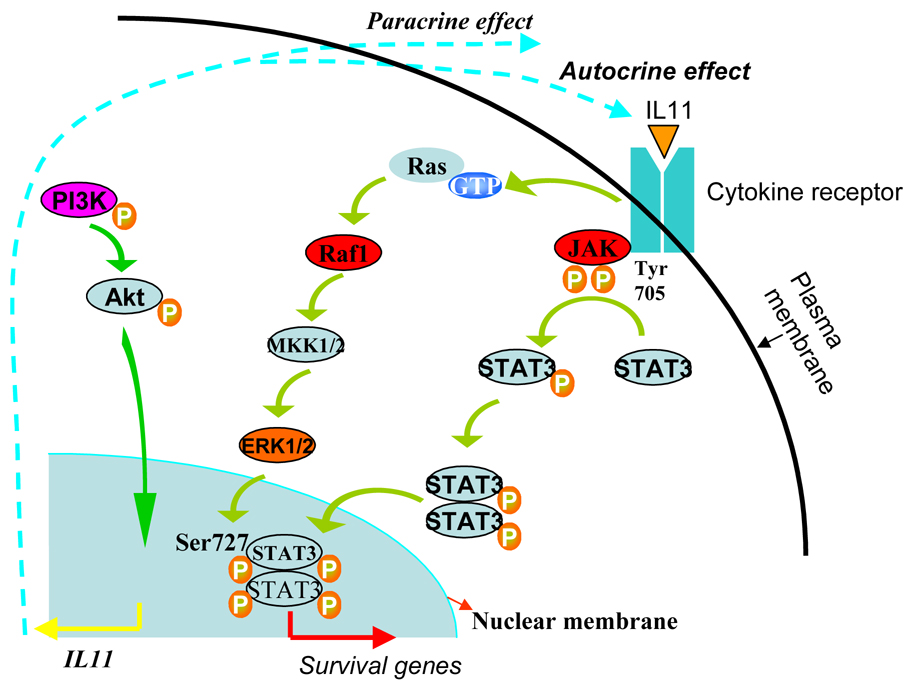
In vitro cellular models are being studied in order to elucidate the underlying molecular mechanisms of tolerance and anti-apoptotic effects after preconditioning 68,79,80. Based on these observations, it is hypothesized that preconditioning initiates survival signaling in the donor cells and set these cells into a “primed state” to encounter the rigorous harsh microenvironment of the ischemic myocardium. The preconditioned cells also release biologically active factors to act in a paracrine manner to exert cytoprotective effects on host myocytes. Depending upon the preconditioning stimulus, signaling pathways thus induced increase proliferation and differentiation of the implanted cells. In a recent study, we have shown that preconditioning of skeletal myoblasts using diazoxide was effective in promoting their survival in the infarcted heart post engraftment 81. Elucidating the possible underlying mechanism, we observed that diazoxide preconditioning of the cells significantly favored Akt phosphorylation; a step which is crucial in survival signaling cascades. We also observed explicit increase of IL11 protein levels in the preconditioned myoblasts which occurred in PI3K/Akt and p42/44 MAPK-STAT3 dependent fashion, thus suggestive of its involvement in survival signaling of preconditioned myoblasts. We thus proposed that the secreted IL11 acted in autocrine and paracrine fashion to promote survival signaling in the preconditioned myoblasts and enhanced their survival under oxidant stress in vitro as well as post engraftment in a rat heart model of acute myocardial infarction (Figure 2). In order to demonstrate the versatility of this approach, we have successfully preconditioned bone marrow derived mesenchymal stem cells to promote their resistance to oxidant stress and achieve improved survival in the infarcted heart 53. Interestingly we observed that preconditioning promoted mesenchymal stem cells survival and proliferation via activation of NFκB downstream of PI3K/Akt signaling. The preconditioned mesenchymal stem cells showed higher activity of phospho Akt as was evident from Akt activity assay and upregualted expression of RelA (member of NFκB). Treatment of cells with wortmannin prior to preconditioning abolished the effects of preconditioning and significantly reduced their survival post engraftment. Our results clearly depict that preconditioning of cells with diazoxide involves more than just K+ channel activity and multiple other molecular mechanisms are involved in the preconditioned cells which improve their resistance to oxidant stress. Moreover, pharmacological preconditioning of stem cells before transplantation will be highly effective in overcoming the problem of donor cell death post engraftment.
Figure 2.
Proposed mechanism involved in pharmacological preconditioning of skeletal myoblasts. Release of IL11 downstream of PI3K/Akt triggers autocrine and paracrine effects on the cells and involves ERK1/2 and STAT3 activation to promote cell survival.
3.2 Heat shock preconditioning
Among other mechanisms, preconditioning involves stressor factors or heat-shock proteins (Hsps) to act as molecular chaperones to regulate newly synthesized proteins to be folded and transported across the membrane, or prevent incorrect protein folding and transportation thus enhancing the ability of stressed cells to cope with increased concentrations of denatured proteins 82,83. Hsps are recognized as molecules that maintain cellular homeostasis during changes in the environment and their role under various pathological conditions has also been elucidated based on their altered gene expression 84. Hsps can be induced under stressful conditions especially by physiologically relevant hyperthermia and are responsible for survival and antiapoptotic signaling in cells. The genomic response of mammalian cells to thermal stress however, is considerably more complex than previously recognized. It is therefore difficult to perform large-scale analysis of the effects of heat shock on normal cells. Hsps encompass a plethora of proteins that range in molecular weight from 10 to 150 kDa and are present in most cells albeit with variable pattern of distribution and expression 85. Of all the Hsp families, Hsp70 family is considered to be induced as the first response to non-physiological conditions and includes the constitutively expressed Hsp73 in normal cells and the highly stress-inducible Hsp72. Hsp70 is positively related with thermotolerance of the cells and has been well studied for its role in stress related cytoprotective effects in general and cardioprotective actions in particular. The potential mechanism responsible for the cytoprotective effects of Hsp70 on stress-induced injury remains undefined. Molecular studies have shown p38 MAPK and c-jun N-terminal kinase (JNK) increase levels of Hsp70 in the heart in response to hypoxia 86. The role of cellular c-jun to promote cytoprotective activity of Hsp has also been studied in relation with Hsp90 87. In addition, induction of Fas-mediated signaling rapidly decreases inducible Hsp70 expression, and activation of Hsp70 suppresses Fas-mediated apoptosis 88. These data imply that the inducible Hsp70 possibly acts as a negative regulator of Fas-mediated apoptosis. More so, the protective effects of Hsps are partly due to prevention of pro-inflammatory cytokine production and down-regulation of NF-κB in several cell types 89.
Keeping in view the cytoprotective nature of Hsps, the approach of heat shock preconditioning may be exploited to promote donor cell survival under oxidant stress in vitro and post engraftment in heart cell therapy. Due to the variable and complex nature of heat shock response from different cells, the preconditioning process needs to be extensively optimized prior to cell engraftment. In a study involving cardiac myoblasts, exposure of the cells to physiologically relevant hyperthermia improved their subsequent resistance to oxidant stress 90. The temperature and duration of exposure are crucial determinants of the quality and the extent of resistance developed in the cells. Whereas chronic exposure to 39°C produced no discernible change in proliferation and intrinsic ATP levels in the cells, it successfully promoted their resistance to subsequent oxidant stress via constitutive upregulation of Hsp70 which served as a first line of defense. Thermal shock also promoted cell division and proliferation during acute phase post engraftment but there was no evidence that such a treatment contributed towards long term improvement in donor cell engraftment 55,91. Studies have shown that the genetically modified myocytes overexpressing Hsp72 were more tolerant to hypoxic stress as compared with their normal counterparts 92. Similar results were later duplicated in Hsp70 transgenic animals in the heart which conferred attenuated infarct size and significant functional recovery subsequent to experimental ischemia and reperfusion injury 93. These results clearly demonstrate a possible role of Hsps as an effector molecule during preconditioning. To date, there is only meager published data investigating the direct consequences of thermic shock to induce differentiation pathways. Short-term chemical stimulation of rat mesenchymal stem cells using 2 mM β-mercaptoethanol for neuronal differentiation induced Hsp72 synthesis which simultaneously increased their survival under oxidant stress 94. Heat shock treatment of telomerase-immortalized human mesenchymal stem cells prior to culture in differentiation medium induced osteoblastic differentiation of the cells 95.
