Abstract
Monoamine releasers constitute one class of drugs currently under investigation as potential agonist medications for the treatment of cocaine dependence. The efficacy and safety of monoamine releasers as candidate medications may be influenced in part by their relative potency to release dopamine and serotonin, and we reported previously that releasers with approximately 30-fold selectivity for dopamine versus serotonin release may be especially promising. The present study examined the effects of the releasers benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine, which have 20- to 48-fold selectivity in vitro for releasing dopamine versus serotonin. In an assay of cocaine discrimination, rhesus monkeys were trained to discriminate 0.4 mg/kg i.m. cocaine from saline in a two-key, food-reinforced procedure. Each of the releasers produced a dose- and time-dependent substitution for cocaine. 4-Benzylpiperidine had the most rapid onset and shortest duration of action. Phenmetrazine and benzylpiperazine had slower onsets and longer durations of action. In an assay of cocaine self-administration, rhesus monkeys were trained to respond for cocaine injections and food pellets under a second order schedule. Treatment for 7 days with each of the releasers produced a dose-dependent and selective reduction in self-administration of cocaine (0.01 mg/kg/injection). The most selective effects were produced by phenmetrazine. Phenmetrazine also produced a downward shift in the cocaine self-administration dose effect curve, virtually eliminating responding maintained by a 30-fold range of cocaine doses (0.0032–0.1 mg/kg/injection) while having only small and transient effects on food-maintained responding. These findings support the potential utility of dopamine-selective releasers as candidate treatments for cocaine dependence.
Cocaine abuse and dependence continue to be significant public health concerns, and no uniformly effective pharmacotherapies are currently available (Vocci and Elkashef, 2005). One strategy for development of medications has been suggested by the relative success of methadone for the treatment of opioid dependence and of nicotine formulations for the treatment of tobacco dependence (Mello and Negus, 1996; Grabowski et al., 2004; Haney and Spealman, 2008). These medications produce effects similar to the abused substance, and they have been referred to as “agonist” medications. The value of agonist medications lies in their ability to reduce consumption of the abused substance, to maintain compliance during treatment, and to act at a given pharmacological target with lower toxicity than the abused substance. For example, nicotine formulations deliver the principle psychoactive component of tobacco (nicotine) without delivering toxic components also contained in tobacco (e.g., carcinogenic tars).
Cocaine binds to monoamine transporters and blocks uptake of dopamine, norepinephrine, and serotonin. The dopaminergic effects of cocaine are thought to be especially important in mediating its abuse-related effects; as a result, research on candidate agonist medications for cocaine abuse and dependence has focused on drugs that function as indirect or direct dopamine agonists (Mello and Negus, 1996; Rothman et al., 2002a; Grabowski et al., 2004; Haney and Spealman, 2008). For example, amphetamine is a monoamine releaser that selectively promotes release of dopamine and norepinephrine versus serotonin (Hoffman, 2001; Rothman et al., 2001). In preclinical studies conducted in rhesus monkeys, chronic treatment with amphetamine produced a dose-dependent and sustained reduction in cocaine self-administration under second order and progressive-ratio schedules while producing smaller and transient effects on responding maintained by food delivery (Negus and Mello, 2003a,b). Moreover, under a concurrent-choice schedule of cocaine and food availability, chronic amphetamine treatment produced a shift in responding away from cocaine choice and toward food choice (Negus, 2003). In a double-blind, placebo-controlled clinical study, amphetamine maintenance dose-dependently reduced cocaine use (Grabowski et al., 2001), and similar findings have been reported in other clinical studies (for review, see Grabowski et al., 2004). Other advantages of amphetamine include its high oral bioavailability, relatively long duration of action, and long-standing clinical availability for the treatment of other disorders including attention deficit-hyperactivity disorder and narcolepsy (Hoffman, 2001).
One clear disadvantage of amphetamine as a candidate medication for cocaine dependence is its high abuse liability, and as with cocaine, the abuse-related effects of amphetamine are thought to be mediated by its actions as an indirect dopamine agonist (Gold et al., 1989). However, the abuse-related effects associated with dopamine release or reuptake inhibition may be attenuated by concurrent serotonin release or reuptake inhibition (Baumann et al., 2000; Czoty et al., 2002; Wee et al., 2005). This suggests that dual dopamine/serotonin releasers might have lower abuse liability than amphetamine and other more selective releasers of dopamine versus serotonin (Rothman et al., 2007). Moreover, withdrawal from cocaine is associated with depression-like behavioral and neurochemical effects that may involve deficits in both dopaminergic and serotonergic systems, and these deficits might be amenable to concurrent increases in dopamine and serotonin levels (Baumann and Rothman, 1998; Rothman et al., 2007). In view of the potential advantages of dual dopamine/serotonin releasers, we recently evaluated effects on cocaine- and food-maintained responding produced by chronic administration of five monoamine releasers that differed in their relative potencies to release dopamine versus serotonin (Rothman et al., 2005; Negus et al., 2007). Dopaminergic selectivities were defined as the ratio of in vitro potencies to promote release of serotonin and dopamine from rat brain synaptosomes, and the calculated selectivities for the five compounds using this method were 80, 30, 6.5, 0.27, and <0.0079. All five releasers produced dose-dependent and sustained decreases in cocaine self-administration; however, the most selective decrease in cocaine- versus food-maintained responding was produced by a releaser with 30-fold selectivity for dopamine versus serotonin release.
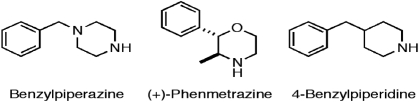
The purpose of the present study was to evaluate the effects of additional releasers with dopamine/serotonin selectivities similar to the optimal value of 30 identified in our previous study. This study specifically focused on the releasers benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine (Fig. 1). These compounds have selectivities for dopamine versus serotonin release of 20, 37, and 48, respectively, as determined in the rat brain synaptosome assay of monoamine release (Table 1). Acute effects of each compound were studied first in an assay of cocaine discrimination to assess potency, time course, and magnitude of cocaine-like abuse-related effects in rhesus monkeys. The effects of each releaser were evaluated subsequently during chronic administration in an assay of cocaine- and food-maintained responding to assess behavioral selectivity to reduce cocaine self-administration. Taken together with previous findings, our results support the hypothesis that releasers with modest biochemical selectivity for promoting dopamine versus serotonin release may produce behaviorally selective reductions in cocaine- versus food-maintained responding. Such compounds seem to retain abuse liability. However, on the basis of their efficacy and behavioral selectivity to reduce cocaine self-administration, they may warrant further consideration as candidates or lead compounds for development of new medications.
Fig. 1.
Structures of benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine.
