Summary
Brain principal glutamatergic neurons synthesize 3α-hydroxy-5α-pregnan-20-one (Allo), a neurosteroid that potently, positively, and allosterically modulates GABA action at GABAA receptors. Cerebrospinal fluid (CSF) Allo levels are decreased in patients with posttraumatic stress disorder (PTSD) and major depression. This decrease is corrected by fluoxetine in doses that improve depressive symptoms. Depression-like behavioral dysfunctions (aggression, fear, and anxiety) associated with a decrease of corticolimbic Allo content can be induced in mice by social isolation. In socially isolated mice, fluoxetine and analogs stereospecifically normalize the decrease of Allo biosynthesis and improve behavioral dysfunctions by a mechanism independent from 5-HT reuptake inhibition. Thus, fluoxetine and related congeners facilitate GABAA receptor neurotransmission and effectively ameliorate emotional and anxiety disorders and depression by acting as selective brain steroidogenic stimulants (SBSSs).
Keywords: allopregnanolone, aggressive behavior, contextual fear conditioning, depression, PTSD, GABAA receptors, selective brain steroidogenic stimulants (SBSSs)
Introduction
Emotional disorders such as impulsivity, irritability, aggression, and anxiety spectrum disorders, including generalized anxiety, panic, and posttraumatic stress disorder (PTSD) are frequently associated with major depression [1, 2].
Although the brain structures responsible for these complex psycho-pathologies are not yet precisely defined, there is growing evidence that these clinical manifestations are associated with functional alterations of monoamine (5-HT, NE, DA) neurotransmitters expressed by specific cortico-limbic circuit neurons [2-5]. For example, the frontal cortex and hippocampus mediate cognitive deficits, feelings of worthlessness, hopelessness, guilt, and suicidality while the corpus striatum, nucleus accumbens, and amygdala are important in processing aversive or reward responses to emotional stimuli, thereby mediating the onset of anhedonia, anxiety, and the reduced behavioral motivation frequently shown in patients with major depression [2].
Reduced cortical GABA levels in depressed patients, determined by positron magnetic resonance [6], and the beneficial effects following the administration of benzodiazepines (BZs) that positively and allosterically modulate GABA action at GABAA receptors [7], suggest that in addition to monoamines, a perturbation of the GABAergic signaling could also play a fundamental role in the pathogenesis of the emotional and anxiety disorders associated with depression.
Here, we will review results from human and rodent studies suggesting that emotional disorders, anxiety disorders, and depression may reflect a cortico-limbic perturbation of GABAergic neurotransmission that may be the result of a reduction of the GABAA receptor-active neurosteroid, 3α-hydroxy-5α-pregnan-20-one (allopregnanolone, abbreviated as Allo).
Neurons expressing neurosteroid biosynthesis
Allo is a potent (nM affinity) positive endogenous allosteric modulator of GABA action that acts at the majority of synaptic and extrasynaptic GABAA receptor subtypes [8**, 9**-13]. Hence, Allo fails to exhibit the receptor subunit selectivity typical of BZs. Importantly, Allo is particularly active (nmol concentrations) at extrasynaptic GABAA receptors expressing subunits α4βxδ or α5βxδ where classical BZs fail to act or act with low affinity [12].
Allo is the most abundant brain neurosteroid acting at GABAA receptors [14**, 15**]. In both human and rodent brains, Allo is synthesized from progesterone by the sequential action of two reducing enzymes: 5α-reductase (5α-R) type I, which transforms progesterone into 5α-DHP, and 3α-hydroxysteroid dehydrogenase (3α-HSD), which transforms 5α-DHP into Allo and vice-versa [15**-17**] (Fig. 1). These two enzymes co-localize and are highly expressed in cortical, hippocampal, and amygdala glutamatergic pyramidal neurons and in olfactory bulb glutamatergic mitral neurons. However, these enzymes are not expressed in glial cells or GABAergic interneurons [17**].
Fig. 1.
