Abstract
We recently showed that poly(ADP-ribose) polymerase (PARP) is activated within atherosclerotic plaques in an animal model of atherosclerosis. Pharmacological inhibition of PARP or reduced expression in heterozygous animals interferes with atherogenesis and may promote factors of plaque stability, possibly reflecting changes in inflammatory and cellular factors consistent with plaque stability. The current study addresses the hypothesis that pharmacological inhibition of PARP promotes atherosclerotic plaque regression. Using a high-fat diet-induced atherosclerosis apolipoprotein E(-/-) mouse model, we demonstrate that administration of the potent PARP inhibitor, thieno[2,3-c]isoquinolin-5-one (TIQ-A), when combined with a regular diet regimen during treatment, induced regression of established plaques. Plaque regression was associated with a reduction in total cholesterol and low-density lipoproteins. Furthermore, plaques of TIQ-A-treated mice were highly enriched with collagen and smooth muscle cells, displayed thick fibrous caps, and exhibited a marked reduction in CD68-positive macrophage recruitment and associated foam cell presence. These changes correlated with a significant decrease in expression of monocyte chemoattractant protein-1 and intercellular cell adhesion molecule-1, potentially as a result of a robust reduction in tumor necrosis factor expression. The PARP inhibitor appeared to affect cholesterol metabolism by affecting acyl-coenzymeA/cholesterol acyltransferase-1 expression but exerted no effect on cholesterol influx or efflux as assessed by an examination of the ATP-binding cassette transporter-1 and the scavenger receptor-A expression levels in the different experimental groups. In accordance, PARP inhibition may prove beneficial not only in preventing atherogenesis but also in promoting regression of preexisting plaques.
Atherosclerosis is a major contributor to morbidity and mortality in developed countries and is the underlying cause of a number of cardiovascular diseases (Strong and McGill, 1963; Libby, 2006). Atherosclerosis is a slowly developing, progressive disease characterized by the accumulation of lipids and fibrous components in the intima, the innermost layer of medium and large arteries. The formation of arterial fatty streaks as a result of lipid accumulation within macrophages and smooth muscle cells (SMCs) is the cellular hallmark of atherogenesis. Attempts by the immune system to clear these fatty streaks lead to the development of a chronic inflammation at the site of the lesion, creating conditions conducive to plaque development and progression that are aided by the existence of risk factors, such as dyslipidemia (Libby, 2006). It is becoming increasingly clear that the most important event in the onset of atherosclerosis-associated cardiovascular diseases is the rupture of such atherosclerotic plaques, with ensuing thrombotic occlusion of arteries (Libby, 2006).
Although stabilization of vulnerable plaques is an urgent proximate clinical objective, the ultimate clinical goal remains atherosclerotic plaque regression (Williams et al., 2008). Management of atherosclerosis is based on the control of primary risk factors, such as dyslipidemia, hypertension, and smoking, and predisposing conditions, such as metabolic syndrome and diabetes; however, there are no drugs that specifically target arterial plaques (Fazio and Linton, 2006). Hence, there is a critical need to develop therapeutic interventions that selectively target one or more features of the atheroma and thereby stimulate the regression of established atherosclerotic lesions.
Poly(ADP-ribose) polymerase (PARP) is family of proteins, of which PARP-1 is an important member. PARP-1 is a DNA repair-associated nuclear enzyme, which is activated in response to DNA damage (Meyer-Ficca et al., 2005). PARP-1 activation catalyzes the covalent coupling of branched chains of ADP-ribose units to various nuclear proteins, such as histones and PARP-1 itself (Meyer-Ficca et al., 2005). Excessive PARP-1 activation after DNA damage upon exposure to proinflammatory agents leads to acute depletion of the metabolic intermediates NAD+ and ATP. This activation leads to cell death because of energy deprivation, which ultimately participates in the overall process of inflammation (Cuzzocrea, 2005; Erdélyi et al., 2005). Although initially implicated in necrotic cell death (Ha and Snyder, 1999), PARP-1 has been found by our laboratory and others to be an active agent in the early stages of the apoptotic process (Boulares et al., 1999; Johnson, 2002).
We recently showed that PARP is activated within atherosclerotic plaques in an animal model of atherosclerosis (Oumouna-Benachour et al., 2007a). Decreasing PARP activity, through pharmacological inhibition or a reduction in PARP-1 gene dosage in PARP-1(+/-) mouse models, interferes with plaque development and may promote factors of plaque stability (Oumouna-Benachour et al., 2007a; Hans et al., 2008). These PARP-1-dependent changes may reflect a reduction in monocyte chemoattractant protein (MCP-1) and cellular changes related to plaque dynamics (Oumouna-Benachour et al., 2007a), including an increase in SMC content, decreased collagen degradation, and increased TIMP-2 expression. Support for these observations is provided by a recent report by von Lukowicz et al. (2008). These reported effects of PARP inhibition on atherosclerotic plaque dynamics suggest that PARP-1 activity may play a role in atherosclerotic plaque progression. In accordance, the current study was conducted to specifically test the hypothesis that PARP inhibition in combination with normolipidemic diet may promote regression of pre-established atherosclerotic plaques.
