Abstract
Background
The restoration of natural accommodation in the presbyopic and cataract affected eye is a subject of intense research effort. A new instrument has been developed to test the viability and efficacy of procedures and methods to restore accommodation ex vivo in animal or human eyes.
Methods
A section of the globe containing the crystalline lens, zonules, ciliary muscle and sclera is bonded into eight curved shoes. After dissecting the sclera between the shoes, even radial load is applied to stretch the zonules and capsular bag to simulate the natural accommodative process. The associated change in optical power is measured using a modified Scheiner’s disk method. Changes in the diameter of the lens and ciliary processes are recorded, as well as zonular load.
Results
No effective change in power was observed for the three presbyopic human eyes under four millimetres diameter stretching; the diameter of the ciliary aperture increased by between 1.8 mm and 2.3 mm, while the maximum increase in lens diameter was 0.19 mm. For the three younger monkey eyes, the diameter of the ciliary aperture increased by 1.4 mm with a corresponding increase in the lens diameter of between 0.50 mm and 0.65 mm. Stretching forces were generally higher for the human than for the monkey eyes, reaching maxima of 35 mN and 52 mN, respectively. The monkey eyes changed power by between 9.1 and 10.1 dioptres. An almost identical, progressive increase for lens diameter, power and stretching force versus stretch distance was found for all three monkey eyes.
Conclusion
The better understanding of the mechanisms and forces involved in the primate accommodative apparatus will assist with the development of accommodating IOLs and other methods to restore accommodation.
Keywords: ciliary muscle, crystalline lens, lens stretcher, simulation of accommodation
Visual accommodation is the mechanism by which the eye changes its optical power to focus on near or distant objects. In most mammalian species, a shift in optical power is achieved by changing the anterior and posterior curvatures of the crystalline lens. According to the Helmholtz theory of accommodation, the zonular tension eases as the ciliary muscle contracts. With less radial tension on the capsular bag, the crystalline lens takes on a more spherical shape, thereby increasing the optical power for focusing at near. This mechanism provides the eye with a continuously variable focusing system, which is controlled by a defocusing signal from the retinal image. This physio-optical system relies on the ability of the crystalline lens to deform elastically under zonular tension and to return to its more curved shape when zonular tension is relaxed. In the young human eye, the lens is both soft and elastic, which provides for an accommodative amplitude of more than 10 dioptres.1
Due to the continuous growth of epithelial cell layers on the inside of the capsular bag, the lens becomes denser and harder.2,3 This is the main cause for the gradual reduction in accommodative power. By the age of about 45 years, the lens has hardened so much that the lack of accommodation is sufficient to impair normal visual requirements for near work or reading, that is, the eye has become presbyopic. Although there has been scepticism about how well the other accommodative components retain their function with age, Glasser4 confirmed that at least in the non-human primate eye, the ciliary muscle and zonule apparatus are still intact. The electro-invasive method he used to stimulate accommodation shortcuts the natural visual neurological feedback mechanism but there is no evidence to suggest that this part of the accommodative stimulation ceases to function or could not be reactivated in the presbyopic eye.
Over the past two years, several methods have been proposed to reverse the effect of presbyopia. Several single and dual element accommodating intraocular lenses (A-IOL) are now commercially available.5,6 They are rigid or semi-rigid lenses, implanted inside the capsular bag with a haptic mechanism to provide axial movement as the ciliary muscle contraction changes the diameter of the bag. Axial motion of lens elements is restricted by the bag’s thickness and available space in the anterior chamber. Ho7 presented a theoretical analysis of the achievable amplitude of accommodation with these devices in 2004. While a single element A-IOL can provide no more than 2.00 dioptres of accommodation, a well designed dual element A-IOL can achieve 5.00 dioptres. None of the clinical investigations has been able to demonstrate a sustained accommodative range of more than about 1.00 dioptre with any of the A-IOLs.
A different approach to regain accommodation is taken by Ripken and colleagues.8 Using a femto second laser, they cut various patterns of discs, stars, cones, layers or cylinders into the crystalline lens with the aim of achieving relative movements of lens segments and an effective change in surface curvature. This attempt is still in the early ex vivo animal experimental stage and feasibility still remains to be verified.
