Abstract
Elevated sensitivity to the euphoric or stimulant effects of ethanol is associated with higher levels of alcohol use in some human populations. Midbrain dopamine neurons are thought to be important mediators of both ethanol reward and locomotor stimulation. Patch-clamp recordings were used to examine the electrical properties of dopamine neurons in a genetic model of heightened (FAST) and reduced (SLOW) sensitivity to the locomotor-activating effects of ethanol. Pacemaker firing of dopamine neurons was faster in FAST than SLOW mice, as was the current density through IH channels. Acute administration of ethanol accelerated the firing of dopamine neurons to a greater extent in recordings from FAST than SLOW mice. Dopamine neurons from FAST mice also exhibited reduced GABAA receptor-mediated synaptic input, compared with SLOW mice. The results suggest that dopamine neuron IH channels, firing rate, and GABAergic input may play a role in sensitivity to the locomotor activation observed at early time points after ethanol administration and could underlie differences in sensitivity to alcohol relevant to risk for alcohol abuse.
Twin and adoption studies support a major genetic component in the predisposition to develop alcoholism, although the specific genes responsible have been elusive (Quickfall and el-Guebaly, 2006). Thus, endophenotypes, which are simpler, more easily measured traits that share a genetic relationship with alcoholism, are being used to investigate the genetic and neurobiological underpinnings of this complex disorder. Simple measures of ethanol sensitivity such as changes in balance and subjective stimulation (e.g., feeling “high” or energized) have shown promise as endophenotypes that predict susceptibility to excessive alcohol use (Schuckit and Smith, 2000; King et al., 2002). The biphasic effects of ethanol (stimulation followed by sedation) in humans are time- and dose-dependent (Addicott et al., 2007), and similar patterns of activity and sedation can be readily modeled in mice (Palmer et al., 2002). Such animal models allow research aimed at gaining a better understanding of the neurophysiological underpinnings of sensitivity to ethanol.
Ethanol has many established targets in the brain, and differential sensitivity of these targets could contribute to individual differences in alcohol-related behaviors. Dopamine neurotransmission originating in the midbrain is critical to the direct and conditioned reinforcing effects of ethanol (Bechtholt and Cunningham, 2005; Doyon et al., 2005) and ethanol-induced stimulation (Di Chiara and Imperato, 1988; Shen et al., 1995). Animals will self-administer ethanol directly into the posterior ventral tegmental area (VTA) (Rodd et al., 2004), and dopamine antagonists administered at the terminal site into the nucleus accumbens attenuate alcohol drinking (Samson et al., 1993). In vitro, acute administration of ethanol accelerates VTA and substantia nigra dopamine neuron firing (Brodie et al., 1999; Okamoto et al., 2006), which may contribute to increases in terminal dopamine release observed in vivo (Di Chiara and Imperato, 1988; Yim et al., 1998). GABA receptor-mediated inhibition of dopamine neurons (Tepper et al., 1998) may modulate the rewarding effects of ethanol (Samson et al., 1987; Melis et al., 2002). Furthermore, behavioral studies suggest that both GABAA and GABAB receptor subtypes play a role in the locomotor response to ethanol (Phillips et al., 1992; Boehm et al., 2002; Palmer et al., 2002; Holstein et al., 2009). Recent work by Okamoto et al. (2006) suggests that ethanol also enhances a nonselective cation conductance (IH) in VTA and substantia nigra dopamine neurons. IH facilitates spontaneous firing of dopamine neurons (Neuhoff et al., 2002; Puopolo et al., 2007; but see Mercuri et al., 1995), suggesting a cellular mechanism by which ethanol could directly modulate locomotion, reward, and other dopamine-related behaviors.
This investigation examined dopamine neurons in the substantia nigra of a genetic mouse model of heightened (FAST) and reduced (SLOW) sensitivity to the acute locomotor-stimulating effects of ethanol. The FAST and SLOW mice are the only lines that have been selectively bred for this ethanol sensitivity trait. The FAST mice also consume more ethanol than SLOW mice (Risinger et al., 1994) and exhibit reduced sensitivity to the sedative effects of ethanol (Phillips et al., 2002). These characteristics make them a compelling model of the cluster of traits associated with risk for alcoholism. The goal of the electrophysiological studies performed herein was to test the hypothesis that cellular mediators of dopamine neuron activity could mediate ethanol-induced stimulation. Dopamine neurons from FAST mice exhibited greater basal firing rate, greater IH current density, and lower levels of GABAergic input. This genetic correlation between the electrophysiological findings and the ethanol sensitivity trait identify specific properties of dopamine neurons that may be controlled by the same genes that influence sensitivity to the stimulant effects of ethanol.
Materials and Methods
All animal protocols were approved by the institutional animal care and use committee at Oregon Health & Science University. Dopamine, ethanol, [Met]5 enkephalin, MK-801, strychnine, 2,3-dihydroxy-6,7-dinitroquinoxaline, prazosin, and picrotoxin were obtained from Sigma-Aldrich (St. Louis, MO). ZD 7288 was purchased from Tocris Bioscience (Ellisville, MO). Baclofen, sulpiride, and hexamethonium were obtained from Sigma/RBI (Natick MA). CGP 56999a was a gift from Novartis (Basel, Switzerland).
