Summary
Naive T lymphocytes acquire a phenotype similar to antigen-experienced memory T cells as a result of proliferation under lymphopenic conditions. Such ‘memory-like’ T cells (TML) constitute a large fraction of the peripheral T cell pool in patients recovering from T cell ablative therapies, HIV patients under highly active antiretroviral therapy and in the elderly population. To generate a model which allows characterization of TML cells without adoptive transfer, irradiation or thymectomy, we developed genetically modified mice which express diphtheria toxin A under control of a loxP flanked stop cassette (R-DTA mice). Crossing these mice to CD4Cre mice resulted in efficient ablation of CD4 single positive thymocytes whereas double positive and CD8 single positive thymocytes were only partially affected. In the periphery the pool of naïve (CD44lo CD62Lhi) T cells was depleted. However, some T cells were resistant to Cre-activity, escaped deletion in the thymus and underwent lymphopenia-induced proliferation resulting in a pool of TML cells that was similar in size and turnover to the pool of CD44hiCD62Llo memory-phenotype T cells in control mice. CD4Cre/R-DTA mice remained lymphopenic despite the large available immunological ‘space’ and normal antigen-induced T cell proliferation. CD4Cre/R-DTA mice showed a biased T cell receptor repertoire indicating oligoclonal T cell expansion. Infection with the helminth Nippostrongylus brasiliensis resulted in diminished effector cell recruitment and impaired worm expulsion demonstrating that TML cells are not sufficient to mediate an effective immune response.
Keywords: Diphtheria toxin, T cell repertoire, homeostasis
Introduction
The generation of T cells in the thymus starts at a late stage during embryonic development and the first thymic emigrants are found in the periphery at day 18 of gestation (1). The pool of peripheral T cells is then filled within 3 weeks after birth mainly by continuous output of newly generated T cells from the thymus with high proliferation rates (2, 3). Thymic output peaks at 4 weeks of age and then steadily decreases at a rate of 3% per year throughout life due to thymic involution (4). However, the total number of peripheral T cells and the ratio of naïve to memory T cells remain remarkably stable until the 7th decade of life (5). Above that age T cell numbers decrease and certain T cell clones expand resulting in a lymphopenic immune system with an oligoclonal repertoire of TCR specificities and a relative increase in the pool of ‘memory phenotype’ T cells (TMP) (6). Similar alterations of the T cell compartment are also found e.g. in patients recovering from radio- or chemotherapy or in HIV patients under highly active antiretroviral therapy (HAART). Oligoclonal T cell repertoires can only recognize a restricted spectrum of antigens which might result in functionally impaired immune responses against newly encountered pathogens.
Peripheral T cell pools are under tight homeostatic control which is critical to maintain both a polyclonal repertoire of naïve T cells capable of responding to newly encountered antigens and a small pool of antigen-experienced memory T cells that provide protection against re-infection with previously encountered pathogens. Homeostasis is regulated by thymic output as well as proliferation and death of peripheral T cells. Three major mechanisms are involved in peripheral T cell homeostasis: (i) availability of homeostatic cytokines like IL-2, IL-7 and IL-15, (ii) low-avidity interaction with self-peptide/MHC molecules and (iii) ‘space’ (reviewed in (7)). The pools of naïve peripheral CD4+ and CD8+ T cells might be coregulated and both T cell subsets can partially utilize each others niches (8). In contrast, homeostasis of the naïve and TMP cell pools are regulated independently (9). In mice the TMP cell pool consists mainly of CD44hiCD62Llo cells and includes antigen-experienced memory T cells specific for foreign antigens (protective clones) or self antigens (autoreactive clones) with the potential to induce autoimmunity (10–12). In addition, antigen-independent expansion of naïve T cells that converted to ‘memory-like’ T cells (TML) during lymphopenia-induced proliferation can substantially contribute to this T cell pool. TML and antigen-experienced memory T cells are basically indistinguishable by phenotype or gene expression profile (7, 13). Since homeostasis of both types of TMP cells is probably regulated by similar mechanisms, they might compete for factors that promote their survival and turnover (14). Studies on T cell homeostasis under lymphopenic conditions have so far been performed using thymectomy, mouse mutants with defects in T cell development, bone marrow chimeras or adoptive transfers of mature T cells into lymphopenic hosts which either genetically lacked endogenous T cells or were irradiated before transfer (9, 14–16). So far there are no mouse models for constant and spontaneous lymphopenia without experimental manipulations that could be used to study certain aspects of immune responses under lymphopenic conditions. Spontaneous mouse models for lymphopenia would be helpful to develop more efficient vaccination strategies for the elderly population and to study peripheral tolerance mechanisms that control the onset of autoimmunity which is often associated with lymphopenic conditions (12).
Here, we describe a newly generated mouse strain, which encodes diphtheria toxin A (DTA) under control of a loxP flanked stop cassette in the ubiquitously expressed ROSA26 locus. Thereby, it can be used in combination with tissue-specific and/or inducible Cre-expressing mouse strains to achieve toxin-mediated cell ablation in vivo. ROSA-DTA mice were crossed to CD4Cre mice in order to generate a spontaneous lymphopenic mouse model with an oligoclonal repertoire of TML cells. We determined their phenotype and turnover under steady-state conditions and analyzed the immune response upon infection with the helminth Nippostrongylus brasiliensis.
Results
Conditional diphtheria toxin A (DTA) expression in T cells induces cell death without bystander toxicity
T cell development and homeostasis was analyzed using a newly generated mouse strain (R-DTA) with conditional expression of the diphtheria toxin A (DTA) gene. R-DTA mice were constructed by homologous recombination of a loxP flanked neomycin resistance gene followed by DTA into the ubiquitously expressed ROSA26 locus (17) (Fig.1A, B). The loxP flanked cassette prevents DTA expression in the absence of Cre activity. Mice carrying the targeted allele were born at the expected Mendelian frequency, were healthy and fertile and could be bred to homozygosity demonstrating that toxin expression was under tight control. To analyze the efficiency of T cell deletion and potential toxicity to bystander T cells, a co-culture was set up with CD4+ T cells from wild-type (Thy1.1+) mice and R-DTA (Thy1.2+) mice, which were stimulated for two days with plate-bound anti-TCR antibody and then transduced with a retrovirus expressing a GFP-Cre fusion protein. At 36 hours after transduction the majority of GFP+ cells from R-DTA mice had died with a further decrease at 60 hours, while GFP+ cells from control mice remained alive (Fig. 1C). The ratio of GFP− T cells from R-DTA and wild-type mice remained stable. This demonstrates that cell death occurs fast after toxin expression and DTA expression in T cells from R-DTA mice is not toxic to bystander T cells.
Figure 1. Generation of R-DTA mice and selective T cell ablation in vitro.
A) Schematic representation of the targeting construct used to generate R-DTA mice which express the diphtheria toxin A (DTA) subunit under control of a loxP-flanked stop cassette in the ubiquitously expressed ROSA26 locus. Cre activity removes the stop cassette and allows DTA expression. B) Southern blot analysis of tail DNA from heterozygous (Het) offspring and wild type (WT) mice. The wild-type allele generates a band at 11 kB and the targeted allele at 3.8 kB. C) Efficient Cre-mediated killing of T cells from R-DTA mice in vitro. A mixed T cell culture with CD4+ T cells from control mice (Thy1.1+) and R-DTA mice (Thy1.2+) was stimulated for 2 days with plate-bound anti-TCR antibody and then transduced with a retrovirus expressing a GFP-Cre fusion protein. 36 and 60 hours after transduction the ratio of Thy1.1/Thy1.2 cells was analyzed among the transduced cells (GFP+) and non-transduced cells (GFP−) by flow cytometry.