Scleral expansion and pharmacological intervention have also been reported9,10 but results have been less than convincing.11
A more promising concept is the replacement of the hardened crystalline lens with a soft gel, which mimics the optical and mechanical properties of a young human lens. It is based on the extra-capsular cataract extraction procedure (ECCE), which has been used for many years to extract cataract lenses through a five to six millimetre diameter capsulorhexis and insertion of a foldable IOL. With the new method, a small approximately one millimetre capsulorhexis is made and closed with a valve. A liquid polymer is injected to refill the capsular bag to its natural size at the age of young adulthood. After refilling, the polymer gel is cured using visible or UV light to form a soft elastic lens.12
The optical and mechanical properties of the cured polymer are of critical importance for the success of this concept for restoring accommodation. To facilitate the material development, a method was developed to simulate the natural accommodative mechanism ex vivo. Based on the concept first presented by Parel and colleagues,13 this instrument is used to dissect the sclera into eight segments and measure pulling force, stretch distance, optical power and lens diameter simultaneously, while stretching the lens.13–17 The ex vivo accommodation simulator (EVAS) was designed to include all the above measurements, but the mechanical construction of the stretching mechanism compromises the achievable accuracy and resolution of this instrument. Only a single actuator and force transducer were used, requiring an elaborate assembly of pulleys and strings to achieve the radial pulling motion for the eight segments. This arrangement introduces significant friction, backlash and slack, making it difficult to obtain precise measurements. The uneven pulling can lead to decentration and optical distortion of the lens, further complicating power measurements.
With stretching forces in the mN range and radial movements of up to three millimetres, a more accurate instrument is required, which can provide precise measurement and control of the lens stretching process. The target for the optical power measurement is to cover a range of 10 to 60 dioptres with a resolution of 0.25 dioptres. The newly developed ex vivo accommodation simulator (EVAS II), incorporating a frictionless and backlash-free design, aims to achieve these objectives.
MATERIALS AND METHODS
Lens stretching
Our method of ex vivo simulation of accommodation uses eight shoes, which are bonded to the outside of a scleral ring, the section of which contains intact ciliary muscle, zonules and crystalline lens as well as the hyaloid membrane and anterior vitreous. The posterior section of the globe, as well as the cornea and the iris, are removed to allow free access to both the anterior and posterior sides of the lens for optical power measurements (Figure 1). The sclera is dissected between the shoes so that radial outward movement of the shoes is not constrained by circumferential tension in the sclera. The ciliary body is purposely left intact, as dissecting it between the shoes is likely to interfere with the intricate zonular meshwork that penetrates the ciliary muscle.18 For some eyes, some or all the zonular fibres are cut at the end of normal stretching experiments to obtain force readings from only the posterior zonules or the circumferential stretching force of the ciliary body.
Figure 1.
Dissected human eye, bonded to stretching shoes, shown in fully stretched position. 1: arms, 2: shoes, 3: sclera, dissected, 4: ciliary body, 5: zonules, 6: crystalline lens
The stretching forces are transmitted from the scleral segments via the ciliary body and muscle to the zonules and capsular bag. As the radial tension on the bag increases, the lens diameter expands and the anterior and posterior lens surfaces flatten, leading to a decrease in optical power. Stretching forces, stretching distance, change in lens diameter and optical power are recorded during the stretching and relaxation phases of the measurement.
A standard measurement cycle consists of eight 0.25 mm radial pull steps to a total of two millimetres radius increase, equivalent to a four millimetres increase in diameter. In between each pull step, there is a four-second pause, during which either power readings or images for diameter analysis are taken. Force readings are recorded continuously. The release cycle is similar to the pulling, with eight steps and four-second intervals.
As demonstrated previously,19 the use of eight segments to generate radial pulling motion is only an approximation of true radial pull for all zonules. The resulting error in force or elongation is less than two per cent and can be neglected. In theory, this segmentation will also lead to higher order optical aberrations in the stretched lens but the effect is not significant enough to affect the optical power measurements.
Instrumentation
The basic design concept of the ex vivo accommodation simulator was described in an earlier publication.19 The key components of the new instrument are eight identical linear motion stages with integrated force transducers (Figure 2). The base plate of each of these stages is mounted onto a motorised linear stage with five millimetres of travel (M110.1DG, Physik Instrumente, Germany). The frictionless force measurement is achieved by a pair of soft parallel leaf springs, which support the top plate and the scleral shoes. The leaf spring arrangement constrains the shoes to only one degree of linear motion. A strain gauge force transducer (FORT100, WPI, Sarasota FL) provides a stiff linkage between the linear stage and the shoe, detecting the push and pull forces, while also transferring the radial motion.
Figure 2.
