Abstract
Pruritus (itch) is a common cause of discomfort by dermatological disorders. Several peripherally and centrally mediated pathologies that induce pruritus do not generally respond to typical allergenic and anti-inflammatory treatments. In accordance, we employed an acute allergenic murine model to determine whether the endogenous cannabinoid system could be targeted to treat pruritus. Subcutaneous administration of the mast cell degranulator compound 48/80 evoked an intense, concentration-dependent scratching response. Systemic Δ9-tetrahydrocannabinol reduced the scratching response, although this effect was accompanied with hypomotility. Complementary genetic and pharmacological approaches to target fatty acid amide hydrolase (FAAH), the primary enzyme responsible for the degradation of the endocannabinoid anandamide, were evaluated in the compound 48/80 model. FAAH(-/-) mice and mice treated with the respective irreversible and reversible FAAH inhibitors, URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) and OL-135 [1-oxo-1-[5-(2-pyridyl)-2-yl]-7-phenylheptane], displayed comparable reductions in scratching to mice treated with common nonsedative allergenic treatments (loratadine and dexamethasone) but without affecting locomotor behavior. The antiscratching phenotype of FAAH-compromised mice was completely blocked by either genetic deletion or pharmacological antagonism of the CB1 receptor. Neural-specific conditional FAAH knockout (FAAH-NS) mice, which have FAAH exclusively restricted to neural tissues, showed a similar magnitude of scratching as wild-type mice. It is important that URB597 reduced compound 48/80-induced scratching in FAAH-NS mice, but it did not produce any further reduction in FAAH(-/-) mice. These findings indicate that neuronal FAAH suppression reduces the scratching response through activation of CB1 receptors. More generally, these are the first preclinical data suggesting that FAAH represents a novel target to treat pruritus without eliciting overt side effects.
Pruritus is the general clinical term for itch, characterized by a generally unpleasant sensation attributed to an area at or just below the skin. The sensation is closely related to the reflex desire and action to scratch the afflicted area. Pruritus can greatly reduce the quality of life of those inflicted and is a common symptom attributed to discomfort from dermatological conditions (Stante et al., 2005). Although there are no collective data on the prevalence of patients seeking treatment for pruritus, the numbers on skin diseases alone estimates an incidence of one in three Americans afflicted at any given moment, costing a yearly $28.3 billion in medical treatment (Bickers et al., 2006). Because of the great variety of pathophysiological conditions (e.g., anemia, parasites, liver/kidney disease, thyroid disorders, HIV) underlying pruritus symptomology, common treatments are not always effective. Recent studies show that pruritus shares much of its neural substrates and signaling characteristics with that of another nocifensive modality, pain. Many of the same receptor systems shown to modulate analgesic actions also are being examined for their effects on itch (e.g., serotonin and opioid), often with antinociception-enhancing perception of itch (for review, see Paus et al., 2006; Schmelz and Handwerker, 2006).
Δ9-Tetrahydrocannabinol (THC), the primary psychoactive component in marijuana, and other cannabinoid receptor agonists produce analgesic effects through the activation of cannabinoid receptors in the periphery, spine, and brain (for review, see Pacher et al., 2006). However, given the occurrence of motor suppression, abuse potential, physical dependence, and psychomimetic effects of THC and other direct-acting cannabinoid agonists, recent efforts in cannabinoid research are focused on examining drugs that prevent the catabolism of endogenous cannabinoids. The endocannabinoid system consists of CB1 and CB2 receptors and several phospholipid-derived endogenous ligands that bind to these receptors. The most characterized endogenous cannabinoid, anandamide, is rapidly degraded predominantly by the enzyme fatty acid amide hydrolase (FAAH). Inactivation or inhibition of FAAH greatly increases anandamide levels in brain, spinal cord, liver, and other tissues (Cravatt et al., 2001; Boger et al., 2005; Fegley et al., 2005). FAAH also degrades several other fatty acid amides with known physiological functions, including oleamide (sleep), N-palmitoyl ethanolamine (PEA; anti-inflammatory), and oleoylethanolamide (satiety) (Cravatt et al., 2001). FAAH(-/-) mice and wild-type mice treated with FAAH inhibitors possess cannabinoid receptor-mediated hypoalgesic and anti-inflammatory phenotypes, without any signs of significant sedation or muscle relaxation (Lichtman et al., 2004a,b).
Although minimal study has been performed in the area of cannabinoids and pruritus, cannabinoid receptor agonists tend to decrease scratching behavior, whereas cannabinoid receptor antagonists, such as rimonabant, elicit a dose-dependent increase in scratching behavior in mice (Darmani and Pandya, 2000). It is interesting that several mixed CB1/CB2 receptor agonists (i.e., THC, WIN55212-2, CP55940, and HU-210) potently reversed rimonabant-induced scratching in an order similar to their potency to elicit known cannabinoid-mediated behavioral effects (Janoyan et al., 2002). In addition, each of these cannabinoids also reduced scratching elicited by the 5-hydroxytryptamine2A/C receptor agonist, 2,5-dimethoxy-4-iodoamphetamine (Darmani, 2001). In limited case reports, dronabinol (oral THC) relieved intractable pruritus resulting from liver cholestasis (Neff et al., 2002), a common clinical cause of severe and prolonged pruritus. Finally, transdermal application of the potent cannabinoid receptor agonist, HU-210, significantly reduced the perception of itch elicited by histamine iontophoresis in a small group of healthy volunteers (Dvorak et al., 2003). Taken together, the above evidence suggests that cannabinoids may provide a novel therapeutic target for both peripheral and central inhibition of pruritus. At present, there are no published studies of which we are aware that have examined the role of FAAH in an animal model of pruritus.
The overall objective of the present study was to elucidate the therapeutic potential of targeting the endocannabinoid system to treat pruritus. A model using the mast cell degranulator, compound 48/80, that directly activates signaling events characteristic of an acute allergenic response was adapted from Sugimoto et al. (1998). Local application of compound 48/80 elicits short-term and predictable levels of scratching behavior in the affected area. Clinical treatments of allergenic pruritus (e.g., antihistamines and glucocorticoids) effectively suppress scratching compound 48/80-induced scratching. The prototypical cannabinoid receptor agonist, THC, was evaluated in the compound 48/80 pruritus model for both scratching attenuation and levels of behavioral locomotor inactivity. FAAH(-/-) mice and wild-type mice treated with FAAH inhibitors were also assessed in the compound 48/80-induced scratching model. In addition, both pharmacological and transgenic tools were employed to elucidate cannabinoid receptor mechanisms and whether blockade of neuronal FAAH was critical in reducing scratching responses.
