Summary
Eyes absent (Eya), named for its role in Drosophila eye development but broadly conserved in metazoa, possesses dual functions as a transcriptional coactivator and protein tyrosine phosphatase. While Eya’s transcriptional activity has been extensively characterized, the physiological requirements for its phosphatase activity remain obscure. In this study, we provide insight into Eya’s participation in phosphotyrosine-mediated signaling networks by demonstrating cooperative interactions between Eya and the Abelson (Abl) tyrosine kinase during development of the Drosophila larval visual system. Mechanistically, Abl-mediated phosphorylation recruits Eya to the cytoplasm, where in vivo studies reveal a requirement for its phosphatase function. Thus we propose a model in which in addition to its role as transcription factor, Eya functions as a cytoplasmic protein phosphotyrosine phosphatase.
Introduction
Organogenesis requires coordinated cell proliferation, differentiation and morphogenesis. The Retinal Determination (RD) gene network, a conserved collection of transcription factors named for their key roles in Drosophila eye specification but that participate in the development of numerous organ systems in both flies and mammals (reviewed by Wawersik and Maas, 2000; Pappu and Mardon, 2004), provides a tractable model for investigating how signaling pathways interact with tissue-specific transcriptional networks to coordinate developmental programs.
Eyes absent (Eya), an RD network member, was first characterized as a novel nuclear factor required for Drosophila eye development. Thus eye-specific loss of eya leads to an “eyeless” phenotype whereas misexpression can induce formation of ectopic eye tissue (Bonini et al., 1993; Bonini et al., 1997; Pignoni et al., 1997). Eya family members are identified by a conserved C-terminal Eya Domain (ED) that mediates its interaction with another RD protein, Sine oculis (So; Six in vertebrates) (Bonini et al., 1993; Pignoni et al., 1997). The Eya-So complex functions as a bipartite transcriptional factor, with Eya providing transactivation and So contributing DNA binding specificity (Ohto et al., 1999; Silver et al., 2003).
The ED also possesses intrinsic protein tyrosine phosphatase activity (Li et al., 2003; Rayapureddi et al., 2003; Tootle et al., 2003). While no physiological substrates have yet been identified, the observation that Eya can be tyrosine phosphorylated in cultured cells and can dephosphorylate itself in vitro suggests it is a target of phosphotyrosine signaling pathways and may have autocatalytic activity (Tootle et al., 2003). Impaired phosphatase activity has been associated with defects in both Drosophila and human development (Rayapureddi et al., 2003; Tootle et al., 2003; Mutsuddi et al., 2005; Rayapureddi and Hegde, 2006), indicating an essential contribution to Eya function.
Given Eya’s well established role within the RD network, we and others proposed that phosphatase activity might directly influence Eya-So transcriptional output (Li et al., 2003; Tootle et al., 2003; Rebay et al., 2005). However, a recent systems level analysis of Eya-So regulation of gene expression found that loss of Eya phosphatase function did not globally impair transcriptional output, suggesting an alternate model in which Eya phosphatase and transcriptional activities make independent and distinct contributions to developmental processes requiring Eya function (Jemc and Rebay, 2007a, b).
Here we describe the results of a set of experiments designed to identify the phosphotyrosine signaling pathways in which Eya participates and to test the hypothesis that Eya phosphatase function can operate independently from its nuclear transcriptional activities. Our findings reveal a novel requirement for Eya phosphatase activity in the cytoplasm and demonstrate that full Eya function can be reconstituted by coexpression of nuclearly and cytoplasmically restricted protein pools. Mechanistically, we describe an enzyme-substrate relationship between the Abelson (Abl) non-receptor tyrosine kinase and Eya such that Abl-mediated phosphorylation relocates Eya from the nucleus to the cytoplasm. Genetic synergy between eya and abl contributes to multiple developmental programs, including axon pathfinding in the embryonic central nervous system (CNS) and larval visual system. Together our data support a new model in which Eya function is partitioned between two independent subcellular sites: the nucleus where it fulfills its well-established role as a transcription factor, and the cytoplasm where it participates in phosphotyrosine signaling mechanisms.
Results
Genetic cooperativity between eya and abl
The discoveries that Eya possesses protein tyrosine phosphatase activity and is tyrosine phosphorylated in cultured cells (Li et al., 2003; Rayapureddi et al., 2003; Tootle et al., 2003) imply participation in phosphotyrosine-mediated signaling. To identify the relevant pathways, we asked whether altered dosage of any tyrosine kinases could dominantly modify the frequency with which Eya overexpression induces ectopic eye formation. The rationale was that if activity of a particular kinase is important for Eya function, then a two-fold alteration in dose might be sufficient to change the level of Eya activity and alter ectopic eye induction efficiency.
While full details of the screen will be reported elsewhere, most striking among the results were interactions with alleles of the Abelson (Abl) non-receptor tyrosine kinase, the Drosophila homolog of the mammalian c-abl oncogene (Figure 1A). Heterozygosity for abl loss of function mutations or coexpression of a kinase dead Abl transgene previously shown to function as a dominant negative (Hsouna et al., 2003) dominantly suppressed Eya’s ectopic eye induction ability. Conversely, while overexpression of Abl alone resulted in minimal phenotypic perturbation, co-overexpression of Eya and Abl led to synthetic lethality.
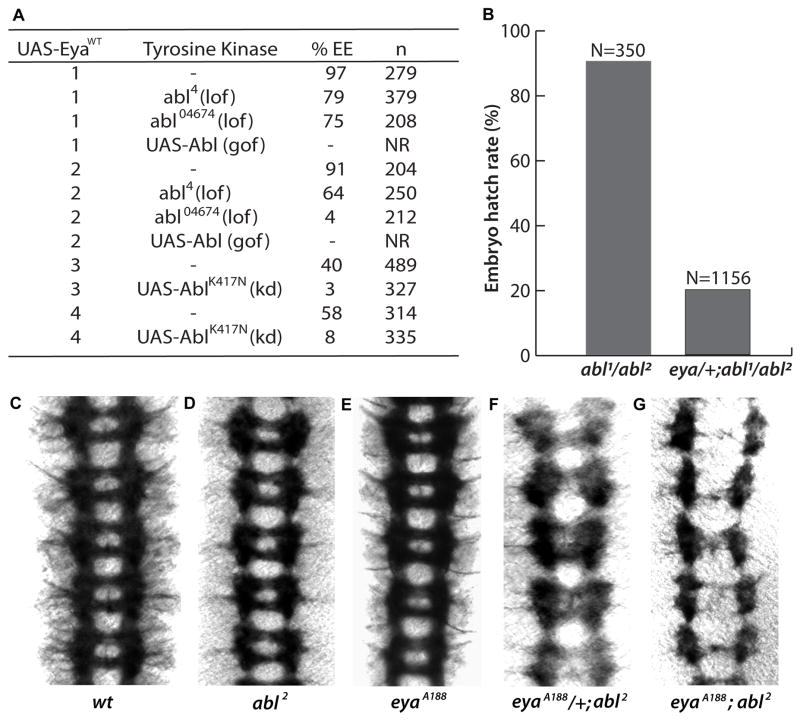
Figure 1. Genetic interactions reveal cooperativity between eya and abl.
(A) Altered abl dosage dominantly modifies Eya’s ectopic eye induction efficiency. Lines 1–4 are independent transgenic lines; lof, loss-of-function; gof, gain-of-function; kd, kinase dead; n, number scored; NR, none recovered. %EE, percent of flies of genotype kinase/+; dpp-Gal4>UAS-Eya with ectopic eye tissue on head.
