Abstract
Dopamine D2-like agonists induce penile erection (PE) and yawning in a variety of species, effects that have been suggested recently to be specifically mediated by the D4 and D3 receptors, respectively. The current studies were aimed at characterizing a series of D2, D3, and D4 agonists with respect to their capacity to induce PE and yawning in the rat and the proerectile effects of apomorphine [(R)-(-)-5,6,6a,7-tetrahydro-6-methyl-4H-dibenzo-[de,g]quinoline-10,11-diol hydrochloride] in wild-type and D4 receptor (R) knockout (KO) mice. All D3 agonists induced dose-dependent increases in PE and yawning over a similar range of doses, whereas significant increases in PE or yawning were not observed with any of the D4 agonists. Likewise, D2, D3, and D4 antagonists were assessed for their capacity to alter apomorphine- and pramipexole (N′-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride)-induced PE and yawning. The D3 antagonist, PG01037 [N-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl-benzamide hydrochloride], inhibited the induction of PE and yawning, whereas the D2 antagonist, L-741,626 [3-[4-(4-chlorophenyl)-4-hydroxypiperidin-l-yl]methyl-1H-indole], reversed the inhibition of PE and yawning observed at higher doses. The D4 antagonist, L-745,870 [3-(4-[4-chlorophenyl]piperazin-1-yl)-methyl-1H-pyrrolo[2,3-b]pyridine trihydrochloride], did not alter apomorphine- or pramipexole-induced PE or yawning. A role for the D3 receptor was further supported because apomorphine was equipotent at inducing PE in wild-type and D4RKO mice, effects that were inhibited by the D3 antagonist, PG01037, in both wild-type and D4R KO mice. Together, these studies provide strong support that D2-like agonist-induced PE and yawning are differentially mediated by the D3 (induction) and D2 (inhibition) receptors. These studies fail to support a role for the D4 receptor in the regulation of PE or yawning by D2-like agonists.
The involvement of dopamine in the regulation of penile erection (PE) has been a long-studied phenomenon (Hyyppä et al., 1970). Systemic administration of the nonselective dopamine agonist, apomorphine, is known to induce PE and yawning in a variety of species, including rats (Benassi-Benelli et al., 1979), mice (Rampin et al., 2003), monkeys (Gisolfi et al., 1980), and man (Lal et al., 1987), suggesting that the receptor regulation of these effects may be similar across species. Several D3-preferring agonists, including 7-OH-DPAT, pramipexole, and quinpirole, have been shown to induce PE over low doses, with inhibition of PE occurring at higher doses (Melis et al., 1987; Ferrari et al., 1993; Ferrari and Giuliani, 1995), as has been demonstrated previously for yawning (e.g., Collins et al., 2005, 2007). D2-like agonist-induced PE and yawning are thought to be centrally mediated because they are inhibited by relatively nonselective, centrally active, D2-like antagonists, such as haloperidol, sulpiride, and clozapine, but not the peripheral D2-like antagonist, domperidone (Benassi-Benelli et al., 1979; Gower et al., 1984; Doherty and Wisler, 1994; Hsieh et al., 2004). Moreover, a significant body of literature supports a common role for the paraventricular nucleus in the induction of PE and yawning by both physiologic and pharmacologic means (e.g., Argiolas and Melis, 1998, 2005; Melis and Argiolas, 1999, 2003); however, the specific receptor(s) mediating the proerectile effects of D2-like agonists have yet to be elucidated.
A specific role for the D4 receptor in the induction of PE by D2-like agonists has been suggested recently. Dose-dependent increases in the percentage incidence of PE were reported after systemic administration of D4-selective agonists (Hsieh et al., 2004). Similar dose-dependent inductions of PE after systemic (Brioni et al., 2004; Enguehard-Gueiffier et al., 2006; Melis et al., 2006) or intra-paraventricular nucleus (Melis et al., 2005, 2006) administration of a variety of D4-selective agonists (e.g., ABT-724, CP226269, PD-168,077, and PIP3EA), with the D4-selective antagonist, L-745,870, reported to block PD-168,077- and PIP3EA-induced PE (Melis et al., 2005, 2006; Enguehard-Gueiffier et al., 2006). Although these findings support a role for the D4 receptor in the mediation of PE, it should be noted that D4-selective agonists generally have been reported to induce fewer erections compared with less selective D2-like agonists such as apomorphine, and L-745,870 has been shown to be ineffective at altering the induction of PE by apomorphine (Melis et al., 2006), suggesting that other receptor(s) are also involved in the mediation of D2-like agonist-induced PE. It is interesting that a variety of D3-preferring agonists [e.g., (+)3-PPP, 7-OH-DPAT, pramipexole, quinelorane, and quinpirole] also have been reported to increase PE (Melis et al., 1987; Ferrari et al., 1993; Doherty and Wisler, 1994; Ferrari and Giuliani, 1995), suggesting that D3 receptors may be involved in the induction of PE by D2-like agonists.
The current studies were aimed at characterizing the roles of the D2, D3, and D4 receptors in the regulation of D2-like agonist-induced PE. Thus, in vitro binding affinities for a series of D2-like agonists and antagonists with varying degrees of selectivity for the D2, D3, and D4 receptors were first determined to compare receptor selectivity. Agonists were then assessed for their capacity to induce PE and yawning, and antagonists were assessed for their capacity to alter the induction of PE and yawning by apomorphine or pramipexole in rats. Likewise, the proerectile effects of apomorphine were evaluated in D4R wild-type (WT) and KO mice alone and in combination with the D3 antagonist, PG01037. Convergent evidence from the characterization of the proerectile effects of D2-like agonists, and the agonist-antagonist interactions in rats and D4R WT and KO mice, supports the notion that the induction of PE and yawning by D2-like agonists used herein are similarly mediated by the D3 receptor, whereas the inhibition of PE and yawning observed at higher doses results from a concomitant activation of the D2 receptor.
Materials and Methods
Subjects. Male Wistar rats (250-275 g) were obtained from Harlan (Indianapolis, IN), whereas WT and D4R KO mice (30-35 g) were derived from the mating of D4R heterozygote mice (129/Ola C57BL/6J) for more than 20 generations (Rubinstein et al., 1997). Rats were housed three to a cage, and mice were singly housed in temperature- and humidity-controlled rooms on a 12-h dark/light cycle, with lights on at 7:00 AM. Food and water were freely available; however, no food or water was available during observations. All studies were performed in accordance with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996), and all procedures were approved by the University of Michigan Committee on the Use and Care of Animals and National Institutes of Health Guidelines under Institutional Animal Care and Use Committee-approved protocols.
