Abstract
An important clinical task is to coherently integrate the use of protein-targeted drugs into pre-existing therapeutic regimens, with the goal of improving treatment efficacy. Constitutive activation of Ras-dependent signaling is important in many tumors, and agents that inhibit this pathway might be useful in numerous therapeutic combinations. The MCP compounds were identified as inhibitors of Ras-Raf interactions, and previously shown to inhibit multiple Ras-dependent transformation phenotypes when used as monoagents in cell culture analyses. In this study, we investigate the ability of the MCP110 compound to synergistically enhance the activity of other therapeutic agents. In both a defined K-Ras-transformed fibroblast model and in human tumor cell lines with mutationally activated Ras, MCP110 selectively synergizes with other agents targeting the MAPK pathway, and with multiple agents (paclitaxel, docetaxel, and vincristine) targeting the microtubule network. The synergistic activity of MCP110 and paclitaxel was further established by experiments showing that in Kaposi's sarcoma oncogenically-transformed cell lines, cellular models for tumors treated with taxanes in the clinic and in which Raf-dependent signaling plays an important role, MCP110 synergizes with paclitaxel and limit growth. Finally, in vivo testing indicate that MCP110 is bioavailable, inhibits the growth of LXFA 629 lung and SW620 colon carcinoma cells in xenograft models, and again strongly synergizes with paclitaxel. Together, these findings indicate that MCP compounds have potential to be effective in combination with other anticancer agents.
Keywords: MCP110, Ras, Raf, taxane, vinca alkaloid
Introduction
Activation of the Ras oncoprotein is a critical element in many different cancers, including pancreatic, breast, and others (reviewed in (1)). In some tumors Ras is directly activated by mutation, while in others the constitutive signaling of upstream regulatory factors such as the epidermal growth factor (EGF) receptor promote deregulated activation of wild type Ras(2). Active Ras promotes tumor growth through its ability to activate multiple downstream effector signaling pathways that promote cell proliferation, survival, migration, and angiogenesis (reviewed in (3, 4)). Among these different pathways, Ras interaction with and activation of Raf serine/threonine kinases (Raf-1, A-Raf, and B-Raf), phosphatidylinositol 3-kinases (PI3Ks), and Ral guanine nucleotide exchange factors (RalGEFs) has been shown to be critical for tumor promotion. Although different tumor types rely to differing degrees on activation of the Raf, PI3K, and RalGEF effector pathways (5), the particular importance of Raf activation has long been appreciated (6). For these reasons, strategies to rationally design chemotherapeutic agents that specifically antagonize the Ras/Raf/MEK/ERK signaling cascade have been considered to be promising (6).
The MCP1 compound was isolated based on its ability to block the interaction of Ras and Raf-1 in a yeast two-hybrid assay (7). In initial characterizations of the efficacy of MCP1 and a more potent derivative, MCP110, both were shown to efficiently reverse multiple Ras-dependent transformation changes in mammalian cells (7, 8). This analysis demonstrated that MCP compounds inhibited Ras-induced activation of the Raf and ERK mitogen-activated protein kinase (MAPK) signaling cascade, Ras-induced cell migration, morphological changes, and anchorage-independent growth, and Ras-regulated expression of matrix metalloproteases and cyclin D1 (7). Based on these results, this class of compounds was selected for further evaluation.
Very few clinical agents are successful as monotherapies: instead, dual or triple-therapies are generally significantly more potent. Modern therapeutic combination strategies fall into four categories. In one approach, a single signaling pathway is “vertically” targeted, with drugs inhibiting multiple steps in a signaling cascade: for example, pretreatment of A549 lung carcinoma cells with the PI-3K inhibitor PX-866, which strongly potentiates the action of the EGFR inhibitor Iressa (9). In a second, “horizontal”, approach, two or more cooperating signaling pathways are targeted in parallel. Synergistic effect has been documented in glioblastoma cells treated with Raf-1 or MEK kinase inhibitors (GW5074 and U0126) together with ILKAS, an antisense oligonucleotide that inhibits the PI-3K-regulated ILK and AKT kinases (10). A third approach is the use of multiple agents for the same target. For example, Cetuximab and Iressa (an antibody and a small molecule inhibitor of EGFR) showed a marked synergistic effect in a phase II clinical trial in colon carcinoma (11). A final approach is the combination of a pathway-targeted drug with a conventional cytotoxic agent. For example, the humanized anti-Her2 antibody herceptin (trastuzumab) productively synergizes with cisplatin and taxanes to treat breast cancer (12, 13).
In this study, we have assessed the efficacy of MCP110 in enhancing the activity of established clinical agents, and probed the mode of action of MCP110. Our data indicate that the MCP110 synergizes both with other small molecules targeting the MAPK pathway, and also with multiple mitotic spindle-targeting agents. This synergy occurs in vitro and in vivo, and is observed in multiple cancer models relevant to activation of Ras signaling. These studies predict that MCP compounds are potentially useful additions to the clinical armamentarium.
Materials and Methods
Cell lines and plasmids
Cells used included SW620 cells (ATCC), EC-vGPCR (14), and NIH3T3 cells stably transfected with the pBabe-puro retrovirus vector, or expressing constitutively activated H-, K-, or N-Ras (15), Raf22W (16), MEK1ΔN3/S222D (15), or the KSHV-GPCR (17).
Compounds
MCP1 and MCP110 were synthesized as described previously (8); structures and structure-activity relationships have also been described for these compounds (8). Sorafenib (18) (Calbiochem), U0126 (Promega), paclitaxel (Biomol), docetaxel (Fox Chase Cancer Center pharmacy; Sanofi-Aventis), vincristine (Sigma), AACOCF3 (Biomol), gemcitabine (Shanghai Sunshine International Tdg., Co., Ltd) and staurosporine (Sigma) were commercially prepared.
Proliferation, anchorage-independence, cell cycle, and apoptosis assays
Proliferation was measured 48 hours after addition of compounds to cells using WST-1 reagent (Roche Applied Sciences, Indianapolis, IN) according to standard protocols. Anchorage-independent growth assays were performed essentially as described (19). 12-21 days after cell seeding, cells were stained with thiazolyl blue tetrazolium bromide (MTT), and colonies >600 μm in diameter were scored using a Nikon SMZ1500 microscope coupled with a Roper Scientific Inc. Cool Snap charge coupled device (CCD) camera with Image Pro-Plus software (Media Cybernetics; Silver Spring, MD). Survival curves were based on at least six concentration points, with values determined in at least three separate experiments, with each assay performed in sextuplicate. All statistical analysis was performed using the Excel software program (ID Business Solution, Inc., NJ, USA), with the exception that drug combinations were investigated for synergy, additive effect or antagonism using Median Dose Effect analysis as in (20), using CalcuSyn software (Biosoft, Ferguson, MO) to establish combination index (CI). Cell cycle compartmentalization and apoptotic index were determined using fluorescence-activated cell sorting (FACS) analysis using FACSII instrument (BD Biosciences, Franklin Lakes, NJ), using standard approaches. Apoptotic cells were detected by staining with antibody to annexin V (BD Bioscience Pharmingen).