Besides Hsp70, all other Hsps in general and the sub-family of small Hsps in particular are involved in cytoprotection 96. Small Hsps constitute a large family of proteins with monomeric molecular weight of 10–30 kDa. Unlike the high molecular weight Hsps which are involved in protein folding in vivo, small Hsps play an important role in protecting the organism from stress. Hsp27 is highly expressed in the heart and acts as an endogenous cytoprotective stress response protein, eliciting cardioprotection via its role as a molecular chaperone. By serving as the terminal substrate of the p38 MAPK cascade, Hsp27 affords a functional link between p38 MAPK and the actin cytoskeleton 97. It also interacts with Akt to maintain the kinase in an active conformation state 98. Transgenic overexpression of Hsp20 in cardiomyocytes was associated with improved contraction and protection against beta-agonist-induced apoptosis. The extent of infarction and apoptotic cell death was significantly reduced in the transgenic hearts with an associated increase in protein ratio of Bcl-2/Bax and reduced caspase-3 activity.
3.3 Cytokine preconditioning of stem cells
Deprivation of trophic factors induces apoptosis in the cells which are dependent on these factors for survival. Treatment of stem cells with growth factor proteins which orchestrate improved survival, differentiation and proliferation may significantly improve their effectiveness in cardiac repair process 99. Cytokine preconditioning basically involves growth factor interaction with growth factor receptors present on the surface of cells to activate downstream signal transduction for cell survival.
Unlike other chemokines which interact with multiple G-protein coupled receptors, SDF-1α mediates its effects through its only known specific receptor CXCR4. The SDF-1α/CXCR4 ligand/receptor system is distributed in different tissues and cell types and modulates several biological functions through signal transduction pathways. These include increased cell growth, proliferation, survival and anti-apoptosis, emigrational and transcriptional activation. We have recently shown that chemokine preconditioning of mesenchymal stem cells by treatment with the recombinant SDF-1α protein enhanced their survival and proliferation under anoxic conditions in vitro and after engraftment in the infarcted heart 51. The pro-survival effects of SDF-1α were abolished by pretreatment of the cells with CXCR4 blocker AMD3100 and were found to involve activation of PI3K/ Akt pathway. The preconditioned mesenchymal stem cells also released antiapoptotic and angiogenic cytokines for paracrine consequences. The cumulative effect of preconditioning on myocyte regeneration, angiogenesis and cell survival in the ischemic heart resulted in reduced infarct size and left ventricle remodeling. Similar results have also been observed with other growth factors. The addition of VEGF2 to endothelial progenitor cell cultures resulted in significant and dose-dependent decreases in endothelial progenitor cell apoptosis. Incidentally, the cells treated with VEGF-2 showed elevated expression of phosphorylated Akt 46. In an interesting study, H9c2 cardiomyoblasts grown on collagen matrix supplemented with VEGF and basic fibroblast growth factor (bFGF) showed improved survival and growth characteristics. The bio-artificial graft thus generated was later used for myocardial restoration 49. The use of bio-artificial graft approach may also promote retention of the transplanted cells as it will overcome the problem of cellular washout which generally accounts for significantly high loss of cells after intramyocardial injection.
Besides anti-apoptotic effects, cytokine preconditioning may stimulate the release of growth factors for paracrine influence on the host myocytes and for paracrine/ autocrine cues to direct the stem cells to adopt certain specific phenotype. Different growth factors act as key mediators of cellular differentiation which is regulated by the multiple factors. Besides other factors, the developmental fate of stem cells is determined by their inherent plastic nature and the extracellular micro environmental cues emanating from growth factors constituting the cardiac milieu. Conversely, an optimal combination of growth factors for directed differentiation of stem cells into the desired lineage remains a challenge. Many of these growth factors exert non-specific effects on stem cell differentiation through the activation of multiple intracellular signaling pathways. Culture of CD133+ cells in VEGF165 and brain-derived nerve growth factor, either alone or together, promoted their myo-endothelial co-differentiation 100. In the same context, we have also performed transduction of embryonic stem cells for VEGF165 over expression which caused their endothelial differentiation besides incurring apoptotic resistance 101. Several growth factor including bone morphogenetic protein-2 and bFGF, have been implicated as critical components of this early cardiomyogenic inductive signaling 102. Pretreatment of bone marrow cells with a cocktail of growth factors promotes their cardiomyogenic differentiation 103. Transforming growth factor (TGF) family includes 30 growth and differentiation factors including TGF-β, activins, inhibins, and bone morphogenetic proteins. TGF-β is important for progression of differentiation in most cells. There is an evidence that TGF-β family proteins are able to redirect differentiation of stem cells that have fully differentiated or are engaged in differentiation along a particular lineage 104.
Most of the above-mentioned studies reflect the cytoprotective effects of growth factors and their ability to direct their differentiation. We have recently shown that IGF-1 preconditioning of stem cells has dual effects of cytoprotection as well as improved integration of the preconditioned cells with the host myocytes post engraftment via upregulated expression of connexin 43. A wide range of studies have shown that IGF-1R has specific anti-apoptotic signaling capacity which enables IGF-1 to mediate intracellular signaling. IGF-1/IGF-1R ligand/receptor interaction causes sequential activation of signal transduction pathways which prevents cell apoptosis 105. Two major pathways activated by IGF-1/IGF-1R interaction included PI3K/Akt and mitogen-activated protein kinase (MAPK)/Erk1/2. The Akt pathway is initiated by the interaction of IGF-1R with one of its major substrates, insulin receptor substrate-1 that activates PI3K and Akt. The MAPK (p44/p42) is another anti-apoptotic pathway of the IGF-1R system and gets activated by mitogenic signals, DNA damage, or oxidative stress. Furthermore, this particular pathway has been implicated in the protection from apoptosis by IGF-1R 106. In one of our recently concluded studies, we performed ex vivo preconditioning of bone marrow derived Sca-1+ cells with IGF-1 which markedly promoted their survival and proliferation in the infarcted heart. Elucidating the mechanism of IGF-1 preconditioning, we found that the preconditioned cells had higher phosphorylation of IGF-1R which activated 5 fold more expression of connexin 43 in the preconditioned cells in a PI3K/Akt dependent manner. Interestingly, when connexin 43 gene silencing studies were performed in the IGF-1 preconditioned cells using connexin 43 specific siRNA, we observed that the survival of the cells was significantly compromised under anoxia in vitro and after engraftment in the infarcted rat heart. These observations clearly depicted a role for connexin 43 in Sca-1+ cell survival downstream of PI3K/Akt. Interestingly, the preconditioned cells continued to overexpress connexin 43 after engraftment which helped these cells to engraft better than their non-preconditioned counterparts. Thus it is clear that IGF-1 preconditioning of stem cells concomitantly met the two fundamental requirements for heart cell therapy; donor cell survival and their engraftment, by upregulated expression of connexin 43 which played a dual role of cytoprotection as well as engraftment of the transplanted cells.
Besides growth factors, delivery of the pro-survival factor cocktail has been attempted to achieve improved survival of the donor cells 107. The full pro-survival factor cocktail containing 50% (vol/vol) growth factor–reduced Matrigel, supplemented with ZVAD (100 mM, benzyloxycarbonyl-Val-Ala-Asp(O-methyl)-fluoromethyl ketone, Calbiochem), Bcl-XL BH4 (cell-permeant TAT peptide, 50 nM, Calbiochem), cyclosporine A (200 nM, Wako Pure Chemicals), IGF-1 (100 ng/ml, Peprotech) and pinacidil (50 mM, Sigma). The constitution of the cocktail was based on an attempt to protect the cells by addressing the most prevalent multiple parallel processes responsible for cell death.
Despite promising results, one impediment in the use of growth factors or proteins from survival signaling pathways is the short biological half-lives of most of the protein factors which necessitates multiple administrations of growth factors to have stable therapeutic effects. This problem may be overcome by genetic modification of stem cells for growth factor expression.
3.4 Genetic modulation of stem cells
Ex-vivo genetic modulation of stem cells prior to transplantation has been extensively studied for delivery of transgenes encoding for growth factors and anti-apoptotic factors to confer apoptotic resistance and enhance cell viability of the donor cells and host myocytes 108. There is evidence in literature that the cells overexpressing angiogenic growth factors show better survival and neovascularization after engraftment and improve cardiac function by limiting the remodeling process in the scar and/or decreasing apoptosis of the hypertrophied myocytes in peri-infarct region 11,44,45. Moreover, the genetically modified cells serve as a reservoir of the therapeutically active transgene expression product which may function in autocrine and paracrine manner to confer therapeutic effects.
