Abstract
The effective treatment of pain is typically limited by a decrease in the pain-relieving action of morphine that follows its chronic administration (tolerance). Therefore, restoring opioid efficacy is of great clinical importance. In a murine model of opioid antinociceptive tolerance, repeated administration of morphine significantly stimulated the enzymatic activities of spinal cord serine palmitoyltransferase, ceramide synthase, and acid sphingomyelinase (enzymes involved in the de novo and sphingomyelinase pathways of ceramide biosynthesis, respectively) and led to peroxynitrite-derive nitroxidative stress and neuroimmune activation [activation of spinal glial cells and increase formation of tumor necrosis factor-α, interleukin (IL)-1β, and IL-6]. Inhibition of ceramide biosynthesis with various pharmacological inhibitors significantly attenuated the increase in spinal ceramide production, nitroxidative stress, and neuroimmune activation. These events culminated in a significant inhibition of the development of morphine antinociceptive tolerance at doses devoid of behavioral side effects. Our findings implicate ceramide as a key upstream signaling molecule in the development of morphine antinociceptive tolerance and provide the rationale for development of inhibitors of ceramide biosynthesis as adjuncts to opiates for the management of chronic pain.
Chronic, severe pain is a significant health problem (Renfrey et al., 2003). One third of Americans suffer from some form of chronic pain, and in over 30% of cases, the pain becomes resistant to analgesic therapy (Renfrey et al., 2003). The economic impact of pain is equally large, at approximately $100 billion annually (Renfrey et al., 2003). Opiate/narcotic analgesics, typified by morphine sulfate, are the most effective treatments for acute and chronic severe pain. However, their clinical utility is often hampered by the development of analgesic tolerance, which necessitates escalating doses to achieve equivalent pain relief (Foley, 1995). This complex pathophysiological cycle contributes to decreased quality of life of patients because of oversedation, reduced physical activity, constipation, respiratory depression, potential for addiction, and other side effects (Foley, 1995). In accordance, there is major interest in new approaches to maintain opiate efficacy during repetitive dosing for chronic pain, without engendering tolerance or unacceptable side effects. Recently, several pathogenic processes that occur at the level of the spinal cord have been implicated. These include nitric oxide and superoxide-derived peroxynitrite production and peroxynitrite-induced nitroxidative stress (Muscoli et al., 2007), neuronal apoptosis (Mayer et al., 1999), and neuroimmune activation, herein defined as glial cell activation and release of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 (Song and Zhao, 2001; Watkins et al., 2007). A link among these processes seems to be at the level of peroxynitrite (Muscoli et al., 2007), the product of the interaction between nitric oxide and superoxide and a potent proinflammatory and proapoptotic reactive species (Salvemini et al., 1998) recently shown to contribute to the development of morphine antinociceptive tolerance through spinal apoptosis and increased production of TNF-α, IL-1β, and IL-6 (Muscoli et al., 2007). In search of the molecular mechanism leading to spinal nitroxidative stress and neuroimmune activation, we reasoned that the sphingolipid ceramide could be a unique signaling candidate because of its potent proinflammatory signaling properties coupled with its implication in the generation of nitroxidative stress. Its involvement in nitroxidative stress has been associated in the pathogenesis of radiation-induced injury (Kolesnick and Fuks, 2003), sepsis (Delogu et al., 1999), acute lung injury (Göggel et al., 2004), emphysema (Petrache et al., 2005), and asthma (Masini et al., 2005), which share with antinociceptive tolerance roles of apoptosis and inflammation in their pathogenesis. Ceramide is generated by enzymatic hydrolysis of sphingomyelin by sphingomyelinases (SMases) (sphingomyelin pathway) and/or from de novo synthesis co-ordinated by serine palmitoyltransferase and ceramide synthase (de novo pathway) (Kolesnick, 2002). The steady-state availability of ceramide is further regulated by ceramidases that convert ceramide to sphingosine by catalyzing hydrolysis of its amide group (Kolesnick, 2002). Ceramide serves as a second messenger to activate downstream effectors, including ceramide-activated protein kinase and ceramide-activated protein phosphatase, and generates other second messengers, such as sphingosine-1-phosphate (Kolesnick, 2002). A potential role of ceramide in peripheral pain sensitization is documented by the observations that intradermal injection of ceramide in rats produces dose-dependent hyperalgesia and that TNF-α-induced thermal hyperalgesia in rats is blocked by GW4869 (Delgado et al., 2006), an inhibitor of neutral SMase (Joseph and Levine, 2004). That ceramide may modulate nociception is underscored by studies of hereditary sensory neuropathy, an autosomal dominant disorder traced to certain missense mutations in serine palmitoyltransferase, the rate-limiting enzyme in generation of ceramide from the de novo pathway. Such mutations increase this enzyme's activity and the levels of ceramide, triggering apoptosis in peripheral sensory neurons and progressive degeneration of dorsal root ganglia and motor neurons (Dawkins et al., 2001). Furthermore, a deficiency of acid ceramidase activity causes the inherited metabolic disorder known as Farber disease (Rother et al., 1992). This sphingolipid storage disease is characterized by a massive accumulation of ceramide in subcutaneous lipid-loaded nodules, ex-cruciating pain in the joints and extremities, marked accumulation of ceramide in lysosomes, and death in approximately 3 to 4 years after birth (Rother et al., 1992).
Collectively, we hypothesize and show using several structurally unrelated specific pharmacological inhibitors of the sphingomyelin and de novo pathways that ceramide generated during repeated administration of morphine contributes to the development of antinociceptive tolerance through downstream pathways of neuroimmune activation. Our results provide a pharmacological basis for developing inhibitors of ceramide biosynthesis as adjunct to opiates for pain management, thus addressing a large and currently unmet medical need with major socioeconomic consequences.
Materials and Methods
Induction of Morphine-Induced Antinociceptive Tolerance in Mice
Male CD-1 mice (24-30 g; Charles River Laboratories, Inc., Wilmington, MA) were housed and cared for in accordance and guidelines of the Institutional Animal Care and Use Committee of the Saint Louis University Medical Center, in accordance with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and the Universities of Rome and Messina in compliance with Italian regulations on protection of animals used for experimental and other scientific purpose (D.M. 116192), and with European Economic Community regulations. The Institutional Animal Care and Use Committee of Saint Louis University Medical Center and the Universities of Rome and Messina approved all studies. The numbers of animals used are the minimum number necessary to achieve statistical significance at p < 0.05 as set forth by the International Society for the Study of Pain guidelines (Covino et al., 1980). Mice were housed five per cage and maintained under identical conditions of temperature (21 ± 1°C) and humidity (65% ± 5%) with a 12-h light/dark cycle and allowed food ad libitum. Nociceptive thresholds were determined by measuring latencies (in seconds) of mice placed in a transparent glass cylinder on a hot-plate (Ugo Basile, Comerio, Italy) maintained at 52°C. Determination of antinociception was assessed between 7:00 and 10:00 AM. All injections were given intraperitoneally or subcutaneously in a volume of 0.1 and 0.3 ml, respectively, at approximately 7:00 AM and 4:00 PM. Responses indicative of nociception included intermittent lifting and/or licking of the hindpaws or escape behavior. Hot-plate latencies were taken in mice from all groups on day 5 before (baseline latency) and 40 min after an acute dose of morphine (0.3-3 mg/kg) or its vehicle (saline) (response latency). Results expressed as percentage of maximum possible antinociceptive effect, which was calculated as follows: (response latency - baseline latency)/(cut-off latency - baseline latency) × 100. A cut-off latency of 20 s was employed to prevent tissue damage. Ten mice per group were used, and all experiments were conducted with the experimenters blinded to treatment conditions. Fumonisin B1 (FB1), a competitive and reversible inhibitor of ceramide synthase (Delgado et al., 2006), myriocin, an inhibitor of serine palmitoyltransferase (Delgado et al., 2006), D609, an inhibitor of the acid sphingomyelinase (Delgado et al., 2006), or their vehicle (saline) were given daily intraperitoneally 15 min before each dose of morphine. The following experimental groups were used.
