Abstract
Repeated, high-dose methamphetamine (METH) administrations cause persistent dopaminergic deficits in rodents, nonhuman primates, and humans. In rats, this treatment also causes the formation of high-molecular mass (greater than approximately 120 kDa) dopamine transporter (DAT)-associated complexes, the loss of DAT monomer immunoreactivity, and a decrease in DAT function, as assessed in striatal synaptosomes prepared 24 h after METH treatment. The present study extends these findings by demonstrating the regional selectivity of DAT complex formation and monomer loss because these changes in DAT immunoreactivity were not observed in the nucleus accumbens. Furthermore, DAT complex formation was not a consequence limited to METH treatment because it was also caused by intrastriatal administration of 6-hydroxydopamine. Pretreatment with the D2 receptor antagonist, eticlopride [S-(-)-3-chloro-5-ethyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-6-hydroxy-2-methoxybenzamide hydrochloride], but not the D1 receptor antagonist, SCH23390 [R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride], attenuated METH-induced DAT complex formation. Eticlopride pretreatment also attenuated METH-induced DAT monomer loss and decreases in DAT function; however, the attenuation was much less pronounced than the effect on DAT complex formation. Finally, results also revealed a negative correlation between METH-induced DAT complex formation and DAT activity. Taken together, these data further elucidate the underlying mechanisms and the functional consequences of repeated administrations of METH on the DAT protein. Furthermore, these data suggest a multifaceted role for D2 receptors in mediating METH-induced alterations of the DAT and its function.
Repeated, high-dose methamphetamine (METH) administrations cause persistent striatal dopaminergic deficits (for review, see Gibb et al., 1994; Brown and Yamamoto, 2003). Although mechanisms contributing to this phenomenon remain to be elucidated fully, dopamine (DA) (Wagner et al., 1983; Schmidt et al., 1985), the DA transporter (DAT) (Schmidt and Gibb, 1985; Fumagalli et al., 1998), hyperthermia (Bowyer et al., 1992), glutamate (Sonsalla et al., 1989; Mark et al., 2004), microglial activation (LaVoie et al., 2004; Thomas et al., 2004), and reactive species (Giovanni et al., 1995; Yamamoto and Zhu, 1998; Gluck et al., 2001; Imam et al., 2001) are among the contributing factors. Repeated, high-dose METH injections also rapidly (within 1 h after the final METH administration) decrease plasmalemmal DA uptake, as assessed in striatal synaptosomes prepared from treated rats. As with METH-induced striatal dopaminergic deficits, DA, reactive species, and hyperthermia contribute to this rapid decrease in DAT function (for review, see Fleckenstein et al., 2000).
In addition to effects described above, repeated, high-dose METH administrations also cause the formation of high molecular mass (greater than approximately 120 kDa) DAT-associated complexes, as assessed 24 h after treatment. This phenomenon is attenuated by either prevention of METH-induced hyperthermia or pretreatment with the DA synthesis inhibitor, α-methyl-p-tyrosine (α-MT) (Baucum et al., 2004). Reactive species also are implicated in DAT complex formation because in vitro exposure to the reducing agent, β-mercaptoethanol, reverses this process (Baucum et al., 2004). Concurrent with METH-induced DAT complex formation, METH treatment causes a loss of DAT monomer immunoreactivity and a decrease in DAT function (Baucum et al., 2004). However, mechanisms underlying these phenomena and their relationship to DAT complex formation remained to be elucidated. Furthermore, the regional specificity of DAT complex formation remained unknown. In accordance, the present study addresses these issues. Results revealed regional specificity in DAT complex formation and that this phenomenon was not exclusive to METH treatment. Furthermore, the data demonstrated a negative correlation between METH-induced complex formation and loss of DAT function. Finally, these studies, taken together with previous reports, suggest a multifaceted role for D2 receptors in affecting METH-induced alterations in the DAT and its function.