Materials and Methods
Animals, Diet, and Treatment Protocols. C57BL/6 ApoE(-/-) (The Jackson Laboratory, Bar Harbor, ME) mice were housed and bred in a pathogen-free animal care facility at the Louisiana State University Health Sciences Center (New Orleans, LA) and allowed full access to laboratory rodent chow and water. The Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) was followed, and experimental protocols were approved by the Louisiana State University Health Sciences Center Animal Care and Use Committee. Six-week-old ApoE(-/-) mice received a high-fat diet (Harlan Teklad, Madison, WI) containing 21% fat by weight (0.15% cholesterol) for 12 or 16 weeks. Some groups received a diet switch in the last 4 weeks with or without receiving intraperitoneal injections of the specific PARP inhibitor thieno[2,3-c]isoquinolin-5-one (TIQ-A) (Sigma-Aldrich, St. Louis, MO) (Chiarugi et al., 2003; Oumouna-Benachour et al., 2007a) of 3 mg/kg three times per week. Details of the different groups are described in Fig. 1A. Mice were fasted, anesthetized with ketamine/xylazine (60 and 3 mg/kg, respectively), and blood was drawn for sera preparation. Plasma cholesterol and LDL were analyzed using a commercially available kit according to the manufacturer's instructions (Cholestech, Hayward, CA).
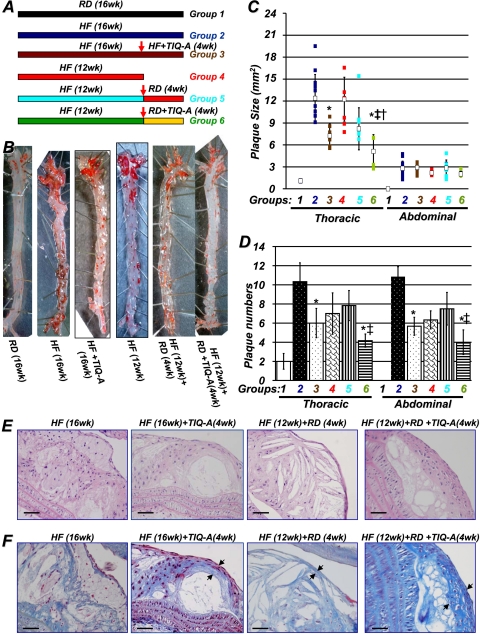
Fig. 1.
PARP inhibition by TIQ-A promotes atherosclerotic plaque regression in an animal model of atherosclerosis. A, diagram of the study. ApoE(-/-) mice (n = 60) were fed an HF diet for 12 weeks, after which one group was sacrificed immediately (n = 12), and the remaining were divided into four groups of at least 12 mice each. One of these groups was continued with the high-fat diet, one group was continued with the high-fat diet and received 3 mg/kg TIQ-A (3 times/week), and the other two were switched to an RD for 4 additional weeks, receiving 3 mg/kg TIQ-A (3 times/week) [HF (12 weeks) + RD + TIQ-A (4 weeks)] (n = 12) or vehicle [HF (12 weeks) + RD (4 weeks)] (n = 12); the control group received a regular diet throughout the 16 weeks (RD; 16 weeks). B, aortas from the different experimental groups were formalin (phosphate-buffered saline)-perfused, and atherosclerotic plaques were visualized by en face Oil Red O staining. Plaque size (C) and number (D) were determined as described previously (Oumouna-Benachour et al., 2007a). *, difference from ApoE(-/-) mice fed a high-fat diet for 16 weeks; ‡, difference from ApoE(-/-) mice fed a high-fat diet for 12 weeks, followed by 4 weeks of the regular diet; p < 0.01; open squares, medians. †, difference from ApoE(-/-) mice fed a high-fat diet and administered TIQ-A for 4 weeks. E, hematoxylin and eosin staining of representative plaques from the different experimental groups; bar, 5 μm. F, trichrome staining of representative plaques from the different experimental groups; bar, 5 μm. Arrows, TIQ-A not only increased collagen content but also caused thickening of fibrous caps.
Histology, Quantitation of Atherosclerosis, Immunohistochemistry, and Quantitation of Immunoreactivity. Perfusion fixed aortas were dissected and prepared for either Oil Red O staining using the Animal Models of Diabetic Complications Consortium protocol with slight modifications or paraffin embedding. Tissues were sectioned and subjected to hematoxylin and eosin or trichrome staining or immunohistochemistry using standard protocols. Lesion areas were assessed as described previously (Oumouna-Benachour et al., 2007a). Immunohistochemistry was conducted using antibodies to murine smooth muscle actin (SMA) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), CD68 (Abcam Inc., Cambridge, MA), ATP-binding cassette transporter (ABCA)-1 (Novus Biologicals, Inc., Littleton, CO), acyl-coenzyme A/cholesterol acyltransferase (ACAT)-1 (Cayman Chemical, Ann Arbor, MI), or SR-A (Abcam Inc.), and immunoreactivity was assessed in captured images of immunostained sections as described previously (Oumouna-Benachour et al., 2007a).
Foam Cell Generation, Treatment, and Immunoblot Analysis. Foam cells were generated from RAW 264.7 mouse macrophage cells as described previously (Oumouna-Benachour et al., 2007a), after which they were treated with the indicated concentrations of 7-ketocholesterol. Protein extracts were prepared and subjected to immunoblot analysis with antibodies to ABCA-1, ACAT-1, or SR-A.
