Abstract
Human melanoma cells are very resistant to treatment with chemotherapeutic agents, and melanoma shows poor response to chemotherapeutic therapy. We describe a strong synergistic proapoptotic effect of the Bcl-2 family inhibitor ABT-737 and the standard antimelanoma drugs, namely, dacarbazine and fotemustine, and the experimental agent, imiquimod. Experiments with human melanoma cells, keratinocytes, and embryonic fibroblasts showed that all three agents activated the mitochondrial apoptosis pathway. ABT-737 on its own was ineffective in melanoma cells unless Mcl-1 was experimentally downregulated. However, ABT-737 strongly enhanced the proapoptotic activity of the chemotherapeutic drugs. Whereas cell death induction by all three agents involved the activity of both BH3-only proteins, Bim and Noxa, the combination with ABT-737 overcame the requirement for Bim. However, the synergism between ABT-737 and imiquimod or dacarbazine required endogenous Noxa, as demonstrated by experiments with Noxa-specific RNAi. Surprisingly, although Bim was activated, it was unable to replace Noxa. Studies of mitochondrial cytochrome c release using BH3 peptides confirmed that a main effect of dacarbazine, fotemustine, and imiquimod was to neutralize Mcl-1, thereby sensitizing mitochondria to the inhibition of other Bcl-2 family members through ABT-737. ABT-737 is thus a promising agent for combination therapy for human melanoma. Importantly, the efficacy of this therapy depends on endogenous Noxa, and the ability of chemotherapeutic drugs to activate Noxa may be a valuable predictor of their synergism with Bcl-2-targeting drugs.
Introduction
Mitochondrial apoptosis is to a large extent regulated by the Bcl-2 family of proteins, which consists of three separate groups [1]. The effector proteins, Bax and Bak, regulate the release of cytochrome c and other apoptogenic factors from mitochondria to the cytosol. Bax and Bak are activated consecutively to the activation of one or several BH3-only proteins, such as Bim, Puma, Bad, or Noxa. The antiapoptotic proteins Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and A1 inhibit apoptosis by binding to BH3-only proteins or Bax/Bak [2]. How BH3-only proteins activate Bax/Bak is still under dispute. The two molecular models that have been put forward to explain this differ in particular on the point whether BH3-only proteins have to activate the downstream effectors Bax and Bak (the “direct activation model”) or whether the role of BH3-only proteins is confined to the neutralization of antiapoptotic Bcl-2 proteins (as posited by the “displacement model”) and apoptosis occurs by the default of these inhibitors [3–5].
The BH3 domain of BH3-only proteins can bind to antiapoptotic Bcl-2 family proteins. Intriguingly, there are very significant differences in affinities between the various pairings. Bim and Puma BH3 domains can bind all antiapoptotic Bcl-2 proteins comparably well, whereas other BH3-only proteins demonstrate a dichotomy in the system: especially Bad can only bind to and neutralize Bcl-2, Bcl-xL, and Bcl-w but not A1 or Mcl-1, whereas Noxa binds only Mcl-1 and A1, not the others [6].
Because the basic cytochrome c release machinery seems intact in at least nearly all tumors, compounds have recently been designed or isolated that mimic BH3-only proteins in that they bind to the BH3 domain docking site in antiapoptotic Bcl-2 proteins and thereby block the antiapoptotic function of these proteins [7–9]. Although a number of such molecules have been described over the past years, not all of them were confirmed as true Bcl-2 binders and antagonists in a recent study [10]. These substances not only show promise as antitumor agents but also proved to be valuable tools in dissecting the mitochondrial apoptotic pathway. One substance, ABT-737, was derived from a screen of small compounds that showed binding affinity for the BH3 domain binding cleft of Bcl-xL [11]. ABT-737 has strong proapoptotic activity as a single agent against a number of B-lymphoid malignancies as well as acute and chronic leukemia cells and against small cell lung cancer cells (for review, see [12,13]). Like Bad, ABT-737 binds with high affinity to antiapoptotic Bcl-2, Bcl-xL, and Bcl-w but with much lower affinity to Mcl-1 or A1 [11]. This suggested that apoptosis resistance because of the high expression of the Bcl-2 would be overcome, whereas high-level expression of Mcl-1 could protect tumor cells against ABT-737. Indeed, a number of recent studies show consistently that Mcl-1 can confer resistance to ABT-737, whereas experimental approaches that downregulate Mcl-1 sensitize tumor cells to ABT-737 (reviewed in [13]). In a number of cell lines, ABT-737 has been found to induce apoptosis synergistically with other chemotherapeutic agents [14]. The molecular mechanisms of this synergism are largely unknown. As ABT-737 targets Bcl-2-like proteins other than Mcl-1 (A1 seems not to be critical in cell lines tested so far), the role of a second, synergizing agent likely involves neutralization of Mcl-1. Such neutralization could for instance be achieved by the activation of Noxa, the displacement of Bim or Bax/Bak from Bcl-2/Bcl-xL/Bcl-w (which should allow a neutralization of Mcl-1 by released Bim), or by the direct targeting of Mcl-1. A recent report suggests that synergism between ABT-737 and a synthetic cytotoxic retinoid in human acute lymphoblastic leukemia cell lines involves the reactive oxygen species-dependent downregulation of Mcl-1 [15].
Disseminated malignant melanoma carries a very poor prognosis. Although patients are routinely given chemotherapy, response rates are typically below 10% [16], and this treatment resistance is associated with very low apoptotic responses of patient-derived melanoma lines in vitro. Very recently, it was reported that human melanoma lines are resistant to ABT-737 and that this resistance can be overcome by tackling the Mcl-1-Noxa axis, either by down-regulating Mcl-1 by RNAi or by up-regulating Noxa using a proteasome inhibitor [17].
We here report strong synergism of ABT-737 with two chemotherapeutic agents in clinical use (dacarbazine and fotemustine) and one experimental agent (imiquimod [18,19]) in apoptosis induction in human melanoma cell lines. Dacarbazine and fotemustine are both alkylating substances from different substance classes and are both in clinical use for disseminated melanoma [20]. Imiquimod is an entirely different molecule that was developed as an immunostimulatory drug but was later found to stimulate Toll-like receptors (TLR) 7 and 8 and to induce apoptosis in a number of skin-derived tumors by an unknown mechanism [21]. In analyzing the molecular mechanism of this synergism between ABT-737 and different drugs, we use knockdown and knockout cell lines (including other cell types) to show that both Bim and Noxa play a role in these forms of apoptosis. Surprisingly, the loss of Bim but much less the loss of Noxa could be compensated for by ABT-737. Synergism of these cytotoxic drugs with ABT-737 thus requires endogenous Noxa and the ability of cytotoxic drugs to activate Noxa may be an important predictor of their synergism with BH3-only protein mimetics.