Materials and Methods
Subjects. Tests utilizing transgenic animals (i.e., FAAH, CB1, and CB2 knockout animals) and their controls were performed on mice from the Center Transgenic Colony at Virginia Commonwealth University (Richmond, VA) backcrossed onto either a C57BL/6J (13 generations) or DBA/1 (six generations) background. All other experiments utilized adult male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME), with the exception of one intravenous study employing male ICR mice (Harlan, Indianapolis, IN). Mice were housed four to six per cage in a temperature (20–22°C)-controlled environment, with food and water available ad libitum while in their home cages. Mice were kept on a 12-h light/dark cycle, with all experiments performed during the animals' light period. All experiments were performed with the approval of the Institutional Animal Care and Use Committee at Virginia Commonwealth University in accordance with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Drugs. THC and rimonabant were obtained from the National Institute on Drug Abuse (Bethesda, MD), and OL-135 was synthesized as described previously (Boger et al., 2005). URB597 was purchased from Cayman Chemical (Ann Arbor, MI), and compound 48/80, diphenhydramine, dexamethasone, and loratadine were purchased from Sigma-Aldrich (St. Louis, MO). Compound 48/80, diphenhydramine, dexamethasone, and loratadine were dissolved in 0.9% saline, whereas all other drugs were dissolved in vehicle mixture of ethanol/alkamuls-620 (Sanofi-Aventis, Bridgewater, NJ)/saline in a ratio of 1:1:18. Drugs were diluted to an injection volume of 10 μl/g body mass and injected via intraperitoneal injection unless otherwise specified. Compound 48/80 was administered as a fixed quantity diluted per 200-μl subcutaneous injection.
All pretreatments and genotypic comparisons were made against a compound 48/80 challenge dose of 30 μg. The CB1/CB2 mixed receptor agonist THC, the first-generation antihistamine diphenhydramine, the nonsedating antihistamine loratadine, and the glucocorticoid dexamethasone, were each administered 30 min before compound 48/80 injection. The reversible FAAH inhibitor OL-135 and the irreversible FAAH inhibitor URB597 were both administered 1 h before compound 48/80, based on previous reports showing anandamide levels peaked at that time (Lichtman et al., 2004a; Fegley et al., 2005). In one study, URB597 was administered intravenously 10 min after application of compound 48/80 to examine reversal of scratching. In all studies utilizing rimonabant, the selective CB1 receptor antagonist was administered 15 min before compound 48/80.
Behavioral Evaluation of Scratching Response. Animals were pretreated with test drugs at times described for individual experiments. All animals were placed into white (for contrast) acrylic chambers (20 × 20 × 20 cm), with a clear acrylic front panel and a mirrored back panel, for 30 min to acclimate to the observational environment. The chambers were enclosed in sound-attenuating cabinets, designed and custom-built at Virginia Commonwealth University, that contained an indirect filtered light-emitting diode light source and fans for air circulation and white noise. At the 30-min time point, animals were briefly removed from the chambers, which were wiped clean with water. Subjects were then given an injection of compound 48/80 under the scruff at the most dorsal point of the back just beneath the head and were returned to the chambers for 30 min. Behavior was recorded through the clear front panel using a series of Fire-i digital cameras (Unibrain, Inc., San Ramon, CA), and the videos were processed and saved using ANY-maze video tracking software (Stoelting Co., Wood Dale, IL). Chambers were fully sanitized at the end of each testing day using ammonia-based cleansers and soap, then left to air dry for at least 2 days to dissipate any odors.
The videos were subsequently placed in randomized order in a separate ANY-maze protocol for a trained observer to score using a keyboard-based behavioral tracking system, blinded to treatment group. Either ANY-maze or ODLog2 (Macropod Software, Armidale, NSW, Australia) software was used to track key presses assigned to specific behavioral endpoints for both time pressed and number of occurrences. The scratching response was tracked as hind leg scratching of the injection site and surrounding areas, excluding behaviors focused at areas within the ears or flicking and pulling at ear tags. Behavioral locomotor inactivity was also monitored, scored as complete lack of voluntary movement for any continuous period longer than 5 s.
Data Analysis. All data are reported as mean ± S.E.M. and represent the total number of seconds a specific behavior was scored from a total observational period of 1800 s. Experiments with only two treatment groups were analyzed for statistical significance using Student's t test. Experiments with more than two groups were analyzed using one- or two-way analysis of variance (ANOVA), with specified appropriate post hoc test. Resulting p values of less than 0.05 were considered significant. ED50 values (with 95% confidence intervals) and the potency ratios derived from the ED50 values were calculated using the least-squares linear regression method. Significant differences in potency were determined by the 95% confidence intervals of the potency ratio, not including a ratio of 1.
Results
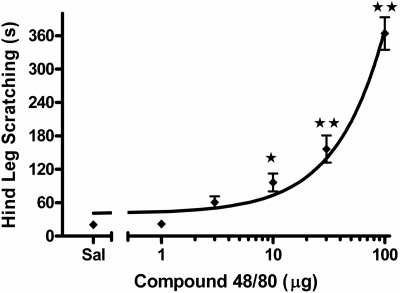
Pruritic Response to Compound 48/80. In an initial experiment, we evaluated the compound 48/80 model as a reliable model of pruritus by examining a similar dose range as used by Sugimoto et al. (1998) in eliciting scratching. Injections of compound 48/80 in caudal regions of the back induced increased grooming behavior (data not shown) and intense scratching with the hind paws directed at the rostral area of the back. As shown in Fig. 1, compound 48/80 dose-dependently increased time mice spent scratching the area of injection more than 100-fold above that of saline control injections [F(5,30) = 55.3, p < 0.001]. Based on these results, 30 μg of compound 48/80 was used in all subsequent experiments because this was the lowest tested dose that significantly differed from saline at p < 0.01 (Dunnett's post hoc test).
Fig. 1.
Dose-response of time spent hind leg scratching induced by subcutaneous compound 48/80 (1, 3, 10, 30, 100 μg). Totals represent time scratching within 30 min postinjection. ⋆, p < 0.05; ⋆⋆, p < 0.01 versus saline (Dunnett's post hoc test). n = 8 mice/dose.
To validate further the allergenic pruritus model, a pair of drugs representing the most common classes of clinically effective treatments for allergenic itch was tested against compound 48/80 (30 μg). The glucocorticoid compound, dexamethasone (2 mg/kg i.p.), significantly reduced scratching compared with saline pretreatment [t(14) = 6.1, p < 0.001]. In an initial study, we attempted to examine the dose-response relationship of diphenhydramine, an over-the-counter and commonly used H1 receptor antagonist. However, a significant reduction in scratching only occurred at doses that also elicited profound hypothermia and almost complete immobility (data not shown). The nonsedating second generation H1 receptor antagonist, loratadine (10 mg/kg i.p.), significantly reduced scratching behavior compared with vehicle controls [t(10) = 4.8, p < 0.001]. No significant effects of behavioral locomotor inactivity were found for either dexamethasone or loratadine. The results of these tests are summarized in Table 1.
TABLE 1.