Materials and Methods
Animals. Male Sprague-Dawley rats (290-400 g; Charles River Laboratories, Inc., Raleigh, NC) were maintained under controlled lighting and temperature conditions, with food and water provided ad libitum. Rats were housed three animals per cage during treatment. METH-treated rats were maintained at warmer temperatures to assure METH-induced hyperthermia. Temperatures were assessed at 1-h intervals beginning 30 min before the first saline or METH administration, and mean temperatures after the first saline or METH administration over the course of treatment were determined for all groups. Rats were sacrificed by decapitation. All procedures were conducted in accordance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the University of Utah Institutional Animal Care and Use Committee.
Drugs and Chemicals. (±)METH hydrochloride was supplied by the Research Triangle Institute (Research Triangle Park, NC). Eticlopride hydrochloride, SCH23390 hydrochloride, and 6-hydroxydopamine (6-OHDA) hydrobromide were purchased from Sigma-Aldrich (St. Louis, MO). Drugs were dissolved in 0.9% saline vehicle. Drug doses were calculated as the free base.
6-OHDA Treatment. Rats were anesthetized with equithesin (3 ml/kg i.p.) and fixed on a stereotaxic frame (David Kopf Instruments, Tujunga, CA) with an incisor bar setting of -1.0 mm. The skull was exposed, and a burr hole was drilled through the skull to introduce a needle for the injection. Unilateral injection of either 6-OHDA (16 μg in 2.8 μl in 0.9% NaCl containing 0.02% ascorbic acid) or vehicle (0.9% NaCl containing 0.02% ascorbic acid) were injected (0.25 μl/min) into the striatum in opposite hemispheres at coordinates 1.0 mm anterior and ±3.0 mm lateral of the midline relative to bregma and 6.0 mm ventral to dura. The needle was left in place for 3 min after infusion and then slowly removed. Animals were sacrificed 24 h after lesion.
Tissue Preparation. DAT complex formation was assessed in synaptosomes as described previously (Baucum et al., 2004). In brief, striatal or nucleus accumbens tissues were homogenized in ice-cold 0.32 M sucrose, pH 7.4, and centrifuged (800g, 12 min; 4°C). The supernatants were then centrifuged (22,000g, 15 min; 4°C), and the resulting pellets were resuspended in ice-cold double-distilled H2O at concentrations of 45 to 100 mg/ml. In some studies, tissue aliquots were added to modified Krebs' assay buffer (126 mM NaCl, 4.8 mM KCl, 1.3 mM CaCl2, 16 mM sodium phosphate, 1.4 mM MgSO4, 11 mM glucose, and 1 mM ascorbic acid, pH 7.4) and retained to assess [3H]DA uptake. The remaining samples were then combined with nonreducing loading buffer (final concentration, 2.25% SDS, 18% glycerol, 180 mM Tris base, pH 6.8, and bromphenol blue) and subjected to Western blot analysis.
Western Blot Analysis. Equal quantities of protein (11-45 μg) were loaded into each well of a 10% or 4 to 16% SDS-polyacrylamide gel electrophoresis or a 4 to 12% NuPAGE Novex Bis-Tris Midi gradient gel (Invitrogen, Carlsbad, CA) and electrophoresed using a Hoefer SE 660 gel apparatus (Amersham Biosciences, Chalfont St. Giles, UK) or XCell4 Surelock Midi-cell (Invitrogen). For data presented in Fig. 1, three times more nucleus accumbens tissue than striatal tissue was subjected to SDS-polyacrylamide gel electrophoresis to have approximately equal levels of DAT monomer immunoreactivity in the saline control lanes. For data presented in Fig. 2, equal volumes of sample were loaded into each well. Samples were then transferred overnight to a polyvinylidene difluoride hybridization transfer membrane (PerkinElmer Life and Analytical Sciences, Waltham, MA). Each membrane was blocked for 30 min with Starting Block Blocking Buffer (Pierce Chemical, Rockford, IL). The membrane was then incubated for 1 h at room temperature or overnight at 4°C with a N-terminal DAT antibody (a generous gift from Dr. Roxanne Vaughan, University of North Dakota; Freed et al., 1995). The polyvinylidene difluoride membrane was then washed five times in Tris-buffered saline with Tween (250 mM NaCl, 50 mM Tris, pH 7.4, and 0.05% Tween 20). The membranes were then incubated for 1 h with a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (BioSource International, Camarillo, CA). After five washes in Tris-buffered saline with Tween, the bands were visualized using Western Lightning Chemiluminescence Reagents Plus (PerkinElmer Life and Analytical Sciences) and were quantified by densitometry using a FluorChem SP Imaging System (Alpha Innotech, San Leandro, CA). DAT immunoreactivity with an apparent molecular mass greater than approximately 120 kDa was defined as DAT complexes. Protein concentrations were determined using the method of Lowry et al. (1951).