Compound formulation for in vivo application in SCID xenografts
Both MCP110 and paclitaxel were administered in a liposomal formulation containing 10% (w/v) phospholipon 90G (American Lecithin Company, Oxford, CT) and 33% (v/v) Myritol 318 (Cognis Corp., Cincinnati, OH). Details of preparation of the formulation are available on request.
Pharmacokinetics and maximum tolerated dose (MTD) assessment in nude mice
To determine the in vivo bioavailability of MCP1 and MCP110 administered through different routes, groups of 12 NMRI nu/nu mice received a single dose of compounds formulated in 100% ethanol and 10% hydroxypropyl-beta-cyclodextrin (HPβCD). For the oral administration route the dose level was chosen at 30 mg/kg, and clinical signs were documented immediately before dosing, and at 0.5, 1, 2 and 5 hours after dosing. Compound plasma concentrations were determined at 0.5, 1, 2, and 5 hours after dosing. For i.p. and i.v. routes, the compounds were tested at a dose level of 3 mg/kg, and clinical signs and compound plasma concentrations were documented before dosing, and 10 min, 20 min, 1 and 3 hours after dosing. For determining the blood plasma concentrations, an internal standard was added to all plasma samples: compounds were detected using HPLC-MS/MS analysis with a PE/SCIEX API3000 instrument.
For MTD assessment, groups of NMRI nu/nu mice received a single i.p. injection of MCP110 at dose levels specified in the results, or vehicle (20% DMSO, 5% Cremophor EL, 75%HPβCD (Yiming Fine Chemicals, Ltd., China), and a solution composed of 10% Myritol 318 (v/v) and 3% Phospholipon 90G (w/v) in water. For 7 day-repetitive dose studies, MCP110 was delivered at 0, 300, 600 and 1200 mg/kg/day, with dosing followed by a 4-day or 16-day observation period. Doses were administered at a volume of 10 ml/kg/day.
Tumor xenograft analysis in athymic (nu/nu) nude and SCID mice
SW620 colon carcinoma and LXFA 629 NSCLC cells were used to induce xenografts in 4-6 week old SCID and athymic nude males. Studies with LXFA 629 cells were performed by Oncotest (Freiburg, Germany). Exponentially growing cells were harvested, washed with PBS and resuspended in DMEM. 2.5 - 5 × 106 cells were transplanted subcutaneously into the right flank of each mouse (5-10 mice per group). Animals were monitored for 3 weeks for tumor formation prior to treatment. During treatment, MCP compounds were injected daily; paclitaxel was included in 8/17 injections (see Results). For analysis, tumor volume was determined by measuring (L*W*W)/2, where L and W represents the longest length and width of the tumor, respectively. Tumor growth inhibition was measured as the median tumor weight of the treated group (T) divided by the median tumor weight of the control group (C) at the time when the median tumor weight in the control group has reached ∼ 700 mg, and expressed as T/C value. A standard Mann-Whitney-Wilcoxon U-test was used to establish significant differences in the ranking of individual tumors.
Results
Synergy of MCP110 with Ras>Raf>MEK1>MAPK pathway inhibitors in Ras-transformed cells
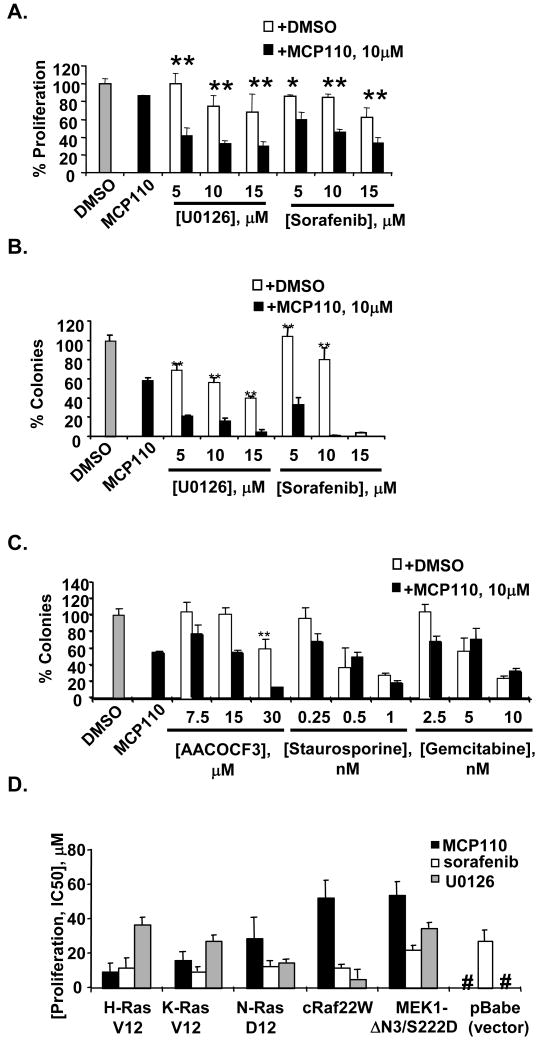
We first evaluated the ability of MCP110 to synergize with agents that vertically target the Ras>Raf>MEK>ERK pathway, versus their ability to synergize with compounds that targeted unrelated signaling pathways. Sorafenib (BAY 43-9006) inhibits Raf kinase (and additional kinases, (21)), and U0126 specifically inhibits MEK1 kinase (22). In the NIH3T3-K-Ras(G12V) model, the IC50s of these compounds used as monoagents are 17 μM (MCP110), 10.3 μM (sorafenib) and 47.6 μM (U0126) in a proliferation assay and 8.1 μM (MCP110), 9.3 μM (sorafenib) and 12.4 μM (U0126) in a soft agar colony formation assay. We observed a dose dependent growth inhibition effect when NIH3T3-K-Ras(G12V) cells were treated with the combination of 10 μM of MCP110 with U0126 or sorafenib, in both proliferation (Figure 1A) and colony formation (Figures 1B) assays, with the colony formation assay showing a more pronounced effect of combination.
Figure 1. Specific synergy between MCP110 and other agents targeting the MAPK pathways in NIH3T3-K-Ras(G12V) cells.
A. The proliferation of cells in DMSO-treated plates was taken as 100%, and the number of cells in compound-treated plates was expressed as % of this value for this and following experiments. Then, proliferation was assessed for NIH3T3-K-Ras(G12V) cells treated with DMSO vehicle (gray bar), MCP110 at 10 μM delivered as monoagent, or with U0126 or sorafenib at 5, 10, or 15 μM in combination with DMSO (white bars) or MCP110 at 10 μM (black bars). P values: * <0.05, **<0.001.
B. Assay design was as in A, except that colony forming potential was assayed. P values: * <0.05, **<0.001.
C. The same experimental design was used as in A, except that the concentrations of AACOCF3, staurosporine, and gemcitabine were as indicated.