Behavioral Observations. On the day of testing, rats were transferred from their home cage to a test chamber (48 × 23 × 20 cm, clear rodent cage; cob bedding present in rat studies and absent in mouse studies) and allowed to habituate for a period of 30 min before vehicle or antagonist pretreatment. After a 30-min pretreatment, one dose of agonist was administered, and the total number of yawns and PEs were recorded for a period of 45 min (rats) or 30 min (mice) thereafter; yawning was not observed in mice. Yawning was defined as a prolonged (∼1 s), wide opening of the mouth followed by a rapid closure, whereas PE was defined as an emerging, engorged penis, usually followed by an upright posture, repeated pelvic thrusts, and genital grooming. All observations of drug-induced behavioral effects were separated by at least 48 h to allow for drug washout.
D2-Like Agonist-Induced Yawning and Penile Erection in Rats. The following D2-like agonists were assessed for their capacity to induce PE and yawning: apomorphine (0.01-0.32 mg/kg), pramipexole (0.01-1.0 mg/kg), PD-128,907 (0.01-0.32 mg/kg), quinpirole (0.0032-0.32 mg/kg), sumanirole (0.1-3.2 mg/kg), ABT-724 (0.001-0.32 mg/kg), PD-168,077 (0.0032-0.32 mg/kg), and PIP3EA (0.0032-0.32 mg/kg). All agonists were investigated in separate groups of eight rats, with each rat receiving each dose of one agonist presented in random order.
Effects of D2-, D3-, and D4-Selective Antagonists on Apomorphine- and Pramipexole-Induced Yawning and Penile Erection in Rats. The following D2-like antagonists were assessed for their capacity to alter the induction of PE and yawning by apomorphine (0.01-0.32 mg/kg) and pramipexole (0.01-1.0 mg/kg): PG01037 (32.0 mg/kg), L-741,626 (1.0 mg/kg), and L-745,870 (1.0 mg/kg). PG01037 and L-741,626 were administered as 30-min pretreatments, whereas L-745,870 was administered 15 min before agonist injection. Each antagonist × agonist combination was assessed in separate groups of eight rats, with each rat receiving all dose combinations in random order.
Effects of D2-Like Antagonists on Pramipexole-Induced Yawning and Penile Erection in Rats. The following series of D2-like antagonists were assessed for their capacity to alter the induction of PE and yawning by pramipexole (0.1 mg/kg): PG01037 (1.0-32.0 mg/kg), SB-277011A (1.0-32.0 mg/kg), raclopride (0.0032-0.1 mg/kg), haloperidol (0.0032-0.1 mg/kg), L-741,626 (0.32-10.0 mg/kg), Ro 61-6270 (1.0-32.0 mg/kg), and L-745,870 (0.32-10.0 mg/kg). Each antagonist was assessed in separate groups of eight rats, with each rat receiving all dose combinations, presented in random order.
Apomorphine-Induced Penile Erection in Wild-Type and D4 Receptor Knockout Mice. The capacity of apomorphine (0.0003-0.032 mg/kg) to induce PE was assessed in WT and D4RKO mice. Each group of mice was comprised of six littermates (one group each of WT and KO mice), with saline injections administered 30 min before apomorphine doses. All mice were exposed to each dose of apomorphine presented in random order.
Effects of PG01037 on Apomorphine-Induced Penile Erection in Wild-Type and D4 Receptor Knockout Mice. The capacity of the D3-selective antagonist, PG01037 (10.0 and 30.0 mg/kg), to alter apomorphine-induced (0.0003-0.032 mg/kg) PE was assessed in both WT and D4R KO mice. PG01037 was administered 30 min before doses of apomorphine or saline injections, with each mouse receiving each combination of doses presented in random order. One WT mouse was euthanized due to health problems after five of the 19 treatments and, therefore, was not included in the analysis.
Binding Analysis. All Ki values were assessed using membranes prepared from cells recombinantly expressing the hD2, hD3, and hD4 receptors. Ligands were assessed for their capacity to inhibit [3H]PD-128,907 (or [3H]spiperone) binding to the D3 receptor or [3H]spiperone binding to the D2 or D4 receptor. Membranes for D2, D3, and D4 receptor binding assays were prepared as described previously (Enguehard-Gueiffier et al., 2006) from hD2 baculovirus-infected insect cells (∼2-5 pmol/mg protein) or SH-SY5Y neuroblastoma cells stably expressing either the hD3 or hD4 receptor (∼1-2 pmol/mg protein). Competitions using [3H]PD-128,907 were performed in a buffer containing 25 mM Tris-HCl, pH 8.0, 0.5 mM EDTA, 1 mM MgSO4, and 1 mM CaCl2, with 5 μg of hD3-SH-SY5Y membranes in the presence of 2 nM [3H]PD-128,907 and varying concentrations of competing ligands (10-11 to 10-4 M, final), whereas competitions using [3H]spiperone for D3 (5-μg membrane), D2 (5-μg membrane), and D4 (10-μg membrane) receptors were performed in 25 mM Tris-HCl, pH 8.0, 75 mM NaCl, 0.5 mM EDTA, 1 mM MgSO4, and 1 mM CaCl2 with 2 nM (D3) or 200 pM (D2 and D4)[3H]spiperone (final volume of 500 μl) in the presence of varying concentrations of competing ligands (10-11 to 10-4 M, final). Radioligand binding assays were performed at room temperature in 96-well microtiter plates and filtered onto GF/B filter plates with radioactivity detected by liquid scintillation counting on a TopCount counter (PerkinElmer Life and Analytical Sciences, Waltham, MA). The IC50 values for inhibition of [3H]spiperone binding to the D2 and D4 receptors were calculated using either a single-site model (for antagonists) or two-site model (for agonists) using GraphPad Prism (GraphPad Software Inc., San Diego, CA). IC50 values for inhibition of [3H]PD-128,907 binding to the D3 receptor were fit to a single-site model. Ki values were derived from the IC50 values according to the Cheng-Prusoff equation (Cheng and Prusoff, 1973) to take into consideration the radioligand concentration and the Kd values for [3H]spiperone on the D2 and D4 receptors and [3H]PD-128,907 on the D3 receptor (data not shown). Note that the IC50 values for agonist inhibition of [3H]spiperone binding to the D2 and D4 receptors determined from a single-site fit are expressed as a K0.5 to reflect the radioligand-corrected value.