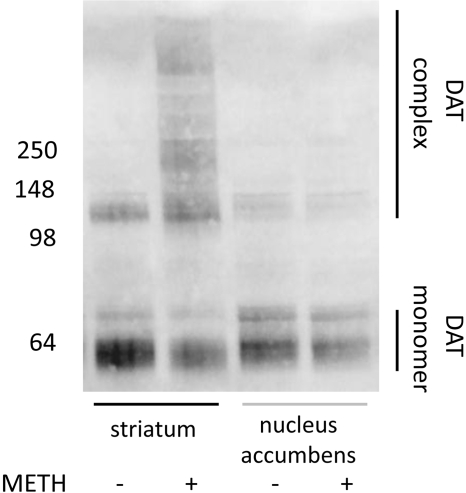
Fig. 1.
METH-induced DAT complexes form in the striatum and not in the nucleus accumbens. Rats received four injections of METH (+; 7.5 mg/kg/injection s.c.; 2-h intervals) or saline vehicle (-; 1 ml/kg/injection s.c.; 2-h intervals) and were sacrificed 24 h later. Molecular masses (kilodaltons) are indicated adjacent to a representative blot.
Fig. 2.
Intrastriatal 6-OHDA infusion causes DAT complex formation. Rats received unilateral intrastriatal injections in opposite hemispheres of either 6-OHDA (+; 16 μg in 2.8 μl of 0.9% NaCl containing 0.02% ascorbic acid) or vehicle (-; 0.9% NaCl containing 0.02% ascorbic acid) and were sacrificed 24 h later. Representative data obtained from two individual rats are shown. Molecular masses (kilodaltons) are indicated adjacent to a representative blot.
Synaptosomal [3H]DA Uptake. [3H]DA uptake was evaluated by incubating retained striatal synaptosomes (as described above) at 37°C for 10 min in assay buffer (126 mM NaCl, 4.8 mM KCl, 1.3 mM CaCl2, 16 mM sodium phosphate, 1.4 mM MgSO4, 11 mM glucose, and 1 mM ascorbic acid, pH 7.4) and 1 μM pargyline. Nonspecific values were determined in the presence of 50 μM cocaine. Samples were incubated for 10 min, and the assays were initiated by the addition of [3H]DA (0.5 nM final concentration). After incubation for 3 min, samples were placed on ice to stop the reaction. Samples were then filtered through GF/B filters (Whatman, Clifton, NJ), soaked previously in 0.05% polyethylenimine. Filters were rapidly washed three times with 3 ml of ice-cold 0.32M sucrose using a filtering manifold (Brandel Inc., Gaithersburg, MD). Radioactivity trapped in filters was counted using a liquid scintillation counter. Protein concentrations were determined using the method of Lowry et al. (1951).
Data Analysis. Statistical analyses among multigroup data were conducted using one-way analysis of variance, followed by a Newman-Keuls post hoc test. Differences among groups were considered significant if the probability of error was less than or equal to 5%. Correlation analysis was used to assess the association between [3H]DA uptake with DAT complex and DAT monomer immunoreactivity. Results were considered significant if the probability of error was less than or equal to 5%. Data were normalized to the mean value of the saline control groups of each experiment. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA).
