Abstract
5-Oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-oxo-ETE) is a metabolite of the 5-lipoxygenase (5-LO) product 5S-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-HETE), formed by the microsomal enzyme 5-hydroxyeicosanoid dehydrogenase (5-HEDH). 5-oxo-ETE is a chemoattractant for neutrophils and eosinophils, both in vitro and in vivo. To examine the substrate selectivity of 5-HEDH and to search for potential inhibitors, we prepared a series of 5S-hydroxy fatty acids (C12 to C20 containing zero to four double bonds) by total chemical synthesis and examined their metabolism by microsomes from monocytic U937 cells. Although most of these fatty acids were oxidized to their 5-oxo metabolites by 5-HEDH, 5-HETE seemed to be the best substrate. However, substrates containing less than 16 carbons, a methylated α-carboxyl group, or a hydroxyl group at the ω-end of the molecule were not substantially metabolized. Some of the fatty acids tested were fairly potent inhibitors of the formation of 5-oxo-ETE by 5-HEDH, in particular 5-hydroxy-6-octadecenoic acid and 5-hydroxy-6-eicosenoic acid. Both substances selectively inhibited 5-oxo-ETE formation by human peripheral blood mononuclear cells incubated with arachidonic acid and calcium ionophore without affecting the formation of leukotriene B4, 12-HETE, or 12-hydroxy-5,8,10-heptadecatrienoic acid. We conclude that the requirements for appreciable metabolism by 5-HEDH include a chain length of at least 16 carbons, a free α-carboxyl group, and a hydrophobic group at the ω-end of the molecule. 5-Hydroxy-Δ6 C18 and C20 fatty acids selectively inhibit 5-HEDH without inhibiting 5-LO, leukotriene A4 hydrolase, 12-lipoxygenase, or cyclooxygenase. Such compounds may be useful in defining the role of 5-oxo-ETE and its mechanism of synthesis.
The 5-lipoxygenase (5-LO) pathway results in the formation of a series of products with potent proinflammatory effects, including leukotrienes (LTs) B4, C4, and D4, and 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-oxo-ETE). LTB4 acts principally via the BLT1 receptor and has potent stimulatory effects on neutrophils, monocytes, and lymphocytes, whereas LTD4 acts through the cysLT1 and cysLT2 receptors and is an important mediator in asthma (Funk, 2001). 5-oxo-ETE is a potent activator of eosinophils, neutrophils, and monocytes, inducing cell migration, calcium mobilization, and actin polymerization (Norgauer et al., 1996; Sozzani et al., 1996; Powell and Rokach, 2005). It also stimulates degranulation and activates the respiratory burst, especially after priming with cytokines such as granulocyte macrophage–colony-stimulating factor (O'Flaherty et al., 1996a,b). It induces transendothelial migration of eosinophils in vitro (Dallaire et al., 2003) and elicits tissue infiltration of eosinophils into the lungs (Stamatiou et al., 1998) and eosinophils and neutrophils into the skin (Muro et al., 2003). 5-oxo-ETE has also been reported to promote the survival of prostate tumor cells (Ghosh and Myers, 1998; Sundaram and Ghosh, 2006). Its actions are mediated by the OXE receptor (Hosoi et al., 2002), which is a G protein-coupled receptor that is highly expressed by eosinophils, neutrophils, and monocytes (Jones et al., 2003) and prostate tumor cells (Sundaram and Ghosh, 2006).
The formation of the above products is initiated by the oxidation of arachidonic acid (AA) to LTA4 and 5S-hydroperoxy-6,8,11,14-eicosatetraenoic acid (5-HpETE) by 5-LO (Borgeat and Samuelsson, 1979), which is abundant in most types of inflammatory cells. LTA4 is converted by LTA4 hydrolase and LTC4 synthase to LTB4 and LTC4, respectively, with the latter being further metabolized to LTD4 by γ-glutamyl transpeptidase (Funk, 2001). 5-HpETE is rapidly reduced to 5S-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-HETE), which is oxidized by 5-hydroxyeicosanoid dehydrogenase (5-HEDH) to 5-oxo-ETE (Powell et al., 1992).
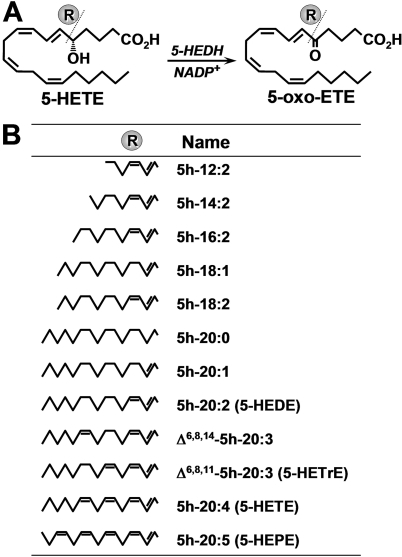
5-HEDH is a microsomal enzyme that requires the cofactor NADP+, which is normally present at only low concentrations within cells, thus limiting the synthesis of 5-oxo-ETE under basal conditions. However, the oxidation of 5-HETE to 5-oxo-ETE is strongly stimulated when intracellular NADP+ levels are increased after exposure of cells to oxidative stress or, in phagocytic cells, activation of the respiratory burst (Powell and Rokach, 2005). 5-HEDH selectively oxidizes eicosanoids containing a hydroxyl group in the S configuration followed by a 6-trans double bond. Although LTB4 has a 5S-hydroxyl group, it is not a substrate for this enzyme because the double bond in the 6-position is in the cis configuration. The impact of chain length and the number of double bonds of the substrate on metabolism by 5-HEDH are not known. Because such information is vital for the design of both 5-HEDH inhibitors and reagents for the purification of the enzyme, which has not yet been cloned, we prepared a series of 5-HETE analogs with different numbers of carbon atoms and double bonds (Fig. 1) and determined whether these compounds could serve as substrates for 5-HEDH and/or inhibit the conversion of 5-HETE to 5-oxo-ETE by this enzyme.
Fig. 1.
Metabolism of 5-hydroxy fatty acids by 5-HEDH. A, conversion of 5-HETE to 5-oxo-ETE by 5-HEDH. B, structures of 5S-hydroxy fatty acids that were tested for substrate and/or inhibitor activity with respect to microsomal 5-HEDH.