Materials and Methods
Cell Lines and Culture Conditions
1205Lu, 451Lu, WM35, and Sbcl2 human melanoma cells representing different stages of tumor progression (1205Lu and 451Lu are from metastatic melanoma, WM35 and Sbcl2 are radial growth phase melanomas [22,23]) were obtained from Dr. Meenhard Herlyn, Wistar Institute, Philadelphia, and cultured in TU2% melanoma medium containing 80% (v/v) MCDB153, 20% (v/v) Leibovitz's L-15, 2% (v/v) fetal calf serum (FCS; Biochrom, Berlin, Germany), 5 µg/ml insulin (Bovine, Sigma,Munich, Germany), 1.68 mM CaCl2, and penicillin/streptomycin (Biochrom). HaCaT keratinocytes [24] were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FCS and 1% (v/v) penicillin/streptomycin. Induction of apoptosis was performed using imiquimod (InvivoGen, San Diego, CA) at concentrations of 10 to 100 µg/ml (41.6–416 µM; 10 mg/ml stock in DMSO). Fotemustine (Muphoran) was from Servier (Germany), dacarbazine (DTIC) was obtained from Sigma. ABT-737 (5 mg/ml stock in DMSO) was provided by Abbott Laboratories (Abbott Park, IL). Mouse embryonic fibroblasts (MEFs) deficient for Noxa had been isolated from Noxa-deficient mice by Dr. Andreas Strasser, Walter and Eliza Hall Institute (WEHI), Melbourne, Australia, and immortalized by Dr. Christoph Borner, Albert-Ludwigs-University Freiburg, Germany). Bax+/- Bak+/-, Bax-/- Bak-/-, Bax+/- Bak-/-, and Bax-/- Bak+/- (immortalized with SV40 large Tantigen) were kindly provided by Dr. David Huang, WEHI. 3T3-MEF cell lines isolated from Bim- or Puma-deficient mice were established by Dr. Susanne Kirschnek, Dr. Andreas Villunger (University of Innsbruck), and Dr. Stefan Paschen. SV40 large T-transformed MEF cells from Mcl-1-deficient mice were generously provided by Dr. Joseph Opferman, St. Jude's Children's Hospital, Memphis. All MEF cells were analyzed in comparison to wild type cells established at the same time and under the same conditions. Mouse embryonic fibroblast cells were cultured in Dulbecco's modified Eagle's medium containing 10% FCS and 50 µM of 2-mercaptoethanol. All cultures were incubated under standard culture conditions (37°C, 5% CO2).
Lentiviral Constructs and Lentiviral Infection
For generation of lentiviral Noxa RNAi constructs, RNAi sequences N7 and N8 specifically targeting Noxa mRNA were cloned from pSUPER-Noxi7 and pSUPER-Noxi8 (a kind gift from Dr. Eric Eldering, Department of Experimental Immunology, Academic Medical Center Amsterdam, the Netherlands [25]) in the GFP-expressing lentiviral vector pLVTHM (Dr. Didier Trono, Lausanne) using restriction enzymes EcoRI/ClaI, yielding the plasmids pLVAWN7 and pLVAWN8. Lentiviral constructs of shBIM and shScrambled have been described previously [26]. Production of lentiviral particles was done by transfecting 293 FT cells (Invitrogen, Basel, Switzerland) together with packaging vectors pMD2.G and psPAX2 (Dr. Didier Trono, Lausanne). HaCaT keratinocytes, 1205Lu and 451Lu melanoma cells were infected with lentivirus carrying either a vector with scrambled shRNA or a vector containing one Bim-specific shRNA fragment or one of two Noxa-specific shRNA fragments. Cells with lentiviral integration of shScrambled, shBim, and shNoxa were sorted for GFP expression by FACS analyses. Knockdown of Mcl-1 was achieved using 20 nM of specific siRNA using the manufacturer's protocol (Lipofectamine RNAiMax; Invitrogen).
Western Blot Analysis
Cells were extracted in buffer containing 1% Triton X-100, and protein concentrations were estimated by the method of Bradford. Protein samples were separated on 12.5% SDS polyacrylamide gels. Antibodies against A1, Bcl-w (both Cell Signaling Technology, Danvers, MA), Bcl-2, Bcl-xL, Mcl-1, cytochrome c (all BD Pharmingen, San Jose, CA), Bim, tubulin (both Sigma), Noxa (Alexis, Farmingdale, NY), Puma (Prosci, Poway, CA), Bax (clone 6A7), Bak (both Upstate Biotechnology, Lake Placid, NY), cytochrome c oxidase subunit IV (CoxIV; MoBiTec, Goettingen, Germany), caspase 8 (1C12 clone; Cell Signaling Technology), and GAPDH (Chemicon, NY) were used as suggested by the manufacturers. Detection was performed with horseradish peroxidase-conjugated secondary antibodies (specific to mouse [Dianova, Hamburg, Germany] or rabbit [Sigma] immunoglobulin G [IgG]) and enhanced chemiluminescence (Amersham Biosciences, Freiburg, Germany).
Detection of Apoptosis and Cell Death
To measure cell death, cells were stimulated using imiquimod, dacarbazine, or fotemustine as indicated, collected, and were directly stained by the addition of 10 mM propidium iodide (PI) and analyzed within 1 hour by flow cytometry (FACSCalibur, Becton Dickinson); positively staining cells were considered dead. Apoptosis was measured by staining for active caspase 3 done with cells fixed for 20 minutes at room temperature in freshly prepared 4% formaldehyde in PBS, washed in PBS containing 0.5% BSA, and incubated with rabbit anti-active caspase 3 antibody (1:500; Abcam, Cambridge, MA) in staining buffer (PBS/0.5% BSA/0.5% saponin) for 30 minutes. Cells were washed three times with PBS/BSA/saponin and stained with Cy5-conjugated goat-antirabbit IgG (1:300; Dianova) secondary antibody for 30 minutes. Cells were washed and analyzed by flow cytometry. For caspase inhibition 50 µM zVAD-fmk (Bachem, Bubendorf, Switzerland) was added 20 minutes before the treatment with imiquimod ± ABT, dacarbazine, or fotemustine to block caspase-dependent apoptosis.