TABLE 1.
EC50 values in nanomolar ± S.E.M. for monoamine releasers to release dopamine, norepinephrine, and serotonin The ratios of 5-hydroxytryptamine (5HT) EC50/dopamine (DA) EC50 and norepinephrine (NE) EC50/DA EC50 are also shown. Higher ratios imply greater selectivity for releasing dopamine. For all compounds, potency to release NE was higher than potency to release DA.
Materials and Methods
Subjects
Studies were conducted in 12 adult male rhesus monkeys (Macaca mulatta) that weighed 6 to 12 kg. Seven monkeys were studied in the drug discrimination experiments, and five monkeys were studied in the drug self-administration experiments. All monkeys had an experimental history involving the evaluation of dopaminergic and/or opioid compounds in assays of drug discrimination or drug self-administration. Monkeys were maintained on a diet of fruit and Lab Diet Jumbo Monkey biscuits (PMI Feeds, Inc., St. Louis, MO) sufficient to maintain stable and healthy weights throughout the course of the experiment. Biscuits and fruit were provided in the afternoon (between 1:00 and 5:00 PM and after daily sessions) for subjects in drug discrimination studies. Biscuits were provided in the morning (9:30–10:30 AM), and fruit was provided in the afternoon (5:00 PM) for subjects in drug self-administration studies. In addition, monkeys could receive 1-g banana-flavored pellets (Precision Primate Pellets Formula L/I Banana Flavor; P.J. Noyes Co., Lancaster, NH) during daily operant sessions as described below. Water was continuously available. For drug discrimination studies, a 12-h light/dark cycle was in effect (lights on from 7:00 AM to 7:00 PM). For drug self-administration studies, lights were on from 8:00 to 11:00 AM, 1:00 to 3:00 PM, and 5:00 to 7:00 PM (i.e., lights on from 7:00 AM to 7:00 PM except during times of experimental components; see below).
Animal maintenance and research were conducted in accordance with the guidelines provided by the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). The facility was licensed by the United States Department of Agriculture, and protocols were approved by the Institutional Animal Care and Use Committee. The health of the monkeys was periodically monitored by consulting veterinarians. Monkeys had visual, auditory, and olfactory contact with other monkeys through-out the study. Operant procedures and foraging toys provided an opportunity for environmental manipulation and enrichment.
Drug Discrimination Procedures
Apparatus. Each monkey was housed individually in a well ventilated, stainless steel chamber (56 × 71 × 69 cm). The home cages of all monkeys were modified to include an operant panel (28 × 28 cm) mounted on the front wall. Three round translucent response keys (5.1 cm in diameter) were arranged 3.5 cm apart in a horizontal row 9 cm from the top of the operant panel. Each key could be transilluminated by red or green stimulus lights (Superbright LEDs; Fairchild Semiconductor, San Jose, CA). In addition, three circular translucent panels (1.9 cm in diameter) were located in a vertical column below the center response key and could be transilluminated by red or green stimulus lights. The operant panel also supported an externally mounted pellet dispenser (model G5210; Ralph Gerbrands, Arlington, MA) that delivered 1-g food pellets to a food receptacle mounted on the cage beneath the operant response panel. Operation of the operant panel and pellet dispenser and data collection were accomplished with custom-written software operating on microprocessors and software purchased from MED Associates (St. Albans, VT) and located in a separate room.
Discrimination Training. Subjects were trained to discriminate cocaine (0.4 mg/kg i.m.) from saline using procedures identical to those used for our previous studies of the effects of monoamine releasers (Negus et al., 2007). Discrimination sessions consisted of multiple, 20-min cycles and were conducted 5 days/week. Each cycle consisted of a 15-min time-out period followed by a 5-min response period. During the time out, all stimulus lights were off, and responding had no scheduled consequences. During the response period, the right and left response keys were transilluminated red or green, and monkeys could receive up to 10 food pellets by responding under a fixed-ratio (FR) 30 schedule of food presentation. For four of the seven monkeys, the left key was illuminated green, and the right key was illuminated red. For the other three monkeys, the colors of the response keys were reversed. The center key was not illuminated at any time, and responding on the center key had no scheduled consequences. If all available food pellets were delivered before the end of the 5-min response period, the stimulus lights transilluminating the response keys were turned off, and responding had no scheduled consequences for the remainder of that response period.
On training days, monkeys were given an intramuscular injection of either saline or 0.40 mg/kg cocaine 5 min after the beginning of each time-out period (i.e., 10 min before the response period). After administration of saline, responding on only the green key (the saline-appropriate key) produced food, whereas after administration of 0.40 mg/kg cocaine, only responding on the red key (the drug-appropriate key) produced food. Responses on the inappropriate key reset the FR requirement. Daily sessions consisted of one to five cycles, and if the training dose of cocaine was administered, it was administered only during the last cycle.
During the response period of each cycle, three dependent variables were determined: 1) percentage of injection-appropriate responding before delivery of the first reinforcer [(injection-appropriate responses emitted before first reinforcer/total responses emitted before first reinforcer) × 100], 2) percentage of injection-appropriate responding for the entire response period [(injection-appropriate responses emitted during response period/total responses emitted during response period) × 100]; and 3) response rate (total responses emitted during response period/total time stimulus lights were illuminated).
Monkeys were considered to have acquired cocaine discrimination when the following three criteria were met for seven of eight consecutive training sessions: 1) the percentage of injection-appropriate responding before delivery of the first reinforcer was greater than or equal to 80% for all cycles, 2) the percentage of injection-appropriate responding for the entire cycle was greater than or equal to 90% for all cycles, and 3) at least one pellet was earned during all training cycles.
Discrimination Testing. Once monkeys met criterion levels of cocaine discrimination, testing began. Test sessions were identical to training sessions except that responding on either key produced food, and test compounds were administered using a time course procedure. In the time course procedure, a single dose of the test compound was administered, and 5-min response periods were scheduled to begin after 10, 30, 100, and 300 min. In some instances, an additional response period was scheduled to occur after 24 h. Benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine were each studied in a group of four monkeys. For comparison, d-amphetamine was studied in a group of three monkeys.
Test sessions were conducted only if the three criteria listed above were met during the training day immediately preceding the 1st test day. Mean data from saline and drug cycles during the training day immediately preceding the initial test day served as the control data for the subsequent test day. If responding did not meet criterion levels of discrimination performance, then training was continued until criterion levels of performance were obtained for at least 2 consecutive days.
Data Analysis. The primary dependent variables were the percentage of cocaine-appropriate responding and the response rate for each response period. For statistical analysis, saline control levels of cocaine-appropriate responding and response rates were compared with the effects of each test drug by two-factor analysis of variance, with test drug dose and time after treatment as the two factors. A significant analysis of variance was followed by individual means comparison using the Bonferroni post hoc test (GraphPad Prism 4.0c for Macintosh; GraphPad Software Inc., San Diego, CA). The criterion for significance was set at p < 0.05.