(B) Reduced eya dosage impairs viability of abl mutant embryos. N, number of animals scored. Dissected ventral nerve cords from Stage 16 embryos stained with BP102 to reveal the pattern of the axon scaffold.
(C) Wild type.
(D) abl2 homozygotes have intact commissures.
(E) eyaA188 homozygotes are indistinguishable from wild type.
(F) eyaA188/+, abl2 mutant embryos have discontinuities along the longitudinal axon bundles with 20% of commissures lost or defective.
(G) In eyaA188;abl2 double homozygotes 77% of commissures are lost and the longitudinal tracts show severe disruptions.
To confirm the eya-abl synergy predicted by the ectopic eye induction results, we first asked whether expression of kinase dead Abl could interfere with the ability of an Eya transgene to rescue the “eyeless” phenotype associated with the eye-specific loss of function allele eya2. Whereas Eya transgenes alone restore eye tissue to 100% of eya2 flies (Tootle et al., 2003; Mutsuddi et al., 2005), co-expression of kinase dead Abl reduced rescue efficiency to 40%. Second, we asked whether reduced eya dosage could alter the pupal lethality associated with zygotic loss of abl. Using two different recessive eya alleles (eyaA188 and eyaG130, Rebay et al., 2000), we found that while 90% of abl1/abl2 transheterozygotes survived until the pupal stage, only 20% of eya/+; abl1/abl2 embryos hatched to the larval stage (Figure 1B); eya/+ animals were indistinguishable from +/+ controls in this assay. Together these genetic results suggest a cooperative interaction between eya and abl.
Interactions with abl reveal a role for eya in embryonic CNS axonogenesis
To elucidate the developmental processes that require eya-abl genetic cooperativity, we asked whether the embryonic lethality of eya/+; abl1/abl2 animals might be caused by defects in the CNS, a tissue in which both Abl and Eya are expressed (Gertler et al., 1989; Bennett and Hoffmann, 1992; Bonini et al., 1998) and in which Abl function has been characterized (Gertler et al., 1989; Fogerty et al., 1999; Moresco and Koleske, 2003). In wild type embryos, the CNS axon scaffold consists of two longitudinal axon bundles connected by segmentally repeated pairs of commissural tracts (Figure 1C); zygotic loss of abl results in mild discontinuities in the longitudinal tracts (Wills et al., 1999a; Grevengoed et al., 2001; Figure 1D). Removal of both maternal and zygotic abl leads to severe disruption of the axon scaffold and fully penetrant embryonic lethality (Grevengoed et al., 2001). Thus if the eya and abl gene products function cooperatively, then reducing eya dose in a zygotic abl background might compromise the function of maternally provided Abl, thereby resulting in exacerbated axonal defects and penetrant embryonic lethality.
While the CNS of eyaA188 homozygous, eyaA188/+ heterozygous or eyaA188; abl2/+ mutant embryos were indistinguishable from wild type, reduction or loss of eya enhanced the abl axon patterning defects such that more pronounced gaps were seen along the longitudinal tracts and the commissures were lost at high penetrance (Figure 1E – G). eyaA188; abl2 embryos phenocopied maternal and zygotic abl mutants (Grevengoed et al., 2001) in that the distance between the two longitudinal axon bundles became irregular, with increased separation apparent in segments where commissures were lost (Figure 1G). Together these results suggest that eya and abl work cooperatively to control axon targeting in the CNS. In this context, eya must function redundantly with other pathway components given that eya single mutants lack obvious axonal defects; the lack of eya expression in the female germline (Bonini et al., 1998) rules out the possibility that maternal rescue masks a zygotic phenotype.
eya and abl are required for photoreceptor axon targeting
We next asked whether eya and abl also control photoreceptor axon targeting. Eya and Abl are both expressed in retinal neurons (Bennett and Hoffmann, 1992; Bonini et al., 1998), yet potential roles in photoreceptor morphogenesis have not been explored. During normal development, axonal projections from the differentiating photoreceptor neurons in each ommatidium of the third instar eye imaginal disc travel together through the optic stalk into the optic lobes of the larval brain where R1–R6 growth cones target the lamina while R7 and R8 axons travel deeper to the medulla.
Larval eye-brain complexes dissected from viable hypomorphic allelic combinations for each gene were examined for photoreceptor axon targeting defects by staining with anti-chaoptin (24B10) to highlight all photoreceptor projections or with anti-β-galactosidase to follow the Ro-lacZtau marker in R2–R5 projections (Garrity, et al. 1999). Instead of forming an even plexus of growth cones at the lamina as seen in wild type, in eya or abl mutant larvae the axons fasciculated aberrantly to produce an irregular pattern of gaps and thickenings in the lamina with a significant percentage of axon bundles failing to terminate properly at the lamina (Figure 2A–C, E–G). To determine whether eya and abl might function synergistically during photoreceptor axon targeting, we investigated dose-sensitive genetic interactions. Although eya2/eyaA188; abl2/+ or eya2/eyaA188; abl1/abl2 were synthetic lethal prior to third instar, we were able to analyze eyaA188/+; abl1/abl2 animals. While eyaA188/+ larvae appeared wild type, heterozygosity for eya dominantly enhanced the abl mistargeting phenotype resulting in a highly disorganized lamina plexus (Figure 2D, H).
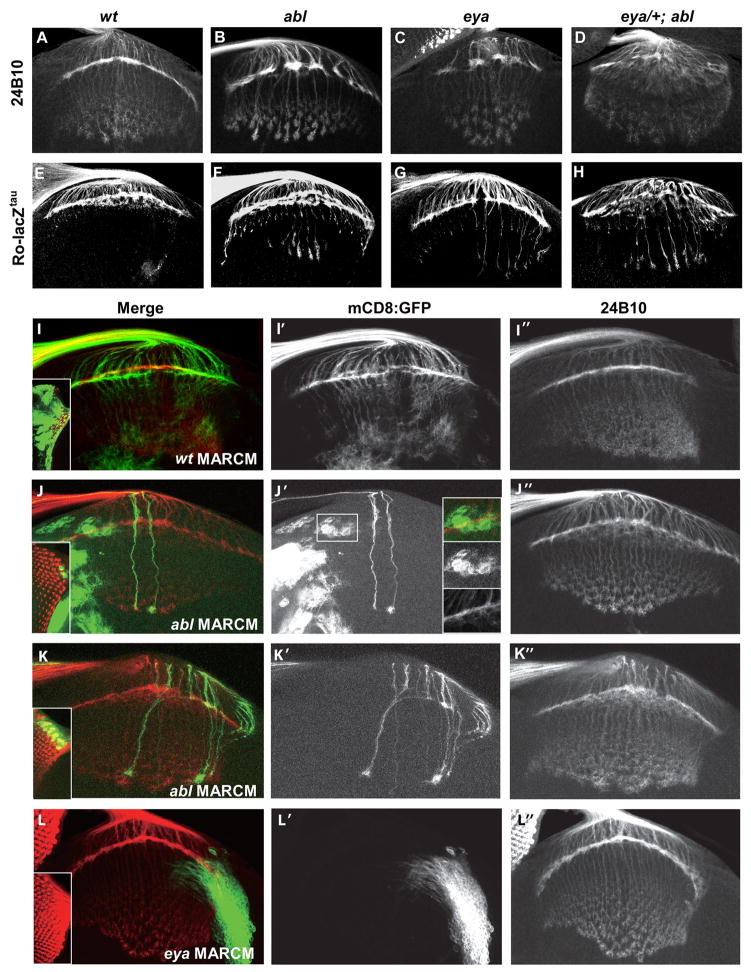
Figure 2. Eya and Abl are required for photoreceptor axon targeting in the brain.