One of eight stretching stages. 1: linear stage with DC servo motor, 2: force transducer, 3: leaf springs, 4: piano wire, 5: scleral shoe
The eight stages are arranged in a symmetric star pattern and connected to a computer. At the centre of the instrument, the lens power measurement assembly can be inserted. It consists of a 0.25 inch CCD camera (GP-CX261V, Panasonic, Japan) mounted on a motorised vertical stage with position feedback. In diameter measurement mode, a white LED is switched on to provide retro-illumination for the second CCD camera positioned above the centre of the instrument to capture images of the changing lens diameter. A diffuser plate has been added above the LED to enhance the contrast of the retro-illuminated diameter images.
Customised control software was developed to control the stretching motion and to acquire the signals from the force sensors, the optical power readings and to capture images for the lens diameter measurements.
Optical power calibration
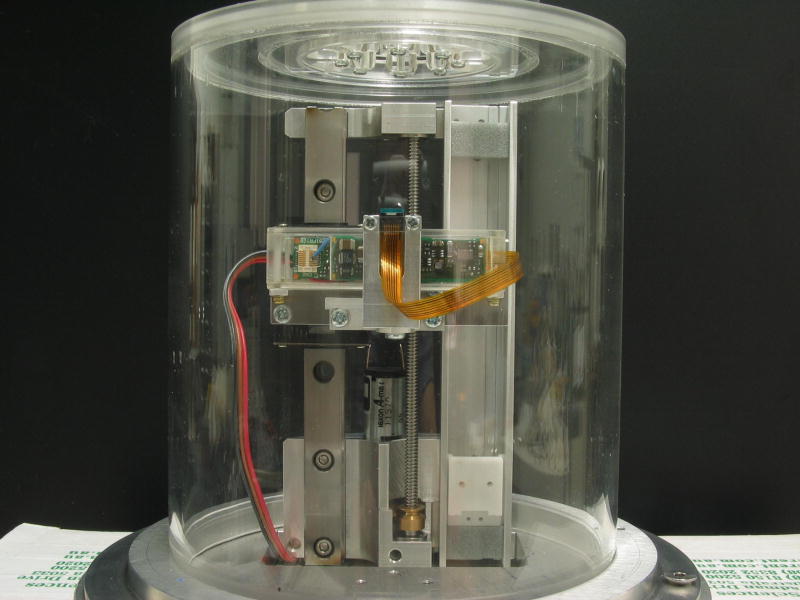
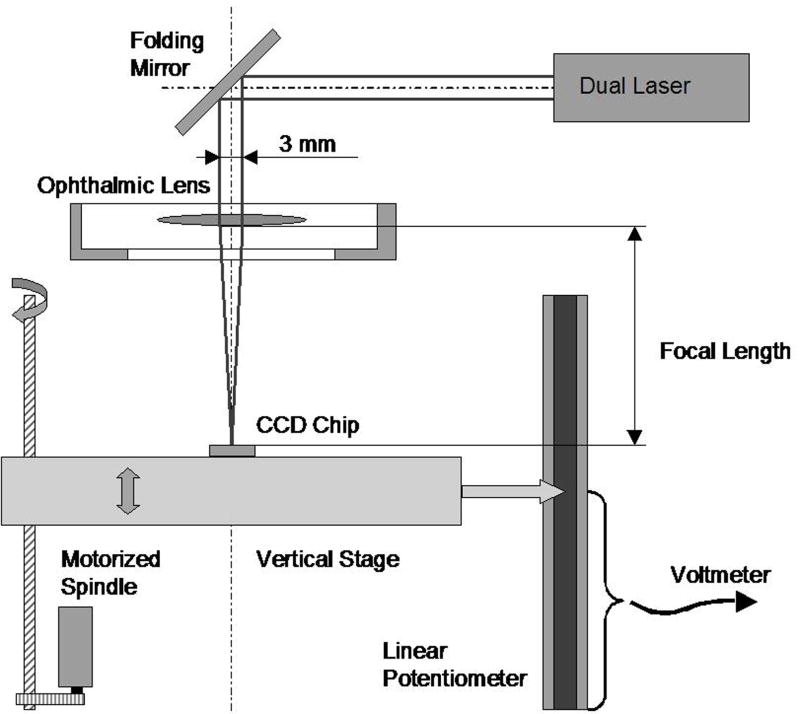
The optical power of the lens under examination is determined by measuring the focal distance of collimated paraxial light passing through the lens and saline solution.20 Two parallel red laser beams of less than one millimetre in diameter and separated by three millimetres are directed through the centre of the lens (Figures 3 and 4). A 0.25 inch CCD camera chip underneath the lens captures the refracted laser beams. The CCD chip is mounted on a motorised vertical stage with position feedback. By moving the CCD camera to the position where the two spots from the laser beams merge into one, the optical power of the lens can be determined. During the lens-stretching procedure, the CCD chip is continuously repositioned to follow the change in focal length. The position of the CCD chip is obtained as an analogue voltage from a linear potentiometer, ranging from zero to +5.00 V. The image from the two laser spots is displayed on a television monitor and observed by the operator who manually controls the positioning motor via a rotary dial. Position readings are either recorded continuously or on manual triggering. To provide the operator with information on the direction to move when out of focus, one of the laser beams is pulsed with a frequency of three Hertz.