Reverse Transcription and Real-Time PCR. RNA was extracted from thoracic aortas, and cDNA was generated by standard methods. Primers for MCP-1 were as follows: forward, 5′-ACT-GAA-GCC-AGC-TCT-CTC-TTC CTC-3′; and reverse, 5′-TCC-TTC-TTG-GGG-TCA-GCA-CAG-AC-3′; for ICAM-1: forward, 5′-TGC-GTT-TTG-GAG-CTA-GCG-GAC-CA-3′; and reverse, 5′-CGA-GGA-CCA-TAC-AGC-ACG-TGC-CAG-3′; for TNF: forward primer, 5′-CAT-CTT-CTC-AAA-ATT-CGA-GTG-ACA-A-3′; and reverse primer, 5′-TGG-GAG-TAG-ACA-AGG-TAC-AAC-CC-3′; for β-actin: forward, 5′-ACC-GTG-AAA-AGA-TGA-CCC-AGA-TC-3′; and reverse, 5′-TAG-TTT-CAT-GGA-TGC-CAC-AGG-3′. The specificity of the primers sets was confirmed in our previous studies (Oumouna-Benachour et al., 2007a,b; Zerfaoui et al., 2008) and as shown in the supplemental data. Amplification, detection, and data analysis were performed with the iCycler real-time PCR system (Bio-Rad, Hercules, CA).
Statistical Analysis. All data are expressed as mean ± S.D. of values from three independent experiments having at least six mice per group. PRISM software (GraphPad Software Inc., San Diego, CA) was used to analyze the differences between experimental groups by two-way analysis of variance. P values < 0.01 were considered significant.
Results
The PARP Inhibitor, TIQ-A, Promotes Regression of Previously Established Atherosclerotic Plaques in High-Fat Diet-Fed ApoE(-/-) Mice. ApoE(-/-) mice were fed a high-fat (HF) diet for 12 weeks to allow active plaques to develop. Mice were then divided into five groups (groups 2-6; for details, see Fig. 1A); group 1 consisted of ApoE(-/-) mice on a regular diet for 16 weeks, animals in group 2 were kept on a high-fat diet for 16 weeks, group 3 received an HF diet for 16 weeks and were given three times weekly injections of TIQ-A (3 mg/kg) for the last 4 weeks, animals in group 4 were sacrificed immediately after 12 weeks, animals in group 5 received HF an diet for 12 weeks and a regular diet for 4 weeks and received injections containing only diluent, and animals in group 6 received an HF diet for 12 weeks and were given three-times-weekly injections of TIQ-A (3 mg/kg) along with regular diet for 4 weeks. The rationale behind this strategy was predicated on the assumption that atherosclerotic patients subjected to plaque regression-promoting therapy would be simultaneously treated with cholesterol-lowering drugs, such as statins, and encouraged to reduce lipid intake. Figure 1B shows that a 12-week high-fat diet regimen induced pronounced plaque formation throughout the aorta, as assessed with Oil Red O staining (group 4). The high-fat diet for an additional 4 weeks further increased the plaque size (Fig. 1C) and number (Fig. 1D; group 2 versus 4). It is important to note that no apparent toxicity was detected in wild-type or ApoE(-/-) mice receiving TIQ-A injections as assessed by measuring hepatic enzymes (data not shown). Plaque size and number (Fig. 1, C and D) decreased slightly but insignificantly in the thoracic region of the aorta after switching to a regular diet during the last 4 weeks of the protocol (group 5 versus 2). However, TIQ-A along with regular diet clearly promoted the regression of established plaques in the thoracic and abdominal regions of the aorta (group 6). It is interesting that TIQ-A administration without the switch to regular diet also promoted plaque regression, albeit to a smaller extent than the latter group (group 3 versus 6). In the abdominal region, TIQ-A administration induced a significant reduction in plaque number (Fig. 1D). However, although lesion size trended lower in this region, these differences were not significant (Fig. 1C).
A microscopic examination of plaques from the different experimental groups revealed that plaques in ApoE(-/-) mice that received a high-fat diet throughout the 16-week experiment (group 2) were advanced, layered lesions with multiple fibrous caps and cholesterol-rich lipid cores within the intimal layer containing distinct large macrophage-derived foam cells and SMCs (Fig. 1E). The plaques from group 4 (HF for 12 weeks) also exhibited similar traits except that the plaques exhibited very thin, single-layered fibrous cap. It is interesting that plaques of group 5 [12 weeks HF + 4 weeks regular diet (RD)] were highly similar to plaques of group 4. In contrast, plaques from mice treated with TIQ-A during the final 4 weeks were much smaller, contained fewer foam cells, and exhibited an SMC-rich fibrous cap (group 6). The plaques in the TIQ-A-treated groups (groups 3 and 6) exhibited well contained lipid cores (Fig. 1E). Trichrome straining of these plaques clearly indicates a much thicker fibrous cap in the TIQ-A-treated experimental groups (Fig. 1F). It is noteworthy that these latter traits are primary features of stable atherosclerotic plaques, suggesting that TIQ-A administration in combination with regular diet regimen not only promoted plaque regression but also promoted factors that contribute to plaque stability.