Results
Results presented in Fig. 1 confirmed findings of Baucum et al. (2004) that repeated METH administrations caused the formation of DAT-associated complexes in rat striatum, as assessed in striatal synaptosomes prepared 24 h after the last administration. In contrast, METH-induced DAT complex formation was not detected in the nucleus accumbens (Fig. 1). DAT complex formation was not a consequence restricted to METH treatment because 6-OHDA, administered at a neurotoxic dose demonstrated to deplete DAT monomer immunoreactivity 3 weeks after treatment (data not shown), caused DAT complex formation as assessed 24 h after treatment (Fig. 2).
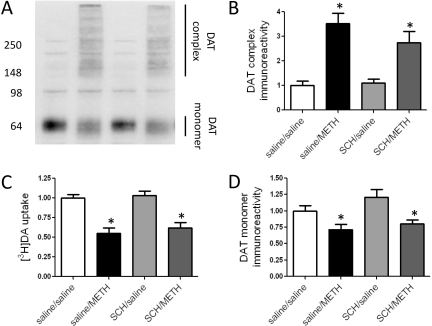
Results presented in Fig. 3, A and B, demonstrate that pretreatment with the D2 receptor antagonist, eticlopride, attenuated the METH-induced DAT complex formation as assessed 24 h after treatment. Eticlopride pretreatment also attenuated, although to a lesser degree, the METH-induced decreases in DAT activity (Fig. 3C) and loss of DAT monomer immunoreactivity (Fig. 3D) at this time point. In contrast, the D1 receptor antagonist, SCH23390, did not prevent either METH-induced DAT complex formation (Fig. 4, A and B), decreases in DAT activity (Fig. 4C), or loss of DAT monomer immunoreactivity (Fig. 4D). In both experiments, elevated body temperatures were maintained in the rats pretreated with either eticlopride (mean temperature of 39.4°C ± 0.1 and 39.3°C ± 0.1 for saline/METH- and eticlopride/METH-treated rats, respectively) or SCH23390 (mean temperature of 39.1°C ± 0.1 and 38.9°C ± 0.2 for saline/METH- and SCH23390/METH-treated rats, respectively) over the course of METH treatment. Finally, using values presented in Figs. 3 and 4 for saline/METH-treated rats, results revealed that [3H]DA uptake was negatively correlated with DAT complex immunoreactivity (r2 = 0.64; p ≤ 0.0001; Fig. 5A) and positively correlated with DAT monomer immunoreactivity (r2 = 0.14; p ≤ 0.05; Fig. 5B).
Fig. 3.
Eticlopride pretreatment attenuates DAT complex formation (B), the decreases in [3H]DA uptake (C), and the DAT monomer loss after METH treatment (D). Rats received four injections of METH (7.5 mg/kg/injection s.c.; 2-h intervals) or saline vehicle (1 ml/kg/injection s.c.; 2-h intervals) and were sacrificed 24 h later. Thirty minutes before each injection, rats were pretreated with eticlopride (ETIC; 0.5 mg/kg/injection i.p.) or saline vehicle (1 ml/kg/injection i.p.). A, representative blot of saline/saline-treated (lane 1), saline/METH-treated (lane 2), ETIC/saline-treated (lane 3), and ETIC/METH-treated (lane 4) samples. Molecular masses (kilodaltons) are indicated adjacent to a representative blot. Columns represent the means ± S.E.M. of 17 to 23 independent determinations. *, values different from saline-treated controls (p ≤ 0.05); †, values different from saline/METH-treated group (p ≤ 0.05).
Fig. 4.