Figure 3.
Optical power measurement unit. 1: CCD camera chip, 2: vertical slider, 3: camera electronics, 4: linear potentiometer, 5: motorised spindle
Figure 4.
Measurement of optical power
To calibrate the correlation between optical power and the CCD position, a reference table was created. As reference standards, 12 different plus powers (10 to 38 dioptres) of 0.5 inch ophthalmic trial set lenses were placed in the stretching chamber. For plus power above 20 dioptres, two lenses had to be stacked, as the range of standard trial set lenses extends only to +20 D. For each lens power, the operator adjusted the CCD position until the best focus position was achieved, as indicated by the merging of the pulsed and continuous laser spots. The calibration table was established and the linearity error obtained by plotting the CCD position versus the focal length of the trial set lenses (see Figure 5). The maximum error between the best fitted line and measured focal length was 4.0 per cent. The calibration table was used to convert CCD position readings into dioptric power. Readings between the table values were interpolated, or extrapolated for readings outside the table range.
Figure 5.
Calibration graph for slide position versus focal length. The linear fit equation for the trial lenses is used to convert slide position readings into focal length and dioptric power.
To verify this calibration data, the measurements were repeated using a selection of intraocular lenses ranging from 16.7 D to 40.3 D. There was no significant difference between the two datasets (Figure 5), both reaching R2 values for the linear line fit of at least 0.9978.
This calibration curve is valid only if the axial position of the posterior pole of the lenses is at the prescribed location. This is ensured by selecting the correct set of shoes to match the eyeball type and size.
Lens and ciliary aperture diameter measurements
Lens power and lens diameter cannot be measured simultaneously on EVAS II. When performing lens diameter measurements, the dual laser beam unit is removed and replaced with a CCD video camera fitted with a macro lens. The camera captures plane view still images of the crystalline lens just before and after each stretching motion. Illumination is provided by a white diffused LED, positioned underneath the lens. In a post-measurement data processing step, all the images are recalled and analysed. In a first step, the image magnification is determined to calculate the scale factor in pixels/mm. A disc of known diameter is placed in the object plane and the image captured. Using two cursors to mark the diameter on the calibration image, the distance in pixels is obtained and the scale factor calculated.
For the lens and ciliary aperture diameter measurements, two pairs of cross-hairs are overlaid on the lens images (Figure 6). The cross-hairs are moved manually by mouse or arrow keys until they touch the edge of lens and ciliary processes in horizontal and vertical directions. An ellipse, fitted into the prescribed rectangle, is displayed for lens and ciliary aperture to assist with the alignment of the cross-hairs. The mean of the vertical and horizontal cursor distances is calculated and recorded as lens and ciliary aperture diameters. All images of one measurement cycle are displayed sequentially for this manual analysis step. At the end of the data processing procedure, the software saves all the summarised results in a spreadsheet.
Figure 6.
Semi-automated image analysis to determine changes in lens and ciliary aperture diameter
To establish the reliability of this manual image analysis, a set of images from one stretching cycle was processed three times and the variability determined. In all, 17 images were analysed. The standard deviation was determined for the three repeats of lens and ciliary aperture diameters and all 17 standard deviations averaged to obtain an estimate of the repeatability. For the lens diameters, the average standard deviation was only 5 μm; for the ciliary aperture, 13 μm. The larger uncertainty for the latter was expected, as the wavy edge profile of the ciliary processes makes it more difficult to align the cross-hair consistently. With absolute changes in lens and ciliary aperture diameters of more than 0.6 mm and 2.1 mm respectively, the repeatability errors are negligible.
Lens specimen
For this study, three human eyes and three eyes from cynomolgus monkeys (Macaca fascicularis) were measured. The human cadaver eyes were obtained from the Lions Eye Bank of Lexington and the Northwest Lions Foundations for Sight and Hearing. The details are listed in Table 1.
Table 1.
