Abstract
The rewarding effect of opioids, the driving force for compulsive behaviors of opioid abuse and addiction, is primarily mediated by the μ-opioid receptor. However, the role of the δ-opioid receptor (DOR) in opioid reward and addiction is still poorly understood. The recently discovered adaptive DOR property of exocytotic translocation in sensory neurons after chronic opioid exposure provides a new avenue of conceptual thoughts to exploring the DOR function in this psychoneurological disease. In this study, we investigated potential adaptive function of DOR in neurons of the central nucleus of the amygdala (CeA), a forebrain structure increasingly recognized for mediating stimulus reward learning in drug addiction. Using whole-cell recordings in CeA slices, we found that in rats displaying morphine-induced behavior of conditioned place preference, a behavioral measure of drug reward, the overall synaptic strength of glutamate synapses in CeA neurons was significantly enhanced. The selective DOR agonist [d-Pen2,d-Pen5]-enkephalin, having no apparent effect on glutamatergic excitatory postsynaptic current (EPSC) in neurons from control rats, produced a significant, dose-dependent inhibition of the synaptic current in neurons from those morphine-treated rats. Detailed analyses of EPSC properties revealed that DOR activation inhibited the EPSC by reducing presynaptic release of glutamate, indicating functional DOR emerging on presynaptic glutamate terminals. The morphine treatment also significantly increased DOR proteins in CeA preparations of synaptosomes. These findings provide functional evidence for an adaptive modulation by presynaptic DOR of a key synaptic activity altered by morphine, thus implying likely important involvement of DOR in opioid reward and addiction.
Opioid compounds such as morphine and heroin are highly addictive and are among commonly abused drugs due to their strong rewarding effect, ultimately leading to opioid dependence and addiction, a psychoneurological disease and disturbing social problem for which effective treatments are still lacking (Nestler, 2004; Woolf and Hashmi, 2004; Koob and Le Moal, 2008). Several brain regions, including the ventral tegmental area and the nucleus accumbens, have been classically implicated in the mechanisms of reward and addiction to many abused drugs, including opioids. In these brain regions, important adaptive changes have been identified that are thought to contribute to the compulsive behavior of opioid abuse induced by repeated exposure to opioid drugs (Williams et al., 2001; Koob and Le Moal, 2008). Recently, the amygdala complex including the central nucleus of the amygdala (CeA), a forebrain structure well known for mediating negative emotional responses such as fear (McKernan and Shinnick-Gallagher, 1997; Rogan et al., 1997; Garcia et al., 1999), has been increasingly recognized as a critical player also in positive emotional responses characterized in the process of positive stimulus-reward learning and in drug-seeking behaviors (Baxter and Murray, 2002; Gottfried et al., 2003; See et al., 2003). Among many targets of long-term opioids, central glutamate synaptic transmission has been identified as a key component that undergoes significant molecular, pharmacological, and physiological adaptations after prolonged opioid exposure (Carlezon and Nestler, 2002; Siggins et al., 2003; Jones and Bonci, 2005).
Opioid actions are mediated primarily by three types of classic opioid receptors: μ-opioid receptor (MOR), δ-opioid receptor (DOR), and κ-opioid receptor (Pan, 1998; Williams et al., 2001; Waldhoer et al., 2004). Previous studies have demonstrated clearly that MOR plays an essential role in all major actions of opioids, including opioid reward and addiction, as genetic deletion or pharmacological blockade of MOR abolishes opioid-induced rewarding effect and associated behaviors of opioid addiction (Matthes et al., 1996; Contet et al., 2004). However, little is known at present about the function of DOR, which is also abundantly expressed in those reward-related brain areas including the CeA, in opioid reward, and in adaptive changes of the endogenous opioid system, leading to compulsive opioid-seeking behaviors and opioid abuse (Kieffer and Gavériaux-Ruff, 2002). Because direct antagonism of MOR in an opioid-dependent state inevitably causes devastating syndromes of opioid withdrawal (Williams et al., 2001), understanding of the adaptive function of DOR and those of other affected signaling systems may provide more appropriate targets to circumvent the problems of opioid dependence and addiction. Therefore, in this study, we investigated the action of DOR and its adaptive changes in CeA neurons from rats that display morphine-induced behavior of conditioned-place preference (CPP), a behavioral measure well established for the rewarding effect of many drugs of abuse in animals (Tzschentke, 2007).
Materials and Methods
All procedures involving the use of animals conformed to the guidelines set by the University of Texas-M.D. Anderson Cancer Center Animal Care and Use Committee. Male, Wistar rats (150–200 g) were used for behavioral CPP tests in vivo and subsequent whole-cell recordings in brain slices in vitro.
