Abstract
SPARC up-regulation is a poor prognostic factor in head and neck cancer. It was hypothesized that because of a SPARC-albumin interaction, tumoral SPARC facilitates the accumulation of albumin in the tumor and increases the effectiveness of albumin-bound paclitaxel (nab-paclitaxel). This hypothesis was tested by correlating the response to nab-paclitaxel and SPARC tumor expression in a retrospective analysis of a 60-patient clinical study of nab-paclitaxel as monotherapy against head and neck cancer. Sixteen tumor specimens were available for analysis. There were 11 responders (CR/PR) and 5 nonresponders (SD/PD) among the 16 nab-paclitaxel-treated patients (12/16 SPARC-positive, 75%). Response to nab-paclitaxel was higher for SPARC-positive patients (10/12, 83%) than SPARC-negative patients (1/4, 25%). The SPARC-negative patients exhibited significantly lower response than the overall response rate among all 60 patients (1/4, 25% vs 45/60, 75%). Although preliminary, data are supportive of the hypothesis that SPARC overexpression may correlate with response to nab-paclitaxel. If confirmed in larger studies, treatment with nab-paclitaxel may convert a poor prognosis SPARC-positive patient population into a group with better clinical outcomes.
Introduction
Head and neck cancer, which includes cancers of the oral cavity, larynx, nasal passages, pharynx, and salivary glands, accounts for approximately 3% of adult malignancies in the United States [1]. In 2008, there were estimated 47,560 new cases of head and neck cancers in the United States, with 11,260 mortalities [1]. For advanced disease, treatment regimens include surgery, radiotherapy, chemotherapy, and potentially targeted therapy. Intra-arterial infusion chemotherapy for head and neck cancer is associated with favorable pharmacokinetics, lower systemic toxicity, and high tumor response rate [2]. However, this route of administration was not available for paclitaxel until the advent of albumin-bound paclitaxel (nab-paclitaxel). Albumin-bound paclitaxel (nab-paclitaxel), also known as Abraxane, or ABI-007, is a Cremophor-free, albumin-bound 130-nm particle form of paclitaxel (Abraxane package insert). It received approval from the Food and Drug Administration in January 2005 for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Compared with conventional paclitaxel formulation known as Taxol (Bristol-Myers Squibb Co, Princeton, NJ), nab-paclitaxel does not require the use of Cremophor-EL, thus avoids the severe toxicities associated with this vehicle.
Clinical studies by Damascelli et al. [3] have demonstrated that the intra-arterial nab-paclitaxel chemotherapy by percutaneous catheterization has great promise in the treatment of advanced squamous cell carcinomas (SCCs) of the head and neck. In a phase 1 study with 31 advanced patients, the maximum tolerated dose (MTD) was determined to be 270 mg/m2 every 4 weeks. The toxicity was acceptable, with the dose-limiting toxicity being myelosuppression with grade 4 neutropenia in one patient. The treatment showed strong antitumor activity with an overall response rate (ORR) of 75.85% (22 of 29 assessable patients, with 3 complete responses [CRs] and 19 partial responses [PRs]). In an ensuing phase 2 trial [4], 60 previously untreated patients with locally advanced SCC of the oral cavity, oropharynx, or hypopharynx were treated with intra-arterial nab-paclitaxel every 3 weeks at an initial dose of 230 mg/m2 and subsequently a reduced dose of 150 mg/m2. The chemotherapy was highly effective and produced complete or partial responses in 45 of 60 patients (ORR: 75%; CR: 15 patients, 25%; PR: 30 patients, 50%). In a group of 23 previously untreated patients with advanced SCC of the tongue [5], treatment with intra-arterial nab-paclitaxel at 150 to 230 mg/m2 every 3 weeks resulted in clinical and radiologic objective responses in 18 patients (ORR: 78%; CR: 6 patients, 26%; PR: 12 patients, 52%) and acceptable toxicity. Overall, intra-arterial nab-paclitaxel demonstrated safety and strong efficacy against SCC of head and neck and consistently exhibited high tumor response rates of approximately 75% in clinical studies.