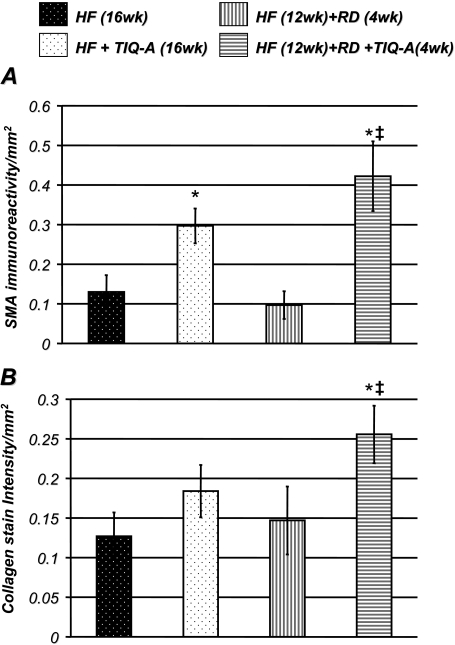
TIQ-A Administration Promotes an Enrichment of SMCs and an Increase in Collagen Content in Regressed Atherosclerotic Plaques. Figure 2A shows that TIQ-A treatment promoted a significant increase in smooth muscle actin immunoreactivity, confirming that SMCs were enriched within these plaques. This SMC enrichment was accompanied by a significant increase in collagen content per area as assessed by trichrome staining (Fig. 2B). It is interesting that although collagen content trended higher in plaques of the high-fat diet-fed experimental group receiving TIQ-A, without a diet switch, compared with their high-fat diet-only counterparts, the difference was not statistically different. It is important to note that, in our experimental model, diet switch did not significantly change SMA and collagen contents, which suggests that the promoted increase in these two traits by the PARP inhibitor was independent of superimposed lipid lowering. These results further support the interpretation that TIQ-A administration not only induces plaque regression but also promotes factors that contribute to plaque stability.
Fig. 2.
TIQ-A administration promotes an enrichment of SMCs and an increase in collagen content in regressed atherosclerotic plaques. Immunohistochemistry analysis with antibodies to SMA (A) or trichrome staining (B) of plaques from the different experimental groups; quantitation of immunoreactivity or trichrome stain was conducted using Image-Pro Plus software (MediaCybernetics, Inc., Bethesda, MD) and expressed as immunoreactivity per millimeter squared. *, difference from ApoE(-/-) mice fed the high-fat diet for 16 weeks; ‡, difference from ApoE(-/-) mice fed the high-fat diet for 12 weeks followed with 4 weeks of regular diet; P < 0.01.
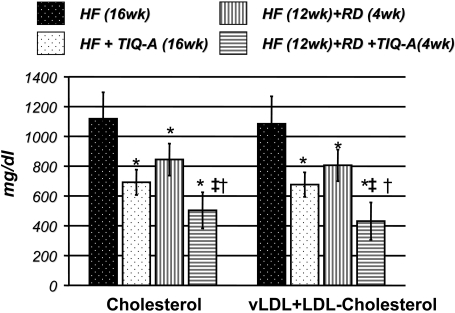
TIQ-A-Dependent Atherosclerotic Plaque Regression Is Associated with Lower Serum Lipid Levels in ApoE(-/-) Mice on a High-Fat-Followed-by-Regular-Diet Regimen. Examination of the lipid profile in sera of the different experimental groups after overnight fasting shows TIQ-A treatment significantly lowered total cholesterol and vLDL + LDL cholesterol levels in comparison with untreated mice (Fig. 3). These results suggest that the regression of pre-existing atherosclerotic plaques caused by TIQ-A administration observed in our experimental model may be due, at least in part, to reductions in lipid content. Regular diet alone for 4 weeks decreased cholesterol and vLDL + LDL levels, but the levels remained higher than TIQ-A treated mice, and the decrease seems to be insufficient to cause significant reduction in plaque size and number.
Fig. 3.
TIQ-A-dependent atherosclerotic plaque regression is associated with significant lowering in total serum cholesterol and LDL in ApoE(-/-) mice on a high-fat-followed-by-regular-diet regimen. The different experimental groups were fasted and anesthetized, and blood was drawn for sera preparation. Plasma cholesterol and LDL (milligrams per deciliter) were analyzed using a commercially available kit; n = 6. *, difference from ApoE(-/-) mice fed the high-fat diet for 16 weeks; ‡, difference from ApoE(-/-) mice fed the high-fat diet for 12 weeks followed by 4 weeks of regular diet; †, difference from ApoE(-/-) mice fed the high-fat diet and administered TIQ-A for 4 weeks. P < 0.01.
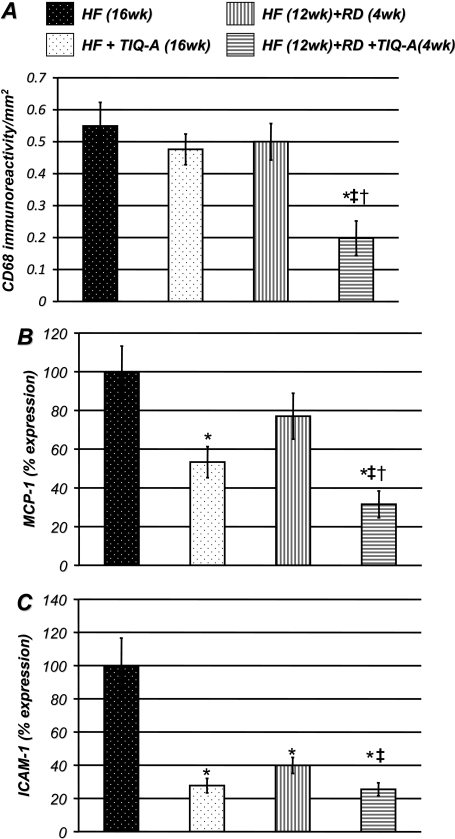
TIQ-A Administration Promotes Plaque Regression, in Part, by Reducing Macrophage Recruitment: Potential Role of Reduced Expression of the Chemokine, MCP-1, and the Adhesion Molecule, ICAM-1. The continuous recruitment of macrophages to lesions is considered a crucial factor in maintaining the size and complexity of atherosclerotic plaques (Libby, 2006). Figure 4A shows that TIQ-A treatment significantly reduced the intensity of CD68 immunoreactivity, suggesting that the population of macrophage-like cells within plaques was reduced compared with the plaques of animals that were not subjected to TIQ-A treatment. These results imply that treatment with the drug may alter the maintenance of the number of CD68-positive and macrophage-like cells within plaques and further suggest that this modulation of the resident CD68-positive cell population may be involved in plaque regression. Such effects may be associated with an alteration in the balance between recruitment of new monocytes and egress and viability of foam cells within atherosclerotic plaques. It is interesting that TIQ-A administration did not induce a lowering of CD-68 content in the high-fat diet-fed animal group without a diet switch. The data provide support for the notion that a combination of TIQ-A administration and a diet switch may be more effective in promoting atherosclerotic plaque regression. Given that very little is known about cell egress out of atherosclerotic plaques, we elected to focus, in our study, on the effects of the PARP inhibitor on cell recruitment.