3.4.1 Genetic modulation of stem cells for growth factor expression
The delivery of angiogenic growth factors by genetic manipulation has given encouraging results 44,109. Transplantation of ex-vivo genetically modified cells for VEGF grants early phase and late phase effects. Firstly, survival signaling is activated in the cells which reduces apoptotic index of donor cells and host myocytes. Secondly, there is improved regional blood flow in the ischemic myocardium through VEGF induced vasodilatation thus leading to preservation of the cell graft as well as myocardial architecture in the ischemic area during the early phase after engraftment. This is followed by late phase angiogenic effect which salvages the host myocardium, in conjunction with the functional benefits of cellular cardiomyoplasty from the donor cell derived neomyogenesis 110.
Heme oxygenase-1 (HO-1), the rate-limiting enzyme of heme catabolism, is known to modulate various cellular functions, including cytokine production, cell proliferation, and apoptosis, in stress-related conditions. HO-1 plasmid modification of graft mesenchymal stem cells protected the cells from subsequent hypoxia injury in vitro when subjected to 1% oxygen for 24 h 111. Engraftment of the HO-1 gene modified cells showed 5-fold increase in cell graft survival in ischemic heart in vivo as compared with the cells without gene modification. The increase in cell survival was attributed to anti-inflammatory and anti-apoptosis activities of HO-1 which reduced infiltration of mononuclear cells and down-regulated the expression of pro-inflammatory cytokines. These findings underscore the role of angiogenic growth factor gene delivery for protection of the cell graft from ischemia/inflammation induced death. Similar results have also been reported with other angiogenic growth factors 112.
In an attempt to enhance cytoprotection with angiogenic gene delivery, multimodal gene therapy approach has been devised in which multiple growth factor genes are concomitantly delivered to the heart for their combined effects. Nevertheless, an optimal combination of multiple genes is required to be determined for their supplemental and synergistic interaction to achieve the desired therapeutic outcome. Yau and colleagues delivered VEGF and IGF-1 encoding transgenes to the infarcted rat heart using mesenchymal stem cells 113. Although, independent expression of VEGF and IGF-I was cytprotective, the combined VEGF and IGF-1 delivery synergistically contributed towards survival of the cell graft. On the same note, we have reported a novel bicistronic vector encoding for VEGF and angiopoietin-1 (Ang-1) which was used for simultaneous delivery of the two genes to infarcted heart in a porcine model of coronary artery ligation 114. The transfected cells co-overexpressed VEGF and Ang-1 at the site of cell graft with resultant attenuation of infarct size expansion and improved regional blood flow in the infarcted myocardium.
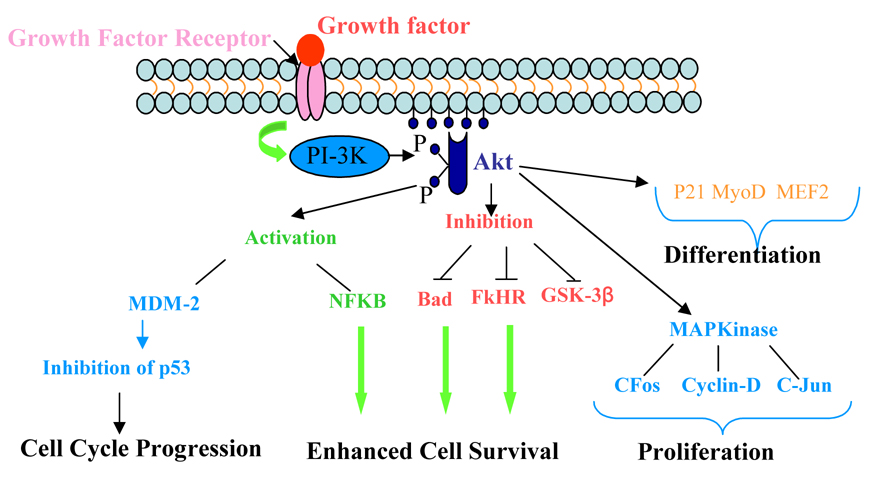
IGF-I is an important extracellular effector molecule which is produced in the heart and other tissues and acts in both autocrine as well as paracrine manner for pleiotropic effects. IGF-1 gene delivery has also been combined with heart cell therapy to promote donor cell survival, engraftment and differentiation in the host myocardium 115,116. In a recently concluded study, we have carried out mesenchymal stem cell based delivery of IGF-1 to the infarcted rat heart and exploited IGF-1/IGF-1 ligand/receptor signaling for distinct beneficial effects on the carrier cells as well as host myocytes to promote their survival and proliferation (our unpublished data). IGF-1 overexpressing mesenchymal stem cells showed significantly higher survival when subjected to 8h anoxia as compared with the non-transfected cells. Molecular studies showed that IGF- 1 overexpression exerted its biological effects by binding to its transmembrane receptors and stimulated Akt phosphorylation at Thr308 and Bcl2 via PI3K signaling. Upon transplantation into infarcted rat heart, the transplanted cells continued to overexpress IGF-1 and protected the host myocytes as was evident from lower TUNEL positivity. Histochemical examination of tissue sections at 7 days post engraftment of the cells showed numerous islands of the surviving host myocytes in the centre of the infarct as a result of IGF-1 overexpression on host myocytes (Figure 3).
Figure 3.
Schematic representation of growth factor interaction with growth factor receptors on the cell surface to initiate PI3K/Akt pathway responsible for cell cycle progression, survival, and proliferation.
Intrinsically produced SDF-1α protein level in the heart remains elevated for 5–7 days after infarction thus signifying its specific role in the repair process 117. Protein and gene delivery of human SDF-1α (hSDF-1α) targeted to the infarcted heart, has been employed for cytoprotection of myocytes in order to attenuate infarct size expansion 51,118. In our subsequent work, overexpression of hSDF-1α supported cell survival during the initial phase after transplantation 119. Our results depicted successful and efficient non-viral vector transfection of skeletal myoblasts with hSDF-1α using FuGene™6 in the presence of ZnCl2 which was effective for indirect gene therapy after myocardial infarction.
3.4.2 Transgenic overexpression of pro-survival signaling molecules
Targeting of pro-survival genes is a novel modality to modulate cell survival signaling for the desired therapeutic effects. Transgenic overexpression of pro-survival signaling molecules promotes resistance of the donor cells under ischemia and supports their survival. PI3-kinase is a critical component in a signal transduction pathway which mechanistically acts through activation of Akt 120. Transduction of cardiomyocytes with PI3-kinase encoding adenoviral vector for overexpression of PI3-kinase significantly enhanced their survival upon subsequent exposure to hypoxia 121. This was attributed to the activation of various protein factors including Akt, a major player in survival signaling cascade in many systems, and its downstream pro-survival signaling molecules 122. Therefore, Akt gene modification of stem cells has been demonstrated to significantly enhance their survival post engraftment in the infarcted heart 45. Intramyocardial injection of 5 × 106 Akt overexpressing MSCs reduced collagen deposition and attenuated infarct size in rat heart model by inhibition of cardiomyocyte apoptosis in the peri-infarct area. There was significant inhibition of the remodeling process and complete normalization of systolic and diastolic functions. Later studies from the same research group showed that Akt-modified mesenchymal stem cells released paracrine factors which immensely contributed towards host myocyte survival and functional recovery of the infarcted heart 14. Using a comprehensive functional genomic strategy, secreted frizzled related protein 2 (Sfrp2) was found as the paracrine factor that mediated myocyte survival and repair of the infarcted heart after ischemic injury via modulation of Wnt signaling 123. However, we argue that engraftment and longer term growth of donor cells may be less meaningful if regional blood flow in the ischemic myocardium is not restored. We opted to genetically modify mesenchymal stem cells for concomitant overexpression of Akt and Ang-1 11. Ang-1 has a pivotal role as the modulator of vascular development by promoting formation of stable and leak resistant blood vessels 124. More importantly, Ang-1 enhances endothelial cell survival via PI3K/Akt pathway 125. A major role has been shown for Akt downstream of Ang-1/Tie2 signaling pathway and is the primary mediator of endothelial cell survival via Akt/FKHR transcription factor 126. Our choice of Ang-1 and Akt transgene combination achieved maximum beneficial effects on cell survival and angiogenesis. When subjected to anoxia for 8h, we observed that cells co-overexpressing Akt and Ang-1 were more resistant to anoxic injury as compared with their cell counterparts transduced with null vector or with either of Akt or Ang-1 alone. We also observed that mesenchymal stem cells co-overexpressing Akt and Ang-1 were able to engraft better after transplantation in an infarcted rat heart and were able to adopt myogenic and endothelial phenotype.