Naive Group. In this group, mice were injected twice a day with an intraperitoneal injection of saline (vehicle used to deliver the drugs to the other groups over 4 days) and a subcutaneous injection of saline (vehicle used to deliver morphine over 4 days). On day 5, mice received an intraperitoneal injection of saline followed 15 min later by a subcutaneous injection of saline.
Naïve + Drug Groups. In these groups, mice were injected twice a day for 4 days with an intraperitoneal injection of the highest dose of FB1 (1 mg/kg/day), myriocin (0.4 mg/kg/day), or D609 (20 mg/kg/day) used and a subcutaneous injection of saline. On day 5, mice received an intraperitoneal injection of FB1 (0.5 mg/kg), myriocin (0.2 mg/kg), or D609 (10 mg/kg) followed 15 min later by a subcutaneous injection of saline.
Vehicle Group. In this group, mice were injected twice a day for 4 days with an intraperitoneal injection of saline and a subcutaneous injection of saline. On day 5, mice received an intraperitoneal injection of saline followed 15 min later by a subcutaneous injection of acute morphine eliciting near-to-maximal antinociception (3 mg/kg).
Vehicle + Drug Groups. In these groups, mice were injected twice a day for 4 days with an intraperitoneal injection of the highest dose of FB1 (1 mg/kg/day), myriocin (0.4 mg/kg/day), or D609 (40 mg/kg/day) used and a subcutaneous injection of saline. On day 5, mice received an intraperitoneal injection of FB1 (0.5 mg/kg), myriocin (0.2 mg/kg), or D609 (20 mg/kg) followed 15 min later by subcutaneous doses of acute morphine giving between 10 and 95% of antinociceptive responses within 40 min of administration (0.1-3 mg/kg).
Morphine Group. In this group, mice were injected twice a day for 4 days with an intraperitoneal injection of saline and subcutaneous injection of morphine (20 mg/kg/day). On day 5, mice received an intraperitoneal injection of saline followed 15 min later by a subcutaneous dose of acute morphine (3 mg/kg).
Morphine + Drug Groups. In these groups, mice were injected twice a day for 4 days with an intraperitoneal injection of FB1 (0.25, 0.5, and 1 mg/kg/day), myriocin (0.1, 0.2, and 0.4 mg/kg/day), or D609 (10, 20, and 40 mg/kg/day) and subcutaneous injection of morphine (20 mg/kg/day). On day 5, mice received an intraperitoneal dose of FB1 (0.5 mg/kg), myriocin (0.2 mg/kg), or D609 (20 mg/kg) followed 15 min later by the subcutaneous doses of acute morphine (3 mg/kg).
In another set of experiments and to address whether FB1, myriocin, or D609 reverse the expression of tolerance, mice were treated twice a day with morphine as described above and on day 5 received a single intraperitoneal dose of FB1 (1 mg/kg), myriocin (0.4 mg/kg), or D609 (40 mg/kg) followed 15 min later by the acute dose of morphine (3 mg/kg). On day 5 and after the behavioral tests, spinal cord tissues from the lumbar enlargement segment of the spinal cord (L4-L6) and dorsal horn tissues were removed, and tissues were processed for immunohistochemical, Western blot, and biochemical analysis.
Rotorod Test
Mice were trained before experimentation for their ability to remain for 120 s on a revolving Rotorod apparatus (accelerating units increase from 3.5-35 rpm in 5 min) as described. Mice (four per group) were injected with an intraperitoneal injection of the highest dose of FB1 (1 mg/kg), myriocin (0.4 mg/kg), or D609 (40 mg/kg) used to block antinociceptive tolerance or its vehicle. Mice (n = 4 per group) were tested and examined for motor impairments on the Rotorod at 15, 30, and 60 min after drug administration as described under Materials and Methods. The latency time to fall off the Rotorod was determined (cut-off time used was 120 s).
Determination of Ceramide Synthase Activity
About 60 to 80 mg of spinal cord homogenates was incubated with [3H]palmitic acid (2.5 μCi/ml; GE Healthcare, Chalfont St. Giles, UK) for 1 h. Lipids were extracted with ice-cold methanol containing 2% acetic acid and 5% chloroform and resolved using thin-layer chromatography. Lipids comigrating with standards were scraped and quantified by lipid scintillation counting as described previously (Castillo et al., 2007).
Determination of Sphingomyelinase Activity
The sphingomyelinase activity was measured using Amplex Red Sphingomyelinase Assay Kit (Molecular Probes, Carlsbad, CA) following the manufacturer's instructions. First, spinal cord tissues were homogenized in buffers for each specific assay as described previously (Dobrowsky and Kolesnick, 2001). For the acid isoforms, Na acetate (100 mM at pH 5.0) lysis buffer was used. EDTA (2 mM) was added to the lysis buffer for detection of the insoluble isoform. For neutral isoform detection, the tissues were homogenized in HEPES (20 mM, pH 7.4) lysis buffer. The kinetics for sphingomyelinase activity was measured in a fluorescence microplate reader for 2 h followed by normalization per protein concentration of the sample. Hydrogen peroxide and sphingomyelinase were used as positive controls.
Determination of Serine Palmitoyl Transferase Activity
Serine palmitoyl transferase (SPT) activity was determined by measuring the incorporation of [3H]serine into 3-ketosphinganine following the method described previously (Williams et al., 1984). The results were normalized by the protein concentration of the samples.
Light Microscopy
Spinal cord tissues (L4-L6 area) were taken on day 5 after morphine treatment. Tissue segments were fixed in 4% (w/v) PBS-buffered paraformaldehyde, and 7-μm sections were prepared from paraffin-embedded tissues. Tissue transversal sections were deparaffinized with xylene, stained with hematoxylin/eosin, and studied using light microscopy (Dialux 22; Leica, Wetzlar, Germany) to study the superficial laminae of the dorsal horn.