D. IC50 values for inhibition of proliferation were determined for each cell line in reference to a DMSO control. #, no significant growth inhibition at > 60 μM.
For comparison, we used the more sensitive colony formation assay to analyze the combination of MCP110 with a set of small molecule agents with molecular targets not directly related to Ras transformation. These included staurosporine (a PKC inhibitor with additional off-target activities), gemcitabine (a DNA synthesis inhibitor), and AACOCF3 (a cPLA2 inhibitor; cPLA2 is a component of a side feedback regulation loop for the MAPK pathway (23)). IC50 values for compounds used as monagents were 37.0μM (AACOCF3), 1.4 nM (staurosporine), and 58.3 nM (gemcitabine). Staurosporine and gemcitabine exhibited neither synergistic nor additive effect with MCP110 (Figure 1A, 1C). AACOCF3 had a weak additive activity, although much less than was seen with sorafenib or U0126.
If MCP110 action is specifically related to its ability to inhibit the Ras-Raf interaction, MCP110 should be potent in cell lines transformed by Ras, but not transformed by oncogenes acting downstream of the Ras-Raf interaction. We analyzed MCP110 activity in NIH3T3 cells transformed by constitutively active H-Ras(G12V), K-Ras(G12V), and N-Ras(G12D). For comparison, we also examined activity of MCP compounds in cells transformed by Raf22W (16), a constitutively activated Raf-1 derivative truncated to lack Ras-interacting sequences, or by catalytically activated MEK1ΔN3/S222D (15); or in NIH3T3 cells containing expression vector (pBabe-puro). This analysis (Figure 1D) showed significantly greater potency of MCP110 in Ras-transformed versus Raf- or MEK1-transformed cell lines, and no activity of MCP110 in the vector-control cell line. This contrasted with sorafenib and U0126, which were active in all transformed cell lines.
Synergy of MCP110 with microtubule-targeting agents
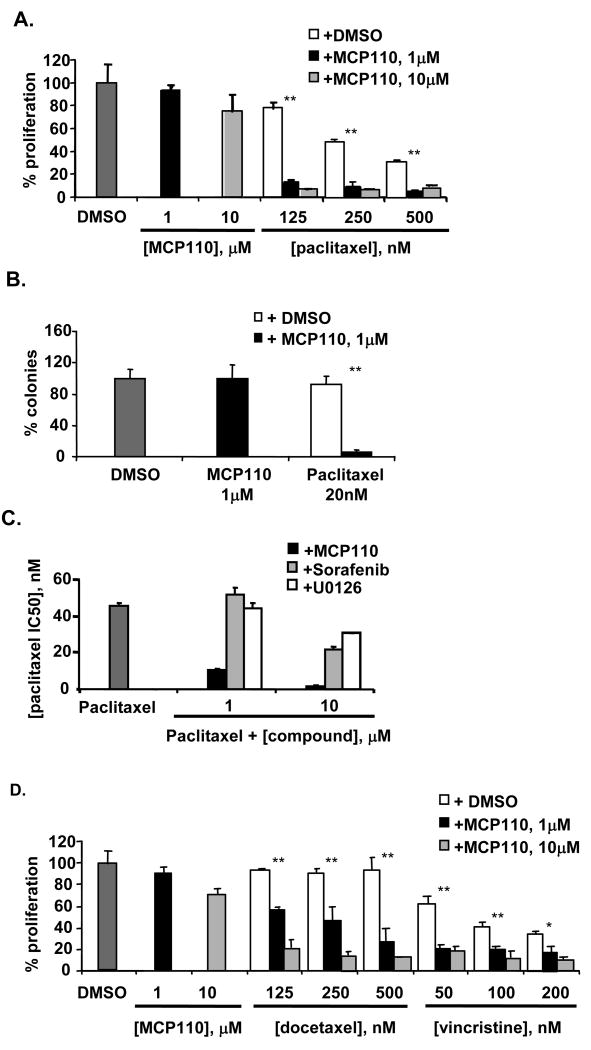
Paclitaxel is widely used in the treatment of lung, ovarian, and breast carcinomas (24). Although a primary mode of action of paclitaxel is as a cytotoxic agent that disrupts microtubule dynamics, paclitaxel also modulates Raf-1 activation, and paclitaxel efficacy has been defined as partially dependent on Raf-1 status (25-31). The paclitaxel IC50 for inhibition of proliferation in NIH3T3-K-Ras(G12V) cells is 930 nM for proliferation and 46 nM for soft agar colony formation. Combination of paclitaxel with low doses of MCP110 produced a very striking reduction of cell proliferation and colony formation (Figures 2A and 2B). In complementary analysis, we generated colony formation IC50 curves for paclitaxel in the presence of 1 or 10 μM MCP110, sorafenib, or U0126 (Figure 2C). Treatment with 1 μM of MCP110 reduced the paclitaxel IC50 from 46 to 10.7 nM; treatment with 10 μM MCP110 reduced the paclitaxel IC50 to 1-1.5 nM (Figure 2C): significantly greater synergy was observed between paclitaxel and MCP110 than with paclitaxel and sorafenib or U0126. MCP110 also strongly potentiated action of two additional microtubule-targeting agents, docetaxel and vincristine (Figure 2D), suggesting a broad utility in combination with this inhibitor class.
Figure 2. MCP1 and MCP110 compounds synergize with paclitaxel and other microtubule-targeting agents in inhibition of anchorage-dependent and -independent growth in NIH3T3-K-Ras(G12V) cells.
A. Proliferation assay was performed in Figure 1A, except using MCP110 and paclitaxel at the values indicated. P values: **<0.001.
B. A colony formation assay was performed as in Figure 1B, except using MCP110 and paclitaxel. P values: **<0.001.
C. Paclitaxel was administered to cells in combination with DMSO (dark gray bar) or MCP110 (black), sorafenib (light gray) and U0126 (white) at 1 or 10 μM, as indicated.
D. Proliferation assay was performed as in Figure 2A, except using docetaxel and vincristine at the indicated concentrations. IC50 values for docetaxel and vincristine used as monoagents were 303 nM and 270 nM, respectively. P values: * <0.05, **<0.001.