Alternatively, the 26 kD antiapoptotic protein Bcl-2 belongs to the Bcl-2 family of proteins and serves as a critical regulator of pathways involved in apoptosis. Bcl-2 regulates the metabolic functions of mitochondria during ischemic conditions and contributes to cytoprotection 127. Mesenchymal stem cells genetically modified to over express Bcl-2 can delay the onset of cell death and modestly augment viable cell growth in the first 48 hours of apoptosis. Cardiomyoblasts overexpressing Bcl-2 showed increased resistance to apoptosis and survived better after engraftment 128. Interestingly, overexpression of Bcl-2 in transgenic mice improved the heart function and inhibited cardiomyocytes apoptosis 129. Bcl-2 gene is known not only for regulating apoptosis but also controls non-apoptotic cell death 130. Recently, transgenic cell lines derived from embryonic stem cells expressing Bcl-2 were found to self-renew continuously, even under serum-free and feeder cell-free conditions 131. A comparison of Bcl-2 overexpression and heat shock treatment for anti-apoptotic effects on smooth muscle cells showed significantly reduced cell loss after transplantation and improved cardiac function after myocardial infarction. Still, Bcl-2 modified cells showed better survival and reduced inflammatory response 132. Despite encouraging results from the studies which involved targeting of survival signaling molecules in the cells, the approach is not without some undesired effects. The downside of this approach is that overexpression of Akt and Bcl-2 genes may predispose the cells to neoplasia and hence, increase the risk of tumorigenesis. Therefore, the level of expression and the time duration for it’s continuation should be optimized to avoid undesired outcome.
4. Concluding remarks
Improvement in cardiac function subsequent to stem cell transplantation is directly related with the number of cells injected and retained at the site of cell graft. The loss of cells due massive cell death and cellular washout is generally compensated by a very high number of cell engraftment. Optimization of cell engraftment conditions with special emphasis on cell survival strategies may significantly enhance the functional outcome of the procedure. Cell death is a multi-factorial phenomenon and may warrant multi-prong strategy to improve cell survival. Addressing one single factor responsible for cell death may not be an effective strategy. Defining approaches to reduce the harsh microenvironment of the host ischemic myocardium and priming the cells to a state of ‘readiness’ by stimulation of their survival signaling pathways may significantly address this issue. Ischemic preconditioning is a powerful protective phenomenon against lethal ischemic injury. Signaling pathways of ischemic preconditioning can be successfully employed for anti-apoptotic measures in cell based therapies. Cytokine treatment prevents left ventricular remodeling and dysfunction after myocardial infarction through increased neovascularization and reduced apoptosis in the border area 133. Cytokine therapy combined with stem cell transplantation for priming the heart prior to stem cell transplantation makes the host myocardial milieu more hospitable for the cells. Alternatively, preconditioning of stem cells with cytokines may upregulate pro-survival proteins and transcription factors to facilitate their directed differentiation to adopt specific cardiac phenotype. Pharmacological preconditioning may be more appropriate to take advantage of molecular mechanisms responsible for protection against ischemic injury during stem cell transplantation and further activating endogenous cellular machinery for cellular regeneration. With the emergence of paracrine factor hypothesis, combining multiple cell types with inherent ability to release paracrine factors, co-administration of cell types dedicated to varying potentials and different functions may help the donor cells survive better 134. The success of this approach however, will be determined by the choice of the donor cells being employed for simultaneous engraftment. Combining cells with angiogenic as well as myogenic potential may provide an ideal combination to develop a biological bypass via neovascularization to restore regional blood flow in the ischemic myocardium and improve contractile function of left ventricle by undergoing myogenic differentiation. Additionally, these cells may be preconditioned to fortify their resistance to ischemia through stimulation of their survival signaling pathways.
Acknowledgements
This work was supported by National Institutes of Health grants # R37-HL074272; HL-23597; HL70062 and HL-080686 (to M.A).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anversa P, Leri A, Rota M, Hosoda T, Bearzi C, Urbanek K, Urbanek J, Bolli R. Concise review: stem cells, myocardial regeneration, and methodological artifacts. Stem Cells. 2007;25:589–601. doi: 10.1634/stemcells.2006-0623. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez A, Garcia-Sancho J. Cardiac repair by stem cells. Cell Death Differ. 2007;14:1258–1261. doi: 10.1038/sj.cdd.4402146. [DOI] [PubMed] [Google Scholar]
- 3.Invernici G, Cristini S, Madeddu P, Brock S, Spillmann F, Bernasconi P, Cappelletti C, Calatozzolo C, Fascio U, Bisleri G, Muneretto C, Alessandri G, Parati EA. Human adult skeletal muscle stem cells differentiate into cardiomyocyte phenotype in vitro. Exp Cell Res. 2007 doi: 10.1016/j.yexcr.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Hagege AA, Marolleau JP, Vilquin JT, Alheritiere A, Peyrard S, Duboc D, Abergel E, Messas E, Mousseaux E, Schwartz K, Desnos M, Menasche P. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation. 2006;114:I108–I113. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe E, Smith DM, Jr, Delcarpio JB, Sun J, Smart FW, Van Meter CH, Jr, Claycomb WC. Cardiomyocyte transplantation in a porcine myocardial infarction model. Cell Transplant. 1998;7:239–246. doi: 10.1177/096368979800700302. [DOI] [PubMed] [Google Scholar]
- 6.Kim WG, Park JJ, Chung DH, Na CY. Autologous cardiomyocyte transplantation in an ovine myocardial infarction model. Int J Artif Organs. 2002;25:61–66. doi: 10.1177/039139880202500110. [DOI] [PubMed] [Google Scholar]
- 7.Etzion S, Barbash IM, Feinberg MS, Zarin P, Miller L, Guetta E, Holbova R, Kloner RA, Kedes LH, Leor J. Cellular cardiomyoplasty of cardiac fibroblasts by adenoviral delivery of MyoD ex vivo: an unlimited source of cells for myocardial repair. Circulation. 2002;106:I125–I130. [PubMed] [Google Scholar]
- 8.Li RK, Jia ZQ, Weisel RD, Merante F, Mickle DA. Smooth muscle cell transplantation into myocardial scar tissue improves heart function. J Mol Cell Cardiol. 1999;31:513–522. doi: 10.1006/jmcc.1998.0882. [DOI] [PubMed] [Google Scholar]
- 9.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haider H, Asjraf M. Bone marrow cell transplantation in clinical perspective. J Mol Cell Cardiol. 2005;38:225–235. doi: 10.1016/j.yjmcc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Jiang S, Haider H, Idris NM, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 12.Tomita S, Mickle DA, Weisel RD, Jia ZQ, Tumiati LC, Allidina Y, Liu P, Li RK. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg. 2002;123:1132–1140. doi: 10.1067/mtc.2002.120716. [DOI] [PubMed] [Google Scholar]
- 13.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 14.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 15.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 16.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 17.Bel A, Messas E, Agbulut O, Richard P, Samuel JL, Bruneval P, Hagege AA, Menasche P. Transplantation of autologous fresh bone marrow into infracted myocardium: a word of caution. Circulation. 2003;108 Suppl 1:II247–II252. doi: 10.1161/01.cir.0000089040.11131.d4. [DOI] [PubMed] [Google Scholar]