CPP. A standard rat CPP apparatus (MED Associates, St. Albans, VT) was used for analysis of morphine-induced reward behavior in rats, as we reported previously (Zhu et al., 2007). The CPP apparatus had two test chambers with distinct environmental cues: a black chamber with a stainless steel rod grid floor and a white chamber with a stainless steel mesh floor. A third center compartment in neutral gray connected the two test chambers with operational doors. Automated data were collected by 15 infrared photobeam detectors on chamber floors and were automatically sent to a computer for storage and analysis. The conditioning procedure consisted of four phases and lasted for a total of 12 consecutive days. For phase 1 (habituation; days 1–2), after an intraperitoneal saline injection, a rat was placed in the center compartment and allowed to move freely between the two test chambers for 30 min each day. For phase 2 (pretest; day 3), after an intraperitoneal saline injection, the rat was placed in the center compartment, and a preference test was conducted by recording the time the rat spent in each of the two test chambers during a 30-min test period. This pretest determined baseline preference and equipment bias. For phase 3 (morphine conditioning; days 4–11), rats were randomly assigned to saline and morphine groups; on day 4, the rat in the morphine group was injected with morphine (10 mg/kg i.p.) and was immediately confined in a chamber for 20 min. Morphine conditioning was paired with the nonpreferred chamber in this study with equipment bias to prevent potential influence of a ceiling effect in CPP tests. CPP was also consistently induced by drugs paired with the preferred chamber for alcohol (Zhu et al., 2007) and morphine (data not shown). On day 5, the rat was injected with saline and confined in the other chamber for 20 min. The same procedure of morphine and saline conditioning on alternate days was repeated on days 6 and 7 through days 10 and 11. In the saline control group, rats received saline injection on all 8 conditioning days. For phase 4, (post-test; day 12), after an intraperitoneal saline injection, the conditioned rat was placed in the center compartment and allowed to move freely between the two test chambers for 30 min, and the time the rat spent in each test chamber was automatically recorded to determine CPP behavior. On day 13, an intraperitoneal injection of saline or naloxone (1.5 mg/kg) was made on a conditioned rat, followed by a CPP test to determine the drug effect on the existing CPP behavior.
Brain Slice Preparations. A rat was anesthetized with inhalation of halothane and then euthanized by decapitation. The brain was removed and cut in a Vibratome slicer in cold (4°C) physiological saline to obtain coronal slices (200–300 μm thick) containing the CeA. A single slice was submerged in a shallow recording chamber and perfused with preheated (35°C) physiological saline (126 mM NaCl, 2.5 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 2.4 mM CaCl2, 11 mM glucose, and 25 mM NaHCO3, saturated with 95% O2 and 5% CO2, pH 7.2–7.4). Slices were maintained at around 35°C throughout a recording experiment.
Whole-Cell Recording. Visualized whole-cell voltage-clamp recordings were obtained from neurons in the medial part of the CeA in a slice. Neurons were visualized through a microscope with infrared illumination (Olympus, Tokyo, Japan). Whole-cell recordings were made with a glass pipette (resistance, 3–5 MΩ) filled with a solution containing 126 mM potassium gluconate, 10 mM NaCl, 1 mM MgCl2, 11 mM EGTA, 10 mM HEPES, 2 mM ATP, and 0.25 mM GTP, pH adjusted to 7.3 with KOH; osmolarity 280 to 290 mOsmol/l. An AxoPatch 1-D amplifier and AxoGraph software (Molecular Devices, Sunnyvale, CA) were used for data acquisition and on-line/off-line data analyses. Holding potential was -70 mV for all recordings. A seal resistance of 1 GΩ or above and an access resistance of 20 MΩ or less were considered acceptable. Series resistance was optimally compensated. The access resistance was monitored periodically throughout the experiment. Junction potential was not corrected. Recordings were made in a morphine-free solution 1 to 4 h after making the slice preparation from morphine- or saline-treated rats on the same day as behavioral tests.
Glutamate Synaptic Currents. Electrical stimuli of constant current (0.25 ms, 0.04–0.5 mA) were used to evoke glutamate-mediated excitatory postsynaptic current (EPSCs) with bipolar stimulating electrodes (FHC Inc., Bowdoinham, ME) placed in the ventrolateral part of the CeA. All EPSCs were recorded in the presence of the GABAA receptor antagonist bicuculline (30 μM). Under our experimental conditions, the EPSC is mediated predominantly by non-N-methyl-d-aspartate receptors, and no glycine or GABAB receptor-mediated synaptic current is detectable in CeA neurons (Zhu and Pan, 2004). The glutamate synaptic strength between control and morphine-treated groups was studied with three stimulus intensities: maximum intensity to elicit a maximum EPSC, two thirds of the maximum intensity, and one third of the maximum. For paired-pulse ratios (PPRs), a pair of EPSCs was evoked by two stimuli with an interval of 40, 60, and 80 ms, and the PPR at each interval was calculated by dividing the second EPSC amplitude by the first one. Six PPRs were averaged to obtain a mean PPR before and during application of a drug in a given cell. The PPR, which has been widely used to determine the involvement of a presynaptic action site (Dobrunz and Stevens, 1997; Ungless et al., 2001; Bie et al., 2005), has an inverse relationship with the probability of presynaptic transmitter release, so that an increased PPR indicates a reduced transmitter release, and vice versa. Miniature EPSCs were obtained in 60-s epochs in the presence of tetrodotoxin (1 μM), and a sliding EPSC template defined with the acquisition software was used to detect and analyze the frequency and amplitude of miniature EPSCs. Drug solutions were made by diluting fresh stock solutions immediately before use and applied through the bath solution.