T cell ablation in CD4Cre/R-DTA mice
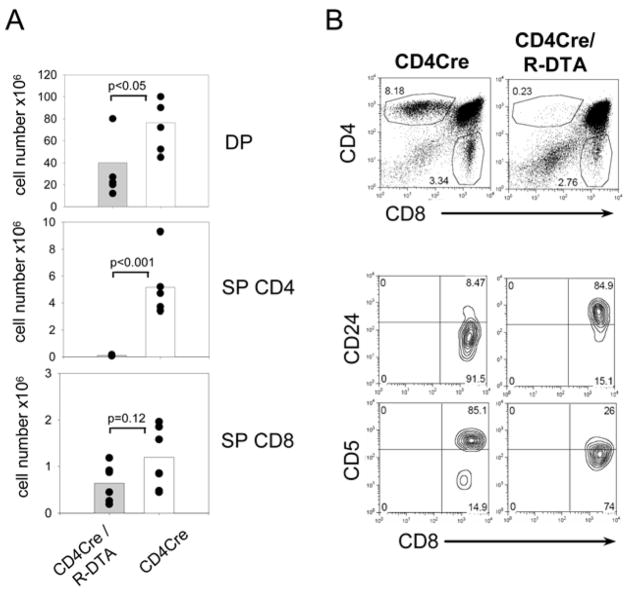
R-DTA mice were crossed to CD4Cre mice to analyze T cell deletion in vivo. The regulatory elements used to generate CD4Cre transgenic mice faithfully reflect expression of the endogenous CD4 gene, which starts at the double negative (DN) 3 stage and continues through the double positive (DP) and CD4 single positive (SP) stage but is shut off in CD8 SP cells (18, 19). Flow-cytometric analysis of the thymus in CD4Cre/R-DTA mice revealed that total numbers of DP thymocytes were reduced by ~50% and CD4 SP cells were almost absent (Fig. 2A, B). In contrast, only partial deletion of CD8 SP cells was observed. The CD8 SP cells had an immature phenotype (CD5loCD24hi) indicating incomplete thymic maturation (Fig. 2B). Subsets of the DN population were not different as compared to control mice (data not shown). Therefore, the toxin starts to be effective at the DP stage and continued expression in the CD4 lineage leads to efficient ablation of CD4 SP thymocytes.
Figure 2. Analysis of thymocyte development in CD4Cre/R-DTA mice.
A) Total numbers of double positive (DP), CD4 single positive (SP) and CD8 SP cells from pooled data of three independent experiments with 6 individual mice per group. B) Total thymocytes from CD4Cre or CD4Cre/R-DTA mice were stained with anti-CD4 and anti-CD8 antibodies and analyzed by flow cytometry. Contour-plots show expression levels of CD5 and CD24 on gated CD8 SP cells from CD4Cre mice (left) and CD4Cre/R-DTA mice (right).
Next, T cell subsets were analyzed in peripheral lymphoid organs. Efficient, although incomplete, deletion of both CD4+ and CD8+ T cells was observed in lymph nodes, spleen and blood (Fig. 3A, B). The reduced number of CD8+ T cells was not due to leaky Cre expression in these cells since no Cre mRNA could be detected in CD8+ T cells of CD4Cre mice by RT-PCR (Fig. 3C). The remaining CD4+ T cells in CD4Cre/R-DTA mice expressed the Cre recombinase, however, they did not recombine the stop cassette in the ROSA26 locus and therefore remained neo positive by genomic PCR analysis (Fig. 3C and D). These results confirm a previous report using CD4Cre mice in combination with conditional diphtheria toxin receptor expression from the ROSA26 locus, where a small fraction of peripheral T cells also retained the stop cassette (20). The reason for this resistance to Cre-recombination is currently unclear and requires further investigation.
Figure 3. Low numbers of peripheral T cells in CD4Cre/R-DTA mice.
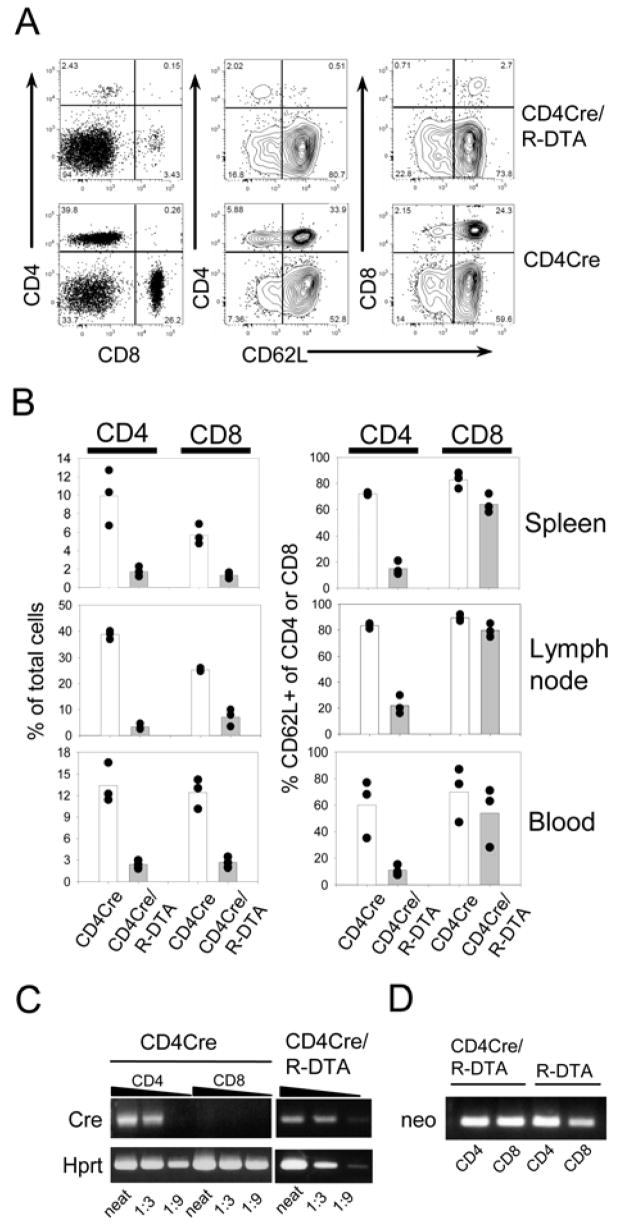
A) Mesenteric lymph node cells from CD4Cre/R-DTA and CD4Cre control mice were stained with anti-CD4, anti-CD8 and anti-CD62L antibodies and analyzed by flow cytometry. B) Blood, spleen and mesenteric lymph nodes of three individual mice were analyzed for the frequency of CD4+ and CD8+ T cells among total cells (left graphs) and the frequency of CD62L+ cells among CD4+ and CD8+ T cells (right graphs). Results are representative of several independent experiments. C) Semiquantitative RT-PCR analysis for Cre- and Hprt-expression of sorted CD4+ and CD8+ T cells from CD4Cre mice (left) and sorted CD4+ T cells from CD4Cre/R-DTA mice (right). D) Genomic PCR of sorted CD4+ and CD8+ T cells from CD4Cre and CD4Cre/R-DTA mice to determine the presence or absence of the loxP flanked neomycin resistance gene.