Detection of Bax and Bak Activation
HaCaT or melanoma cells were harvested after stimulation and were stained using the activation-specific anti-active Bax antibody (Clone3, 1:100; BD Pharmingen [27]). Fluorescein isothiocyanate (FITC)-conjugated goat-antimouse IgG (1:300; Dianova) was used as secondary antibody. For shNoxa, shBim, and shScrambled RNAi knockdown cells expressing GFP, Cy5-conjugated goat-antimouse IgG (1:300; Dianova) was used as secondary antibody. Staining for active Bak was done using mAb1 (1:50; Calbiochem, Darmstadt, Germany). Secondary antibodies and procedure were as previously mentioned. Flow cytometry was performed with a FACSCalibur.
Subcellular Fractionation
Cells were harvested 24 hours after stimulation in mitochondrial buffer, and the mitochondria were isolated as described earlier [28]. Subcellular localization of Bcl-2 family members was analyzed by loading equal volumes of the mitochondria-enriched fraction, cytosolic fraction, and the pellet fraction after 1 hour of ultracentrifugation (UZ-pellet, 4°C at 260,000g) on 12.5% SDS polyacrylamide gels.
Cytochrome c Release Assay
Untreated or stimulated 1205Lu melanoma cells (0.15–0.2 x 106 cells per sample) were harvested by trypsinization, washed with PBS, and resuspended in 50 µl lysis buffer (20 mM HEPES pH 7.2, 100 mM KCl, 5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 250 mM sucrose, 1x Roche Protease Inhibitor Mix) including 25 to 150 µg/ml digitonin. Cells were incubated for 60 minutes at 30°C in the absence or presence of indicated BH3 peptides (100 µM) or ABT-737 (50 µM). Cells were then centrifuged for 10 minutes at 13,000g to separate pellets and supernatants. Six times SDS sample loading buffer was added to the supernatants, and the pellets were resuspended in equal volumes of 1 x SDS sample loading buffer. The samples were heated for 10 minutes at 95°C before the separation by SDS-PAGE. After the protein transfer, the presence of cytochrome c in both pellets and supernatants was detected with anti-cytochrome c antibody (BD Pharmingen) and visualized by ECL Plus Western blot analysis detection system from GE Healthcare (Munich, Germany). The BH3 peptides Bim, mNoxa, and Bad (confirmed by matrix-assisted laser desorption/ionization time of flight and >90% pure) were obtained from Biosynthan GmbH (Berlin, Germany). Peptides were dissolved in 100% DMSO and stored as 10 mM stock solutions at -80°C. The amino acid sequences used were MRPEIWIAQELRRIGDEFNA (Bim), LWAAQRYGRELRRMSDEFVDSFKKG (Bad), and ELPPEFAAQLRKIGDKVYCT (Noxa).
Results
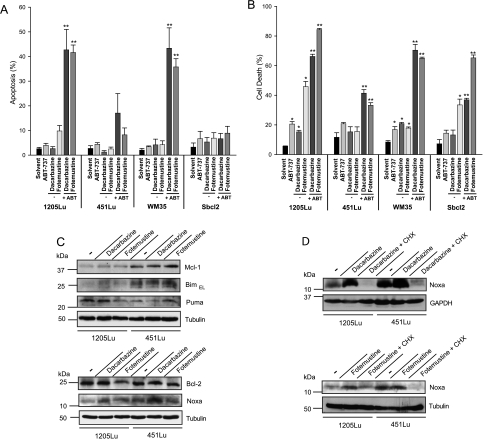
Four melanoma cell lines previously established from human tumors were first tested for susceptibility to ABT-737 alone or in combination treatment. Analysis of the expression levels of Bcl-2 family proteins showed expression of all major antiapoptotic proteins, of Bax and Bak, and the BH3-only proteins Bim, Puma, and Noxa. No obvious differences between the cell lines were seen, perhaps with the exception that Noxa and Mcl-1 were detectable in all four lines, but their levels were noticeably lower in 1205Lu cells. Bim expression also varied somewhat between the cell lines, and Bim levels were lower in all melanoma lines compared with the levels in HaCaT human keratinocytes (Figure W1, A and B). All melanoma lines showed considerable resistance to apoptosis induction by dacarbazine and fotemustine with very little apoptosis after 24 hours of treatment. Two cell lines displayed substantial cell death on 48 hours of fotemustine treatment (cell death induction was statistically significant for three of the four lines tested). Even relatively high concentrations of ABT-737 also induced only little apoptosis in the melanoma cell lines, with the strongest response around 20% of cell death in 1205Lu cells after 48 hours (Figure 1, A and B). However, the combination of either dacarbazine or fotemustine and ABT-737 was active in inducing apoptosis in all cell lines. Although there was some variation in efficiency, substantial cell death was seen in all melanoma lines, ranging from approximately 40% to more than 80% cell death at 48 hours of treatment (Figure 1, A and B).
Figure 1.
Synergistic killing of melanoma cells by dacarbazine or fotemustine and ABT-737. 1205Lu, 451Lu, WM35, and Sbcl2 melanoma cells were either incubated with solvent or with ABT-737 (ABT; 1 µM) alone or together with dacarbazine (300 µg/ml) or fotemustine (50 µg/ml) for 24 (A) or 48 hours (B). Apoptosis/cell death was measured by staining for active caspase 3 (24 hours) and PI (48 hours). Data represent means ± SEM of three independent experiments. *P < .05 compared with solvent control; **P < .05 compared with the corresponding dacarbazine or fotemustine concentration alone. In two independently performed experiments, PI staining could be completely blocked by adding 50 µM zVAD-fmk before treatment with ABT-737 (1 µM) plus dacarbazine or fotemustine (data not shown). (C) Western blot analysis of whole-cell lysates showing the levels of Bim, Noxa, Puma, Bcl-2, and Mcl-1 expression in 1205Lu and 451Lu melanoma cells 24 hours untreated or treated with dacarbazine (300 µg/ml) or fotemustine (50 µg/ml). The immunoblots show representative results from three independent experiments. (D) Western blot analysis of whole-cell lysates from 1205Lu and 451Lu melanoma pretreated for 2.5 hours with cycloheximide (CHX, 2.5 µg/ml) before adding dacarbazine (300 µg/ml) or fotemustine (50 µg/ml) for additional 24 hours. Tubulin and GAPDH were used as a loading control. The immunoblots show representative results from two independent experiments.