SCH3390 pretreatment does not attenuate DAT complex formation (B), the decreases in [3H]DA uptake (C), or the DAT monomer loss after METH-treatment (D). Rats received four injections of METH (7.5 mg/kg/injection s.c.; 2-h intervals) or saline vehicle (1 ml/kg/injection s.c.; 2-h intervals) and were sacrificed 24 h later. Thirty minutes before each injection, rats were pretreated with SCH23390 (SCH; 0.5 mg/kg/injection i.p.) or saline vehicle (1 ml/kg/injection i.p.). A, representative blot of saline/saline-treated (lane 1), saline/METH-treated (lane 2), SCH/saline-treated (lane 3), and SCH/METH-treated (lane 4) samples. Molecular masses (kilodaltons) are indicated adjacent to a representative blot. Columns represent the means ± S.E.M. of 12 to 14 independent determinations. *, values different from saline-treated controls (p ≤ 0.05).
Fig. 5.
DAT complex immunoreactivity negatively correlates (A; r2 = 0.64; p ≤ 0.0001; n = 32), and DAT monomer immunoreactivity positively correlates (B; r2 = 0.14; p ≤ 0.05; n = 32) with [3H]DA uptake. Data points represent values from Figs. 3 and 4 for the saline/METH-treated rats.
Discussion
Many studies have examined the impact of amphetamines, including METH, on DAT function. Some reports have focused on the effects of amphetamine application in vitro and suggested that internalization of the DAT occurs after drug application (Saunders et al., 2000; Gulley et al., 2002). Other studies have examined the impact of in vivo METH administration on DAT function and have demonstrated that repeated, high-dose injections of METH, administered in a pattern designed to mimic “bingeing” in METH abusers, rapidly (within 1 h) decreases striatal DAT function (Metzger et al., 2000) and that this decrease remains even 24 h after treatment (Kokoshka et al., 1998; Baucum et al., 2004; Chu et al., 2008).
In addition to these previous studies, other studies, including the current report, have demonstrated the formation of high-molecular mass DAT-associated complexes, as assessed in striatal synaptosomes prepared 24 to 48 h after METH treatment (Baucum et al., 2004). This 24- to 48-h time frame is of particular interest because METH treatment also causes microglial activation during this period (LaVoie et al., 2004; Thomas et al., 2004). Furthermore, activated microglia can cause reactive species formation and oxidative stress (for review, see Block and Hong, 2007). It is noteworthy that DAT complex formation was a consequence not restricted to METH treatment because intrastriatal injection of 6-OHDA, an agent that causes oxidative stress and is used commonly to model aspects of Parkinson's disease (Smith and Cass, 2007), also caused DAT complex formation as assessed 24 h after administration.
Oxidative stress has been indirectly implicated in DAT complex formation as in vitro exposure to the reducing agent, β-mercaptoethanol, reversed this process (Baucum et al., 2004). Given that oxidative stress may contribute to DAT complex formation and that DAT activity can be decreased by exposure to reactive oxygen species (Berman et al., 1996; Fleckenstein et al., 1997; Huang et al., 2003), the correlation between the magnitude of DAT complex formation/monomer loss and impairment of DAT activity after METH treatment was examined. Results revealed a negative correlation between DAT complex immunoreactivity and DAT activity (r2 = 0.64) and a positive correlation between DAT monomer immunoreactivity and DAT activity (r2 = 0.14). Although both correlations were statistically significant, the relationship between DA uptake and DAT monomer immunoreactivity was much weaker than that between DA uptake and DAT complex immunoreactivity. This disparity permits speculation that the DAT monomers may have varying degrees of functionality 24 h after METH treatment, realizing that DAT activity can be altered by either changes in DA transport kinetics or in DAT protein levels. Both correlations suggest that at the 24-h time point, DAT complex formation/monomer loss and the deficits in DA transport are associated and may result from common mechanisms, possibly including oxidative stress. It is important to note that many additional factors may be affecting uptake. For example, the direct interaction of the DAT with D2 receptors enhances DAT activity (Lee et al., 2007); disruption of such an interaction would lead to decreased DAT activity.