Materials. ABT-724 was synthesized by Dr. Kenner Rice (Chemical Biology Research Branch, National Institute on Drug Abuse, Bethesda, MD). Apomorphine, haloperidol, PD-128,907, and quinpirole were obtained from Sigma-Aldrich (St. Louis, MO). L-741,626, L-745,870, PD-168,077, and raclopride were obtained from Tocris Bioscience (Ellisville, MO). PG01037 was synthesized by Drs. Amy Newman and Peter Grundt (Medicinal Chemistry Section, National Institute on Drug Abuse, Baltimore, MD). PIP3EA was synthesized by Drs. Alain Gueiffier and Cécile Enguehard-Gueiffier (Francois-Rabelais Universite, Tours, France). Pramipexole and SB-277011A were synthesized by Drs. Shaomeng Wang and Jianyong Chen (University of Michigan, Ann Arbor, MI). Ro 61-6270 was provided by F. Hoffman-La Roche (Basel, Switzerland). Sumanirole was synthesized by Drs. Stephen Husbands and Benjamin Greedy (University of Bath, Bath, UK). All drugs were dissolved in sterile water, with the exceptions of PG01037 and SB-277,011A, which were dissolved in 10% β-cyclodextrin, and haloperidol, L-741,626, PD-168,077, and PIP3EA, which were dissolved in 5% ethanol and sterile water. In the rat studies, all drugs were administered subcutaneously in a volume of 0.1 ml/kg, with the exception of L-745,870, which was administered intraperitoneally. In the mouse studies, apomorphine and PG01037 were administered intraperitoneally in a volume of 0.1 ml/kg. The cDNAs for the human dopamine (hD2, hD3, and hD4) receptors were generously provided by Drs. Olivier Civelli (University of California, Irvine, CA), Pierre Sokoloff (Institut National de la Santé et de la Recherche Médicale, Paris, France), and Dr. Hubert VanTol (University of Toronto, Toronto, ON, Canada).
Data Analysis. Radioligand binding data were analyzed using nonlinear regression and analyzed for one- or two-site inhibition curves (GraphPad Prism). All yawning and PE studies were conducted with eight rats per group, with results expressed as the mean number of yawns or PE observed over 45 min ± S.E.M. Percentage incidence represents the number of rats displaying at least one PE during the 45-min observation period. Mouse studies were conducted with six littermates per group, and results are expressed as the mean number of PE observed over 30 min ± S.E.M. Because of the fact that the event occurs on less than one occasion per test session and, thus, is not normally distributed, the significant effects of agonists on the induction of PE or antagonists on agonist-induced PE were determined using Mann-Whitney U tests (GraphPad Prism). One-way, repeated-measures ANOVA with post hoc Dunnett's tests was used to determine significant levels of agonist-induced yawning (GraphPad Prism), whereas significant effects of antagonists on apomorphine- and pramipexole-induced yawning were determined using two-way ANOVA with post hoc Bonferroni tests (SPSS; SPSS Inc., Chicago, IL). One-way repeated-measures ANOVA with post hoc Dunnett's tests were used to determine significant effects of antagonists on pramipexole-induced yawning. (GraphPad Prism).
Results
In Vitro Binding Analysis. Because a comparison of binding affinities of the ligands used in these studies at the D2, D3, and D4 receptors has not been reported previously in a single study, these data were obtained for each compound against recombinantly expressed human hD2, hD3, and hD4 receptors and were directly compared using radioligand filter binding assays. The capacity of all of the agonists and antagonists to displace the antagonist, [3H]spiperone, was assessed for each receptor subtype, whereas displacement of the D3-preferring agonist, [3H]PD-128,907, was also assessed for the D3 receptor subtype. Most ligands displaced radioactive probes with a single-phase inhibition, consistent with a one-site model; only agonist binding to D2 receptors displayed biphasic inhibition curves (composed of a low-affinity state and a guanine nucleotide-sensitive high-affinity state). Binding affinities and selectivity ratios for ligands binding to the D2 and D3 receptors (D2/D3) and D4 and D3 receptors (D4/D3) are shown in Table 1; note that the more relevant comparisons with the D2high state and D3 receptors (D2high/D3) are also shown. The individual Ki and K0.5 values obtained in this study are within the range of previously reported values from several studies, using different assay conditions and different radioligand probes. The data presented here, all assayed under similar conditions, provide an appropriate comparison of the receptor subtype selectivity of the D2-like ligands used in the behavioral studies reported herein. The absence of a strong correlation to in vivo potency has been described previously (e.g., Levant, 1997) and is duly noted.
TABLE 1.
In vitro binding affinities and selectivity ratios at D2, D3, and D4 receptors for D2-like agonists and antagonists
| D2: [3H]Spip: K0.5 | D2: [3H]Spip: Khigh | D2: [3H]Spip: Klow | D3: [3H]PD-128907: Ki | D3: [3H]Spip: Ki | D4: [3H]Spip: K0.5 | D2/D3 Ratiob | D2 high/D3 Ratiob | D4/D3 Ratiob | |
|---|---|---|---|---|---|---|---|---|---|
| nM | |||||||||
| Agonist | |||||||||
| Pramipexole | >10,000 | N.D. | N.D. | 0.5 | 10.2 | 194 | N.A.c | N.A.c | 388 |
| PD-128,907 | 931 | 3.5 (29%) | >10,000 | 1.9 | 9.7 | 2430 | 490 | 1.8 | 1280 |
| Quinpirole | 118 | 10 (55%) | 3250 | 6 | 9.4 | 109 | 20 | 1.7 | 18 |
| Apomorphine | 19 | 3.6 (50%) | 570 | 75 | 231 | 3.4 | 0.3 | 0.05 | 0.05 |
| ABT-724 | >10,000 | N.D. | N.D. | >10,000 | 947 | 58 | N.A.c | N.A.c | N.A.c |
| PD-168,077 | 4250 | N.D. | N.D. | 1400 | 726 | 23 | 3.04 | N.A.c | 0.02 |
| PIP3EA | 32 | 1.7 (42%) | 950 | 1720 | 1910 | 3.7 | 0.02 | 9.9 × 10−04 | 2.2 × 10−03 |
| Sumanirole | 144 | 0.2 (42%) | 256 | 613 | 493 | >10,000 | 0.2 | 3.3 × 10−04 | N.A.c |
| Antagonist | |||||||||
| PG01037 | 52 | N.D. | N.D. | 0.06 | 0.03 | 760 | 867 | N.A.c | 1.3 × 104 |
| SB-277011A | 527 | N.D. | N.D. | 78 | 74 | 3600 | 6.8 | N.A.c | 46 |
| Raclopride | 2.2 | N.D. | N.D. | 79 | 8.8 | 5030 | 0.03 | N.A.c | 64 |
| Haloperidol | 3 | N.D. | N.D. | 16 | 33 | 2.1 | 0.2 | N.A.c | 0.1 |
| L-741,626 | 18.1 | N.D. | N.D. | 604 | 271 | 260 | 0.03 | N.A.c | 0.4 |
| L-745,870 | 3600 | N.D. | N.D. | 3020 | 872 | 0.5 | 1.2 | N.A.c | 1.7 × 10−04 |
| Ro 61-6270 | 1450 | N.D. | N.D. | 5470 | 793 | 0.5 | 0.3 | N.A.c | 9.1 × 10−05 |
N.A., not applicable; N.D., not determined.