Synaptosome Preparations. The protocol for preparing synaptosomes was generally based on previous reports (Sbrenna et al., 2000; Dunkley et al., 2008). CeA tissues from saline- and morphine-treated rats were gently homogenized in ice-cold 0.32 M sucrose buffer at pH 7.4, and then centrifuged for 10 min at 1000g (4°C). The supernatant was collected and centrifuged for 20 min at 10,000g (4°C), and then the synaptosomal pellet was resuspended in the lysis buffer (0.1% Triton X-100, 150 mM NaCl, 25 mM KCl, 10 mM Tris-HCl, pH 7.4, with protease inhibitors) at 4°C for 10 min. For total protein preparations, as we described previously (Ma et al., 2006), CeA tissues from saline- and morphine-treated rats were homogenized on ice for 10 min in the lysis buffer containing 50 mM Tris-Cl, 150 mM NaCl, 0.02 mM NaN2, 100 μg/ml phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, and 1% Triton X-100. The lysates were centrifuged at 14,000 rpm for 10 min at 4°C, and the supernatant was used for SDS-polyacrylamide gel electrophoresis. Protein concentrations were determined by using the Bio-Rad (Hercules, CA) protein assay kit.
Western Blotting. The samples were treated with SDS sample buffer at 95°C for 5 min, loaded on a 10% SDS-polyacrylamide gel, and blotted to a nitrocellulose membrane. The blots were incubated overnight at 4°C with a goat polyclonal anti-DOR primary antibody (1:250; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and monoclonal anti-synaptophysin antibody (1:1000; Millipore Bioscience Research Reagents, Temecula, CA) or monoclonal anti-β-actin antibody (1:250; Santa Cruz Biotechnology, Inc.). The membranes were washed extensively with Tris-buffered saline and incubated with horseradish peroxidase-conjugated anti-mouse IgG antibody (1:10,000; GE Healthcare, Chalfont St. Giles, UK) and anti-goat IgG antibody (1:10,000; Jackson ImmunoResearch Laboratories Inc., West Grove, PA). The immunoreactivity was detected using enhanced chemiluminescence (ECL Advance Kit; Amersham Biosciences). The intensity of bands was captured digitally and analyzed quantitatively with Eastman Kodak (Rochester, NY) 1D software, version 3.5.4. The immunoreactivity of DOR in the synaptosomes was normalized to that of synaptophysin, and immunoreactivity of total DOR was normalized to that of β-actin.
Data Analysis and Statistical Tests. General numerical data of evoked EPSCs were statistically tested with a paired or unpaired Student's t test and presented as mean ± S.E.M. Data of miniature EPSCs were tested by Statview software with the Kolmogorov-Smirnov test. CPP data were presented as percentage of the time a rat spent in the morphine-paired chamber versus the sum of times spent in the two test chambers; CPP behavior was determined by comparing times the same rat spent in the morphine-paired chamber between pretest and post-test. Paired or unpaired Student's t tests were used to statistically analyze CPP data. A p value <0.05 was considered statistically significant.
Materials. Morphine, [d-Pen2,d-Pen5]-enkephalin (DPDPE), and naltrindole were kindly supplied by the National Institute on Drug Abuse (Rockville, MD). All other drugs were purchased from Tocris Bioscience (Ellisville, MO) or from Sigma-Aldrich (St. Louis, MO).
Results
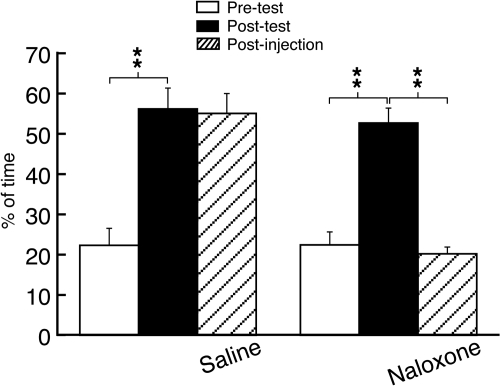
Morphine Conditioning Induces Reward Behavior through Opioid Receptors. To demonstrate a rat model of opioid reward, we conditioned rats with repeated morphine, a strong rewarding opioid, or saline and performed a behavioral test of CPP, a commonly used behavioral measure for the rewarding effect of opioids and other abused drugs in animals (Tzschentke, 2007). Morphine conditioning induced consistent CPP behavior in all conditioned rats (n = 15; Fig. 1), consistent with a brief report in our previous study of alcohol reward (Zhu et al., 2007). One day after the postconditioning test, we conducted another CPP test on the same animal without additional morphine administration and found that the CPP behavior persisted without apparent decline in magnitude after administration of saline. However, when naloxone (1.5 mg/kg i.p.), a nonselective opioid receptor antagonist, was instead administered before the test, the CPP behavior was completely reversed, suggesting that the reward behavior of morphine generally requires activation of opioid receptors (Fig. 1).
Fig. 1.
Conditioning treatment with repeated morphine induces the reward behavior of CPP through opioid receptors. Data were expressed as percentage of time the rat spent in the morphine-paired chamber versus the sum of the times spent in both morphine- and saline-paired chambers. Data of pretest (before morphine conditioning) and post-test (after morphine conditioning) were compared to determine the CPP behavior. One day after the post-test, a postinjection test was performed after an intraperitoneal injection of saline (n = 7 rats) or naloxone (1.5 mg/kg, n = 8 rats). **, p < 0.01.