Immunohistochemistry for Ceramide, Glial Fibrillary Acidic Protein, and IBa1
For ceramide staining, endogenous peroxidase was quenched with 0.3% (v/v) hydrogen peroxide in 60% (v/v) methanol for 30 min after deparaffinization. Nonspecific adsorption was minimized by incubating the section in 2% (v/v) normal goat serum in PBS for 20 min. Endogenous biotin or avidin binding sites were blocked by sequential incubation for 15 min with biotin and avidin, respectively. Sections were incubated overnight with anticeramide antibody [1:50 (v/v) in PBS; Sigma-Aldrich, St. Louis, MO]. Sections were washed with PBS and incubated with secondary antibody. The counter stain was developed with a biotin-conjugated goat anti-rabbit IgG and avidin-biotin peroxidase complex (brown color) and nuclear fast red (red background). Positive staining was detected as a brown color. To verify the binding specificity for ceramide, some sections were also incubated with only the primary antibody (no secondary) or with only the secondary antibody (no primary). In these situations, no positive staining was found in the sections, indicating that the immunoreactions were positive in all the experiments carried out. For glial fibrillary acidic protein (GFAP) and IB1a staining, frozen sections were used. In brief, mice were anesthetized with halothane (Sigma-Aldrich) and intracardially perfused with a fresh solution of 4% paraformaldehyde in phosphate buffer (0.1 M sodium phosphate, pH 7.4). After perfusion, the spinal cord lumbar enlargement was quickly removed and postfixed in the same fixative overnight. Tissues were then sunk in solution of 30% (w/v) sucrose in phosphate buffer at 4°C until the tissues were processed for sectioning. Transverse spinal sections (20 μm) were cut in a cryostat and mounted on polylysine-coated slides and processed for immunohistochemistry. All of the sections were blocked with 2% goat serum in 0.3% Triton X-100 for 1 h at room temperature (RT). For immunofluorescent staining, the sequential spinal sections were incubated with primary antibody, either polyclonal rabbit anti-GFAP (GFAP, astrocyte marker, 1:500; Dako North America, Inc., Carpinteria, CA) or anti-IBa1 (microglia marker, 1:500; Wako Pure Chemicals, Osaka, Japan) overnight at 4°C, followed by incubation with fluorescein isothiocyanate-conjugated (for GFAP) or Texas red-conjugated (for IBa1) secondary antibodies (1:500) for 2 h at RT in the dark. After washing, the stained sections were examined with a fluorescence microscope (Fluovert; Leica), and images were captured with a Sony DX500 digital camera (Sony, Tokyo, Japan). All images were taken at the same exposure settings. To determine the specificity of immunoreaction, the negative control sections were processed as in the above procedures but omitting the primary antibody.
Immunoprecipitation and Western Blot
Animals were rapidly sacrificed (<1 min) in a CO2 chamber, and the dorsal portion of the spinal cord lumbar region enlargement was removed and stored at -80°C until used. Cytosolic and nuclear extracts were prepared as described previously (Bethea et al., 1998), with minor modifications. In brief, tissues from each mouse were suspended in extraction buffer A (0.2 mM phenylmethylsulfonyl fluoride, 0.15 μM pepstatin A, 20 μM leupeptin, 1 mM sodium orthovanadate), homogenized for 2 min, and centrifuged at 1000g for 10 min at 4°C. Supernatants were collected as the cytosolic fraction. The pellets containing nuclei were resuspended in buffer B (1% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl, pH 7.4, 1 mM EGTA, 1 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 20 μm leupeptin, 0.2 mM sodium orthovanadate). After centrifugation for 30 min at 15,000g at 4°C, the supernatants were collected as nuclear extracts and then stored at -80°C for further analysis. The levels of IκB-α and phospho-NF-κB p65 (Ser536) were quantified in cytosolic fraction from spinal cord tissue, whereas NF-κB p65 levels were quantified in nuclear fraction. The membranes were blocked with 5% (w/v) nonfat dried milk in 1× PBS for 40 min at room temperature and subsequently probed with specific anti-IκB-α (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), phospho-NF-κB p65 (Ser536) (1:1000; Cell Signaling Technology Inc., Danvers, MA), GFAP, or IBa1 with 5% (w/v) nonfat dried milk in 1× PBS and 0.1% Tween 20 at 4°C overnight, followed by incubations with either peroxidase-conjugated bovine anti-mouse IgG secondary antibody or peroxidase-conjugated goat anti-rabbit IgG (1:2000; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for 1 h at room temperature. Manganese superoxide dismutase (MnSOD) nitration was determined with Western blot analysis of immunoprecipitated protein complex in total lysates using antibodies specific to these proteins. In brief, the immunoprecipitated proteins were resolved in 12% SDS-polyacrylamide gel electrophoresis mini and proteins transferred to nitrocellulose membranes. Membranes were blocked for 1 h at RT in 1% bovine serum albumin/0.1% thimerosal in 50 mM Tris-HCl, pH 7.4/150 mM NaCl/0.01% Tween 20 (TBS/T), then incubated with rabbit polyclonal antibodies for MnSOD (1:2000; Upstate Biotechnology, Billerica, MA) followed by incubation of secondary antibodies conjugated with peroxidase for 1 h at room temperature. Protein bands were visualized by enhanced chemiluminescence (Amersham Biosciences). After stripping, all membranes were reprobed with either monoclonal anti-β-actin or α-tubulin antibody (1:20,000; Sigma-Aldrich) as a loading control. The relative expression of the protein levels as the band density for IκB-α (∼37 kDa), phospho-NF-κB (65 kDa), NF-κB p65 (75 kDa), Mn-SOD (∼29 kDa), GFAP (∼50 kDa), and Iba1 (∼17 kDa) was quantified by scanning of the X-ray films with GS-700 Imaging Densitometer (Bio-Rad, Hercules, CA) and a computer program (Molecular Analyst; IBM, New York, NY).
Measurement of Mn and Cu,Zn-SOD Activities
Dorsal half of the spinal cord lumbar region enlargement (L4-L6) was homogenized with 10 mM phosphate-buffered saline, pH 7.4, in a Polytron homogenizer and then sonicated on ice for 1 min (20 s, three times). The sonicated samples were subsequently centrifuged at 1100g for 10 min, and SOD activity was measured in the supernatants as described previously (Wang et al., 2004). In brief, a competitive inhibition assay was performed that used xanthine-xanthine oxidase-generated superoxide to reduce nitroblue tetrazolium to blue tetrazolium salt. The reaction was performed in sodium carbonate buffer (50 mM, pH 10.1) containing EDTA (0.1 mM), nitroblue tetrazolium (25 μM), xanthine, and xanthine-oxidase (0.1 mM and 2 nM, respectively; Boehringer Ingelheim GmbH, Ingelheim, Germany). The rate of nitroblue tetrazolium reduction was monitored spectrophotometrically (PerkinElmer Lambda 5 Spectrophotometer; PerkinElmer Life and Analytical Sciences, Waltham, MA) at 560 nm. The amount of protein required to inhibit the rate of NTB reduction by 50% was defined as 1 unit of enzyme activity. Cu,Zn-SOD activity was inhibited by performing the assay in the presence of 2 mM NaCN after preincubation for 30 min. Enzymatic activity was expressed in units per milligram of protein (Wang et al., 2004).