Selectivity ratios were based on radioligand-corrected values (K0.5) for D2 and D4 using [3H]Spiperone and values for D3 using [3H]PD128-907. Selectivity ratios for D2 (high) and D2 (low) were calculated based on a two-site model (using Prism) assuming that the Kd for [3H]spiperone is identical for both sites.
Selectivity ratio could not be calculated.
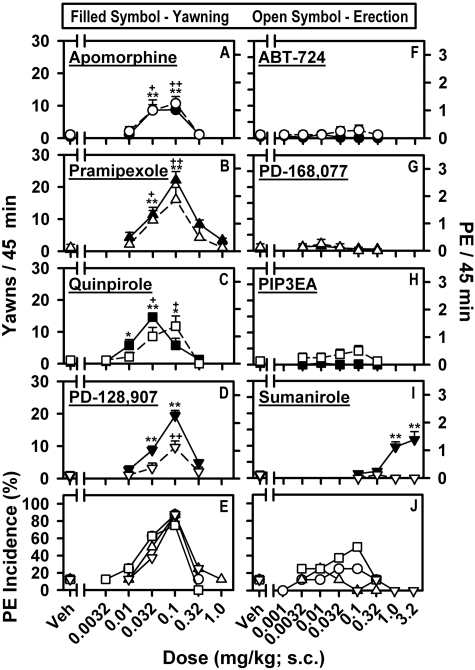
D2-Like Agonist-Induced Yawning and Penile Erection in Rats. Dose-dependent increases in PE and yawning were observed for the nonselective D2-like agonist, apomorphine, and the D3-preferring agonists, PD-128,907, pramipexole, and quinpirole, with inhibition of both responses occurring at higher doses, resulting in inverted U-shaped dose-response curves for PE and yawning (Fig. 1). Peak levels of PE and yawning were observed at the same dose for apomorphine (0.1 mg/kg), pramipexole (0.1 mg/kg), and PD-128,907 (0.1 mg/kg), whereas doses of 0.032 and 0.1 mg/kg quinpirole induced peak levels of yawning and PE, respectively. Apomorphine, pramipexole, and PD-128,907 induced at least one PE over the 45 min in 87.5% of rats, whereas the maximal percentage incidence of PE for quinpirole was 75%. None of the D4-selective agonists induced significant levels of PE or yawning (Fig. 1). PIP3EA induced at least one PE in 50% of rats at a dose of 0.1 mg/kg, whereas the maximal percentage incidence of PE for PD-168,077, and ABT-724 was 25%. Although significant levels of yawning were observed with the D2-preferring agonist, sumanirole, PE was not induced (Fig. 1).
Fig. 1.
Dose-response curves for D2-like agonist-induced PE and yawning. Characterization of PE and yawning induced by apomorphine (A), pramipexole (B), quinpirole (C), PD-128,907 (D), ABT-724 (F), PD-168,077 (G), PIP3EA (H), and sumanirole (I) was conducted in separate groups of rats with data presented as mean (±S.E.M.), n = 8, number of PEs and yawns observed in 45 min. E and J, percentage of rats displaying at least one PE over 45 min. *, p < 0.05; **, p < 0.01, significant differences in agonist-induced yawning as determined using one-way, repeated-measures ANOVA with post hoc Dunnett's tests; +, p < 0.05; ++, p < 0.01, agonist-induced PE as determined by Mann-Whitney U test compared with vehicle-treated animals.
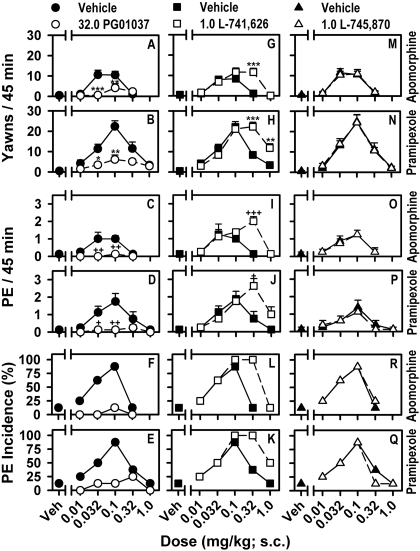
D3-, D2-, and D4-Selective Antagonism of Apomorphine- and Pramipexole-Induced Yawning and Erection in Rats. The effects of the D3-selective antagonist, PG01037, the D2-selective antagonist, L-741,626, and the D4-selective antagonist, L-745,870, on apomorphine- and pramipexole-induced PE and yawning are shown in Fig. 2. Significant inhibition of the induction of both PE and yawning by apomorphine and pramipexole was observed after a dose of 32.0 mg/kg PG01037, whereas the inhibition of PE or yawning observed at higher doses was unaffected (Fig. 2, A-D). PG01037 also reduced the maximal percentage incidence of PE for apomorphine from 87.5 to 12.5% and from 87.5 to 25% for pramipexole (Fig. 2, E and F). Unlike with PG01037, the D2-selective antagonist, L-741,626 (1.0 mg/kg), selectively reversed the inhibition of PE and yawning observed at higher doses of apomorphine and pramipexole at a dose that did not affect the induction of yawning or PE at lower doses (Fig. 2, G-J). Pretreatment with L-741,626 not only increased the maximal number of PEs and yawns observed but also shifted the peaks of the PE and yawning dose-response curves for apomorphine and pramipexole [1/2] log unit to the right. L-741,626 also shifted the descending limb of the dose-response curves for the percentage incidence of PE for apomorphine and pramipexole resulting in 100% of rats exhibiting at least one PE at doses of 0.1 and 0.32 mg/kg (Fig. 2, K and L). When given at a behaviorally active dose of 1.0 mg/kg (Enguehard-Gueiffier et al., 2006), L-745,870 failed to modify apomorphine- or pramipexole-induced PE or yawning and, furthermore, did not alter the percentage incidence of PE for either apomorphine or pramipexole (Fig. 2, M-R).