The effectiveness of nab-paclitaxel as a single agent against head and neck cancer in these trials prompted the question whether tumor-secreted SPARC, because of its albumin-binding properties, could enhance the accumulation of albumin in tumor tissue and hence influence the response to nab-paclitaxel [6]. SPARC was first identified as a glycosylated 43-kDa secreted protein with high binding affinity to albumin [7]. SPARC modulates the cell and extracellular matrix interactions and acts as a key regulator of critical cellular functions such as proliferation, survival, and cell migration [8]. In normal tissues, SPARC expression is limited to bone and tissues undergoing development, remodeling, and repair [9]. However, SPARC expression is known to be upregulated in many different tumors and plays important roles in tumor progression [10]. SPARC can be produced by both cancer cells and the reactive stromal cells and is found to be highly expressed in the tumor-stroma interface of the invading tumors and induced by hypoxia and acidity [11]. Overexpression of SPARC is associated with increased tumor invasion and metastasis, leading to poor prognosis in multiple tumor types, including breast, prostate, esophagus, gastric, colorectal, liver, lung, kidney, melanoma, bladder, head and neck, thyroid, and brain cancers [10]. Specifically for head and neck cancer, SPARC overexpression has been demonstrated to be a prognostic factor for short disease-free survival and poor overall survival (OS) [12,13].
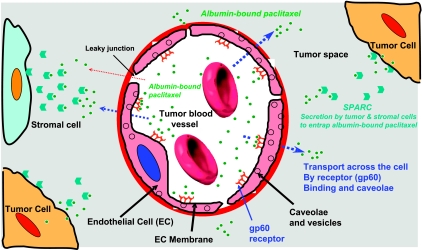
Previously, we have shown that SPARC in the tumor can facilitate the accumulation of albumin-bound drugs [6]. Various proliferating tumors are known to accumulate albumin as a nutrition and nitrogen source for de novo protein synthesis [14]. nab-Paclitaxel takes advantage of this important aspect of tumor biology by using the gp60 and caveolae-mediated albumin transport pathway to traverse the blood vessel endothelial lining into the tumor wherein it is preferentially retained by tumoral SPARC. The proposed mechanism of action for nab-paclitaxel as shown in Figure 1 explains the observed almost doubling of response to nab-paclitaxel compared with Taxol during a phase 3 clinical trial in metastatic breast cancer [15]. The purpose of this retrospective clinical study was to investigate the correlation between SPARC tumoral expression and response to nab-paclitaxel as a monotherapy against head and neck cancer to further explain the observed high response rate of solid tumors to nab-paclitaxel.
Figure 1.
Mechanisms for the transport and accumulation of albumin-bound paclitaxel in tumors. The transport of albumin-bound paclitaxel complexes across the endothelial barrier of tumor microvasculature is facilitated by gp60 receptor and caveolin-1 mediated transcytosis. The accumulation of albumin-bound paclitaxel in tumor is enhanced by the presence of albumin-binding protein SPARC in the tumor interstitium. Entry of paclitaxel into the cells (tumor or stromal) likely occurs by rapid exchange of albumin-bound paclitaxel to the lipidic components of the cell membrane. The mechanism for cellular uptake remains to be elucidated.
Materials and Methods
Tumor and Normal Tissues
Human head and neck tumor tissue array and normal human tissues were obtained from Cybrdi (Frederick, MD). Head and neck tumor tissues (N = 119) were taken from various regions including cheek (n = 18), jaws (n = 6), larynx (n = 33), tongue (n = 27), lip (n = 6), gingival (n = 3), nasopharynx (n = 3), ethmoid sinus (n = 3), and nose (n = 20). Normal tissues (N = 25) were taken from regions adjacent to tumors and consisted of gingival (n = 9), tongue (n = 6), and tonsil (n = 10). Biopsies from 16 head and neck cancer patients receiving intra-arterial nab-paclitaxel treatment were provided by Dr. Bruno Damascelli (Istituto Nazionale Tumori, Milano, Italy) [4]. Tumors (N = 16) consisted of tongue (n = 6), tonsil (n = 6), palate (n = 2), gum (n = 1), and oropharynx (n = 1). As control, normal adult human head and neck tissue array was obtained from Cybrdi (N = 16), consisted of samples from tonsil (n = 10) and tongue (n = 6).