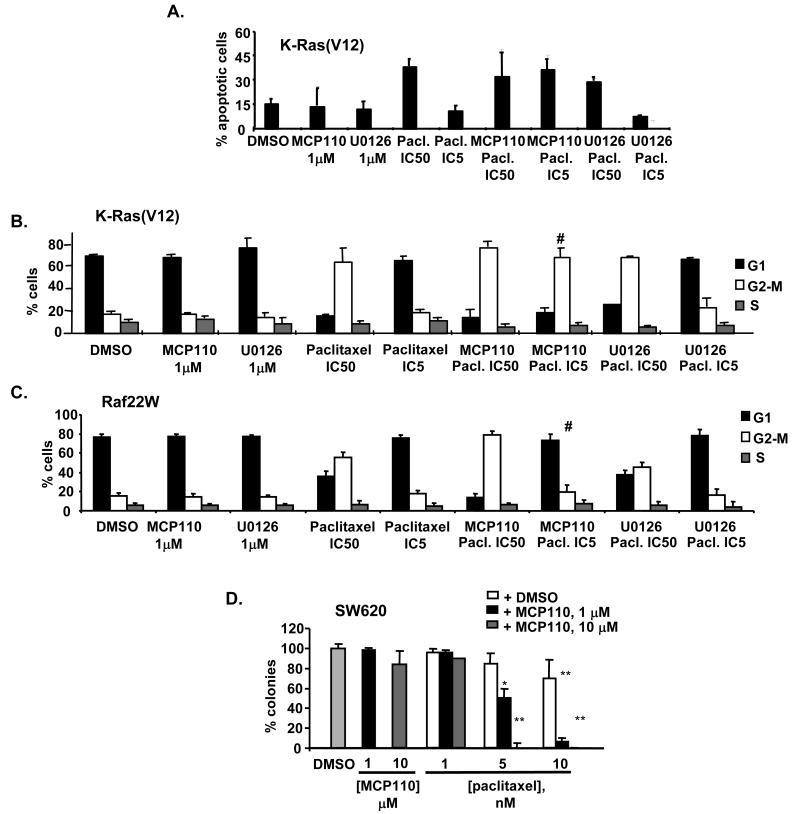
We have found that significantly more annexin V-positive, apoptotic cells were found following combined MCP110 and paclitaxel-treatment, than in cells treated with either agent alone (Figure 3A). Induction of cell death by microtubule-targeting agents is most potent when treated cells are in mitosis (32, 33). Using FACS analysis, we analyzed the cell cycle compartmentalization of NIH3T3-K-Ras(G12V) cells treated with MCP110, paclitaxel, or both (Figure 3B). For reference, we compared cell cycle compartmentalization of NIH3T3-K-Ras(G12V) cells treated with U0126 and paclitaxel (Figure 3B), or the NIH3T3-Raf22W cell lines in which MCP110 compounds were inactive with both (Figure 3C). Intriguingly, MCP110 administered in combination with paclitaxel significantly increased the percentage of cells in the G2/M compartment even though MCP110 used as monoagent did not affect cell cycle compartmentalization at the concentrations tested, and this effect was specific to the K-Ras(G12V)-transformed cell line. The combination of U0126 and paclitaxel did not have this effect in either cell line.
Figure 3. Selective action of MCP110 and paclitaxel in Ras versus Raf-transformed cells.
A. NIH3T3-K-Ras(G12V) cells were stained with antibody to annexin V and analyzed by FACS analysis. For combination experiments, MCP110 and U0126 at indicated concentrations were mixed with paclitaxel at predetermined IC50 and IC5 values for the cell line.
B. Cell cycle compartmentalization of NIH3T3-K-Ras(G12V) cells treated with compounds at the concentrations noted. # indicates G2 accumulation of MCP110/paclitaxel-treated cells.
C. Experiment as in B, except using NIH3T3-Raf22W cells. # indicates no G2 accumulation of MCP110/paclitaxel-treated cells.
D. Colony-forming potential of SW620 cells was determined in cells treated with DMSO (light gray), MCP110 at 1 or 10 μM delivered as a monoagent, or with paclitaxel at 1, 5, or 10 nM in combination with MCP110 at concentrations indicated. Values show percent reduction of colonies in compound-treated cells in reference to DMSO-treated cells. P values: * <0.05, **<0.001.
Sensitivity of human cancer cell lines to MCP compounds, paclitaxel, and MCP- paclitaxel combination
Requirements for Ras-dependent signaling in transformation differ between humans and mice, and between fibroblasts and epithelial cells (5, 34, 35). To determine whether MCP compounds are effective as mono- and combination agents in human cancers as well as in defined transformation models, we analyzed a number of human cancer cell lines with known activating mutations in Ras, including SW620, HCT116, MDA-MB231, NCI-460, and A549. For each of these cell lines, IC50 values for MCP110 ranged between 10-15 μM. We used the SW620 colorectal adenocarcinoma cell line, which contained an activating K-Ras(G12V) mutation, for further detailed studies.
Preparatory to in vivo analysis, we wished to determine in detail the level of synergy between MCP110, paclitaxel, sorafenib and U0126 compounds. SW620 cells were treated simultaneously with MCP110 and other drugs at a series of fixed ratios for 48 hours, in both proliferation (WST-1) or in colony formation in soft agar (SA) assays (Table 1). We also measured whether 1 and 10 μM of MCP110 sensitized SW620 cells to 1, 5 and 10 nM of paclitaxel in soft agar colony formation (Figure 3D), using the general approach used in Figures 1 and 2. All approaches clearly showed a strong synergistic effect of MCP110 and paclitaxel on growth of these cells in both assays in different concentration ratios, and synergistic or strong additive effects between MCP110 and sorafenib and U0126. Although the highest degree of synergy was observed between MCP110 and paclitaxel assessed in soft agar, a significant effect was also seen in the proliferation assay. Similar results were obtained in additional cell lines with activated Ras (e.g., A549 and NCI-460 cells; results not shown), supporting the idea that these compounds would combine well in primary human tumors.
Table 1. Synergy of MCP110 with U0126, sorafenib, and paclitaxel in SW620 human colon carcinoma cells.
Anchorage-dependent (WST-1) and –independent (soft agar, SA) growth assays were performed after treatment with compounds at the indicated constant ratios. The combination index (CI) was calculated for ED50, ED75 and ED90 (see Methods); average CI value for each combination is also shown. A CI of <0.3 indicates a strong synergistic effect, 0.3<CI<0.7 a synergistic effect, 0.7<CI<0.9 moderate synergy, 0.9<CI<1 additive effect and CI>1 antagonistic effect, respectively. Concentrations of compounds analyzed were selected based on discussion in (20, 45, 46).