Statistics
For paired group analysis, Student's t test was performed. For paired multiple groups, analysis of variance followed by Student-Newman-Keuls test was employed to analyze the data. Results are expresses as mean ± S.E.M. for n animals. A statistical significant difference was defined as a p value < 0.05.
Results
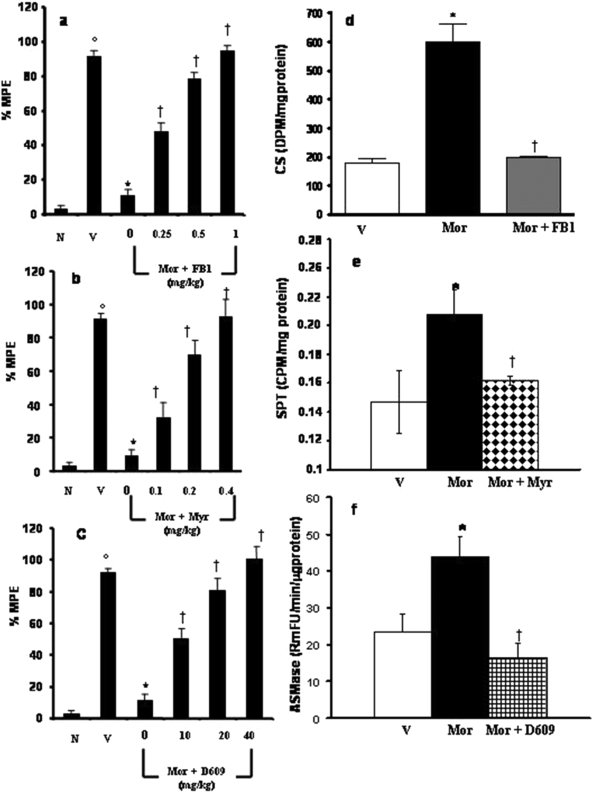
Inhibition of Ceramide Biosynthesis Blocks Morphine Antinociceptive Tolerance without Affecting Motor Function. Compared with animals receiving an equivalent injection of saline (naive group), acute injection of morphine (3 mg/kg) in animals that received saline over 4 days (vehicle group) produced a significant near-maximal antinociceptive response [percentage maximal possible antinociceptive effect (%MPE), ranging from 90-95%] (Fig. 1, a-c). On the other hand, repeated administration of morphine over the same time course (morphine group) led to the development of antinociceptive tolerance, as evidenced by a significant loss of its antinociceptive response (Fig. 1, a-c). Antinociceptive tolerance was associated with increased enzymatic activity of ceramide synthase (CS; Fig. 1d), SPT (Fig. 1e), and the insoluble form of acid sphingomyelinase (ASMase; Fig. 1f) and was also associated with the appearance of ceramide in the superficial layers of the dorsal horn, as detected by immunohistochemistry (arrows, Fig. 2, b-b3). Activities of the soluble form of ASMase and the neutral SMase were not changed compared with vehicle (data not shown). Baseline latencies in vehicle and morphine groups were statistically insignificant from each other and ranged between 6 and 8 s (n = 10).
Fig. 1.
The development of morphine antinociceptive tolerance is prevented by inhibitors of ceramide biosynthesis. On day 5. acute injection of morphine (3 mg/kg) in animals that received saline over 4 days [vehicle group (V)] produced a significant antinociceptive response compared with responses observed in animals that received an equivalent volume of saline [naïve group (N)] (a-c). On the other hand, a significant loss to the antinociceptive effect of the acute injection of morphine was observed in tolerant animals [morphine (Mor) group] (a-c). The development of tolerance was associated with increased activity of CS (d), SPT (e), and ASMAse (insoluble form). Inhibitors of CS, SPT, and SMAse, namely FB1 (1 mg/kg/day, n = 10), Myr (0.4 mg/kg/day, n = 10), and D609 (40 mg/kg/day, n = 10), blocked the increased enzymatic activities of their respective enzymes (d-f). Finally, coadministration of morphine over 4 days with FB1 (0.25-1 mg/kg/day, a), Myr (0.1-0.4 mg/kg/day, b), or D609 (10-40 mg/kg/day, c) inhibited the development of tolerance in a dose-dependent manner. Results are expressed as mean ± S.E.M. for 10 animals. ○, p < 0.001 for vehicle versus naive; *, p < 0.001 for morphine alone versus vehicle; †, p < 0.001 for morphine plus drug versus morphine alone.
Fig. 2.
Immunohistochemical detection of ceramide. No positive staining for ceramide was observed in the dorsal horn compared with ventral horn tissues of control groups (a-a3). Five days after morphine treatment, a marked appearance of positive staining for ceramide (brown) was observed in the dorsal horn compared with the ventral horn (b-b3, see arrows). FB1 treatment abolished the presence of positive staining for ceramide (c-c3). Tissue sections were stained using 3,3′-diaminobenzidine. Representative of at least three experiments performed on different days. Tissues from the dorsal and ventral spinal cord were taken on the same day and processed together.
To investigate whether the increased ceramide synthesis had a functional role in the development of morphine's antinociceptive tolerance, morphine was coadministered with specific inhibitors of both de novo and sphingomyelinase pathways. Coadministration of morphine with FB1 (1 mg/kg/day, n = 10), a competitive and reversible inhibitor of ceramide synthase (Delgado et al., 2006), attenuated, as expected, the increase in CS activity (Fig. 1d) and ceramide immunostaining (Fig. 2, c-c3) and attenuated in a dose-dependent manner (0.1-1 mg/kg/day, n = 10) the development of tolerance (Fig. 1a). Similar results were obtained with another inhibitor of the de novo pathway, myriocin, which targets the rate-limiting, most upstream enzyme, serine palmitoyltransferase (Delgado et al., 2006). Coadministration of morphine with myriocin (0.4 mg/kg/day, n = 10) blocked, as expected, the activation of SPT (Fig. 1e), the increase in ceramide immunostaining (data not shown), and the development of antinociceptive tolerance in a dose-dependent manner (0.1-0.4 mg/kg/day, n = 10) (Fig. 1b). The role of the SMase pathway was determined by treating animals with D609 (10-40 mg/kg/day, n = 10), an inhibitor of this enzyme (Delgado et al., 2006). When coadministered with morphine, D609 (40 mg/kg/day, n = 10) blocked the increased activity of ASMase (Fig. 1f) and ceramide immunostaining (data not shown) and blocked in a dose-dependent manner (10-40 mg/kg/day, n = 10) the development of tolerance (Fig. 1c). Because the inhibitory activities of D609 are not limited to SMase but may also include sphingomyelin synthase, it is possible that inhibition of both enzymes accounted for the overall beneficial action of D609 against tolerance development (Delgado et al., 2006).