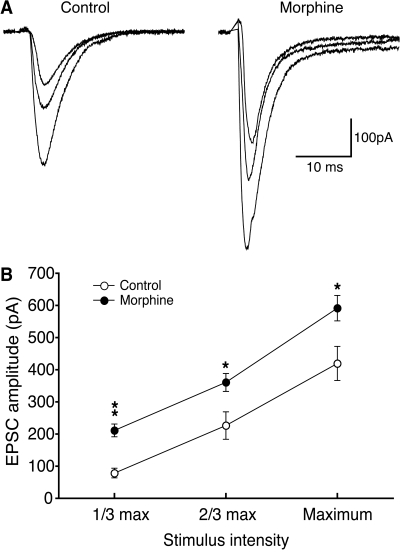
Morphine Enhances Glutamate Synaptic Transmission. We then prepared CeA slices from those rats treated with morphine and displaying resultant CPP behavior to investigate morphine-induced adaptive changes in glutamate synaptic activity in CeA neurons under whole-cell voltage-clamp conditions in vitro. To assess a potential change in general synaptic strength of CeA glutamate synapses, we compared input-output responses of evoked synaptic currents from those morphine-treated rats versus saline-treated control rats. Under our recording conditions in the presence of the GABAA receptor antagonist bicuculline (30 μM), the evoked EPSC is mediated predominantly by non-N-methyl-d-aspartate glutamate receptors because it is nearly completely blocked by 6-cyano-2,3-dihydroxy-7-nitroquinoxaline (10 μM) (Zhu and Pan, 2004). As shown in Fig. 2, glutamate synaptic efficacy measured by the averaged amplitude of evoked EPSCs was significantly enhanced in morphine-treated rats, indicating strengthened glutamate synaptic transmission in CeA neurons after the morphine treatment.
Fig. 2.
Morphine increases glutamate synaptic strength in neurons of the CeA. A, representative EPSCs evoked by an electrical stimulus at three intensities (maximum, one third maximum, and two thirds maximum) in a CeA neuron from a saline-treated control rat (control group) and from a morphine-treated rat with CPP behavior (morphine group). B, group data of the evoked EPSCs in neurons of the control group (n = 14) and of the morphine group (n = 12). *, p < 0.05; **, p < 0.01.
To further evaluate changes in the glutamate synaptic properties of CeA neurons, we used the paradigm of PPR, a commonly used synaptic assessment for changes in presynaptic transmitter release and a synaptic property that is less variable among cells in a slice preparation, allowing feasible comparison between two different groups of cells (Bie et al., 2005). In control slices, a pair of stimuli at three different intervals all evoked a PPR of >1, indicating a consistent synaptic facilitation under these conditions (Fig. 3). In neurons from morphine-treated rats, the same simulation also evoked consistent synaptic facilitation, but the value of PPRs was significantly reduced at all three stimulus intervals. This suggests a stimulus interval-independent reduction in the PPR of glutamate EPSCs. Given the well documented inverse relationship between PPR and presynaptic release of neurotransmitters (Dobrunz and Stevens, 1997; Bie et al., 2005; Zhu et al., 2007), the reduced PPR indicates that synaptic release of glutamate in CeA neurons is functionally increased by morphine, contributing to the strengthened neurotransmission of glutamate synapses in the CeA of morphine-treated rats.
Fig. 3.
Morphine increase of glutamate synaptic transmission involves a presynaptic site. A, representative pairs of EPSCs evoked by two consecutive stimuli at an interval of 40 ms in a CeA neuron from a control rat and from a morphine-treated rat. The inlet shows the same two EPSC pairs scaled to the amplitude of the first EPSC, illustrating morphine-induced reduction in the paired-pulse ratio. B, paired-pulse ratios at three between-stimulus intervals as indicated in CeA neurons of the control group (n = 28 at 40 ms and n = 11 at 60 and 80 ms) and of the morphine group (n = 9 at each interval). *, p < 0.05.
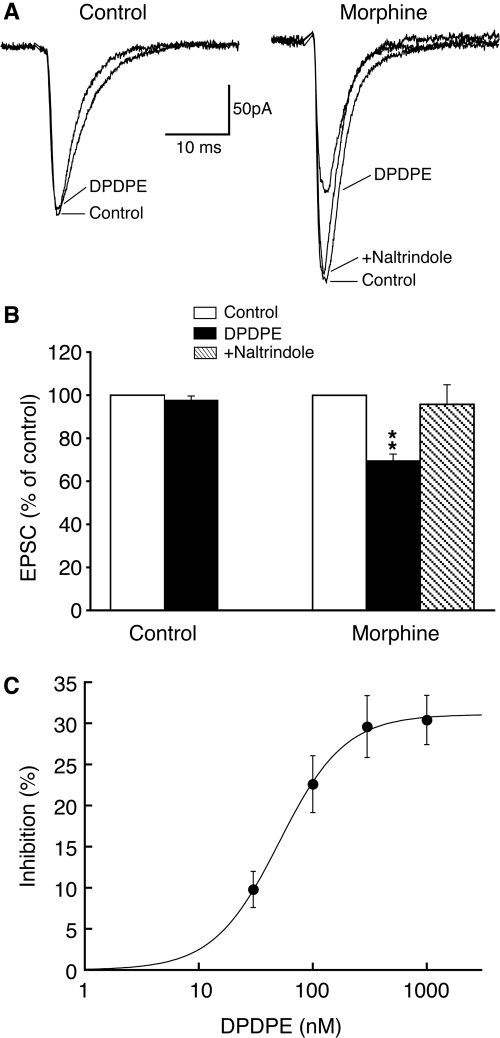