Immunohistochemical Staining
Expression of SPARC in head and neck cancer and normal tissues was examined by immunohistochemical staining, performed as described previously [16]. Briefly, paraffin-embedded sections were washed thrice for 10 minutes each with Hemo De (Fisher Scientific, Pittsburgh, PA), rinsed in absolute ethanol, and then treated with methanol containing 0.5% hydrogen peroxide for 30 minutes. The slides were then washed in water, incubated in PBS-Tween (0.1% Tween 20 in PBS) for 10 minutes, followed by blocking in Dulbecco's modified Eagle's medium containing 10% FBS for 1 hour. The slides were then incubated with 10 µg/ml of polyclonal antihuman SPARC antibody (R&D Systems, Minneapolis, MN) in PBS-Tween for 1 hour, washed twice in PBS-Tween for 5 minutes each, and incubated for 1 hour with 1:100 dilution of antirabbit horseradish peroxidase-conjugated antibody (Pierce, Rockford, IL). Sections were washed in PBS-Tween and incubated for 10 minutes in the substrate solution (100 µl of 2.4 mg/ml 3-amino-9-ethylcarbazole in N′N′-dimethyl formamide, 1 ml of acetate buffer, pH 5.2, and 5 µl of 30% wt/wt hydrogen peroxide), counterstained with Mayer's hematoxylin, and mounted with Crystal Mount (BioMeda, Corp, Foster City, CA). The staining was scored on a 0 to 4 scale (0 = no staining, 4 = strong positive). Positive SPARC expression was defined as ≥2+ staining and negative SPARC expression was defined as <2+ staining. The immunohistochemical scoring was performed blinded by a trained pathologist. The SPARC scoring in this study was an overall score of the tumor, comprising both the tumor and stromal cells, as both tumoral SPARC and stromal fibroblast SPARC have been shown to be important factors determining prognosis.
Patient Group and nab-Paclitaxel Treatment
Tumor blocks from 16 patients analyzed retrospectively for SPARC status were part of the phase 2 clinical study with 60 patients by Damascelli et al. [4]. The 16 randomly chosen patients all had biopsy-proven SCC of the oral cavity, oropharynx, or hypopharynx (stage T3/T4, any nodal stage). As described previously, each patient received a minimum of two to a maximum of four intra-arterial infusions of nab-paclitaxel (dose range, 150–230 mg/m2), with an interval of 3 weeks between each infusion. The intra-arterial administration was performed by percutaneous catheterization of the femoral artery. After intra-arterial nab-paclitaxel chemotherapy, patients received definitive treatment, which included surgery, chemotherapy, radiotherapy, or combination therapy.
Outcome Evaluation and Statistical Analysis
Response to treatment was evaluated by physical examination and CT performed before each intra-arterial infusion and 3 weeks after the last treatment cycle. Objective response was assessed with the clinical and radiological method that provided the best evidence. The response was classified as complete response (CR, complete disappearance of all clinical and radiologic evidence of disease), partial response (PR, ≥50% decrease in tumor size), stable disease (SD, <50% decrease or <25% increase in tumor size), or tumor progression (PD, >25% increase in tumor size). The correlation between tumor SPARC expression status and clinical response to nab-paclitaxel was analyzed by Fisher's exact test using the Prism statistical program (GraphPad, San Diego, CA).
Results
SPARC Is Overexpressed in Head and Neck Tumors
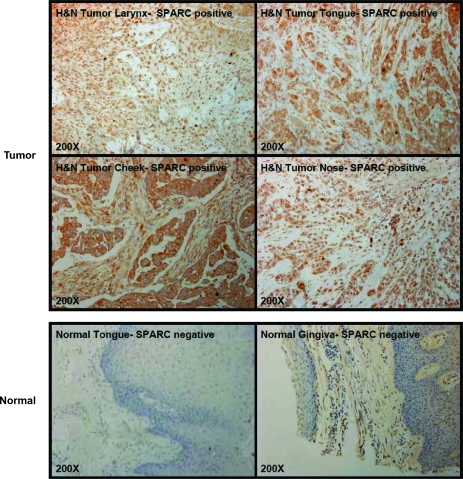
To confirm previous findings of SPARC overexpression in head and neck cancer, we examined SPARC expression by immunohistochemical staining of human head and neck tumor tissue array. In the tissue array, 72 (61%) of 119 head and neck cancer tissues were SPARC-positive (immunostaining score, ≥2+) compared with 0 (0%) of 25 normal tissues (P < .0001, Fisher's exact test). Among head and neck cancers, SPARC expression was higher in tumor tissues from tongue than those from other regions: tongue (22/27, 81% positive) versus cheek (12/18, 68%), ethmoid sinus (2/3, 67%), larynx (17/33, 52%), jaw (3/6, 50%), lip (3/6, 50%), nose (9/20, 45%), gingiva (0/3, 0%), and nasopharynx (0/3, 0%). Representative immunohistochemical stainings are shown in Figure 2.