Drug Self-Administration
Apparatus. Each monkey was housed individually in a well ventilated stainless steel chamber (64 × 64 × 79 cm). The home cages of all monkeys were modified to include an operant panel and pellet dispenser identical to those described above for drug discrimination studies. In addition, a double-lumen catheter was surgically implanted into each monkey under aseptic conditions as described previously (Negus and Mello, 2003b). The intravenous catheter was protected by a tether system consisting of a custom-fitted nylon vest connected to a flexible stainless steel cable and fluid swivel (Lomir Biomedical Inc., Malone, NY). Two syringe pumps (model B5P-lE; Braintree Scientific, Inc., Braintree, MA; or model 980210, Harvard Apparatus, South Natick, MA) were mounted above each cage for delivery of saline or drug solutions through the two lumen of the intravenous catheters. One syringe pump (the self-administration pump) was used to deliver self-administered cocaine injections through one lumen of the double-lumen catheter. The second syringe pump (the treatment pump) was used for noncontingent delivery of saline or test drugs through the second lumen of the double-lumen catheter. The treatment pump delivered injections every 20 min from 10:30 AM each day until 9:30 AM the next morning for a total of 3 injections/h and 69 injections/day. No treatment injections were delivered between 9:30 AM and 10:30 AM. During this period, monkeys received their morning ration of food, and their health status was evaluated by the technical staff. Catheter patency was periodically evaluated by intravenous administration of ketamine (5 mg/kg) or the short-acting barbiturate methohexital (3 mg/kg) through the catheter lumen. The catheter was considered to be patent if intravenous administration of ketamine or methohexital produced a loss of muscle tone within 10 s. Operation of the operant panel, pellet dispenser and drug pumps and data collection were accomplished with custom-written software operating on microprocessors and software purchased from MED Associates and located in a separate room.
Training Procedure. Procedures for the evaluation of cocaine- and food-maintained responding were identical to those used in our previous studies of monoamine releasers (Negus and Mello, 2003b; Negus et al., 2007). Studies were conducted 7 days/week, and each day, there were four components of food availability and four components of drug availability. Food components began at 11:00 AM, 3:00 PM, 7:00 PM, and 6:00 AM the next morning, and drug components began at noon, 4:00 PM, 8:00 PM, and 7:00 AM the next morning. At all other times, responding had no scheduled consequences. Each food and drug component lasted 1 h or until 25 food pellets or 20 injections had been delivered, whichever occurred first. Thus, monkeys could earn a maximum of 100 food pellets/day and 80 injections/day.
Periods of food and cocaine availability were associated with different colored stimulus lights projected on the center response key of the operant response panel. Red stimulus lights signaled food availability, and green stimulus lights signaled the availability of cocaine injections (delivered in a volume of 0.1 ml in 1 s). Under the terminal schedule, the completion of a variable ratio of 16 responses on the center response key resulted in the illumination for 1 s of an appropriately colored stimulus light (red for food, green for drug) underneath the center key (VR16:S). In addition, completion of this VR response requirement a fixed ratio of two times (FR2) resulted in delivery of the available reinforcer and the initiation of a 10-s time-out period, during which the stimulus light illuminating the center response key was turned off, and responding had no scheduled consequences. This terminal second order schedule is designated as FR2(VR16:S). The two side keys were not transilluminated during sessions of food and cocaine availability, and responding on these keys had no scheduled consequences.
During initial training, responding was maintained by the delivery of 1-g food pellets during food components and by 0.032 mg/kg/injection cocaine injections during drug components. Training continued until monkeys met the following criteria for stable food and cocaine self-administration under the terminal schedule: 1) 3 consecutive days during which the number of drug injections/day differed by no more than 20% from the mean number of drug injections/day during those 3 days and there was no upward or downward trend; and 2) during the same 3 consecutive days, the mean number of both drug injections per day and food pellets per day was greater than 50.
Testing Procedures. Once cocaine- and food-maintained responding stabilized, testing began. Testing was conducted in two phases. The first phase of testing evaluated the effects of varying doses of benzylpiperazine, phenmetrazine, and 4-benzylpiperidine on cocaine- and food-maintained responding during availability of a fixed dose of 0.01 mg/kg/injection cocaine. Each dose of each drug was tested for a period of 7 consecutive days. During the 7-day test period, the unit dose of cocaine was changed from the maintenance dose of 0.032 mg/kg/injection to a test dose of 0.01 mg/kg/injection, and saline or a dose of a test drug was administered by the treatment pump through the treatment lumen of the double-lumen catheter as described above (one injection every 20 min from 10:30 AM each day until 9:30 AM the next day). A unit dose of 0.01 mg/kg/injection cocaine was used for initial studies because previous studies have demonstrated that this is the lowest dose to reliably maintain high rates of cocaine self-administration in all monkeys and because behavior maintained by this unit dose of cocaine is sensitive to the effects of pretreatment compounds (Negus and Mello, 2003b; Negus et al., 2007). The dose ranges for each test drug were as follows: benzylpiperazine (0.32–1.0 mg/kg/h), (+)phenmetrazine (0.1–0.56 mg/kg/h), and 4-benzylpiperidine (0.56–1.8 mg/kg/h). These dose ranges were empirically determined to cover a range from doses that produced little or no effect to doses that decreased rates of cocaine self-administration to less than 20% of control. The infusion rate for test drugs was identical to that used in our previous studies with amphetamine and other monoamine releasers. A similar infusion rate was employed in the present study to permit direct comparison with those previous studies (Negus and Mello, 2003b; Negus et al., 2007). At the conclusion of each test period, the maintenance dose of cocaine (0.032 mg/kg/injection) and saline control treatment were reinstated for a period of at least 4 days and until the number of reinforcers per day maintained by cocaine and food returned to baseline levels. This interval between successive treatments was designed to reduce the possibility of carryover effects from one treatment condition to the next. The effects of each test drug on cocaine- and food-maintained responding were evaluated in groups of three to four monkeys. In general, all doses of one drug were tested in a given monkey before initiation of studies with another drug. Both the sequence of drug doses and the sequence of drugs were mixed across monkeys.