Morphine Recruits Functionalδ-Opioid Receptors. Although MOR plays an essential role in opioid reward and addiction, the function of DOR remains largely unclear (Contet et al., 2004). To determine whether DOR had a role in this morphine reward-related synaptic adaptation, we examined DOR actions on glutamate synaptic activity in CeA neurons from morphine-treated rats and control rats. In CeA slices from control rats, the selective DOR agonist DPDPE (1 μM) had no apparent effect on the amplitude of evoked EPSCs in all cells tested (control, 229 ± 17 pA; DPDPE, 227 ± 21 pA; n = 18 from 10 rats; p > 0.05; Fig. 4, A and B), consistent with our previous report showing a lack of functional DOR on glutamate synapses in CeA neurons from normal rats (Zhu and Pan, 2005). In contrast, in CeA slices from morphine-treated rats, we found that DPDPE (1 μM) significantly inhibited the EPSC amplitude in 12 of 17 cells generally surveyed (control, 258 ± 37 pA; DPDPE, 185 ± 32 pA; n = 12; p < 0.01; Fig. 4, A and B). The DOR agonist did not induce a significant effect in the remaining five cells. The DPDPE inhibition of EPSCs was predominantly mediated by DOR because it was nearly completely reversed by the selective DOR antagonist naltrindole (1 μM) (control, 206 ± 29 pA; DPDPE, 136 ± 22 pA; p < 0.01; DPDPE + naltrindole, 198 ± 31 pA; p > 0.05 compared with the control; n = 6 of a separate cell group). In addition, the DOR agonist inhibited the EPSC in a dose-dependent manner, with a near maximum inhibition of 30.4 ± 3.0% of control at 1 μM and an EC50 of 50.7 nM (Fig. 4C). This finding indicates that repeated morphine treatment that causes the reward-related CPP behavior recruits new functional DOR on the majority of glutamate synapses in a brain area importantly involved in opioid reward and drug addiction.
Fig. 4.
Morphine recruits new functional DORs on CeA glutamate synapses. A, representative EPSCs in a CeA neuron, from the control and morphine groups, under conditions of control, in the presence of the selective DOR agonist, DPDPE, and DPDPE plus naltrindole, a selective DOR antagonist. B, group data summarizing the effects of DPDPE and naltrindole addition in the two cell groups. C, dose-response curve of the DPDPE inhibition on EPSCs. The estimated EC50 for the DPDPE effect is 50.7 nM (n = 6–11 for each data point). **, p < 0.01.
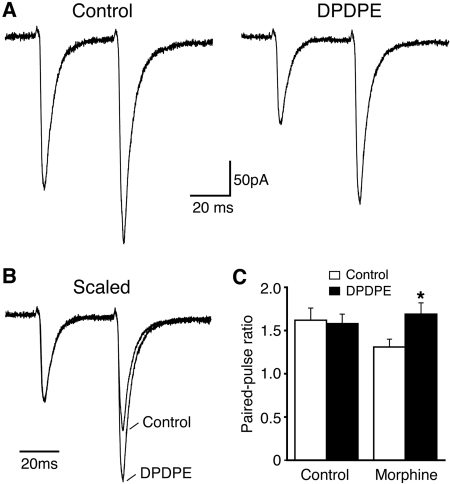
DOR Inhibition of EPSCs Is Presynaptic. We next determined whether the EPSC inhibition by the newly emerged DOR involved a presynaptic site, a postsynaptic site, or both, using synaptic analyses of both PPR and miniature EPSCs. Consistent with a lack of functional DOR on CeA glutamate synapses under normal conditions, DPDPE (1 μM) failed to alter the PPR of EPSCs in CeA slices from control rats (control, 1.62 ± 0.14; DPDPE, 1.58 ± 0.11; n = 6; p > 0.05; Fig. 5). In CeA slices from morphine-treated rats, however, the averaged PPR was significantly increased by DPDPE (1 μM) (control, 1.31 ± 0.09; DP-DPE, 1.69 ± 0.13; n = 6; p < 0.01; Fig. 5). This result of an increased PPR indicates that DOR inhibition of glutamate EP-SCs probably involves a presynaptic site with reduced probability of glutamate release. This notion was supported by the following experiments on spontaneous, action potential-independent miniature EPSCs in CeA neurons. The effect of the DOR agonist on miniature EPSCs was examined in the presence of tetrodotoxin (1 μM) in slices from morphine-treated rats. As shown in Fig. 6, DPDPE (1 μM), having little effect on the amplitude of miniature EPSCs, significantly reduced the frequency, a direct measure of release rate for synaptic glutamate (amplitude: control, 19.7 ± 1.4 pA; DPDPE, 18.2 ± 2.1 pA; p > 0.05; frequency: control, 11.2 ± 2.3 Hz; DPDPE, 7.3 ± 1.9 pA; p < 0.05, n = 6). Postsynaptically, DPDPE (1 μM) induced no significant change in membrane current on neurons from morphine-treated rats (2.3 ± 1.2 pA, n = 11), further supporting the DOR presence on presynaptic glutamate terminals.
Fig. 5.