Dissected eye-brain complexes from third-instar larvae stained with anti-chaoptin 24B10 (A–D, I–K) or with anti β-galactosidase to visualize the R2–R5 specific Ro-lacZtau marker (E–H).
(A,E) In wild type, R1–R6 axons form a normal lamina plexus, and R7 and R8 axons are arranged in regular staggered rows within the medulla. R2–R5 axons mostly stop in the lamina.
(B,F) In abl1/abl2 trans-heterozygotes the lamina plexus is discontinuous, with gaps in the plexus, and thicker axon bundles beneath. A subset of R2–R5 axons fail to stop in the lamina and extend into the medulla.
(C,G) eya2/eyaA188 mutants phenocopy abl mutants.
(D,H′) eya A188/+;abl mutants have a highly disorganized lamina with thick bundles of R2–R5 axons failing to stop in the lamina.
(I–K) MARCM clones of eya and abl labeled with 24B10, red. GFP, green, marks the mutant axons.
(I-I″) Photoreceptor axons of large wild type clones (I inset) exhibit normal targeting to the brain.
(J-J″) Axonal projections appear normal in small abl clones. Targeting of wild type axons to mutant brain tissue appears normal (J′ insets).
(K-K″) Larger abl clones show fasciculation defects and laminar gaps.
(L-L″) Wild type axons exhibit normal targeting to eya mutant brain tissue.
In order to determine if defects observed in eya and abl mutants resulted from loss of gene function in the photoreceptors, the brain, or in both tissues, we used the MARCM (mosaic analysis with a repressible cell marker) approach (Lee et al., 2001) to generate mutant clones positively marked with a membrane-tethered GFP. While both wild type control and small abl2 clones exhibited normal axon targeting, larger abl2 clones exhibited aberrant fasciculation and targeting to wild type brain tissue (Figure 2I–K); eya mutant photoreceptor clones were unrecoverable due to apoptosis (Bonini et al., 1993). Wild type photoreceptors exhibited normal targeting to either eya or abl mutant brain tissue (Figure 2J′ insets, 2L), suggesting both abl and eya are required autonomously in the photoreceptors for proper terminal differentiation.
While abl loss does not perturb retinal induction or photoreceptor specification (Supplementary Figure 1A), eya has well established roles in these processes (Bonini, et al., 1993). To investigate whether eya axon targeting defects resulted from earlier disruptions in eye induction and photoreceptor specification, we used GMR-Gal4 driven expression of an RNAi transgene to knock down eya posterior to the morphogenetic furrow. Significant reduction of Eya protein levels was observed without apparent defects in photoreceptor specification and patterning (Supplementary Figure 1). Using the Ro-lacZtau marker to assess targeting defects, EyaRNAi discs showed a clear “shoot-through” phenotype, with an average of 18.2 overshooting axon bundles per brain, compared to 8.4 in control discs (Figure 3A, B and J). The mistargeted axon bundles in the eya knockdown were generally thicker than those in the control, suggesting the scoring scheme underestimates the severity of the eya defects.
Figure 3. Eya and Abl interact in post-mitotic photoreceptor cells to regulate axon targeting.
Dissected eye-brain complexes from third-instar larvae stained with anti β-galactosidase to visualize the Ro-lacZtau marker.
(A) GMR-Gal4/+; Ro-lacZtau/+ controls show a few thin overshooting bundles.
(B) UAS-EyaRNAi/+; GMR-Gal4/+; Ro-lacZtau/+ larvae have significant mistargeting defects.
(C) UAS-AblRNAi/+; GMR-Gal4/+; Ro-lacZtau/+ was similar to control.
(D) Double knockdown of abl and eya (UAS-EyaRNAi, UAS-AblRNAi/+; GMR-Gal4/+; Ro-lacZtau/+) causes multiple thick axon bundles to overshoot the lamina.
(E) Increased Eya expression (UAS-Eya/GMR-Gal4; Ro-lacZtau/+) causes targeting defects.
(F) Reduced abl expression suppresses Eya overexpression phenotypes (UAS-AblRNAi/+; UAS-Eya/GMR-Gal4; Ro-lacZtau/+).
(G) Increased cytoplasmic Eya perturbs axon targeting (GMR-Gal4/+; Ro-lacZtau/UAS-Myr-EyaWT).
(H) Expression of phosphatase-dead Myr-tagged Eya (GMR-Gal4/+; Ro-lacZtau/UAS-Myr-EyaK699Q) shows only mild targeting defects.
(I) Reduced abl expression suppresses Myr-EyaWT phenotypes (UAS-AblRNAi/+; UAS-Myr-EyaWT/GMR-Gal4; Ro-lacZtau/+).
(J) Summary of the average number of overshooting axon bundles per brain for each genotype ± standard deviation. Scoring was performed blind to the genotype. n, number of brains scored. Statistical significance (p values) were calculated using Excel’s built-in TTEST function after performing an unpaired, one-tailed T test for each pair of genotypes. All phenotypes were significant compared to control (p<.001) except that of AblRNAi (†). p-values for other relevant comparison pairs are indicated next to brackets.
Confirming cooperative cell autonomous interactions in the differentiating photoreceptors, GMR-Gal4 driven coexpression of a weak abl RNAi transgene that on its own has no phenotype enhanced the EyaRNAi targeting defects (21.8 axon bundles/brain, compared to 18.2 in EyaRNAi alone; Figure 3B – D, J). Finally, RNAi-mediated abl knockdown dominantly suppressed the overshooting phenotypes observed upon GMR-Gal4 driven overexpression of UAS-EyaWT (12.4 overshooting axon bundles/brain versus 25.7 for EyaWT alone; Figure 3E, F, J).
Abl directly phosphorylates Eya
To elucidate the molecular mechanisms underlying eya-abl genetic synergy, we asked whether Eya might be a substrate of the Abl tyrosine kinase. Using anti-phosphotyrosine immunoblotting of Eya immunoprecipitated from transfected S2 cells, we found that coexpression of wild type Abl, but not kinase dead Abl, increased Eya tyrosine phosphorylation (Figure 4A). Treatment with lambda phosphatase removed the phosphotyrosine signal (Figure 4B). Co-overexpression of Eya and Abl transgenes in embryos and eye imaginal discs confirmed that expression of Abl but not kinase dead Abl increased the phosphotyrosine signal on Eya in Drosophila tissues (Figure 4C, D).
Figure 4. Eya is a substrate of Abl.
(A–D, F) Immunoblots of immunoprecipitated Flag-Eya double labeled with anti-Flag, red, and anti-phosphotyrosine (anti-pY), green. Reduced sensitivity of anti-Flag relative to anti-pY may explain the lack of complete overlap (yellow) of the two signals.
(A) In transiently transfected S2 cells, Eya pY signal increases in the presence of Abl.
(B) Treatment with lambda phosphatase removes the pY signal.
(C) Actin-Galr4 driven coexpression of AblWT but not kinase dead AblKD, increases tyrosine phosphorylation of Flag-Eya in embryos.
(D) GMR-Gal4 driven coexpression of Abl increases tyrosine phosphorylation of Flag-Eya in 3rd instar eye discs.
(E) Schematic of Eya deletion constructs.