Phenmetrazine produced the most selective reduction in cocaine- versus food-maintained responding during the first phase of drug self-administration studies; as a result, a second phase of studies was conducted to evaluate the effects of (+)phenmetrazine on cocaine- and food-maintained responding during availability of a range of cocaine doses. During each 7-day test period, either saline or a test unit dose of cocaine (0.0032–0.1 mg/kg/injection) was substituted for the maintenance dose of 0.032 mg/kg/injection cocaine, and either saline or a dose of (+)phenmetrazine was delivered by the treatment pump through the treatment lumen of the double-lumen catheter. The dose of (+)phenmetrazine was individually selected for each monkey as the lowest dose to reduce rates of 0.01 mg/kg/injection cocaine self-administration to less than 10% of control values during the first phase of the study (0.32 mg/kg/h in two monkeys, 0.56 mg/kg/h in one monkey). After each 7-day test, the maintenance dose of cocaine was reinstated for a period of at least 4 days and until responding recovered to baseline levels. Cocaine doses were tested in a mixed order, and the cocaine dose-effect curve during saline treatment was determined before the cocaine dose-effect curve during phenmetrazine treatment.
Phenmetrazine effects were not evaluated during availability of different magnitudes of the food reinforcer. However, in previous studies using progressive-ratio and concurrent-choice procedures (Negus, 2003; Negus et al., 2003a), we found that the reinforcing efficacy of a single food pellet was roughly equivalent to the reinforcing efficacy of a unit dose of 0.032 mg/kg/injection cocaine. As a consequence, phenmetrazine effects were evaluated on self-administration of cocaine unit doses (0.0032–0.1 mg/kg/injection) with efficacies less than, approximately equal to, and greater than the reinforcing efficacy of a single food pellet.
Data Analysis. The primary dependent variables were the total injections per day and total pellets per day delivered during the last 3 days of each 7-day test period. For statistical analysis in the first phase of the study, values for cocaine- and food-maintained responding during drug treatments were expressed as a percentage of control values for cocaine- and food-maintained responding obtained during saline treatment. Test drug effects were then analyzed by two-factor analysis of variance, with test drug dose as one factor and reinforcer type (cocaine or food) as the other factor. A significant analysis of variance was followed by individual means comparison using the Bonferroni post hoc test (GraphPad Prism 4.0c for Macintosh; GraphPad Software Inc.). The criterion for significance was set at p < 0.05.
Drugs. Cocaine HCl was obtained from the National Institute on Drug Abuse (National Institutes of Health, Bethesda, MD) and was dissolved in sterile saline. Benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine (provided by B. Blough of Research Triangle Institute, Research Triangle Park, NC) and d-amphetamine HCl (Sigma-Aldrich, St. Louis, MO) were dissolved in sterile water. All drug solutions were filter-sterilized using a 0.22-μm Millipore filter (Millipore Corporation, Billerica, MA). Doses were calculated using the salt forms of the drugs given above. All drugs were administered intramuscularly in the assay of cocaine discrimination and intravenously in the assay of cocaine self-administration.
Results
Effects of Monoamine Releasers in Monkeys Trained to Discriminate Cocaine from Saline. During the training days preceding test days, monkeys responded almost exclusively on the saline key during saline cycles (mean saline-appropriate responding ± S.E.M. = 99.98 ± 0.02) and almost exclusively on the cocaine key during cocaine training (mean percentage of cocaine-appropriate responding ± S.E.M. = 99.72 ± 0.22). Mean response rates (± S.E.M.) were 2.15 (±0.24) and 2.63 (±0.30) responses/s during saline and drug training cycles, respectively.
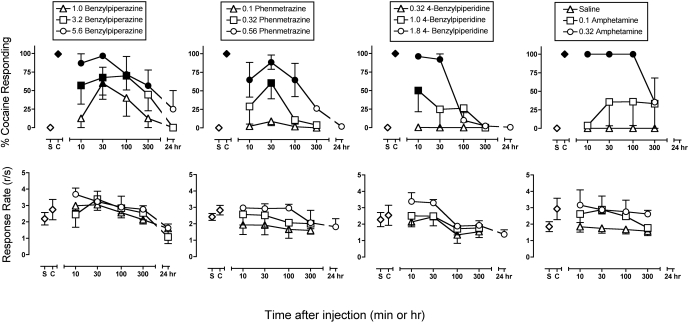
Figure 2 shows the time courses for the cocaine-like discriminative stimulus effects of benzylpiperazine, phenmetrazine, 4-benzylpiperidine, and d-amphetamine. All four drugs produced a dose- and time-dependent substitution for the training dose of 0.4 mg/kg cocaine in all monkeys. Amphetamine and phenmetrazine were roughly equipotent, and a dose of 0.32 mg/kg was the lowest dose to produce a significant increase in cocaine-appropriate responding. A higher dose of 1.0 mg/kg was required to produce a significant increase in cocaine-appropriate responding for benzylpiperazine and 4-benzylpiperidine.
Fig. 2.
Time course of the cocaine-like discriminative stimulus effects and rate-altering effects of benzylpiperazine, phenmetrazine, 4-benzylpiperidine, and amphetamine. Abscissae, time after drug administration in minutes or hours. Points over “S” and “C” show control data during saline and cocaine training cycles, respectively, on the days preceding test days. Top ordinates, percentage of cocaine-appropriate responding. Bottom ordinates, response rate in responses per second. All points show mean ± S.E.M. in three (amphetamine) or four (other drugs) monkeys. Filled points, significant difference from saline control (p < 0.05).
The drugs also differed in the time courses of their discriminative stimulus effects. Both 4-benzylpiperidine and amphetamine had rapid onsets of action and produced peak effects within 10 min, whereas benzylpiperazine and phenmetrazine had slower onsets of action and typically did not produce peak effects until 30 min after their administration. Benzylpiperazine phenmetrazine and amphetamine all had comparably long durations of action, with significant effects sustained for 100 to 300 min. In contrast, 4-benzylpiperidine had a shorter duration of action, with significant effects sustained for only 10 to 30 min.
Across the dose ranges tested, none of the drugs significantly altered response rates. There was a tendency for the highest doses of each drug to increase response rates in some monkeys, but this effect was not observed in all monkeys and did not achieve statistical significance.