Fig. 4.
TIQ-A administration promotes plaque regression, in part, by reducing macrophage recruitment: potential role of reduced expression of the chemokine, MCP-1, and the adhesion molecule, ICAM-1. A, immunohistochemistry analysis with antibodies to CD68 of plaques from ApoE(-/-) mice on different experimental protocols; quantitation of immunoreactivity was conducted using Image-Pro Plus software and expressed as immunoreactivity per millimeter squared. B, thoracic aortas isolated from the four experimental groups (n = 6) described in A were collected and subjected to RNA extraction and cDNA generation. cDNA was subjected to real-time PCR, with primers specific to murine MCP-1 (B) or ICAM-1 (C); β-actin was used as an internal control for normalization of expression values. *, difference from ApoE(-/-) mice fed the high-fat diet for 16 weeks; ‡, difference from ApoE(-/-) mice fed the high-fat diet for 12 weeks followed with 4 weeks of the regular diet; †, difference from ApoE(-/-) mice fed the high-fat diet and administered TIQ-A for 4 weeks. P < 0.01.
MCP-1 is a potent chemotactic factor that is up-regulated at sites of inflammation and control monocyte/macrophage trafficking (Libby, 2006). To test the hypothesis that the observed decrease in CD68-positive cells was associated with a reduction in the expression of chemotactic factors, we used quantitative RT-PCR to monitor TIQ-A-dependent changes in MCP-1 expression. Figure 4B shows that the expression of MCP-1 gene in the thoracic aorta, assessed using primers specific for murine MCP-1, and normalized to β-actin expression levels, was significantly reduced by TIQ-A administration (Fig. 4B). The reduction in MCP-1 gene expression was correlated with a decrease in MCP-1 immunoreactivity within plaques of TIQ-A-treated mice (data not shown). It is noteworthy that the combination of a diet switch along with TIQ-A administration achieved a much greater reduction in MCP-1 expression, clearly suggesting that the two approaches exert additive effects.
Expression of ICAM-1 has been shown to be critically involved in the later stages of atherosclerotic plaque progression (Libby, 2006). TIQ-A treatment significantly reduced expression of ICAM-1 in the thoracic aortas of treated mice as assessed by quantitative RT-PCR (Fig. 4C). Overall, the current study indicates that TIQ-A administration promotes the regression of previously established atherosclerotic plaques and suggests that it may do so, in part, by stalling the continuous recruitment of monocytes/macrophages and reducing retention of recruited macrophages/foam cells that are required for the maintenance of advanced plaques.
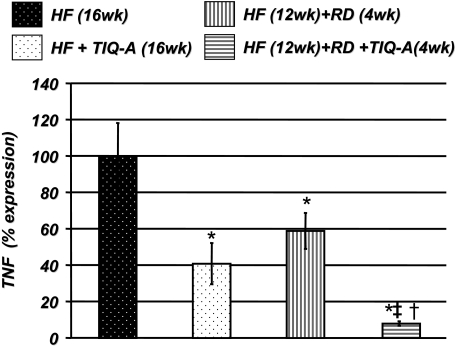
TIQ-A-Induced Reduction in the Expression of Chemokines and Adhesion Molecules Is Associated with a Significant Reduction in TNF Expression. A number of studies have shown that the expression of MCP-1 and adhesion molecules is highly associated with that of TNF (Libby, 2006). Figure 5 shows that TIQ-A administration significantly down-regulated TNF expression, confirming that TIQ-A-induced changes in the expression of inflammatory factors were accompanied by corresponding changes in TNF. The combination of TIQ-A administration and a diet switch exerted a more pronounced effect on TNF expression than that achieved by the two approaches individually.
Fig. 5.
TIQ-A-induced reduction in the expression of chemokines and adhesion molecules is associated with a modulation of TNF expression. Thoracic aortas isolated from the four experimental groups (n = 6) were collected and subjected to RNA extraction and cDNA generation. cDNA was subjected to real-time PCR with primers specific to murine TNF or β-actin. *, difference from ApoE(-/-) mice fed high-fat diet for 16 weeks; ‡, difference from ApoE(-/-) mice fed the high-fat diet for 12 weeks followed by 4 weeks of the regular diet; †, difference from ApoE(-/-) mice fed the high-fat diet and administered TIQ-A for 4 weeks. P < 0.01.