Materials and Methods
Materials. 5-HETE (Zamboni and Rokach, 1983), 5-oxo-ETE (Khanapure et al., 1998), and 5S,18-dihydroxy-6E,8Z-octadecadienoic acid (5,18-diOH-18:2) (Cossette et al., 2008) were prepared by total organic synthesis. 5-HETE methyl ester was prepared by treatment of 5-HETE with diazomethane and was purified by reversed-phase high-performance liquid chromatography (RP-HPLC). 13S-Hydroxy-9Z,11E-octadecadienoic acid (13-HODE) was prepared by incubating linoleic acid (Nu-Chek Prep, Inc., Elysian, MN) with soybean lipoxygenase (Sigma-Aldrich, St. Louis, MO) (Hamberg and Samuelsson, 1967). 5h-20:5 (5-HEPE) and NADP+ were purchased from Cayman Chemical (Ann Arbor, MI) and Roche Diagnostics (Indianapolis, IN), respectively. Phorbol 12-myristate 13-acetate (PMA) and t-butyl hydroperoxide (tBuOOH) were obtained from Sigma-Aldrich, whereas A23187 was purchased from Calbiochem (San Diego, CA).
Chemical Synthesis of 5-HETE Analogs. Except for 5-HETE and 5h-20:5 (see above), all of the 5-hydroxy fatty acids used in this study were prepared by total chemical synthesis by methods that will be described separately. All structures were confirmed by NMR and high-resolution mass spectrometry. The purity of all products was checked by HPLC before use, and if any degradation products were present, they were repurified by RP-HPLC.
Differentiated U937 Cells. U937 cells were obtained from American Type Culture Collection (Manassas, VA) and were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum, l-glutamine (2 mM), sodium bicarbonate (2.5 g/l), penicillin (100 U/ml), and streptomycin (100 μg/ml). To induce differentiation into macrophage-like cells, 106 cells/ml were incubated with PMA (18 nM) for 4 days (Erlemann et al., 2007). At this time, the medium was replaced by PBS, and the cells were suspended by scraping with a rubber policeman. After washing by centrifugation, the cells were resuspended in PBS.
Preparation of Microsomal Fractions. Differentiated U937 cells (2 × 108) or human neutrophils, prepared as described below, were suspended in 20 ml of PBS supplemented with 1 mM phenylmethylsulfonyl fluoride and disrupted by sonication at a setting of 40 cycles/s (model 4710 ultrasonic homogenizer; Sonics & Materials, Inc., Newtown, CT) on ice for 4 × 8 s with 1-min intervals for cooling. The disrupted cells were successively centrifuged at 4°C at 1500g for 10 min, 10,000g for 10 min, and 150,000g for 120 min, and the final pellet was resuspended in 20 mM sodium phosphate buffer, pH 7.4. Protein was measured with the Bio-Rad (Hercules, CA) DC protein assay kit.
Preparation of Neutrophils and Peripheral Blood Mononuclear Cells. Blood from healthy subjects was treated with Dextran 500 (Sigma-Aldrich) for 45 min at 4°C, followed by centrifugation over Ficoll-Paque (GE Healthcare, Piscataway, NJ). Peripheral blood mononuclear cells, which were present at the interface, were washed by centrifugation and resuspended in PBS at a concentration of 5 × 106 cells/ml. Neutrophils were obtained by resuspending the pellet in PBS and washing by centrifugation. CaCl2 (1.8 mM) and MgCl2 (1 mM) were added to intact peripheral blood mononuclear cells (PBMCs) 5 min before incubation with AA.
Measurement of Oxo-Eicosanoids by Precolumn Extraction-RP-HPLC. Incubations with 5-hydroxy fatty acids were terminated by the addition of 0.65 ml of methanol (microsomes) or 0.65 ml of methanol containing 0.15% trifluoroacetic acid (intact cells) and cooling to 0°C. The concentration of methanol in each sample was then adjusted to 30% by addition of water and 13-HODE (100 ng) was added as an internal standard. In the cases of 5h-12:2 and 5h-14:2, the final concentration of methanol was 15%. Eicosanoids were analyzed by precolumn extraction/RP-HPLC (Powell, 1987) using a modified Waters Alliance system (Waters, Milford, MA). The stationary phase was a column (150 × 3.9 mm) of octadecylsilyl-silica (4-μm particle size Novapak C18 column; Waters). The mobile phases used are indicated in Table 1 and the legend to Fig. 6. Products were identified on the bases of their UV spectra and comparison of their retention times (tRs) with those of authentic standards (Table 1). They were quantitated by comparing the areas of their peaks of UV absorbance at their λmax with that of the internal standard, 13-HODE, and were corrected for differences in extinction coefficients. The extinction coefficients used were as follows: 13-HODE, 23,000; 5-HETE, 27,000; 5-oxo-ETE, 22,000; and 5-oxo-18:1 and 5-oxo-20:1, 13,800. The extinction coefficients of other 5-oxo-fatty acids were assumed to be identical to that of 5-oxo-ETE. All solvents were of HPLC grade.
TABLE 1.
Chromatographic properties of 5-hydroxy fatty acids and their 5-oxo metabolites Fatty acids were analyzed by automated precolumn extraction/RP-HPLC as described under Materials and Methods. All of the analogs contain a Δ6 trans-double bond and either a 5S-hydroxyl or a 5-oxo group as indicated. The tR values for the 5-oxo products were confirmed by comparison with authentic standards.
|
Analog
|
tR
|
Precolumn Solvent (%
MeOH)a
|
Gradientb
|
|
|---|---|---|---|---|
| 5-Hydroxy | 5-Oxo | |||
| min | ||||
| 12:2 | 5.8 | 7.05 | 15 | 1 |
| 14:2 | 8.3 | 9.6 | 15 | 1 |
| 16:2 | 10.8 | 11.8 | 30 | 1 |
| 18:1 | 14.1 | 15.4 | 30 | 2 |
| 18:2 | 13.3 | 14.2 | 30 | 1 |
| 20:1 | 17.6 | 18.4 | 30 | 2 |
| 20:2 | 15.7 | 16.5 | 30 | 1 |
| Δ6,8,14-20:3 | 13.9 | 14.8 | 30 | 1 |
| Δ6,8,11-20:3 | 14.3 | 15.2 | 30 | 1 |
| 20:4 | 12.8 | 13.7 | 30 | 1 |
| 20:5 | 11.5 | 12.4 | 30 | 1 |
| 20:4-Me | 11.9 | 13.1 | 30 | 3 |
| 18h-18:2 | 5.6 | 6.8 | 15 | 4 |
The solvent used to equilibrate the precolumn was MeOH/water containing 2.5 mM H3PO4
All mobile phases were linear gradients between two solvents: gradient 1 [25–92% solvent B (acetonitrile/acetic acid (100/0.02)] in solvent A [water/acetic acid (100:0.02)] over 16 min; gradient 2 (40–95% B over 20 min); gradient 3 (45–99% acetonitrile in water over 16 min); and gradient 4 (35–75% B over 15 min)
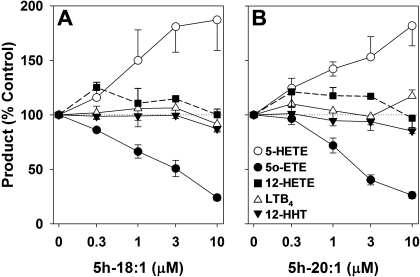
Fig. 6.