Changes in Bcl-2 family proteins were monitored in the two cell lines from metastatic melanoma, 451Lu and 1205Lu. We observed a small increase in Bim levels by treatment with dacarbazine but not with fotemustine in 1205Lu cells. In 1205Lu cells, substantial Noxa induction was detectable on dacarbazine and fotemustine treatment, whereas in 451Lu cells, Noxa was only induced on dacarbazine treatment after 24 hours (Figure 1C). Noxa induction occurred through de novo gene induction because it was blocked by the translational inhibitor cycloheximide (Figure 1D). There was no consistent change in Puma levels. An inverse correlation of the levels of Noxa and Mcl-1 has, in many cases, been observed. However, in most experiments, no reduction of Mcl-1 was seen on treatment with either agent (Figure 1C).
A similar pattern was observed in cells treated with imiquimod. Imiquimod was developed as a small molecule immune modifier that was found to activate myeloid cells through TLR7 and TLR8 [29] but was later described to be able to induce apoptosis independently of these receptors [18]. Imiquimod shows great promise for the local treatment of some types of skin cancer and may even be useful for the topical treatment of melanoma [21].
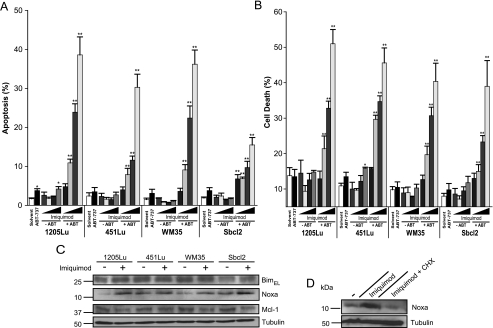
Although imiquimod has been reported to induce apoptosis in some melanoma cells [19], we found only insubstantial induction of cell death in our panel of cell lines treated with imiquimod alone. However, high levels of apoptosis were observed in all cell lines when treated with the combination of imiquimod and ABT-737 (Figure 2, A and B). When the levels of Bim and Noxa were monitored during treatment with imiquimod, a slight induction of Bim levels was observed in two cell lines (1205Lu and Sbcl2) but not the other two. More consistently, a small up-regulation of Noxa levels was seen in all four lines (Figure 2C). Induction of Noxa was again blocked by cycloheximide in the tested 1205Lu melanoma cell line (Figure 2D). A corresponding decrease in Mcl-1 levels was observed in three of the four lines (Figure 2C). These results indicated that up-regulation of endogenous Noxa by chemotherapeutic agents might contribute to the sensitization of melanoma cells to ABT-737. The slight up-regulation of Bim might indicate that this protein is also involved.
Figure 2.
Synergistic killing of melanoma cells by imiquimod and ABT-737. 1205Lu, 451Lu, WM35, and Sbcl2 melanoma cells were either incubated with solvent (DMSO, 0.95%) or with ABT-737 (ABT; 1 µM) alone or with increasing concentrations (from left to right 5, 25, 50, 75 µg/ml) of imiquimod as indicated. After 6 (A) or 24 hours (B) of treatment, cells were collected and apoptosis/cell death was measured by either staining for active caspase 3 (A) or staining for PI-positive cells (B), followed by flow cytometric analysis. Data represent means ± SEM of at least four (A) or three (B) experiments. *P < .05 compared with solvent control; **P < .05 compared with the corresponding imiquimod concentration alone. (C) Western blot analysis of whole-cell lysates showing the levels of Bim, Noxa, and Mcl-1 expression in melanoma cells 24 hours after stimulation ± 75 µg/ml imiquimod. Tubulin was used as a loading control. In two independently performed experiments, PI staining could be blocked by adding 50 µM zVAD-fmk before treatment with ABT-737 (1 µM) plus 75 µg/ml imiquimod (data not shown). (D) Western blot analysis of whole-cell lysates from 1205Lu melanoma pretreated for 2.5 hours with cycloheximide (CHX, 2.5 µg/ml) before adding imiquimod (90 µg/ml) for additional 24 hours.
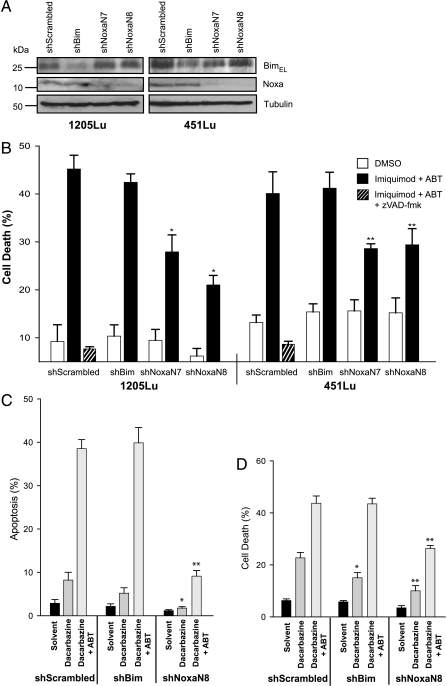
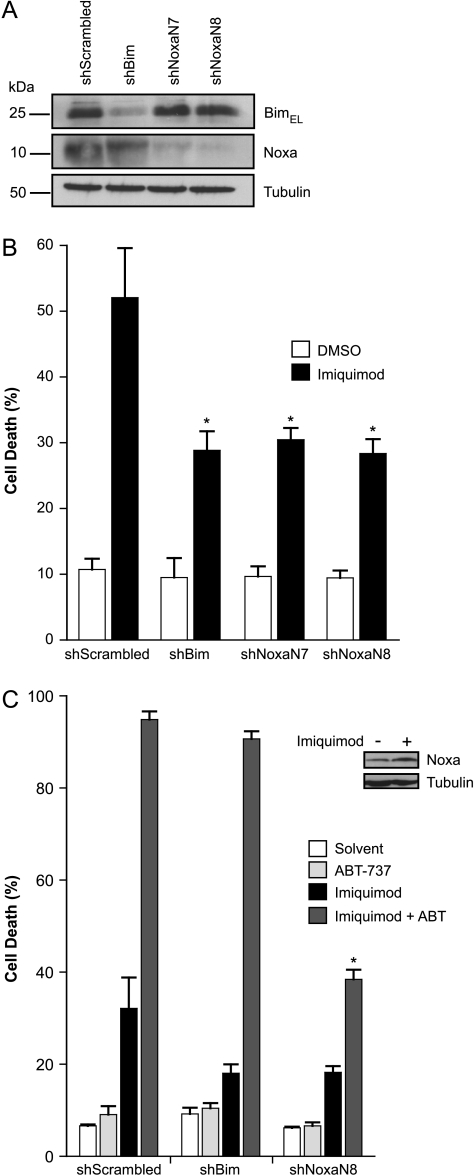
To test for the contribution of Bim and Noxa directly, polyclonal cell lines with Bim- or Noxa-specific shRNA were established for the cell lines 451Lu and 1205Lu. Substantial reduction in the protein expression levels was achieved by the expression of the shRNA specific for Bim or Noxa (Figure 3A). As shown in Figure 3B, there was no protection by the reduction in Bim levels against apoptosis induced by imiquimod plus ABT-737. However, both cell lines showed significant protection against apoptosis induced by imiquimod/ABT-737 when Noxa-specific shRNA was expressed (Figure 3B). This protection was reflected by a reduced activation of Bax and Bak in cells expressing Noxa-specific but not Bim-specific shRNA (Figure W2). Dacarbazine alone induced relatively little apoptosis in these cells (Figure 3, C and D). However, a contribution of both Bim and Noxa to dacarbazine-induced cell death was detectable at 48 hours of treatment (Figure 3D). Intriguingly, including ABT-737 completely removed the requirement for Bim but not for Noxa because the Noxa knockdown still showed protection against the combination but not the Bim knockdown (Figure 3, C and D). The synergism of ABT-737 with these two drugs therefore required the activity of endogenous Noxa protein.