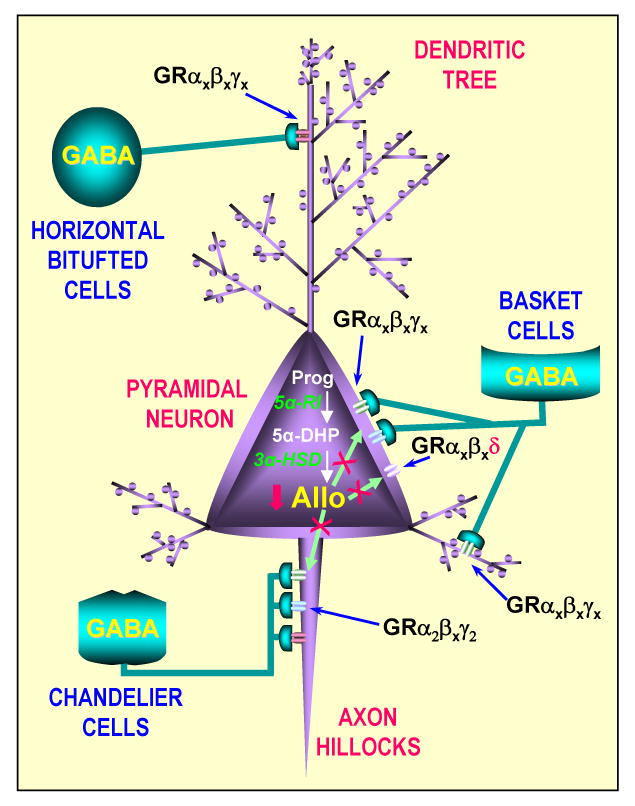
Simplified cortical circuitry that depicts the action of Allo on GABAA receptors (GR) expressed on the cell body, dendrites, or axon hillocks of a pyramidal neuron.
Allo is synthesized in pyramidal neurons by the action of 5α-R-type I and 3α-HSD.
Allo diffuses (indicated by  ) to cell membranes and facilitates the action of GABA at synaptic and extrasynaptic GABAA receptors.
) to cell membranes and facilitates the action of GABA at synaptic and extrasynaptic GABAA receptors.
 , denotes Allo biosynthesis downregulation in pyramidal neurons of socially isolated mice.
, denotes Allo biosynthesis downregulation in pyramidal neurons of socially isolated mice.
 , denotes a decrease of Allo levels reaching synaptic or extrasynaptic GABAA receptors located on pyramidal neurons in socially isolated mice.
, denotes a decrease of Allo levels reaching synaptic or extrasynaptic GABAA receptors located on pyramidal neurons in socially isolated mice.
GRαxβx , extrasynaptic GABAA receptors that express δ subunits.
, extrasynaptic GABAA receptors that express δ subunits.
Taken together, these considerations suggest that Allo synthesized by glutamatergic neurons of the olfactory bulb, frontal cortex, hippocampus, and amygdala modulates GABA action at synaptic or extrasynaptic GABAA receptors. These receptors are located on dendritic shafts or cell bodies of the above-mentioned glutamatergic neurons by an autocrine mechanism or more likely by this neurosteroid reaching GABAA receptor intracellular sites through lateral membrane diffusion (Fig. 1) [17**, 18].
Allo is decreased in cerebrospinal fluid (CSF) of depressed and PTSD patients
Based on the observation made in our laboratory, that fluoxetine and paroxetine increase the content of Allo in neurons of various rat brain areas (olfactory bulb > frontal cortex > hippocampus > striatum > cerebellum) [19**], we hypothesized that by normalizing brain Allo levels in depressed and in PTSD patients, administration of selective serotonin reuptake inhibitors (SSRIs) may alleviate both the anxiety and dysphoria symptomatology of these psychiatric disorders [20].
As proof of concept, we measured Allo levels in the cerebrospinal fluid (CSF) of patients with psychiatric disorders [21**, 22*], based on the assumption that the amount of Allo in the CSF is a reliable index of brain Allo levels. We found that the concentration of Allo in the CSF of non-psychiatric subjects (∼40 fmol/ml) was approximately 2-fold higher than that measured in the CSF of depressed patients [21**].
To support the hypothesis that this decrease in the CSF Allo levels of depressed patients reflects a decrease of brain Allo content, we compared the expression of 5α-R type I mRNA in samples (N=12) of the prefrontal-cortical area (BA9) from depressed patients that were age- and sex-matched with non-psychiatric subjects. In depressed patients, the level of 5α-R type I mRNA was dramatically decreased (about 50%) compared to that of non-psychiatric subjects. However, 5α-R type I mRNA expression failed to change in the cerebellum of the same patients (Agis-Balboa, personal communication).
In a recent human study, we also reported that in PTSD patients, Allo level downregulation in the CSF was in keeping with an increase of PTSD re-experiencing and comorbid depressive symptoms [22*]. Also, Allo levels were decreased in all PTSD patients but were lowest in those patients with PTSD and comorbid depression [22*].