Effects of 5h-18:1 and 5h-20:1 on the formation of AA metabolites by human PBMCs. PBMCs (5 × 106 cells in 1 ml) were preincubated for 1 min with either 5h-18:1 (n = 6) (A) or 5h-20:1 (n = 4) (B) and then incubated with AA (20 μM), A23187 (5 μM), and tBuOOH (100 μM) for 10 min. The amounts of 5-HETE (○), 12-HETE (▪), 12-HHT (▾), LTB4 (▵), and 5-oxo-ETE (•) were analyzed by precolumn extraction/RP-HPLC as described under Materials and Methods using a linear gradient prepared from solvents A (water), B (acetonitrile), and C (methanol), all containing 0.02% acetic acid, as follows: 0 min, 46.5% A, 28.5% B, 25% C; and 24 min, 11.1% A, 37.6% B, 51.3% C at a flow rate of 1 ml/min. All values are means ± S.E.
Results
Metabolism of 5-Hydroxy Fatty Acids by 5-HEDH in U937 Cell Microsomes. We examined the ability of 5-HEDH to metabolize a series of analogs of 5-HETE in the presence of NADP+ (100 μM). To obtain an approximation of the maximal rates of metabolism of these compounds, a relatively high substrate concentration (3 μM) was used in the presence of a relatively low concentration of microsomal protein (15 μg/ml protein). The 5-oxo metabolites could readily be detected on the bases of their UV absorbance and chromatographic properties. With the exception of 5h-12:2, all of the analogs containing the conjugated Δ6,8-diene chromophore were converted to the corresponding Δ6,8-dienones, resulting in increases in the λmax from ∼235 to ∼280 nm and increased retention times (Table 1). The identities of the 5-oxo products were all confirmed by comparison of their chromatographic properties and UV spectra with those of authentic chemically synthesized compounds. The retention times of the synthetic 5-hydroxy fatty acids and their 5-oxo metabolites are shown in Table 1.
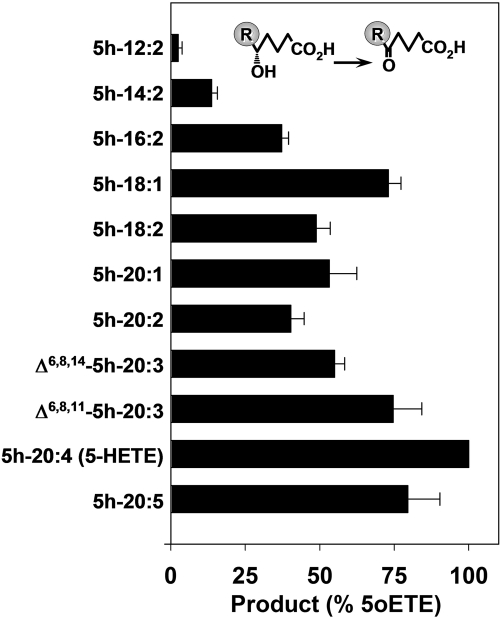
Among the compounds tested, 5-HETE was the best substrate for 5-HEDH. 5h-20:5 and Δ6,8,11-5h-20:3 were also good substrates, being metabolized to approximately 80% the extent of 5-HETE (Fig. 2). Δ6,8,14-5h-20:3 was metabolized at a somewhat lower rate than Δ6,8,11-5h-20:3 (approximately 55% the extent of 5-HETE). Among the series of Δ6,8 5-hydroxy-dienoic acids tested, those with chain lengths between 16 and 20 were oxidized by approximately 40 to 50% the extent of 5-HETE. Further reduction of the chain length of the fatty acid to 14 carbons (5h-14:2) considerably reduced the degree of metabolism by 5-HEDH to approximately 14% that of 5-HETE, whereas little or no metabolism was observed for the 12-carbon analog 5h-12:2.
Fig. 2.
Metabolism of 5-hydroxy fatty acids by microsomal 5-HEDH. Microsomal fractions (15 μg/ml) from PMA-differentiated U937 cells were incubated with 5-hydroxy fatty acids (3 μM) in the presence of NADP+ (100 μM) for 10 min at 37°C. The amounts of 5-oxo products formed were determined by RP-HPLC (Table 1). The values are means ± S.E. of four independent experiments.
It is somewhat surprising that 5h-18:1 was a somewhat better substrate for 5-HEDH than 5h-18:2. Although 5h-18:1 and 5h-20:1 did not contain a UV chromophore, the 5-oxo-products could readily be quantitated because of their UV absorbance at 225 nm because of the 5-oxo-6-ene chromophore. Because we were successful in synthesizing a relatively large amount of 5-oxo-18:1, it was possible to reliably determine its molar extinction coefficient, which was calculated to be 13,800.
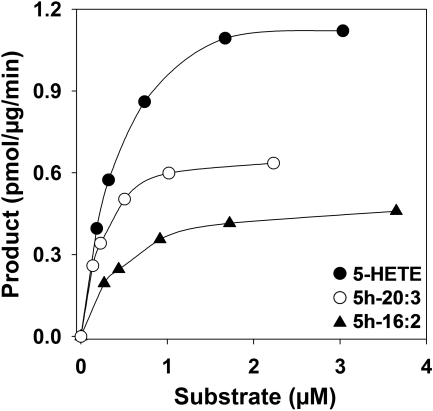
We further investigated the metabolism of those 5-hydroxy fatty acids that could be formed from endogenous substrates, including 5h-18:2, Δ6,8,11-5h-20:3, and 5h-20:5, which can be formed from sebaleic acid (Cossette et al., 2008), Mead acid (Patel et al., 2008), and 5,8,11,14,17-eicosapentaenoic acid (Powell et al., 1995), respectively. We also included 5h-16:2 because it is the shortest 5-hydroxy-fatty acid that was appreciably metabolized. The Km and Vmax values for the above substrates were determined by nonlinear regression (Fig. 3; Table 2). The Vmax values for 5-HETE and 5h-20:5 were significantly higher than those for the other three compounds tested, which were in the order Δ6,8,11-5h-20:3 > 5h-18:2 ≈ 5h-16:2. The Km value for 5h-18:2 was significantly lower than those of all of the other compounds tested, which were in the order 5h-16:2 ≈ Δ6,8,11-5h-20:3 ≈ 5-HETE <5h-20:5. Overall, 5-HETE was the best substrate, whereas 5h-16:2 was the poorest substrate among this series of compounds.