(F) Abl primarily targets the PST-rich region of Eya. Lane numbering matches construct number in (E). Molecular Weight (MW) standards are indicated in Kd on left. Arrowheads on right point out IgG bands. Asterisks indicate constructs run with expected mobility in the absence of Abl.
(G) In vitro kinase assay using recombinant mammalian c-Abl. Coomassie staining in left panel shows fusion protein amounts. Right panel is phosphoimager exposure of same blot showing signal in GST-EyaPST lane.
(H) In vitro kinase assays using immunoprecipitated Myc-tagged Drosophila Abl from transfected S2 cells phosphorylates GST-EyaPST. Left panel, Coomassie stain; middle panel, phosphoimager exposure of same blot. Right panels show that immunoprecipitated kinase dead Abl (KD) does not phosphorylate GST-EyaPST: top, Coomassie; middle, P32 exposure; bottom, anti-Myc immunoblot; quantitation of relative intensity of signals is indicated.
To determine which region of Eya becomes tyrosine phosphorylated, we transfected S2 cells with a set of Eya deletion constructs (Silver et al., 2003; Figure 4E) in the presence or absence of Abl. Deletion of the Eya Domain (ED) increased the phosphotyrosine signal, while deletion of the PST-rich region reduced it (Figure 4F, Lanes 4 and 3, respectively). Comparable results were obtained using either wild type or phosphatase dead Eya constructs, indicating the changes do not simply reflect loss or hyperactivity of Eya phosphatase activity. Examination of smaller deletions within the 215 amino acid PST-rich domain demonstrated that Abl can phosphorylate multiple residues across the region (data not shown).
To ask whether Eya can be a direct substrate of Abl, in vitro kinase assays were performed using recombinant c-Abl and GST-Eya fusion proteins expressing either the PST-rich region, the ED or GST alone (GST-EyaPST, GST-EyaED and GST; GST-EyaED was inactive as a phosphatase in the assay conditions). Consistent with the S2 cell deletion analysis results, GST-EyaPST was directly phosphorylated by c-Abl, whereas GST-EyaED and GST were not (Figure 4G). Comparable results were obtained using Drosophila Abl isolated by immunoprecipitation from transfected S2 cells, but not with kinase dead Drosophila Abl (Figure 4H). Thus Abl-mediated tyrosine phosphorylation of the PST-rich region of Eya is likely direct.
Abl-mediated tyrosine phosphorylation relocalizes Eya to the cytoplasm
Eya has been extensively characterized as a nuclear transcription factor, while Drosophila Abl is a cytoplasmic/membrane-associated tyrosine kinase (Bennett and Hoffmann, 1992; Bonini et al., 1998; Fox and Peifer, 2007). To investigate how the two proteins might interact and the potential consequences of Abl-mediated phosphorylation of Eya, we examined the subcellular localization of Eya and Abl in cotransfected S2 cells. Although Eya is predominantly nuclear, cytoplasmic signal is often apparent (Figure 5A, E). Treatment with Leptomycin B, an inhibitor of Crm-1 dependent nuclear export, resulted in exclusively nuclear Eya localization (Supplementary Figure 2), suggesting a dynamic nuclear export/import cycle. In Eya/Abl cotransfected cells, while Abl’s subcellular distribution remained constant, Eya showed a marked increase in cytoplasmic accumulation (Figure 5B–E). Kinase dead Abl did not alter Eya subcellular distribution (Figure 5E), consistent with Abl-mediated phosphorylation of Eya triggering this effect. Cotransfection of So blocked Abl-induced relocation of Eya to the cytosol (Supplementary Figure 3). Finally, indicating Abl can trigger a comparable localization shift in Drosophila tissues, Eya/Abl coexpression in the wing imaginal disc resulted in significant cytoplasmic Eya accumulation (Figure 5F, G) whereas Eya expressed alone or in combination with kinase dead Abl appeared exclusively nuclear (Figure 5H).
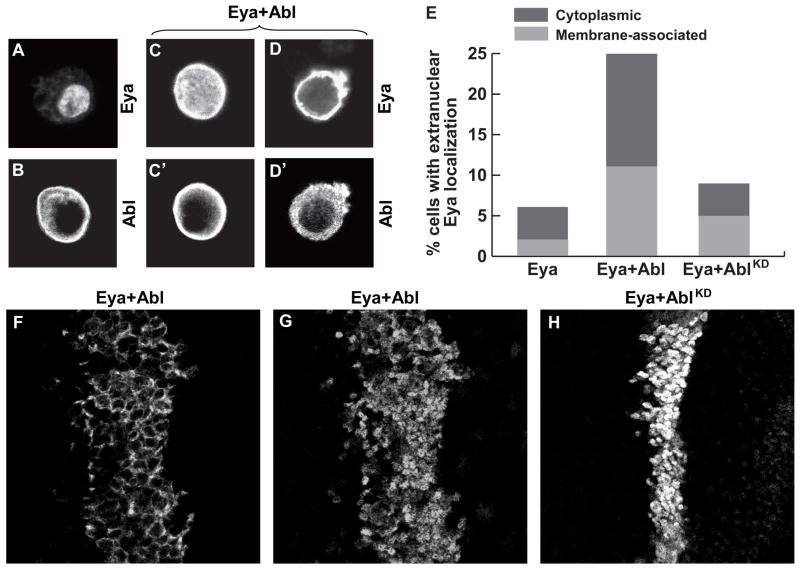
Figure 5. Abl expression relocalizes Eya to the cytoplasm.
(A–D) Indirect immunofluorescence of transfected S2 cells stained with anti-Flag to recognize Flag-Eya and/or Anti-Myc to detect Abl-Myc.
(A) Eya is predominantly nuclear, although cytoplasmic staining can be seen.
(B) Abl localizes to the cytoplasm with enrichment at the plasma membrane.
(C–D) Cells coexpressing Eya and Abl double labeled with anti-Flag (C,D) and anti-Myc (C′,D′).
(C-C′) Example of Abl expressing cell with uniform distribution of Eya.
(D-D′) Example of Abl expressing cell with exclusive cytoplasmic Eya localization and membrane enrichment.
(E) Quantitation of Eya localization. ~300 cells were counted for each condition.
(F–H) Anti-Eya staining of wing imaginal discs coexpressing Eya and Abl transgenes with Ptc-Gal4.
(F–G) Different optical sections of the same disc coexpressing Eya and Abl reveals expression of Eya in both cytoplasm (F) and nucleus (G).
(H) No cytoplasmic localization was detected upon coexpression of Eya and kinase dead AblKD.
Extranuclear Eya localization is required for function
The discovery that Abl promotes cytoplasmic accumulation of Eya suggests extranuclear Eya localization might be physiologically relevant; if so, then nuclear restriction of Eya should compromise function. To test this hypothesis, we inserted the SV40 nuclear localization sequence (NLS; Kalderon et al., 1984) into Eya and tested its efficacy by examining NLS-Eya localization in S2 cells. Expressed alone or with Abl, NLS-Eya was exclusively nuclear whereas the control protein containing a non-functional mutant NLS (NLSMUT-Eya) relocalized to the cytosol as efficiently as untagged Eya in response to Abl activation (Supplementary Figure 3). NLS-Eya retained wild type transactivation ability (Supplementary Figure 4), indicating the NLS insertion does not compromise nuclear function.