Maximal effect of scratching attenuation by nonsedating pruritus treatments
| Drug Pretreatment | Vehicle Control | Control Scratching | Treatment Scratching | Scratching Reduction | Behavioral Locomotor Inactivity |
|---|---|---|---|---|---|
| Mean ± S.E.M. | % | p | |||
| 2 mg/kg Dexamethasone | Saline | 279.7 ± 14.1 | 150.6 ± 16.0 | 46 | 0.40 |
| 10 mg/kg Loratadine | Saline | 299.0 ± 14.9 | 143.5 ± 15.4 | 52 | 0.33 |
| 10 mg/kg URB597a | 1:1:18 | 256.7 ± 14.7 | 133.2 ± 8.5 | 48 | 0.96 |
| 30 mg/kg OL-135 | 1:1:18 | 264.5 ± 32.2 | 116.5 ± 17.1 | 56 | 0.27 |
Data from four experiments with identical treatment conditions were combined
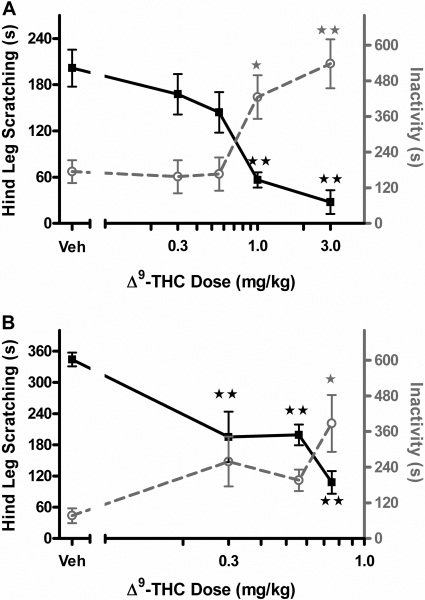
Evaluation of THC Treatment. Intraperitoneal administration of THC dose-dependently attenuated compound 48/80-induced scratching [F(4,35) = 15.3, p < 0.001; Fig. 2A]. The resulting curve shows that THC possessed great efficacy and potency in suppression of scratching behavior (ED50 = 0.66 mg/kg, 95% CI 0.52 to 0.82 mg/kg). However, this attenuation of scratching was accompanied with a concurrent significant suppression in behavioral locomotor activity [F(4,35) = 7.9, p < 0.001]. To determine whether the therapeutic profile improves with local administration, THC was given subcutaneously at the site of compound 48/80 administration (injection concentration was increased to 5 μl/g body mass). As seen in Fig. 2B, local administration of THC (0.3, 0.56, 0.75 mg/kg) produced a significant reduction in compound 48/80-induced scratching [F(3,20) = 11.4, p < 0.001] (p < 0.01, Dunnett's post hoc test), indicating an increase in potency compared with the intraperitoneal route of administration. In addition, significant hypomotility [F(3,20) = 3.7, p < 0.05] occurred only at the highest doses tested, demonstrating a dissociation between the suppressive effects of THC on scratching and its locomotor depressive effects.
Fig. 2.
Evaluation of THC in attenuating scratching response induced by compound 48/80. ▪ and solid line, hind leg scratching time, quantified on the left axis; ○ and gray dashed line, time animals spent inactive quantified on the right axis. A, THC dose-response (0.3, 0.56, 1, 3 mg/kg) after 30-min intraperitoneal pretreatment. B, THC dose-response (0.3, 0.56, 0.75 mg/kg) after 30-min subcutaneous pretreatment. ⋆, p < 0.05; ⋆⋆, p < 0.01 versus vehicle (Dunnett's post hoc test). n = 6 to 8 mice/dose.
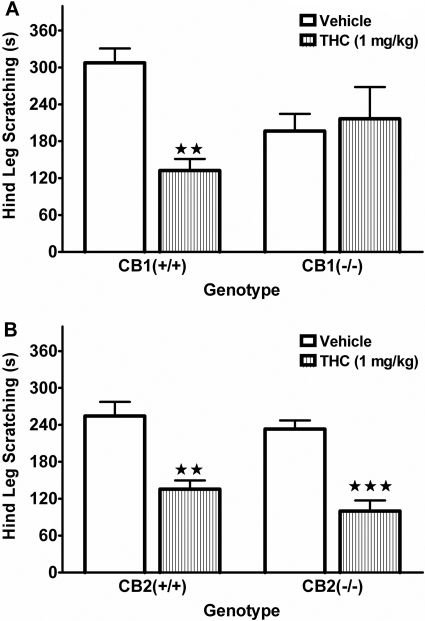
Because THC binds to both CB1 and CB2 receptors, we evaluated its receptor mechanism of action using mice lacking functional CB1 or CB2 receptors. As shown in Fig. 3A, THC (1 mg/kg) significantly reduced scratching behavior in the CB1(+/+) mice but did not cause any significant changes in the CB1(-/-) mice [F(1,28) = 8.9, p < 0.01 for interaction between genotype and THC pretreatment]. Compound 48/80 produced significantly less scratching behavior in CB1(-/-) mice than in CB1(+/+) mice (p < 0.01, Tukey's post hoc test). In addition, there was a significant interaction between genotype and THC on the locomotor activity data [F(1,28) = 15.5, p < 0.001]. THC more than doubled the time that the CB1(+/+) mice spent inactive (vehicle, 208 ± 62 s; THC, 532 ± 46 s) but did not significantly affect the CB1(-/-) mice. However, CB1(-/-) mice were significantly less active than the CB1(+/+) mice under vehicle conditions (p < 0.01, Tukey's post hoc test). In contrast, CB2(-/-) mice exhibited similar scratching responses and levels of inactivity compared with CB2(+/+) mice under vehicle treatment (see Fig. 3B). In addition, both genotypes showed equivalent responses to treatment with THC with respect to both scratching suppression and behavioral inactivity, with only a resulting main effect of THC treatment for scratching suppression [F(1,20) = 52.3, p < 0.001].
Fig. 3.
Effect of THC (1 mg/kg) on attenuating scratching response induced by compound 48/80 in CB1 and CB2 knockout animals and wild-type controls. A, THC (striped bars) significantly attenuated scratching response compared with 1:1:18 vehicle control in CB1(+/+), but not in CB1(-/-), mice. CB1(-/-) mice also show attenuated (p ≤ 0.05) scratching response with vehicle pretreatment. B, THC elicits a similar attenuation of compound 48/80-induced scratching in CB2(+/+) and CB2(-/-). ⋆⋆, p < 0.01; ⋆⋆⋆, p < 0.001 versus corresponding vehicle-treated mice. n = 6 to 8 mice/group.
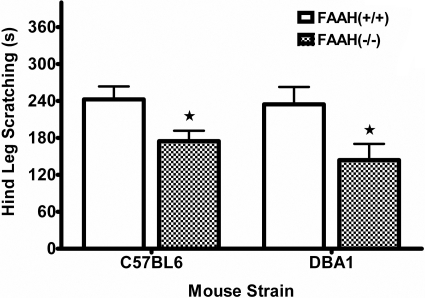
Evaluation of FAAH(-/-) Mice Scratching Response. FAAH(-/-) mice display impaired catabolic activity to anandamide and other fatty acid amides, resulting in at least 10-fold increases in endogenous levels of these lipid signaling molecules (Cravatt et al., 2001). The effects of compound 48/80 challenge in FAAH(-/-) mice backcrossed onto either C57BL/6 (C57) or DBA/1 (DBA) genetic backgrounds are shown in Fig. 4. FAAH(-/-) mice demonstrated a significantly reduced scratching response to compound 48/80 compared with FAAH(+/+) mice in both C57 [t(14) = 2.5, p < 0.05] and DBA [t(14) = 2.3, p < 0.05] strains. Neither the C57 nor DBA strains of FAAH(-/-) mice showed any differences in time spent inactive during testing (Table 2). These two background strains were examined because C57 mice were used in most other behavioral tests presented here, and the neural-specific conditional FAAH knockout (i.e., FAAH-NS) mice available for this research were developed on the DBA background.