Figure 2.
Overexpression of SPARC in human head and neck tumor tissue arrays. SPARC expression was analyzed by immunohistochemical staining in human head and neck tumor and normal head and neck tissue arrays. SPARC was overexpressed in head and neck cancer of different sites but not in normal tissues.
SPARC Is Overexpressed in Head and Neck Cancer Patients Enrolled in the Current Trial
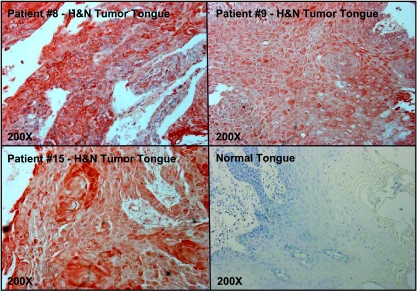
We further evaluated SPARC status in tumor samples from 16 head and neck cancer patients enrolled in the phase 2 intra-arterial nab-paclitaxel clinical trial conducted by Damascelli et al. [4]. A large percentage of the tumors (12 of 16 patients, 75%) were SPARC-positive, compared with 0% of normal tissues (0 of 16 samples from Cybrdi normal head and neck tissue array; P < .0001, Fisher's exact test). There was no correlation between SPARC status and tumor/nodal stage. For tumors at specific sites, 83.3% of tongue tumors (5/6) overexpressed SPARC, compared with 0% of normal tongue (0/6; P = .015, Fisher's exact test). SPARC was overexpressed in 83.3% of tonsil tumors (5/6), compared with 0% of normal tonsil (0/10; P = .001, Fisher's exact test). Representative immunohistochemical stainings are shown in Figure 3.
Figure 3.
SPARC overexpression in head and neck cancer patients (tongue cancer subgroup). SPARC expression was analyzed by immunohistochemical staining in biopsy samples from patients with tongue cancers. Normal tongue tissue served as a control.
SPARC Expression Correlates with Response to Albumin-Bound Paclitaxel
The ORR to nab-paclitaxel among 60 patients in the study was 75% (45/60), with 25% CR (15 patients) and 50% PR (30 patients) [4]. The 16 patients with available SPARC status included 11 responders (3 CR, 8 PR) and 5 nonresponders (2 SD, 3 PD). Detailed results from the 16 patients are summarized in Table 1. Response to nab-paclitaxel (CR and PR) was higher for SPARC-positive patients (10 responders in 12 patients, 83%) compared with SPARC-negative patients (1 responder in 4 patients, 25%), P = .06, Fisher's exact test. Furthermore, the SPARC-negative patients exhibited significantly lower response rate compared with the ORR in the study (1/4 = 25% vs 45/60 = 75%, P < .05, Fisher's exact test). No correlation was observed between tumor response and tumor/nodal stage. Overall, there was correlation between positive SPARC expression and favorable response to nab-paclitaxel in this group of 16 head and neck cancer patients (Table 2).
Table 1.
Patient Data, SPARC Expression, and Response to nab-Paclitaxel in 16 Head and Neck Cancer Patients.
| Patient No. | Site | Staging | Response | SPARC |
| 1 | Left tonsil | T3 N0 | Complete | Positive |
| 2 | Tongue | T4 N2 A | Partial | Positive |
| 3 | Tongue | T3 N2 A | Partial | Negative |
| 4 | Tongue | T3 N0 | Partial | Positive |
| 5 | Gum | T3 N0 | Partial | Positive |
| 6 | Tonsil | T3 N0 | Complete | Positive |
| 7 | Tonsil | T3 N0 | Complete | Positive |
| 8 | Tongue | T3 N1 | Partial | Positive |
| 9 | Tongue | T3 N0 | Partial | Positive |
| 10 | Tonsil | T3 N0 | Partial | Positive |
| 11 | Tonsil | T4 N2 B | Partial | Positive |
| 12 | Oropharynx | T3 N0 | Progression | Positive |
| 13 | Tonsil | T3 N0 | Progression | Negative |
| 14 | Palate | T4 N0 | Stable | Negative |
| 15 | Tongue | T3 N2 B | Stable | Positive |
| 16 | Palate | T4 N0 | Progression | Negative |
Table 2.