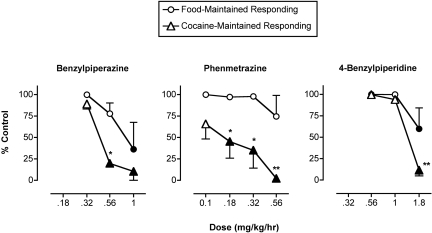
Effects of Monoamine Releasers on Cocaine- and Food-Maintained Responding during Availability of 0.01 mg/kg/Injection Cocaine. Figure 3 shows mean data from the last 3 days of 7-day treatments with benzylpiperazine, phenmetrazine, and 4-benzylpiperidine on responding maintained by 0.01 mg/kg/injection cocaine and food. Control levels of cocaine- and food-maintained responding during saline treatment were 79.7 ± 0.3 cocaine injections/day (of a maximum of 80 injections/day) and 100 ± 0 food pellets/day (of a maximum of 100 pellets/day). All three monoamine releasers produced a dose-dependent decrease in cocaine self-administration. However, phenmetrazine was the most potent of the three monoamine releasers, and it produced the most selective reductions in cocaine- versus food-maintained responding. Specifically, phenmetrazine at doses of 0.18 to 0.56 mg/kg/h significantly decreased cocaine self-administration but not food-maintained responding, and across this same dose range, cocaine self-administration was decreased more than food-maintained responding. There were individual differences in the sensitivity of individual monkeys to phenmetrazine effects (see Table 2); however, in each monkey, at least one dose of phenmetrazine reduced cocaine self-administration by more than 90%, whereas it had little or no effect on food-maintained responding.
Fig. 3.
Effects of treatment with benzylpiperazine, phenmetrazine, and 4-benzylpiperidine on responding maintained by 0.01 mg/kg/injection cocaine and food. Abscissae, dose of test drug in milligrams per kilogram per hour, log scale. Ordinates, percentage control levels of cocaine- and food-maintained responding. All points show mean ± S.E.M. in four (phenmetrazine) or three (other drugs) monkeys from the last 3 days of each 7-day treatment. Filled points indicate a significant difference from control levels of cocaine- or food-maintained responding (p < 0.05). Asterisks, significant difference between levels of cocaine- and food-maintained responding at that dose of the test drug (*, p < 0.05; **, p < 0.01).
TABLE 2.
Effects of phenmetrazine (0.1–0.56 mg/kg/h) on responding maintained by 0.01 mg/kg/injection cocaine (Coc) and food (Fd) in individual monkeys Values show the percentage control rates of cocaine- and food-maintained responding during the last 3 days of 7-day treatment with each phenmetrazine dose.
|
Monkey Identification Number
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
21714
|
96D278
|
92L
|
95C291
|
|||||
| Dose | Coc | Fd | Coc | Fd | Coc | Fd | Coc | Fd |
| 0.1 | 16.9 | 100 | 71.6 | 100 | 74.1 | 100 | 100 | 100 |
| 0.18 | 17.8 | 92.0 | 17.5 | 100 | 45.4 | 96.7 | 100 | 100 |
| 0.32 | 0 | 91.7 | 0 | 100 | 57.5 | 100 | 82.5 | 100 |
| 0.56 | 0 | 2.0 | 0.4 | 100 | 0.9 | 100 | 7.1 | 100 |
Benzylpiperazine significantly decreased cocaine self-administration at doses of 0.56 to 1.0 mg/kg/h, but the higher dose of 1.0 mg/kg/h also significantly decreased food-maintained responding. Only a dose of 0.56 mg/kg/h benzylpiperazine reduced cocaine self-administration more than food-maintained responding. 4-Benzylpiperidine significantly reduced both cocaine- and food-maintained responding at a dose of 1.8 mg/kg/h; however, cocaine self-administration was reduced significantly more than food-maintained responding.
Overt behavioral effects of monoamine releaser treatments were not formally monitored; however, the highest doses of benzylpiperazine (1.0 mg/kg/h), phenmetrazine (0.56 mg/kg/h), and 4-benzylpiperidine (1.8 mg/kg/h) increased activity expressed either as increased whole-body locomotion (e.g., circling in cage) or increased hand/arm movements (e.g., grooming or manipulation of cage features). In one monkey, an incident of self-biting occurred on day 6 of treatment with 4-benzylpiperidine; as a result, this treatment was terminated 1 day early in this monkey.
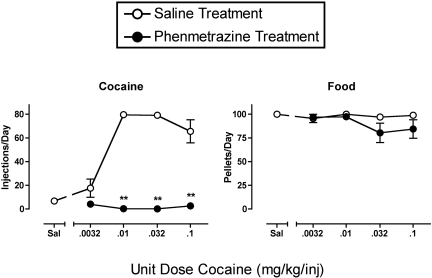
Effects of Phenmetrazine on the Cocaine Self-Administration Dose-Effect Curve. Figure 4 shows mean data from the last 3 days of 7-day treatment with either saline or phenmetrazine on responding maintained by food and a range of cocaine doses. During saline treatment, the cocaine self-administration dose-effect curve displayed the usual inverted-U shape. A low dose of 0.0032 mg/kg/injection cocaine maintained low self-administration rates similar to those maintained by saline delivery. Doses of 0.01 and 0.032 mg/kg/injection maintained peak rates of cocaine self-administration, and self-administration declined slightly during availability of a higher unit dose of 0.1 mg/kg/injection cocaine. Rates of food-maintained responding were similar during availability of saline and all unit doses of cocaine.
Fig. 4.
Effects of treatment with phenmetrazine on the cocaine dose-effect curve. Abscissae, unit dose of cocaine in milligrams per kilogram per injection available during drug components of each daily session, log scale. Points above “Sal” show data obtained when saline was the solution available for self-administration. Left ordinate, number of injections per day (maximum = 80). Right ordinate, number of food pellets per day (maximum = 100). All points show mean ± S.E.M. in three monkeys from the last 3 days of each 7-day treatment. Asterisks, significant difference between saline and phenmetrazine treatment conditions at a given unit dose of cocaine (**, p < 0.01). The phenmetrazine dose was individually selected for each monkey as the lowest dose to decrease rates of 0.01 mg/kg/injection cocaine self-administration by >90%. This dose was 0.32 mg/kg/h in two monkeys (21714 and 96D278) and 0.56 mg/kg/h in one monkey (92L).
Phenmetrazine treatment virtually eliminated cocaine self-administration across a 30-fold range of cocaine doses from 0.0032 to 0.1 mg/kg/injection. These phenmetrazine-induced reductions in cocaine self-administration were statistically significant at cocaine unit doses of 0.01 to 0.1 mg/kg/injection. Phenmetrazine treatment had little or no effect on food-maintained responding.
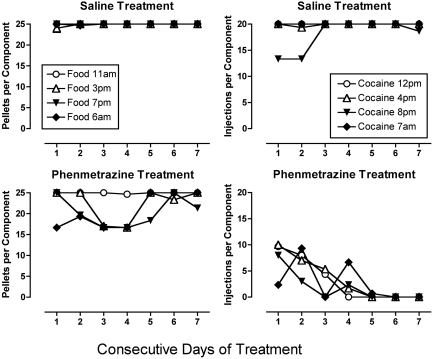
Figure 5 shows rates of cocaine- and food-maintained responding during each day of saline or phenmetrazine treatment when unit doses of 0.01, 0.032, and 0.1 mg/kg/injection cocaine were available. During saline treatment, monkeys usually responded at stable rates for ≥60 injections/day and >75 food pellets/day throughout the 7-day test period. Treatment with phenmetrazine produced a gradual and virtually complete elimination of cocaine self-administration during the first 1 to 4 days, and this reduction in cocaine self-administration was sustained for the remainder of the 7-day treatment period in all monkeys. Phenmetrazine also decreased food-maintained responding in some monkeys, and these reductions in food-maintained responding were usually greatest on days 3 to 5 of treatment. However, reductions in food-maintained responding dissipated during the last days of treatment, and responding for food recovered to baseline levels by the end of the 7-day treatment.