Animals. Genetic selection of the FAST and SLOW mouse lines has been described previously (Phillips et al., 2002). In brief, mice from a heterogeneous stock (the product of an eight-way cross of genetically divergent inbred mouse strains) were tested for spontaneous locomotor activity for 4 min beginning 2 min after an injection of 2 g/kg ethanol i.p. Locomotor data after saline injection were collected at a 24-h interval from the ethanol response data, using the same procedure, and were subtracted from the ethanol response data to obtain an ethanol-induced stimulation score. For 37 generations, mice with high locomotor stimulation scores were selectively bred to each other and designated as FAST mice, whereas mice with low stimulation scores were interbred and designated as SLOW mice. Selection was subsequently relaxed (i.e., breeding was performed within line by arbitrary choice of breeders) because the additive genetic variance appeared to have been exhausted for several previous generations, suggesting that selection trait-relevant alleles had been homozygously fixed. In the approximately 50 generations hence, FAST and SLOW lines have been maintained without selection pressure but have retained a large phenotypic difference (Palmer et al., 2002). Second replicate FAST and SLOW mice from S37G75–93 (where S37 represents 37 selection generations and GXX represents the total number of breeding generations) were used in these studies. Data were obtained from a total of 41 SLOW and 33 FAST alcohol-naive male mice. On most days, recordings were made from one FAST and one SLOW mouse and the order in which the animals were euthanized varied across days.
Brain Slice Electrophysiology. Mice (32–130 days old) were sacrificed under halothane anesthesia, and their brains were quickly removed and immediately placed into an ice-cold isotonic solution. Horizontal slices of the ventral midbrain (220 μm thick) were obtained with a vibrating microtome and stored in a physiological salt solution (containing 126 mM NaCl, 2.5 mM KCl, 1.2 mM MgCl2, 2.4 mM CaCl2, 1.4 mM NaH2PO4, 25 mM NaHCO3, 0.4 mM ascorbic acid, and 11 mM d-glucose) at 35°C for at least 30 min before recordings were initiated. Slices were perfused at a rate of 1.5 to 2.0 ml/min and maintained at 35°C throughout the experiment. Dopamine neurons of the substantia nigra were identified visually by their appearance and position in relation to the medial terminal nucleus of the accessory optic tract and electrophysiologically by their slow spontaneous pacemaker firing and the presence of the time-dependent hyperpolarization-induced cation conductance IH. Substantia nigra neurons were chosen for this investigation because they have an established role in locomotion, respond to ethanol, and are more homogeneous than VTA neurons in their synaptic inputs and firing properties. Loose cell-attached patch-clamp experiments were performed with an internal pipette solution made up almost entirely of sodium HEPES plus 20 mM NaCl and 290 mOsm, pH 7.35. Whole-cell patch-clamp experiments were performed in voltage clamp at -60 mV. Experiments measuring GIRK-mediated currents used a pipette filling solution that contained 115 mM potassium methylsulfonate, 20 mM NaCl, 1.5 mM MgCl2, 2 mM ATP, 0.2 mM GTP, 10 mM phosphocreatine, 10 mM potassium HEPES, and 10 mM BAPTA, pH 7.30 to 7.35, 268 to 275 mOsm. Experiments recording GABAA-mediated currents used a pipette filling solution that contained 57.5 mM KCl, 57.5 mM potassium gluconate, 20 mM NaCl, 1.5 mM MgCl2, 2 mM ATP, 0.2 mM GTP, 10 mM phosphocreatine, 10 mM potassium HEPES, and 10 mM BAPTA, 275 mOsm, pH 7.35. The synaptic blockers used throughout this study to isolate specific receptor systems were sulpiride (200 nM, dopamine D2), strychnine (10 μM, glycine), CGP 56999a (100 nM, GABAB), picrotoxin (100 μM, GABAA), MK-801 (10 μM, N-methyl-d-aspartate), 2,3-dihydroxy-6,7-dinitroquinoxaline (10 μM, AMPA), and prazosin (100 nM, α1 adrenergic). GABAB receptors were blocked during all experiments measuring GABAA receptor currents (see Ariwodola and Weiner, 2004).
Ethanol, [Met]5 enkephalin, baclofen, and dopamine were applied by bath perfusion. Dopamine and GABA were also applied by iontophoresis. Iontophoretic pipettes were pulled from thin-wall glass to have a resistance of approximately 100 MΩ, then filled with 1 M dopamine or GABA. A backing current of -0.2 to -4.0 nA was applied to prevent passive leakage. The tip of the pipette was placed within 20 μm of the cell being recorded, and dopamine or GABA was ejected as a cation with a pulse of +190 nA for 1 to 5 s until the measured current reached a maximal plateau.
Synaptic currents were evoked by electrical stimulation with a bipolar platinum-stimulating electrode placed 50 to 150 μm caudal to the cell being recorded. GABAA currents were evoked with a single stimulation, and a train of five pulses was used to evoke GABAB and dopamine D2 receptor-mediated synaptic currents. The experimenter was blinded to the mouse line and the presence or absence of tetrodotoxin for the analysis of miniature and spontaneous GABAA inhibitory postsynaptic currents (mIPSCs and sIPSCs, respectively), which was done using Axograph 4.5.1 (John Clements). Cell capacitance and membrane resistance were also estimated by Axograph 4.5.1 in response to a 10-mV test pulse. Experiments were designed to be short so that virtually every cell recorded was included in the final analysis.
Statistical Analysis. Data were collected and quantified on a Macintosh G4 computer using Axograph 4.5.1 and Chart 4.0.1 (Molecular Devices, Sunnyvale, CA). One- and two-way analyses of variance were used to assess effects of independent variables, followed by Tukey's post hoc test, when appropriate. When recordings from FAST and SLOW mice were compared on single dependent measures, analyses were made with unpaired two-tailed Student's t tests. Statistical significance was defined a priori with α= 0.05. Data are presented as mean ± S.E., with n designating the number of cells obtained in each experimental group.