In 15 depressed patients affected by major depression, treatment with fluoxetine and fluvoxamine (8-10 weeks, doses of 0.8-4.8 for fluoxetine and 1.7-9.1 μmol/kg for fluvoxamine) normalized the CSF Allo content [21**]. Moreover, a statistically significant correlation existed between symptomatic improvement (Hamilton Rating Scale for Depression Score) and the increase of CSF Allo elicited by fluoxetine or fluvoxamine. Similar results were reported when Allo or 5α-tetrahydrodeoxycorticosterone levels were measured in the plasma of depressed patients treated with SSRIs [23].
Taken together, these data suggest that among the molecular mechanisms underlying major depression and PTSD symptomatology must be included a deficit of GABAergic neurotransmission likely caused by a downregulation of brain Allo biosynthesis.
Downregulation of neurosteroid biosynthesis in cortico-limbic circuits mediates aggression, anxiety, and fear induced in mice by social isolation
To examine whether a downregulation of GABA action at GABAA receptors is related to the emotional or anxiety spectrum disorders observed in depressed or PTSD patients, we and others [24, 25*, 26*, 27, 28*, 29*-32] studied rodents (mice and rats) exposed to a protracted period (3-4 weeks) of social isolation stress. It is known that in mice this condition causes i) aggression [24, 27, 28*, 30, reviewed in 32], ii) an enhanced contextual fear response to stressful stimuli [26*, reviewed in 32], and iii) a decreased response to barbiturates and BZs and other GABA-mimetic drugs [24, 30, 33**]. In these socially isolated mice, the behavioral abnormalities were associated with a marked decrease of brain Allo content caused by a decrease of 5α-R type I mRNA and protein expression [25*, 26*, 27, 28*, 29*-32]. In socially isolated mice, the intensity of aggression and the enhancement of fear are inversely related to the extent of Allo content downregulation measured in the olfactory bulb, frontal cortex, hippocampus, and amygdala [26*, 28*]. Moreover, when Allo was administered subcutaneously to socially isolated mice, it attenuated their aggression and fear responses to stressful stimuli (i.e. mild electric foot shock) in a dose-dependent manner [26*, 28*, 32]. These doses of Allo failed to alter gross behavioral patterns or trends of locomotor activity in group-housed mice (used as control).
To provide further evidence that the decrease of brain Allo content in socially isolated mice is responsible for the altered behavioral responses, we induced decreased brain Allo content by administering the potent 5α-R type I inhibitor, 17β-(N,N-diisopropylcarbamoyl)-androst-3,5diene-3-carboxylic acid (SKF 105,111) to group-housed mice [26*, 32, 33**]. The subcutaneous administration of SKF 105,111 (1-80 umol/kg) induced a fast occurring (30-60 min delay) and marked (∼80%) reduction of brain Allo content that lasted for at least 6 hrs [11, 16, 26*, 32].
The SKF 105,111-induced decrease of brain Allo content by an extent comparable to the Allo content decrease induced by social isolation can be related to the shorter duration of pentobarbital-induced sedation, increased aggressiveness, and enhanced expression of contextual fear after exposure to a conditioning stimulus [25*, 26*, 27, 28*, 29*-32, 33**].
Measurements of Allo and 5α-R type I mRNA and protein levels show that the neurosteroid biosynthesis reduction in socially isolated mice does not occur uniformly in every brain areas but is greater in cortico-limbic circuits, which are known to regulate the levels of emotions and anxiety, and particularly in neurons that express the highest levels of 5α-R type I [26*, 27, 28*, 29*, 30, 32].
It has been suggested that the neuronal networks that underlie the expression of aggression and fear conditioning responses include excitatory glutamatergic projections from the medial frontal (prelimbic and infralimbic) cortex (mFC) and hippocampus (CA1) (Fig. 2) to the basolateral nucleus of the amygdala [34-38]. In the basolateral amygdala (BLA), cortical and hippocampal projections establish excitatory synapses with GABAergic interneurons and also pyramidal-like glutamatergic output neurons. These project either directly to the neurons of the central amygdala (CeA) or to the intercalated (ITC) inhibitory GABAergic neurons located on the capsule surrounding the central amygdaloid nucleus. The CeA spiny output neurons (presumably GABAergic) project to the brainstem and hypothalamus, thereby modulating inter alia the intensity of emotional responses to environmental stimuli (Fig. 2). In socially isolated mice, the expression of 5α-R type I and Allo is downregulated selectively in layer V/VI glutamatergic pyramidal neurons of the mFC and in glutamatergic pyramidal-like neurons of the BLA (Fig. 2) [29*]. Hence, a selective decrease of Allo in cortical layer V/VI pyramidal neurons or BLA glutamatergic output neurons may reduce the inhibitory potency of GABA at GABAA receptors located on dendrites or cell bodies of these principal neurons. In functional terms, this may represent the molecular mechanisms that underlie the decreased plasticity of the cortico-limbic pathways converging on the ITC and CeA spiny neurons in socially isolated mice, ultimately resulting in an altered output from the CeA neurons projecting into the hypothalamic and brainstem nuclei.