Fig. 4.
FAAH(-/-) mice display a phenotypic reduction in compound 48/80-induced scratching. Graph shows a reduced response in mice lacking FAAH enzyme activity (checkered bars) bred onto either C57BL/6 or DBA/1 background strains. Significance was determined between genotypes for each strain using Student's t test; ⋆, p < 0.05 versus respective FAAH(+/+) group (Tukey's post hoc test). n = 8 to 12 mice/group.
TABLE 2.
Behavioral inactivity for experiments investigating FAAH mechanism of action Mean time spent inactive (±S.E.M.) for each group in the studies examining the mechanism of action of the FAAH reduction of pruritus. p Values are given for the appropriate ANOVA or Student's t test of each experiment.
| Time Inactive | p | |
|---|---|---|
| s ± S.E.M. | ||
| FAAH genotype comparison | ||
| C57 FAAH(+/+) | 84.4 ± 42.3 | 0.66 |
| C57 FAAH(—/—) | 58.4 ± 39.1 | |
| DBA FAAH(+/+) | 36.7 ± 24.9 | 0.82 |
| DBA FAAH(—/—) | 43.6 ± 17.6 | |
| Rimonabant FAAH reversal | 0.20 (Interaction between genotype and rimonabant) | |
| FAAH(+/+), vehicle | 131.4 ± 36.5 | |
| FAAH(+/+), rimonabant (1 mg/kg) | 171.4 ± 35.5 | |
| FAAH(—/—), vehicle | 273.0 ± 51.1 | |
| FAAH(—/—), rimonabant (1 mg/kg) | 183.8 ± 66.3 | |
| Neuronal FAAH comparison | 0.034 | |
| FAAH(+/—) | 126.2 ± 65.0 | |
| FAAH(—/—) | 143.1 ± 44.3 | |
| FAAH-NS | 364.2 ± 87.6a | |
| URB597-treated FAAH transgenic mice | 0.51 (Interaction between URB597 and genotype) | |
| FAAH(+/—) | ||
| Vehicle | 217.4 ± 33.7 | |
| URB597 (10 mg/kg) | 319.5 ± 47.2 | |
| FAAH(—/—) | ||
| Vehicle | 143.4 ± 35.8 | |
| URB597 (10 mg/kg) | 262.5 ± 46.9 | |
| FAAH-NS | ||
| Vehicle | 320.8 ± 56.8 | |
| URB597 (10 mg/kg) | 344.1 ± 35.8 | |
| URB597-treated CB1 and CB2(—/—) mice | ||
| CB1(+/+) | 0.84 (Interaction between URB597 and genotype) | |
| Vehicle | 140.5 ± 45.7 | |
| URB597 (10 mg/kg) | 197.4 ± 46.5 | |
| CB1(—/—) | ||
| Vehicle | 418.7 ± 89.6b | |
| URB597 (10 mg/kg) | 447.9 ± 87.2b | |
| CB2(+/+) | 0.70 (Interaction between URB597 and genotype) | |
| Vehicle | 247.1 ± 49.7 | |
| URB597 (10 mg/kg) | 247.4 ± 25.5 | |
| CB2(—/—) | ||
| Vehicle | 283.8 ± 39.5 | |
| URB597 (10 mg/kg) | 311.3 ± 17.7 | |
| Rimonabant reversal of URB597 | 0.98 (Interaction between URB597 and Rimonabant) | |
| Vehicle, vehicle | 252.6 ± 47.2 | |
| Vehicle, rimonabant (1 mg/kg) | 214.8 ± 21.2 | |
| URB597 (10 mg/kg), Vehicle | 273.0 ± 51.1 | |
| URB597 (10 mg/kg), rimonabant (1 mg/kg) | 226.8 ± 47.3 |
p < 0.05 vs. FAAH(+/—)
p < 0.001 (CB1 genotype)
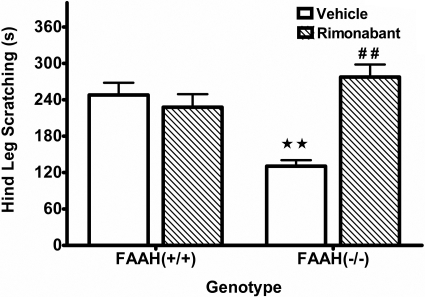
Although numerous biologically activated fatty acid amides are elevated in mice lacking functional FAAH activity, anandamide is the only mediator that has shown high affinity for activating cannabinoid receptors. To test whether anandamide and CB1 receptors contribute to the FAAH(-/-) phenotypic reduction in scratching, a moderate dose of rimonabant (1 mg/kg i.p.) was given 15 min before compound 48/80. As seen in Fig. 5, the vehicle-treated FAAH(-/-) mice displayed a significant reduction in scratching behavior compared with the vehicle-treated FAAH(+/+) mice. The dose of rimonabant did not affect scratching in FAAH(+/+) mice but significantly reversed the FAAH(-/-) phenotype [F(1, 28) = 20.0, p < 0.001 for interaction between rimonabant and genotype], indicating a CB1 receptor mechanism. There were no significant effects of either FAAH genotype or rimonabant pretreatment on locomotor activity (Table 2).
Fig. 5.
The FAAH(-/-) antipruritic phenotype is mediated through a CB1 receptor mechanism of action. FAAH(+/+) and FAAH(-/-) mice on a C57BL/6 background were given an intraperitoneal injection of rimonabant (1 mg/kg) 15 min before compound 48/80. The rimonabant (hatched bars) did not affect scratching behavior in FAAH(+/+) mice compared with 1:1:18 vehicle controls but reversed the phenotypic reduction in FAAH(-/-) mice. ⋆⋆, p < 0.01 versus FAAH(+/+) vehicle group; ##, p < 0.01 versus FAAH(-/-) vehicle group (Tukey's post hoc test). n = 8 mice/group.