- 18.Bartunek J, Vanderheyden M, Wijns W, Timmermans F, Vandekerkhove B, Villa A, Sanchez PL, Arnold R, San Roman JA, Heyndrickx G, Fernandez-Aviles F. Bone-marrow-derived cells for cardiac stem cell therapy: safe or still under scrutiny? Nat Clin Pract Cardiovasc Med. 2007;4 Suppl 1:S100–S105. doi: 10.1038/ncpcardio0744. [DOI] [PubMed] [Google Scholar]
- 19.Singla DK, Hacker TA, Ma L, Douglas PS, Sullivan R, Lyons GE, Kamp TJ. Transplantation of embryonic stem cells into the infarcted mouse heart: formation of multiple cell types. J Mol Cell Cardiol. 2006;40:195–200. doi: 10.1016/j.yjmcc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Gallo P, Condorelli G. Human embryonic stem cell-derived cardiomyocytes: inducing strategies. Regen Med. 2006;1:183–194. doi: 10.2217/17460751.1.2.183. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–I54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan X, Zhang H, Wei YJ, Hu SS. Embryonic stem cell transplantation for the treatment of myocardial infarction: Immune privilege or rejection. Transpl Immunol. 2007;18:88–93. doi: 10.1016/j.trim.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Swijnenburg RJ, Sheikh AY, Robbins RC. Comment on "Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response". Faseb J. 2007;21:1290. doi: 10.1096/fj.07-0502ufm. author reply 1291. [DOI] [PubMed] [Google Scholar]
- 24.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barile L, Chimenti I, Gaetani R, Forte E, Miraldi F, Frati G, Messina E, Giacomello A. Cardiac stem cells: isolation, expansion and experimental use for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2007;4 Suppl 1:S9–S14. doi: 10.1038/ncpcardio0738. [DOI] [PubMed] [Google Scholar]
- 26.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Ye L, Haider H, Sim EK. Adult stem cells for cardiac repair: a choice between skeletal myoblasts and bone marrow stem cells. Exp Biol Med (Maywood) 2006;231:8–19. doi: 10.1177/153537020623100102. [DOI] [PubMed] [Google Scholar]
- 28.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 29.Khan M, Kutala VK, Vikram DS, Wisel S, Chacko SM, Kuppusamy ML, Mohan IK, Zweier JL, Kwiatkowski P, Kuppusamy P. Skeletal myoblasts transplanted in the ischemic myocardium enhance in situ oxygenation and recovery of contractile function. Am J Physiol Heart Circ Physiol. 2007;293:H2129–H2139. doi: 10.1152/ajpheart.00677.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkins BZ, Hueman MT, Meuchel J, Hutcheson KA, Glower DD, Taylor DA. Cellular cardiomyoplasty improves diastolic properties of injured heart. J Surg Res. 1999;85:234–242. doi: 10.1006/jsre.1999.5681. [DOI] [PubMed] [Google Scholar]
- 31.Guarita-Souza LC, Carvalho KA, Woitowicz V, Rebelatto C, Senegaglia A, Hansen P, Miyague N, Francisco JC, Olandoski M, Faria-Neto JR, Brofman P. Simultaneous autologous transplantation of cocultured mesenchymal stem cells and skeletal myoblasts improves ventricular function in a murine model of Chagas disease. Circulation. 2006;114:I120–I124. doi: 10.1161/CIRCULATIONAHA.105.000646. [DOI] [PubMed] [Google Scholar]
- 32.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 33.Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 35.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, Dinsmore JH, Wright S, Aretz TH, Eisen HJ, Aaronson KD. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 36.Hodgetts SI, Beilharz MW, Scalzo AA, Grounds MD. Why do cultured transplanted myoblasts die in vivo? DNA quantification shows enhanced survival of donor male myoblasts in host mice depleted of CD4+ and CD8+ cells or Nk1.1+ cells. Cell Transplant. 2000;9:489–502. doi: 10.1177/096368970000900406. [DOI] [PubMed] [Google Scholar]
- 37.Khoynezhad A, Jalali Z, Tortolani AJ. Apoptosis: pathophysiology and therapeutic implications for the cardiac surgeon. Ann Thorac Surg. 2004;78:1109–1118. doi: 10.1016/j.athoracsur.2003.06.034. [DOI] [PubMed] [Google Scholar]
- 38.Baldi A, Abbate A, Bussani R, Patti G, Melfi R, Angelini A, Dobrina A, Rossiello R, Silvestri F, Baldi F, Di Sciascio G. Apoptosis and post-infarction left ventricular remodeling. J Mol Cell Cardiol. 2002;34:165–174. doi: 10.1006/jmcc.2001.1498. [DOI] [PubMed] [Google Scholar]
- 39.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 40.Yamaoka M, Yamaguchi S, Suzuki T, Okuyama M, Nitobe J, Nakamura N, Mitsui Y, Tomoike H. Apoptosis in rat cardiac myocytes induced by Fas ligand: priming for Fas-mediated apoptosis with doxorubicin. J Mol Cell Cardiol. 2000;32:881–889. doi: 10.1006/jmcc.2000.1132. [DOI] [PubMed] [Google Scholar]
- 41.Logue SE, Gustafsson AB, Samali A, Gottlieb RA. Ischemia/reperfusion injury at the intersection with cell death. J Mol Cell Cardiol. 2005;38:21–33. doi: 10.1016/j.yjmcc.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 43.Shirai T, Rao V, Weisel RD, Ikonomidis JS, Li RK, Tumiati LC, Merante F, Mickle DA. Preconditioning human cardiomyocytes and endothelial cells. J Thorac Cardiovasc Surg. 1998;115:210–219. doi: 10.1016/s0022-5223(98)70459-3. [DOI] [PubMed] [Google Scholar]
- 44.Yau TM, Kim C, Ng D, Li G, Zhang Y, Weisel RD, Li RK. Increasing transplanted cell survival with cell-based angiogenic gene therapy. Ann Thorac Surg. 2005;80:1779–1786. doi: 10.1016/j.athoracsur.2005.04.079. [DOI] [PubMed] [Google Scholar]
- 45.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 46.Shintani S, Kusano K, Ii M, Iwakura A, Heyd L, Curry C, Wecker A, Gavin M, Ma H, Kearney M, Silver M, Thorne T, Murohara T, Losordo DW. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med. 2006;3 Suppl 1:S123–S128. doi: 10.1038/ncpcardio0430. [DOI] [PubMed] [Google Scholar]
- 47.Rosengart TK, Chedrawy EG, Patejunas G, Retuarto M. Vascular endothelial growth factor before cells. J Thorac Cardiovasc Surg. 2005;129:696. doi: 10.1016/j.jtcvs.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 48.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 49.Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY, Hendry SL, Pearl J, Hoyt G, Sista R, Yang PC, Blau HM, Gambhir SS, Robbins RC. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114:I167–I173. doi: 10.1161/CIRCULATIONAHA.105.001297. [DOI] [PubMed] [Google Scholar]
- 50.Niagara MI, Haider H, Jiang S, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 51.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning Enhances Cell Survival and Differentiation of Stem Cells during Transplantation in Infarcted Myocardium. Cardiovasc Res. 2007 doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 52.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 53.Afzal R, Haider H, Niagara MI, Jiang S, Ashraf M. Preconditioning promotes survival and proliferation of mesenchymal stem cells in the infarcted rat heart via activation of NFκB downstream of PI3K/Akt signaling. Circulation. 2007;116:68. [Google Scholar]
- 54.Cui JH, Park SR, Park K, Choi BH, Min BH. Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formation in vivo. Tissue Eng. 2007;13:351–360. doi: 10.1089/ten.2006.0080. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki K, Smolenski RT, Jayakumar J, Murtuza B, Brand NJ, Yacoub MH. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102:III216–III221. doi: 10.1161/01.cir.102.suppl_3.iii-216. [DOI] [PubMed] [Google Scholar]