Fig. 5.
Time course of phenmetrazine effects on responding maintained by food and cocaine (0.01–0.1 mg/kg/injection). Data obtained during availability of 0.01, 0.032, and 0.1 mg/kg/injection cocaine are shown in the top, middle, and bottom rows of graphs, respectively. Data obtained during saline treatment are shown in the left column, and data obtained during phenmetrazine treatment are shown on the right. Abscissae, consecutive days of treatment. All treatments were implemented for a period of 7 days. The shaded region in the middle right shows cocaine- and food-maintained responding during the 7 days after termination of phenmetrazine treatment (post-treatment days 1–7, P1–P7). Left ordinates, number of injections per day (maximum = 80). Right ordinates, number of food pellets per day (maximum = 100). All points show mean ± S.E.M. in three monkeys.
Figure 5 also shows recovery of responding for 0.032 mg/kg/injection cocaine after the termination of phenmetrazine treatment. Cocaine self-administration gradually recovered to control levels in all monkeys after 7 days. Food-maintained responding was disrupted in one monkey on the 2nd day after termination of phenmetrazine treatment, but in general, termination of phenmetrazine treatment had little effect on responding for food. Note that recovery data are presented only from phenmetrazine effects during availability of 0.032 mg/kg/injection cocaine because this was the maintenance cocaine dose that was reinstated at the conclusion of each treatment.
Figure 6 shows a more detailed view of effects produced by saline and phenmetrazine treatment on responding for food and 0.01 mg/kg/injection cocaine. Specifically, data are shown for each of the daily food and drug components. During saline treatment, monkeys usually responded for the maximum number of food pellets and cocaine injections during each component. The only exception was that one monkey did not self-administer cocaine during the 8:00 PM session on the first 2 days of 0.01 mg/kg/injection cocaine availability. During phenmetrazine treatment, food-maintained responding was not affected in any monkey during the 11:00 AM component, and transient decreases were observed in one monkey during the other three components. In contrast, cocaine self-administration was eliminated in all monkeys during all components by day 5 of phenmetrazine treatment. There was no clear diurnal trend in the gradual phenmetrazine-induced decrease in cocaine self-administration during the first 4 days of treatment.
Fig. 6.
Diurnal pattern of effects produced by saline and phenmetrazine treatment on responding maintained by food and cocaine (0.01 mg/kg/injection). Abscissae, consecutive days of treatment with saline (top) or phenmetrazine (bottom). Left ordinates, number of food pellets delivered during each of the four daily food components (maximum = 25/component). Right ordinates, number of cocaine injections delivered during each of the four daily drug components (maximum = 20/component). All points show mean data from three monkeys. Error bars are omitted for clarity.
Discussion
The main finding of this study was that phenmetrazine produced a dose-dependent, sustained, and relatively selective reduction in cocaine- versus food-maintained responding in rhesus monkeys. The other monoamine releasers benzylpiperazine and 4-benzylpiperidine also produced dose-dependent, sustained, and selective decreases in cocaine self-administration, although these compounds displayed a lower degree of behavioral selectivity across a narrower range of doses than phenmetrazine. Overall, these results support the more general finding that monoamine releasers with modest biochemical selectivity to release dopamine versus serotonin may also display relatively high behavioral selectivity to decrease cocaine- versus food-maintained responding (Negus and Mello, 2003b; Negus et al., 2007). The relatively high efficacy and behavioral selectivity of these compounds to decrease cocaine self-administration suggests that they may warrant further consideration either as candidate medications in their own right or as lead compounds for the development of new drugs (e.g., with slower onset to attenuate abuse liability).
Cocaine-Like Discriminative Stimulus Effects of Monoamine Releasers. All the monoamine releasers examined in this study produced full substitution for cocaine in the drug discrimination assay. There were marginal differences in their relative potencies and time courses, but their shared ability to produce cocaine-like discriminative stimulus effects is consistent with their classification as candidate agonist medications for cocaine dependence.
These findings also agree with previous reports that benzylpiperazine and phenmetrazine produced cocaine-like stimulant effects in both laboratory animals and humans. Regarding benzylpiperazine, both cocaine and benzylpiperazine substituted for the discriminative stimulus effects of bupropion in rats (Jones et al., 1980), and like cocaine, benzylpiperazine produced amphetamine-like discriminative stimulus effects and maintained drug self-administration in rhesus monkeys (Fantegrossi et al., 2005). In humans, benzylpiperazine has emerged as a drug of abuse used either alone or with the serotonergic drug l-(m-trifluoromethylphenyl)piperazine to mimic the neurochemical and behavioral effects of methylenedioxymethamphetamine (Ecstasy) (Baumann et al., 2005; Fantegrossi et al., 2005). Benzylpiperazine is currently a schedule I drug in the United States. Phenmetrazine is a schedule II stimulant that was once approved as an appetite suppressant, but its use was discontinued due to high abuse potential (Silverstone, 1992). Phenmetrazine substituted for the discriminative stimulus effects of cocaine in rats and amphetamine in monkeys, maintained self-administration in monkeys and humans, and produced prototypical stimulant-like subjective effects in humans (Griffiths et al., 1976; Chait et al., 1987; de la Garza and Johanson, 1987; Wood and Emmett-Oglesby, 1988). To our knowledge, this is the first report of the cocaine-like effects of 4-benzylpiperidine; however, its robust cocaine-like discriminative stimulus effects and rapid onset of action suggest that it too would have high abuse liability in humans.
These findings are also consistent with our previous evaluation of the cocaine-like discriminative stimulus effects of monoamine releasers (Negus et al., 2007). In that study, full substitution for cocaine was obtained with releasers that displayed ≥30-fold selectivity in vitro for promoting release of dopamine versus serotonin, and a reduction in maximal levels of cocaine-appropriate responding was observed at dopamine versus serotonin selectivity levels ≤6.5.