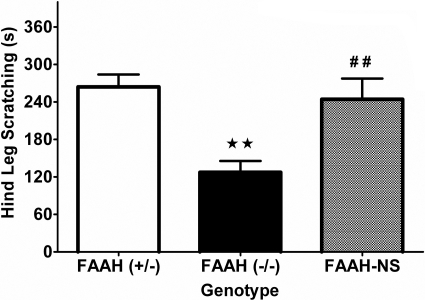
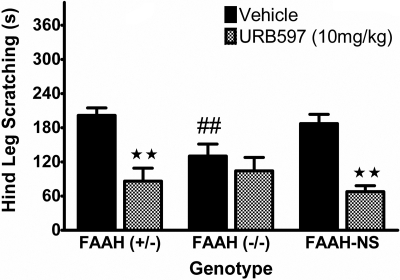
Because CB1 receptors are expressed in both the CNS and periphery, we next evaluated the contribution of FAAH activity in neural tissues. FAAH-NS mice are conditional knockin mice with FAAH expression linked to the promoter for neural-specific enolase, resulting in mice that express FAAH exclusively in neuronal tissue (i.e., brain, spinal cord, and dorsal root ganglia). Thus, these mice possess wild-type levels of anandamide and noncannabinoid fatty acid amide levels in brain and spinal cord but have greatly elevated levels of these lipid signaling molecules in peripheral organs (Cravatt et al., 2004). A comparison of FAAH-NS mice to FAAH(-/-) and FAAH(+/-) controls (on a DBA/1 background strain) is shown in Fig. 6. FAAH(+/-) mice exhibited a similar response to compound 48/80 as wild-type animals, whereas FAAH(-/-) mice continued to display a significant reduction in scratching [F(2, 26) = 9.7, p < 0.001; Fig. 6A]. The observation that the FAAH-NS mice showed a similar magnitude of scratching to compound 48/80 as wild-type mice indicates that neuronal fatty acid amides regulate this response. Despite scratching significantly more than FAAH(-/-) mice and the same as FAAH(+/-) mice, FAAH-NS showed a significant increase in time spent inactive (Table 2). This pattern of findings shows that scratching and hypomotility can be functionally uncoupled.
Fig. 6.
Mice that only express FAAH in neural tissues do not display a phenotypic reduction in compound 48/80-induced scratching. Heterozygous mice (+/-; white bar), global FAAH knockout mice (-/-; black bar), and conditional knockout mice expressing FAAH isolated to neural tissues (NS; gray bar). FAAH(-/-) mice display typical phenotypic reduction in scratching response (⋆⋆, p < 0.01; Tukey's post hoc test) compared with FAAH(+/-) controls; however, this phenotype is absent in mice with FAAH expression active in neural tissues (##, p < 0.01 versus FAAH(-/-) group). n = 10 mice/group.
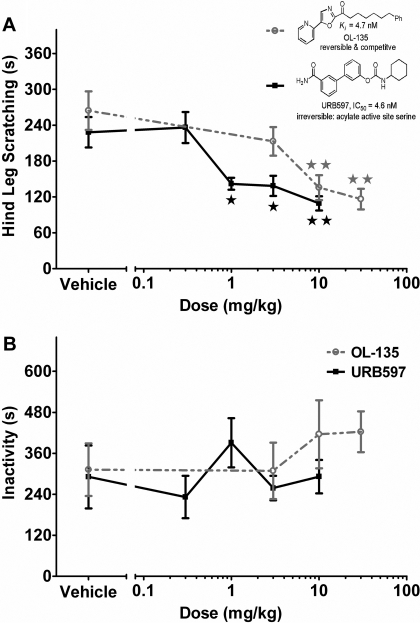
Treatment of Scratching by FAAH Inhibitors. In the final series of experiments, we evaluated the effects of the selective FAAH inhibitors, URB597 and OL-135, in the compound 48/80 pruritus model. As seen in Fig. 7 and summarized in Table 1, both compounds significantly reduced scratching responses, with no significant effects on behavioral activity. URB597, shown in the black line, significantly reduced the scratching response compared with vehicle at doses above 1 mg/kg [F(4,35) = 8.9, p < 0.001], with an ED50 dose of 0.8 mg/kg (95% CI, 0.4–1.6 mg/kg). Increasing doses of URB597 had no significant effect on behavioral locomotor inactivity in the mice (shown in Fig. 7B), including doses that elicited significant attenuation of scratching (ANOVA, p = 0.50). Likewise, OL-135, shown in the gray line, significantly reduced scratching compared with vehicle [F(3,28) = 8.1, p < 0.001], with an ED50 dose of 3.9 mg/kg (95% CI, 2.1–7.2 mg/kg). OL-135 also did not affect locomotor activity (Fig. 7B; ANOVA, p = 0.62). Both FAAH inhibitors displayed equivalent efficacy in reducing scratching behavior. The potency ratio (95% confidence limits) for the antiscratching effects between URB597 and OL-135 was 4.1 (95% CI, 1.8–9.1), indicating a significant difference because the confidence limits did not include the ratio of 1.
Fig. 7.
FAAH inhibitors dose-dependently reduce compound 48/80 scratching without hypomotility. A, dose-response of FAAH inhibitors in attenuating scratching response to compound 48/80. The irreversible FAAH inhibitor URB597 (0.3, 1, 3, and 10 mg/kg i.p.) was given 1 h before compound 48/80, shown as closed black squares (▪) with solid black line. The reversible FAAH inhibitor OL-135 (1, 3, 10, and 30 mg/kg i.p.) was also given 1 h before compound 48/80, shown as open gray circles (○) with dashed gray line. Inset, structure and descriptions of both FAAH inhibitors. B, dose response of behavioral inactivity of the FAAH inhibitors in response to compound 48/80. ⋆, p < 0.05; ⋆⋆, p < 0.01 versus vehicle (Dunnett's post hoc test). n = 8 mice/dose.
Because pruritic conditions are treated after persistent itching and are not treated prophylactically, we next investigated whether URB597 would reverse scratching after the allergenic response was already initiated. In accordance, ICR mice were given compound 48/80 and monitored before and after either vehicle or URB597 (1 mg/kg) intravenous treatment. As shown in Table 3, both groups showed equivalent levels of scratching response over the initial 10 min after compound 48/80 (i.e., before drug treatment). At the conclusion of the first 10-min time block, the mice were removed from the observation cage, immediately given an injection of either vehicle or URB597 (10 mg/kg) into the tail vein, and returned to the test chamber by 11 min. Mice were then scored again for the final 10 min of the compound 48/80 scratching (i.e., from 20–30 min after compound 48/80 administration), allowing 10 min without scoring for onset of drug action. After the intravenous injection, both groups showed less scratching behavior than during the first 10-min time block, in line with a typical compound 48/80 time course. It is important that URB597 injected after the induction of compound 48/80-induced scratching significantly attenuated scratching behavior compared with vehicle-treated mice. In addition, the vehicle- and URB597-treated groups displayed a similar degree of hypomotility at each respective time block.
TABLE 3.