DOR inhibition of EPSCs involves a presynaptic site. A, representative EPSC pairs at the 40-ms interval before and after the addition of DPDPE in a CeA neuron from the morphine group. B, same EPSC pairs as in A, but scaled to the amplitude of first EPSC, illustrating the DPDPE-mediated increase in the paired-pulse ratio. C, group data of the DPDPE effect in the two cell groups. *, p < 0.05.
Fig. 6.
DOR activation reduces presynaptic release of glutamate. A, current traces with spontaneous events of miniature EPSCs in the absence and presence of DPDPE from a CeA neuron of the morphine group. B, graphs showing cumulative probability of the frequency and amplitude of miniature EPSCs in control and in DPDPE for the neuron in A. C, group data of the DPDPE effect on miniature EPSC frequency and amplitude in neurons from the morphine group. *, p < 0.05.
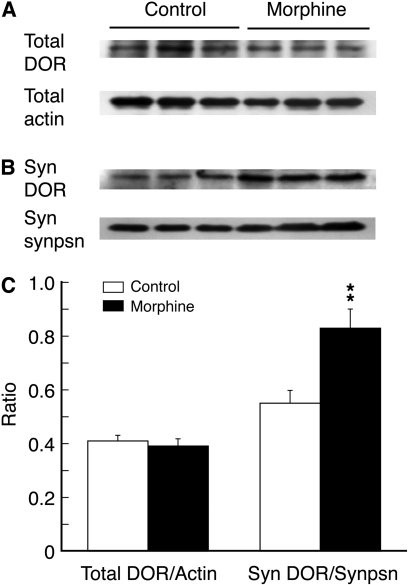
Morphine Increases DOR Proteins in CeA Synaptosomes. Finally, we explored the potential mechanism for the DOR emergence on CeA glutamatergic terminals. Given the previous evidence for morphine-induced exocytotic membrane trafficking of intracellular DOR in pain-modulating neurons (Morinville et al., 2004; Ma et al., 2006; Zhang et al., 2006), we proposed that a similar mechanism of DOR membrane trafficking could account for the DOR appearance on glutamate terminals in CeA neurons. To demonstrate a potential increase in the functional expression of DOR proteins on surface membrane of synaptic terminals, we used CeA preparations of synaptosomes, which are thought to be largely free of cell body contents and have a greatly reduced amount of intraterminal contents (Ghijsen et al., 2003; Dunkley et al., 2008), including presumably intraterminal DOR. Compared with CeA tissues from control rats (n = 6), the total DOR protein in CeA tissues taken from the morphine-treated rats (n = 6) remained unchanged in our Western blot analysis (Fig. 7, A and C), consistent with our previous report on brainstem neurons (Ma et al., 2006). However, in CeA synaptosomal preparations of synaptic terminals as identified by the specific synaptic terminal marker synaptophysin, the amount of DOR protein was significantly increased by the morphine treatment (Fig. 7, B and C, n = 6 rats in both control and morphine groups). This result indicates that repeated morphine probably increases the expression of DOR proteins on terminal membrane without significant new synthesis of DOR proteins in the CeA.
Fig. 7.
Morphine increases DOR protein expression in CeA synaptosomes. A, representative lanes of Western blots of total DOR proteins and total proteins of β-actin in CeA tissues taken from a saline-treated control rat and from a morphine-treated rat. B, Western blot lanes of DOR proteins and synaptophysin, a synaptic terminal marker, in CeA preparations of synaptosomes from a rat of the two indicated groups. C, summary graph showing changes in the expression ratios of total DOR proteins versus β-actin and synaptosomal DOR proteins versus synaptosomal synaptophysin (n = 6 rats for each group). **, p < 0.01. Syn, synaptosome; synpsn, synaptophysin.
Discussion
In this study, we have shown that repeated morphine-conditioning treatment, which induces the CPP behavior related to morphine reward, enhances glutamate synaptic strength and recruits new functional DOR on glutamate synapses in neurons of the CeA, an important forebrain structure implicated in the rewarding effect of and addiction to many abused drugs, including opioids. It seems that the DOR emerges on terminal membrane of presynaptic glutamatergic synapses onto CeA neurons, and its activation inhibits glutamate synaptic activity, which has been enhanced by the morphine treatment.
Mechanisms for DOR Induction. Increasing evidence has emerged from a number of recent studies showing that under normal conditions, DOR is predominantly localized in intracellular compartments, such as cytoplasmic large dense-core vesicles and Golgi/endoplasmic reticulum-associated structures, in many types of central neurons in the brain and spinal cord (Zhang et al., 2006; Cahill et al., 2007). Intracellular localization of nonfunctional DOR is consistent with recent reports that DOR agonists lack a significant effect on brainstem and midbrain neurons under control conditions (Hack et al., 2005; Ma et al., 2006) and with our observation in this study of the lack of a DOR agonist effect in control CeA neurons, although a DOR-mediated hyperpolarization was reported in a small population of normal brainstem neurons after acute application of MOR agonists (Marinelli et al., 2005). It is interesting that chronic, but not acute, morphine has been shown to induce exocytotic membrane trafficking of intracellular DOR from cytoplasmic compartments to surface membrane with detailed anatomical and molecular evidence (Morinville et al., 2004; Guan et al., 2005; Hack et al., 2005; Ma et al., 2006).