| Combination | Ratio | Assay | CI(ED50) | CI(ED75) | CI(ED90) | Avgd. CI |
|---|---|---|---|---|---|---|
| MCP110-U0126 | 2.1/1 | WST-1 | 0.74 | 0.47 | 0.35 | 0.52 |
| MCP110-Sorafenib | 5.5/1 | WST-1 | 0.85 | 0.78 | 0.72 | 0.78 |
| MCP110-Paclitaxel | 2200/1 | WST-1 | 0.64 | 0.41 | 0.31 | 0.45 |
| MCP110-Paclitaxel | 1000/1 | WST-1 | 0.69 | 0.48 | 0.35 | 0.51 |
| MCP110-Paclitaxel | 1000/1 | SA | 0.39 | 0.30 | 0.24 | 0.31 |
| MCP110-Paclitaxel | 300/1 | SA | 0.28 | 0.22 | 0.18 | 0.23 |
Synergy of MCP compounds with sorafenib and paclitaxel in cell models for KSHV transformation
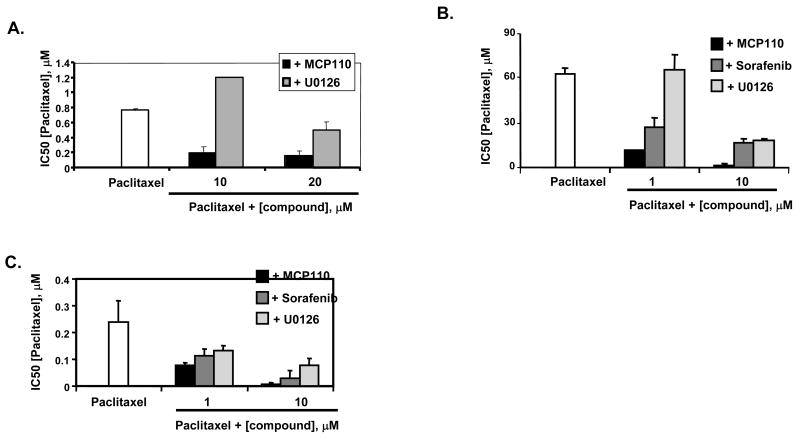
Oncogenic viruses act in part by activating pro-transformation cellular signaling pathways, with a number of such viruses inducing or dependent on Ras-pathway activation. KSHV transformation requires Ras-Raf-dependent signaling (36, 37), and paclitaxel is one of the few treatments currently approved for advanced stages of KSHV infection (38). We assessed whether MCP110 can block the cell transformation induced by the KSHV G-protein coupled receptor (vGPCR; ORF74), as the KSHV-vGPCR an important contributor to KSHV transforming potential (14, 39) and the activity of this protein requires Ras-dependent signaling (39, 40). We established IC50 for MCP110 and paclitaxel as single agents for proliferation (19.7 μM and 750 nM, respectively) and soft agar colony formation (13.8 μM and 65.5 nM, respectively) in NIH3T3-vGPCR cells (17). Figures 4A and 4B demonstrate that MCP110 markedly reduced the IC50 of paclitaxel in these cells. KSHV-induced cancers are marked by action of the vGPCR in promotion of vascular endothelial growth factor (VEGF)-driven angiogenesis: we also analyzed MCP110 action in the endothelial cell (EC)-vGPCR KSHV model (14). IC50 for MCP110 and paclitaxel in proliferation assays were 20.2 μM and 250 nM, respectively, in EC-vGPCR cells. Figure 4C shows similar potent action of MCP110 in reducing paclitaxel IC50 in this cell line. In these activities, MCP110 was more potent than sorafenib, and significantly more potent than U0126 used at similar concentrations (Figures 4A-4C).
Figure 4. MCP110 effectively synergizes with paclitaxel to inhibit anchorage-dependent and independent growth of two distinct KSHV-GPCR transformed cell lines.
A. Proliferation assays were performed in NIH3T3-KSHV cells treated with paclitaxel alone (white), or in combination with MCP110 (black) or U0126 (light gray) at concentrations indicated. IC50 values determined for U0126 as monoagent was 20 μM.
B. Colony formation assays were performed in NIH3T3-KSHV cells treated with paclitaxel alone (white), or in combination with MCP110 (black), sorafenib (dark gray), or U0126 (light gray). IC50 values determined for sorafenib and U0126 used as monoagents were 15 and 20 μM, respectively.
C. Paclitaxel IC50 values were determined in proliferation assays performed in EC-vGPCR cells incubated with paclitaxel alone (white) or in combination with MCP110 (black), sorafenib (dark gray) or U0126 (gray) at 1 and 10 μM. IC50 values determined for sorafenib and U0126 used as monoagents were 15 and 54 μM, respectively. Note, EC-vGPCR cells do not form colonies in soft agar, so anchorage-independent growth could not be investigated.
MCP compound bioavailability and maximum tolerated dose (MTD) assessment
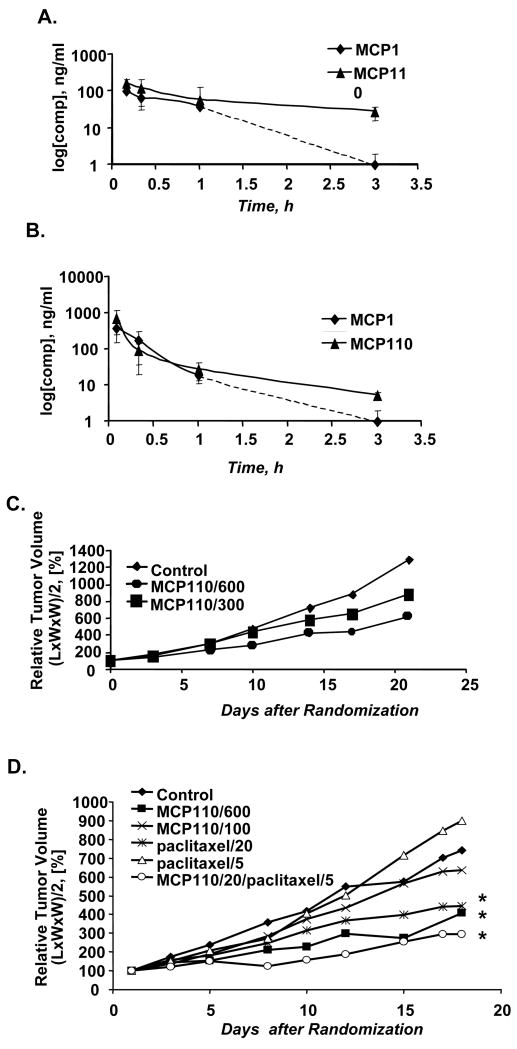
To support the clinical development of an MCP-based analog, we first established the bioavailability and MTD for the prototype lead compound MCP110, using the related compound MCP1 as a reference. MCP110 was well tolerated after oral, intravenous (i.v.) and intraperitoneal (i.p.) administration in nude mice, and i.v. and i.p. bioavailability values ranged from moderate to almost complete (64% to 100%) (Figure 5A, 5B). MCP110 persisted in the plasma at 16.6% of initially detected levels 3 hours following administration by i.p., and 0.72% of initially detected levels when it was administrated by i.v. By contrast, the plasma concentration of MCP1 dropped sharply by 1 hour following injection, and MCP1 was undetectable by 3 hours post-injection.
Figure 5. MCP110 is bioavailable in vivo, nontoxic, and synergizes with paclitaxel to inhibit tumor growth.
A.,B. The concentrations of MCP1 and MCP110 compounds in mouse plasma were determined after compound administration via (A) intraperitoneal (i.p.), or (B) intravenous (i.v.) routes.
C. LXFA 629 Nude mice bearing xenografts of LXFA 629 cells were randomized and treated via an i.p. route with vehicle control, or MCP110 at doses of 300 mg/kg or 600 mg/kg (see Methods). Relative tumor volume was calculated based on the formula: (L*W*W)/2, and taken as 100% at the day of randomization. Mean values of groups of 5-12 animals were plotted for each dose.