URB597 reversal of compound 48/80-induced scratching URB597 (10 mg/kg) reverses scratching when given intravenously 10 min after administration of compound 48/80. All values are represented as mean ± S.E.M. (n = 7 mice/group).
|
Scratching
|
Behavioral Inactivity
|
|||
|---|---|---|---|---|
| Preinjection | Postinjection | Preinjection | Postinjection | |
| s | ||||
| Vehicle | 38.6 ± 4.4 | 19.5 ± 4.1 | 4.5 ± 2.9 | 33.7 ± 10.2 |
| URB597 | 45.1 ± 7.2 | 4.7 ± 0.2** | 1.1 ± 0.8 | 47.0 ± 14.7 |
P < 0.01 versus vehicle-treated postinjection group
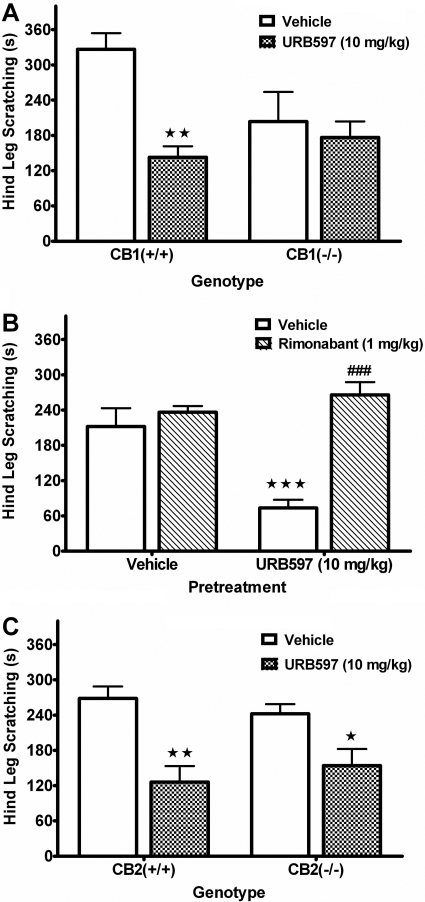
To examine the receptor mechanism of action, we evaluated the effects of URB597 on compound 48/80-induced scratching in CB1(-/-) and CB2(-/-) mice (see Fig. 8). URB597 significantly decreased scratching in CB1(+/+) mice but was without effect in CB1(-/-) mice [F(1,30) = 5.9, p < 0.05 for interaction between URB597 and genotype; Fig. 8A]. There was also a significant increase in behavioral locomotor inactivity, regardless of treatment, in the CB1(-/-) mice [F(1,30) = 15.0, p < 0.001 for genotype; Table 2]. Because vehicle-treated CB1(-/-) mice displayed a reduced scratching response compared with vehicle-treated CB1(+/+) mice (p = 0.05, Tukey's post hoc test), we next evaluated whether rimonabant would block the reduction of scratching behavior caused by URB597. As depicted in Fig. 8B, rimonabant completely reversed the reduction of scratching caused by URB597 [F(1, 20) = 16.2, p < 0.001 for interaction between rimonabant and URB597]. Rimonabant and URB597 did not alter behavioral activity levels either alone or in combination (see Table 2). In contrast, URB597 elicited a similar attenuation of scratching in both CB2(+/+) and CB2(-/-) mice [F(1,20) = 23.9, p < 0.001 for main effect of URB597; Fig. 8C]. In addition, both CB2(+/+) and CB2(-/-) mice displayed a similar degree of scratching behavior and activity levels (Table 2). These data indicate that CB1 receptors are critical for URB597's attenuation of compound 48/80-induced scratching.
Fig. 8.
The FAAH inhibitor, URB597, attenuates compound 48/80-induced scratching through a CB1 receptor mechanism of action. Mice were given either 1:1:18 vehicle or 10 mg/kg URB597 1 h before compound 48/80. A, URB597 reduced scratching in CB1(+/+), but not CB1(-/-), mice. B, rimonabant (1 mg/kg) blocked the antiscratching effects of URB597. C, URB597 reduced scratching in both CB2(+/+) and CB2(-/-) mice. ⋆, p < 0.05; ⋆⋆, p < 0.01; ⋆⋆⋆, p < 0.001 versus each respective vehicle control group; ###, p < 0.001 versus URB597 with vehicle pretreatment (Tukey's post hoc test). n = 6 to 8 mice/group.
Finally, to validate the specificity of both the transgenic and pharmacological tools, the effects of vehicle or URB597 (10 mg/kg) were evaluated in FAAH(+/-), FAAH(-/-), and FAAH-NS mice to ascertain the locus of action. In a replication of the previous experiment shown in Fig. 6, FAAH(-/-) mice showed a phenotypic attenuation in scratching compared with FAAH(+/-) controls, whereas FAAH-NS mice displayed wild-type levels of compound 48/80-induced scratching (Fig. 9). URB597-treated FAAH(-/-) mice exhibited no further suppression of scratching beyond the phenotypic reduction, but this FAAH inhibitor significantly reduced scratching behavior in both FAAH(+/-) and FAAH-NS mice [F(2,43) = 3.92, p < 0.05 for interaction between genotype and URB597]. Thus, systemic pharmacological inhibition of FAAH by URB597, in mice with either ubiquitous FAAH activity or only neuronal FAAH activity, produces a similar magnitude in the suppression of scratching in response to compound 48/80. This scratching suppression is equivalent to that seen as a phenotypic characteristic of FAAH(-/-) animals, mice with globally inactive FAAH.
Fig. 9.
URB597 reduced scratching in FAAH-NS and FAAH(+/-) mice to the same magnitude as the FAAH(-/-) antiscratching phenotypic response. However, URB597 did not further reduce scratching in FAAH(-/-) mice. ⋆⋆, p < 0.01 versus the corresponding vehicle group for each respective genotype; ##, p < 0.01 versus FAAH(+/-) vehicle controls (Tukey's post hoc test). n = 8 mice/group.
Discussion
Pruritus represents a significant clinical problem that can produce great suffering in affected patients that includes 70% of patients suffering from liver cholestasis and approximately 20% in kidney failure and most patients with atopic dermatitis. In fact, upwards of 50% of all pruritus cases requiring medical treatment may result from a systemic, chronic underlying pathology (Bernhard, 1994). None of the aforementioned conditions have pharmacotherapies available that consistently alleviate the pruritus. The research presented in the present manuscript is the first to examine the therapeutic and mechanistic roles of the endogenous cannabinoid system in the modulation of pruritus. Here, we demonstrate that THC and pharmacological blockade or genetic deletion of FAAH reduce scratching in a murine model of pruritus.
FAAH(-/-) mice and mice treated with FAAH inhibitors displayed a significant decrease in compound 48/80-induced scratching with the absence of motor suppression. The converging evidence from FAAH(-/-) mice and two separate classes of FAAH inhibitors suggests that inactivation of this enzyme, resulting in elevated levels of several fatty acid amides, leads to inhibition of pruritic responses. The ED50 values of URB597 and OL-135 in attenuation of scratching response closely matched those previously reported for inhibition of FAAH and/or enhancement of activity from exogenously administered anandamide (Lichtman et al., 2004a; Piomelli et al., 2006). The FAAH inhibitors URB597 and OL-135 show similar efficacy in scratching suppression as current treatments of choice for allergenic pruritus (Table 1). It is important that neither of these agents elicited hypomotility. Intravenous administration of URB597 demonstrated rapid reversal of pruritic activity after initiation of mast cell degranulation and allergenic response, suggesting further a nonimmune blockade of itch signaling with potential therapeutic application. Reversal of scratching is of clinical relevance because pruritus is rarely treated prophylactically.