CD4Cre/R-DTA mice contain a normal-sized pool of ‘memory phenotype’ T cells and remain lymphopenic over time
Interestingly, the remaining CD4+ T cells in CD4Cre/R-DTA mice lost expression of CD62L, whereas most CD8+ T cells were CD62L+ (Fig. 3A, B). To analyze whether the CD62L+ CD8+ T cells belong to the naïve T cell pool or the TMP cell pool, cell suspensions were stained for CD44, which is expressed at increased levels on activated T cells and TMP cells but not on naïve T cells. Both CD4+ and CD8+ T cells were mainly CD44hi indicating that they had acquired a memory T cell phenotype probably due to lymphopenia induced proliferation in these mice (Fig. 4A). CD62L expression is usually lost after activation but can be re-expressed on memory CD8+ T cells and marks them as central memory T cells (TCM) with the capacity to enter lymph nodes via high endothelial venules (21). Thus, the majority of remaining CD8+ T cells appeared to have a TCM phenotype, whereas most CD4+ T cells differentiated to T cells with an effector memory (TEM) phenotype (Fig. 4A).
Figure 4. CD4Cre/R-DTA mice lack naïve T cells but show comparable numbers of memory phenotype T cells.
A) Analysis of CD4+ and CD8+ T cell subsets in mesenteric lymph node, spleen and blood. Cells were stained with anti-CD4, anti-CD8, anti-CD62L and anti-CD44 and analyzed by four color flow cytometry. B) Analysis of Treg cells in thymus and mesenteric lymph nodes. The dot plots show the frequency of CD4+Foxp3+ Treg cells among total live cells. Results are representative of 3–4 independently analyzed mice. C) Frequency of CD4+ and CD4− NKT cells (αβTCR+ cells) in the liver. Dot plots are gated on NK1.1+ cells. D) Smaller T cell zones in lymph node and spleen of CD4Cre/R-DTA mice as compared to R-DTA control mice. Sections of spleen and mesenteric lymph nodes were stained with anti-Thy1.2 (yellow) and anti-B220 (blue) antibodies. Original magnification was 80x. E) Number of total (left) and CD44hi ‘memory phenotype’ (right) T cells in inguinal lymph nodes of six week old CD4Cre/R-DTA mice (filled bars) or R-DTA mice (open bars). Graphs show combined results from two independent experiments with four mice total per group. Error bars indicate standard deviation. F) The frequency of T cells in the peripheral blood of individual CD4Cre/R-DTA mice (filled symbols) and R-DTA mice (open symbols) was monitored over 32 weeks by flow cytometry.
Analysis of natural T regulatory (Treg) cells (CD4+Foxp3+ T cells) revealed that thymic and peripheral Treg cells were reduced but not completely absent in CD4Cre/R-DTA mice compared to control mice (Fig. 4B). CD4Cre/R-DTA mice further showed selective loss of the CD4+ subset of NKT cells in the liver (Fig. 4C). Histological analysis of spleen and lymph nodes showed smaller T cell zones as compared to wild-type mice but the overall architecture with defined T and B cell zones remained intact which indicates that naïve T cells are not required to establish these structures (Fig. 4D). Strikingly, the size of the TMP cell pool in CD4Cre/R-DTA mice was comparable to control mice, despite the drastic reduction of total peripheral T cell numbers (Fig. 4E). The available ‘space’ in CD4Cre/R-DTA mice was not filled and they remained lymphopenic over time (Fig. 4F). Even the few naïve CD8+ T cells (CD44loCD62L+) did not increase, although it has been shown that mixed bone marrow chimeras with limited thymic output could reconstitute peripheral T cells in Rag2-deficient hosts and that naïve CD8+ T cells could expand using the niche of naïve CD4+ T cells and vice versa (9, 22).
Turnover, survival and functionality of TMP cells in CD4Cre/R-DTA mice
To compare the turnover of TMP cells in CD4Cre/R-DTA mice and control mice, BrdU-containing drinking water was administered for 7 days. BrdU incorporation was determined in CD44hi T cells in both groups of mice to directly compare the turnover within the TMP cell pool. CD4+CD44hi T cells from CD4Cre/R-DTA mice showed a slightly higher turnover in comparison to CD4+CD44hi T cells from wild-type mice, whereas no significant difference was observed for the CD8+CD44hi T cell populations (Fig. 5A). We further compared the replicative history of T cell subsets in both mice by staining for KLRG1, a marker for replicative senescent T cells which is expressed after numerous rounds of replication (23). KLRG1 was expressed with comparable frequency among CD62L− effector/memory phenotype T cells of both CD4Cre/R-DTA and control mice indicating a similar replicative history (Fig. 5B). This illustrates that homeostatic proliferation and turnover of T cells within the memory phenotype pool is comparable in lymphopenic CD4Cre/R-DTA mice and normal control mice. When T cells from CD4Cre/R-DTA mice were cultured in vitro for 5 days in the presence of 20 ng/ml IL-2 they increased in total numbers (Fig. 5C) which further demonstrates that T cells that escaped Cre-recombination during development were not deleted at later stages. Furthermore, T cells from CD4Cre/R-DTA mice responded with vigorous proliferation upon in vitro stimulation for 3 days with plate-bound anti-TCR antibodies demonstrating their responsiveness to TCR-mediated stimulation (Fig. 5D). CFSE-labeled T cells from CD4Cre/R-DTA mice underwent homeostatic proliferation after transfer into irradiated recipient mice which indicates that they survived and proliferated in vivo (Fig. 5E). Finally, T cells from CD4Cre/R-DTA mice could be polarized to Th1 and Th2 cells demonstrating that TML cells from these mice can become functional effector T cells (Fig. 5F).
Figure 5. Remaining T cells in CD4Cre/R-DTA mice are functional and show normal homeostatic proliferation.
A) Turnover of ‘memory phenotype’ T cells was analyzed by BrdU labeling in the drinking water for 7 days and staining for CD4, CD8, CD44 and BrdU. The graph shows the percentage of BrdU+ cells among CD44hi gated CD4+ or CD8+ T cells from three R-DTA mice (open bars) and four CD4Cre/R-DTA mice (filled bars). B) KLRG1 expression was analyzed on splenic CD4+ and CD8+ T cells of CD4Cre/R-DTA mice (upper panel) or R-DTA mice (lower panel) by staining for CD62L, KLRG1 and CD4 or CD8. Percentages indicate the frequency of KLRG1+ cells among CD62L− cells. C) Expansion of T cells from CD4Cre/R-DTA or control mice in vitro. Single cell suspensions of spleen and mesenteric lymph nodes were cultured in vitro for 5 days in the presence of 20 ng/ml IL-2. The total number of CD4+ and CD8+ T cells was determined on indicated days after setup of the culture. The results show the mean from two individual mice per group. D) TCR-mediated in vitro proliferation. Single cell suspensions of spleen and mesenteric lymph nodes were labeled with CFSE, incubated with 0.2 μg/ml anti-TCR (H57) and 0.2 μg/ml anti-CD28 antibody in the presence of 20 ng/ml IL-2 and analyzed three days later. The experiment has been repeated with comparable results. E) Spontaneous in vivo proliferation. Single cell suspension from spleen and mesenteric lymph nodes of CD4Cre/R-DTA or control mice were labeled with CFSE and transferred into 600 rad irradiated recipient mice. 7 days later mice were analyzed for spontaneous proliferation of transferred T cells. F) Quantitative RT-PCR to determine the expression of IL-4 and IFN-γ in polarized T cell cultures from CD4Cre/R-DTA (filled bars) and control mice (open bars). Error bars show s.d. of triplicate samples.