Fig. 3.
Effects of substrate concentration on the amounts of 5-oxo products formed by microsomal 5-HEDH. Microsomal fractions from PMA-differentiated U937 cells were incubated with different concentrations of 5h-16:2 (▴), Δ6,8,11-5h-20:3 (○), or 5-HETE (•) as described in the legend to Fig. 2, and the amounts of 5-oxo products formed were determined by RP-HPLC (Table 1). The values are from a single experiment and are representative of five independent experiments with similar results.
TABLE 2.
Km and Vmax values for the metabolism of some 5S-hydroxy fatty acids by 5-HEDH-containing U937 cell microsomes Various concentrations of 5-hydroxy fatty acids were incubated with U937 cell microsomes (15 μg/ml protein) for 10 min at 37°C. The amounts of 5-oxo products formed were determined by RP-HPLC as shown in Table 1. The values were determined by nonlinear regression using GraFit software and are means ± S.E. (n = 5).
| Substrate | Km | Vmax |
|---|---|---|
| nM | pmol / min / mg | |
| 5h-16:2 | 435 ± 114 | 383 ± 42 |
| 5h-18:2 | 275 ± 99 | 445 ± 59 |
| Δ6,8,11-5h-20:3 | 436 ± 158 | 608 ± 66 |
| 5-HETE | 516 ± 190 | 1062 ± 147 |
| 5h-20:5 | 679 ± 151 | 872 ± 146 |
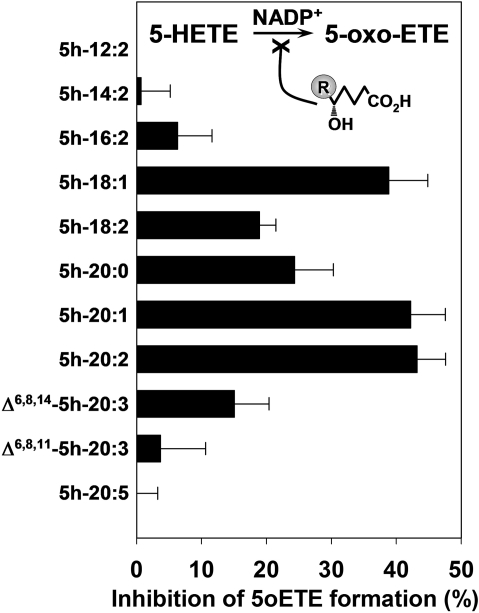
Inhibition of 5-oxo-ETE Formation by 5-Hydroxy Fatty Acids. We investigated the capability of 5-hydroxy fatty acids (0.3 μM) to inhibit the oxidation of 5-HETE (1 μM) to 5-oxo-ETE (Fig. 4). The use of a low inhibitor concentration permitted better discrimination of the abilities of these compounds to inhibit 5-HETE oxidation. It is interesting that the relative abilities of 5-hydroxy fatty acids to inhibit 5-oxo-ETE formation were quite different from their abilities to act as substrates for 5-HEDH. The best 5-HEDH substrates (5h-20:5 and Δ6,8,11-5h-20:3) exhibited little or no inhibitory activity under these conditions, although at higher concentrations, they did inhibit 5-oxo-ETE formation. Likewise, the poorest substrates for 5-HEDH (5h-12:2 and 5h-14:2) did not display any detectable inhibitory activity. The most active 5-HEDH inhibitors were 5h-18:1, 5h-20:1, and 5h-20:2, which all inhibited 5-oxo-ETE formation by approximately 40 to 45% when present at a concentration less than one-third that of the substrate 5-HETE.
Fig. 4.
Inhibition of 5-HEDH by 5-hydroxy fatty acids. Microsomal fractions (15 μg/ml) from PMA-differentiated U937 cells were incubated with 5-HETE (1 μM) in the presence of various 5-hydroxy fatty acids (0.3 μM) and NADP+ (100 μM) for 10 min at 37°C. The amounts of 5-oxo-ETE formed were determined by RP-HPLC. The values are means ± S.E. of four independent experiments.
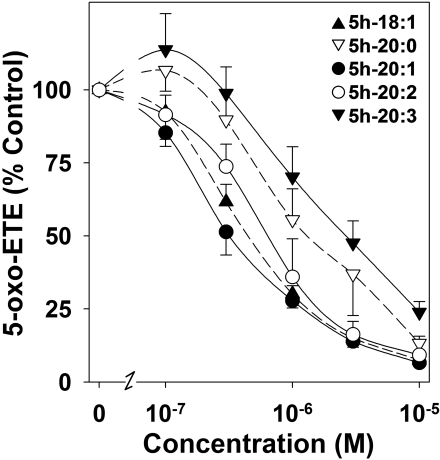
To investigate in more detail the inhibitory effects of some of the 5-HETE analogs, different concentrations were preincubated for 5 min with U937 cell microsomes, followed by incubation with 5-HETE (1 μM) in the presence of NADP+ for 10 min. Concentration-response curves for these 5-hydroxy fatty acids are shown in Fig. 5. 5h-20:1 (IC50, 0.37 ± 0.08 μM) and 5h-18:1 (IC50, 0.47 ± 0.08 μM) had similar potencies in inhibiting 5-oxo-ETE formation, whereas 5-hydroxy-20:2 (IC50, 0.67 ± 0.13 μM) seemed to be slightly less potent. 5h-20:0 (IC50, 2.1 ± 1.1 μM) and Δ6,8,11-5h-20:3 (IC50, 2.9 ± 1.0 μM) were less potent 5-HEDH inhibitors.
Fig. 5.