Figure 3.
A and B: Noxa but not Bim knockdown by RNAi protects melanoma cells against apoptosis induced by imiquimod or dacarbazine in the presence of ABT-737. (A) Immunoblot showing levels of Bim and Noxa expression in polyclonal 1205Lu and 451Lu cell lines expressing shRNA specific for Bim or Noxa (two different sequences, N7 and N8) after 24 hours of stimulation with 50 µg/ml imiquimod plus 1 µM ABT-737. Tubulin was used as a loading control. (B) Cells shown under A were treated for 24 hours with DMSO (0.7%) or 50 µg/ml imiquimod plus ABT-737 (ABT; 1 µM), and cell death was assessed by staining with PI, followed by flow cytometric analysis. 1205Lu- and 451Lu shScrambled controls were treated with 50 µM zVAD-fmk before stimulation with imiquimod plus ABT. Data represent means ± SEM of five experiments. *P < .006; **P < .025 compared with scrambled control line. C and D: Apoptosis induction and requirement for Bim or Noxa in 1205Lu melanoma cells treated with dacarbazine and the combination with ABT-737. (C) Cells were treated with ABT-737 (ABT; 1 µM) for 24 hours together with dacarbazine (300 µg/ml), and cell death was assessed by staining for active caspase 3, followed by flow cytometric analysis. Data represent means ± SEM of at least three experiments. *P < .05; **P < .005 compared with scrambled control. (D) Cells were treated for 48 hours with 300 µg/ml dacarbazine ± ABT-737 (ABT; 1 µM), and cell death was measured by staining with PI and subsequent flow cytometry. Data represent means ± SEM of five experiments. *P < .05; **P < .005 compared with scrambled control.
The results were similar although not identical for apoptosis induction with fotemustine. At 24 hours of treatment, knockdown of either Bim or Noxa led to significant protection when fotemustine was used on its own (Figure W3B). However, the requirement for Noxa in the combination treatment of fotemustine plus ABT-737 was seen only at very early times (at 6 hours of treatment) but not at 24 hours (Figure W3).
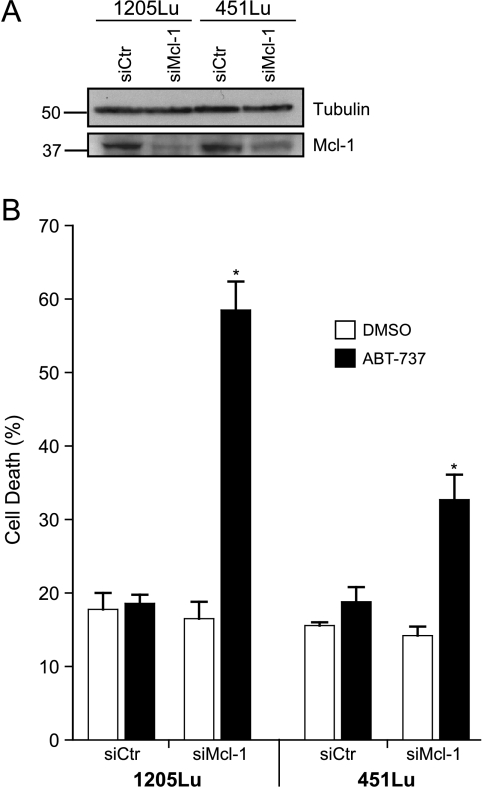
ABT-737 can effectively neutralize Bcl-2, Bcl-xL, and Bcl-w but not Mcl-1 or A1 [11]. The observed sensitization of melanoma cells to ABT-737 by dacarbazine, fotemustine, and imiquimod therefore suggested that the contribution of these latter drugs to the synergistic effect is the neutralization of Mcl-1. Indeed, knockdown of Mcl-1 in the two melanoma lines tested was sufficient to sensitize the cells to single-agent treatment with ABT-737 (Figure 4A). This further supports a model where dacarbazine, fotemustine, and imiquimod cause the inhibition of antiapoptotic Bcl-2 family members, critically including Mcl-1. The combination with ABT-737 then can inhibit the complete group of antiapoptotic Bcl-2 family proteins.
Figure 4.
Knockdown of Mcl-1 sensitizes 1205Lu and 451Lu melanoma cells to ABT-737. Cells were transiently transfected with control (Ctr) and Mcl-1-specific siRNA. At 24 hours after transfection, cells were treated without (DMSO) or with 1 µM ABT-737 for 24 hours and assayed for Mcl-1 knockdown by Western blot analysis (A) or PI-positive cells (B). Data represent means ± SEM of at least three experiments. *P < .04 compared with control plus ABT-737. Note that in two independently performed experiments, PI staining could be completely blocked by adding 50 µM zVAD-fmk before treatment with ABT-737 (1 µM, data not shown).
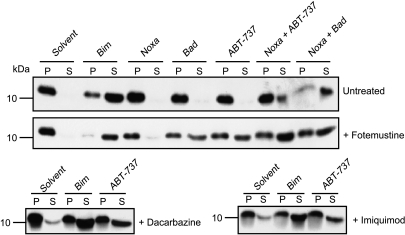
To test this hypothesis directly at the mitochondrial level, we used peptides consisting of the BH3 domains of BH3-only proteins and analyzed mitochondrial sensitivity of permeabilized melanoma cells (the cell line 1205Lu was used for these experiments). In untreated 1205Lu cells, only the BH3 peptide derived from Bim (which can target all antiapoptotic Bcl-2 proteins [6]) caused the release of cytochrome c. Neither Bad peptide (targeting Bcl-2, Bcl-xL, and Bcl-w) nor Noxa peptide (targeting Mcl-1 and A1) had this activity. ABT-737 on its own likewise had no effect, whereas the combination of Bad or ABT-737 together with Noxa was able to induce release (Figure 5). Cytochrome c release from mitochondria of melanoma cells was thus achieved by a combination of agents that bound to all antiapoptotic Bcl-2 proteins.