The TCR repertoire in CD4Cre/R-DTA mice is oligoclonal and allows spontaneous proliferation of adoptively transferred T cells
It has been shown that an established pool of TMP cells generated by spontaneous proliferation of adoptively transferred naïve T cells into Rag-deficient mice blocks spontaneous proliferation of a second wave of transferred naïve T cells but does not inhibit the establishment of a naïve T cell pool by endogenously generated thymic emigrants (14, 16, 24). Spontaneous proliferation after adoptive transfer of naïve CD4+ T cells was only observed when the repertoire of TCR specificities from the host was incomplete (16). To determine the TCR repertoire in CD4Cre/R-DTA mice, the pattern of the Vβ chain usage was analyzed by flow cytometry of peripheral blood samples. As indicated in Fig. 6A CD4+ and CD8+ T cells from CD4Cre/R-DTA mice showed biased Vβ chain usage as compared to their negative littermates indicating an incomplete repertoire of TCR specificities. To analyze whether this incomplete TCR repertoire allows spontaneous proliferation of adoptively transferred CD4+ T cells, purified naïve wild-type CD4+ T cells were labeled with CFSE and transferred into non-irradiated CD4Cre/R-DTA recipient mice. 7 days later a large fraction of transferred T cells had undergone spontaneous proliferation and acquired a memory phenotype (CD44hiCD62Llo) (Fig. 6B and data not shown). This result demonstrates that the TCR repertoire of endogenous T cells in CD4Cre/R-DTA mice was indeed incomplete.
Figure 6. Biased Vβ repertoire indicates TCR repertoire incompleteness.
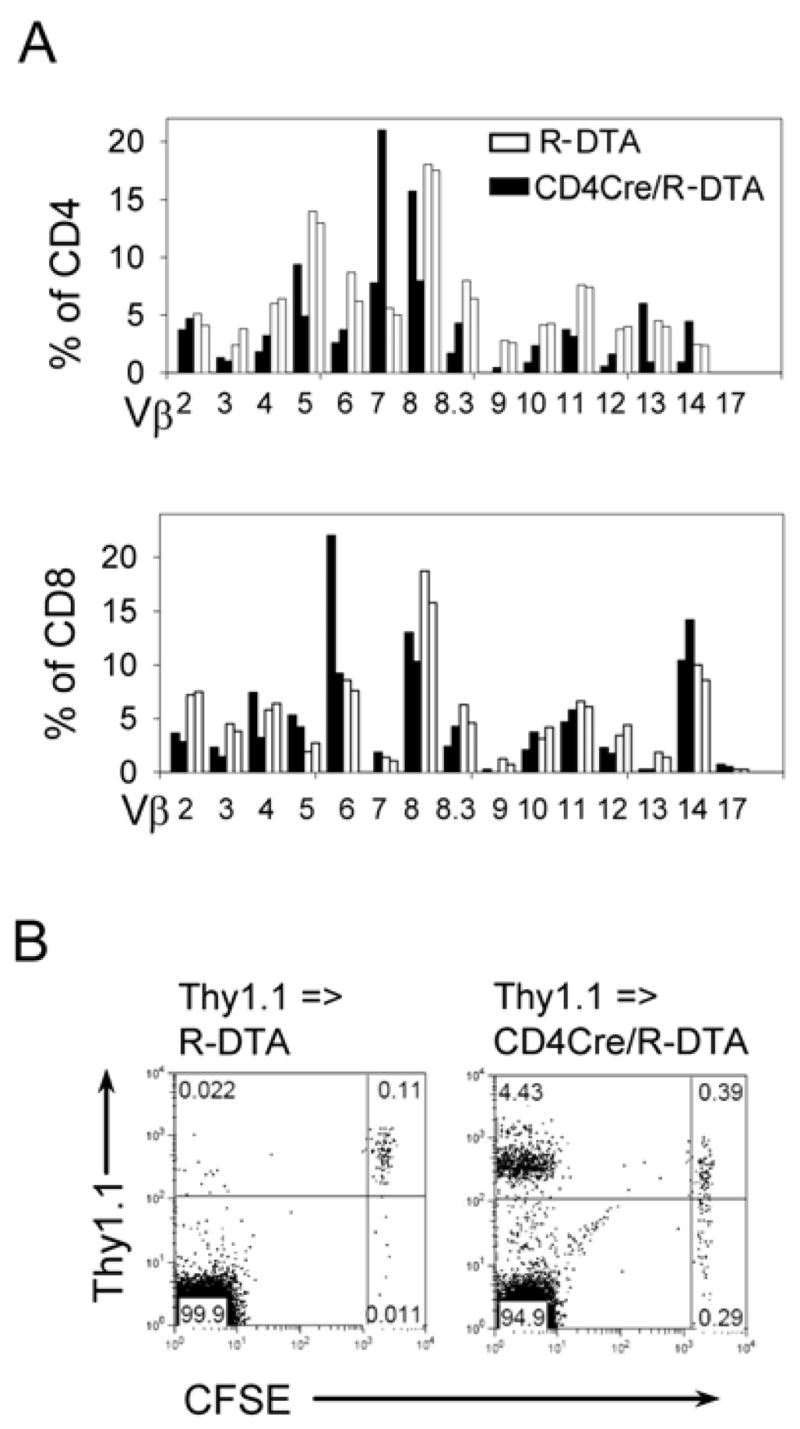
A) The peripheral blood of two CD4Cre/R-DTA mice (filled bars) and two age- and sex-matched control R-DTA mice (open bars) was analyzed by staining for CD4, CD8 and individual TCR-Vβ domains. The relative increase/decrease of the frequency of certain Vβ domains in CD4Cre/R-DTA mice indicates a biased TCR repertoire. The experiment has been repeated with similar results. B) Sorted naïve CD4+ T cells from wild-type Thy1.1 mice were labeled with CFSE and transferred into non-irradiated R-DTA or CD4Cre/R-DTA mice. 7 days later mice were analyzed for proliferation of transferred CD4+ T cells which only occurs in hosts with an incomplete TCR repertoire.