Concentration-response curves for the inhibition by 5-hydroxy fatty acids of 5-oxo-ETE formation by microsomal 5-HEDH. Microsomal fractions (15 μg/ml) from PMA-differentiated U937 cells were preincubated with various concentrations of 5h-18:1 (▴), 5h-20:1 (•), 5h-20:2 (○), 5h-20:0 (▿), and Δ6,8,11-5h-20:3 (5h-20:3, ▾) for 5 min followed by incubation with 5-HETE (1 μM) in the presence of NADP+ (100 μM) for 10 min. The amounts of 5-oxo-ETE formed were determined by RP-HPLC. The values are means ± S.E. (n = 4).
Effects of 5h-18:1 and 5h-20:1 on Eicosanoid Formation by PBMCs. To determine whether 5h-18:1 and 5h-20:1 could selectively block 5-oxo-ETE formation in intact cells, we incubated human PBMCs with AA (20 μM) and A23187 (5 μM) in the presence of tBuOOH (100 μM) and different concentrations of the above Δ6 5-hydroxy fatty acids (Fig. 6). Neither 5h-18:1 (Fig. 6A) nor 5h-20:1 (Fig. 6B) inhibited the formation of the 5-LO product LTB4, the 12-LO product 12-HETE, or the cyclooxygenase product 12-hydroxy-5,8,10-heptadecatrienoic acid (12-HHT). In contrast, formation of the 5-LO/5-HEDH product 5-oxo-ETE was inhibited in a concentration-dependent manner by both of the above hydroxy-fatty acids, whereas the amounts of its endogenously generated substrate, 5-HETE, increased correspondingly. The IC50 values for the inhibitory effects of 5h-18:1 and 5h-20:1 on 5-oxo-ETE formation were 2.7 ± 0.6 and 2.3 ± 0.5 μM, respectively.
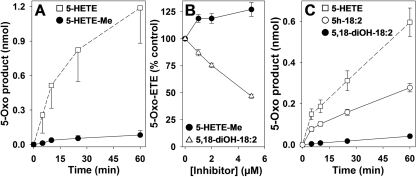
Metabolism of 5-HETE Methyl Ester by 5-HEDH. To determine whether a free carboxyl group is required for metabolism by 5-HEDH, we examined the metabolism of the methyl ester derivative of 5-HETE (5-HETE-Me). Although only very small amounts of 5-oxo-ETE methyl ester (5-oxo-ETE-Me) were formed from 5-HETE-Me by U937 cell microsomes, substantial amounts of unesterified 5-oxo-ETE and 5-HETE were detected (data not shown), presumably because of hydrolysis of 5-HETE-Me by esterase(s), followed by oxidation of free 5-HETE to 5-oxo-ETE. The high esterase activity of U937 cells complicated interpretation of the results because we could not exclude the possibility that at least part of the large amount of 5-oxo-ETE that we detected was formed by hydrolysis of 5-HEDH-derived 5-oxo-ETE-Me rather than from esterase-derived 5-HETE.
In contrast to U937 cell microsomes, neutrophil microsomes displayed relatively little 5-HETE-Me esterase activity, so we used neutrophils as a source of 5-HEDH to investigate the metabolism of this substrate. The time courses for the oxidation of 5-HETE and 5-HETE-Me are compared in Fig. 7A. 5-HETE-Me is a very poor substrate for 5-HEDH, with only approximately 4% being converted to 5-oxo-ETE-Me after 60 min, compared with approximately 60% conversion of free 5-HETE to 5-oxo-ETE. Only small amounts of free 5-oxo-ETE (∼2.7%) and 5-HETE (∼0.3%) were formed from 5-HETE-Me under these conditions (data not shown). We also investigated the possibility that 5-HETE-Me could inhibit the formation of 5-oxo-ETE from 5-HETE by 5-HEDH. As shown in Fig. 7B, when 5-HETE (1 μM) was incubated with a 5-fold excess of 5-HETE-Me, there was actually an increase in 5-oxo-ETE formation (p < 0.01), possibly because of the hydrolysis of 5-HETE-Me and oxidation of the resulting free 5-HETE by 5-HEDH as noted above.
Fig. 7.
Importance of the α and ω ends of the substrate for metabolism by 5-HEDH. A, time courses for the metabolism of 5-HETE (2 μM) to 5-oxo-ETE (□) and 5-HETE-Me (2 μM) to 5-oxo-ETE-Me (•) by microsomal fractions (30 μg/ml protein) from human neutrophils in the presence of NADP+ (100 μM). B, concentration-response relationships for the effects of 5-HETE-Me (•) and 5,18-diOH-18:2 (▵) on the conversion of 5-HETE (1 μM) to 5-oxo-ETE by neutrophil and U937 cell microsomes, respectively, in the presence of NADP+ (100 μM). Microsomal fractions (30 μg/ml protein) were incubated with 5-hydroxy fatty acids for 15 min. C, time courses for the metabolism of 5-HETE to 5-oxo-ETE (□), 5h-18:2 to 5-oxo-18:2 (○), and 5,18-diOH-18:2 to 5-oxo-18-HODE (•) by microsomal fractions (30 μg/ml protein) from PMA-differentiated U937 cells in the presence of NADP+ (100 μM). All values are means ± S.E. (n = 4).
Effect of ω-Oxidation on 5-HEDH-Catalyzed Oxidation. To investigate the effect of ω-oxidation on metabolism by 5-HEDH, we used 5,18-diOH-18:2, which we had prepared previously by total chemical synthesis in connection with a study on the metabolism of sebaleic acid (5,8-octadecadienoic acid) (Cossette et al., 2008). Because preliminary experiments suggested that it is a very poor substrate for 5-HEDH, we examined the time course of its metabolism by U937 cell microsomes (Fig. 7C). In agreement with the data shown in Fig. 2, 5h-18:2 is converted to 5-oxo-18:2 at approximately one-half the rate of 5-HETE. However, 5,18-diOH-18:2 is oxidized by 5-HEDH (to 5-oxo-18-HODE) at only approximately one-sixth the rate of 5h-18:2. The ability of 5,18-diOH-18:2 to inhibit the formation of 5-oxo-ETE from 5-HETE was also determined by adding increasing concentrations of the dihydroxy compound to a fixed concentration (1 μM) of 5-HETE (Fig. 7B). At the highest concentration tested (5 μM), 5,18-diOH-18:2 inhibited the formation of 5-oxo-ETE by approximately 50%.