Much of our current knowledge on DOR anatomical localization and exocytotic translocation comes from previous studies dealing with postsynaptic DOR in the cell body, which allows translocation analysis with confocal microscopy (Morinville et al., 2004; Guan et al., 2005; Patwardhan et al., 2005). Demonstrating DOR translocation in synaptic terminals is more challenging technically, and our own confocal study in brainstem neurons has shown morphine-induced increase in DOR-immunoreactive varicosities apposing postsynaptic membrane (presumably synaptic contacts), consistent with the mechanism of DOR membrane trafficking (Ma et al., 2006). In this study of CeA neurons, we show a morphine-induced selective increase in DOR proteins in CeA synaptosomes, of which the preparation procedures have removed most cell body contents and cytoplasmic contents of synaptic terminals, such as endoplasmic reticulum, small synaptic vesicles, and, probably, intraterminal DOR contained in large dense-core vesicles, while keeping terminal membrane-bound proteins such as receptors and ion channels intact (Gottfried et al., 2003; Dunkley et al., 2008). This observation of increased DOR expression on terminal membrane, but not total DOR proteins in CeA neurons, further supports the notion that a similar mechanism of DOR membrane trafficking also occurs in central synaptic terminals and accounts for the morphine-induced recruitment of new functional DOR for synaptic modulation. The molecular determinants and signaling pathways that mediate chronic morphine-induced DOR membrane trafficking in central neurons are still unknown at present.
DOR Inhibition of Glutamate Neurotransmission. For presynaptic DOR, a previous study and our previous report have identified the induction of new functional DOR on GABAergic terminals in midbrain and brainstem neurons after exposure to chronic morphine (Hack et al., 2005; Ma et al., 2006). It is noticeable that all these observations of morphine induction of both pre- and postsynaptic DOR were made in central and peripheral neurons involved in pain sensation and opioid analgesia, showing a functional consequence of enhanced analgesic effects of DOR through its increased surface expression (Ma et al., 2006; Zhang et al., 2006; Cahill et al., 2007; Sykes et al., 2007). The current study provides evidence for the occurrence of this adaptive DOR function on central glutamate terminals in the CeA, suggesting that chronic exposure to opioids may have much broader influence on synaptic plasticity and opioid receptor functions across many brain functions and, particularly, extending the DOR adaptation to a brain area increasingly implicated in opioid reward and drug addiction. Given the critical roles of central glutamate synaptic activity and CeA functions in the brain's adaptive responses to opioids and other abused drugs in drug addiction (Baxter and Murray, 2002; See et al., 2003; Siggins et al., 2003), the present findings may stimulate new mechanistic pursuits in future studies on DOR functions in opioid reward and drug addiction in the context of prolonged exposure to MOR agonists.
Morphine Induction of DOR. Unlike previous studies of pain-modulating neurons with morphine administration in a noncontingent environment, the DOR function in this study was induced by morphine administration associated with a distinct environment through conditioning, a procedure required for CPP behavior. This raises an interesting question: Is the pharmacological effect of repeated morphine itself, without environmental conditioning, sufficient for induction of the presynaptic DOR function in CeA neurons? Based on those previous studies of morphine-induced DOR function in pain modulation, which apparently does not require conditioning, we propose that noncontingent morphine treatment would also induce the DOR function in amygdala neurons. However, this does not necessarily preclude a DOR role in the initiation and maintenance of CPP behavior because it is still unknown what are the differences between the same morphine treatments with and without conditioning in the extent, time course, and cellular distribution of the induced DOR function in CeA synapses, nor is the impact of preventing this DOR function on CPP behavior. Identifying the specific effect of environmental conditioning on the DOR function may reveal crucial information on the stimulus-reward learning function of the CeA and mechanisms for the development and maintenance of CPP behavior.
Functional Considerations. We have shown recently that alcohol- and morphine-induced enhancement of glutamate synaptic activity in CeA neurons is required to maintain the CPP behavior (Zhu et al., 2007). Our present findings demonstrate that it also requires a general activation of opioid receptors and suggest a likely role of the emerged DOR in this behavior of opioid reward through modulation of CeA glutamate synaptic transmission. Detailed synaptic and cellular mechanisms for this DOR function in opioid reward are unknown, and demonstrating such mechanisms in the CeA to account for the behavior still presents a significant challenge at present. This is mainly because we still know little about the overall impact of this new DOR function on neuronal and network activity in the CeA and, especially, about whether similar DOR adaptation also occurs on other synaptic sites in the CeA. Also unknown is the functional relationship between glutamate synaptic activity in the CeA and what drives the CPP behavior. Nevertheless, because MOR is essential for induction and maintenance of compulsive behaviors of opioid abuse, and abrupt inactivation or antagonism of MOR inevitably leads to devastating, aversive symptoms of opioid withdrawal (Williams et al., 2001; Contet et al., 2004), it is important to illustrate the brain's adaptive responses to abused drugs and underlying mechanisms to develop potential therapeutic targets for the treatment of drug addiction. The current study may provide a useful clue for future investigations of DOR roles in initiation and maintenance of opioid reward- and opioid dependence-related behaviors. As more is revealed regarding the adaptive changes and functional impacts of DOR in the CeA and in other reward-related brain areas, DOR and its trafficking-regulating components might serve as such a therapeutic target to reduce the rewarding properties of abused opioid compounds and other drugs of abuse in the treatment of drug addiction.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA023069]; and the University of Texas-M.D. Anderson Cancer Center [Institutional Research Grant].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.148908.