Results from the present study revealed that pretreatment with the D2 receptor antagonist, eticlopride, but not the D1 receptor antagonist, SCH23390, attenuated METH-induced DAT complex formation. These data extend previous findings of a role for DA in mediating DAT complex formation because prior treatment with the DA-depleting agent, α-MT, attenuates this phenomenon (Baucum et al., 2004). It is important that METH-induced hyperthermia was maintained in these experiments because its prevention attenuates METH-induced striatal DAT complex formation (Baucum et al., 2004). In contrast to the attenuation of DAT complex formation, eticlopride pretreatment had a much less pronounced effect on METH-induced DAT monomer loss and decreases in DAT function. One possible explanation for this disparity may be that the DAT activity is more susceptible to a particular D2 receptor-mediated insult than the DAT is prone to form DAT complexes. In such a scenario, attenuation of that D2 receptor-mediated insult might greatly reduce DAT complex formation while only slightly attenuating decreases in DAT function.
Previous studies have demonstrated that D1 and D2 receptor antagonist treatments attenuated the acute decreases in DAT activity, as assessed 1 h after multiple, high-dose METH treatment (Metzger et al., 2000). Findings that there was no or only a modest effect of D1 or D2 receptor antagonist pretreatment on METH-induced decreases in DA uptake when assessed at 24 h, permit speculation that the D1/D2 receptor-mediated attenuation of the METH-induced decrease in DAT activity observed at 1 h may have been nearly or completely reversed by 24 h. Furthermore, because DAT complexes are not observed until 12 to 24 h after METH treatment (Baucum et al., 2004), and their formation is attenuated by D2 antagonist pretreatment, a second D2 receptor-mediated event may be contributing to complex formation by a mechanism independent of the earlier (within 1 h) effect on DAT function. In either scenario, the complex role of D2 receptors in regulating the impact of METH on DAT is apparent.
Baucum et al. (2004) were the first to suggest that DAT complex formation may be associated with the persistent dopaminergic deficits caused by METH because either administration α-MT or prevention of hyperthermia attenuates both complex formation (Baucum et al., 2004) and the persistent dopaminergic deficits caused by the stimulant (Schmidt et al., 1985; Bowyer et al., 1992). Further support for this assertion comes from findings that in addition to preventing DAT complex formation, pretreatment with a D2, but not a D1, receptor antagonist attenuates METH-induced persistent dopaminergic deficits in rats maintained hyperthermic throughout METH treatment (Broening et al., 2005). It is noteworthy that previous studies have shown that pretreatment with D1 receptor antagonists (O'Dell et al., 1993; Albers and Sonsalla, 1995; Angulo et al., 2004) and D2 receptor antagonists (O'Dell et al., 1993; Albers and Sonsalla, 1995) attenuate METH-induced dopaminergic deficits. However, these studies did not assess the ability of the D1 and D2 receptor antagonists to attenuate the persistent dopaminergic deficits independent of their attenuation of METH-induced hyperthermia.
Additional data suggesting an association between complex formation and long-term dopaminergic deficits come from findings that METH treatment does not cause DAT complex formation in the nucleus accumbens, a brain region that is less susceptible than the striatum to deficits caused by METH treatment (Eisch et al., 1992; Cass, 1997; Haughey et al., 1999; but see also findings that DA neurons in the core of the nucleus accumbens are susceptible to METH-induced dopaminergic deficits; Broening et al., 1997; Brown and Molliver, 2000). Still, the precise role of DAT complex formation in the development of METH-induced persistent dopaminergic deficits remains to be determined.