Figure 5.
Pretreatment of 1205Lu cells with fotemustine, dacarbazine, and imiquimod sensitizes mitochondria for cytochrome c release by ABT-737. 1205Lu cells were pretreated for 24 hours with 50 µg/ml fotemustine, 300 µg/ml dacarbazine, 50 µg/ml imiquimod, or solvent. Digitonin-permeabilized cells were incubated for 60 minutes at 30°C with solvent (DMSO), BH3 peptides (100 µM), or ABT-737 (50 µM). Reaction were centrifuged, and supernatant (S, containing released cytochrome c) and pellet (P) fractions (containing mitochondria-bound cytochrome c) were probed with an anti-cytochrome c antibody.
Cells were then pretreated with dacarbazine, fotemustine, or imiquimod for 24 hours and subjected to the same assay. In all cases, these pretreated, permeabilized cells were sensitive to ABT-737 on its own (and to Bad peptide, which was tested for fotemustine-pretreated cells; Figure 5). Taken together, these data provide strong evidence that dacarbazine, fotemustine, and imiquimod can neutralize Mcl-1 through endogenous Noxa (and these agents may also contribute to the inactivation of other antiapoptotic Bcl-2 proteins). ABT-737 can then neutralize the remaining Bcl-2-like proteins, leading to the release of cytochrome c.
These results showed a surprisingly important role for endogenous Noxa in sensitizing melanoma cells to apoptosis induced by ABT-737. The situation in melanoma is complicated by the fact that single agent shows only little apoptosis-inducing activity, and we therefore looked for other cell types where apoptosis would be induced in the absence of ABT-737 and the contributing BH3-only proteins and found that both MEFs and HaCaT human keratinocytes died when treated with imiquimod alone. The contribution of Bim and Noxa to imiquimod-induced apoptosis was first confirmed in MEFs. Mouse embryonic fibroblast cells from mice lacking either Bim or Noxa but not cells from mice lacking Puma were significantly protected against imiquimod-induced apoptosis, whereas the loss of Mcl-1 increased sensitivity (Figure W4A). Although MEF cells lacking both Bax and Bak were protected against imiquimod-induced apoptosis, cells expressing only either Bax or Bak died in a way comparable to wild type MEF cells (Figure W4B). Imiquimod is a known ligand for TLR7 and TLR8. To confirm the suspected independence of imiquimod-induced apoptosis of TLR, we also tested MEF cells lacking the essential signaling adaptor of TLR7/8, MyD88. These cells showed normal sensitivity toward imiquimod (Figure W4B); imiquimod thus induces apoptosis independently of TLR.
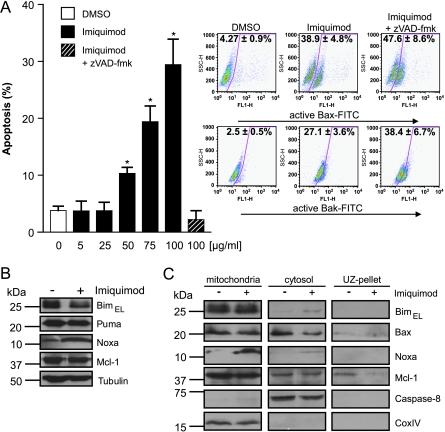
As shown in Figure 6A, imiquimod on its own induced substantial apoptosis in HaCaT human keratinocytes. Induction of apoptosis was accompanied by a clear induction of Noxa protein but a reduction of Bim levels. Mcl-1 levels did not change (Figure 6B). Subcellular fractionation showed an accumulation of Noxa at mitochondria, coincident with a cytosolic depletion of Bax (Figure 6C), suggesting an involvement of Noxa in imiquimod-induced apoptosis also in HaCaT cells. Stable cell lines were then generated that expressed shRNA directed against Bim or Noxa. These cell lines showed a clear reduction of the target proteins as assessed by Western blot analysis (Figure 7A). As shown in Figure 7B, although up-regulation was seen only for Noxa but not for Bim (Figure 6B), the loss of either Bim or Noxa conferred significant protection against imiquimod treatment. Reduction in either Bim or Noxa by RNAi reduced the imiquimod-dependent activation of both Bax and Bak (Figure W5) suggesting that both Bax and Bak were activated consecutively to the activation of Bim or Noxa by imiquimod. As in melanoma cells, ABT-737 was ineffective on its own but showed strong synergism with imiquimod in terms of apoptosis induction in HaCaT cells. Again, the requirement for Bim was overcome by ABT-737 but not the requirement for Noxa (Figure 7C). These results thus confirm in a different cellular context that endogenous Noxa is required for synergistic apoptosis induction by ABT-737 and imiquimod. Whereas both Bim and Noxa were involved, ABT-737 was able to replace Bim but not Noxa, suggesting the surprising conclusion that Noxa fulfills a molecular function that cannot be performed by Bim in this situation.
Figure 6.
Induction of apoptosis and levels and localization of Bcl-2 proteins in HaCaT keratinocytes during imiquimod treatment. (A) Cells were treated with DMSO alone (solvent control, 1%) or with varying concentrations of imiquimod (10–100 µg/ml) for 24 hours, and apoptosis was assessed by staining for active caspase 3, followed by flow cytometric analysis. zVAD-fmk (50 µM) was added 20 minutes before the addition of 100 µg/ml imiquimod. Data represent means ± SEM of three experiments. *P < .05 compared with solvent control. In parallel, Bax activation was monitored by flow cytometry after staining with anti-active Bax/FITC-conjugated goat-antimouse IgG. Bak activation was detected by staining with anti-active Bak/FITC-conjugated goat-antimouse IgG. Representative dot blots are shown. Values represent ± SEM of at least four independent experiments. (B) Western blot analysis showing levels of BimEL, Puma, Noxa, and Mcl-1 in whole-cell lysates after 24 hours of treatment with 100 µg/ml imiquimod or with 1% DMSO (solvent control). Tubulin was used as a loading control. Three separate experiments gave similar results. (C) Localization of Bcl-2 family proteins in HaCaT cells after imiquimod treatment. Cells were incubated without (1% DMSO) or with 100 µg/ml imiquimod. At 24 hours later, cells were collected and subjected to fractionation experiments. Caspase 8 was used as a cytosolic, CoxIV as a mitochondrial loading control. The immunoblots are representatives of three separate experiments.