Effects of Monoamine Releasers on Cocaine- and Food-Maintained Responding. The present results are consistent with our previous findings to suggest that monoamine releasers can produce dose-dependent, sustained, and selective decreases in cocaine self-administration (Negus et al., 2007). In our previous study, the most selective reductions in cocaine- versus food-maintained responding were achieved with methamphetamine, which had a 30-fold selectivity for promoting dopamine versus serotonin release in the rat brain synaptosome assay. The present study examined a series of compounds with similar pharmacological selectivities for dopamine versus serotonin release, and all three releasers produced selective reductions in cocaine versus food-maintained responding. In particular, the behavioral selectivity of phenmetrazine was similar to that of methamphetamine, and treatment with appropriate doses of either drug nearly eliminated self-administration of 0.01 mg/kg/injection cocaine but had little or no effect on concurrent food-maintained responding. Moreover, in the present study, phenmetrazine nearly eliminated self-administration of two higher cocaine doses (0.032 and 0.1 mg/kg/injection) without increasing low rates of responding maintained by a low cocaine dose (0.0032 mg/kg/injection). Thus, reductions in high-dose cocaine self-administration reflected a robust downward shift in the cocaine self-administration dose-effect curve while having much smaller and more transient effects on food-maintained responding.
These results with phenmetrazine and related monoamine releasers contrast sharply with effects produced in this same procedure by other candidate medications from other drug classes, including serotonin-selective releasers (Negus et al., 2007), monoamine uptake inhibitors with varying degrees of selectivities for dopamine and serotonin transporters (Negus et al., 1999, 2009), dopamine receptor antagonists (Negus et al., 1996), δ and κ opioid receptor agonists (Negus et al., 1997; Mello and Negus, 1998; Pereira-Do Carmo et al., 2006), selective and high-efficacy μ opioid receptor agonists (Negus and Mello, 2002), and antagonists for corticotropin releasing factor-1 receptors (Mello et al., 2006). We have argued previously that optimal medications for the treatment of cocaine dependence might be those that produce sustained and selective decreases in cocaine self-administration across a broad range of cocaine doses (Mello and Negus, 1996). Overall, our results suggest that monoamine releasers with modest biochemical selectivity to release dopamine versus serotonin may produce this profile of effects more reliably than many other classes of candidate medications.
Pharmacological Mechanisms of Monoamine Releaser Effects. In the present study and our previous study (Negus et al., 2007), monoamine releasers were selected for evaluation in rhesus monkeys based on their pharmacological selectivity to promote release of dopamine versus serotonin in an in vitro assay of monoamine release from rat brain synaptosomes (Rothman et al., 2001, 2002b, 2005; Wee et al., 2005). Our results provide preliminary support for the hypothesis that behavioral selectivity to reduce cocaine- versus food-maintained responding is influenced by biochemical selectivity to release dopamine versus serotonin. To be more specific, optimal behavioral selectivity has been associated with a 30- to 40-fold biochemical selectivity to release dopamine versus serotonin. However, a causal relationship between biochemical and behavioral selectivity is not yet possible for at least three reasons.
First, it is not clear what magnitudes of biochemical selectivity can be reliably differentiated for correlation with behavioral results. In vitro potencies to release individual monoamines are associated with some degree of variability (S.E.M. values, ∼10% of mean values; see Table 1), and this variability is compounded in the ratios that are used to specify selectivity. As a result, relatively small differences in calculated selectivity values (e.g., the differences in selectivities of the three releasers evaluated in the present study) may not be reliable or statistically significant. Second, data on selectivity for monoamine release were collected using rodent tissue tested under a specified set of experimental conditions, and it is not clear whether these selectivity values would be preserved under other in vitro assay conditions, let alone under conditions of in vivo testing in another species. Finally, biochemical selectivity to release dopamine versus serotonin reflects only one dimension of pharmacological action along which these compounds may differ, and other drug actions on other targets may also influence behavioral selectivity to reduce cocaine- versus food-maintained responding. Overall, then, the present results provide only preliminary evidence to support the hypothesis that biochemical selectivity to release dopamine versus serotonin might function as a determinant of behavioral selectivity to reduce cocaine- versus food-maintained responding. However, these results do provide an empirical rationale for further studies to examine this hypothesis in greater detail.
Implications for Clinical Development of Monoamine Releasers as Agonist Medications. The present results contribute to a growing literature supporting the proposition that monoamine releasers may be useful as agonist medications for treatment of cocaine abuse and dependence. The most extensive body of data exists for amphetamine. A double-blind, randomized clinical trial found that amphetamine maintenance reduced cocaine use with few undesirable effects (Grabowski et al., 2001). Similar results have been obtained in other clinical trials with less rigorous experimental designs (for review, see Grabowski et al., 2004). These clinical findings agree with preclinical results. For example, chronic amphetamine treatment produced selective and sustained reductions in cocaine self-administration in rats (Peltier et al., 1996) and rhesus monkeys (Negus, 2003; Negus and Mello, 2003a,b). In addition, a recent human laboratory study found that amphetamine maintenance for 3 to 5 days reduced the subjective effects of intranasal cocaine (Rush et al., 2009). Although the abuse liability of amphetamine may limit its potential for drug abuse treatment, novel formulations, such as the prodrug l-lysine-d-amphetamine, may serve to slow the release and reduce the abuse liability of amphetamine (Jasinski and Krishnan, 2006).
Like amphetamine, phenmetrazine has a history of use as an anorectic agent; however, its use was discontinued due to high abuse liability, and it has not been used for other clinical applications, such as narcolepsy or attention deficit hyperactivity disorder (Silverstone, 1992). For these reasons, phenmetrazine itself may be difficult to rehabilitate for use as a medication for treatment of cocaine abuse and dependence. However, a promising alternative might be phendimetrazine, a schedule III anorectic that is metabolized by hepatic enzymes to phenmetrazine in humans (Rothman et al., 2002b). This requirement for hepatic metabolism may not only slow the onset of stimulant effects after oral administration but also retard the ability of phendimetrazine to produce stimulant effects after more problematic routes of parenteral administration. For example, intragastric phendimetrazine substituted for the discriminative stimulus effects of intragastric amphetamine in rhesus monkeys, but intravenous phendimetrazine failed to increase striatal dopamine levels in rats and failed to maintain self-administration in rhesus monkeys (Corwin et al., 1987; de la Garza and Johanson, 1987; Rothman et al., 2002b). Taken together, these results suggest that phendimetrazine may serve as a prodrug with lower abuse liability than phenmetrazine (Rothman et al., 2002b). As a result, phendimetrazine may be viable as a candidate medication.
Acknowledgments
We thank Peter A. Fivel, Cara Sylvester, Annie Mills, and Melissa Timm for outstanding technical support.