Fig. 2.
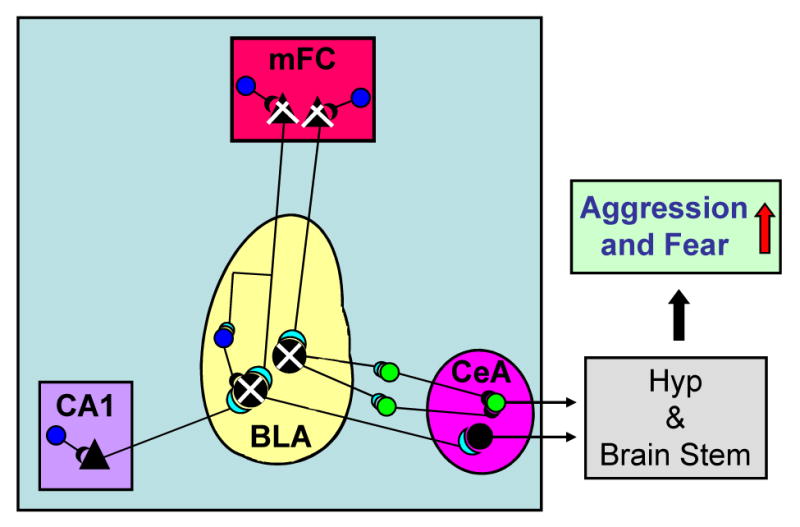
Schematic representation of the main intrinsic connections of the basolateral (BLA) and central (CeA) nuclei of the amygdala and extrinsic projections from the medial frontal cortex (mFC) (layer V/VI) and hippocampus (CA1) pyramidal neurons to the BLA.
In socially isolated mice, the decrease in Allo biosynthesis in layer V/VI pyramidal glutamatergic neurons of the mFC and in pyramidal-like glutamatergic neurons of the BLA (indicated by  ) downregulates the inhibitory potency of GABAergic interneurons (indicated by
) downregulates the inhibitory potency of GABAergic interneurons (indicated by  ) impinging on these pyramidal neurons. This Allo content decrease results in an increased excitatory output from BLA to the intercalated (ITC) neurons or to neurons of the CeA nucleus, which project to the hypothalamus (Hyp) and brain stem, enhancing fear and aggression (indicated by
) impinging on these pyramidal neurons. This Allo content decrease results in an increased excitatory output from BLA to the intercalated (ITC) neurons or to neurons of the CeA nucleus, which project to the hypothalamus (Hyp) and brain stem, enhancing fear and aggression (indicated by  ).
).
 Pyramidal-like glutamatergic neurons expressing Allo
Pyramidal-like glutamatergic neurons expressing Allo
 Pyramidal glutamatergic neurons expressing Allo
Pyramidal glutamatergic neurons expressing Allo
 Inhibitory GABAergic interneurons
Inhibitory GABAergic interneurons
 Intercalated (ITC) GABAergic neuron
Intercalated (ITC) GABAergic neuron
 Decreased Allo biosynthesis in mFC pyramidal and BLA pyramidal-like glutamatergic neurons
Decreased Allo biosynthesis in mFC pyramidal and BLA pyramidal-like glutamatergic neurons
Modified from Sah and Westbrook [45].
Therefore, by altering the function of cortico-amygdaloid circuits, the reduction of 5α-R type I expression and consequently that of the Allo levels in glutamatergic neurons of FC and BLA may be involved in the increased aggressive behavior and in the enhancement of the contextual fear responses and anxiety-like behaviors observed in socially isolated mice.
Effects of fluoxetine and norfluoxetine on neurosteroid biosynthesis are unrelated to their efficacy as 5-HT reuptake inhibitors
To address the question of whether the mechanisms whereby SSRIs increase brain and CSF Allo levels and improve clinical symptoms are dependent on changes in 5-HT neurotransmission, we tested whether fluoxetine, norfluoxetine, and other specific SSRIs stereoselectively upregulate brain neurosteroid content, reduce aggression, or prevent the enhancement of fear expression at doses capable of inhibiting 5-HT reuptake in socially isolated mice.