To establish whether these inhibitors when tested at the highest dose shown to block antinociceptive tolerance cause motor function impairment, mice were treated with myriocin (0.4 mg/kg), FB1 (1 mg/kg), or D609 (40 mg/kg) and then tested on the Rotorod for potential motor function deficits at 15, 30, and 60 min after drug administration. Compared with the vehicle-treated group, these drugs did not show signs of Rotorod deficits over the observed time frame (n = 4, data not shown).
Inhibition of Ceramide Biosynthesis Does Not Affect the Acute Antinociceptive Effects to Morphine. The inhibitory effects of FB1, myriocin, or D609 were not attributable to acute antinociceptive interactions among FB1, myriocin, or D609 and morphine because the responses to acute morphine (0.3-3 mg/kg, n = 10) in animals treated with the highest dose of FB1 (1 mg/kg/day, n = 10), Myr (0.4 mg/kg/day, n = 10), D609 (40 mg/kg/day, n = 10), or their vehicle over 5 days were statistically insignificant (Fig. 3). These results suggest that ceramide is not involved in spinal neurotransmission and antinociceptive signaling in response to brief administration of morphine. When tested alone, at the highest dose, FB1, myriocin, or D609 had no antinociceptive effects. Thus, on day 5, hot-plate latencies after a subcutaneous injection of saline in the vehicle group or in animals that received the highest dose of FB1, myriocin, or D609 were statistically insignificant and ranged between 6 and 7 s (n = 10; data not shown).
Fig. 3.
Lack of effect of ceramide inhibitors on antinociceptive responses to acute morphine in nontolerant animals. On day 5, acute injection morphine (0.3-3 mg/kg) in animals that received saline over 4 days produced a dose-dependent significant antinociceptive response compared with responses obtained in animals receiving an equivalent volume of its vehicle. The antinociceptive response to morphine was not altered in animals that were treated over 4 days with Myr (0.4 mg/kg/day), FB1 (1 mg/kg/day), or D609 (40 mg/kg/day), indicating lack of an acute interaction between morphine and ceramide synthesis inhibitors. Results are expressed as mean ± S.E.M. for 10 animals. *, p < 0.001 for vehicle plus morphine versus vehicle alone.
Inhibition of Ceramide Biosynthesis Does Not Reverse Established Morphine Tolerance. Loss of the antinociceptive effect of morphine observed on day 5 in the morphine group was not restored by a single administration of the highest dose of FB1 (1 mg/kg, n = 6), myriocin (0.4 mg/kg/day, n = 6), or D609 (40 mg/kg, n = 6) used and given by intraperitoneal injection 15 min before the acute dose of morphine (3 mg/kg). Thus, the %MPE was 96 ± 3, 10 ± 2, 7 ± 3, 13 ± 2, and 11 ± 2% for the vehicle, morphine, morphine plus FB1, morphine plus myriocin, and morphine plus D609 groups, respectively (n = 6, p < 0.5 for all groups). These results suggest that these pharmacological agents inhibit the development of, and not the expression of, tolerance.
The profound and equal inhibitory effect of myriocin, FB1, and D609 indicated that controlling ceramide levels in the dorsal horn of the spinal cord is paramount to preventing antinociceptive tolerance, regardless of the enzymatic pathway by which it is synthesized. Therefore, only FB1 was chosen as an effective and well characterized inhibitor of ceramide biosynthesis in subsequent mechanistic studies aimed to understand the downstream pathophysiological effects initiated by an increase in spinal cord ceramide.
Inhibition of Ceramide Biosynthesis Attenuates Peroxynitrite-Derived Nitroxidative Stress.
We have reported recently that peroxynitrite is a key player in the development of morphine antinociceptive tolerance and have provided data to show that formation of 3-nitrotyrosine (NT) in the superficial layers of the dorsal horn during morphine antinociceptive tolerance originates from spinal production of peroxynitrite (Muscoli et al., 2007). Therefore, detection of NT in this setting can be reliably used as a marker of peroxynitrite. We now show that the appearance of NT staining in tolerant mice (Fig. 4b) was blocked by coadministration of morphine with FB1 (1 mg/kg/day; Fig. 4c), suggesting the contribution of ceramide in the production of spinal peroxynitrite. Post-translational nitration and enzymatic inactivation of MnSOD in the spinal cord are important sources for sustaining high levels of spinal peroxynitrite during the development of central sensitization associated with morphine antinociceptive tolerance (Muscoli et al., 2007). As can be seen in Fig. 4, FB1 (1 mg/kg/day) prevented post-translational nitration of mitochondrial MnSOD as shown by immunoprecipitation (from 400 ± 50 to 850 ± 70 densitometry units ± S.E.M. for vehicle and morphine, respectively, n = 5, p < 0.001; and from 850 ± 70 to 350 ± 45 for morphine and morphine plus FB1, respectively, n = 5, p < 0.001; a representative gel of five animals is shown in Fig. 4d) and restored in a dose-dependent manner (0.25-1 mg/kg/day, n = 5), the loss of its enzymatic activity as measured spectrophotometrically (Fig. 4f). Total levels of MnSOD protein did not change among the three groups (a representative gel of five animals is shown in Fig. 4e).
Fig. 4.
The development of nitroxidative stress during morphine antinociceptive tolerance is blocked by an inhibitor of ceramide biosynthesis, FB1. On day 5, acute injection of morphine did not lead to the appearance of NT staining in the dorsal horn (a). On the other hand, acute administration of morphine on day 5 after repeated administration of morphine led to significant protein nitration, as detected by immunohistochemistry (b, see arrows), post-translational nitration (d), and enzymatic inactivation of MnSOD (f). Coadministration of morphine over 4 days with FB1 (1 mg/kg/day) attenuated NT staining (c), prevented Mn-SOD nitration (d), and restored its enzymatic activity in a dose-dependent manner (0.125-1 mg/kg/day, f; n = 5 and 10, respectively). Total protein levels did not change among groups as measured by Western blotting analysis (e). Gels shown in d and e are representative of gel results obtained from six animals. Micrographs (×10 magnification) are representative of at least five from different animals performed on different days and are taken from the superficial layers of the dorsal horn, the anatomical site that stains for NT during tolerance (Muscoli et al., 2007). *, p < 0.001 for morphine versus vehicle; †, p < 0.05; ††, p < 0.001 for morphine plus FB1 versus morphine alone.