Naïve T cells from CD4Cre/R-DTA mice can be sustained when the peripheral T cell compartment has been filled with wild-type T cells
To determine whether T cells with a naïve phenotype can be found in CD4Cre/R-DTA mice during the recovery from peripheral T cell depletion, the remaining CD4+ T cells in CD4Cre/R-DTA mice were depleted with anti-CD4 monoclonal antibody and the phenotype of newly generated thymic emigrants in the peripheral blood was analyzed by flow cytometry (Fig. 7A). Interestingly, naïve phenotype T cells could be observed during an early phase between day 5 and 7 after antibody administration before the size of the TMP cell pool was reestablished (Fig. 7B). This transient population of naïve T cells most likely represents recent thymic emigrants. Due to the lymphopenic environment, these cells underwent spontaneous proliferation and converted to a memory phenotype (CD62LloCD44hi). However, thymic output was too low to fill the naïve T cell pool. To further substantiate these findings, mixed bone marrow chimeras were generated with bone marrow from wild-type (Ly5.2+Thy1.1+) and CD4Cre/R-DTA mice (Ly5.2+Thy1.2+) and transferred at a ratio of 1:10 into lethally irradiated wild-type (Ly5.1+) recipient mice. The first detectable peripheral CD4+ T cells at 4 weeks after reconstitution were derived from bone marrow of CD4Cre/R-DTA mice reflecting the higher inoculum of bone marrow cells from CD4Cre/R-DTA mice. However, from week 6 to week 8 after reconstitution the frequency of T cells derived from cotransferred wild-type bone marrow increased and outcompeted the T cells derived from bone marrow of CD4Cre/R-DTA mice (Fig. 7C). Thymocyte development at 10 weeks after reconstitution clearly showed that the vast majority of CD4 SP thymocytes was derived from the relatively small inoculum of wild-type bone marrow cells further supporting the notion that CD4+ T cells are deleted during transition from the DP to CD4 SP stage of thymic development without causing bystander toxicity (Fig. 7D). In peripheral lymph nodesof these mixed chimeras,10–17% of donor-derived CD4 + T cells were of CD4Cre/R-DTA origin and this population consisted to more than 50% of naïve (CD44lo) T cells which is comparable to the frequency of CD44lo T cells of wild-type origin and several fold higher than in CD4Cre/R-DTA mice (Fig. 7D, E). Therefore, we conclude that wild-type T cells established a peripheral TMP cell pool with a complete repertoire of TCR-specificities which prevented further conversion of naïve T cells to TMP cells and therefore allowed recent thymic emigrants of CD4Cre/R-DTA origin to remain in the naïve T cell pool.
Figure 7. Naïve CD4+ T cells from CD4Cre/R-DTA mice can be detected shortly after CD4-depletion or when the peripheral T cell pool has been replenished by wild-type T cells.
A) The frequency of CD4+ T cells in the peripheral blood of CD4Cre/R-DTA mice was analyzed in two individual mice at different days after intraperitoneal administration of 200 μg anti-CD4 monoclonal antibody (GK1.5). The experiment was repeated once with similar results. B) Analysis of the frequency of naïve (CD62L+CD44lo) CD4+ T cells at 0, 7, 12 and 18 days after anti-CD4 depletion. C) Percentage of wild-type (Thy1.1+) CD4+ T cells among total donor-derived (Ly5.2+) CD4+ T cells in the peripheral blood at 4, 6 and 9 weeks after bone marrow reconstitution of lethally irradiated Ly5.1+ recipient mice with 106 total bone marrow cells from Thy1.2+Ly5.2+ CD4Cre/R-DTA mice and 105 total bone marrow cells from Thy1.1+Ly5.2+ wild-type mice. D) Left: frequency of total thymocytes derived from wild-type bone marrow (Thy1.1+) or CD4Cre/R-DTA bone marrow (Thy1.1−). Middle: frequency of thymocyte subsets derived from wild-type precursors. Right: frequency of thymocyte subsets derived from CD4Cre/R-DTA precursors. Lower panel: Frequency of naïve (CD44lo) and activated/memory phenotype (CD44hi) CD4+ T cells from wild-type (Thy1.1+) and CD4Cre/R-DTA (Thy1.1−) donors in spleen and lymph node. Data are representative of 4 individual mice. E) Frequency of naïve (CD44lo) CD4+ T cells derived from wild-type bone marrow or CD4Cre/R-DTA bone marrow in mixed bone marrow chimeras (MC) in comparison to the frequency of naïve CD4+ T cells in wild-type and CD4Cre/R-DTA mice.
Infection of CD4Cre/R-DTA mice with Nippostrongylus brasiliensis results in impaired effector cell recruitment and worm expulsion
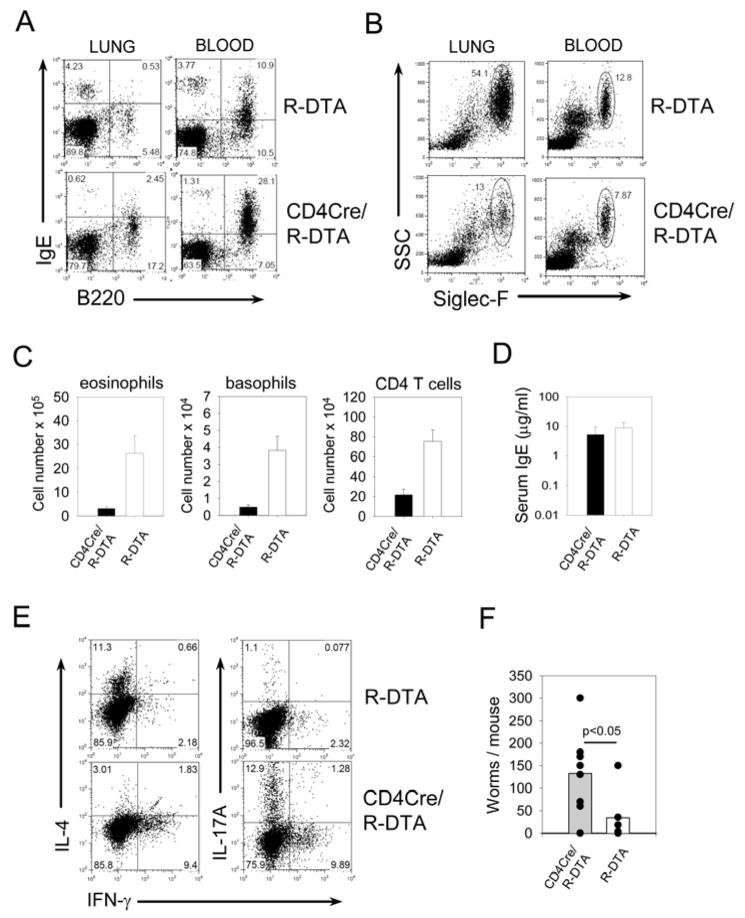
To address the functional consequences of an immune system with an oligoclonal T cell repertoire, we infected CD4Cre/R-DTA mice and R-DTA control mice with the helminth parasite Nippostrongylus brasiliensis. In this model, worm expulsion from the intestine and recruitment of eosinophils and basophils to the lung is strictly dependent on conventional CD4+ T cells (25, 26). On day 9 after infection CD4Cre/R-DTA mice showed reduced frequencies of basophils (IgE+B220−) and eosinophils (Siglec-F+SSChi) in the blood and lung compared to R-DTA mice (Fig. 8A and B). Total numbers of eosinophils, basophils and CD4+ T cells in the lung were 5–10 fold lower (Fig. 8C). Interestingly, the serum IgE levels were comparable between CD4Cre/R-DTA and R-DTA mice (Fig. 8D), which indicates that TMP cells could recognize N. brasiliensis antigen and induced a strong humoral immune response. Intracellular cytokine staining of CD4+ T cells isolated from the mesenteric lymph nodes of infected mice revealed that CD4+ T cells from wild-type mice had preferentially differentiated towards Th2 cells whereas T cells from CD4Cre/R-DTA mice were polarized towards Th1 and Th17 cells (Fig. 8E). This inappropriate T cell response might explain why worm expulsion from the small intestine was significantly impaired with four-fold higher worm counts in CD4Cre/R-DTA mice compared to R-DTA mice (Fig. 8F).