Fig. 2.
D3-, D2-, and D4-selective antagonists on apomorphine- and pramipexole-induced PE and yawning. Effects of the D3-selective antagonist PG01037 (32.0 mg/kg) on apomorphine- and pramipexole-induced yawning (A and B), PE (C and D), and percentage incidence of PE (E and F). Effects of the D2-selective antagonist L-741,626 (1.0 mg/kg) on apomorphine- and pramipexole-induced yawning (G and H), PE (I and J), and percentage incidence of PE (K and L). Effects of the D4-selective antagonist L-745,870 (1.0 mg/kg) on apomorphine- and pramipexole-induced yawning (M and N), PE (O and P), and percentage incidence of PE (Q and R). Data are presented as mean (±S.E.M.), n = 8, number of PEs and yawns observed in 45 min. *, p < 0.05; **, p < 0.01; ***, p < 0.001, significant effect of antagonist on agonist-induced yawning as determined by a two-way ANOVA with post hoc Bonferroni tests. +, p < 0.05; ++, p < 0.01; +++, p < 0.001, significant effect of antagonist on agonist-induced PE as determined by Mann-Whitney U test.
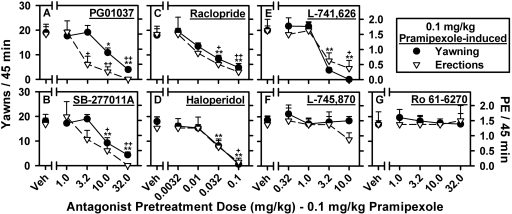
D3, D2, and D4 Antagonism of Pramipexole-Induced Yawning and Penile Erection in Rats. The effects of a series of D2-like antagonists, with varying degrees of selectivity for the D2, D3, and D4 receptors, on PE and yawning induced by the maximally effective dose of pramipexole (0.1 mg/kg) are shown in Fig. 3. Dose-dependent inhibition of pramipexole-induced PE and yawning was observed with both of the D3-selective antagonists, PG01037 and SB-277011A (Fig. 3, A and B); however, there were slight differences in the relative potencies with PG01037 inhibiting PE at a dose (3.2 mg/kg) [1/2] log unit lower than that required to inhibit yawning (10.0 mg/kg), whereas SB-277011A was equipotent at inhibiting the induction of yawning and PE (10.0 mg/kg). Similar to SB-277,011A, inhibition of pramipexole-induced yawning and PE was observed at the same dose of the nonselective D2/D3 antagonist, raclopride (0.032 mg/kg; Fig. 3C), whereas the relatively nonselective D2-like antagonist, haloperidol, and the D2-selective antagonist, L-741,626, produced a dose-dependent inhibition of pramipexole-induced PE and yawning with a significant inhibition of yawning observed at a dose [1/2] log unit lower than was required to inhibit the induction of PE (Fig. 3, D and E). Unlike all other D2-like antagonists tested, the D4-selective antagonists, L-745,870 (Fig. 3F) and Ro 61-6270 (Fig. 3G), did not alter the induction of either PE or yawning by pramipexole, although a slight, but not significant, reduction of pramipexole-induced PE was observed after a dose of 10.0 mg/kg L-745,870.
Fig. 3.
Effects of a series of D2-like antagonists with a range of selectivities for the D3, D2, and D4 receptors on PE and yawning induced by 0.1 mg/kg pramipexole. Effects of the D3-selective antagonists PG01037 (1.0-32.0 mg/kg) (A) and SB-277011A (1.0-32.0 mg/kg) (B), the nonselective D2/D3 antagonist raclopride (0.0032-0.1 mg/kg) (C), the nonselective D2-like antagonist haloperidol (0.0032-0.1 mg/kg) (D), the D2-selective antagonist L-741,626 (0.32-10.0 mg/kg) (E), and the D4-selective antagonists L-745,870 (0.32-10.0 mg/kg) (F) and Ro 61-6270 (1.0-32.0 mg/kg) (G). *, p < 0.05; **, p < 0.01, one-way repeated-measures ANOVA with post hoc Dunnett's tests were used to determine significant effects of antagonists on pramipexole-induced yawning. +, p < 0.05; ++, p < 0.01. Mann-Whitney U tests were used to determine significant effects of antagonists on pramipexole-induced PE.
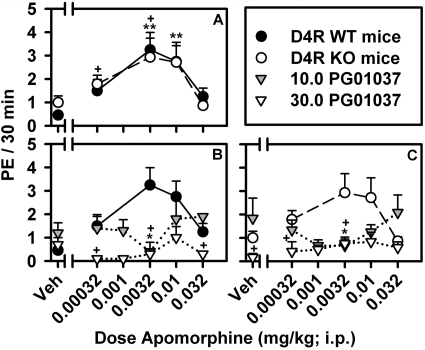
Apomorphine-Induced Penile Erection in Wild-Type and D4 Receptor Knockout Mice. Similar to the effects of apomorphine in rats, a dose-dependent increase in PE was observed over low doses of apomorphine, with inhibition of PE occurring at higher doses resulting in an inverted U-shaped dose-response curve for apomorphine-induced PE in both WT and D4R KO mice (Fig. 4A). No significant differences in the potency or effectiveness of apomorphine to induce PE were observed between the WT and D4R KO mice, with peak levels of PE observed at a dose of 0.0032 mg/kg apomorphine in both genotypes. Likewise, the effects of the D3-selective antagonist, PG01037, in WT and D4R KO mice were similar to the effects observed in rats. Pretreatment with PG01037 resulted in a dose-dependent inhibition of apomorphine-induced PE in both WT and D4R KO mice, with a dose of 30.0 mg of PG01037 producing an almost complete inhibition of apomorphine-induced PE (Fig. 4, B and C).
Fig. 4.
Dose-response curves for apomorphine-induced PE in D4RWT and KO mice. A, apomorphine-induced PE in D4R WT and D4R KO mice was conducted in groups of six littermates with data presented as mean (±S.E.M.). Effects of PG01037 (10.0 and 30.0 mg/kg) on apomorphine-induced PE in D4R WT (B) and D4R KO (C) mice. Significant differences in apomorphine-induced PE in D4RWT(*, p < 0.05; **, p < 0.01) and D4R KO (+, p < 0.05; ++, p < 0.01) as determined by Mann-Whitney U test compared with vehicle-treated animals. Significant effects of PG01037 (10.0 mg/kg, *, p < 0.05; and 30.0 mg/kg, +, p < 0.05) on apomorphine-induced PE compared with vehicle-treated mice as determined by Mann-Whitney U tests.