Results
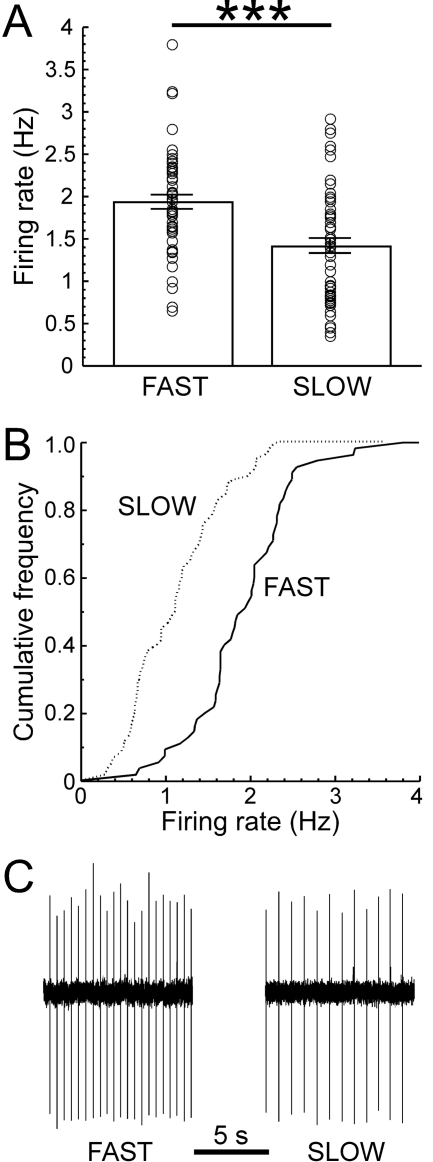
Dopamine Neuron Spontaneous Firing Rate, Ethanol, and IH. Dopamine neurons in the pars compacta of the substantia nigra were identified by their size, anatomical location, and width of their action potentials. Firing of these neurons was recorded using the loose cell-attached patch configuration. In acutely prepared brain slices, glutamate inputs are severed, and dopamine neurons fire almost exclusively in a rhythmic, pacemaker pattern. The spontaneous pacemaker firing rate of dopamine neurons was significantly higher in neurons obtained from FAST mice (1.93 ± 0.08 Hz) than those obtained from SLOW mice (1.41 ± 0.09 Hz, Fig. 1). In addition, a number of neurons from SLOW mice were firing in couplets rather than in a pacemaker pattern, which was not the case in FAST mice. These neurons were not included in the analysis of firing rate.
Fig. 1.
Dopamine neurons from FAST mice exhibit a more rapid spontaneous firing rate. Midbrain dopamine neurons exhibit a tonic, regular firing pattern when observed in vitro in brain slice preparations. Examination of firing rate using the loose patch cell-attached configuration revealed that the dopamine cell basal firing rate is significantly faster in slices from FAST compared with SLOW mice [A and B, t(113) = 4.35, P < 0.0001, n = 55–60 cells from 17–18 mice per group]. Sample recordings in slices taken from a FAST and a SLOW mouse illustrate the difference in pacemaker firing rate (C). The data presented are predominantly from an independent experiment but do include neurons also presented in Figs. 2C and 3A.
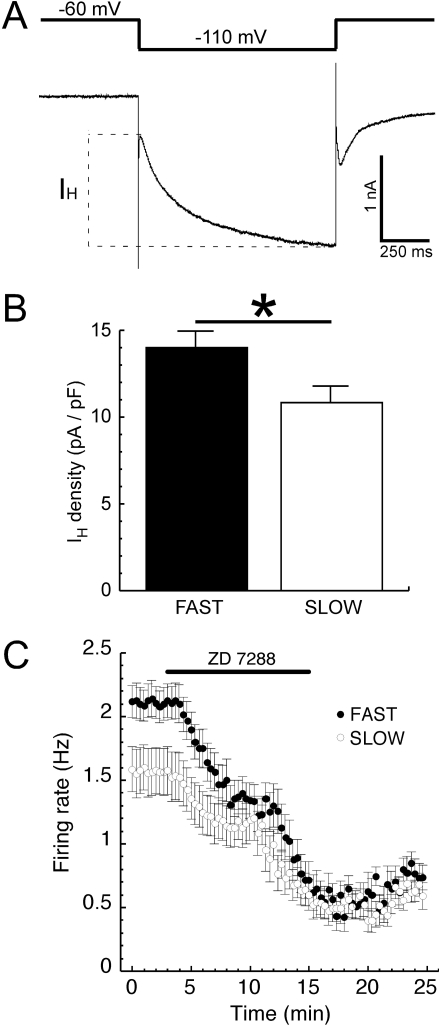
Dopamine neuron firing rate and pattern are critical to the behavioral consequences of terminal dopamine release. One determinant of spontaneous firing rate in dopamine neurons is a hyperpolarization-induced, nonselective cation conductance termed IH (Puopolo et al., 2007), recently identified as a putative target of ethanol action (Okamoto et al., 2006). Results obtained with a 50-mV hyperpolarization for 1 s using whole-cell patch clamp indicated that dopamine neurons from FAST mice had a significantly higher IH current density than those obtained from SLOW mice (Fig. 2). This was a combined effect of slightly larger raw IH values from FAST mice (441 ± 33 versus 400 ± 38 pA, P = 0.41), with significantly smaller membrane capacitance values (31.6 ± 1.1 versus 36.3 ± 1.1 pF, P = 0.004). Dopamine neurons from FAST mice also exhibited significantly higher input resistance (239 ± 24 versus 180 ± 9.8 MΩ, n = 39–43, P = 0.03, data not shown). When the dopamine neuron firing rate was once again directly measured in the loose cell-attached configuration, blockade of IH with 30 μM ZD 7288 eliminated the line difference in the dopamine neuron firing rate (Fig. 2C). This concentration of ZD 7288 has been shown previously to produce complete blockade of IH in dopamine neurons from C57BL/6J mice (Neuhoff et al., 2002).
Fig. 2.