Figure 8. Infection of CD4Cre/R-DTA mice with N. brasiliensis reveals the functionally impaired immune response.
A) and B) Frequency of basophils (IgE+B220−) and eosinophils (Sigelc-F+SSChi) in lung and peripheral blood on day 9 after N. brasiliensis infection. Dot plots are gated on CD4−autofluorescence− cells and are representative of 4 independent experiments. C) Total numbers of eosinophils, basophils and CD4 T cells in the lung of R-DTA (n=5) and CD4Cre/R-DTA (n=6) mice on day 9 after N. brasiliensis infection. The graph shows pooled results from 3 independent experiments. Error bars indicate standard deviation. Differences between groups were statistically highly significant (p<0.01, Student’s T-test). D) Serum IgE levels of N. brasiliensis-infected R-DTA (n=7) and CD4Cre/R-DTA (n=8) mice from 4 independent experiments. The difference is not significant (p=0.13, Student’s T-test). E) Intracellular cytokine staining of CD4+ T cells isolated from mesenteric lymph nodes of indicated mice on day 9 after N. brasiliensis infection. The experiment was repeated with comparable results. F) The number of adult worms in the small intestine was determined on day 9 after infection of R-DTA (n=6) or CD4Cre/R-DTA (n=8) mice. The graph shows pooled results from 3 independent experiments.
Taken together, DTA expression in T cells resulted in efficient elimination of naïve CD4+ and CD8+ T cells. Low thymic output and lymphopenia-induced proliferation lead to rapid conversion of recent thymic emigrants to TML cells with an incomplete TCR repertoire. The size and turnover of this T cell pool was comparable to the normal TMP cell pool found in wild-type mice indicating that homeostasis of the TMP cell pool is regulated independently of the available immunological ‘space’. Infection with N. brasiliensis resulted in diminished effector cell recruitment and worm expulsion demonstrating the insufficiency of TML cells to orchestrate an effective primary type 2 immune response.
Discussion
The TMP cell pool is filled shortly after birth by expansion of the first thymic emigrants which enter a lymphopenic environment, proliferate and convert to TML cells (27). The size of the TMP cell pool is remarkably stable throughout life and antigen-experienced memory T cells are thought to use the same survival niches as TML cells. In fact, transient lymphopenia, a common phenomenon associated with viral or bacterial infections, often results in attrition of the numbers of antigen-experienced memory T cells due to competition with newly generated TML cells which proliferate under lymphopenic conditions (28). Furthermore, competition among TML cells results in oligoclonal T cell expansion, reduces the diversity of the T cell repertoire, limits the responsiveness of the immune system against newly encountered antigens and is thought to be a major cause of morbidity and mortality associated with infectious diseases in patients with such alterations of the T cell compartment and the elderly population (29). Lymphopenia can result in autoimmunity, since autoreactive clones that escaped negative selection in the thymus might expand and/or survive better than T cells that are tolerant to self-antigens (10, 30). This fatal situation might be triggered by high levels of IL-21 which is thought to co-stimulate proliferation of autoreactive T cells (10). Lymphopenia-induced T cell expansion has further been shown to be a barrier for solid organ transplantation since lymphopenia-induced ‘memory-like’ T cells (TML) cells cannot be tolerized by antibodies against co-stimulatory molecules (31). On the other hand, lymphopenia has been shown to be beneficial for tumor surveillance probably due to better priming or expansion of tumor-specific T cells under such conditions (32, 33). These examples illustrate that a better understanding of TML cells with regard to their turnover, effector function and in vivo migration is critically needed.
Using a newly generated mouse model we could show here that the turnover of endogenously generated TML cells is comparable to ‘memory phenotype’ T cells (TMP) in normal mice. Our results clearly demonstrate that the size of the TMP cell pool is regulated independently of the naïve T cell pool and remains constant even in the complete absence of naïve T cells. One elegant study described T cell homeostasis in chimeric mice which were generated by reconstitution of lethally irradiated Rag2-deficient mice with bone marrow from wild-type and T cell-deficient mice mixed at different ratios (15). Using this approach, it could be demonstrated that the size of peripheral T cell pools are maintained even when the thymic output falls below 10% of normal. However, an even lower thymic output resulted in lymphopenia and was associated with a strong bias towards T cells with a memory phenotype and an oligoclonal TCR repertoire. Therefore, efficient continuous thymic output seems to be required but might not be sufficient to establish and maintain normal numbers of naïve peripheral T cells (15, 24). Interestingly, when the remaining peripheral CD4+ T cells in CD4Cre/R-DTA mice were depleted with a monoclonal antibody, a transient wave of naïve phenotype T cells was observed at day 7 after depletion indicating that new thymic migrants were detected which had not yet converted to a memory phenotype. The low thymic output in CD4Cre/R-DTA mice is not sufficient to establish a peripheral pool of naïve T cells. However, mixed chimeras with bone marrow from wild-type and CD4Cre/R-DTA mice revealed that peripheral T cells with a naïve phenotype could be generated from CD4Cre/R-DTA mice when the peripheral T cell pools had been filled with T cells derived from wild-type bone marrow. Interestingly, spontaneous proliferation of adoptively transferred naïve CD4+ T cells in lymphopenic hosts could be blocked by pre-existing TMP cells (14, 16). However, proliferation could still occur when the TCR repertoire of the TMP population was oligoclonal (16). This indicates that the repertoire diversity and not the size of the TMP cell pool controls spontaneous proliferation of naïve T cells. The biased TCR repertoire in CD4Cre/R-DTA mice suggests oligoclonal expansion of T cells that might be specific for autoantigens or commensal antigens. Further studies including the generation of gnotobiotic mice are required to distinguish between both possibilities.
The behavior of T lymphocytes under lymphopenic conditions has been studied mainly by adoptive cell transfers into irradiated or genetically lymphopenic mice (7, 34). The spontaneous lymphopenic mouse model described here avoids ex vivo handling of T cells which has been shown to change the expression level of about 200 genes (13). CD4Cre/R-DTA mice might help to complete our understanding of the functionality of lymphopenic immune systems in fighting tumors and infections. As we show here, a lymphopenic immune system with a strong bias towards TMP cells cannot mount an efficient primary immune response against N. brasiliensis, although TML cells could be polarized to Th1 and Th2 cells in vitro. The inefficient immune response in CD4Cre/R-DTA mice could be due to an insufficient precursor frequency of N. brasiliensis-specific CD4+ T cells, which is indicated by the reduced TCR diversity and the low total number of T cells. It is also possible that T cell activation under lymphopenic conditions results mainly in Th1/Th17 polarization and prevents differentiation towards Th2 cells. Further experiments are required to address this point.