Correlation of SPARC Expression with Response to nab-Paclitaxel in 16 Head and Neck Cancer Patients.
| SPARC Status | Patients Responding | Patients Nonresponding |
| Positive (n = 12) | 10/12 (83%) | 2/12 (17%) |
| Negative (n = 4) | 1/4 (25%) | 3/4 (75%) |
Correlation was also observed in a subgroup of tongue and tonsil cancer patients among the 16 head and neck cancer patients. Among the six patients with cancer of the tongue, five patients (83.3%) responded to nab-paclitaxel treatment (0 CR/5 PR). Five patients were SPARC-positive and four of them were responders. Among the six patients with cancer of the tonsil, five patients (83.3%) responded to nab-paclitaxel treatment (3 CR/2 PR). There were five SPARC-positive patients in this subgroup, and all of them were responders.
Discussion
SPARC has been proposed as an important factor affecting the drug accumulation and tumor response of albumin-bound drugs including nab-paclitaxel through the albumin-SPARC interaction [15,17]. To better understand the reasons behind the improved response rate for nab-paclitaxel, we analyzed SPARC expression levels in head and neck cancer tissue array and patient biopsy samples and correlated the SPARC status with clinical response. This study was unique as it allowed us to examine response to nab-paclitaxel in a monotherapy setting, without confounding factors from other anticancer agents that are frequently used in combination with paclitaxel. In a previous study by Borad et al. [18], SPARC was identified by microarray across multiple tumor types as the gene most differentially expressed in patients with advanced cancer, with overexpression over normal tissues being observed in 68% of 112 tumors. Tumor types with a particularly high level of SPARC positivity were melanoma (88%), pancreatic cancer (81%), and breast (67%). Consistent with this result, our study showed that there was overexpression of SPARC in a large percentage of head and neck cancers but not in normal tissues. In the tumor tissue array, 61% of head and neck cancer tissues were SPARC-positive compared with 0% of normal tissues. Further, in 16 head and neck cancer patients in our clinical study, 75% were SPARC-positive, compared with 0% of samples from Cybrdi normal tissue array. For the subgroup of six patients with tongue tumors, 83.3% overexpressed SPARC, compared with 0% of normal tongue tissues. Our data expanded on the nearly universal observation that SPARC is predominantly overexpressed in solid tumors including head and neck cancer [10].
In previous studies, a positive SPARC status was a strong independent prognostic indicator for short disease-free interval (DFI) and poor OS in head and neck cancer patients [12], and SPARC positivity in Stage II tongue carcinoma was also a poor prognostic factor for OS [13]. In a study by Chin et al. [12], SPARC mRNA levels were identified by microarray to be upregulated in tumor tissues compared with matching normal tissues. Immunohistochemistry confirmed SPARC expression in tumor cells as well as stromal fibroblasts both within the tumor and adjacent to it. In a group of 62 head and neck cancer patients, SPARC-positive patients (n = 18) was associated with shorter DFI (mean, 28.3 months) compared with SPARC-negative patients (n = 44; mean, 59.4 months; P < .002). SPARC positivity was also an indicator for poor OS (mean, 34.94 months for SPARC-positive vs 59.84 months for SPARC-negative patients; P = .018). The association of SPARC status with DFI and OS was even more significant (P < .001) when analyzed in combination with PAI-1 and uPA expression. In a separate study by Kato et al. [13] with 86 patients of tongue carcinoma, 38 patients were diffusely or partly SPARC-positive, whereas 48 patients were SPARC-negative. The 5-year OS rate was 37.5% for the patients intensively positive for SPARC, compared with 63.2% for partly SPARC-positive patients and significantly lower than the survival rate of 70.7% for SPARC-negative cases (P < .05, Wilcoxon test). In 23 stage II cases, the 5-year survival rate was significantly lower in the intensively and partly SPARC-positive cases (28.6%) than in the SPARC-negative cases (91.7%; P < .001, Wilcoxon test), whereas the frequency of the postoperative metastasis was significantly higher in SPARC-positive cases (5/8, 62.5%) than in the negative cases (1/15, 6.7%; P < .01, χ2 test).