In these studies, we showed that intraperitoneal doses of fluoxetine (1.4-2.9 μmol/kg) correct the brain Allo level decrease and reduce the behavioral deficits associated with prolonged social isolation. Further, fluoxetine continues to do so in socially isolated mice in which brain 5-HT synthesis was inhibited by pretreatment with p-chlorophenyalanine (1.2 mmol/kg i.p. at 72, 48, and 24 hr before measurement) that reduces brain 5-HT content by 80% [25*].
Since fluoxetine is an S and R racemic mixture that is metabolized into S or R norfluoxetine [40], we used S- or R-fluoxetine and S- or R-norfluoxetine in a dose-response study to evaluate their stereospecificity in modifying brain Allo content and related behavioral responses. We also tested whether neurosteroidogenic doses differ from the doses of these compounds that inhibit 5-HT reuptake.
We found [27, 28*, 41**] that fluoxetine and norfluoxetine in subμmolar doses and in a stereospecific manner (S-isomers > R-isomers) reverse the decrease of brain Allo levels and at the same doses, correct the behavioral deficits expressed by socially isolated mice. Importantly, these actions of S-fluoxetine and S-norfluoxetine cannot be related to their intrinsic SSRI activity because to normalize pentobarbital-induced sedation, to reduce aggression, and to upregulate brain Allo levels in socially isolated male mice, the EC50s are at least 10-to-50 times lower than the EC50 required to inhibit 5-HT reuptake (Fig. 3). More importantly, the 5-HT reuptake inhibition is not stereospecific (Table 1).
Fig. 3.
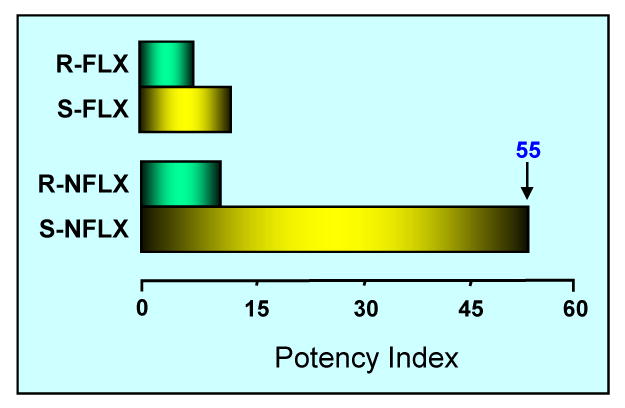
The stereospecific potency of S-norfluoxetine required to stimulate Allo biosynthesis is 55 times higher than 5-HT reuptake inhibition. Data on the x-axis (potency index) represent the ratios between the EC50 doses that inhibit 5-HT reuptake and the EC50 doses that stimulate Allo biosynthesis. Each value is the mean of four to six socially isolated mice (data from Table 1).
Table 1. Fluoxetine and norfluoxetine stereoisomers induce normalization of pentobarbital (PTB) right reflex loss (RRL), reduce the duration of attacks against an intruder (Aggression), activate neurosteroidogenesis (Allo) at doses that fail to affect 5-HT reuptake.
| Mice | PTB-RRL (EC50, μmol/kg) |
Aggression (EC50, μmol/kg) |
Allo (EC50, μmol/kg) |
5-HT Reuptake (EC50, μmol/kg) |
|---|---|---|---|---|
| S-Fluoxetine | 0.70±0.2 * | 0.71±0.03 * | 0.80±0.07 * | 10.5±2.4 |
| R-Fluoxetine | >1.80 | 1.30±0.02 | >1.80 | 13.7±3.2 |
| S-Norfluoxetine | 0.25±0.1 ** | 0.20±0.08 ** | 0.15±0.03 ** | 8.3±3.1 |
| R-Norfluoxetine | 1.70±0.3 | 1.53±0.20 | >0.9 | 10.1±3.8 |
Drugs were administered 30 min before behavioral tests and [14C]5-HT reuptake measurement. Data represent the mean ± SEM of four to six mice socially isolated for 4 weeks before testing.
P<0.01 when S-fluoxetine is compared with R-fluoxetine.