To determine the functional consequences of nuclear restriction during eye development, transgenic lines were generated, expressed under control of the dpp-Gal4 driver and assessed in both ectopic eye induction and genetic rescue assays. NLS-EyaWT transgenes exhibited an average 20% frequency of ectopic eye induction and 48% rescue efficiency, significantly lower than the 49% and 100% respective averages obtained from comparable analysis of untagged EyaWT (Figure 6A–D; Hsiao et al., 2001; Tootle et al., 2003). Ruling out the possibility that reduced activity might be attributed solely to insertion of the tag, NLSMUT-EyaWT lines exhibited almost twice the activity of NLS-EyaWT (Figure 6A, B).
Figure 6. Coexpression of membrane-tethered and nuclearly-restricted Eya reveals a cytoplasmic requirement for Eya phosphatase activity.
(A) Average activities of Eya transgenes in ectopic eye induction assay (see Supplementary Table 1 for details). n, number of transgenic lines tested; N, total number of flies scored.
(B) Average activities of Eya transgenes in genetic rescue assay (see Supplementary Table 2 for details). n, number of transgenic lines tested; N, total number of flies scored.
(C – F) Adult heads representative of: eyeless phenotype of eya2; modest rescue by NLS-EyaWT; strong rescue by coexpressed NLS-EyaWT + Myr-EyaWT; and modest rescue by coexpressed NLS-EyaWT + Myr-EyaK699Q.
(G) Average ectopic eye induction efficiency of recombinant lines obtained by systematic crossing of four NLS-EyaWT lines with multiple Myr-EyaWT and Myr-EyaK699Q transgenes (see Supplementary Table 3 for details). n, number of independent recombinant lines tested; N, total number of flies scored; student t-test determined was applied to determine the p values between groups. No significant difference is determined between NLS-EyaWT and NLS-EyaWT+Myr-EyaK699Q (p=0.48).
Coexpression of membrane-tethered and nuclearly-restricted Eya reconstitutes full function
Our finding that nuclear tethering of Eya compromised its activity led us to consider how cytoplasmic Eya might contribute to overall function. In one scenario, nucleocytoplasmic shuttling might allow Eya to be “activated” in the cytoplasm in a manner critical for proper nuclear function. Alternatively, nucleocytoplasmic shuttling might establish two physically and functionally separate pools of Eya protein: a nuclear pool devoted to transcriptional regulation and a cytosolic pool that participates in cytoplasmic signaling events. It should be noted that to date only nuclear Eya has been visualized in developing tissues (Bonini et al., 1998). Accordingly, we predict low endogenous cytoplasmic Eya concentrations will prove sufficient for its cytoplasmic functions.
To generate the cytoplasmically restricted pool of Eya needed to distinguish between these models, we inserted the Src myristoylation tag (Cross et al., 1984) at the Eya N-terminus and showed that Myr-EyaWT was effectively excluded from the nucleus both in S2 cells and in Drosophila tissues (Supplementary Figures 5B and 6B). When tested in either the ectopic eye or genetic rescue assays, Myr-EyaWT transgenes lacked activity, consistent with the essential requirement for Eya transcriptional activity during retinal specification (Supplementary Tables 1 and 2, Supplementary Figure 7).
We then asked whether coexpression of Myr-EyaWT and NLS-EyaWT, which results in two distinct protein pools targeted and restricted to different subcellular compartments (Supplementary Figures 5C and 6), could reconstitute full Eya function. Four NLS-EyaWT transgenes selected based on an ectopic eye induction frequency that approximated the average value (20%) were recombined with Myr-EyaWT transgenes that expressed comparable levels of cytosolic Eya protein (Supplementary Figure 8). When tested in the ectopic eye induction assay, a consistent trend emerged in which coexpression of NLS-EyaWT and Myr-EyaWT resulted in an approximate two-fold increase in activity relative to expression of NLS-EyaWT alone (Figure 6G, and Supplementary Table 3). Increased activity was also apparent in the genetic rescue assay where the size of rescued eye tissue was markedly enhanced (Figure 6D, E). Because Myr-EyaWT alone lacked activity in these assays (Supplementary Tables 1 and 2), the activity increase obtained from coexpressing NLS- and Myr- tethered Eya proteins reflects strong synergy rather than additivity. Excluding the possibility that the increased activity might result from NLS-EyaWT recruiting Myr-EyaWT to the nucleus, immunostaining revealed both nuclear and cytoplasmic Eya localization in the NLS-EyaWT + Myr-EyaWT expressing cells, with the expression level in each compartment comparable to that observed when either transgene was expressed alone (Supplementary Figure 6). Taken together, these results support a model in which cytoplasmic Eya and nuclear Eya have separate roles such that full Eya function can be reconstituted from coexpression of two spatially restricted protein pools.
Eya phosphatase activity is required in the cytoplasm
The complementation assay described above provides an ideal system to test whether Eya phosphatase activity might be important for cytoplasmic function. We therefore generated additional Myr-Eya transgenes carrying the K699Q missense mutation that ablates in vitro phosphatase activity, retains productive interactions with So, and exhibits reduced activity in both ectopic eye and genetic rescue assays (Tootle et al., 2003). As previously shown for the untagged versions (Tootle et al., 2003), Myr-EyaWT and Myr-EyaK699Q lines expressed comparable protein levels (Supplementary Figure 8). Like Myr-EyaWT, Myr-EyaK699Q transgenes alone had no activity in ectopic eye or genetic rescue assays (Supplementary Tables 1 and 2). However, in contrast to Myr-EyaWT, Myr-EyaK699Q showed very limited synergy when coexpressed with NLS-EyaWT, with only an ~10% increase in ectopic eye induction relative to NLS-EyaWT alone (Figure 6G). Similarly, in the genetic rescue experiment, the NLS-EyaWT+Myr-EyaK699Q recombinants restored smaller eye fields compared to the NLS-EyaWT+Myr-EyaWT recombinants (Figure 6E, F).
To begin to investigate whether cytoplasmic Eya activity might be relevant to photoreceptor morphogenesis, we asked whether GMR-Gal4 driven expression of Myr-EyaWT could perturb axon targeting. Expression of Myr-EyaWT caused an average of 19.0 axon bundles/brain to overshoot the lamina (Figure 3G), significantly higher than the 8.4 average obtained in controls. In contrast, expression of phosphatase-dead Myr-EyaK699Q resulted in only 11.8 axon bundles/brain overshooting the lamina (Figure 3H and Supplementary Figure 9). Reduction in abl dose dominantly suppressed the targeting defects associated with expression of Myr-EyaWT, further implicating Eya-Abl synergy in this context (Figure 3I).
Discussion
The discovery that Eya possesses intrinsic protein tyrosine phosphatase activity suggests prior studies of its nuclear transcriptional functions within the RD network may have revealed only a partial picture of the signaling pathways and developmental contexts in which it operates. The data presented in this paper lead us to propose a new model in which Eya, in addition to operating as a nuclear transcription factor, participates independently as a phosphatase in cytoplasmic signaling events important for eye development.
Our analysis of the subcellular compartmentalization of Eya function has revealed a novel requirement for Eya activity in the cytoplasm. Specifically, although nuclearly restricted NLS-Eya appears fully competent as a coactivator as judged by cultured cell transcriptional reporter assays, it exhibits a reduced ability to induce eye tissue in either wild type or eya loss-of-function backgrounds. Coexpression of cytoplasmically restricted Myr-Eya restores a wild-type level of eye inducing activity to the NLS-Eya background, supporting the interpretation that NLS-Eya is fully competent with respect to transcription but cannot perform the essential function normally provided by cytoplasmic Eya. Eya phosphatase activity appears to contribute to cytosolic function, as phosphatase-dead versions of cytoplasmically restricted Eya transgenes fail to complement the NLS-Eya background effectively. Thus we propose that while regulation of gene expression by the core RD network relies primarily on nuclear Eya function, other signaling events important for retinal development may rely on transcription-independent functions of the cytoplasmic Eya phosphatase.