D. SCID mice with xenografts of SW620 cells were randomized and treated via an i.p. route with vehicle control, 100 mg/kg or 600 mg/kg of MCP110, 20 or 5 mg/kg of paclitaxel, or with a combination of MCP110 at 20 mg/kg and paclitaxel at 5 mg/kg. Relative tumor volume was calculated as in (C). Average values of groups of 5-12 animals were plotted for each dose. P values: * <0.05.
Acute dose toxicity and repeated dose toxicity were established. NMRI nu/nu mice received a single i.p. injection of MCP110 in vehicle at dose levels of 0, 600 or 1000 mg/kg, at a consistent dose volume of 10 ml/kg. Observation of mice for 14 days subsequent to injection indicated that mice tolerated a single i.p. dose of up to 1000 mg/kg of MCP110. Based on this finding, a 7-day repeat dose study using MCP110 at 0, 300, 600 and 1200 mg/kg/day in constant dose volumes was performed, followed by 16-days observation. Treatment-related changes in body weights were observed during the treatment period in mice that received 1200 and 600 mg/kg/day, but not 300 mg/kg/day. These changes were dose-dependent and resulted in an absence of body weight gain at 600 mg/kg/day, and weight loss at 1200 mg/kg/day. 3 of 4 males and 1 of 4 females treated with MCP110 at 1200 mg/kg/day survived until day 9 for scheduled sacrifice. Microscopically, there were no changes observed in the surviving female treated at 1200 mg/kg/day. The males treated at 1200 mg/kg/day presented minimal to slight changes in the kidney and testes. Shortly after cessation of treatment, all surviving animals for each dose used showed a normal body weight gain. Thus, the maximum tolerated repeat dose of MCP 110 was established between 600 and 1200 mg/kg/day and dose levels of 600 mg/kg/day or lower were used for future repeat dose studies with MCP 110.
MCP110 inhibits the growth of LXFA 629 lung adenocarcinoma xenografts in nude mice
MCP110 was administered i.p. to male NMRI nu/nu mice bearing an established xenograft of the lung adenocarcinoma cell line LXFA 629. A control group received vehicle only on days 0-20 (Group 1). Dose levels (in mg/kg/day) were 600 mg/kg on days 0-5 and 14-18 (Group 2); and 300 mg/kg on days 0-18 (Group 3). Tumor volumes were measured at 3-day intervals for up to 21 days. A reduction in the growth rate of LXFA 629 tumor xenografts relative to the growth rate in the vehicle control group was observed by day 17, with T/C values (ratio of median relative tumor volumes in test and control groups) of 49.3 and 73.8% for Groups 2 and 3, respectively (Figure 5C). A Mann-Whitney-Wilcoxon U-test demonstrated significant differences in the ranking of individual tumors according to size between groups 2 and 3, and between both MCP110-treatment groups and the vehicle control group. Other arguments in favor of significant compound-mediated tumor inhibition are (i) a continuous increase of T/C values during periods of treatment and (ii) dose dependent anti-tumor activity apparent from comparison of T/C values beginning by the first week of treatment.
MCP110 potently sensitizes SW620 human colorectal carcinoma cells to paclitaxel in vivo
To assess MCP110/paclitaxel synergy in vivo, we first analyzed the effect of in vivo treatment of SW620 xenografts with MCP110 or paclitaxel alone. SW620 cells were implanted into the right flank of male SCID mice. Palpable tumors of 5-14 mm diameter appeared after 2-3 weeks, after which animals were randomized to treatment groups, and tumor volume measured at 3-day intervals for up to 18 days to assess treatment efficacy (Figure 5D). Control group mice were injected i.p. with vehicle daily for 17 days starting from the time of animal randomization (“Control”). Group 2 (MCP110/600) received 600 mg/kg/day of MCP110 daily during 17 days of the study. Similarly, animals were treated with 300 mg/kg/day (Group 3, data not shown) and 100 mg/kg/day of MCP110 (Group 4, MCP110/100). In Groups 5 and 6, animals were treated with 20 mg/kg/day or 5 mg/kg/day of paclitaxel on days 1, 3, 5, 8, 10, 12, 15 and 17. The data indicated that 100 mg/kg/day of MCP110 and 5 mg/kg/day of paclitaxel did not significantly limit tumor growth (p >.5), while both 300 and 600 mg/kg/day MCP110 strongly limited tumor growth (p < 0.05). Paclitaxel at 20 mg/kg moderately reduced tumor growth, although not to the same extent as 600 mg/kg MCP100; animals dosed with this level of paclitaxel showed signs of distress, including descending colon (by autopsy). T/C values of 55, 68, 86, 60 and 121% were observed for Groups 2, 3, 4, 5, and 6, respectively.
Based on these results, we selected concentrations of 20 mg/kg of MCP110 and 5 mg/kg of paclitaxel to evaluate potential combination synergy. In Group 7, MCP110-20/ paclitaxel -5 (Figure 4D) MCP110 was administered daily for 17 days, with paclitaxel administered on days 1, 3, 5, 8, 10, 12, 15 and 17. Within 9 days, a striking reduction in the growth rate of SW620 tumor xenografts relative to the vehicle control group was observed, with T/C values of 40% at 18 days after initiation of dosing (Figure 5D). This synergistic effect clearly exceeded that seen with either drug used as monoagent at 4-5 fold higher concentrations (MCP110-20/paclitaxel-5 versus paclitaxel-20, P = 0.039).
Discussion
These results indicate that MCP110 demonstrate useful synergies with other agents vertically targeting the Ras-dependent MAPK signaling pathway, and with 3 microtubule targeting agents. These synergies were identified in defined mouse model cell lines for Ras and KSHV-GPCR transformation, and cell lines derived from human cancers containing activating Ras mutations (Figures 1-3). In NIH3T3-K-Ras(G12V) cells treated with MCP110, paclitaxel more effectively caused cells to enter G2/M and to undergo apoptosis (Figure 3). Although different cell line models yield differing results (e.g. (25, 26)), a number of studies have indicated that inhibition of the Raf/MAPK signaling pathway specifically increases the sensitivity of cells to taxol (41, 42) suggesting one mechanism for MCP110 is through its prevention of Ras-Raf interaction and hence inhibition of Ras/Raf/MEK/MAPK signaling. Data supporting this interpretation include 1) our previous biochemical analyses demonstrating MCP110 inhibition of this pathway (7, 8), 2) the fact that MCP110 does not synergize with pathway-irrelevant compounds such as staurosporine or gemcitabine (Figure 2), 3) the fact that MCP110 is selectively active in cells transformed with Ras, but not in cells transformed with Raf22W or MEK, or in untransformed cells (Figure 1D), and 4) the fact that G2/M accumulation is not seen in MCP110/paclitaxel treated Raf22W-transformed cells (Figure 3).