Dopamine neurons from FAST mice exhibit a higher IH current density. The nonselective cation conductance IH was examined in voltage clamp with a hyperpolarization from -60 to -110 mV, measured as the time-dependent component illustrated by the dotted lines (A). Measured as a function of cell capacitance, dopamine neurons from FAST mice exhibited a higher IH density compared with those from SLOW mice [B, t(67) = 2.37, P = 0.02, n = 33–36 cells from six mice per group], which could contribute to their faster firing rate. When IH channels were blocked with ZD 7288 (30 μM), spontaneous firing was more substantially slowed in cells from FAST mice [C, t(22) = 2.53, P = 0.019, n = 12 cells from four to six mice per group].
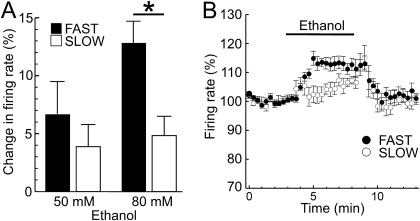
Acutely, ethanol directly accelerates dopamine neuron firing (Brodie et al., 1999) and also increases IH in a cAMP-dependent manner (Okamoto et al., 2006). If IH is an important determinant of initial locomotor response to ethanol, ethanol-induced firing may differ between the FAST and SLOW lines. Consistent with previous reports, bath perfusion of a pharmacologically relevant concentration of ethanol (50–80 mM; Harris et al., 2008) modestly increased spontaneous firing of dopamine neurons (Fig. 3). However, this increase was significantly more pronounced in dopamine neurons from FAST animals. Taken together, the results suggest that IH-induced firing of dopamine neurons is genetically correlated with the selection trait and provide evidence that IH could contribute to the acute locomotor response to ethanol.
Fig. 3.
Ethanol increases the dopamine neuron firing rate to a greater extent in recordings from FAST mice. Ethanol (50–80 mM) produced a subtle increase in pacemaker firing of dopamine neurons (A). This increase was significantly larger in recordings from FAST mice [F(1,38) = 6.84, P = 0.013 for main effect of line, n = 9–11 cells from four mice per group]. The time course of the effect of ethanol (80 mM) is illustrated in B (P = 0.035, Tukey's post hoc test for 80 mM ethanol applications between recordings from FAST versus SLOW mice).
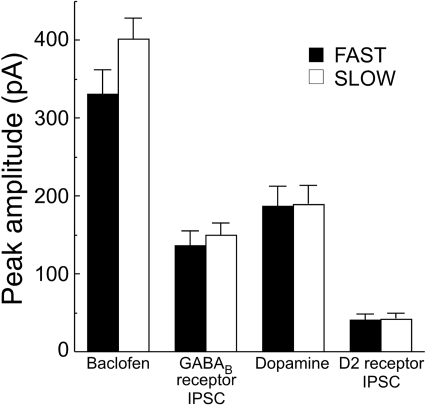
No Differences Observed in GIRK Channel-Mediated Conductances. A second determinant of firing rate that may have been affected by selection of the FAST and SLOW mice is potassium signaling via dopamine D2- and GABAB receptor activation. Neurotransmission through these receptors activates an inhibitory G-protein coupled potassium conductance (GIRK) that has direct effects on dopamine neuron firing (Beckstead et al., 2004). This GIRK conductance has also been identified as a potential direct target of ethanol action in the brain (McDaid et al., 2008). Thus, GIRK-mediated conductances were examined in dopamine neurons from FAST and SLOW mice. Recordings using whole-cell patch clamp suggested that there was no line difference in currents obtained by synaptic activation of GABAB or D2 receptors or in maximal currents elicited by pharmacological activation of these receptors with exogenously applied agonists (Fig. 4). This provides evidence that GIRK channels in dopamine neurons are not involved in the early locomotor response to ethanol and probably do not contribute to the differences observed in cell firing.
Fig. 4.
GIRK channel-mediated inhibitory transmission does not differ between FAST and SLOW mice. Experiments using whole-cell patch clamp of dopamine neurons revealed no difference in GIRK channel-mediated currents in slices obtained from FAST and SLOW mice. GIRK conductance was examined through activation of metabotropic GABAB and dopamine D2 receptors, both through application of exogenous agonists (30 μM baclofen and dopamine applied through iontophoresis) and by evoking IPSCs in the presence of synaptic blockers (n = 9–14 cells from three to six mice per group; baclofen, P = 0.095; GABAB IPSC, P = 0.59; dopamine, P = 0.87; D2 IPSC, P = 0.92).
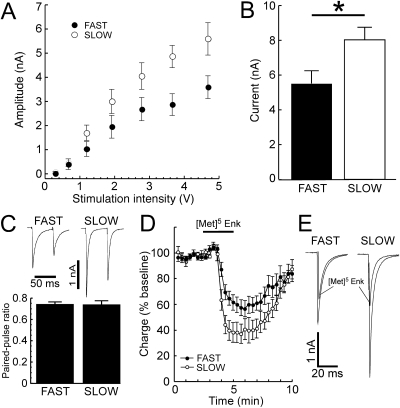
Line Differences in GABAA Receptor-Mediated Transmission. GABAA receptors in the midbrain have been implicated in the neuronal effects of ethanol (Samson et al., 1987; Melis et al., 2002). If dopamine neuron firing is involved in ethanol-induced locomotor activation, then FAST and SLOW mice may exhibit differential GABAA receptor-mediated input onto dopamine neurons. GABAB, glycine, and ionotropic glutamate receptors were pharmacologically antagonized, dopamine neurons were patch clamped, and GABAA receptor-mediated currents were evoked electrically. The amplitude of GABAA inhibitory postsynaptic currents (IPSCs) was significantly larger in dopamine neurons obtained from SLOW mice (Fig. 5). Maximal GABAA receptor-mediated currents activated by iontophoresis of exogenous GABA were also larger in slices from SLOW mice, suggesting a postsynaptic component for this effect. The μ opioid receptor agonist [Met]5 enkephalin is known to presynaptically inhibit GABA release onto dopamine neurons (Johnson and North, 1992). The effect of [Met]5 enkephalin on evoked GABAA IPSCs was significantly larger in recordings obtained from SLOW mice, suggesting that alterations in the presynaptic release of GABA onto dopamine neurons may also be controlled by the same genes responsible for ethanol-induced locomotor activation. However, no difference in paired-pulse ratio was observed between recordings from FAST and SLOW mice (Fig. 5C), indicating that probability of release was not affected by genetic selection.