P<0.001 when S-norfluoxetine is compared with R-norfluoxetine and S-fluoxetine. The EC50 were calculated from dose-response curves analyzed by the “quantal dose-response: probits test” [46] equipped with a statistical package. Statistical comparisons among the different IC50 values were performed by using the COHORT package. For details see Pinna et al., [27, 28, 41]
For the first time, these studies provide evidence suggesting that fluoxetine upregulates endogenous brain stores of Allo and regulates GABAergic tone and related behaviors by a mechanism that may be independent from modifications of 5-HT reuptake mechanisms.
Selective and potent action of S-fluoxetine, S-norfluoxetine, and other SSRIs on neurosteroid biosynthesis
The mechanisms by which fluoxetine and norfluoxetine [27, 28*, 41**] and other SSRIs (i.e., paroxetine, fluvoxamine, sertraline) [19**-21**, 42, 43] cause a rapid (minutes) increase of brain Allo levels in rodents remain unclear.
A possible hypothesis is that fluoxetine or norfluoxetine correct the brain Allo level decrease in socially isolated mice via a direct action on 5α-R type I. However, studies in vitro using recombinant rat 5α-R type I or 3α-HSD showed that fluoxetine, paroxetine, or sertraline in concentrations as high as 50 μM failed to activate 5α-R type I. In contrast, these drugs directly activated 3α-HSD, decreasing the Km of this enzyme for 5α-DHP by 100-fold and thereby favoring the reduction of 5α-DHP into Allo [44].
When the results of these in vitro studies [44] are compared to those of our in vivo studies [27, 28*, 41**], it becomes evident that in mice the doses of fluoxetine and norfluoxetine that cause a rapid increase in brain Allo levels do not exceed brain concentrations in the low nanomolar range, whereas the fluoxetine concentrations that directly activate 3α-HSD in vitro are in the μmolar range. Moreover, the high potency and stereospecificity of fluoxetine and norfluoxetine in decreasing aggressive behavior and normalizing brain Allo content during social isolation (see Table 1, and Fig. 3) support the notion that these compounds facilitate the action of 5α-R type I or 3α-HSD by an unidentified indirect mechanism, which is very likely perturbed by protracted social isolation.
Thus, these drugs, which originally were termed “SSRI” antidepressants, may be beneficial in psychiatric disorders because in doses that are inactive on 5-HT reuptake mechanisms, they increase the bioavailability of neuroactive GABAergic steroids [27]. Based on these considerations, we now propose that the term “SSRIs” should be changed to the more appropriate term “selective brain steroidogenic stimulants” (SBSSs), which more accurately defines the pharmacological mechanisms expressed by fluoxetine and its congeners [27].
Conclusions
The pharmacology of the S stereoisomers of fluoxetine and norfluoxetine appears to be prototypic for molecules that possess specific neurosteroidogenic activity. The doses of S-fluoxetine and S-norfluoxetine required to normalize brain Allo content downregulation, pentobarbital action, aggressiveness, and anxiety in socially isolated mice are between 10- to 50-fold lower than those required to induce SSRI activity. However, the precise mechanisms of action by which S-fluoxetine and S-norfluoxetine increase neurosteroids remain to be investigated.
Derivatives of S-fluoxetine and S-norfluoxetine, acting with high potency and specificity on brain neurosteroid expression at doses devoid of significant action on brain 5-HT reuptake mechanisms, may represent a new class of pharmacological tools important for the management of anxiety, related mood disorders, dysphoria, fear, and impulsive aggression.
Based on these data, new drugs devoid of SSRI activity but that are potent neurosteroidogenic agents should be developed for the treatment of psychiatric disorders that result from the downregulation of neurosteroid expression, including major depression, and in the prevention of PTSD.