Discussion
We previously showed that the presence of a polar hydroxyl group at either C12 (6-trans isomers of LTB4) or C15 (5,15-diHETE) does not dramatically reduce metabolism by 5-HEDH (Powell et al., 1992), which initially suggested that the ω-end of the substrate may not be important for binding to the enzyme. The present study demonstrates that 5S-hydroxy-6-trans-fatty acids with chain lengths between 16 and 20 carbons are all reasonably good substrates for 5-HEDH. However, further reduction of the chain length to 14 or 12 carbons dramatically reduces (5h-14:2) or nearly eliminates (5h-12:2) oxidation by 5-HEDH, indicating that at least part of the hydrophobic ω-end of the molecule is required for recognition and metabolism by 5-HEDH. Further evidence for the importance of this region of the substrate is the very slow metabolism of 5,18-diOH-18:2, which is the major metabolite of 5h-18:2 by unstimulated neutrophils, in contrast to PMA-activated neutrophils, which produce approximately equivalent amounts of 5,18-diOH-18:2 and 5-oxo-18:2 (Cossette et al., 2008). The hydrophobic ω-end of 5-oxo-ETE is also important for activation of the OXE receptor because 5-oxo-ETE analogs with chain lengths of less than 18 carbons (Patel et al., 2008) or with an ω-hydroxyl group (Powell et al., 1996) have relatively little biological activity.
Although 5-HETE seems to be the best substrate for 5-HEDH, there is not a strict requirement for four double bonds because 5-hydroxy C20 fatty acids containing between one and five double bonds were all oxidized to their 5-oxo derivatives. This is consistent with our previous findings that 5h-20:5 is a good substrate for 5-HEDH (Powell et al., 1995) and that 5,8,11-eicosatrienoic acid (Mead acid) is converted to 5-oxo-20:3 by neutrophils (Patel et al., 2008). It was somewhat surprising that 5-hydroxy fatty acids with only a single double bond are relatively good substrates. We were unable to investigate the metabolism of fatty acids lacking any double bonds (e.g., 5h-20:0) because the corresponding 5-oxo products would not contain a UV-absorbing chromophore. It would seem likely that the 6-trans double bond is important for substrate recognition in view of the complete lack of metabolism of LTB4 because of its 6,7-double bond being in the cis, rather than the trans, configuration (Powell et al., 1992). In contrast, 6-trans-LTB4 is a relatively good substrate for 5-HEDH (Powell et al., 1992). On the other hand, 5h-20:0 does inhibit 5-HEDH activity, so it must at least have some affinity for the enzyme, whether or not it is metabolized.
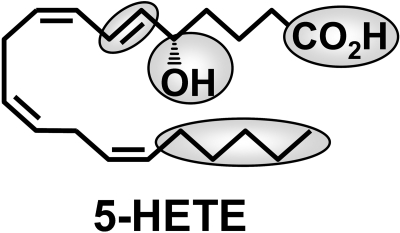
The most important structural feature for metabolism by 5-HEDH is clearly the 5S-hydroxyl group (Fig. 8) because we have shown previously that fatty acids with hydroxyl groups in other positions or in the 5R configuration are not significantly metabolized (Powell et al., 1992). Although we did not determine whether 5R-hydroxy fatty acids can inhibit the oxidation of 5S-HETE by microsomal 5-HEDH in the present study, this seems unlikely, at least in the case of 5R-HETE, because we previously found that 5RS-HETE is converted to 5-oxo-ETE by microsomal 5-HEDH. 5S-HETE was nearly completely depleted under the conditions used, whereas the concentration of 5R-HETE was nearly unaltered (Powell et al., 1992). Thus, 5R-HETE was unable to prevent the metabolism of 5S-HETE by 5-HEDH.
Fig. 8.
Structure of 5-HETE, showing the regions of the molecule that are important for metabolism by 5-HEDH.
The very slow rate of metabolism of 5-HETE-Me indicates that a free carboxyl group is also required to obtain a substantial degree of metabolism by 5-HEDH, suggesting that this group is also bound to the active site of the enzyme. This is not surprising, given its proximity to the 5S-hydroxyl group. This contrasts with another eicosanoid-metabolizing enzyme, 15-hydroxyprostaglandin dehydrogenase, which is not substantially affected by methylation of the carboxyl group of the substrate (Ohno et al., 1978), presumably because it is a greater distance from the hydroxyl group being oxidized. A free carboxyl group is also important for interaction of 5-oxo-ETE with its receptor because methylation results in a 20-fold reduction in potency (Powell et al., 1996).
5-HETE has been shown previously to be incorporated into cellular lipids and to be formed as a result of peroxidation of membrane lipids in red cells in the presence of tBuOOH (Hall and Murphy, 1998b). Although 5-HETE and 5-oxo-ETE, like isoprostanes, can be formed by nonenzymatic autoxidation of AA-containing lipids, it is unlikely that 5-HEDH could oxidize esterified 5-HETE because it requires a substrate with a free carboxyl group. Therefore, when it is formed during an autoxidation reaction, 5-oxo-ETE presumably would be formed by nonenzymatic dehydration of 5-HpETE rather than by oxidation of 5-HETE (Hall and Murphy, 1998a).
The relative potencies of 5-hydroxy fatty acids in inhibiting 5-HETE metabolism by 5-HEDH were considerably different from their relative rates of metabolism. The most potent inhibitors were 5h-20:1, 5h-18:1, and 5h-20:2. In contrast, Δ6,8,11-5h-20:3 and 5h-20:5 were at least as good substrates but were not very potent inhibitors of enzyme activity. To determine whether some of these compounds could selectively inhibit 5-HEDH in intact cells, we investigated their effects on eicosanoid synthesis by PBMCs treated with A23187 to activate cPLA2 and 5-LO. We also added tBuOOH, which increases the rate of 5-oxo-ETE synthesis by raising NADP+ levels as a result of its metabolism by glutathione peroxidase and the NADPH-dependent reduction of GSSG to GSH by glutathione reductase (Erlemann et al., 2004). PB-MCs have high 5-LO activity and convert endogenous AA to LTB4, 5-HETE, and 5-oxo-ETE. 12-HETE is also formed, principally because of the presence of adherent platelets, which are present in substantial numbers, unless specific steps are taken to remove them (Pawlowski et al., 1983). These cells also convert AA to the cyclooxygenase product 12-HHT, which has been shown recently to be a natural ligand for the BLT2 receptor (Okuno et al., 2008). Both 5h-18:1 and 5h-20:1 selectively inhibited 5-oxo-ETE formation. This resulted in the accumulation of its substrate 5-HETE, which is not metabolized by PBMCs by the alternate pathway of ω-oxidation as it is in neutrophils (Powell et al., 1992). In contrast, there were no changes in the levels of LTB4, 12-HETE or 12-HHT. This indicates that these monoene 5-hydroxy fatty acids selectively block 5-HEDH without inhibiting 5-LO, LTA4 hydrolase, 12-LO, or cyclooxygenase.