Mechanistically, we propose Eya traffics dynamically between nuclear and cytoplasmic compartments, with its final localization determined by its phosphorylation state and interactions with specific signaling partners. Thus in contexts in which Abl signaling is activated, Abl-mediated phosphorylation may provide a cytoplasmic retention signal that targets Eya to its appropriate site of action, presumably through interactions with specific phosphotyrosine binding proteins. Autocatalytic Eya phosphatase activity (Tootle et al., 2003) would play a critical positive role with respect to overall Eya function by returning Eya to the nucleus to prevent depletion of the nuclear pool needed to carry out essential transcriptional programs. Although cytosolic Eya substrates have not yet been identified, the fact that phosphatase-dead cytoplasmically-restricted Eya was less active than the wild type version in an assay in which dynamic shuttling between nuclear and cytoplasmic compartments was not relevant suggests that Eya-mediated dephosphorylation of substrates other than itself is likely important.
While Eya has been primarily characterized as a nuclear protein, several previous observations are consistent with our proposed model of extranuclear function. First, in mammalian cultured cells, Eya nuclear localization and/or retention requires the presence of its binding partner Six, such that in its absence Eya localizes to the cytosol (Ohto et al., 1999; Zhang et al., 2004). Second, protein-protein interactions with several membrane-associated and cytoplasmic proteins have been demonstrated in two-hybrid screens, although only one interaction has been further investigated (Fan et al., 2000; Embry et al., 2004; Li et al., 2004). In this example, interactions between Eya and the G-protein Gαi can recruit Eya to the cytoplasm of cultured cells and a balance between binding to G-proteins and Six has been proposed to regulate Eya distribution and function (Fan et al., 2000; Embry et al., 2004).
To what aspects of eye development might cytosolic Eya activity contribute? While identification of Eya substrates and elucidation of the specific signaling events regulated by cytoplasmic Eya activity will be required to answer this question definitively, several intriguing models are worth considering. First, the ectopic eye induction and genetic rescue assays used to characterize the complementation between Myr-Eya and NLS-Eya transgenes imply a requirement for extra-nuclear Eya in retinal specification. In considering this context, it is important to note that while a great deal is understood about how transcriptional hierarchies such as the RD network drive retinal induction, much less is known about how specific differentiation programs are coordinated with the morphogenetic events that pattern the tissue. In Drosophila, specification of retinal fates is immediately preceded by adhesive and morphological changes in and posterior to the morphogenetic furrow (reviewed by Carthew, 2007). Although phosphotyrosine signaling at the morphogenetic furrow has not been extensively studied, its importance to cell adhesion and epithelial morphogenesis in other contexts is well-documented. For example, recent work studying the invagination of the ventral furrow during Drosophila gastrulation demonstrated that Abl signaling acting in parallel to the Rho activator RhoGEF2 regulates actin organization to drive apical cell constriction (Fox and Peifer, 2007). Given the importance of cell constriction in the retinal furrow, it will be interesting to investigate whether similar signaling mechanisms operate in this context and whether cytoplasmic Eya phosphatase activity is involved. Encouragingly, loss of eya impairs morphogenetic furrow propagation (Pignoni et al., 1997), suggesting investigation of defects in epithelial remodeling and reorganization of cell-cell contacts at the furrow in eya mutants could be fruitful.
Another possibility is that cytoplasmic Eya phosphatase function might provide critical feedback regulation on other signaling pathways during retinal specification. Indeed, a complex web of interactions between multiple signaling networks including the Wingless, Notch, Hedgehog, and EGFR pathways has been shown to be critical for RD network function and retinal induction (Reviewed by Pappu and Mardon, 2004; Silver and Rebay, 2005). Thus if cytosolic Eya phosphatase activity were absent or mislocalized, the resulting signaling imbalances could potentially compromise eye specification and development.
Finally, cytoplasmic Eya function could be important for neuronal morphogenesis, perhaps through involvement in Abl-mediated signaling events. Because Abl signaling has not yet been explored in the retina, determining which downstream branches of the pathway operate in this developmental context will be important for elucidating the molecular and cellular defects underlying the phenotypes and interactions we have reported. For example, the photoreceptor axon targeting defects observed in eya or abl mutants, or upon Myr-EyaWT expression, could reflect impaired receiving or processing of attractive signals from either brain cells or adjacent retinal neurons, weakening of repulsive signaling between axons, or strengthening of adhesive properties between the axons such that they fail to spread properly as they exit the optic stalk.
In considering the mechanistic possibilities whereby Eya might interact with the Abl signaling network, it is important to reiterate that while our genetic analyses indicate eya and abl function cooperatively, the two genes encode proteins with opposing catalytic functions. Thus a simple relationship whereby Eya dephosphorylates Abl or its substrates is unlikely to offer a suitable explanation since this would most likely be reflected as antagonism rather than synergy. Instead, Abl phosphorylation and recruitment of Eya to the cytoplasm may facilitate formation of protein complexes important for Abl signaling and/or promote interactions with components of other phosphotyrosine signaling pathways, which together would target Eya phosphatase activity toward appropriate substrates. Finally, our results do not preclude Eya’s nuclear transcriptional activities from also contributing to Abl signaling; thus it will be important to investigate further the complex spatio-temporal requirements for Eya, other members of the RD network, and the Abl signaling pathway during retinal development.
Experimental Procedures
Fly strains
We used the following fly stocks: hsFLP, Elav-Gal4, UAS-mCD8GFP;tub-Gal80FRT80B/TM6Tb; hsFLP, Elav-Gal4, UAS-mCD8GFP;tub-Gal80FRT40A/Cyo; Ptc-Gal4; Apt-Gal4; Act-Gal4; GMR-Gal4(Zipursky); dpp-Gal440C6; Ro-lacZtau; eyaA188; eyaG130; eya2; eyaClift;abl1; abl2; abl4; abl04674.
eya and abl MARCM clones were generated by heat-shocking hsFLP, Elav-Gal4, UAS-mCD8GFP; eyaCliftFRT40A/tub-Gal80FRT40A animals and hsFLP, Elav-Gal4, UAS-mCD8GFP; abl2FRT80B/tub-Gal80FRT80B animals at 37°C for 2 hr on the second day after embryos were laid.
For CNS studies, abl2/TM3TwiGFP, eyaA188/CyoTwiGFP and eyaA188/CyoTwiGFP; abl2/TM3TwiGFP flies were crossed as appropriate. Non-GFP expressing embryos were selected for immunostaining.
UAS-EyaWT, UAS-NLS-EyaWT, UAS-Myr-EyaWT, UAS-Myr-EyaK699Q, UAS-NLSMUT-EyaWT, UAS-MyrMUT-EyaWT transgenics were generated by standard P element-mediated transformation. In the ectopic eye assay ~200 flies of the genotype dpp-Gal4>UAS-Eya were examined for each line for the presence of pigmented eye-like tissue under the antennae. For ease of genetic manipulation, only lines with 3rd chromosome inserts were tested in the rescue assay and ~100 flies of the genotype eya2; dpp-Gal4/UAS-Eya were scored for recovery of eye tissue. Recombinants carrying two different Eya transgenes were confirmed by immunostaining. To examine the R2–R5 overshooting phenotype, GMR-Gal4/CyoGFP; Ro-lacZtau flies were crossed to GFP-balanced lines of UAS-EyaWT, UAS-EyaRNAi/y, UAS-AblRNAi/y, UAS-EyaRNAi, UAS-AblRNAi/y, UAS-AblRNAi/y;UAS-EyaWT, UAS-Myr-EyaWT, UAS-Myr-EyaK699Q or UAS-AblRNAi/y; UAS-Myr-EyaWT. Quantitation of mistargeted axon bundles was performed blind as to genotype. 10–40 brains were scored for each genotype, and an average number of overshooting bundles/brain calculated.