Discussion
These studies were aimed at characterizing the receptors involved in the regulation of the proerectile effects of D2-like agonists in rats and mice. Convergent evidence from the pharmacologic evaluation of the effects of a series of D2-like agonists with varying degrees of selectivity for the D2, D3, and D4 receptors alone and in combination with D2-, D3-, and D4-selective antagonists suggest that the induction of PE is mediated by an activation of the D3 receptor, whereas the inhibition of PE observed at higher doses results from the concomitant activation of the D2 receptor, as has been described previously for D2-like agonist-induced yawning (Collins et al., 2005, 2007). These studies failed to support a role for the D4 receptor in the mediation of D2-like agonist-induced PE because D4-selective agonists failed to induce PE and D4-selective antagonists failed to inhibit PE in rats, whereas apomorphine was equally effective at inducing PE in WT and D4R KO mice.
In agreement with previous reports, apomorphine, pramipexole, and quinpirole induced PE and yawning with inverted U-shaped dose-response curves and 75 to 87.5% of rats displaying at least one PE at the peak dose; however, these studies are the first to report a similar proerectile effect for the D3-preferring agonist, PD-128,907. The results of these studies suggest that the capacity of these agonists to induce PE is related to their activity at the D3, but not the D4, receptor because increases in yawning and PE were observed over a similar range of low doses, even though large differences exist between their in vitro selectivities for the D3 compared with the D4 receptor (e.g., apomorphine D4/D3 ≈ 0.05 and PD-128,907 D4/D3 ≈ 1280; Table 1). In agreement with this notion but contrary to previous findings (Brioni et al., 2004; Melis et al., 2005; Enguehard-Gueiffier et al., 2006), all of the highly selective D4 agonists failed to induce PE. It should be noted, however, that the maximal PE responses for apomorphine, quinpirole, and pramipexole were lower than some previous reports (e.g., Melis et al., 2006), suggesting procedural differences may have affected the PE response. Nevertheless, the percentage incidences of PE for apomorphine and the D3-preferring agonists were similar to previous reports (e.g., Hsieh et al., 2004), suggesting that any procedural differences only affected the maximal number of PEs observed, not the absolute capacity of the agonists to induce PE.
The effects of D2-, D3-, and D4-selective antagonists on apomorphine- and pramipexole-induced PE and yawning further support specific roles for the D3 and D2 receptors in the mediation of D2-like agonist-induced PE. When given at behaviorally active doses (Collins et al., 2005, 2007; Enguehard-Gueiffier et al., 2006), the D3-selective antagonist, PG01037, and the D2-selective antagonist, L-741,626, differentially affected apomorphine- and pramipexole-induced PE and yawning, whereas the D4-selective antagonist, L-745,870, did not alter the induction or inhibition of PE or yawning. Similar to the effects of the D3 and D2 antagonists on yawning, PG01037 produced a selective rightward and/or downward shift of the ascending limb, whereas L-741,626 produced a selective rightward shift of the descending limb of the PE dose-response curves for apomorphine and pramipexole with respect to both the absolute number and percentage incidence of PE. Together with previous reports describing specific roles for the D3 and D2 receptors in the regulation of D2-like agonist-induced yawning (Collins et al., 2005, 2007), the differential and selective effects of the D3 and D2 antagonists on PE, combined with the fact that both apomorphine and a variety of D3-preferring agonists were equipotent at inducing PE and yawning, suggest that the induction of PE and yawning by D2-like agonists is mediated by the D3 receptor, whereas the inhibition of PE and yawning observed at higher doses results from a concomitant activation of the D2 receptor. It should be noted, however, that unlike pramipexole, apomorphine also has activity at D1-like receptors that also may influence the PE response, although the precise role of D1 receptors in the modulation of PE is currently unclear (Melis et al., 1987; Zarrindast and Jamshidzadeh, 1992; D'Aquila et al., 2003; Hsieh et al., 2004) and may involve peripheral rather than central D1 receptors (El-Din et al., 2007).
A role for the D3 receptor in the induction of PE and yawning is further supported by the dose-response analysis of a series of D2-like antagonists on pramipexole-induced PE and yawning. Dose-dependent inhibition of pramipexole-induced PE was observed after pretreatment with D3-selective (PG01037 and SB-277011A), nonselective D2/D3 (raclopride), nonselective D2-like (haloperidol), and D2-selective (L-741,626) antagonists, an effect that was correlated with their capacity to inhibit yawning but not observed with the D4-selective antagonists (L-745,870 and Ro 61-6270). Furthermore, all of the D2-like antagonists inhibited PE and yawning with similar potencies, regardless of the fact that large differences exist with respect to their in vitro selectivity for D3 compared with D4 receptors (e.g., PG01037, D4/D3 ≈ 1.3 × 104; raclopride, D4/D3 ≈ 64; and haloperidol, D4/D3 ≈ 0.1; Table 1), whereas antagonists highly selective for D4 compared with D3 receptors (e.g., L-745,870, D4/D3 ≈ 1.7 × 10-4; and Ro 61-6270, D4/D3 ≈ 9.1 × 10-5; Table 1) failed to alter pramipexole-induced PE or yawning. Although Ro 61-6270 has not been characterized extensively (Clifford and Waddington, 2000), L-745,870 has been shown to possess favorable pharmacokinetics (0.3 mg/kg p.o. is thought to be sufficient to occupy ∼90% of D4 receptors; Patel et al., 1997) and has been shown to inhibit PD-168,077- and PIP3EA-induced PE at a dose of 1.0 mg/kg (Enguehard-Gueiffier et al., 2006; Melis et al., 2006), suggesting that the doses used in the current studies were sufficient to block D4 receptors. Together with previous reports that L-745,870 was unable to alter apomorphine-induced PE (Melis et al., 2006), the current studies suggest that the proerectile effects of D2-like agonists (e.g., apomorphine and pramipexole) are mediated by activation of the D3, but not the D4, receptor.
Despite the distinct and differential effects of PG01037 and L-741,626 observed in the current studies, the fact that relatively large doses of the D3-selective antagonists (PG01037 and SB-277011A) were required to inhibit pramipexole-induced yawning and PE, whereas similar effects were observed with relatively low doses of nonselective (raclopride and haloperidol) and selective (L-741,626) D2 antagonists, effects that may suggest that the inhibition of PE is mediated by antagonist activity at receptor(s) other than the D3 receptor. These are not, however, the first studies to suggest a disconnect between the in vitro and in vivo potencies of the D3-antagonists, PG01037 and SB-277011A. In fact, a number of previous studies have reported similar in vivo potencies when these antagonists have been evaluated in a variety of operant procedures (3.2-24.0 mg/kg) (Andreoli et al., 2003; Xi et al., 2004; Gilbert et al., 2005; Cervo et al., 2007). Moreover, previous studies, aimed at characterizing the in vivo selectivity of D2-like agonists and antagonists, suggest that PG01037 and SB-277011A are devoid of significant D2, cholinergic and serotonergic antagonist activities at doses up to 56.0 mg/kg, whereas L-741,626 displays a much more limited in vivo D2 selectivity with significant D3 antagonist activity observed at doses as low as 3.2 mg/kg (Collins et al., 2005, 2007).