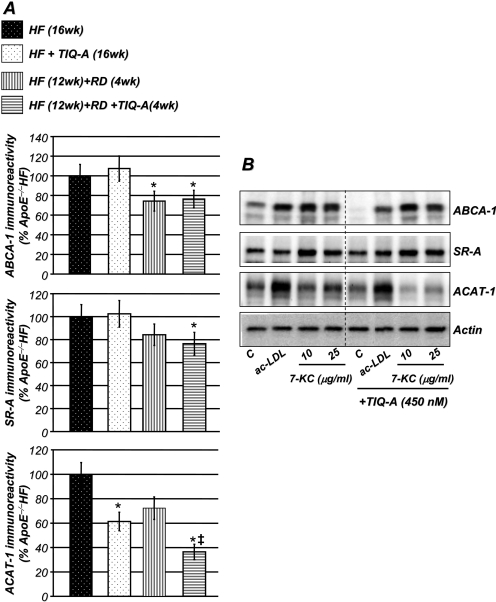
Treatment with the PARP Inhibitor, TIQ-A, Decreases ACAT-1 without Altering ABCA-1 or SR-A Expression in Vivo and in Lipid-Treated RAW 264.7-Derived Foam Cells. Alteration in cholesterol trafficking is considered as a possible mechanism responsible for lesion shrinkage and potential plaque regression (Williams et al., 2008). Thus, we examined the effects of TIQ-A treatment on the ABCA-1, a regulator of cholesterol efflux, SR-A, a macrophage scavenger receptor, and ACAT-1, a cholesterol-converting enzyme in plaques of the different experimental groups. Figure 6A shows that although TIQ-A treatment exerted no effect on ABCA-1 or SR-A expression, it significantly reduced ACAT-1 expression in atherosclerotic plaques compared with levels detected in plaques of ApoE(-/-) mice fed a high-fat diet for 16 weeks. Using an in vitro system with RAW 264.7-derived foam cells treated with either acetylated-LDL or 7-ketocholesterol, TIQ-A treatment exerted similar effects on expression of ABCA-1, SR-A, and ACAT-1 (Fig. 6B). The latter results were confirmed at the mRNA level by RT-PCR (data not shown). In accordance, the in vitro data support our observation using our animal model. Altogether, it is tempting to speculate that the PARP inhibitor affects intracellular cholesterol metabolism by affecting ACAT-1 expression but exerts no effect on SR-A-mediated cholesterol influx or ABCA-1-mediated cholesterol efflux.
Fig. 6.
Effects of TIQ-A on ABCA-1, SR-A, and ACAT-1 expression in vivo and in vitro. A, immunohistochemistry analysis with antibodies to ABCA-1, SR-A, or ACAT-1 of plaques from the different experimental groups; quantitation of immunoreactivity was conducted using Image-Pro Plus software and expressed as percentage immunoreactivity detected in plaques of ApoE(-/-) fed high-fat diet for 16 weeks. *, difference from ApoE(-/-) mice fed the high-fat diet for 16 weeks; ‡, difference from ApoE(-/-) mice fed the high-fat diet for 12 weeks followed by 4 weeks of regular diet; P < 0.01. B, foam cells were generated through an incubation with acetylated-LDL (10 μg/ml) as described previously (Oumouna-Benachour et al., 2007a). Cells were then treated with acetylated-LDL (10 μg/ml) or 7-ketocholesterol for an additional 12 h. Cell extracts were prepared and subjected to immunoblot analysis with antibodies to ABCA-1, SR-A, ACAT-1, or actin. These are representative blots for four different experiments.
Discussion
A number of strategies have been proposed to achieve the ultimate clinical objective of atherosclerotic plaque regression, but currently available options fall short of this goal (Williams et al., 2008). HMG-CoA reductase inhibitors (statins), which prevent or slow the progression of atherosclerosis (Frisinghelli and Mafrici, 2007), are the drugs of choice for the treatment of hypercholesterolemia. However, these drugs only reduce the risk of coronary heart disease morbidity or mortality by ∼30% (Frisinghelli and Mafrici, 2007). It is clear that the unmet need for drugs that specifically promote atherosclerotic plaque regression justifies an intense drug development effort. The current study, which grew out of our recent identification of PARP-1 as an important player in atherogenesis in a high-fat diet-based mouse model of atherosclerosis (Oumouna-Benachour et al., 2007a), was designed with this goal in mind. The maintenance of inflammation is a complicated process that involves the production of chemokines, such as MCP-1, growth factors, and the induction of cell death, all of which are required for atherosclerotic plaque progression and instability (Libby, 2006). Because of the potentially close connection between PARP-1 and some of these events and the finding that pharmacological inhibition or genetic reduction of PARP promotes factors of atherosclerotic plaque stability, we surmised that treatment with the PARP inhibitor would interfere with these key events and promote plaque regression. In this study, we present evidence to support the hypothesis that administration of the PARP inhibitor, TIQ-A, promotes plaque regression. We also provide an initial insight into the potential mechanism by which the PARP inhibitor might achieve its effects. Our results specifically suggest that TIQ-A might promote atherosclerotic plaque regression by modulating several factors required for the maintenance of advanced atherosclerotic plaques, including adhesion molecules, inflammatory factors, such as MCP-1 and TNF, and through a correction of dyslipidemia and effects on the survival of vascular cells, including CD-68-positive lipid-laden foam cells. It is noteworthy that TIQ-A administration, without a diet switch, partially increased SMC content and decreased lipid levels and MCP-1 and TNF expression but maintained CD-68 content in plaques of high-fat diet-fed ApoE(-/-) mice. The lack of an effect of the PARP inhibitor on CD-68 content may be associated with a combination of continued influx of monocytes/macrophages and/or SMCs taking up foam cell phenotypic appearance (Rong et al., 2003). It is important to mention that treatment of PARP-1-deficient ApoE(-/-) mice with TIQ-A did not cause any additive effect on plaque size and structure and on sera lipid profile after a high-fat diet regimen similar to the one used for this study (data not shown). In accordance, the observed effect of TIQ-A treatment in our experimental model may be attributed, specifically, to its inhibitory effect on PARP.