Abbreviations
- 3α-HSD
3α-hydroxysteroid dehydrogenase
- 5α-RI
5α-reductase type I
- Allo
allopregnanolone
- BLA
baso-lateral nuclei of the amygdala
- mFC
medial prefrontal cortex
- SBSSs
selective brain steroidogenic stimulants
- CSF
cerebrospinal fluid
- PTSD
posttraumatic stress disorder
- SSRI
selective serotonin reuptake inhibitors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diagnostic Statistical Manual of Mental Disorders. American Psychiatric Press; Washington DC: 2000. [Google Scholar]
- 2.Berton O, Nestler IJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 3.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 4.Wong ML, Licinio J. From monoamines to genomic targets: a paradigm shift for drug discovery in depression. Nat Rev Drug Discov. 2004;3:136–151. doi: 10.1038/nrd1303. [DOI] [PubMed] [Google Scholar]
- 5.Morilak D, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- 6.Kugaya A, Sanacora G, Verhoeff NP, Fujita M, Mason GF, Seneca NM, Bozkurt A, Khan SA, Anand A, Degen K, et al. Cerebral benzodiazepine receptors in depressed patients measured with [123I]iomazenil SPECT. Biol Psychiatry. 2003;54:792–799. doi: 10.1016/s0006-3223(02)01788-2. [DOI] [PubMed] [Google Scholar]
- 7.Costa E, Guidotti A. Benzodiazepines on trial: a research strategy for their rehabilitation. TIPS. 1996;17:192–200. doi: 10.1016/0165-6147(96)10015-8. [DOI] [PubMed] [Google Scholar]
- 8.Bellelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]; ** A review of how pregnane steroids positively and allosterically potentiate the action of GABA at several GABAA receptor subtypes, mediate ‘fast’ synaptic inhibition in the mammalian brain, and consequently have anxiolytic, analgesic, anticonvulsant, sedative, hypnotic and anesthetic properties.
- 9.Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]; ** Demonstrated that 3α, 5α-pregnane neurosteroids potentiate GABA-activated Cl- currents recorded from a human cell line transfected with various human GABAA receptor subunits. These steroids are active at nanomolar concentrations in potentiating GABA-activated Cl- currents.
- 10.Puia G, Ducic I, Vicini S, Costa E. Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Receptor Channels. 1993;1:135–142. [PubMed] [Google Scholar]
- 11.Puia J, Mienville JM, Matsumoto K, Costa E, Guidotti A. On the putative physiological role of allopregnanolone on GABAA receptor function. Neuropharmacology. 2003;44:49–55. doi: 10.1016/s0028-3908(02)00341-6. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D. Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem Int. 2008;52:588–595. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheney DL, Uzunov D, Costa E, Guidotti A. Gas chromatographic-mass fragmentographic quantitation of 3 alpha-hydroxy-5 alpha-pregnan-20-one (allopregnanolone) and its precursors in blood and brain of adrenalectomized and castrated rats. J Neurosci. 1995;15:4641–4650. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Measured subpicomolar concentrations of allopregnanolone in brain by gas chromatography-mass spectrometry and suggested that the brain is a steroidogenic organ that produces allopregnanolone in physiologically relevant concentrations.
- 15.Baulieu EE. Steroid hormones in the brain: several mechanisms. In: Fuxe K, Gustafson JA, Wettenberg L, editors. Steroid hormone regulation of the brain. Pergamon; Elmsford, NY: 1981. pp. 3–14. [Google Scholar]; ** Reported for the first time that the biosynthesis of neurosteroids in the rat brain is independent of the supply by peripheral endocrine glands. Several biological functions have been proposed for neurosteroids.
- 16.Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Watanabe H, Costa E, Guidotti A. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agis-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of neurons that express enzymes mediating brain neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Demonstrated that 5α-RI and 3α-HSD colocalize in cortical, hippocampal, olfactory bulb, amygdala, and thalamus glutamatergic principal neurons but not in glial cells or in GABAergic interneurons, suggesting that allopregnanolone acts at GABAA receptors located on dendritic shafts and spines or somata of glutamatergic neurons by reaching GABAA receptor intracellular sites.
- 18.Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covery DF, Mennerick S. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci USA. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Fluoxetine dose-dependently increased the content of allopregnanolone in several brain regions of the rat suggesting that a fluoxetine stimulation of brain allopregnanolone biosynthesis might be operative in the anxiolytic and antidysphoric actions of this drug.
- 20.Guidotti A, Costa E. Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3 alpha, 5 alpha-tetrahydroprogesterone (allopregnanolone) availability? Biol Psychiatry. 1998;44:865–873. doi: 10.1016/s0006-3223(98)00070-5. [DOI] [PubMed] [Google Scholar]
- 21.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** CSF allopregnanolone content was lower in patients with major unipolar depression. The normalization of CSF allopregnanolone content in SSRI-treated depressed patients appears to be sufficient to mediate the anxiolytic and antidysphoric actions of SSRIs via its positive allosteric modulation of the action of GABA at GABAA receptors.
- 22.Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]; * This study suggests that a dysfunction of GABAergic neurotransmission in PTSD may be induced by lower allopregnanolone brain levels.