Although 5h-18:1 and 5h-20:1 selectively inhibit 5-HEDH, the fact that they are oxidized to significant amounts of the corresponding 5-oxo compounds by this enzyme could limit their usefulness. However, we have recently shown that the 5-oxo compounds derived from these compounds (i.e., 5-oxo-18:1 and 5-oxo-20:1) have only weak biological activities, compared with 5-oxo-ETE. 5-Oxo-18:1 is ∼100 times less potent than 5-oxo-ETE in activating neutrophils and eosinophils, whereas 5-oxo-20:1 is approximately 200 to 300 times less potent (Patel et al., 2008). Modification of the structures of these compounds could lead to the development of an inhibitor that binds tightly to the active site of 5-HEDH but is resistant to oxidation by the enzyme. Because no 5-oxo-ETE receptor antagonist is currently available, such an inhibitor could be very useful in helping to define the pathophysiological role of this substance in inflammatory diseases such as asthma. It could also be useful in investigating the mechanism of formation of 5-oxo-ETE. Although this enzyme is present in most types of human inflammatory cells and in at least some structural cells, its activity has not been detected in mouse macrophages, which can synthesize 5-oxo-ETE by dehydration of 5-LO-derived 5-HpETE (Zarini and Murphy, 2003). The fact that 5-oxo-ETE synthesis is selectively inhibited by 5-HEDH inhibitors in human monocytes provides further evidence that in these cells, it is principally formed by oxidation of 5-HETE by 5-HEDH rather than by dehydration of 5-HpETE.
We conclude that the structural requirements for metabolism of eicosanoids by 5-HEDH are a free carboxyl group, a 5S-hydroxyl group followed by a 6-trans double bond, and a hydrophobic group at the ω-end of the substrate (Fig. 8). The formation of 5-oxo-ETE, the biologically active product of this enzyme, can be inhibited selectively by 5-hydroxy-Δ6-long-chain fatty acids. Inhibitors of this nature could be useful in investigating the biological role of 5-oxo-ETE and its mechanism of formation.
Acknowledgments
We thank the National Science Foundation for the AMX-360 and Bruker 400 MHz NMR instruments.
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL81873]; the Canadian Institutes of Health Research [Grant MOP-6254]; and the Quebec Heart and Stroke Foundation.
The Meakins-Christie Laboratories-MUHC-RI are supported in part by the Le Fonds de la Recherche en Santé du Québec [center grant] and the J.T. Costello Memorial Research Fund.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.143453.
ABBREVIATIONS: 5-LO, 5-lipoxygenase; LT, leukotriene; 5-oxo-ETE, 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid; AA, arachidonic acid; 5-HpETE, 5S-hydroperoxy-6,8,11,14-eicosatetraenoic acid; 5-HETE, 5S-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid; 5-HEDH, 5-hydroxyeicosanoid dehydrogenase; 5,18-diOH-18:2, 5S,18-dihydroxy-6E,8Z-octadecadienoic acid; RP-HPLC, reversed-phase high-performance liquid chromatography; 13-HODE, 13S-hydroxy-9Z,11E-octadecadienoic acid; 5h-20:5 (5-HEPE), 5-hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoic acid; PMA, phorbol 12-myristate 13-acetate; tBuOOH, t-butyl hydroperoxide; PBS, phosphate-buffered saline; PBMC, peripheral blood mononuclear cell; 5h-12:2, 5S-hydroxy-6E,8Z-dodecadienoic acid; 5h-14:2, 5S-hydroxy-6E,8Z-tetradecadienoic acid; tR, retention time; 5-oxo-18:1, 5-oxo-6E-octadecenoic acid; 5-oxo-20:1, 5-oxo-6E-eicosenoic acid; 5h-18:1, 5S-hydroxy-6E-octadecenoic acid; 5h-18:2, 5S-hydroxy-6E,8Z-octadecadienoic acid; 5h-20:1, 5S-hydroxy-6E-eicosenoic acid; Δ6,8,11-5h-20:3, 5S-hydroxy-6E,8Z,11Z-eicosatrienoic acid; 5h-16:2, 5S-hydroxy-6E,8Z-hexadecadienoic acid; 5h-20:2, 5S-hydroxy-6E,8Z-eicosadienoic acid; 5h-20:0, 5-hydroxyeicosanoic acid; 12-HHT, 12-hydroxy-5,8,10-heptadecatrienoic acid; Δ6,18,14-5h-20:3, 5-hydroxy-6E,8Z,14Z-eicosatrienoic acid; 5-HETE-Me, 5-HETE methyl ester; 5-oxo-ETE-Me, 5-oxo-ETE methyl ester.