- 56.Gurke L, Marx A, Sutter PM, Frentzel A, Salm T, Harder F, Seelig J, Heberer M. Ischemic preconditioning improves post-ischemic skeletal muscle function. Am Surg. 1996;62:391–394. [PubMed] [Google Scholar]
- 57.Maulik N, Yoshida T, Engelman RM, Deaton D, Flack JE, 3rd, Rousou JA, Das DK. Ischemic preconditioning attenuates apoptotic cell death associated with ischemia/reperfusion. Mol Cell Biochem. 1998;186:139–145. [PubMed] [Google Scholar]
- 58.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 59.Das S, Engelman RM, Maulik N, Das DK. Angiotensin preconditioning of the heart: evidence for redox signaling. Cell Biochem Biophys. 2006;44:103–110. doi: 10.1385/CBB:44:1:103. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki YJ, Nagase H, Day RM, Das DK. GATA-4 regulation of myocardial survival in the preconditioned heart. J Mol Cell Cardiol. 2004;37:1195–1203. doi: 10.1016/j.yjmcc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Xu M, Wang Y, Ayub A, Ashraf M. Mitochondrial K(ATP) channel activation reduces anoxic injury by restoring mitochondrial membrane potential. Am J Physiol Heart Circ Physiol. 2001;281:H1295–H1303. doi: 10.1152/ajpheart.2001.281.3.H1295. [DOI] [PubMed] [Google Scholar]
- 62.Takashi E, Ashraf M. Pathologic assessment of myocardial cell necrosis and apoptosis after ischemia and reperfusion with molecular and morphological markers. J Mol Cell Cardiol. 2000;32:209–224. doi: 10.1006/jmcc.1999.1067. [DOI] [PubMed] [Google Scholar]
- 63.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 64.Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, Losordo DW. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via "imported" nitric oxide synthase activity. Circulation. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 65.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Addya S, Shiroto K, Turoczi T, Zhan L, Kaga S, Fukuda S, Surrey S, Duan LJ, Fong GH, Yamamoto F, Maulik N. Ischemic preconditioning-mediated cardioprotection is disrupted in heterozygous Flt-1 (VEGFR-1) knockout mice. J Mol Cell Cardiol. 2005;38:345–351. doi: 10.1016/j.yjmcc.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 67.Debska G, Kicinska A, Skalska J, Szewczyk A, May R, Elger CE, Kunz WS. Opening of potassium channels modulates mitochondrial function in rat skeletal muscle. Biochim Biophys Acta. 2002;1556:97–105. doi: 10.1016/s0005-2728(02)00340-7. [DOI] [PubMed] [Google Scholar]
- 68.Kicinska A, Szewczyk A. Protective effects of the potassium channel opener-diazoxide against injury in neonatal rat ventricular myocytes. Gen Physiol Biophys. 2003;22:383–395. [PubMed] [Google Scholar]
- 69.Kis B, Nagy K, Snipes JA, Rajapakse NC, Horiguchi T, Grover GJ, Busija DW. The mitochondrial K(ATP) channel opener BMS-191095 induces neuronal preconditioning. Neuroreport. 2004;15:345–349. doi: 10.1097/00001756-200402090-00027. [DOI] [PubMed] [Google Scholar]
- 70.Busija DW, Katakam P, Rajapakse NC, Kis B, Grover G, Domoki F, Bari F. Effects of ATP-sensitive potassium channel activators diazoxide and BMS-191095 on membrane potential and reactive oxygen species production in isolated piglet mitochondria. Brain Res Bull. 2005;66:85–90. doi: 10.1016/j.brainresbull.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 71.Sato T, Li Y, Saito T, Nakaya H. Minoxidil opens mitochondrial K(ATP) channels and confers cardioprotection. Br J Pharmacol. 2004;141:360–366. doi: 10.1038/sj.bjp.0705613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skak K, Gotfredsen CF, Lundsgaard D, Hansen JB, Sturis J, Markholst H. Improved beta-cell survival and reduced insulitis in a type 1 diabetic rat model after treatment with a beta-cell-selective K(ATP) channel opener. Diabetes. 2004;53:1089–1095. doi: 10.2337/diabetes.53.4.1089. [DOI] [PubMed] [Google Scholar]
- 73.Rajapakse N, Kis B, Horiguchi T, Snipes J, Busija D. Diazoxide pretreatment induces delayed preconditioning in astrocytes against oxygen glucose deprivation and hydrogen peroxide-induced toxicity. J Neurosci Res. 2003;73:206–214. doi: 10.1002/jnr.10657. [DOI] [PubMed] [Google Scholar]
- 74.Takashi E, Wang Y, Ashraf M. Activation of mitochondrial K(ATP) channel elicits late preconditioning against myocardial infarction via protein kinase C signaling pathway. Circ Res. 1999;85:1146–1153. doi: 10.1161/01.res.85.12.1146. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Takashi E, Xu M, Ayub A, Ashraf M. Downregulation of protein kinase C inhibits activation of mitochondrial K(ATP) channels by diazoxide. Circulation. 2001;104:85–90. doi: 10.1161/01.cir.104.1.85. [DOI] [PubMed] [Google Scholar]
- 76.Kudo M, Wang Y, Xu M, Ayub A, Ashraf M. Adenosine A(1) receptor mediates late preconditioning via activation of PKC-delta signaling pathway. Am J Physiol Heart Circ Physiol. 2002;283:H296–H301. doi: 10.1152/ajpheart.01087.2001. [DOI] [PubMed] [Google Scholar]
- 77.Dzeja PP, Bast P, Ozcan C, Valverde A, Holmuhamedov EL, Van Wylen DG, Terzic A. Targeting nucleotide-requiring enzymes: implications for diazoxide-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2003;284:H1048–H1056. doi: 10.1152/ajpheart.00847.2002. [DOI] [PubMed] [Google Scholar]
- 78.Ahmad N, Wang Y, Haider KH, Wang B, Pasha Z, Uzun O, Ashraf M. Cardiac protection by mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria during late preconditioning. Am J Physiol Heart Circ Physiol. 2006;290:H2402–H2408. doi: 10.1152/ajpheart.00737.2005. [DOI] [PubMed] [Google Scholar]
- 79.Hillion JA, Takahashi K, Maric D, Ruetzler C, Barker JL, Hallenbeck JM. Development of an ischemic tolerance model in a PC12 cell line. J Cereb Blood Flow Metab. 2005;25:154–162. doi: 10.1038/sj.jcbfm.9600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kohin S, Stary CM, Howlett RA, Hogan MC. Preconditioning improves function and recovery of single muscle fibers during severe hypoxia and reoxygenation. Am J Physiol Cell Physiol. 2001;281:C142–C146. doi: 10.1152/ajpcell.2001.281.1.C142. [DOI] [PubMed] [Google Scholar]
- 81.Niagara MI, Haider H, Jiang S, Ashraf M. Short and long term fate of preconditioned skeletal myoblasts in the infarcted heart and the role of IL11 in cytoprotection of preconditioned cells. Circulation. 2007;116 doi: 10.1161/01.RES.0000258460.41160.ef. II-133. [DOI] [PubMed] [Google Scholar]
- 82.Muchowski PJ, Schaffar G, Sittler A, Wanker EE, Hayer-Hartl MK, Hartl FU. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci U S A. 2000;97:7841–7846. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Friant S, Meier KD, Riezman H. Increased ubiquitin-dependent degradation can replace the essential requirement for heat shock protein induction. Embo J. 2003;22:3783–3791. doi: 10.1093/emboj/cdg375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitamura Y, Nomura Y. Stress proteins and glial functions: possible therapeutic targets for neurodegenerative disorders. Pharmacol Ther. 2003;97:35–53. doi: 10.1016/s0163-7258(02)00301-7. [DOI] [PubMed] [Google Scholar]
- 85.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 86.Rafiee P, Shi Y, Pritchard KA, Jr, Ogawa H, Eis AL, Komorowski RA, Fitzpatrick CM, Tweddell JS, Litwin SB, Mussatto K, Jaquiss RD, Baker JE. Cellular redistribution of inducible Hsp70 protein in the human and rabbit heart in response to the stress of chronic hypoxia: role of protein kinases. J Biol Chem. 2003;278:43636–43644. doi: 10.1074/jbc.M212993200. [DOI] [PubMed] [Google Scholar]
- 87.Lu C, Chen D, Zhang Z, Fang F, Wu Y, Luo L, Yin Z. Heat Shock Protein 90 Regulates the Stability of c-Jun in HEK293 Cells. Mol Cells. 2007;24:210–214. [PubMed] [Google Scholar]