In conclusion, the present study provides further evidence for a role of reactive species in DAT complex formation. The functional relevance of DAT complex formation/monomer loss in the striatum was also suggested by the correlation between these phenomena and the METH-induced decrease in DAT function. A multifaceted role for D2 receptors in mediating the impact of METH on DAT was also suggested by the present findings. Finally, the current data support the previous suggestion of an association between DAT complex formation and the persistent dopaminergic deficits caused by the stimulant. These findings contribute to the understanding of the effects of METH on the DAT and may lead to a better appreciation of the etiology and treatment of DA-related disorders.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA00869, DA04222, DA13367, DA11389, DA019447, DA00378].
doi:10.1124/jpet.108.145631.
ABBREVIATIONS: METH, methamphetamine; DA, dopamine; DAT, dopamine transporter; α-MT, α-methyl-p-tyrosine; eticlopride, S-(-)-3-chloro-5-ethyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-6-hydroxy-2-methoxybenzamide hydrochloride; SCH23390, R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride; 6-OHDA, 6-hydroxydopamine.
References
- Albers DS and Sonsalla PK (1995) Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther 275 1104-1114. [PubMed] [Google Scholar]
- Angulo JA, Angulo N, and Yu J (2004) Antagonists of the neuorokinin-1 or dopamine D1 receptors confer protection from methamphetamine on dopamine terminals of the mouse striatum. Ann N Y Acad Sci 1025 171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucum AJ 2nd, Rau KS, Riddle EL, Hanson GR, and Fleckenstein AE (2004) Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine- and hyperthermia-associated mechanism. J Neurosci 24 3436-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SB, Zigmond MJ, and Hastings TG (1996) Modification of dopamine transporter function: effect of reactive oxygen species and dopamine. J Neurochem 67 593-600. [DOI] [PubMed] [Google Scholar]
- Block ML and Hong JS (2007) Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans 35 1127-1132. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W Jr, Ali SF, and Holson RR (1992) The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther 260 817-824. [PubMed] [Google Scholar]
- Broening HW, Morford LL, and Vorhees CV (2005) Interactions of dopamine D1 and D2 receptor antagonists with d-methamphetamine-induced hyperthermia and striatal dopamine and serotonin reductions. Synapse 56 84-93. [DOI] [PubMed] [Google Scholar]
- Broening HW, Pu C, and Vorhees CV (1997) Methamphetamine selectively damages dopaminergic innervation to the nucleus accumbens core while sparing the shell. Synapse 27 153-160. [DOI] [PubMed] [Google Scholar]
- Brown P and Molliver ME (2000) Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity. J Neurosci 20 1952-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM and Yamamoto BK (2003) Effects of amphetamines on mitochondrial function: role of free radicals and oxidative stress. Pharmacol Ther 99 45-53. [DOI] [PubMed] [Google Scholar]
- Cass WA (1997) Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Pharmacol Exp Ther 280 105-113. [PubMed] [Google Scholar]
- Chu PW, Seferian KS, Birdsall E, Truong JG, Riordan JA, Metcalf CS, Hanson GR, and Fleckenstein AE (2008) Differential regional effects of methamphetamine on dopamine transport. Eur J Pharmacol 590 105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Gaffney M, Weihmuller FB, O'Dell SJ, and Marshall JF (1992) Striatal subregions are differentially vulnerable to the neurotoxic effects of methamphetamine. Brain Res 598 321-326. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Gibb JW, and Hanson GR (2000) Differential effects of stimulants on monoaminergic transporters: Pharmacological consequences and implications for neurotoxicity. Eur J Pharmacol 406 1-13. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Beyeler ML, Gibb JW, and Hanson GR (1997) Oxygen radicals diminish dopamine transporter function in rat striatum. Eur J Pharmacol 334 111-114. [DOI] [PubMed] [Google Scholar]
- Freed C, Revay R, Vaughan RA, Kriek E, Grant S, Uhl GR, and Kuhar MJ (1995) Dopamine transporter immunoreactivity in rat brain. J Comp Neurol 359 340-349. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Valenzano KJ, and Caron MG (1998) Role of dopamine transporter in methamphetamine-induced neurotoxicity: evidence from mice lacking the transporter. J Neurosci 18 4861-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb JW, Hanson GR, and Johnson M (1994) Neurochemical mechanisms of toxicity, in Amphetamine and Its Analogs: Psychopharmacology, Toxicology, and Abuse (Cho AK and Segal DS eds) pp 269-295, Academic Press, San Diego, CA.