Perhaps the strongest evidence in support of a specific role for the D3 receptor in the induction of PE by D2-like agonists was provided by the evaluation of apomorphine-induced PE in the WT and D4R KO mice. Not only was apomorphine equally effective at inducing PE in the WT and D4R KO mice, but the proerectile effect of apomorphine was also dose-dependently inhibited by the D3-selective antagonist, PG01037, in both the WT and D4R KO genotypes. Although species differences precluded comparisons of the effects of agonists and antagonists on yawning and PE to be made in mice because D2-like agonists do not induce yawning in mice (S.M. Li, G.T. Collins, N.M. Paul, P. Grundt, A.H. Newman, M. Xu, D.K. Grandy, J.H. Woods, and J.L. Katz, unpublished data), when taken together with the pharmacologic data collected in rats, these data provide strong support for a role for the D3, but not the D4, receptor in the induction of PE by D2-like agonists in rodents.
To summarize, a series of D2-like agonists and antagonists with varying degrees of selectivity for the D2, D3, or D4 receptors were assessed for their capacity to modulate PE and yawning in rats. Similar to the effects of apomorphine, all D3-preferring agonists induced dose-dependent increases in PE and yawning over a similar range of low doses, with the inhibition of PE and yawning occurring at higher doses; D4-selective agonists failed to induce PE or yawning. The D3-selective antagonist, PG01037, and D2-selective antagonist, L-741,626, had similar effects on PE and yawning, with PG01037 selectively shifting the ascending limbs and L-741,626 selectively shifting the descending limbs of the dose-response curves for apomorphine- and pramipexole-induced PE and yawning. In addition, dose-dependent inhibition of pramipexole-induced PE was observed with a series of D2-like antagonists with a wide range of selectivities for the D3 and D2 receptors, an effect that corresponded to their capacity to inhibit pramipexole-induced yawning but was not observed with D4-selective antagonists. Furthermore, the pharmacologic evaluation of the proerectile effects of D2-like agonists was validated in D4R KO mice. Not only was apomorphine equally effective at inducing PE in both WT and D4R KO mice, but the induction of PE by apomorphine was dose-dependently inhibited by the D3-selective antagonist, PG01037, in both genotypes. In conclusion, although inferences with regard to the receptors mediating the proerectile effects of D4-selective agonists could not be made, these studies provide convergent evidence in support of a role for the D3 receptor in the induction of PE by D2-like agonists, with the inhibition of PE observed at higher doses resulting from the concomitant activation of the D2 receptor.
Acknowledgments
We thank Davina Barron, Nhu Truong, Katherine L. Suchland, Dawn French-Evans, and Marika B. Cohen for excellent technical work throughout the course of these studies and Dr. Pierre Sokoloff (Institut National de la Santé et de la Recherche Médicale, Paris, France) and the late Dr. Hubert Van Tol (University of Toronto, Toronto, ON, Canada) for generously providing the cDNAs for the D3 and D4 receptors, respectively.
This work was supported in part by the National Institutes of Health [Grants DA020669, F013771, GM068603, MH67497]; by the Intramural Research program of the National Institutes of Health National Institute on Drug Abuse and National Institutes of Health National Institute on Alcohol Abuse and Alcoholism; and by the University of Michigan Biological Sciences Scholars Program.
doi:10.1124/jpet.108.144048.
ABBREVIATIONS: PE, penile erection; apomorphine, (R)-(-)-5,6,6a,7-tetrahydro-6-methyl-4H-dibenzo[de,g]quinoline-10,11-diol hydrochloride; pramipexole, N′-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride; quinpirole, trans-(-)-(4aR)-4,4a,5,6,7,8,8a,9-octahydro-5-propyl-1H-pyrazolo[3,4-g]quinoline hydrochloride; haloperidol, 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-1-butanone hydrochloride; ABT-724, 2-[[4-pyridin-2-yl)piperazin-1-yl]methyl]-1H-benzimidazole; PD-168,077, N-(methyl-4-(2-cyanophenyl)piperazinyl-3-methylbenzamide maleate; PIP3EA, 2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]imidazo[1,2-a]pyridine; L-745,870, 3-(4-[4-chlorophenyl]piperazin-1-yl)-methyl-1H-pyrrolo[2,3-b]pyridine trihydrochloride; R, receptor; WT, wild type; KO, knockout; PG01037, N-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl-benzamide hydrochloride; PD-128,907, (S)-(+)-(4aR,10bR)-3,4,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano-[4,3-b]-1,4-oxazin-9-ol hydrochloride; sumanirole, (5R)-5,6-dihydro-5-(methylamino) 4H-imidazo[4,5,1-ij]quinolin-2(1H)-one (2Z)-2-butenedioate; L-741,626, 3-[4-(4-chlorophenyl)-4-hydroxypiperidin-l-yl]methyl-1H-indole; SB-277011A, trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclo-hexyl]-4-quinolinecarboxamide; raclopride, 3,5-dichloro-N-(1-ethylpyrrolidin-2-ylmethyl)-2-hydroxy-6-methoxybenzamide tartrate salt; Ro 61-6270, 2-amino-benzoic acid-1-benzyl-piperidin-4-yl-ester; ANOVA, analysis of variance; 7-OH-DPAT, (±)-7-hydroxy-2-dipropylaminotetralin; CP226269, 5-fluoro-2-[[4-(2-pyridinyl)-1-piperazinly]methyl]-1H-indole; (+)3-PPP, (+)-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine.