The effects of PARP inhibition, pharmacologically or by gene knockout, on expression of adhesion molecules, MCP-1, and TNF can be achieved independently of lipid lowering using both in vitro and animal model (Kraus and Lis, 2003; Zerfaoui et al., 2008). Thus, it is very plausible to predict that PARP inhibition may be promoting atherosclerotic plaque regression by inhibiting these inflammatory factors, in part, through lipid-lowering independent mechanisms. The latter needs to be confirmed using an in vivo system that does not rely heavily on exaggerated dyslipidemia, such as the scavenger receptor class B type I-deficient ApoE(-/-) mouse model developed by Krieger's group (Braun et al., 2002; Zhang et al., 2005). Although our results show that TIQ-A administration did not affect cholesterol trafficking, such an effect cannot be ruled out given the fact that the drug markedly decreased ACAT-1 expression. More experimentation is undoubtedly required to clarify the participation of cholesterol metabolism in the effect of the PARP inhibitor on plaque dynamics in our experimental model.
Macrophages may respond to TIQ-A-promoted reductions in chemokine expression by emigrating out of the plaques and thereby contribute to the process of atherosclerotic plaque regression. PARP inhibition, pharmacologically or by gene knockout, not only reduces the expression of MCP-1 and adhesion molecules but also exerts important and differential roles in the death of vascular cells that constitute atherosclerotic plaques (Oumouna-Benachour et al., 2007a; Hans et al., 2008). Although PARP inhibition protects against the death of endothelial cells and SMCs in response to a variety of inflammatory factors, including oxidized cholesterol, it also sensitizes lipid-laden foam cells to the cytotoxic effect of the oxidized cholesterol, 7-ketocholesterol (Hans et al., 2008). In accordance, the reduction in CD68-positive cells within atherosclerotic plaques after TIQ-A administration may be due, in part, to sensitization to oxidized cholesterol.
TIQ-A administration significantly reduced total cholesterol and vLDL + LDL. We reported recently (Oumouna-Benachour et al., 2007a) that TIQ-A treatment in high-fat diet-fed ApoE(-/-) mice caused a trend toward lower serum lipid levels compared with similarly fed animals that did not receive the drug, although the differences did not reach statistical significance. The significant reduction in serum lipids caused by TIQ-A treatment in the current study may be attributable to the switch to a regular diet and assessment of lipid profiles after an overnight fasting. It is noteworthy that regular diet alone did not promote a dramatic regression of plaques in our animal model (Fig. 1), which potentially can be attributed to the intrinsic high lipid levels in these mice, even while under a regular diet regimen (Fig. 3). The persistence of high levels of cholesterol and the combined vLDL, and LDL in ApoE(-/-) mice that were switched to regular diet is consistent with those recently reported by Glaros et al. (2008) showing only a 15% decrease in plasma cholesterol when ApoE(-/-) mice were switched from a 30-day high-fat diet to a regular diet for 60 days. Similar findings were reported by Wu et al. (2006) using LDL-R(-/-) mice that were subjected to a similar regimen. Although the mechanism(s) by which PARP inhibition reduces lipid content is unclear, it is worth noting that the essential requirement for plaque regression is a robust reduction in the plasma proatherogenic lipoprotein, ApoB, or an increase in the efficiency of reverse lipid transport from plaque into the liver (Williams et al., 2008). Whether PARP inhibition affects these processes remains to be examined. A recent study by Fujiwara et al. (2007) reported that esculeoside A, a spirosolane-type glycoside, reduces lipid contents, in a model identical to ours, by inhibiting expression of ACAT-1. Whether the effect of PARP inhibition on ACAT-1 expression is, in part, responsible for the lipid-lowering trait of TIQ-A administration remains to be determined.
In conclusion, our results suggest that administration of the PARP inhibitor, TIQ-A, not only interferes with atherosclerotic plaque development but also promotes regression of previously established plaques, potentially through a reduction in inflammatory factors and serum lipid levels. Thus, the use of PARP inhibitors may prove beneficial not only in preventing atherogenesis but also in promoting the regression of previously established atherosclerotic plaques.
Supplementary Material
Acknowledgments
We thank Drs. Patrick Delafontaine and Gray Malcom for critical reading of the manuscript.
This work was supported by the National Institutes of Health [Grants HL072889, 1P20RR18766] (to A.H.B.; overall Principal Investigator, D. Kapusta); and by the American Heart Association [Postdoctoral Fellowship 0825470E] (to C.P.H.).
doi:10.1124/jpet.108.145938.