ABBREVIATIONS: CeA, central nucleus of the amygdala; MOR, μ-opioid receptor; DOR, δ-opioid receptor; CPP, conditioned-place preference; EPSC, excitatory postsynaptic current; PPR, paired-pulse ratio; DPDPE, [d-Pen2,d-Pen5]-enkephalin.
References
- Baxter MG and Murray EA (2002) The amygdala and reward. Nat Rev Neurosci 3 563-573. [DOI] [PubMed] [Google Scholar]
- Bie B, Peng Y, Zhang Y, and Pan ZZ (2005) cAMP-mediated mechanisms for pain sensitization during opioid withdrawal. J Neurosci 25 3824-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, and Morinville A (2007) Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci 28 23-31. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr and Nestler EJ (2002) Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci 25 610-615. [DOI] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, and Befort K (2004) Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol 14 370-378. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE and Stevens CF (1997) Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18 995-1008. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, and Robinson PJ (2008) A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc 3 1718-1728. [DOI] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, and Thompson RF (1999) The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature 402 294-296. [DOI] [PubMed] [Google Scholar]
- Ghijsen WE, Leenders AG, and Lopes da Silva FH (2003) Regulation of vesicle traffic and neurotransmitter release in isolated nerve terminals. Neurochem Res 28 1443-1452. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, and Dolan RJ (2003) Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301 1104-1107. [DOI] [PubMed] [Google Scholar]
- Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, et al. (2005) Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell 122 619-631. [DOI] [PubMed] [Google Scholar]
- Hack SP, Bagley EE, Chieng BC, and Christie MJ (2005) Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. J Neurosci 25 3192-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S and Bonci A (2005) Synaptic plasticity and drug addiction. Curr Opin Pharmacol 5 20-25. [DOI] [PubMed] [Google Scholar]
- Kieffer BL and Gavériaux-Ruff C (2002) Exploring the opioid system by gene knock-out. Prog Neurobiol 66 285-306. [DOI] [PubMed] [Google Scholar]
- Koob GF and Le Moal M (2008) Addiction and the brain antireward system. Annu Rev Psychol 59 29-53. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang Y, Kalyuzhny AE, and Pan ZZ (2006) Emergence of Functional {delta}-Opioid Receptors Induced by Long-Term Treatment with Morphine. Mol Pharmacol 69 1137-1145. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Connor M, Schnell SA, Christie MJ, Wessendorf MW, and Vaughan CW (2005) delta-opioid receptor-mediated actions on rostral ventromedial medulla neurons. Neuroscience 132 239-244. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, et al. (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383 819-823. [DOI] [PubMed] [Google Scholar]
- McKernan MG and Shinnick-Gallagher P (1997) Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390 607-611. [DOI] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Aibak H, Rymar VV, Pradhan A, Hoffert C, Mennicken F, Stroh T, Sadikot AF, O'Donnell D, et al. (2004) Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord. J Neurosci 24 5549-5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2004) Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci 25 210-218. [DOI] [PubMed] [Google Scholar]
- Pan ZZ (1998) mu-Opposing actions of the kappa-opioid receptor. Trends Pharmacol Sci 19 94-98. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, and Hargreaves KM (2005) Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci 25 8825-8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, Stäubli UV, and LeDoux JE (1997) Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390 604-607. [DOI] [PubMed] [Google Scholar]
- Sbrenna S, Marti M, Morari M, Calo' G, Guerrini R, Beani L, and Bianchi C (2000) Modulation of 5-hydroxytryptamine efflux from rat cortical synaptosomes by opioids and nociceptin. Br J Pharmacol 130 425-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, and McLaughlin J (2003) Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci 985 294-307. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, and De Lecea L (2003) Glutamatergic transmission in opiate and alcohol dependence. Ann N Y Acad Sci 1003 196-211. [DOI] [PubMed] [Google Scholar]
- Sykes KT, White SR, Hurley RW, Mizoguchi H, Tseng LF, and Hammond DL (2007) Mechanisms responsible for the enhanced antinociceptive effects of micro-opioid receptor agonists in the rostral ventromedial medulla of male rats with persistent inflammatory pain. J Pharmacol Exp Ther 322 813-821. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12 227-462. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, and Bonci A (2001) Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411 583-587. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Bartlett SE, and Whistler JL (2004) Opioid receptors. Annu Rev Biochem 73 953-990. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, and Manzoni O (2001) Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81 299-343. [DOI] [PubMed] [Google Scholar]
- Woolf CJ and Hashmi M (2004) Use and abuse of opioid analgesics: potential methods to prevent and deter non-medical consumption of prescription opioids. Curr Opin Investig Drugs 5 61-66. [PubMed] [Google Scholar]
- Zhang X, Bao L, and Guan JS (2006) Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci 27 324-329. [DOI] [PubMed] [Google Scholar]
- Zhu W, Bie B, and Pan ZZ (2007) Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci 27 289-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W and Pan ZZ (2004) Synaptic properties and postsynaptic opioid effects in rat central amygdala neurons. Neuroscience 127 871-879. [DOI] [PubMed] [Google Scholar]
- Zhu W and Pan ZZ (2005) Mu-opioid-mediated inhibition of glutamate synaptic transmission in rat central amygdala neurons. Neuroscience 133 97-103. [DOI] [PubMed] [Google Scholar]