It will be interesting to study CD8+ T cell responses against viral infections and tumors in a lymphopenic environment with a special focus on the homeostasis of antigen-specific memory T cell populations which have to compete with TML cells for survival in the TMP cell pool. These studies might help to develop novel vaccination strategies for elderly people as well as for patients with chronic viral infections or cancer patients recovering from chemotherapy who have in common that their peripheral T cell pool consists mainly of TMP cells with an oligoclonal TCR repertoire.
Materials and Methods
Mice
To generate R-DTA mice, the diphtheria toxin alpha chain (DTA) was amplified by PCR from pKO SelectDT plasmid (Lexicon Genetics Inc.) with the following primer pairs: 5′DTA: 5′-gtcgacctgcaggtcctcgccatgg-3′ and 3′DTA: 5′-ctcgagtttgtccaattatgtcac-3′, and subcloned after SalI/XhoI digest into the pBigT plasmid behind the loxP flanked neomycin resistance (neoR) cassette and in front of the bovine growth hormone polyA sequence (35). The modified pBigT plasmid was cut with PacI/AscI and cloned into the PacI/AscI digested pROSA26-1 plasmid (http://www.fhcrc.org/labs/soriano/vectors/pROSA26-1.html) to generate the final targeting vector. 30 μg targeting vector were linearized with KpnI and electroporated into E14 ES cells (129/Sv background). Southern blots were performed with EcoRV digested DNA using a probe (337 bp) generated by NotI digest of the pROSA26promoter plasmid (http://www.fhcrc.org/labs/soriano/vectors/pROSA26promoter.html). 8 of 57 ES cell colonies showed homologous recombination. Mice were generated by blastocyst injection in the transgenic core facility at UCSF.
CD4Cre mice (C57BL/6NTac-TgN ) (36) were purchased from Taconic farms (Germantown, NY) and bred to R-DTA mice on a mixed 129/Sv x C57BL/6 background. Thy1.1 mice (B6.PL-Thy1a/CyJ) and Ly5.1 mice (B6.SJL-Ptprca Pepcb/BoyJ) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed under specific pathogen free conditions according to institutional guidelines.
Retroviral transduction of T cells
CD4+ T cells from R-DTA mice (Thy1.2) and control Thy1.1 mice were isolated from spleen and mesenteric lymph nodes by negative selection using a CD4+ T cell isolation kit (Miltenyi, Germany), stimulated for 2 days with plate-bound anti-TCR (H57) and anti-CD28 antibody (BD Pharmingen) and transduced by spin-infection with a MSCV-based retrovirus containing a GFP-Cre fusion protein (kindly provided by Kevin Shannon, UCSF).
Flow-cytometry and cell sorting
Single-cell suspensions were incubated with anti-CD16/CD32 blocking antibody (2.4G2) for 5 min at room temperature and stained with the corresponding antibody mixtures. The following monoclonal antibodies were purchased from Caltag (Invitrogen, Carlsbad, CA), unless otherwise indicated: APC labeled anti-CD8, PE-Alexa700 labeled anti-CD4, FITC labeled anti-CD62L, PE labeled anti-CD44, APC labeled anti-Thy1.2, Alexa647 labeled anti-B220, APC labeled anti-CD45.1 (Southern Biotechnology), biotinylated anti-CD5 (BD Pharmingen), biotinylated anti-CD24 (BD Pharmingen), FITC labeled anti-BrdU (BD Pharmingen), FITC-labeled anti-TCR screening panel (BD Pharmingen), PE labeled anti-Siglec-F (BD Pharmingen), biotinylated anti-IgE (Southern Biotech), PE labeled anti-Thy1.1 (eBioscience), biotinylated anti-KLRG1 (eBioscience), PE-labeled streptavidin (Southern Biotech) and APC-labeled streptavidin (Southern Biotech). Intracellular staining for Foxp3 was performed with the anti-mouse/rat Foxp3 staining set (eBioscience). Intracellular cytokine staining was performed with FITC-labeled anti-IFN-γ (XMG1.2, eBioscience), PE-labeled anti-IL-4 (BVD6-24G2, Invitrogen) and Alexa647-labeled anti-IL-17A (eBioTC11-18H10.1, eBioscience) after cells had been stimulated for 4h with 1μg/ml ionomycin and 40 ng/ml PMA with Brefeldin A added at 5μg/ml for the last 2h. Naïve CD4 + T cells (CD62L+CD25−) were sorted from spleen and lymph node of Thy1.1 mice using a FACS-Aria® high speed cell sorter (BD Immunocytometry Systems, San Jose, CA) with a purity of >98%. CFSE labeling was done by incubation of cell suspensions with 0.5 μM CFSE at 5×106 cells/ml for 10 min at 37°C. Cells were analyzed on a FACSCalibur instrument (BD Immunocytometry Systems).
In vitro T cell polarization
Untouched CD4+ T cells were purified from spleen and lymph nodes by MACS technology (Miltenyi) and cultured for 5 days under Th1 (5 ng/ml IL-12, 20 μg/ml anti-IL4 (11B11)) or Th2 (20 ng/ml IL-4, 20 μg/ml anti-IFN-γ (XMG1.2)) conditions with plate-bound anti-αβTCR (H57, 0.2 μg/ml) and anti-CD28 (0.2 μg/ml) in the presence of 20 ng/ml IL-2. On day 5 cells were restimulated for 4 hours with 1 μg/ml ionomycin and 40 ng/ml PMA and subjected to quantitative RT-PCR analysis.
Mixed bone marrow chimeras
Bone marrow from CD4Cre/R-DTA mice (Ly5.2+Thy1.2+) was mixed at a 10:1 ratio with bone marrow from wild-type mice (Ly5.2+Thy1.1+) and 2×106 cells were injected into lethally irradiated (2 × 600rad) wild-type Ly5.1+ mice. Chimeras were kept with antibiotica containing drinking water (2 g/l Neomycin sulfate and 100 mg/l Polymyxin B). Mice were analyzed at the indicated time points after reconstitution.
Histology
Frozen tissue from mesenteric lymph nodes and spleen was cut in 5 μm thick sections and stained with biotinylated anti-Thy1.2 (Caltag) followed by Cy3-labeled Streptavidin (Jackson ImmunoResearch, Cambridgeshire, UK) and Alexa647-labeled anti-B220 (Caltag). Pictures were acquired on a Leica DM RXA microscope. Original magnification was 80x.
PCR analysis
For semiquantitative RT-PCR analysis CD4+ and CD8+ T cells were sorted on a FACSAria cell sorter (BD Immunocytometry Systems, San Jose, CA) with >96% purity. RNA was isolated using a total RNA isolation kit (Fluka, Switzerland) and transcribed in cDNA with Superscript II ™ reverse transcriptase (Invitrogen). Cre expression was determined on serial dilutions of cDNA samples using the following primer pairs: Cre1 5′-tgatagctggctggtggcagatgg-3′ and Cre2 5′-tgctgtttcactggttatgcggcgg-3′. Hprt expression was analyzed with Hprt1 5′-gttggatacaggccagactttgttg-3′ and Hprt2 5′-gagggtaggctggcctataggct-3′ primers.