ABBREVIATIONS: SMC, smooth muscle cell; PARP, poly(ADP-ribose) polymerase; MCP, monocyte chemoattractant protein; Apo, apolipoprotein; TIQ-A, thieno[2,3-c]isoquinolin-5-one; LDL, low-density lipoprotein; SMA, smooth muscle actin; ABCA, ATP-binding cassette transporter; ACAT, acyl-coenzyme A/cholesterol acyltransferase; SR, scavenger receptor; PCR, polymerase chain reaction; ICAM, intercellular cell adhesion molecule; TNF, tumor necrosis factor; HF, high fat; RD, regular diet; vLDL, very low-density lipoprotein; RT, reverse transcription.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
References
- Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, and Smulson M (1999) Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis: caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem 274 22932-22940. [DOI] [PubMed] [Google Scholar]
- Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, and Krieger M (2002) Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res 90 270-276. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Meli E, Calvani M, Picca R, Baronti R, Camaioni E, Costantino G, Marinozzi M, Pellegrini-Giampietro DE, Pellicciari, et al. (2003) Novel isoquinolinone-derived inhibitors of poly(ADP-ribose) polymerase-1: pharmacological characterization and neuroprotective effects in an in vitro model of cerebral ischemia. J Pharmacol Exp Ther 305 943-949. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S (2005) Shock, inflammation and PARP. Pharmacol Res 52 72-82. [DOI] [PubMed] [Google Scholar]
- Erdélyi K, Bakondi E, Gergely P, Szabó C, and Virág L (2005) Pathophysiologic role of oxidative stress-induced poly(ADP-ribose) polymerase-1 activation: focus on cell death and transcriptional regulation. Cell Mol Life Sci 62 751-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio S and Linton M (2006) Failure of ACAT inhibition to retard atherosclerosis. N Engl J Med 354 1307-1309. [DOI] [PubMed] [Google Scholar]
- Frisinghelli A and Mafrici A (2007) Regression or reduction in progression of atherosclerosis, and avoidance of coronary events, with lovastatin in patients with or at high risk of cardiovascular disease: a review. Clin Drug Investig 27 591-604. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Kiyota N, Hori M, Matsushita S, Iijima Y, Aoki K, Shibata D, Takeya M, Ikeda T, Nohara T, et al. (2007) Esculeogenin A, a new tomato sapogenol, ameliorates hyperlipidemia and atherosclerosis in ApoE-deficient mice by inhibiting ACAT. Arterioscler Thromb Vasc Biol 27 2400-2406. [DOI] [PubMed] [Google Scholar]
- Glaros EN, Kim WS, Quinn CM, Jessup W, Rye KA, and Garner B (2008) Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J Lipid Res 49 324-331. [DOI] [PubMed] [Google Scholar]
- Ha HC and Snyder SH (1999) Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A 96 13978-13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans CP, Zerfaoui M, Naura AS, Catling A, and Boulares AH (2008) Differential effects of PARP inhibition on vascular cell survival and ACAT-1 expression favouring atherosclerotic plaque stability. Cardiovasc Res 78 429-439. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Johnson IT (2002) Anticarcinogenic effects of diet-related apoptosis in the colorectal mucosa. Food Chem Toxicol 40 1171-1178. [DOI] [PubMed] [Google Scholar]
- Kraus WL and Lis JT (2003) PARP goes transcription. Cell 113 677-683. [DOI] [PubMed] [Google Scholar]
- Libby P (2006) Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 83 456S-460S. [DOI] [PubMed] [Google Scholar]
- Meyer-Ficca ML, Meyer RG, Jacobson EL, and Jacobson MK (2005) Poly(ADP-ribose) polymerases: managing genome stability. Int J Biochem Cell Biol 37 920-926. [DOI] [PubMed] [Google Scholar]
- Oumouna-Benachour K, Hans CP, Suzuki Y, Naura A, Datta R, Belmadani S, Fallon K, Woods C, and Boulares AH (2007a) Poly(ADP-ribose) polymerase inhibition reduces atherosclerotic plaque size and promotes factors of plaque stability in apolipoprotein E-deficient mice: effects on macrophage recruitment, nuclear factor-kappaB nuclear translocation, and foam cell death. Circulation 115 2442-2450. [DOI] [PubMed] [Google Scholar]
- Oumouna-Benachour K, Oumouna M, Zerfaoui M, Hans C, Fallon K, and Boulares AH (2007b) Intrinsic resistance to apoptosis of colon epithelial cells is a potential determining factor in the susceptibility of the A/J mouse strain to dimethylhydrazine-induced colon tumorigenesis. Mol Carcinog 46 993-1002. [DOI] [PubMed] [Google Scholar]
- Rong JX, Shapiro M, Trogan E, and Fisher EA (2003) Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci U S A 100 13531-13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong JP and McGill HC Jr (1963) The natural history of aortic atherosclerosis: relationship to race, sex, and coronary lesions in New Orleans. Exp Mol Pathol 52 (Suppl 1): 15-27. [PubMed] [Google Scholar]
- von Lukowicz T, Hassa PO, Lohmann C, Borén J, Braunersreuther V, Mach F, Odermatt B, Gersbach M, Camici GG, Stähli BE, et al. (2008) PARP1 is required for adhesion molecule expression in atherogenesis. Cardiovasc Res 78 158-166. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Feig JE, and Fisher EA (2008) Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med 5 91-102. [DOI] [PubMed] [Google Scholar]
- Wu L, Vikramadithyan R, Yu S, Pau C, Hu Y, Goldberg IJ, and Dansky HM (2006) Addition of dietary fat to cholesterol in the diets of LDL receptor knockout mice: effects on plasma insulin, lipoproteins, and atherosclerosis. J Lipid Res 47 2215-2222. [DOI] [PubMed] [Google Scholar]
- Zerfaoui M, Suzuki Y, Naura AS, Hans CP, Nichols C, and Boulares AH (2008) Nuclear translocation of p65 NF-κB is sufficient for VCAM-1, but not ICAM-1, expression in TNF-stimulated smooth muscle cells: differential requirement for PARP-1 expression and interaction. Cell Signal 20 186-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Picard MH, Vasile E, Zhu Y, Raffai RL, Weisgraber KH, and Krieger M (2005) Diet-induced occlusive coronary atherosclerosis, myocardial infarction, cardiac dysfunction, and premature death in scavenger receptor class B type I-deficient, hypomorphic apolipoprotein ER61 mice. Circulation 111 3457-3464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.