- 88.Schett G, Metzler B, Kleindienst R, Amberger A, Recheis H, Xu Q, Wick G. Myocardial injury leads to a release of heat shock protein (hsp) 60 and a suppression of the anti-hsp65 immune response. Cardiovasc Res. 1999;42:685–695. doi: 10.1016/s0008-6363(99)00012-7. [DOI] [PubMed] [Google Scholar]
- 89.Chen D, Pan J, Du B, Sun D. Induction of the heat shock response in vivo inhibits NF-kappaB activity and protects murine liver from endotoxemia-induced injury. J Clin Immunol. 2005;25:452–461. doi: 10.1007/s10875-005-5636-3. [DOI] [PubMed] [Google Scholar]
- 90.Su CY, Chong KY, Chen J, Ryter S, Khardori R, Lai CC. A physiologically relevant hyperthermia selectively activates constitutive hsp70 in H9c2 cardiac myoblasts and confers oxidative protection. J Mol Cell Cardiol. 1999;31:845–855. doi: 10.1006/jmcc.1998.0923. [DOI] [PubMed] [Google Scholar]
- 91.Maurel A, Azarnoush K, Sabbah L, Vignier N, Le Lorc'h M, Mandet C, Bissery A, Garcin I, Carrion C, Fiszman M, Bruneval P, Hagege A, Carpentier A, Vilquin JT, Menasche P. Can cold or heat shock improve skeletal myoblast engraftment in infarcted myocardium? Transplantation. 2005;80:660–665. doi: 10.1097/01.tp.0000172178.35488.31. [DOI] [PubMed] [Google Scholar]
- 92.Heads RJ, Yellon DM, Latchman DS. Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J Mol Cell Cardiol. 1995;27:1669–1678. doi: 10.1016/s0022-2828(95)90722-x. [DOI] [PubMed] [Google Scholar]
- 93.Hutter JJ, Mestril R, Tam EK, Sievers RE, Dillmann WH, Wolfe CL. Overexpression of heat shock protein 72 in transgenic mice decreases infarct size in vivo. Circulation. 1996;94:1408–1411. doi: 10.1161/01.cir.94.6.1408. [DOI] [PubMed] [Google Scholar]
- 94.Cizkova D, Rosocha J, Vanicky I, Radonak J, Galik J, Cizek M. Induction of mesenchymal stem cells leads to HSP72 synthesis and higher resistance to oxidative stress. Neurochem Res. 2006;31:1011–1020. doi: 10.1007/s11064-006-9107-x. [DOI] [PubMed] [Google Scholar]
- 95.Norgaard R, Kassem M, Rattan SI. Heat shock-induced enhancement of osteoblastic differentiation of hTERT-immortalized mesenchymal stem cells. Ann N Y Acad Sci. 2006;1067:443–447. doi: 10.1196/annals.1354.063. [DOI] [PubMed] [Google Scholar]
- 96.Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, Jones WK, Chu G, Kranias EG. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111:1792–1799. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- 97.Zhao TC, Taher MM, Valerie KC, Kukreja RC. p38 Triggers late preconditioning elicited by anisomycin in heart: involvement of NF-kappaB and iNOS. Circ Res. 2001;89:915–922. doi: 10.1161/hh2201.099452. [DOI] [PubMed] [Google Scholar]
- 98.Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278:27828–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- 99.Vandervelde S, van Luyn MJ, Tio RA, Harmsen MC. Signaling factors in stem cell-mediated repair of infarcted myocardium. J Mol Cell Cardiol. 2005;39:363–376. doi: 10.1016/j.yjmcc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 100.Shmelkov SV, Meeus S, Moussazadeh N, Kermani P, Rashbaum WK, Rabbany SY, Hanson MA, Lane WJ, St Clair R, Walsh KA, Dias S, Jacobson JT, Hempstead BL, Edelberg JM, Rafii S. Cytokine preconditioning promotes codifferentiation of human fetal liver CD133+ stem cells into angiomyogenic tissue. Circulation. 2005;111:1175–1183. doi: 10.1161/01.CIR.0000157155.44008.0F. [DOI] [PubMed] [Google Scholar]
- 101.Rufaihah AJ, Haider HK, Heng BC, Ye L, Toh WS, Tian XF, Lu K, Sim EK, Cao T. Directing endothelial differentiation of human embryonic stem cells via transduction with an adenoviral vector expressing the VEGF(165) gene. J Gene Med. 2007;9:452–461. doi: 10.1002/jgm.1034. [DOI] [PubMed] [Google Scholar]
- 102.Nakamura T, Schneider MD. The way to a human's heart is through the stomach: visceral endoderm-like cells drive human embryonic stem cells to a cardiac fate. Circulation. 2003;107:2638–2639. doi: 10.1161/01.CIR.0000074240.87740.BE. [DOI] [PubMed] [Google Scholar]
- 103.Bartunek J, Croissant JD, Wijns W, Gofflot S, de Lavareille A, Vanderheyden M, Kaluzhny Y, Mazouz N, Willemsen P, Penicka M, Mathieu M, Homsy C, De Bruyne B, McEntee K, Lee IW, Heyndrickx GR. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H1095–H1104. doi: 10.1152/ajpheart.01009.2005. [DOI] [PubMed] [Google Scholar]
- 104.Wang XJ, Dong Z, Zhong XH, Shi RZ, Huang SH, Lou Y, Li QP. Transforming growth factor-beta1 enhanced vascular endothelial growth factor synthesis in mesenchymal stem cells. Biochem Biophys Res Commun. 2007 doi: 10.1016/j.bbrc.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 105.Humbert S, Bryson EA, Cordelieres FP, Connors NC, Datta SR, Finkbeiner S, Greenberg ME, Saudou F. The IGF-1/Akt pathway is neuroprotective in Huntington's disease and involves Huntingtin phosphorylation by Akt. Dev Cell. 2002;2:831–837. doi: 10.1016/s1534-5807(02)00188-0. [DOI] [PubMed] [Google Scholar]
- 106.Parrizas M, Saltiel AR, LeRoith D. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3'-kinase and mitogen-activated protein kinase pathways. J Biol Chem. 1997;272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- 107.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 108.Dzau VJ, Gnecchi M, Pachori AS. Enhancing stem cell therapy through genetic modification. J Am Coll Cardiol. 2005;46:1351–1353. doi: 10.1016/j.jacc.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 109.Matsumoto R, Omura T, Yoshiyama M, Hayashi T, Inamoto S, Koh KR, Ohta K, Izumi Y, Nakamura Y, Akioka K, Kitaura Y, Takeuchi K, Yoshikawa J. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2005;25:1168–1173. doi: 10.1161/01.ATV.0000165696.25680.ce. [DOI] [PubMed] [Google Scholar]
- 110.Ye L, Haider H, Jiang S, Ling LH, Ge R, Law PK, Sim EK. Reversal of myocardial injury using genetically modulated human skeletal myoblasts in a rodent cryoinjured heart model. Eur J Heart Fail. 2005;7:945–952. doi: 10.1016/j.ejheart.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 111.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 112.Azarnoush K, Maurel A, Sebbah L, Carrion C, Bissery A, Mandet C, Pouly J, Bruneval P, Hagege AA, Menasche P. Enhancement of the functional benefits of skeletal myoblast transplantation by means of coadministration of hypoxia-inducible factor 1alpha. J Thorac Cardiovasc Surg. 2005;130:173–179. doi: 10.1016/j.jtcvs.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 113.Yau TM, Kim C, Li G, Zhang Y, Weisel RD, Li RK. Maximizing ventricular function with multimodal cell-based gene therapy. Circulation. 2005;112:I123–I128. doi: 10.1161/CIRCULATIONAHA.104.525147. [DOI] [PubMed] [Google Scholar]
- 114.Ye L, Haider H, Jiang S, Tan RS, Ge R, Law PK, Sim EK. Improved angiogenic response in pig heart following ischaemic injury using human skeletal myoblast simultaneously expressing VEGF165 and angiopoietin-1. Eur J Heart Fail. 2007;9:15–22. doi: 10.1016/j.ejheart.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 115.Kofidis T, de Bruin JL, Yamane T, Balsam LB, Lebl DR, Swijnenburg RJ, Tanaka M, Weissman IL, Robbins RC. Insulin-like growth factor promotes engraftment, differentiation, and functional improvement after transfer of embryonic stem cells for myocardial restoration. Stem Cells. 2004;22:1239–1245. doi: 10.1634/stemcells.2004-0127. [DOI] [PubMed] [Google Scholar]