This work was supported in part by the Intramural Research program of the National Institutes of Health [National Institute on Drug Abuse]; and by the National Institutes of Health [Grants R01-DA02519, R01-DA12970, P01-DA14528, K05-DA00101].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.143701.
ABBREVIATIONS: FR, fixed ratio; VR, variable ratio.
References
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, and Rothman RB (2000) Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse 36 102-113. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Budzynski AG, Partilla JS, Blough BE, and Rothman RB (2005) N-Substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or “Ecstasy”). Neuropsychopharmacology 30 550-560. [DOI] [PubMed] [Google Scholar]
- Baumann MH and Rothman RB (1998) Alterations in serotonergic responsiveness during cocaine withdrawal in rats: similarities to major depression in humans. Biol Psychiatry 44 578-591. [DOI] [PubMed] [Google Scholar]
- Chait LD, Uhlenhuth EH, and Johanson CE (1987) Reinforcing and subjective effects of several anorectics in normal human volunteers. J Pharmacol Exp Ther 242 777-783. [PubMed] [Google Scholar]
- Corwin RL, Woolverton WL, Schuster CR, and Johanson CE (1987) Anorectics: effects on food intake and self-administration in rhesus monkeys. Alcohol Drug Res 7 351-361. [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, and Howell LL (2002) Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 300 831-837. [DOI] [PubMed] [Google Scholar]
- de la Garza R and Johanson CE (1987) Discriminative stimulus properties of intragastrically administered d-amphetamine and pentobarbital in rhesus monkeys. J Pharmacol Exp Ther 243 955-962. [PubMed] [Google Scholar]
- Do Carmo GP, Mello NK, Rice KC, Folk JE, and Negus SS (2006) Effects of the selective delta opioid agonist SNC80 on cocaine- and food-maintained responding in rhesus monkeys. Eur J Pharmacol 547 92-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Winger G, Woods JH, Woolverton WL, and Coop A (2005) Reinforcing and discriminative stimulus effects of 1-benzylpiperazine and trifluoromethylphenylpiperazine in rhesus monkeys. Drug Alcohol Depend 77 161-168. [DOI] [PubMed] [Google Scholar]
- Gold LH, Geyer MA, and Koob GF (1989) Neurochemical mechanisms involved in behavioral effects of amphetamines and related designer drugs. NIDA Res Monogr 94 101-126. [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, and Moeller FG (2001) Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol 21 522-526. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, and Negus SS (2004) Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29 1439-1464. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Winger G, Brady JV, and Snell JD (1976) Comparison of behavior maintained by infusions of eight phenylethylamines in baboons. Psychopharmacology (Berl) 50 251-258. [DOI] [PubMed] [Google Scholar]
- Haney M and Spealman R (2008) Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 199 403-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B (2001) Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists, in The Pharmacological Basis of Therapeutics (Hardman JG and Limbird LE eds) pp 215-268, McGraw-Hill, New York.
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Jasinski D and Krishnan S (2008) Human pharmacology of intravenous lisdexamfetamine dimesylate: abuse liability in adult stimulant abusers. J Psychopharmacol doi: 10.1177/0269881108093841. [DOI] [PubMed]
- Jones CN, Howard JL, and McBennett ST (1980) Stimulus properties of antidepressants in the rat. Psychopharmacology (Berl) 67 111-118. [DOI] [PubMed] [Google Scholar]
- Mello NK and Negus SS (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14 375-424. [DOI] [PubMed] [Google Scholar]
- Mello NK and Negus SS (1998) Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J Pharmacol Exp Ther 286 812-824. [PubMed] [Google Scholar]
- Mello NK, Negus SS, Rice KC, and Mendelson JH (2006) Effects of the CRF1 antagonist antalarmin on cocaine self-administration and discrimination in rhesus monkeys. Pharmacol Biochem Behav 85 744-751. [DOI] [PubMed] [Google Scholar]
- Negus SS (2003) Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupentixol. Neuropsychopharmacology 28 919-931. [DOI] [PubMed] [Google Scholar]
- Negus SS, Brandt MR, and Mello NK (1999) Effects of the long-acting monoamine reuptake inhibitor indatraline on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther 291 60-69. [PubMed] [Google Scholar]
- Negus SS and Mello NK (2002) Effects of mu-opioid agonists on cocaine- and food-maintained responding and cocaine discrimination in rhesus monkeys: role of mu-agonist efficacy. J Pharmacol Exp Ther 300 1111-1121. [DOI] [PubMed] [Google Scholar]
- Negus SS and Mello NK (2003a) Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 167 324-332. [DOI] [PubMed] [Google Scholar]
- Negus SS and Mello NK (2003b) Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend 70 39-52. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, and Rothman RB (2007) Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther 320 627-636. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Kimmel HL, Howell LL, and Carroll FI (2009) Effects of the monoamine uptake inhibitors RTI-112 and RTI-113 on cocaine- and food-maintained responding in rhesus monkeys. Pharmacol Biochem Behav 91 333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Lamas X, and Mendelson JH (1996) Acute and chronic effects of flupentixol on the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther 278 879-890. [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, and Lin CE (1997) Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther 282 44-55. [PubMed] [Google Scholar]
- Peltier RL, Li DH, Lytle D, Taylor CM, and Emmett-Oglesby MW (1996) Chronic d-amphetamine or methamphetamine produces cross-tolerance to the discriminative and reinforcing stimulus effects of cocaine. J Pharmacol Exp Ther 277 212-218. [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, and Partilla JS (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39 32-41. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, and Baumann MH (2002a) Appetite suppressants as agonist substitution therapies for stimulant dependence. Ann N Y Acad Sci 965 109-126. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, and Baumann MH (2007) Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. AAPS J 9 E1-E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, Roth BL, and Baumann MH (2005) Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther 313 1361-1369. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Katsnelson M, Vu N, Partilla JS, Dersch CM, Blough BE, and Baumann MH (2002b) Interaction of the anorectic medication, phendimetrazine, and its metabolites with monoamine transporters in rat brain. Eur J Pharmacol 447 51-57. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, and Hays LR (2009) Cocaine effects during d-amphetamine maintenance: a human laboratory analysis of safety, tolerability and efficacy. Drug Alcohol Depend 99 261-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone T (1992) Appetite suppressants: a review. Drugs 43 820-836. [DOI] [PubMed] [Google Scholar]
- Vocci FJ and Elkashef A (2005) Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry 18 265-270. [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, and Woolverton WL (2005) Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther 313 848-854. [DOI] [PubMed] [Google Scholar]
- Wood DM and Emmett-Oglesby MW (1988) Substitution and cross-tolerance profiles of anorectic drugs in rats trained to detect the discriminative stimulus properties of cocaine. Psychopharmacology (Berl) 95 364-368. [DOI] [PubMed] [Google Scholar]