References
- Borgeat P and Samuelsson B (1979) Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids. Proc Natl Acad Sci U S A 76 3213-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossette C, Patel P, Anumolu JR, Sivendran S, Lee GJ, Gravel S, Graham FD, Lesimple A, Mamer OA, Rokach J, et al. (2008) Human neutrophils convert the sebum-derived polyunsaturated fatty acid sebaleic acid to a potent granulocyte chemoattractant. J Biol Chem 283 11234-11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire MJ, Ferland C, Pagé N, Lavigne S, Davoine F, and Laviolette M (2003) Endothelial cells modulate eosinophil surface markers and mediator release. Eur Respir J 21 918-924. [DOI] [PubMed] [Google Scholar]
- Erlemann KR, Cossette C, Grant GE, Lee GJ, Patel P, Rokach J, and Powell WS (2007) Regulation of 5-hydroxyeicosanoid dehydrogenase activity in monocytic cells. Biochem J 403 157-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlemann KR, Rokach J, and Powell WS (2004) Oxidative stress stimulates the synthesis of the eosinophil chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid by inflammatory cells. J Biol Chem 279 40376-40384. [DOI] [PubMed] [Google Scholar]
- Funk CD (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294 1871-1875. [DOI] [PubMed] [Google Scholar]
- Ghosh J and Myers CE (1998) Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci U S A 95 13182-13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LM and Murphy RC (1998a) Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids [In Process Citation]. J Am Soc Mass Spectrom 9 527-532. [DOI] [PubMed] [Google Scholar]
- Hall LM and Murphy RC (1998b) Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids. J Am Soc Mass Spectrom 9 527-532. [DOI] [PubMed] [Google Scholar]
- Hamberg M and Samuelsson B (1967) On the specificity of the oxygenation of unsaturated fatty acids catalyzed by soybean lipoxidase. J Biol Chem 242 5329-5335. [PubMed] [Google Scholar]
- Hosoi T, Koguchi Y, Sugikawa E, Chikada A, Ogawa K, Tsuda N, Suto N, Tsunoda S, Taniguchi T, and Ohnuki T (2002) Identification of a novel eicosanoid receptor coupled to Gi/o. J Biol Chem 277 31459-31465. [DOI] [PubMed] [Google Scholar]
- Jones CE, Holden S, Tenaillon L, Bhatia U, Seuwen K, Tranter P, Turner J, Kettle R, Bouhelal R, Charlton S, et al. (2003) Expression and characterization of a 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils. Mol Pharmacol 63 471-477. [DOI] [PubMed] [Google Scholar]
- Khanapure SP, Powell WS, and Rokach J (1998) The total synthesis of 5-oxo-12(S)-hydroxy-6(E),8(Z),10(E),14(Z)-eicosatetraenoic acid and its 8,9-trans-isomer and their identification in human platelets. J Org Chem 63 8976-8982. [Google Scholar]
- Muro S, Hamid Q, Olivenstein R, Taha R, Rokach J, and Powell WS (2003) 5-oxo-6,8,11,14-eicosatetraenoic acid induces the infiltration of granulocytes into human skin. J Allergy Clin Immunol 112 768-774. [DOI] [PubMed] [Google Scholar]
- Norgauer J, Barbisch M, Czech W, Pareigis J, Schwenk U, and Schröder JM (1996) Chemotactic 5-oxo-icosatetraenoic acids activate a unique pattern of neutrophil responses: analysis of phospholipid metabolism, intracellular Ca2+ transients, actin reorganization, superoxide-anion production and receptor up-regulation. Eur J Biochem 236 1003-1009. [DOI] [PubMed] [Google Scholar]
- O'Flaherty JT, Kuroki M, Nixon AB, Wijkander J, Yee E, Lee SL, Smitherman PK, Wykle RL, and Daniel LW (1996a) 5-oxo-eicosanoids and hematopoietic cytokines cooperate in stimulating neutrophil function and the mitogen-activated protein kinase pathway. J Biol Chem 271 17821-17828. [DOI] [PubMed] [Google Scholar]
- O'Flaherty JT, Kuroki M, Nixon AB, Wijkander J, Yee E, Lee SL, Smitherman PK, Wykle RL, and Daniel LW (1996b) 5-Oxo-eicosatetraenoate is a broadly active, eosinophil-selective stimulus for human granulocytes. J Immunol 157 336-342. [PubMed] [Google Scholar]
- Ohno H, Morikawa Y, and Hirata F (1978) Studies on 15-hydroxyprostaglandin dehydrogenase with various prostaglandin analogues. J Biochem 84 1485-1494. [DOI] [PubMed] [Google Scholar]
- Okuno T, Iizuka Y, Okazaki H, Yokomizo T, Taguchi R, and Shimizu T (2008) 12(S)-Hydroxyheptadeca-5Z,8E,10E-trienoic acid is a natural ligand for leukotriene B4 receptor 2. J Exp Med 205 759-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Cossette C, Anumolu JR, Gravel S, Lesimple A, Mamer OA, Rokach J, and Powell WS (2008) Structural requirements for activation of the 5-oxo-6E,8Z, 11Z,14Z-eicosatetraenoic acid (5-oxo-ETE) receptor: identification of a mead acid metabolite with potent agonist activity. J Pharmacol Exp Ther 325 698-707. [DOI] [PubMed] [Google Scholar]
- Pawlowski NA, Kaplan G, Hamill AL, Cohn ZA, and Scott WA (1983) Arachidonic acid metabolism by human monocytes: studies with platelet-depleted cultures. J Exp Med 158 393-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell WS (1987) Precolumn extraction and reversed-phase high-pressure liquid chromatography of prostaglandins and leukotrienes. Anal Biochem 164 117-131. [DOI] [PubMed] [Google Scholar]
- Powell WS, Gravel S, and Gravelle F (1995) Formation of a 5-oxo metabolite of 5,8,11,14,17-eicosapentaenoic acid and its effects on human neutrophils and eosinophils. J Lipid Res 36 2590-2598. [PubMed] [Google Scholar]
- Powell WS, Gravelle F, and Gravel S (1992) Metabolism of 5(S)-hydroxy-6,8,11,14-eicosatetraenoic acid and other 5(S)-hydroxyeicosanoids by a specific dehydrogenase in human polymorphonuclear leukocytes. J Biol Chem 267 19233-19241. [PubMed] [Google Scholar]
- Powell WS, MacLeod RJ, Gravel S, Gravelle F, and Bhakar A (1996) Metabolism and biologic effects of 5-oxoeicosanoids on human neutrophils. J Immunol 156 336-342. [PubMed] [Google Scholar]
- Powell WS and Rokach J (2005) Biochemistry, biology and chemistry of the 5-lipoxygenase product 5-oxo-ETE. Prog Lipid Res 44 154-183. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Zhou D, Locati M, Bernasconi S, Luini W, Mantovani A, and O'Flaherty JT (1996) Stimulating properties of 5-oxo-eicosanoids for human monocytes: synergism with monocyte chemotactic protein-1 and -3. J Immunol 157 4664-4671. [PubMed] [Google Scholar]
- Stamatiou P, Hamid Q, Taha R, Yu W, Issekutz TB, Rokach J, Khanapure SP, and Powell WS (1998) 5-Oxo-ETE induces pulmonary eosinophilia in an integrin-dependent manner in Brown Norway rats. J Clin Invest 102 2165-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S and Ghosh J (2006) Expression of 5-oxoETE receptor in prostate cancer cells: critical role in survival. Biochem Biophys Res Commun 339 93-98. [DOI] [PubMed] [Google Scholar]
- Zamboni R and Rokach J (1983) Stereospecific synthesis of 5S-HETE, 5R-HETE and their transformation to 5(±)HPETE. Tetrahedron Lett 24 999-1002. [Google Scholar]
- Zarini S and Murphy RC (2003) Biosynthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid from 5-hydroperoxyeicosatetraenoic acid in the murine macrophage. J Biol Chem 278 11190-11196. [DOI] [PubMed] [Google Scholar]