Although high levels of SPARC are generally associated with poor prognosis in multiple tumor types, SPARC can also serve as a target for albumin-based chemotherapies such as nab-paclitaxel because of its albumin-binding activity. In vitro solid-phase binding assay has revealed that SPARC binds to albumin in a saturable and specific manner [6]. In both MX-1 breast tumor cells and tumor xenografts, SPARC was shown to colocalize with albumin [19]. nab-Paclitaxel achieved 33% higher intratumor paclitaxel concentration compared with an equal dose of Cremophor-based paclitaxel in MX-1 tumor xenografts [20]. Further, high-level SPARC expression is linked to response to nab-paclitaxel in tumor xenograft models. In a study with multiple tumor xenografts, the relative efficacy of nab-paclitaxel compared with polysorbate-based docetaxel seemed to increase with increasing SPARC expression in HER2-positive tumors [21]. nab-Paclitaxel at sub-MTD was equal to or better than polysorbate-based docetaxel at its MTD in PC3 prostate and HT29 colon tumors with medium to high SPARC levels but not in MDA-MB-231/HER2+ breast tumors with low SPARC expression. In another study, SPARC-overexpressing xenograft PC3/SP exhibited enhanced response to nab-paclitaxel compared with wild type PC3 prostate tumor xenograft (97% vs 84% tumor growth inhibition, and 36 vs 25 days in tumor growth delay, P < .0001, analysis of variance) [6].
Consistent with preclinical results showing correlation between SPARC and response to nab-paclitaxel, this study suggests that SPARC could also be used to predict a favorable tumor response to nab-paclitaxel treatment in a clinical setting in head and neck tumors and in a subgroup of tongue and tonsil tumors. SPARC-positive patients showed higher response to nab-paclitaxel compared with SPARC-negative patients. The response rate among SPARC-negative patients was also significantly lower than the ORR in the entire group of 60 patients. The preclinical results above along with current data showing overexpression of SPARC in head and neck cancers and clinical response support a potential correlation of response to nab-paclitaxel with SPARC positivity in head and neck cancer. Although this initial study of the SPARC correlation to clinical response was limited by the small number of patients, a similar correlation has also been observed in other tumor types. In particular, SPARC is also associated with improved response to nab-paclitaxel and gemcitabine with promising results in patients with advanced pancreatic cancer in a disease-specific modified phase 1 trial [22]. The use of SPARC as a predictive biomarker for nab-paclitaxel treatment requires further verification, and several larger ongoing clinical studies in multiple tumor types are investigating this correlation.
Acknowledgments
Shihe Hou's expert editorial and writing assistance of this manuscript is greatly appreciated.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Robbins KT, Storniolo AM, Kerber C, Vicario D, Seagren S, Shea M, Hanchett C, Los G, Howell SB. Phase I study of highly selective supradose cisplatin infusions for advanced head and neck cancer. J Clin Oncol. 1994;12:2113–2120. doi: 10.1200/JCO.1994.12.10.2113. [DOI] [PubMed] [Google Scholar]
- 3.Damascelli B, Cantu G, Mattavelli F, Tamplenizza P, Bidoli P, Leo E, Dosio F, Cerrotta AM, Di Tolla G, Frigerio LF, et al. Intraarterial chemotherapy with polyoxyethylated castor oil free paclitaxel, incorporated in albumin nanoparticles (ABI-007): phase II study of patients with squamous cell carcinoma of the head and neck and anal canal: preliminary evidence of clinical activity. Cancer. 2001;92:2592–2602. doi: 10.1002/1097-0142(20011115)92:10<2592::aid-cncr1612>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Damascelli B, Patelli G, Ticha V, Di Tolla G, Frigerio LF, Garbagnati F, Lanocita R, Marchiano A, Spreafico C, Mattavelli F, et al. Feasibility and efficacy of percutaneous transcatheter intraarterial chemotherapy with paclitaxel in albumin nanoparticles for advanced squamous-cell carcinoma of the oral cavity, oropharynx, and hypopharynx. J Vasc Interv Radiol. 2007;18:1395–1403. doi: 10.1016/j.jvir.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Damascelli B, Patelli GL, Lanocita R, Di Tolla G, Frigerio LF, Marchiano A, Garbagnati F, Spreafico C, Ticha V, Gladin CR, et al. A novel intraarterial chemotherapy using paclitaxel in albumin nanoparticles to treat advanced squamous cell carcinoma of the tongue: preliminary findings. AJR Am J Roentgenol. 2003;181:253–260. doi: 10.2214/ajr.181.1.1810253. [DOI] [PubMed] [Google Scholar]