The mechanism underlying FAAH influence on pruritus is not directly clear because of the wide variety of receptor systems and lipid mediators affected by FAAH inactivation. Although inhibition of FAAH leads to increased endogenous anandamide levels, it also increases content of other fatty acid amides important to cellular signaling (Cravatt et al., 2001). It is noteworthy that topical creams containing a noncannabinoid substrate of FAAH, PEA, suppressed ratings of pruritus in a small clinical study of volunteers with various chronic dermatological conditions (Ständer et al., 2006). Although no mechanistic studies were performed, most evidence points to PEA providing anti-inflammatory action through peroxisome proliferator-activated receptor α nuclear receptors (Lo Verme et al., 2005). The FAAH(-/-) phenotypic reduction in scratching was completely reversed by the CB1 antagonist, 1 mg/kg rimonabant. Likewise, the FAAH inhibitor, URB597, failed to attenuate scratching behavior in CB1(-/-) mice or rimonabant-treated wild-type mice. Conversely, URB597 continued to elicit full antipruritic effects in CB2(-/-) mice. Moreover, these findings are consistent with the notion that elevated levels of anandamide mediate this phenotype because it is the only known substrate of FAAH that binds to CB1 receptors.
Through the use of FAAH-NS mice, we evaluated whether FAAH activity in the nervous system plays an important role in modulating scratching behavior in the compound 48/80 model. It is interesting that FAAH-NS mice that express wild-type levels of fatty acid amides in the nervous system but elevated levels in peripheral organs (Cravatt et al., 2004) exhibited a similar scratching response as wild-type mice. Thus, despite possessing elevated levels of fatty acid amides in the peripheral organs, these mice have no phenotypic attenuation of pruritus. Furthermore, administration of URB597 to FAAH-NS mice, which inhibited the remaining active FAAH in the nervous tissues, suppressed the scratching response in a fashion similar to wild-type controls. These suppressed scratching responses mimicked that of FAAH knockout mice in magnitude, and no further reductions of URB597 were seen in global FAAH knockout mice. The above results show the apparent specificity of the scratching phenotype of both the drug and transgenic models to their FAAH activity and indicate that FAAH inhibition within the nervous system is essential for antipruritic action. These findings suggest that this enzyme may be targeted for therapeutic gain to treat a generalized pruritus from a wide variety of etiologies, including immunological, neuropathic, and psychogenic.
This study also demonstrated that even though systemic THC is capable of near full suppression of pruritic scratching response to compound 48/80, its effects are accompanied with overt CNS behavioral effects. Subcutaneous THC administered locally to the site of compound 48/80 potently suppressed scratching response, supporting previous evidence that locally applied cannabinoid agonists have antipruritic action (Dvorak et al., 2003), although suppression was observed in a narrow dose range before also eliciting central inhibitory effects. The profile of THC in the compound 48/80 model is similar to animals pretreated with systemic diphenhydramine, a well established antihistamine that has sedative and adverse CNS effects, limiting its use when patient sedation and cognition are considerations (for review, see Banerji et al., 2007). Pretreatment with dexamethasone and loratadine, representing commonly used nonsedative treatments of allergenic pruritus, produced effective suppression of scratching response to compound 48/80 with minimal effects on locomotor activity and sedation. The suppression of scratching observed after THC administration was absent in CB1 knockout mice but equally effective in CB2 knockout mice, suggesting that at least most of THC's antipruritic actions are attributable to activation of CB1 receptors. However, the observation that THC also increased hypomotility suggests that at least a portion of the suppression seen may be attributed to central side effects at higher doses.
Therapeutic use of THC is hindered by many issues associated with chronic use. THC has been shown to have potential for abuse because it is self-administered by nonhuman primates (Justinova et al., 2008) and shows a withdrawal syndrome indicative of physical dependence in humans (Haney et al., 1999). In contrast, the FAAH inhibitor URB597 seems to lack abuse potential, with no conditioned place preference in rodents (Gobbi et al., 2005) and no self-administration in primates (Justinova et al., 2008). In addition, URB597 does not seem to elicit cannabimimetic subjective effects because it fails to substitute for THC in drug discrimination tests in rodents (Solinas et al., 2007). However, FAAH inhibition has therapeutic efficacy in numerous preclinical models of anxiety, depression, pain, and inflammation (for review, see Cravatt and Lichtman, 2004; Piomelli et al., 2006).
CB1(-/-) mice generally displayed phenotypic reductions in compound 48/80-induced scratching response and increases in time spent inactive under vehicle conditions, complicating interpretation of the results. This CB1(-/-) phenotypic reduction in scratching may be the result of compensatory adaptations across ontogeny, epistasis, in which the effect of gene disruption is modified by the genetic background on which it is placed, or pleiotropic effects, in which other consequences of gene disruption indirectly affect the behavior of interest (Mogil and Grisel, 1998). It is important that the CB1 receptor antagonist, rimonabant, also blocked phenotypic decreases in compound 48/80-induced scratching caused by genetic deletion or pharmacological inhibition of FAAH. These complementary approaches indicate that CB1 receptor activation is a crucial downstream signaling event for the antipruritic phenotype of FAAH-compromised mice.
Previous studies show that pruritus may be gated by inhibitory input from nociceptive pathways. Noxious cooling and heating, along with scratching itself, produce increases in pain ratings with associated decreases in itch (Ward et al., 1996; Yosipovitch et al., 2005, 2007). Compared with either histamine or noxious cold alone, human subjects had selective neural activation of the periaqueductal gray (PAG) during induction of both pruritus and pain together (Mochizuki et al., 2003). The PAG having such a pronounced role in the inhibition of nociception suggests that this brain region may also inhibit pruritus (Waters and Lumb, 2008). Considering that opioid (Rosenfeld, 1994) and cannabinoid (Lichtman et al., 1996) receptor activation in the PAG plays a prominent role in antinociception, their respective roles in modulation of pruritus are similarly anticipated. Opioid drugs such as the μ-receptor antagonist naltrexone and the κ-receptor agonist nalfurafine are being tested clinically as modulators of pruritus (Wikström et al., 2005; Mansour-Ghanaei et al., 2006; Bigliardi et al., 2007). Unlike the μ-opioid receptor, CB1 receptor activation seems to have the benefit of inhibiting both nociceptive and pruriceptive signals. Further study of the important neural substrates in endocannabinoid inhibition of pruriception is necessary to understand the endocannabinoid system's differential function.
In conclusion, the present study demonstrates that activation of the CB1 cannabinoid receptor, via either direct-acting receptor agonist (i.e., THC) or inhibiting degradation of anandamide, can suppress scratching response to a pruritic agent. Although direct agonists show cannabimimetic central effects that included hypomotility, elevating endogenous cannabinoids through FAAH inhibition produces efficacious suppression of scratching response. Suppression of compound 48/80-induced scratching through FAAH inactivation is comparable with currently available treatments, with no apparent effect on sedation and coordination. FAAH produces scratching suppression through activation of CB1 receptors within neural tissues, representing a potential therapeutic target for blockade of pruritic signaling. The potential of a signaling-based neural target to treat pruritus, regardless of the causal pathology, makes FAAH inhibitors a promising prospective tool for the treatment of itch.
Acknowledgments
We thank Ashley Crockett and Darby Fleming for technical assistance in performing video observations for some of the experiments, all of the members of Lichtman laboratory group for creative input and feedback on this project, and a dear friend and mentor, the late Dr. Billy R. Martin, for great insight and guidance that helped shape the project.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants P01-DA017259, DA15197, DA03672, P50-DA005274, T32-DA007027, DA15648].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.150136.