Our observation that MCP110 synergizes with paclitaxel in NIH3T3-vGPCR cells, while U0126 does not (Figure 3), suggests that the productive activity of MCP110 involves more than inhibition of the MEK/ERK signaling. Recent studies have suggested a specific importance of activation of the Raf kinase in anti-apoptotic signaling that extends beyond its ability to activate MEK/ERK (e.g., (43)). MCP110, acting higher in the Ras signaling pathway than MEK-targeted agents, may be particularly able to block such pro-tumorigenic functions. Because the MCP compound class was identified based on its activity as a protein interaction-inhibitor (7, 8), it is more likely to have a specific target than an active site-targeted kinase inhibitor. Nevertheless, our data do not rigorously exclude the possibility that MCP110 has additional “off-target” activities that contribute to its efficacy (as do many drugs, including the sorafenib analyzed here, (21)); this remains to be determined.
Importantly, MCP110 was bioavailable following i.p. or i.v. administration and was well-tolerated in vivo. MCP110 had a measurable anti-tumor activity in LXFA 629 xenografted mice, when administered at dose levels near the MTD, and treatment with MCP110 as a single agent also caused clear dose-dependent inhibition of tumor growth in SW620 (K-RasV12) SCID xenografts. The most striking result of the study was the drastic reduction in SW620 tumor volume achieved using a combination of MCP110 and paclitaxel, at concentration levels at which neither of the compounds produced significant tumor growth inhibition, indicating a clear synergistic response in vivo. The low dose of MCP110 required for synergy with paclitaxel in vivo (20 mg/kg) compares favorably with doses of sorafenib used in previous studies examining combination potential of this agent (40 mg/kg (44)): notably, sorafenib has been successfully developed as a clinical agent. In summary, the obtained data strongly imply that MCP compounds and their analogs are excellent targets for further development towards the clinic.
Acknowledgments
We are grateful to M. Hollingshead (NCI) for assessments of MCP compounds in the NCI 60 panel, and A. Lerro of the FCCC Laboratory Animal Facility for assistance with xenograft experiments. We acknowledge Y. Lu, S. Sakamuri and Q.-Z. Chen for synthesis of structural analogs of MCP1. We are grateful to S. Gutkind, N. Ahn, and D. Dadke for providing the EC-vGPCR, MEK1ΔN3/S222D, and NIH3T3-KSHV cell lines, respectively. We are grateful to S. Per and G. Hudes for support, advice and critical reading of the manuscript. This work was supported by the Ben Franklin Technology Partners of Pennsylvania, by RO1 CA63366 (to EAG), and by NIH core grant CA06927 and an appropriation from the Commonwealth of Pennsylvania (to Fox Chase Cancer Center).
Abbreviations
- EGF
epidermal growth factor
- GEF
guanine nucleotide exchange factor
- FACS
fluorescence-activated cell sorting
- GPCR
G-protein coupled receptor
- HPβCD
Hydroxypropyl-beta-cyclodextrin
- IC
inhibitory concentration
- i.p.
intraperitoneal
- i.v.
intravenous
- KSHV
Kaposi's sarcoma herpesvirus
- MAPK
mitogen-activated protein kinase
- MTD
maximum tolerated dose
- T/C
tumor/control
- VEGF
vascular endothelial growth factor
References
- 1.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 2.Janes PW, Daly RJ, deFazio A, Sutherland RL. Activation of the Ras signalling pathway in human breast cancer cells overexpressing erbB-2. Oncogene. 1994;9:3601–3608. [PubMed] [Google Scholar]
- 3.Campbell PM, Der CJ. Oncogenic Ras and its role in tumor cell invasion and metastasis. Semin Cancer Biol. 2004;14:105–114. doi: 10.1016/j.semcancer.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Cox AD, Der CJ. The dark side of Ras: regulation of apoptosis. Oncogene. 2003;22:8999–9006. doi: 10.1038/sj.onc.1207111. [DOI] [PubMed] [Google Scholar]
- 5.Hamad NM, Elconin JH, Karnoub AE, et al. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 2002;16:2045–2057. doi: 10.1101/gad.993902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolch W. Ras/Raf signalling and emerging pharmacotherapeutic targets. Expert Opin Pharmacother. 2002;3:709–718. doi: 10.1517/14656566.3.6.709. [DOI] [PubMed] [Google Scholar]
- 7.Kato-Stankiewicz J, Hakimi I, Zhi G, et al. Inhibitors of Ras/Raf-1 interaction identified by two-hybrid screening revert Ras-dependent transformation phenotypes in human cancer cells. Proc Natl Acad Sci U S A. 2002;99:14398–14403. doi: 10.1073/pnas.222222699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Y, Sakamuri S, Chen QZ, et al. Solution phase parallel synthesis and evaluation of MAPK inhibitory activities of close structural analogues of a Ras pathway modulator. Biorg Med Chem Letters. 2004;14:3957–3962. doi: 10.1016/j.bmcl.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 9.Ihle NT, Paine-Murrieta G, Berggren MI, et al. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Mol Cancer Ther. 2005;4:1349–1357. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards LA, Verreault M, Thiessen B, et al. Combined inhibition of the phosphatidylinositol 3-kinase/Akt and Ras/mitogen-activated protein kinase pathways results in synergistic effects in glioblastoma cells. Mol Cancer Ther. 2006;5:645–654. doi: 10.1158/1535-7163.MCT-05-0099. [DOI] [PubMed] [Google Scholar]
- 11.Ciardiello F, De Vita F, Orditura M, Comunale D, Galizia G. Cetuximab in the treatment of colorectal cancer. Future Oncol. 2005;1:173–181. doi: 10.1517/14796694.1.2.173. [DOI] [PubMed] [Google Scholar]
- 12.Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 13.Plosker GL, Keam SJ. Spotlight on Trastuzumab in the management of HER2-positive metastatic and early-stage breast cancer. BioDrugs. 2006;20:259–262. doi: 10.2165/00063030-200620040-00007. [DOI] [PubMed] [Google Scholar]
- 14.Montaner S, Sodhi A, Molinolo A, et al. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell. 2003;3:23–36. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- 15.Mansour SJ, Matten WT, Hermann AS, et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 16.Stanton VP, Jr, Nichols DW, Laudano AP, Cooper GM. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989;9:639–647. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dadke D, Fryer BH, Golemis EA, Field J. Activation of p21-activated kinase 1-nuclear factor kappaB signaling by Kaposi's sarcoma-associated herpes virus G protein-coupled receptor during cellular transformation. Cancer Res. 2003;63:8837–8847. [PubMed] [Google Scholar]