Figure 7.
Bim- or Noxa-specific RNAi protects HaCaT cells from imiquimod-induced apoptosis. (A) Immunoblot of HaCaT cells showing Bim and Noxa expression in polyclonal lines stably expressing shRNA directed against Bim or Noxa (two different sequences, N7 and N8) or a control shRNA (scrambled anti-Bim sequence) after 24 hours of stimulation with 100 µg/ml imiquimod. Tubulin was used as a loading control. (B) The cells shown under A were treated for 24 hours with DMSO (1%) or 100 µg/ml imiquimod, and cell death was assayed by staining with PI, followed by flow cytometric analysis. Data represent means ± SEM of five experiments. *P = .04, specific constructs versus scrambled control. (C) HaCaT RNAi cells were treated for 18 hours with DMSO (0.95%, solvent), ABT-737 (ABT; 1 µM), imiquimod (75 µg/ml), or imiquimod plus 1 µM ABT-737. Cell death was assessed by PI staining followed by flow cytometric analysis. Data represent means ± SEM of three experiments. *P < .0002, shNoxa versus shBim cells. Western blots (inset) show induction of Noxa with 75 µg/ml imiquimod 18 hours after induction in HaCaT. Tubulin served as a loading control.
Discussion
ABT-737 has shown efficacy in a range of tumor cells. This study shows strong proapoptotic synergism of ABT-737 and two standard drugs and one experimental agent in melanoma cells. This is encouraging from a clinical point of view as disseminated melanoma is almost completely resistant to chemotherapy, and the inclusion of ABT-737 in a treatment regimen is promising. Remarkably, endogenous Noxa protein made a significant contribution to this synergistic effect and was indispensable for full apoptosis induction not only by the single agents but also by the combination of ABT-737 and either imiquimod or dacarbazine. In the case of fotemustine single treatment, Noxa contributed early on but was dispensable to later time points. One important point is thus that Noxa can do something that Bim is unable to do. This was clearest in the case of HaCaT cells (where imiquimod killed in the absence of ABT-737) but similar in melanoma cells. Knockdown of either Bim or Noxa conferred similar protection against imiquimod (or dacarbazine in melanoma), clearly indicating the involvement of both proteins. However, ABT-737 could replace Bim but not Noxa.
Noxa is an intriguing BH3-only protein in that it seems to bind exclusively to Mcl-1 and A1 (A1 is expressed at low levels only in many tumor cells [10], although it was easily detectable in melanoma cells; Figure W1). Noxa-deficient mice have, as far as investigated, only relatively subtle defects, although a role of Noxa in apoptosis induced by adriamycin in transformed MEF cells [30] to UV light-induced apoptosis in the skin [31] and in apoptosis in activated T cells [25,32] has been established. Noxa can bind to and neutralize Mcl-1, and this binding can trigger the proteasomal destruction of Mcl-1 [33], whereas other Mcl-1-neutralizing ligands fail to trigger degradation [34,35]. The most important function of Noxa may thus be the neutralization/degradation of Mcl-1. Noxa was involved in apoptosis induced by imiquimod and dacarbazine, whereas its role was less pronounced in fotemustine-induced apoptosis. In case of imiquimod in three of the four tested melanomas, we could see a small reduction of Mcl-1, whereas dacarbazine and fotemustine induced degradation of Mcl-1 in some but not all the experiments. Because Noxa is very likely induced and codegraded with Mcl-1, the levels of both proteins will probably be determined not only by their synthesis but also by the rate of their degradation. The actual level of the proteins may therefore not be a perfect indicator of the role of both Noxa and Mcl-1.
Although Bim clearly plays a role in apoptosis induced by imiquimod, dacarbazine, and fotemustine, it could be replaced by ABT-737. Because ABT-737 cannot neutralize Mcl-1, this implies that Bim is also unable to neutralize Mcl-1 in this situation. This is very surprising because Bim has been consistently found to be able to bind to Mcl-1 (the Bim BH3 domain has in fact a 10-fold higher affinity to Mcl-1 compared with the Noxa BH3 domain [6]). When Mcl-1 was immunoprecipitated from cells untreated or treated with dacarbazine with or without ABT-737, Noxa was coprecipitated but not Bim (data not shown). This suggests that Bim, in this situation, is unable to bind to Mcl-1. Although this was unexpected, the results are consistent in the sense that Bim cannot functionally replace Noxa because it cannot bind to Mcl-1. What exactly Bim does when activated by these drugs is not clear. It should be pointed out that there was no induction of Bim by imiquimod or fotemustine and only some induction by dacarbazine in one of two tested cell lines. Bim therefore contributes to apoptosis induced by these agents without an increase in its levels. Although Bim-induced apoptosis is often accompanied by an increase in Bim abundance [36], this is not essential, and situations have even been identified where an increase of Bim levels was associated with a reduction in apoptosis [37]. It seems therefore likely that Bim activation includes some unidentified posttranslational activation event.
Our data are consistent with the view that two separate legs of the Bcl-2 machinery have to be targeted before Bax and Bak are activated [4,6]. This was particularly clear in the experiments where we used BH3 peptides to study mitochondrial cytochrome c release. Whereas in untreated cells the combination of Noxa peptide and ABT-737 (or Bad peptide) was required for cytochrome c release, pretreatment with dacarbazine, fotemustine, or imiquimod rendered mitochondria susceptible to ABT-737 alone. All results thus suggest that, in terms of synergism with ABT-737, the most important functional element of these drugs is to activate Noxa and thereby to neutralize Mcl-1. The reliance on Noxa was different between the agents used, with fotemustine requiring Noxa contribution in the absence but not the presence of ABT-737 (although Noxa was required for full apoptosis at early stages). Perhaps fotemustine is able to activate additional BH3-only proteins that would contribute to the neutralization of Mcl-1 or more effectively inactivate other Bcl-2-like proteins.
It is very difficult to achieve a treatment response in disseminated human melanoma, and this difficulty is reflected by the poor apoptotic response to chemotherapeutic agents of melanoma cell lines in vitro. However, combination of such drugs, including the experimental agent imiquimod, with ABT-737 shows promise for future therapy protocols. Our data provide evidence that the ability of a drug to activate endogenous Noxa is a predictor of its synergism with the new class of agents targeting Bcl-2/Bcl-XL/Bcl-w, such as ABT-737. For future combination therapies with these new agents, it may well be worthwhile to reexamine therapeutic drugs that are inefficient on their own. Potent Noxa inducers might be uncovered in such a screen, which are ineffective as single agents but could be very effective when combined with Bcl-2-targeting drugs.