- Giovanni A, Liang LP, Hastings TG, and Zigmond MJ (1995) Estimating hydroxyl radical content in rat brain using systemic and intraventricular salicylate: impact of methamphetamine. J Neurochem 64 1819-1825. [DOI] [PubMed] [Google Scholar]
- Gluck MR, Moy LY, Jayatilleke E, Hogan KA, Manzino L, and Sonsalla PK (2001) Parallel increases in lipid and protein oxidative markers in several mouse brain regions after methamphetamine treatment. J Neurochem 79 152-160. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Doolen S, and Zahniser NR (2002) Brief, repeated exposure to substrates down-regulates dopamine transporter function in Xenopus oocytes in vitro and rat dorsal striatum in vivo. J Neurochem 83 400-411. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Fleckenstein AE, and Hanson GR (1999) Differential regional effects of methamphetamine on the activities of tryptophan and tyrosine hydroxylase. J Neurochem 72 661-668. [DOI] [PubMed] [Google Scholar]
- Huang CL, Huang NK, Shyue SK, and Chern Y (2003) Hydrogen peroxide induces loss of dopamine transporter activity: a calcium-dependent oxidative mechanism. J Neurochem 86 1247-1259. [DOI] [PubMed] [Google Scholar]
- Imam SZ, el-Yazal J, Newport GD, Itzhak Y, Cadet JL, Slikker W Jr, and Ali SF (2001) Methamphetamine-induced dopaminergic neurotoxicity: role of peroxynitrite and neuroprotective role of antioxidants and peroxynitrite decomposition catalysts. Ann N Y Acad Sci 939 366-380. [DOI] [PubMed] [Google Scholar]
- Kokoshka JM, Vaughan RA, Hanson GR, and Fleckenstein AE (1998) Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur J Pharmacol 361 269-275. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, and Hastings TG (2004) Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol 187 47-57. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, and Liu F (2007) Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J 26 2127-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, and Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193 265-275. [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, and Yamamoto BK (2004) High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci 24 11449-11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger RR, Haughey HM, Wilkins DG, Gibb JW, Hanson GR, and Fleckenstein AE (2000) Methamphetamine-induced rapid decrease in dopamine transporter function: role of dopamine and hyperthermia. J Pharmacol Exp Ther 295 1077-1085. [PubMed] [Google Scholar]
- O'Dell SJ, Weihmuller FB, and Marshall JF (1993) Methamphetamine-induced dopamine overflow and injury to striatal dopamine terminals: attenuation by dopamine D1 or D2 antagonists. J Neurochem 60 1792-1799. [DOI] [PubMed] [Google Scholar]
- Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, and Galli A (2000) Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci U S A 97 6850-6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, and Gibb JW (1985) Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther 233 539-544. [PubMed] [Google Scholar]
- Schmidt CJ and Gibb JW (1985) Role of the dopamine uptake carrier in the neurochemical response to methamphetamine: effects of amfonelic acid. Eur J Pharmacol 109 73-80. [DOI] [PubMed] [Google Scholar]
- Smith MP and Cass WA (2007) Oxidative stress and dopamine depletion in an intrastriatal 6-hydroxydopamine model of Parkinson's disease. Neuroscience 144 1057-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonsalla PK, Nicklas WJ, and Heikkila RE (1989) Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science 243 398-400. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, and Kuhn DM (2004) Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther 311 1-7. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Lucot JB, Schuster CR, and Seiden LS (1983) Alpha-methyltyrosine attenuates and reserpine increases methamphetamine-induced neuronal changes. Brain Res 270 285-288. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK and Zhu W (1998) The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther 287 107-114. [PubMed] [Google Scholar]