- 6.Trieu V, Hwang J, Desai N. New Targets and Delivery System for Cancer Diagnosis and Treatment (SKCC) San Diego, CA: 2007. Nanoparticle Albumin-bound (nab) technology may enhance antitumor activity via targeting of SPARC protein. Abstract 53. [Google Scholar]
- 7.Sage H, Johnson C, Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984;259:3993–4007. [PubMed] [Google Scholar]
- 8.Brekken RA, Sage EH. SPARC, a matricellular protein: at the cross-roads of cell-matrix communication. Matrix Biol. 2001;19:816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 9.Porter PL, Sage EH, Lane TF, Funk SE, Gown AM. Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem. 1995;43:791–800. doi: 10.1177/43.8.7622842. [DOI] [PubMed] [Google Scholar]
- 10.Podhajcer OL, Benedetti LG, Girotti MR, Prada F, Salvatierra E, Llera AS. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008;27:523–537. doi: 10.1007/s10555-008-9135-x. [DOI] [PubMed] [Google Scholar]
- 11.Koukourakis MI, Giatromanolaki A, Brekken RA, Sivridis E, Gatter KC, Harris AL, Sage EH. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 2003;63:5376–5380. [PubMed] [Google Scholar]
- 12.Chin D, Boyle GM, Williams RM, Ferguson K, Pandeya N, Pedley J, Campbell CM, Theile DR, Parsons PG, Coman WB. Novel markers for poor prognosis in head and neck cancer. Int J Cancer. 2005;113:789–797. doi: 10.1002/ijc.20608. [DOI] [PubMed] [Google Scholar]
- 13.Kato Y, Nagashima Y, Baba Y, Kawano T, Furukawa M, Kubota A, Yanoma S, Imagawa-Ishiguro Y, Satake K, Taguchi T, et al. Expression of SPARC in tongue carcinoma of stage II is associated with poor prognosis: an immunohistochemical study of 86 cases. Int J Mol Med. 2005;16:263–268. [PubMed] [Google Scholar]
- 14.Stehle G, Sinn H, Wunder A, Schrenk HH, Stewart JC, Hartung G, Maier-Borst W, Heene DL. Plasma protein (albumin) catabolism by the tumor itself—implications for tumor metabolism and the genesis of cachexia. Crit Rev Oncol Hematol. 1997;26:77–100. doi: 10.1016/s1040-8428(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 15.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O'Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 16.Trieu VN, Narla RK, Myers DE, Uckun FM. EGF-genistein inhibits neointimal hyperplasia after vascular injury in an experimental restenosis model. J Cardiovasc Pharmacol. 2000;35:595–605. doi: 10.1097/00005344-200004000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7:1041–1053. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 18.Borad M, Penny R, Bittner M, Gardiner J, Shack S, Campbell E, Taverna D, Love R, Trent J, Von Hoff DD. First AACR International Conference on Molecular Diagnostics in Cancer Therapeutic Development. Chicago, IL: 2006. Molecular profiling using immunohistochemistry (IHC) and DNA microarray (DMA) as a tool to determine potential therapeutic targets in patients who have progressed on multiple prior therapies. Abstract B82. [Google Scholar]
- 19.Trieu V, Frankel T, Labao E, Soon-Shiong P, Desai N. American Association for Cancer Research (AACR) Annual Meeting. Anaheim, CA: 2005. SPARC expression in breast tumors may correlate to increased tumor distribution of nanoparticle albumin-bound paclitaxel (ABI-007) vs taxol. Abstract 5584. [Google Scholar]
- 20.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of Cremophor-free, albumin-bound paclitaxel, ABI-007, compared with Cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 21.Desai NP, Trieu V, Hwang LY, Wu R, Soon-Shiong P, Gradishar WJ. Improved effectiveness of nanoparticle albumin-bound (nab) paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a function of HER2 and SPARC status. Anticancer Drugs. 2008;19:899–909. doi: 10.1097/CAD.0b013e32830f9046. [DOI] [PubMed] [Google Scholar]
- 22.Von Hoff DD, Borad M, Ramanathan RK, Smith LS, Drengler RL, Wood TE, Laheru D, Hidalgo M. American Association for Cancer Research (AACR) Annual Meeting. San Diego, CA: 2008. Promising clinical activity of a NAB paclitaxel plus gemcitabine combination in a disease-specific phase I in patients with advanced pancreatic cancer. Abstract 4179. [Google Scholar]