Fig. 5.
GABAA receptor input to dopamine neurons is larger in recordings from SLOW mice. Endogenous GABA release was evoked with a bipolar-stimulating electrode placed caudal to the dopamine neuron being recorded. A preliminary investigation found that the amplitude of the inhibitory synaptic current was significantly larger in slices obtained from SLOW compared with FAST mice (P = 0.011, n = 30–33 cells from six to seven mice in each group, data not shown). Full stimulus-response curves were subsequently constructed with varying stimulus intensities (A), again demonstrating significantly larger IPSCs in recordings made from SLOW mice [F(1,18) = 36.7, P = 0.038 for main effect of line; F(6,108) = 4.71, P = 0.0003 for line-stimulus interaction, n = 9–11 cells from three mice per group]. GABAA receptor-mediated currents were also larger in recordings from SLOW mice (B) when activated with iontophoresis of exogenous GABA [1 M, t(20) = 2.44, P = 0.024, n = 11 cells from three mice per group]. There was no difference in the paired-pulse ratio of evoked GABAA receptor currents in brain slices taken from FAST versus SLOW mice (C). Care was taken while conducting the experiments for data shown in A to C to use identical slice configuration, stimulator depth and position, and iontophoretic pipette position relative to each neuron being recorded. Next, GABA release was presynaptically inhibited by activation of μ opioid receptors with the agonist [Met]5 enkephalin (10 μM). The inhibition produced by enkephalin was significantly larger in recordings from SLOW mice [D and E, main effect of line, F(1,20) = 4.76; P = 0.041, n = 10–12 cells from three mice per group].
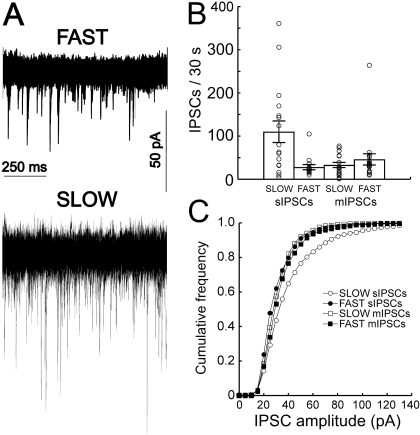
To further investigate these actions, the release of GABA was studied in the absence (sIPSCs) or the presence (mIPSCs) of tetrodotoxin, which eliminates action potentials by blocking voltage-gated sodium channels. There was substantially greater frequency of GABAA receptor-mediated sIPSCs in recordings obtained from SLOW animals (Fig. 6). The sIPSCs also exhibited larger amplitudes in recordings obtained from SLOW mice. These effects were eliminated in the presence of tetrodotoxin, suggesting that line differences in spontaneous GABAergic transmission are action potential dependent. Perfusion of the GABAA receptor antagonist picrotoxin (100 μM) did not affect dopamine neuron firing rate in brain slices taken from SLOW mice (+0.09 ± 1.74%, n = 7 cells from three mice, data not shown). Thus, differences in GABAA receptor-mediated input are probably not responsible for the observed differences in pacemaker firing (e.g., Fig. 1).
Fig. 6.
Spontaneous GABAA IPSCs are larger and more frequent in SLOW mice. In the presence of pharmacological blockers of glycine, N-methyl-d-aspartate, AMPA, and GABAB receptors, spontaneous and miniature GABAA IPSCs were recorded in dopamine neurons of slices from FAST and SLOW mice. Tonic GABAergic input was more pronounced in cells from SLOW mice (A, 30 1-s sweeps overlaid). SLOW mice exhibited a greater frequency of sIPSCs (B), and this effect was blocked in the presence of tetrodotoxin (300 nM, mIPSCs). sIPSCs from SLOW mice also exhibited a larger amplitude (C) (n = 15–20 cells from three to four mice per group, Tukey's post hoc test, P = 0.02 for differences in frequency between SLOW sIPSCs and the other groups, P < 0.0001 for differences in amplitude).
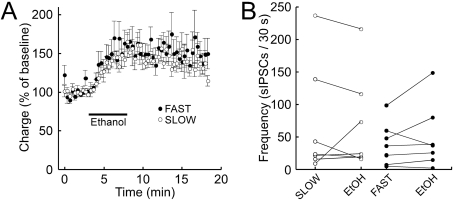
Ethanol acutely enhances GABA transmission in dopamine neurons (Melis et al., 2002). To investigate the contribution of this effect on initial stimulation, GABA transmission was studied in the absence and presence of ethanol (80 mM). Although ethanol did increase the amplitude and the area under the curve of evoked GABAA IPSCs, there was no difference in this effect between dopamine neurons obtained from FAST and SLOW mice (Fig. 7A). Furthermore, ethanol did not have an obvious effect on the frequency of sIPSCs in FAST or SLOW mice (Fig. 7B) or on their amplitude (data not shown).
Fig. 7.