Immunoprecipitation and in vitro kinase assay
S2 cells were cultured at 25°C in Schneider’s medium (Invitrogen) supplemented with 10% Insect Medium Supplement (Sigma), penicillin (1U/ml), and streptomycin (1μg/ml). Cells were transfected with 0.7ug of each plasmid using dimethyldioctadecylammonium bromide (DDAB; Sigma) liposome transfection and induced after 24 hours with .7mM CuS04. Pervanadate treatment with 100 μM NaVO3 and 200 μM H2O2 was performed for 15 minutes prior to fixation or lysis.
For immunoprecipitation studies, cells were lysed in whole cell lysis buffer (100mMNaCl, 50mM Tris pH 7.5, 2mM EDTA, 2mM EGTA, 1%NP-40) with protease inhibitors (Roche), incubated with 20ul anti-Flag agarose (Sigma) for 1 hr at 4°C, washed 2x with buffer and 2x with TBS, boiled in SDS loading buffer, and resolved on 8% SDS-PAGE gels.
Proteins were visualized by immunoblotting using mouse anti-Flag (1:1000, Sigma), rabbit anti-phosphotyrosine (1:400, Upstate), mouse anti-Myc (1:500, Santa Cruz Biotechnology), and IRDye secondary antibodies (1:5000, Li-COR Biosciences) with the Odyssey Infrared Imaging System (Li-COR Biosciences). Dephosphorylation of immunoprecipitated Eya was achieved by incubation with 400 units of Lambda phosphatase (New England Biolab).
In vitro kinase assays were performed using mammalian c-Abl (NEB P6050S) and purified recombinant GST-EyaPST, GST-EyaED and GST as substrates. 20μl reactions containing 1μg of GST-fusion protein, kinase buffer (NEB: 50mM Tris-HCl, 10mM MgCl2, 1mM EGTA, 2mM DTT, .01% Brij 35, pH 7.5@25°C, supplemented with 20M cold ATP), 1μl gamma-32P ATP (Perkin Elmer BLU502H, SA 3000Ci) and either 0.5–1μl NEB Abl or immunoprecipitated Drosophila Abl, were incubated for 30 minutes at 30°C, mixed with 20μl 2X SDS gel loading buffer and boiled before loading onto polyacrylamide gels. Gels were either Coomassie stained or transferred to nitrocellulose for exposure on a Storm phosphoimager.
Immunostaining and antibodies
Imaginal discs dissected from late 3rd instar larvae were fixed in 4% paraformaldehyde in PBT (0.1% Triton X-100 in PBS) for 10 min. Genotyped stage 16 embryos (GFP negative) were dechorionated in 50% bleach and fixed in a 1:1 mix of heptane and 4% paraformaldehyde in PBS for 20 minutes. Tissues were blocked in 1% normal goat serum PBS, incubated with primary antibodies at 4°C overnight, washed 3x in PBT, and incubated with Cy3 or FITC conjugated secondary antibodies (Jackson Immunoresearch, 1:2000) for 1 hr at room temperature, washed and mounted in Prolong antifade (Invitrogen). Fluorescent images were taken using a Zeiss 510 confocal microscope. Antibodies: guinea pig anti-Eya (1:10000), mouse anti-Myc (1:1000, Santa Cruz Biotechnology), mouse anti-Elav, anti-lamin, BP102, and 24B10 (1:10, Developmental Studies Hybridoma Bank), and rabbit anti-β-gal (1:10000, Promega).
Supplementary Material
Acknowledgments
We thank J. Jemc, C. Wrobel, R. Fehon, and E. Ferguson for comments on the manuscript, Rebay and Fehon lab members for discussions, K. Nyberg & M. DiMarco for confocal assistance, J. Weinberg for help with genetics, and M. Seeger, M. Peifer, and D. Van Vactor for reagents. We acknowledge the Bloomington and VDRC stock centers for flies, and the DSHB for antibodies. This research was supported by NIH grant R01 EY12549 to I.R., a Women’s Board Fellowship of the U. of Chicago to W. X. and the Medical Scientist NRSA 5T32 GM07281 to N.D..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelhak S, Kalatzis V, et al. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet. 1997;15(2):157–64. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- Bennett RL, Hoffmann FM. Increased levels of the Drosophila Abelson tyrosine kinase in nerves and muscles: subcellular localization and mutant phenotypes imply a role in cell-cell interactions. Development. 1992;116(4):953–66. doi: 10.1242/dev.116.4.953. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Bui QT, et al. The Drosophila eyes absent gene directs ectopic eye formation in a pathway conserved between flies and vertebrates. Development. 1997;124(23):4819–26. doi: 10.1242/dev.124.23.4819. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Leiserson WM, et al. The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell. 1993;72(3):379–95. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Leiserson WM, et al. Multiple roles of the eyes absent gene in Drosophila. Dev Biol. 1998;196(1):42–57. doi: 10.1006/dbio.1997.8845. [DOI] [PubMed] [Google Scholar]
- Boyle M, Bonini N, DiNardo S. Expression and function of clift in the development of somatic gonadal precursors within the Drosophila mesoderm. Development. 1997;124(5):971–82. doi: 10.1242/dev.124.5.971. [DOI] [PubMed] [Google Scholar]
- Burroughs AM, Allen KN, et al. Evolutionary genomics of the HAD superfamily: understanding the structural adaptations and catalytic diversity in a superfamily of phosphoesterases and allied enzymes. J Mol Biol. 2006;361(5):1003–34. doi: 10.1016/j.jmb.2006.06.049. [DOI] [PubMed] [Google Scholar]
- Carthew RW. Pattern formation in the Drosophila eye. Curr Opin Genet Dev. 2007;17(4):309–13. doi: 10.1016/j.gde.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer AR, Ahern-Djamali SM, et al. Phosphorylation of Enabled by the Drosophila Abelson tyrosine kinase regulates the in vivo function and protein-protein interactions of Enabled. Mol Cell Biol. 1998;18(1):152–60. doi: 10.1128/mcb.18.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross FR, Garber EA, et al. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984;4(9):1834–42. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embry AC, Glick JL, et al. Reciprocal signaling between the transcriptional cofactor Eya2 and specific members of the Galphai family. Mol Pharmacol. 2004;66(5):1325–31. doi: 10.1124/mol.104.004093. [DOI] [PubMed] [Google Scholar]
- Fan X, Brass LF, et al. The alpha subunits of Gz and Gi interact with the eyes absent transcription cofactor Eya2, preventing its interaction with the six class of homeodomain-containing proteins. J Biol Chem. 2000;275(41):32129–34. doi: 10.1074/jbc.M004577200. [DOI] [PubMed] [Google Scholar]
- Fogerty FJ, Juang JL, et al. Dominant effects of the bcr-abl oncogene on Drosophila morphogenesis. Oncogene. 1999;18(1):219–32. doi: 10.1038/sj.onc.1202239. [DOI] [PubMed] [Google Scholar]
- Fox DT, Peifer M. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development. 2007;134(3):567–78. doi: 10.1242/dev.02748. [DOI] [PubMed] [Google Scholar]
- Garrity PA, Lee CH, et al. Retinal axon target selection is regulated by a receptor protein tyrosine phosphatase. Neuron. 1999;22(4):707–717. doi: 10.1016/s0896-6273(00)80730-8. [DOI] [PubMed] [Google Scholar]
- Gertler FB, Bennett RL, et al. Drosophila abl tyrosine kinase in embryonic CNS axons: a role in axonogenesis is revealed through dosage-sensitive interactions with disabled. Cell. 1989;58(1):103–13. doi: 10.1016/0092-8674(89)90407-8. [DOI] [PubMed] [Google Scholar]