Inhibition of Ceramide Biosynthesis Attenuates Neuroimmune Activation. On day 5, when compared with the vehicle group, acute injection of morphine (3 mg/kg, n = 10) in the morphine group led to a significant activation of NF-κB, as demonstrated by IκB-α degradation (Fig. 5, a and a1), increased Ser536 phosphorylation (Fig. 5, b and b1), and increased total NF-κB p65 nuclear expression (Fig. 5, c and c1). Furthermore, acute injection of morphine in the morphine group increased glial cell activation determined by enhanced spinal expression of GFAP (a cellular marker for astrocytes; from 5455.13 ± 0.514 to 7343.95 ± 0.527 densitometry units, n = 5, p < 0.01; Fig. 6b) and IBa1 [ionized calcium-binding adaptor molecule 1; a cellular marker for microglia (Narita et al., 2006), from 241.66 ± 0.039 to 541.29 ± 0.073 densitometry units ± S.E.M., n = 5, p < 0.001; Fig. 6e], measured by immunohistochemistry and Western blotting (data not shown). Finally, acute injection of morphine in the morphine group increased immunoreactivity for TNF-α, IL-1β, and IL-6 in the dorsal horn of the lumbar spinal cord, as measured by enzyme-linked immunosorbent assay (n = 10, Fig. 7, a-c). NF-κB activation was attenuated by FB1 (1 mg/kg/day) (Fig. 5, a-c), as was the activation of astrocytes (from 7343.95 ± 0.527 to 4627.38 ± 0.483 densitometry units ± S.E.M., n = 5, p < 0.001; Fig. 6c) and microglial cell (from 541.29 ± 0.073 to 275.53 ± 0.053 densitometry units ± S.E.M., n = 5, p < 0.001; Fig. 6f). Fumonisin B1 (0.25-1 mg/kg/day, n = 10) reduced in a dose-dependent fashion increased release of TNF-α, IL-1β, and IL-6 (Fig. 7, a-c).
Fig. 5.
NF-κB activation during morphine antinociceptive tolerance is blocked by an inhibitor of ceramide biosynthesis, FB1. On day 5, compared with responses to acute morphine in the vehicle group, repeated administration of morphine over the same time course (Mor group) led to NF-κB activation as evidenced by: 1) a decrease in the basal level of IκB-α (a and a1), 2) a significant increase in phosphorylation of Ser536 (b and b1), and 3) an increase in the nuclear levels of the NF-κB p65 (c and c1); these were events blocked by FB1 (1 mg/kg/day, a-c, a1-c1). Results are expressed as mean ± S.E.M. for n = 5 animals. *, p < 0.001 for morphine alone versus vehicle; †, p < 0.05 and ††, p < 0.05 for morphine plus drug versus morphine alone.
Fig. 6.
Activation of spinal glial cells during morphine antinociceptive tolerance is blocked by an inhibitor of ceramide biosynthesis, FB1. Compared with vehicle (a and d), acute administration of morphine in tolerant mice led to neuroimmune activation, as evidenced by: 1) increased GFAP (marker of activated astrocytes, b) and IBa1 (marker of activated microglial cells, and e) immunoreactivity in the superficial layers of the dorsal horn, the activation of which was blocked by FB1 (1 mg/kg/day, c and f). Micrographs (×20 magnification) are representative of at least three from different animals performed on different days.
Fig. 7.
Increased spinal production of proinflammatory and pronociceptive cytokines are blocked by an inhibitor of ceramide biosynthesis, FB1. On day 5, when compared with responses to acute morphine in the vehicle group, repeated administration of morphine over the same time course (Mor group) led to a significant increase in TNF-α, IL-1β, and IL-6 levels in dorsal horn tissues (a-c), which were reduced by FB1 in a dose-dependent manner (0.25-1 mg/kg/day, n = 10; a-c). Results are expressed as mean ± S.E.M. for n = 10 animals. *, p < 0.001 for morphine alone versus vehicle; †, p < 0.05 and ††, p < 0.05 for morphine plus drug versus morphine alone.
Discussion
The objectives of our study were to establish whether ceramide plays a role in the development of morphine antinociceptive tolerance and, if so, by what mechanism(s). Our results revealed a novel mechanism triggered by repeated administration of morphine, which increased the activity of enzymes involved in the biosynthesis of ceramide from both the de novo and sphingomyelinase pathways; pharmacological inhibition of both pathways blocked the development of antinociceptive tolerance (Fig. 8). Thus, these enzymatic pathways were functionally responsible for both spinal cord ceramide synthesis and antinociceptive tolerance to morphine. The critical role of ceramide in the control of neural apoptosis has been attributed to its generation through both sphingomyelin hydrolysis by neutral (Brann et al., 2002) and/or acid sphingomyelinases and de novo synthesis (Blázquez et al., 2000). The findings that the activity of the soluble and neutral forms of SMAse did not increase in response to repeated morphine administration can be interpreted to suggest that either these enzyme isoforms do not contribute to the development of tolerance or that they do but that their enzymatic activity returned to baseline levels at the time of assay. Addressing the relative contributions of each isoform will be done reliably as selective inhibitors are developed. The profound and equal inhibitory effect of the three pharmacological inhibitors myriocin, FB1, and D609 on the antinociceptive tolerance to morphine indicated that controlling ceramide levels in the dorsal horn of the spinal cord is paramount to preventing tolerance, regardless of the enzymatic pathway by which it is synthesized. Therefore, only FB1 was chosen as an effective and well characterized inhibitor of ceramide biosynthesis in subsequent mechanistic studies aimed to understand the downstream pathophysiological effects initiated by an increase in spinal cord ceramide. The upstream events that link repeated morphine administration with the activation of ceramide biosynthesis remain to be elucidated.
Fig. 8.
Cartoon summarizing the key findings of this study. Formation of ceramide in the spinal cord during repeated administration of morphine plays a critical role in the development of morphine-induced antinociceptive tolerance through formation of peroxynitrite-mediated nitroxidative stress and neuroimmune activation (glial cell activation and release of proinflammatory cytokines) after activation of NF-κB. The overall pathway can, in turn, be amplified through feedback activation of ceramide biosynthesis by several nitroxidative species, including peroxynitrite. Inhibition of ceramide by inhibitors of the ceramide metabolic pathway such as FB1 blocked these pathways, leading to inhibition of antinociceptive tolerance. The ceramide metabolic pathway is an attractive target for novel approaches in the management of pain.