Ethanol effects on GABAergic input to dopamine neurons do not exhibit a line difference. GABAA receptor-mediated IPSCs were evoked in midbrain dopamine neurons. Bath perfusion of ethanol (80 mM) increased the area under the curve (A) of the IPSCs. However, there was no difference between the FAST and SLOW lines [n = 14 cells from six to seven mice per group, main effect of line, F(1,25) = 0.0025, P = 0.96]. Bath perfusion of ethanol had no obvious effects on the frequency (B) or amplitude (data not shown) of sIPSCs recorded from FAST and SLOW mice (n = 7 cells from three mice per group).
Discussion
Alcohol is very commonly abused, and a multitude of targets have been identified as putative mediators of its central effects. This investigation sought not to identify new targets but to use the combination of electrophysiology and a behavioral genetic tool to identify which previously identified targets could be determinants of a specific ethanol-related behavior: locomotor stimulation. Ethanol-induced locomotor stimulation can be easily measured in mice and involves some of the same neuroanatomical pathways shown to influence the rewarding properties of drugs of abuse (Wise and Bozarth, 1987; Di Chiara and Imperato, 1988; Boehm et al., 2002). Moreover, elevated initial sensitivity to the euphoric or stimulatory effects of ethanol is associated with higher levels of alcohol consumption and may be a predictor of the propensity to develop alcoholism in some individuals (King et al., 2002; Gabbay, 2005). The FAST and SLOW mouse lines were selectively bred for differential sensitivity to locomotor activation at an early time point after a single injection of ethanol. Much of the research using these lines has attempted to identify the neurophysiological and neurochemical substrates important for the initial sensitivity difference by looking for pleiotropic effects of the genes involved (i.e., genetic correlation). Measurable differences between the FAST and SLOW lines for alternative (nonselected) traits would suggest that common alleles influence both traits. The present study describes the first electrophysiological analysis of the FAST and SLOW lines and the first attempt to look for differences in the synaptic inputs to specific neurons in these mice. Results using this combination of electrophysiological, behavioral, and genetic tools suggest that dopamine neuron firing rate, IH current density, and GABAA receptor-dependent input could be controlled by some of the same genes responsible for differences in sensitivity to ethanol-induced locomotor activation.
IH-Dependent Firing of Dopamine Neurons. Dopamine neurons in the ventral midbrain were examined in this study because of their dual role in locomotion and ethanol-related reinforcement (Wise and Bozarth, 1987; Samson et al., 1993; Shen et al., 1995; Rodd et al., 2004; Bechtholt and Cunningham, 2005). FAST mice exhibit stronger preference for ethanol than SLOW mice (Risinger et al., 1994), and behavioral studies suggest that dopaminergic mechanisms are important for the expression of ethanol-induced locomotor stimulation in FAST mice (Shen et al., 1995). FAST mice are more sensitive than SLOW mice to the locomotor stimulant effects of drugs that increase extracellular dopamine (Meyer et al., 2009). As measured with in vivo microdialysis, extracellular dopamine levels are increased in response to most drugs of abuse, including ethanol (Di Chiara and Imperato, 1988; Yim et al., 1998), and FAST mice show a greater dopamine response than SLOW mice after both ethanol and cocaine treatment (Meyer et al., 2009). This ethanol-induced increase is blunted in dopamine D2 receptor knockout mice (Job et al., 2006), which also exhibit reduced ethanol-induced conditioned place preference (Cunningham et al., 2000), decreased ethanol preference (Phillips et al., 1998), and decreased operant responding for ethanol (Risinger et al., 2000). However, D2 receptor knockout mice also display heightened ethanol-induced locomotor stimulation (Palmer et al., 2003), opposite to what would be predicted from the dopamine and reward data, suggesting that the relationships among these traits are not completely straight-forward and may be dependent upon genetic background. The preponderance of evidence that dopaminergic mechanisms contribute to the neuropharmacological effects of ethanol suggests that the correlated endophenotypes identified herein are not related to the accidental fixation of selection trait-irrelevant genes. Rather, they suggest the pleiotropic influence of genes on sensitivity to ethanol-induced stimulation and dopamine-mediated mechanisms.
Acutely, ethanol produces a modest but reproducible increase in dopamine neuron firing (Brodie et al., 1999). This effect may be partially dependent on IH channels, which increase dopamine neuron firing and have also been identified in mice (but not rats; see McDaid et al., 2008) as being an important target of ethanol action (Okamoto et al., 2006). The observation that dopamine neuron spontaneous firing rate is higher in FAST than SLOW mice suggests that firing may be an important determinant of the initial stimulation to ethanol. The higher spontaneous firing rate is also consistent with the slightly higher basal locomotion that has been observed sometimes in FAST mice (Holstein et al., 2005). A line difference was also observed in IH current density, offering a plausible mechanism by which firing could be altered. Blocking IH with ZD 7288 eliminated the line difference in firing rate, and acute administration of ethanol increased the firing rate to a greater extent in FAST than SLOW mice. It is impossible to say for certain what effect a subtle increase in firing observed in vitro would have in vivo, where firing patterns are much more complex. However, 2 g/kg ethanol induces locomotion, despite reports that similar concentrations produce merely a ∼10% increase in firing rate in vitro (Okamoto et al., 2006) and a 30% increase in nucleus accumbens dopamine in FAST mice in vivo (Meyer et al., 2009). Taken together with the line difference in behavior, these differential acute effects provide evidence that IH-induced dopamine neuron firing could contribute to ethanol-induced locomotion.