For genomic PCR analysis DNA was isolated from sorted CD4+ and CD8+ T cells by Proteinase K digest and isopropanol precipitation. The primers for amplification of the neo cassette were neo1 5′-cttgggtggagaggctattc - 3′ and neo2 5′-aggtgagatgacaggagatc-3′. All PCR reactions were performed with 35 cycles, 56°C annealing temperature and 60 sec extension time at 72°C.
To determine the expression of cytokines in T cell cultures quantitative RT-PCR was performed using the following primer pairs: IL-4 fwd 5′-agctagttgtcatcctgctc-3′ and IL-4 rev 5′-tggtggctcagtactacgag-3′, IFN-γ fwd 5′-acgctacacactgcatcttg-3′ and IFN-γ rev 5′-tcggatgagctcattgaatg-3′, Hprt1 and Hprt2. Triplicate samples were run on a LightCycler PCR machine (Roche, Switzerland) with the DyNAmo SYBR green qPCR kit (Finnzymes, Espoo, Finland).
Bromodeoxyuridin (BrdU) analysis
Mice were given 0.8 mg/ml BrdU in the drinking water for 7 days. Single cell suspensions of the spleen were labeled with anti-CD4, anti-CD8 and anti-CD44 antibodies, fixed and permeabilized. Then genomic DNA was fragmented with DNAse I (Sigma-Aldrich, St. Louis, MO) and stained with FITC labeled anti-BrdU antibody (BD Pharmingen).
IgE ELISA
Serum IgE levels were determined by standard ELISA technique using the monoclonal antibody R35–72 (BD Pharmingen) for coating and the biotinylated monoclonal antibody R35–118 (BD Pharmingen) for detection.
N. brasiliensis infection
Third-stage larvae (L3) of N. brasiliensis were recovered from the cultured feces of infected rats, washed extensively in 0.9% saline (37°C) and injected (500 organisms) into mice subcutaneously at the base of the tail. Mice were treated with antibiotic-containing water (2 g/l neomycin sulfate, 100 mg/l polymyxin B sulfate; Sigma-Aldrich, St. Louis, MO) for the first 5 days after infection. Worm expulsion was determined by counting adult worms in the small intestine on day 9 after infection.
Statistical analysis
P values were determined with Student’s t-test using Sigma-Plot software (SPSS Inc.). p<0.05 was considered statistically significant.
Acknowledgments
We thank K. Shannon and P. Soriano for providing plasmids, N. Killeen for blastocyst injection, N. Flores, A. Bol and W. Mertl for animal husbandry, L. Stowring and A.-M. Knorn for technical assistance and L. Reinhardt and T. Brocker for helpful comments.
Footnotes
This work was supported in part by NIH grant AI30663 and the Howard Hughes Medical Institute, the Sandler Asthma Basic Research Center (to R.M.L.) and the Emmy Noether Program of the Deutsche Forschungsgemeinschaft (grant Vo944/2 to D.V.).
References
- 1.Fadel S, Sarzotti M. Cellular immune responses in neonates. Int Rev Immunol. 2000;19:173–193. doi: 10.3109/08830180009088504. [DOI] [PubMed] [Google Scholar]
- 2.Modigliani Y, Coutinho G, Burlen-Defranoux O, Coutinho A, Bandeira A. Differential contribution of thymic outputs and peripheral expansion in the development of peripheral T cell pools. Eur J Immunol. 1994;24:1223–1227. doi: 10.1002/eji.1830240533. [DOI] [PubMed] [Google Scholar]
- 3.Schonland SO, Zimmer JK, Lopez-Benitez CM, Widmann T, Ramin KD, Goronzy JJ, Weyand CM. Homeostatic control of T-cell generation in neonates. Blood. 2003;102:1428–1434. doi: 10.1182/blood-2002-11-3591. [DOI] [PubMed] [Google Scholar]
- 4.Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 5.Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 7.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 8.Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 9.Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- 10.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 11.Krupica T, Jr, Fry TJ, Mackall CL. Autoimmunity during lymphopenia: a two-hit model. Clin Immunol. 2006;120:121–128. doi: 10.1016/j.clim.2006.04.569. [DOI] [PubMed] [Google Scholar]
- 12.Khoruts A, Fraser JM. A causal link between lymphopenia and autoimmunity. Immunol Lett. 2005;98:23–31. doi: 10.1016/j.imlet.2004.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldrath AW, Luckey CJ, Park R, Benoist C, Mathis D. The molecular program induced in T cells undergoing homeostatic proliferation. Proc Natl Acad Sci U S A. 2004;101:16885–16890. doi: 10.1073/pnas.0407417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourgeois C, Kassiotis G, Stockinger B. A major role for memory CD4 T cells in the control of lymphopenia-induced proliferation of naive CD4 T cells. J Immunol. 2005;174:5316–5323. doi: 10.4049/jimmunol.174.9.5316. [DOI] [PubMed] [Google Scholar]
- 15.Almeida AR, Borghans JA, Freitas AA. T cell homeostasis: thymus regeneration and peripheral T cell restoration in mice with a reduced fraction of competent precursors. J Exp Med. 2001;194:591–599. doi: 10.1084/jem.194.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min B, Foucras G, Meier-Schellersheim M, Paul WE. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proc Natl Acad Sci U S A. 2004;101:3874–3879. doi: 10.1073/pnas.0400606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 18.Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 19.Wolfer A, Bakker T, Wilson A, Nicolas M, Ioannidis V, Littman DR, Lee PP, Wilson CB, Held W, MacDonald HR, Radtke F. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nat Immunol. 2001;2:235–241. doi: 10.1038/85294. [DOI] [PubMed] [Google Scholar]
- 20.Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 21.Lefrancois L, Marzo AL. The descent of memory T-cell subsets. Nat Rev Immunol. 2006;6:618–623. doi: 10.1038/nri1866. [DOI] [PubMed] [Google Scholar]
- 22.Stockinger B, Barthlott T, Kassiotis G. The concept of space and competition in immune regulation. Immunology. 2004;111:241–247. doi: 10.1111/j.1365-2567.2004.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 24.Tanchot C, Rocha B. The peripheral T cell repertoire: independent homeostatic regulation of virgin and activated CD8+ T cell pools. Eur J Immunol. 1995;25:2127–2136. doi: 10.1002/eji.1830250802. [DOI] [PubMed] [Google Scholar]
- 25.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 27.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 28.Stockinger B, Kassiotis G, Bourgeois C. Homeostasis and T cell regulation. Curr Opin Immunol. 2004;16:775–779. doi: 10.1016/j.coi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shih FF, Mandik-Nayak L, Wipke BT, Allen PM. Massive thymic deletion results in systemic autoimmunity through elimination of CD4+ CD25+ T regulatory cells. J Exp Med. 2004;199:323–335. doi: 10.1084/jem.20031137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, Sayegh MH, Turka LA. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu HM, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62:3914–3919. [PubMed] [Google Scholar]
- 34.Singh NJ, Schwartz RH. The lymphopenic mouse in immunology: from patron to pariah. Immunity. 2006;25:851–855. doi: 10.1016/j.immuni.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]