ABBREVIATIONS: THC, Δ9-tetrahydrocannabinol; FAAH, fatty acid amide hydrolase; PEA, N-palmitoyl ethanolamine; rimonabant, N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide HCl; OL-135, 1-oxo-1-[5-(2-pyridyl)-2-yl]-7-phenylheptane; URB597, cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester; ANOVA, analysis of variance; C57, C57BL/6J mouse strain; DBA, DBA/1 mouse strain; FAAH-NS, neural-specific conditional FAAH knockout; CNS, central nervous system; CI, confidence interval; PAG, periaqueductal gray; WIN55,212-2, (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-d,e]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone; CP55940, (-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)-phenyl]-trans-4-(3-hydroxy-propyl)-cyclohexanol; HU-210, (6aR)-trans-3-(1,1-dimethylheptyl)-6a,7,10,10a-tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[b,d]pyrano-9-methanol.
References
- Banerji A, Long AA, and Camargo CA Jr (2007) Diphenhydramine versus nonsedating antihistamines for acute allergic reactions: a literature review. Allergy Asthma Proc 28 418-426. [DOI] [PubMed] [Google Scholar]
- Bernhard JD (1994) Itch: Mechanisms and Management of Pruritus, McGraw-Hill, New York.
- Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, Gould C, Gemmen E, and Dall T (2006) The burden of skin diseases: 2004: a Joint Project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 55 490-500. [DOI] [PubMed] [Google Scholar]
- Bigliardi PL, Stammer H, Jost G, Rufli T, Büchner S, and Bigliardi-Qi M (2007) Treatment of pruritus with topically applied opiate receptor antagonist. JAm Acad Dermatol 56 979-988. [DOI] [PubMed] [Google Scholar]
- Boger DL, Miyauchi H, Du W, Hardouin C, Fecik RA, Cheng H, Hwang I, Hedrick MP, Leung D, Acevedo O, et al. (2005) Discovery of a potent, selective, and efficacious class of reversible alpha-ketoheterocycle inhibitors of fatty acid amide hydrolase effective as analgesics. J Med Chem 48 1849-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, and Lichtman AH (2001) Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A 98 9371-9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF and Lichtman AH (2004) The endogenous cannabinoid system and its role in nociceptive behavior. J Neurobiol 61 149-160. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Saghatelian A, Hawkins EG, Clement AB, Bracey MH, and Lichtman AH (2004) Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc Natl Acad Sci U S A 101 10821-10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA (2001) Cannabinoids of diverse structure inhibit two DOI-induced 5-HT(2A) receptor-mediated behaviors in mice. Pharmacol Biochem Behav 68 311-317. [DOI] [PubMed] [Google Scholar]
- Darmani NA and Pandya DK (2000) Involvement of other neurotransmitters in behaviors induced by the cannabinoid CB1 receptor antagonist SR 141716A in naive mice. J Neural Transm 107 931-945. [DOI] [PubMed] [Google Scholar]
- Dvorak M, Watkinson A, McGlone F, and Rukwied R (2003) Histamine induced responses are attenuated by a cannabinoid receptor agonist in human skin. Inflamm Res 52 238-245. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, and Piomelli D (2005) Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 313 352-358. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, et al. (2005) Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A 102 18620-18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, and Fischman MW (1999) Abstinence symptoms following oral THC administration to humans. Psychopharmacology (Berl) 141 385-394. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Janoyan JJ, Crim JL, and Darmani NA (2002) Reversal of SR 141716A-induced head-twitch and ear-scratch responses in mice by delta 9-THC and other cannabinoids. Pharmacol Biochem Behav 71 155-162. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, et al. (2008) Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry 64 930-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, and Martin BR (1996) Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther 276 585-593. [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, and Cravatt BF (2004a) Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther 311 441-448. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, and Cravatt BF (2004b) Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain 109 319-327. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, and Piomelli D (2005) The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 67 15-19. [DOI] [PubMed] [Google Scholar]
- Mansour-Ghanaei F, Taheri A, Froutan H, Ghofrani H, Nasiri-Toosi M, Bagherzadeh AH, Farahvash MJ, Mirmomen S, Ebrahimi-Dariani N, Farhangi E, et al. (2006) Effect of oral naltrexone on pruritus in cholestatic patients. World J Gastroenterol 12 1125-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, and Yanai K (2003) Imaging of central itch modulation in the human brain using positron emission tomography. Pain 105 339-346. [DOI] [PubMed] [Google Scholar]
- Mogil JS and Grisel JE (1998) Transgenic studies of pain. Pain 77 107-128. [DOI] [PubMed] [Google Scholar]
- Neff GW, O'Brien CB, Reddy KR, Bergasa NV, Regev A, Molina E, Amaro R, Rodriguez MJ, Chase V, Jeffers L, et al. (2002) Preliminary observation with dronabinol in patients with intractable pruritus secondary to cholestatic liver disease. Am J Gastroenterol 97 2117-2119. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, and Kunos G (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58 389-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R, Schmelz M, Bíró T, and Steinhoff M (2006) Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest 116 1174-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, and Putman D (2006) Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597). CNS Drug Rev 12 21-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JP (1994) Interacting brain stem components of opiate-activated, descending, pain-inhibitory systems. Neurosci Biobehav Rev 18 403-409. [DOI] [PubMed] [Google Scholar]
- Schmelz M and Handwerker HO. (2006) Itch, in Wall and Melzack's Textbook of Pain (McMahon SB and Koltzenburg M eds) pp 219-227, Churchill Livingstone/Elsevier, Philadelphia, PA.
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, and Goldberg SR (2007) The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther 321 370-380. [DOI] [PubMed] [Google Scholar]
- Ständer S, Reinhardt HW, and Luger TA (2006) Topical cannabinoid agonists. an effective new possibility for treating chronic pruritus. Hautarzt 57 801-807. [DOI] [PubMed] [Google Scholar]
- Stante M, Hanna D, and Lotti T (2005) Itch, pain, and metaesthetic sensation. Dermatol Ther 18 308-313. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Umakoshi K, Nojiri N, and Kamei C (1998) Effects of histamine H1 receptor antagonists on compound 48/80-induced scratching behavior in mice. Eur J Pharmacol 351 1-5. [DOI] [PubMed] [Google Scholar]
- Ward L, Wright E, and McMahon SB (1996) A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain 64 129-138. [DOI] [PubMed] [Google Scholar]
- Waters AJ and Lumb BM (2008) Descending control of spinal nociception from the periaqueductal grey distinguishes between neurons with and without C-fibre inputs. Pain 134 32-40 [DOI] [PubMed] [Google Scholar]
- Wikström B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, Ogasawara M, Kawashima Y, Ueno K, Mori A, and Ueno Y (2005) Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol 16 3742-3747. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Duque MI, Fast K, Dawn AG, and Coghill RC (2007) Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. Br J Dermatol 156 629-634. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Fast K, and Bernhard JD (2005) Noxious heat and scratching decrease histamine-induced itch and skin blood flow. J Invest Dermatol 125 1268-1272. [DOI] [PubMed] [Google Scholar]