GABA Receptor-Mediated Transmission. Behavioral studies performed on FAST and SLOW mice implicate GABAA receptor signaling in ethanol-induced locomotor activation (Phillips et al., 1992; Palmer et al., 2002). Systemically administered GABAA receptor ligands have differential effects on FAST and SLOW mice (Shen et al., 1998; Palmer et al., 2002), and differential sensitivities to barbiturates and benzodiazepines were among the first correlated traits identified during selection (Phillips et al., 1992). The present study provides additional evidence that GABAA receptor signaling is important to initial locomotor stimulation and identifies dopamine neurons as one potential locus of this effect. Acute administration of ethanol equivocally increased the amplitude of evoked GABAA IPSCs in recordings from both FAST and SLOW mice. The observation that acute ethanol had no differential line effect argues against a line difference in subunit composition, as does the lack of a line difference in IPSC kinetics (data not shown).
SLOW mice did exhibit a larger basal frequency and amplitude of spontaneous GABAA IPSCs, an effect that was eliminated when voltage-sensitive sodium channels were blocked with tetrodotoxin. However, because the frequency of GABAA sIPSCs was unaffected by acute ethanol, it is unlikely that release probability is critical to initial locomotor stimulation. This assertion is bolstered by the absence of a line difference in paired-pulse ratio of evoked GABAA IPSCs. Both the frequency and amplitude of sIPSCs were higher in recordings from SLOW mice, typically interpreted as dual pre- and postsynaptic effects. However, because there was no difference between sIPSCs from FAST mice and mIPSCs from either line, it is more likely that this form of spontaneous transmission is simply absent in FAST mice. Regardless of the anatomical and physiological factors involved, the present results suggest that the genes that control GABAergic input to dopamine neurons could also be determinants of initial sensitivity to ethanol. Given the dual importance of dopamine neurons in both ethanol reinforcement and locomotor activation, individual variation in GABAA transmission probably contributes to the propensity for higher levels of ethanol use.
Previous evidence suggests that GABAB receptors are involved in ethanol-induced locomotor stimulation. When administered systemically or into the posterior region of the VTA, the GABAB receptor agonist baclofen blocks ethanol stimulation in FAST mice (Shen et al., 1998; Boehm et al., 2002), although there is no line difference in GABAB receptor density in either the substantia nigra or VTA (Boehm et al., 2002). No difference in GABAB receptor signaling was observed presently, whether receptors were activated synaptically or through the exogenous application of baclofen. There was also no observable difference in dopamine D2 receptor signaling in these neurons, suggesting that GIRK channels in the substantia nigra are not critical to ethanol-induced activation at early time points. Dopamine D2 receptor-mediated IPSCs are also not affected by acute administration of 80 mM ethanol (our unpublished observation).
In summary, the current results provide evidence that dopamine neurons are critical to the genetically determined difference in sensitivity to the initial locomotor response to ethanol. This is the first evidence that two previously identified targets of ethanol action, the nonselective cation conductance IH and dopamine neuron firing rate (Brodie et al., 1999; Okamoto et al., 2006), could be determinants of the initial locomotor response. These findings also affirm that GABAA receptor-mediated transmission modulates this initial stimulation and suggest that one potential locus of this effect is in the substantia nigra. The results presented herein are correlational and would be bolstered by replication in future studies using the other replicate set of FAST and SLOW lines. However, given the previously identified role of dopamine in reward and locomotion and the multiple distinct effects observed presently on the same neurons, it is reasonable to conclude that dopamine neurons play a part in mediating locomotor activation after ethanol administration. Ethanol-induced locomotor stimulation is a complex trait that involves many neurophysiological substrates. Because genetic differences in initial sensitivity to ethanol may have predictive value for the risk to develop alcoholism, the correlated neurophysiological pathways identified herein could be legitimate candidate targets for alcoholism treatment regimens.
Acknowledgments
We thank Stephanie Gantz and Drs. John T. Williams, Cheryl Reed, Shane Hentges, and Amanda Sharpe for helpful contributions.
This work was supported in part by the Department of Veterans Affairs; the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant AA10760]; and the National Institutes of Health National Institute on Drug Abuse [Grant DA21699].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.146316.
ABBREVIATIONS: VTA, ventral tegmental area; MK-801, dizocilpine maleate; G protein-coupled inwardly rectifying potassium channel; mIPSC, miniature inhibitory postsynaptic current; sIPSC, spontaneous inhibitory postsynaptic current; IPSC, inhibitory postsynaptic current; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; ZD 7288, 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyridinium chloride; CGP 56999a, [3-{[1-R-(3-carboxyphenyl)ethyl]amino}-2-(S)-hydroxy-propyl]-cyclohexyl-methyl-phosphonic acid.