Our data implicate ceramide as an upstream signaling mediator in one of two major patho-biochemical mechanisms for development of morphine antinociceptive tolerance, namely peroxynitrite-mediated nitroxidative stress and neuroimmune activation (Fig. 8). Considerable evidence implicates peroxynitrite-mediated nitroxidative stress in the development of pain of several etiologies and importantly in opiate antinociceptive tolerance, caused by the presence of superoxide (Salvemini, 2001; Muscoli et al., 2007), nitric oxide (Pasternak, 1995), and, more recently, peroxynitrite (Muscoli et al., 2007). Ceramide stimulates the formation of reactive nitroxidative species, including superoxide and nitric oxide (Pahan et al., 1998; Goldkorn et al., 2005). In turn, superoxide, nitric oxide, and peroxynitrite can increase steady-state concentrations of ceramide by activating sphingomyelinases and by increasing the degradation of ceramidases, the enzymes responsible for the degradation of ceramide (Pautz et al., 2002). These data support the close and reciprocal interaction between the nitroxidative and ceramide metabolic pathways; such close interplay contributes to the overall increase in the levels of ceramide and thus ceramide-mediated damage. The findings in the present study that inhibition of ceramide biosynthesis blocked peroxynitrite suggest that ceramide is an important signaling event in its formation, further supporting the intimal relationship between the ceramide metabolic and the nitroxidative pathways as observed in other pathological settings (Delogu et al., 1999; Kolesnick, 2002; Göggel et al., 2004; Masini et al., 2005; Petrache et al., 2005). A biologically relevant feature of peroxynitrite is post-translational tyrosine nitration and consequent modification of protein function (Radi, 2004), as exemplified by MnSOD, the enzyme that normally keeps concentrations of superoxide under tight control (McCord and Fridovich, 1969). Peroxynitrite-mediated nitration of MnSOD inactivates the enzyme, leading to an increase in superoxide levels, thereby favoring peroxynitrite formation in several disease states (Yamakura et al., 1998, 2001; MacMillan-Crow et al., 2001), including in the development of morphine tolerance (Muscoli et al., 2007) and hyperalgesia associated with acute inflammation and in response to N-methyl-d-aspartate receptor activation (Wang et al., 2004; Muscoli et al., 2007). As shown in this study, inhibition of ceramide biosynthesis by attenuating the formation of peroxynitrite mediated blocked nitration of MnSOD, thus restoring the enzymatic activity of this enzyme. Thus, FB1 interrupted a potentially vicious circle known to influence the presence of nitroxidative stress.
Neuroimmune activation contributes to morphine antinociceptive tolerance, as shown in both preclinical (Song and Zhao, 2001; Watkins et al., 2007) and clinical (Lu et al., 2004) studies. Thus, anticytokine approaches and/or inhibitors of glial cell metabolism block morphine-induced hyperalgesia and antinociceptive tolerance (Song and Zhao, 2001; Watkins et al., 2007). Ceramide activates, through mechanisms ill-defined, several redox-sensitive transcription factors, including NF-κB, which in turn regulate the production of many inflammatory and pronociceptive cytokines; inhibition of ceramide biosynthesis with inhibitors of the sphingomyelinase or de novo pathways blocks NF-κB activation and synthesis of TNF-α, IL-1β, and IL-6 in animal models of acute and chronic inflammation (Delogu et al., 1999; Kolesnick, 2002; Göggel et al., 2004; Masini et al., 2005; Petrache et al., 2005). For the first time, our results identify ceramide as a signaling mediator in neuroimmune activation and suggest that activation of NF-κB is a key step in this process. Inhibition of ceramide biosynthesis by FB1 prevented NF-κB activation, blocked astrocytic and microglial cell activation, and suppressed the increase in TNF-α, IL-1β, and IL-6 in dorsal horn tissues blocking antinociceptive tolerance. We propose that a likely mechanism through which ceramide activates NF-κB is via peroxynitrite. This is supported by the fact that: 1) inhibition of ceramide biosynthesis blocks spinal formation of peroxynitrite (this study), 2) peroxynitrite activates several redox-sensitive transcriptions, including NF-κB and AP-1, and mitogen-activated protein kinase kinases such as p38 kinase to release TNF-α, IL-1β, and IL-6 (Matata and Galiñanes, 2002; Ndengele et al., 2005); and 3) peroxynitrite contributes to the development of antinociceptive tolerance through release of spinal TNF-α, IL-1β, and IL-6 (Muscoli et al., 2007). It is important that glial cells activation can generate several nitroxidative species implicated in the development of morphine antinociceptive tolerance, including superoxide (Salvemini, 2001; Muscoli et al., 2007), nitric oxide (Pasternak, 1995), and peroxynitrite (Muscoli et al., 2007). On a final note, it is important to recognize that ceramide is a potent proapoptotic signaling lipid, and spinal apoptosis has been linked to antinociceptive tolerance (Mayer et al., 1999; Lim et al., 2005). In this context, whether ceramide contributes to the formation of dorsal horn “dark neurons” (Mayer et al., 1999) observed in antinociceptive tolerance is a viable possibility that needs to be explored in future studies.
Our focus addressed the involvement of ceramide within the dorsal horn of the spinal cord; thus, our data cannot exclude the contribution of ceramide in antinociceptive tolerance at supraspinal sites. This exciting possibility will be pursued in future studies. In summary, results derived from our studies have defined, for the first time, the importance of ceramide in the development of antinociceptive tolerance and have provided evidence for the contribution of at least two mechanistic pathways through which this sphingolipid exerts its actions, namely peroxynitrite-derived nitroxidative stress and neuroimmune activation (Fig. 8). These data provide a pharmacological basis validating the approach of developing inhibitors of the ceramide metabolic pathway as adjuncts to opiates in the management of pain.
Acknowledgments
We thank Giovanni Politi (University of Rome, Rome, Italy) and Leesa A. Bryant (Saint Louis University, St. Louis, MO) for technical support.
This work was supported by the Saint Louis University Seed Fund; the National Institutes of Health [Grant RO1-HL077328]; COFIN 2005 [Grant 2005057404004]; Istituto di Ricovero e Cura a Carattere Scientifico Centro Neurolesi “Bonino-Pulejo” grant; and the University of Florence (Florence, Italy).
M.M.N. and S.C. contributed equally to this work.
doi:10.1124/jpet.108.146290.
ABBREVIATIONS: TNF, tumor necrosis factor; IL, interleukin; SMase, sphingomyelinase; FB1, fumonisin B1; D609, tricyclodecan-9-xanthogenate; SPT, serine palmitoyl transferase; PBS, phosphate-buffered saline; GFAP, glial fibrillary acidic protein; RT, room temperature; NF, nuclear factor; MnSOD, manganese superoxide dismutase; %MPE, percentage maximal possible antinociceptive effect; CS, ceramide synthase; ASMase, acid sphingomyelinase; NT, 3-nitrotyrosine; Mor, morphine.