- Grevengoed EE, Loureiro JJ, et al. Abelson kinase regulates epithelial morphogenesis in Drosophila. J Cell Biol. 2001;155(7):1185–98. doi: 10.1083/jcb.200105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson IM. Mammalian homologues of the Drosophila eye specification genes. Semin Cell Dev Biol. 2001;12(6):475–84. doi: 10.1006/scdb.2001.0271. [DOI] [PubMed] [Google Scholar]
- Heanue TA, Reshef R, et al. Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev. 1999;13(24):3231–43. doi: 10.1101/gad.13.24.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkemeyer MJ, Gertler FB, et al. The Drosophila Abelson proto-oncogene homolog: identification of mutant alleles that have pleiotropic effects late in development. Cell. 1987;51(5):821–8. doi: 10.1016/0092-8674(87)90105-x. [DOI] [PubMed] [Google Scholar]
- Hsouna A, Kim YS, et al. Abelson tyrosine kinase is required to transduce midline repulsive cues. J Neurobiol. 2003;57(1):15–30. doi: 10.1002/neu.10232. [DOI] [PubMed] [Google Scholar]
- Jemc J, Rebay I. The Eyes Absent Family of Phosphotyrosine Phosphatases: Properties and Roles in Developmental Regulation of Transcription. 2007a;76:513–538. doi: 10.1146/annurev.biochem.76.052705.164916. [DOI] [PubMed] [Google Scholar]
- Jemc J, Rebay I. Identification of transcriptional targets of the dual-function transcription factor/phosphatase eyes absent. Dev Biol. 2007b;310(2):416–29. doi: 10.1016/j.ydbio.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D, Roberts BL, et al. A short amino acid sequence able to specify nuclear location. Cell. 1984;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kochhar A, Fischer SM, et al. Branchio-oto-renal syndrome. Am J Med Genet A. 2007;143(14):1671–8. doi: 10.1002/ajmg.a.31561. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24(5):251–4. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Li S, Armstrong CM, et al. A map of the interactome network of the metazoan C. elegans. Science. 2004;303(5657):540–3. doi: 10.1126/science.1091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Oghi KA, et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426(6964):247–54. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Miguel-Aliaga I, Allan DW, Thor S. Independent roles of the dachshund and eyes absent genes in BMP signaling, axon pathfinding and neuronal specification. Development. 2004;131(23):5837–48. doi: 10.1242/dev.01447. [DOI] [PubMed] [Google Scholar]
- Moresco EM, Koleske AJ. Regulation of neuronal morphogenesis and synaptic function by Abl family kinases. Curr Opin Neurobiol. 2003;13(5):535–44. doi: 10.1016/j.conb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Mutsuddi M, Chaffee B, et al. Using Drosophila to decipher how mutations associated with human branchio-oto-renal syndrome and optical defects compromise the protein tyrosine phosphatase and transcriptional functions of eyes absent. Genetics. 2005;170(2):687–95. doi: 10.1534/genetics.104.039156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto H, Kamada S, et al. Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol. 1999;19(10):6815–24. doi: 10.1128/mcb.19.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu KS, Mardon G. Genetic control of retinal specification and determination in Drosophila. Int J Dev Biol. 2004;48(8–9):913–24. doi: 10.1387/ijdb.041875kp. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Hu B, et al. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91(7):881–91. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Rayapureddi JP, Hegde RS. Branchio-oto-renal syndrome associated mutations in Eyes Absent 1 result in loss of phosphatase activity. FEBS Lett. 2006;580(16):3853–9. doi: 10.1016/j.febslet.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Rayapureddi JP, Kattamuri C, et al. Characterization of a plant, tyrosine-specific phosphatase of the aspartyl class. Biochemistry. 2005;44(2):751–8. doi: 10.1021/bi0481794. [DOI] [PubMed] [Google Scholar]
- Rayapureddi JP, Kattamuri C, et al. Eyes absent represents a class of protein tyrosine phosphatases. Nature. 2003;426(6964):295–8. doi: 10.1038/nature02093. [DOI] [PubMed] [Google Scholar]
- Rebay I, Chen F, et al. A genetic screen for novel components of the Ras/Mitogen-activated protein kinase signaling pathway that interact with the yan gene of Drosophila identifies split ends, a new RNA recognition motif-containing protein. Genetics. 2000;154(2):695–712. doi: 10.1093/genetics/154.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebay I, Silver SJ, et al. New vision from Eyes absent: transcription factors as enzymes. Trends Genet. 2005;21(3):163–71. doi: 10.1016/j.tig.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Silver SJ, Davies EL, et al. Functional dissection of eyes absent reveals new modes of regulation within the retinal determination gene network. Mol Cell Biol. 2003;23(17):5989–99. doi: 10.1128/MCB.23.17.5989-5999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Carraway KL, 3rd, et al. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature. 1995;373(6514):536–9. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Hatano S, et al. Homologs of animal eyes absent (eya) genes are found in higher plants. Mol Gen Genet. 1999;262(1):131–8. doi: 10.1007/s004380051067. [DOI] [PubMed] [Google Scholar]
- Tootle TL, Silver SJ, et al. The transcription factor Eyes absent is a protein tyrosine phosphatase. Nature. 2003;426(6964):299–302. doi: 10.1038/nature02097. [DOI] [PubMed] [Google Scholar]
- Treisman JE. A conserved blueprint for the eye? Bioessays. 1999;21(10):843–50. doi: 10.1002/(SICI)1521-1878(199910)21:10<843::AID-BIES6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Maas RL. Vertebrate eye development as modeled in Drosophila. Hum Mol Genet. 2000;9(6):917–25. doi: 10.1093/hmg/9.6.917. [DOI] [PubMed] [Google Scholar]
- Wills Z, Marr L, et al. Profilin and the Abl tyrosine kinase are required for motor axon outgrowth in the Drosophila embryo. Neuron. 1999a;22(2):291–9. doi: 10.1016/s0896-6273(00)81090-9. [DOI] [PubMed] [Google Scholar]
- Wills Z, Bateman J, et al. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron. 1999b;22(2):301–12. doi: 10.1016/s0896-6273(00)81091-0. [DOI] [PubMed] [Google Scholar]
- Xu PX, Adams J, et al. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23(1):113–7. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Knosp BM, et al. A comparative study of Eya1 and Eya4 protein function and its implication in branchio-oto-renal syndrome and DFNA10. J Assoc Res Otolaryngol. 2004;5(3):295–304. doi: 10.1007/s10162-004-4044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JE, Bui QT, et al. Cloning and characterization of two vertebrate homologs of the Drosophila eyes absent gene. Genome Res. 1997;7(2):128–41. doi: 10.1101/gr.7.2.128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.