References
- Addicott MA, Marsh-Richard DM, Mathias CW, and Dougherty DM (2007) The biphasic effects of alcohol: comparisons of subjective and objective measures of stimulation, sedation, and physical activity. Alcohol Clin Exp Res 31 1883-1890. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ and Weiner JL (2004) Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci 24 10679-10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtholt AJ and Cunningham CL (2005) Ethanol-induced conditioned place preference is expressed through a ventral tegmental area-dependent mechanism. Behav Neurosci 119 213-223. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, and Williams JT (2004) Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron 42 939-946. [DOI] [PubMed] [Google Scholar]
- Boehm SL 2nd, Piercy MM, Bergstrom HC, and Phillips TJ (2002) Ventral tegmental area region governs GABA(B) receptor modulation of ethanol-stimulated activity in mice. Neuroscience 115 185-200. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, and Appel SB (1999) Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res 23 1848-1852. [PubMed] [Google Scholar]
- Cunningham CL, Howard MA, Gill SJ, Rubinstein M, Low MJ, and Grandy DK (2000) Ethanol-conditioned place preference is reduced in dopamine D2 receptor-deficient mice. Pharmacol Biochem Behav 67 693-699. [DOI] [PubMed] [Google Scholar]
- Di Chiara G and Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85 5274-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, and Gonzales RA (2005) Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem 93 1469-1481. [DOI] [PubMed] [Google Scholar]
- Gabbay FH (2005) Family history of alcoholism and response to amphetamine: sex differences in the effect of risk. Alcohol Clin Exp Res 29 773-780. [DOI] [PubMed] [Google Scholar]
- Harris RA, Trudell JR, and Mihic SJ (2008) Ethanol's molecular targets. Sci Signal 1: re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein SE, Dobbs L, and Phillips TJ (2009) Attenuation of the stimulant response to ethanol is associated with enhanced ataxia for a GABAA, but not a GABAB, receptor agonist. Alcohol Clin Exp Res 33 108-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein SE, Pastor R, Meyer PJ, Phillips TJ (2005) Naloxone does not attenuate the locomotor effects of ethanol in FAST, SLOW, or two heterogeneous stocks of mice. Psychopharmacology (Berl) 182 277-289. [DOI] [PubMed] [Google Scholar]
- Job MO, Ramachandra V, Anders S, Low MJ, and Gonzales RA (2006) Reduced basal and ethanol stimulation of striatal extracellular dopamine concentrations in dopamine D2 receptor knockout mice. Synapse 60 158-164. [DOI] [PubMed] [Google Scholar]
- Johnson SW and North RA (1992) Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol 450 455-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, and Schuster A (2002) Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res 26 827-835. [PubMed] [Google Scholar]
- McDaid J, McElvain MA, and Brodie MS (2008) Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: involvement of barium-sensitive potassium currents. J Neurophysiol 100 1202-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, and Bonci A (2002) Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci 22 2074-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Calabresi P, Stefani A, and Bernardi G (1995) Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur J Neurosci 7 462-469. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Meshul CK, and Phillips TJ (2009) Ethanol- and cocaine-induced locomotion are genetically related to increases in accumbal dopamine. Genes Brain Behav, doi: 10.1111/j.1601-183x.2009.00481.x. [DOI] [PMC free article] [PubMed]
- Neuhoff H, Neu A, Liss B, and Roeper J (2002) I (h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci 22 1290-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, and Morikawa H (2006) Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol 95 619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Low MJ, Grandy DK, and Phillips TJ (2003) Effects of a Drd2 deletion mutation on ethanol-induced locomotor stimulation and sensitization suggest a role for epistasis. Behav Genet 33 311-324. [DOI] [PubMed] [Google Scholar]
- Palmer AA, McKinnon CS, Bergstrom HC, and Phillips TJ (2002) Locomotor activity responses to ethanol, other alcohols, and GABA-A acting compounds in forward- and reverse-selected FAST and SLOW mouse lines. Behav Neurosci 116 958-967. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly MA, Rubinstein M, Grandy DK, and Low MJ (1998) Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci 1: 610-615. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Burkhart-Kasch S, Gwiazdon CC, and Crabbe JC (1992) Acute sensitivity of FAST and SLOW mice to the effects of abused drugs on locomotor activity. J Pharmacol Exp Ther 261 525-533. [PubMed] [Google Scholar]
- Phillips TJ, Shen EH, McKinnon CS, Burkhart-Kasch S, Lessov CN, and Palmer AA (2002) Forward, relaxed and reverse selection for reduced and enhanced sensitivity to ethanol's locomotor stimulant effects in mice. Alcohol Clin Exp Res 26 593-602. [PubMed] [Google Scholar]
- Puopolo M, Raviola E, and Bean BP (2007) Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J Neurosci 27 645-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quickfall J and el-Guebaly N (2006) Genetics and alcoholism: how close are we to potential clinical applications? Can J Psychiatry 51 461-467. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Freeman PA, Rubinstein M, Low MJ, and Grandy DK (2000) Lack of operant ethanol self-administration in dopamine D2 receptor knockout mice. Psychopharmacology (Berl) 152 343-350. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Malott DH, Prather LK, Niehus DR, and Cunningham CL (1994) Motivational properties of ethanol in mice selectively bred for ethanol-induced locomotor differences. Psychopharmacology (Berl) 116 207-216. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, and McBride WJ (2004) Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci 24 1050-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Hodge CW, Tolliver GA, and Haraguchi M (1993) Effect of dopamine agonists and antagonists on ethanol-reinforced behavior: the involvement of the nucleus accumbens. Brain Res Bull 30 133-141. [DOI] [PubMed] [Google Scholar]
- Samson HH, Tolliver GA, Pfeffer AO, Sadeghi KG, and Mills FG (1987) Oral ethanol reinforcement in the rat: effect of the partial inverse benzodiazepine agonist RO15–4513. Pharmacol Biochem Behav 27 517-519. [DOI] [PubMed] [Google Scholar]
- Schuckit MA and Smith TL (2000) The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J Stud Alcohol 61 827-835. [DOI] [PubMed] [Google Scholar]
- Shen EH, Crabbe JC, and Phillips TJ (1995) Dopamine antagonist effects on locomotor activity in naive and ethanol-treated FAST and SLOW selected lines of mice. Psychopharmacology (Berl) 118 28-36. [DOI] [PubMed] [Google Scholar]
- Shen EH, Dorow J, Harland R, Burkhart-Kasch S, and Phillips TJ (1998) Seizure sensitivity and GABAergic modulation of ethanol sensitivity in selectively bred FAST and SLOW mouse lines. J Pharmacol Exp Ther 287 606-615. [PubMed] [Google Scholar]
- Tepper JM, Paladini CA, and Celada P (1998) GABAergic control of the firing pattern of substantia nigra dopaminergic neurons. Adv Pharmacol 42 694-699. [DOI] [PubMed] [Google Scholar]
- Wise RA and Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94 469-492. [PubMed] [Google Scholar]
- Yim HJ, Schallert T, Randall PK, and Gonzales RA (1998) Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res 22 367-374. [PubMed] [Google Scholar]