Supplementary Material
Acknowledgments
The authors thank Saul Rosenberg and Steve Elmore, Abbott Laboratories, for providing ABT-737. The authors thank Meenhard Herlyn (Wistar Institute, Philadelphia), Carola Berking, and Robert Besch (Ludwig Maximilians University, Munich) for providing melanoma cell lines; Robert Besch for providing Mcl-1-specific siRNA; and Dr. Eric Eldering (Academic Medical Centre, Amsterdam) for providing the shNoxa templates. The authors thank Daria Loos for experimental help.
Footnotes
This work was supported by the Wilhelm-Sander Stiftung (grant 2004.030.2 to G.H.) and by the Austrian Science Fund (SFB-F021 to C.P.).
This article refers to supplementary materials, which are designated by Figures W1 to W5 and are available online at www.transonc.com.
References
- 1.Cory S, Adams JM. Killing cancer cells by flipping the Bcl-2/Bax switch. Cancer Cell. 2005;8:5–6. doi: 10.1016/j.ccr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 3.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Baell JB, Huang DC. Prospects for targeting the Bcl-2 family of proteins to develop novel cytotoxic drugs. Biochem Pharmacol. 2002;64:851–863. doi: 10.1016/s0006-2952(02)01148-6. [DOI] [PubMed] [Google Scholar]
- 8.Rutledge SE, Chin JW, Schepartz A. A view to a kill: ligands for Bcl-2 family proteins. Curr Opin Chem Biol. 2002;6:479–485. doi: 10.1016/s1367-5931(02)00352-6. [DOI] [PubMed] [Google Scholar]
- 9.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 10.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 12.Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai Y, Grant S. Targeting multiple arms of the apoptotic regulatory machinery. Cancer Res. 2007;67:2908–2911. doi: 10.1158/0008-5472.CAN-07-0082. [DOI] [PubMed] [Google Scholar]
- 14.Labi V, Grespi F, Baumgartner F, Villunger A. Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy. Cell Death Differ. 2008;15:977–987. doi: 10.1038/cdd.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang MH, Wan Z, Kang YH, Sposto R, Reynolds CP. Mechanism of synergy of N-(4-hydroxyphenyl)retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. J Natl Cancer Inst. 2008;100:580–595. doi: 10.1093/jnci/djn076. [DOI] [PubMed] [Google Scholar]
- 16.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- 17.Miller LA, Goldstein NB, Johannes WU, Walton CH, Fujita M, Norris DA, Shellman YG. BH3 mimetic ABT-737 and a proteasome inhibitor synergistically kill melanomas through Noxa-dependent apoptosis. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.327. 2008 November 6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Schon MP, Schon M. Immune modulation and apoptosis induction: two sides of the antitumoral activity of imiquimod. Apoptosis. 2004;9:291–298. doi: 10.1023/b:appt.0000025805.55340.c3. [DOI] [PubMed] [Google Scholar]
- 19.Schon M, Bong AB, Drewniok C, Herz J, Geilen CC, Reifenberger J, Benninghoff B, Slade HB, Gollnick H, Schon MP. Tumor-selective induction of apoptosis and the small-molecule immune response modifier imiquimod. J Natl Cancer Inst. 2003;95:1138–1149. doi: 10.1093/jnci/djg016. [DOI] [PubMed] [Google Scholar]
- 20.Buzaid AC. Biochemotherapy for advanced melanoma. Crit Rev Oncol Hematol. 2002;44:103–108. doi: 10.1016/s1040-8428(01)00223-2. [DOI] [PubMed] [Google Scholar]
- 21.Schon MP, Schon M. Imiquimod: mode of action. Br J Dermatol. 2007;157(Suppl 2):8–13. doi: 10.1111/j.1365-2133.2007.08265.x. [DOI] [PubMed] [Google Scholar]
- 22.Smalley KS, Contractor R, Haass NK, Kulp AN, Atilla-Gokcumen GE, Williams DS, Bregman H, Flaherty KT, Soengas MS, Meggers E, et al. An organometallic protein kinase inhibitor pharmacologically activates p53 and induces apoptosis in human melanoma cells. Cancer Res. 2007;67:209–217. doi: 10.1158/0008-5472.CAN-06-1538. [DOI] [PubMed] [Google Scholar]
- 23.Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL, Van Belle P, Elder DE, Herlyn M. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- 24.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alves NL, Derks IA, Berk E, Spijker R, van Lier RA, Eldering E. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity. 2006;24:703–716. doi: 10.1016/j.immuni.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Ploner C, Rainer J, Niederegger H, Eduardoff M, Villunger A, Geley S, Kofler R. The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia. 2008;22:370–377. doi: 10.1038/sj.leu.2405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dewson G, Snowden RT, Almond JB, Dyer MJ, Cohen GM. Conformational change and mitochondrial translocation of Bax accompany proteasome inhibitor-induced apoptosis of chronic lymphocytic leukemic cells. Oncogene. 2003;22:2643–2654. doi: 10.1038/sj.onc.1206326. [DOI] [PubMed] [Google Scholar]
- 28.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 30.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 31.Naik E, Michalak EM, Villunger A, Adams JM, Strasser A. Ultraviolet radiation triggers apoptosis of fibroblasts and skin keratinocytes mainly via the BH3-only protein Noxa. J Cell Biol. 2007;176:415–424. doi: 10.1083/jcb.200608070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer A, Villunger A, Labi V, Fischer SF, Strasser A, Wagner H, Schmid RM, Hacker G. The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells. Proc Natl Acad Sci USA. 2006;103:10979–10984. doi: 10.1073/pnas.0603625103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee EF, Czabotar PE, van Delft MF, Michalak EM, Boyle MJ, Willis SN, Puthalakath H, Bouillet P, Colman PM, Huang DC, et al. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J Cell Biol. 2008;180:341–355. doi: 10.1083/jcb.200708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czabotar PE, Lee EF, van Delft MF, Day CL, Smith BJ, Huang DC, Fairlie WD, Hinds MG, Colman PM. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci USA. 2007;104:6217–6222. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 37.Bauer A, Kirschnek S, Hacker G. Inhibition of apoptosis can be accompanied by increased Bim levels in T lymphocytes and neutrophil granulocytes. Cell Death Differ. 2007;14:1714–1716. doi: 10.1038/sj.cdd.4402185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.