References
- Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, and Yezierski RP (1998) Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci 18 3251-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez C, Galve-Roperh I, and Guzmán M (2000) De novo-synthesized ceramide signals apoptosis in astrocytes via extracellular signal-regulated kinase. FASEB J 14 2315-2322. [DOI] [PubMed] [Google Scholar]
- Brann AB, Tcherpakov M, Williams IM, Futerman AH, and Fainzilber M (2002) Nerve growth factor-induced p75-mediated death of cultured hippocampal neurons is age-dependent and transduced through ceramide generated by neutral sphingomyelinase. J Biol Chem 277 9812-9818. [DOI] [PubMed] [Google Scholar]
- Castillo SS, Levy M, Wang C, Thaikoottathil JV, Khan E, and Goldkorn T (2007) Nitric oxide-enhanced caspase-3 and acidic sphingomyelinase interaction: a novel mechanism by which airway epithelial cells escape ceramide-induced apoptosis. Exp Cell Res 313 816-823. [DOI] [PubMed] [Google Scholar]
- Covino B, Dubner R, Gybels J, Losterlitz HW, Liebsking JC, Sternbach RA, Vylicky L, Yamamura H, and Zuimmerman M (1980) Ethical standards for investigations of experimental pain in animals. Pain 9 141-143.7454381 [Google Scholar]
- Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, and Nicholson GA (2001) Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat Genet 27 309-312. [DOI] [PubMed] [Google Scholar]
- Delgado A, Casas J, Llebaria A, Abad JL, and Fabrias G (2006) Inhibitors of sphingolipid metabolism enzymes. Biochim Biophys Acta 1758 1957-1977. [DOI] [PubMed] [Google Scholar]
- Delogu G, Famularo G, Amati F, Signore L, Antonucci A, Trinchieri V, Di Marzio L, and Cifone MG (1999) Ceramide concentrations in septic patients: a possible marker of multiple organ dysfunction syndrome. Crit Care Med 27 2413-2417. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT and Kolesnick RN (2001) Analysis of sphingomyelin and ceramide levels and the enzymes regulating their metabolism in response to cell stress. Methods Cell Biol 66 135-165. [DOI] [PubMed] [Google Scholar]
- Foley KM (1995) Misconceptions and controversies regarding the use of opioids in cancer pain. Anticancer Drugs 6 (Suppl 3): 4-13. [DOI] [PubMed] [Google Scholar]
- Göggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, Brade H, Ehlers S, Slutsky AS, Schütze S, et al. (2004) PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med 10 155-160. [DOI] [PubMed] [Google Scholar]
- Goldkorn T, Ravid T, and Khan EM (2005) Life and death decisions: ceramide generation and EGF receptor trafficking are modulated by oxidative stress. Anti-oxid Redox Signal 7 119-128. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th Ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Joseph EK and Levine JD (2004) Caspase signalling in neuropathic and inflammatory pain in the rat. Eur J Neurosci 20 2896-2902. [DOI] [PubMed] [Google Scholar]
- Kolesnick R (2002) The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest 110 3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick R and Fuks Z (2003) Radiation and ceramide-induced apoptosis. Oncogene 22 5897-5906. [DOI] [PubMed] [Google Scholar]
- Lim G, Wang S, Lim JA, and Mao J (2005) Activity of adenylyl cyclase and protein kinase A contributes to morphine-induced spinal apoptosis. Neurosci Lett 389 104-108. [DOI] [PubMed] [Google Scholar]
- Lu CH, Chao PC, Borel CO, Yang CP, Yeh CC, Wong CS, and Wu CT (2004) Preincisional intravenous pentoxifylline attenuating perioperative cytokine response, reducing morphine consumption, and improving recovery of bowel function in patients undergoing colorectal cancer surgery. Anesth Analg 99: 1465-1471; table of contents. [DOI] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, and Thompson JA (2001) Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol Med 31 1603-1608. [DOI] [PubMed] [Google Scholar]
- Masini E, Bani D, Vannacci A, Pierpaoli S, Mannaioni PF, Comhair SA, Xu W, Muscoli C, Erzurum SC, and Salvemini D (2005) Reduction of antigen-induced respiratory abnormalities and airway inflammation in sensitized guinea pigs by a superoxide dismutase mimetic. Free Radic Biol Med 39 520-531. [DOI] [PubMed] [Google Scholar]
- Matata BM and Galiñanes M (2002) Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-kappa B DNA binding activity. J Biol Chem 277 2330-2335. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Mao J, Holt J, and Price DD (1999) Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A 96 7731-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM and Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244 6049-6055. [PubMed] [Google Scholar]
- Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini E, Matuschak GM, and Salvemini D (2007) Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance. J Clin Invest 117 3530-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Yoshida T, Nakajima M, Narita M, Miyatake M, Takagi T, Yajima Y, and Suzuki T (2006) Direct evidence for spinal cord microglia in the development of a neuropathic pain-like state in mice. J Neurochem 97 1337-1348. [DOI] [PubMed] [Google Scholar]
- Ndengele MM, Muscoli C, Wang ZQ, Doyle TM, Matuschak GM, and Salvemini D (2005) Superoxide potentiates NF-kappaB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock 23 186-193. [DOI] [PubMed] [Google Scholar]
- Pahan K, Sheikh FG, Khan M, Namboodiri AM, and Singh I (1998) Sphingomyelinase and ceramide stimulate the expression of inducible nitric-oxide synthase in rat primary astrocytes. J Biol Chem 273 2591-2600. [DOI] [PubMed] [Google Scholar]
- Pasternak GW (1995) Nitric oxide and opioid tolerance. NIDA Res Monogr 147 182-194. [PubMed] [Google Scholar]
- Pautz A, Franzen R, Dorsch S, Böddinghaus B, Briner VA, Pfeilschifter J, and Huwiler A (2002) Cross-talk between nitric oxide and superoxide determines ceramide formation and apoptosis in glomerular cells. Kidney Int 61 790-796. [DOI] [PubMed] [Google Scholar]
- Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, and Tuder RM (2005) Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 11 491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R (2004) Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A 101 4003-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfrey S, Downton C, and Featherstone J (2003) The painful reality. Nat Rev Drug Discov 2 175-176. [DOI] [PubMed] [Google Scholar]
- Rother J, van Echten G, Schwarzmann G, and Sandhoff K (1992) Biosynthesis of sphingolipids: dihydroceramide and not sphinganine is desaturated by cultured cells. Biochem Biophys Res Commun 189 14-20. [DOI] [PubMed] [Google Scholar]
- Salvemini D (2001) inventor; G.D. Searle & Co., assignee. Analgesic methods using synthetic catalysts for the dismutation of superoxide radicals. U.S. patent 6,180620. 1998 April 9.
- Salvemini D, Wang ZQ, Stern MK, Currie MG, and Misko TP (1998) Peroxynitrite decomposition catalysts: therapeutics for peroxynitrite-mediated pathology. Proc Natl Acad Sci U S A 95 2659-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P and Zhao ZQ (2001) The involvement of glial cells in the development of morphine tolerance. Neurosci Res 39 281-286. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, et al. (2004) A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther 309 869-878. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, and Maier SF (2007) Norman Cousins lecture: glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun 21 131-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RD, Wang E, and Merrill AH Jr (1984) Enzymology of long-chain base synthesis by liver: characterization of serine palmitoyltransferase in rat liver microsomes. Arch Biochem Biophys 228: 282-291. [DOI] [PubMed] [Google Scholar]
- Yamakura F, Matsumoto T, Fujimura T, Taka H, Murayama K, Imai T, and Uchida K (2001) Modification of a single tryptophan residue in human Cu,Zn-superoxide dismutase by peroxynitrite in the presence of bicarbonate. Biochim Biophys Acta 1548 38-46. [DOI] [PubMed] [Google Scholar]
- Yamakura F, Taka H, Fujimura T, and Murayama K (1998) Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem 273 14085-14089. [DOI] [PubMed] [Google Scholar]