- 116.Kanemitsu N, Tambara K, Premaratne GU, Kimura Y, Tomita S, Kawamura T, Hasegawa K, Tabata Y, Komeda M. Insulin-like growth factor-1 enhances the efficacy of myoblast transplantation with its multiple functions in the chronic myocardial infarction rat model. J Heart Lung Transplant. 2006;25:1253–1262. doi: 10.1016/j.healun.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 117.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 118.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 119.Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 121.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 122.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 125.Kim I, Kim HG, Moon SO, Chae SW, So JN, Koh KN, Ahn BC, Koh GY. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res. 2000;86:952–959. doi: 10.1161/01.res.86.9.952. [DOI] [PubMed] [Google Scholar]
- 126.DeBusk LM, Hallahan DE, Lin PC. Akt is a major angiogenic mediator downstream of the Ang1/Tie2 signaling pathway. Exp Cell Res. 2004;298:167–177. doi: 10.1016/j.yexcr.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 127.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 128.Kutschka I, Kofidis T, Chen IY, von Degenfeld G, Zwierzchoniewska M, Hoyt G, Arai T, Lebl DR, Hendry SL, Sheikh AY, Cooke DT, Connolly A, Blau HM, Gambhir SS, Robbins RC. Adenoviral human BCL-2 transgene expression attenuates early donor cell death after cardiomyoblast transplantation into ischemic rat hearts. Circulation. 2006;114:I174–I180. doi: 10.1161/CIRCULATIONAHA.105.001370. [DOI] [PubMed] [Google Scholar]
- 129.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 130.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 131.Yamane T, Dylla SJ, Muijtjens M, Weissman IL. Enforced Bcl-2 expression overrides serum and feeder cell requirements for mouse embryonic stem cell self-renewal. Proc Natl Acad Sci U S A. 2005;102:3312–3317. doi: 10.1073/pnas.0500167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nakamura Y, Yasuda T, Weisel RD, Li RK. Enhanced cell transplantation: preventing apoptosis increases cell survival and ventricular function. Am J Physiol Heart Circ Physiol. 2006;291:H939–H947. doi: 10.1152/ajpheart.00155.2006. [DOI] [PubMed] [Google Scholar]
- 133.Ohtsuka M, Takano H, Zou Y, Toko H, Akazawa H, Qin Y, Suzuki M, Hasegawa H, Nakaya H, Komuro I. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. Faseb J. 2004;18:851–853. doi: 10.1096/fj.03-0637fje. [DOI] [PubMed] [Google Scholar]
- 134.Bonaros N, Rauf R, Werner E, Schlechta B, Rohde E, Kocher A, Bonatti J, Laufer G. Neoangiogenesis after combined transplantation of skeletal myoblasts and angiopoietic progenitors leads to increased cell engraftment and lower apoptosis rates in ischemic heart failure. Interact Cardiovasc Thorac Surg. 2007 doi: 10.1510/icvts.2007.162917. [DOI] [PubMed] [Google Scholar]
- 135.Nicolls MR, Gill RG. LFA-1 (CD11a) as a therapeutic target. Am J Transplant. 2006;6:27–36. doi: 10.1111/j.1600-6143.2005.01158.x. [DOI] [PubMed] [Google Scholar]
- 136.Guerette B, Skuk D, Celestin F, Huard C, Tardif F, Asselin I, Roy B, Goulet M, Roy R, Entman M, Tremblay JP. Prevention by anti-LFA-1 of acute myoblast death following transplantation. J Immunol. 1997;159:2522–2531. [PubMed] [Google Scholar]
- 137.Guerette B, Asselin I, Skuk D, Entman M, Tremblay JP. Control of inflammatory damage by anti-LFA-1: increase success of myoblast transplantation. Cell Transplant. 1997;6:101–107. doi: 10.1177/096368979700600203. [DOI] [PubMed] [Google Scholar]
- 138.Giannoukakis N, Rudert WA, Ghivizzani SC, Gambotto A, Ricordi C, Trucco M, Robbins PD. Adenoviral gene transfer of the interleukin-1 receptor antagonist protein to human islets prevents IL-1beta-induced beta-cell impairment and activation of islet cell apoptosis in vitro. Diabetes. 1999;48:1730–1736. doi: 10.2337/diabetes.48.9.1730. [DOI] [PubMed] [Google Scholar]
- 139.Tellez N, Montolio M, Estil-les E, Escoriza J, Soler J, Montanya E. Adenoviral overproduction of interleukin-1 receptor antagonist increases beta cell replication and mass in syngeneically transplanted islets, and improves metabolic outcome. Diabetologia. 2007;50:602–611. doi: 10.1007/s00125-006-0548-1. [DOI] [PubMed] [Google Scholar]
- 140.Hodgetts SI, Grounds MD. Complement and myoblast transfer therapy: donor myoblast survival is enhanced following depletion of host complement C3 using cobra venom factor, but not in the absence of C5. Immunol Cell Biol. 2001;79:231–239. doi: 10.1046/j.1440-1711.2001.01006.x. [DOI] [PubMed] [Google Scholar]
- 141.Camirand G, Caron NJ, Turgeon NA, Rossini AA, Tremblay JP. Treatment with anti-CD154 antibody and donor-specific transfusion prevents acute rejection of myoblast transplantation. Transplantation. 2002;73:453–461. doi: 10.1097/00007890-200202150-00021. [DOI] [PubMed] [Google Scholar]
- 142.Skuk D, Goulet M, Roy B, Tremblay JP. Efficacy of myoblast transplantation in nonhuman primates following simple intramuscular cell injections: toward defining strategies applicable to humans. Exp Neurol. 2002;175:112–126. doi: 10.1006/exnr.2002.7899. [DOI] [PubMed] [Google Scholar]
- 143.Haider H, Jiang SJ, Ye L, Aziz S, Law PK, Sim EK. Effectiveness of transient immunosuppression using cyclosporine for xenomyoblast transplantation for cardiac repair. Transplant Proc. 2004;36:232–235. doi: 10.1016/j.transproceed.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 144.Kinoshita I, Vilquin JT, Tremblay JP. Pretreatment of myoblast cultures with basic fibroblast growth factor increases the efficacy of their transplantation in mdx mice. Muscle Nerve. 1995;18:834–841. doi: 10.1002/mus.880180806. [DOI] [PubMed] [Google Scholar]
- 145.Iwasaki Y, Shiojima T, Ikeda K, Tagaya N, Kobayashi T, Kinoshita M. Acidic and basic fibroblast growth factors enhance neurite outgrowth in cultured rat spinal cord neurons. Neurol Res. 1995;17:70–72. doi: 10.1080/01616412.1995.11740289. [DOI] [PubMed] [Google Scholar]
- 146.Buckley S, Shi W, Barsky L, Warburton D. TGF-{beta} signaling promotes survival and repair in rat alveolar epithelial type 2 cells during recovery after hyperoxic injury. Am J Physiol Lung Cell Mol Physiol. 2008;294:l739–l748. doi: 10.1152/ajplung.00294.2007. [DOI] [PubMed] [Google Scholar]
- 147.Park SM, Jung JS, Jang MS, Kang KS, Kang SK. Transforming growth factor-beta1 regulates the fate of cultured spinal cord-derived neural progenitor cells. Cell Prolif. 2008;41:248–264. doi: 10.1111/j.1365-2184.2008.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ogilvie M, Yu X, Nicolas-Metral V, Pulido SM, Liu C, Ruegg UT, Noguchi CT. Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J Biol Chem. 2000;275:39754–39761. doi: 10.1074/jbc.M004999200. [DOI] [PubMed] [Google Scholar]
- 149.Zaka M, Rafi MA, Rao HZ, Luzi P, Wenger DA. Insulin-like growth factor-1 provides protection against psychosine-induced apoptosis in cultured mouse oligodendrocyte progenitor cells using primarily the PI3K/Akt pathway. Mol Cell Neurosci. 2005;30:398–407. doi: 10.1016/j.mcn.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 150.Ye L, Haider H, Guo C, Sim EK. Cell-based VEGF delivery prevents donor cell apoptosis after transplantation. Ann Thorac Surg. 2007;83:1233–1234. doi: 10.1016/j.athoracsur.2006.04.008. author reply 1234. [DOI] [PubMed] [Google Scholar]
- 151.Germani A, Di Carlo A, Mangoni A, Straino S, Giacinti C, Turrini P, Biglioli P, Capogrossi MC. Vascular endothelial growth factor modulates skeletal myoblast function. Am J Pathol. 2003;163:1477–1428. doi: 10.1016/S0002-9440(10)63499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Qu Z, Huard J. Matching host muscle and donor myoblasts for myosin heavy chain improves myoblast transfer therapy. Gene Ther. 2000;7:428–437. doi: 10.1038/sj.gt.3301103. [DOI] [PubMed] [Google Scholar]
- 153.Wano C, Kita K, Takahashi S, Sugaya S, Hino M, Hosoya H, Suzuki N. Protective role of HSP27 against UVC-induced cell death in human cells. Exp Cell Res. 2004;298:584–592. doi: 10.1016/j.yexcr.2004.04.048. [DOI] [PubMed] [Google Scholar]