References
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, and Heidbreder CA (2003) Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology 28 1272-1280. [DOI] [PubMed] [Google Scholar]
- Argiolas A and Melis MR (1998) The neuropharmacology of yawning. Eur J Pharmacol 343 1-16. [DOI] [PubMed] [Google Scholar]
- Argiolas A and Melis MR (2005) Central control of penile erection: role of the paraventricular nucleus of the hypothalamus. Prog Neurobiol 76 1-21. [DOI] [PubMed] [Google Scholar]
- Benassi-Benelli A, Ferrari F, and Quarantotti BP (1979) Penile erection induced by apomorphine and N-n-propyl-norapomorphine in rats. Arch Int Pharmacodyn Ther 242 241-247. [PubMed] [Google Scholar]
- Brioni JD, Moreland RB, Cowart M, Hsieh GC, Stewart AO, Hedlund P, Donnelly-Roberts DL, Nakane M, Lynch JJ 3rd, Kolasa T, et al. (2004) Activation of dopamine D4 receptors by ABT-724 induces penile erection in rats. Proc Natl Acad Sci U S A 101 6758-6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Cocco A, Petrella C, and Heidbreder CA (2007) Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. Int J Neuropsychopharmacol 10 167-181. [DOI] [PubMed] [Google Scholar]
- Cheng Y and Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22 3099-3108. [DOI] [PubMed] [Google Scholar]
- Clifford JJ and Waddington JL (2000) Topographically based search for an “Etho-gram” among a series of novel D(4) dopamine receptor agonists and antagonists. Neuropsychopharmacology 22 538-544. [DOI] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, and Woods JH (2007) Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 193 159-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, and Woods JH (2005) Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther 314 310-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aquila PS, Panin F, Cossu M, Peana AT, and Serra G (2003) Dopamine D1 receptor agonists induce penile erections in rats. Eur J Pharmacol 460 71-74. [DOI] [PubMed] [Google Scholar]
- Doherty PC and Wisler PA (1994) Stimulatory effects of quinelorane on yawning and penile erection in the rat. Life Sci 54 507-514. [DOI] [PubMed] [Google Scholar]
- El-Din MM, Senbel AM, Daabees TT, and Sharabi FM (2007) Peripheral modulation of dopaminergic receptors affects erectile responses in rats. Basic Clin Pharmacol Toxicol 100 225-232. [DOI] [PubMed] [Google Scholar]
- Enguehard-Gueiffier C, Hübner H, El Hakmaoui A, Allouchi H, Gmeiner P, Argiolas A, Melis MR, and Gueiffier A (2006) 2-[(4-Phenylpiperazin-1-yl)methyl]imidazo-(di)azines as selective D4-ligands: induction of penile erection by 2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]imidazo[1,2-a]pyridine (PIP3EA), a potent and selective D4 partial agonist. J Med Chem 49 3938-3947. [DOI] [PubMed] [Google Scholar]
- Ferrari F and Giuliani D (1995) Behavioural effects of the dopamine D3 receptor agonist 7-OH-DPAT in rats. Pharmacol Res 32 63-68. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Pelloni F, and Giuliani D (1993) Behavioural evidence that different neurochemical mechanisms underlie stretching-yawning and penile erection induced in male rats by SND 919, a new selective D2 dopamine receptor agonist. Psychopharmacology (Berl) 113 172-176. [DOI] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR Jr, Heidbreder CA, Pak AC, Peng XQ, and Xi ZX (2005) Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse 57 17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisolfi CV, Mora F, and Wall PT (1980) Dopamine and temperature regulation in the primate: effects of apomorphine and pimozide. Brain Res Bull 5 349-352. [DOI] [PubMed] [Google Scholar]
- Gower AJ, Berendsen HG, Princen MM, and Broekkamp CL (1984) The yawning-penile erection syndrome as a model for putative dopamine autoreceptor activity. Eur J Pharmacol 103 81-89. [DOI] [PubMed] [Google Scholar]
- Hsieh GC, Hollingsworth PR, Martino B, Chang R, Terranova MA, O'Neill AB, Lynch JJ, Moreland RB, Donnelly-Roberts DL, Kolasa T, Mikusa JP, McVey JM, Marsh KC, Sullivan JP, and Brioni JD (2004) Central mechanisms regulating penile erection in conscious rats: the dopaminergic systems related to the proerectile effect of apomorphine. J Pharmacol Exp Ther 308 330-338. [DOI] [PubMed] [Google Scholar]
- Hyyppä M, Rinne UK, and Sonninen V (1970) The activating effect of l-dopa treatment on sexual functions and its experimental background. Acta Neurol Scand 46 (Suppl 43): 223. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Lal S, Laryea E, Thavundayil JX, Nair NP, Negrete J, Ackman D, Blundell P, and Gardiner RJ (1987) Apomorphine-induced penile tumescence in impotent patients: preliminary findings. Prog Neuropsychopharmacol Biol Psychiatry 11 235-242. [DOI] [PubMed] [Google Scholar]
- Levant B (1997) The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev 49 231-252. [PubMed] [Google Scholar]
- Melis MR and Argiolas A (1999) Yawning: role of hypothalamic paraventricular nitric oxide. Zhongguo Yao Li Xue Bao 20 778-788. [PubMed] [Google Scholar]
- Melis MR and Argiolas A (2003) Central oxytocinergic neurotransmission: a drug target for the therapy of psychogenic erectile dysfunction. Curr Drug Targets 4 55-66. [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A, and Gessa GL (1987) Apomorphine-induced penile erection and yawning: site of action in brain. Brain Res 415 98-104. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Mascia MS, and Argiolas A (2005) PD-168077, a selective dopamine D4 receptor agonist, induces penile erection when injected into the paraventricular nucleus of male rats. Neurosci Lett 379 59-62. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Sanna F, Melis T, Mascia MS, Enguehard-Gueiffier C, Hubner H, Gmeiner P, Gueiffier A, and Argiolas A (2006) PIP3EA and PD-168077, two selective dopamine D4 receptor agonists, induce penile erection in male rats: site and mechanism of action in the brain. Eur J Neurosci 24 2021-2030. [DOI] [PubMed] [Google Scholar]
- Patel S, Freedman S, Chapman KL, Emms F, Fletcher AE, Knowles M, Marwood R, Mcallister G, Myers J, Curtis N, et al. (1997) Biological profile of L-745,870, a selective antagonist with high affinity for the dopamine D4 receptor. J Pharmacol Exp Ther 283 636-647. [PubMed] [Google Scholar]
- Rampin O, Jérôme N, and Suaudeau C (2003) Proerectile effects of apomorphine in mice. Life Sci 72 2329-2336. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, et al. (1997) Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell 90 991-1001. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr., Hagan JJ, Heidbreder CA, and Gardner EL (2004) Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology (Berl) 176 57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR and Jamshidzadeh A (1992) Inhibitory effect of morphine on yawning induced by cholinoceptor and dopamine D2 receptor activation in rats. Br J Pharmacol 105 675-678. [DOI] [PMC free article] [PubMed] [Google Scholar]