- 23.Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trend Neurosci. 1999;9:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- 24.Valzelli L. In: Aggressive Behavior, Aggressive behavior induced by isolation. Garattini S, Sigg SB, editors. Excerpta Medica Foundation; Amsterdam: 1969. pp. 70–76. [Google Scholar]
- 25.Matsumoto K, Uzunova V, Pinna G, Taki K, Uzunov DP, Watanabe H, Mienville JM, Guidotti A, Costa E. Permissive role of brain allopregnanolone content in the regulation of pentobarbital-induced righting reflex loss. Neuropharmacology. 1999;38:955–963. doi: 10.1016/s0028-3908(99)00018-0. [DOI] [PubMed] [Google Scholar]; * Established an association between the decrease of cortical allopregnanolone content and the shortening of pentobarbital sedation. Fluoxetine normalization of these parameters suggests that brain allopregnanolone plays a physiological permissive role in the modulation of GABA-gated Cl- channel function.
- 26.Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc Natl Acad Sci USA. 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Protracted social isolation of mice combined with contextual fear conditioning exposure may be a suitable model to study the neurochemical correlates of emotion and anxiety spectrum disorders relating to PTSD. Selective activation of neurosteroidogenesis may be a useful intervention in PTSD therapy.
- 27.Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses inactive on 5-HT reuptake. Psychopharmacology. 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]; * This review provides evidence on new mechanisms of pharmacological action of SSRI and concludes that the term “SSRI” may be misleading in defining the pharmacological profile of fluoxetine and its congeners. The term “selective brain steroidogenic stimulants” (SBSSs) is proposed.
- 28.Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci USA. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]; * In socially isolated mice, fluoxetine and norfluoxetine stereospecifically reverse brain allopregnanolone downregulation and reduce aggression. The effects of fluoxetine and norfluoxetine on 5-HT reuptake occurs for doses that are 50-fold higher and are not stereospecific.
- 29.Agís-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Downregulation of neurosteroid biosynthesis in cortico-limbic circuits mediates social isolation induced behavior in mice. Proc Natl Acad Sci USA. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The expression of 5a-RI mRNA is specifically down-regulated in glutamatergic pyramidal neurons that converge on the amygdala from cortical and hippocampal regions and may account for the appearance of behavioral disorders such as anxiety, aggression, and cognitive dysfunction.
- 30.Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5alpha-dihydroprogesterone in psychiatric disorders. Brain Res Rev. 2001;37:110–115. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- 31.Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio GJ. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- 32.Pinna G, Pibiri F, Agis-Balboa R, Nelson M, Guidotti A, Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochemical Research. 2008;33:1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- 33.Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville JM, Costa E, Guidotti A. Brain allopregnanolone regulates the potency of the GABAA receptor agonist muscimol. Neuropharmacology. 2000;39:440–448. doi: 10.1016/s0028-3908(99)00149-5. [DOI] [PubMed] [Google Scholar]; ** Demonstrated for the first time that endogenous allopregnanolone physiologically upregulates GABAergic tone and by such a mechanism plays a permissive/facilitatory role on the muscimol-induced loss of the righting reflex.
- 34.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 35.Milad MR, Quirk GJ, Pitman RK, Orr Sp, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 36.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 37.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–158. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 38.Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–34. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 40.Potts BD, Parli J. Analysis of the enantiomers of fluoxetine and norfluoxetine in plasma and tissue using chiral derivatization and normal-phase liquid chromatography. J Liquid Chromatography. 1992;15:665–681. [Google Scholar]
- 41.Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically facilitate pentobarbital sedation by increasing neurosteroids. Proc Natl Acad Sci USA. 2004;101:6222–6225. doi: 10.1073/pnas.0401479101. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Normalization of pentobarbital-induced sedation elicited by the S-isomers of fluoxetine and norfluoxetine is related to the reversal of the downregulation of brain Allo content and is independent of SSRI activity.
- 42.Uzunova V, Wrynn AS, Kinnunen A, Ceci M, Kohler C, Uzunov DP. Chronic antidepressants reverse cerebrocortical allopregnanolone decline in the olfactory-bulbectomized rat. Eur J Pharmacol. 2004;486:31–34. doi: 10.1016/j.ejphar.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3alpha-reduced neurosteroids to depression and antidepressant action. Psychopharmacology. 2006;186:351–361. doi: 10.1007/s00213-005-0201-6. [DOI] [PubMed] [Google Scholar]
- 44.Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci USA. 1999;96:13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sah P, Westbrook RF. Behavioural neuroscience: The circuit of fear. Nature. 2008;454:589–590. doi: 10.1038/454589a. [DOI] [PubMed] [Google Scholar]
- 46.Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations with computer programs. 2nd. Springer; New York: 1987. [Google Scholar]