- 18.Lyons JF, Wilhelm S, Hibner B, Bollag G. Discovery of a novel Raf kinase inhibitor. Endocr Relat Cancer. 2001;8:219–225. doi: 10.1677/erc.0.0080219. [DOI] [PubMed] [Google Scholar]
- 19.Cox AD, Der CJ. Biological assays for cellular transformation. Methods Enzymol. 1994;238:277–294. doi: 10.1016/0076-6879(94)38026-0. [DOI] [PubMed] [Google Scholar]
- 20.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Gollob JA. Sorafenib: scientific rationales for single-agent and combination therapy in clear-cell renal cell carcinoma. Clin Genitourin Cancer. 2005;4:167–174. doi: 10.3816/CGC.2005.n.028. [DOI] [PubMed] [Google Scholar]
- 22.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhalla US, Ram PT, Iyengar R. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science. 2002;297:1018–1023. doi: 10.1126/science.1068873. [DOI] [PubMed] [Google Scholar]
- 24.Mekhail TM, Markman M. Paclitaxel in cancer therapy. Expert Opin Pharmacother. 2002;3:755–766. doi: 10.1517/14656566.3.6.755. [DOI] [PubMed] [Google Scholar]
- 25.Blagosklonny MV, Schulte T, Nguyen P, Trepel J, Neckers LM. Taxol-induced apoptosis and phosphorylation of Bcl-2 protein involves c-Raf-1 and represents a novel c-Raf-1 signal transduction pathway. Cancer Res. 1996;56:1851–1854. [PubMed] [Google Scholar]
- 26.Blagosklonny MV, Giannakakou P, el-Deiry WS, et al. Raf-1/bcl-2 phosphorylation: a step from microtubule damage to cell death. Cancer Res. 1997;57:130–135. [PubMed] [Google Scholar]
- 27.Blagosklonny MV. Sequential activation and inactivation of G2 checkpoints for selective killing of p53-deficient cells by microtubule-active drugs. Oncogene. 2002;21:6249–6254. doi: 10.1038/sj.onc.1205793. [DOI] [PubMed] [Google Scholar]
- 28.Britten RA, Perdue S, Opoku J, Craighead P. Paclitaxel is preferentially cytotoxic to human cervical tumor cells with low Raf-1 kinase activity: implications for paclitaxel-based chemoradiation regimens. Radiother Oncol. 1998;48:329–334. doi: 10.1016/s0167-8140(98)00084-x. [DOI] [PubMed] [Google Scholar]
- 29.Rasouli-Nia A, Liu D, Perdue S, Britten RA. High Raf-1 kinase activity protects human tumor cells against paclitaxel-induced cytotoxicity. Clin Cancer Res. 1998;4:1111–1116. [PubMed] [Google Scholar]
- 30.Britten RA, Perdue S, Eshpeter A, Merriam D. Raf-1 kinase activity predicts for paclitaxel resistance in TP53mut, but not TP53wt human ovarian cancer cells. Oncol Rep. 2000;7:821–825. doi: 10.3892/or.7.4.821. [DOI] [PubMed] [Google Scholar]
- 31.Lee M, Koh WS, Han SS. Down-regulation of Raf-1 kinase is associated with paclitaxel resistance in human breast cancer MCF-7/Adr cells. Cancer Lett. 2003;193:57–64. doi: 10.1016/s0304-3835(02)00722-x. [DOI] [PubMed] [Google Scholar]
- 32.Abal M, Andreu JM, Barasoain I. Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets. 2003;3:193–203. doi: 10.2174/1568009033481967. [DOI] [PubMed] [Google Scholar]
- 33.Ferlini C, Distefano M, Pignatelli F, et al. Antitumour activity of novel taxanes that act at the same time as cytotoxic agents and P-glycoprotein inhibitors. Br J Cancer. 2000;83:1762–1768. doi: 10.1054/bjoc.2000.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shields JM, Rogers-Graham K, Der CJ. Loss of transgelin in breast and colon tumors and in RIE-1 cells by Ras deregulation of gene expression through Raf-independent pathways. J Biol Chem. 2002;277:9790–9799. doi: 10.1074/jbc.M110086200. [DOI] [PubMed] [Google Scholar]
- 35.Pruitt K, Pestell RG, Der CJ. Ras inactivation of the retinoblastoma pathway by distinct mechanisms in NIH 3T3 fibroblast and RIE-1 epithelial cells. J Biol Chem. 2000;275:40916–40924. doi: 10.1074/jbc.M006682200. [DOI] [PubMed] [Google Scholar]
- 36.Hamden KE, Whitman AG, Ford PW, Shelton JG, McCubrey JA, Akula SM. Raf and VEGF: emerging therapeutic targets in Kaposi's sarcoma-associated herpesvirus infection and angiogenesis in hematopoietic and nonhematopoietic tumors. Leukemia. 2005;19:18–26. doi: 10.1038/sj.leu.2403532. [DOI] [PubMed] [Google Scholar]
- 37.Hamden KE, Ford PW, Whitman AG, et al. Raf-induced vascular endothelial growth factor augments Kaposi's sarcoma-associated herpesvirus infection. J Virol. 2004;78:13381–13390. doi: 10.1128/JVI.78.23.13381-13390.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tulpule A, Groopman J, Saville MW, et al. Multicenter trial of low-dose paclitaxel in patients with advanced AIDS-related Kaposi sarcoma. Cancer. 2002;95:147–154. doi: 10.1002/cncr.10634. [DOI] [PubMed] [Google Scholar]
- 39.Bais C, Santomasso B, Coso O, et al. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 40.Sodhi A, Montaner S, Patel V, et al. Akt plays a central role in sarcomagenesis induced by Kaposi's sarcoma herpesvirus-encoded G protein-coupled receptor. Proc Natl Acad Sci U S A. 2004;101:4821–4826. doi: 10.1073/pnas.0400835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jazirehi AR, Vega MI, Chatterjee D, Goodglick L, Bonavida B. Inhibition of the Raf-MEK1/2-ERK1/2 signaling pathway, Bcl-xL down-regulation, and chemosensitization of non-Hodgkin's lymphoma B cells by Rituximab. Cancer Res. 2004;64:7117–7126. doi: 10.1158/0008-5472.CAN-03-3500. [DOI] [PubMed] [Google Scholar]
- 42.Cheung HW, Ling MT, Tsao SW, Wong YC, Wang X. Id-1-induced Raf/MEK pathway activation is essential for its protective role against taxol-induced apoptosis in nasopharyngeal carcinoma cells. Carcinogenesis. 2004;25:881–887. doi: 10.1093/carcin/bgh087. [DOI] [PubMed] [Google Scholar]
- 43.O'Neill EE, Matallanas D, Kolch W. Mammalian sterile 20-like kinases in tumor suppression: an emerging pathway. Cancer Res. 2005;65:5485–5487. doi: 10.1158/0008-5472.CAN-05-1453. [DOI] [PubMed] [Google Scholar]
- 44.Carter CA, Chen C, Brink C, et al. Sorafenib is efficacious and tolerated in combination with cytotoxic or cytostatic agents in preclinical models of human non-small cell lung carcinoma. Cancer Chemother Pharmacol. 2006 doi: 10.1007/s00280-006-0257-y. [DOI] [PubMed] [Google Scholar]
- 45.Figul M, Soling A, Dong HJ, Chou TC, Rainov NG. Combined effects of temozolomide and the ribonucleotide reductase inhibitors didox and trimidox in malignant brain tumor cells. Cancer Chemother Pharmacol. 2003;52:41–46. doi: 10.1007/s00280-003-0611-2. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds CP, Maurer BJ. Evaluating response to antineoplastic drug combinations in tissue culture models. Methods Mol Med. 2005;110:173–183. doi: 10.1385/1-59259-869-2:173. [DOI] [PubMed] [Google Scholar]