Human eyes
| Specimen | Age | Male/Female | Left/Right |
|---|---|---|---|
| 0010-07 | 71 y | Female | RE |
| 07-0160-OS | 60 y | Male | LE |
| 07-0164-OS | 48 y | Female | LE |
All eyes were measured less than 48 hours post-mortem. For this preliminary study, no attempts were made to obtain pre-presbyopic eyes. Young donor eyes are almost always used for transplantation and using them for ex vivo experimentation could not be justified ethically at this stage.
The three monkey eyes were obtained via a University of Miami Tissue Sharing protocol (Table 2).
Table 2.
Monkey eyes
| Specimen | Age | Left/Right |
|---|---|---|
| 113-217 | 8 y 4 m | LE |
| 105-74 | 4 y 11 m | RE |
| 105-74 | 4 y 11 m | LE |
All eyes were measured less than 24 hours post-mortem. Onset of presbyopia for cynomolgus monkeys occurs at about 18 years of age.
Animal eyes were obtained post-mortem following approved institutional animal care guidelines and the study was performed in accordance with a University of Miami approved ACUC protocol. All human eyes were obtained and used in compliance with the guidelines of the Declaration of Helsinki for research involving the use of human tissue.
RESULTS
Because the optical power and the lens diameter measurements cannot be performed simultaneously, several stretch cycles are required to obtain both results as well as repeat data for statistical analysis of measurement variability. Both the power and diameter measurements were repeated three times, providing an estimate on instrument repeatability and more consistent mean values. Of the human eyes, two samples were too cloudy to obtain reliable power measurements.
The standard measurement procedure consisted of a two millimetre radial stretch (four millimetre diameter) divided into steps of 0.25 mm with a four seconds pause after each step. The release motion followed the same pattern.
Human lenses
FORCE
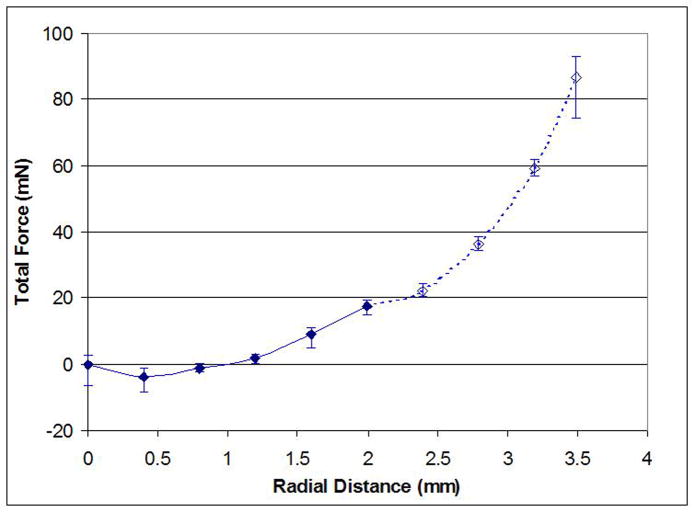
For all measurements, the stretching force was non-linear with respect to the stretched diameter. All measurements were started with the zonules relaxed and stretching forces close to zero. Initially, there is a very slow increase in force, steepening towards the two millimetre stretch distance and rising even further when stretching distances of up to 3.5 mm were attempted (Figure 7). Results are summarised as force difference between start and two millimetre stretch position and listed in Table 3. For the last sample 07-0164, all the zonules were cut after the last of the standard measurements and the measurement repeated without the lens. Only the circumferential tension of the ciliary body resists the radial stretching and this force is significantly lower than the radial force generated by the capsular elasticity and exerted by the zonules.
Figure 7.
Example of stretching force profile (07-0164-OS, human 48 years). The error bars indicate minimum and maximum force reading for each stretching step.
Table 3.
Stretching forces at 2 mm radial pull for human samples
| Sample | age years | zonules intact | zonules cut | zonules intact–zonules cut | ||
|---|---|---|---|---|---|---|
| AVG* mN | STDEV*mN | AVG* mN | STDEV*mN | AVG mN | ||
| 0010-07-01 OD | 71 | 52.0 | 3.4 | |||
| 07-0160 OS | 60 | 30.4 | 1.7 | |||
| 07-0164 OS | 48 | 41.3 | 0.9 | 8.8 | 1.5 | 32.5 |
Average of three repeat measurements
POWER
The increase in lens power for the only sample where power measurements were possible, a 48-year-old donor, is less than 0.2 dioptres (Figure 8). Statistically, this is not significant.
Figure 8.
Lens 07-0164, change in lens power. Error bars indicate minimum and maximum from three repeats.
DIAMETER
Similar to the lens power, the changes in lens diameter were very small and not statistically significant in these presbyopic eyes (Table 4).
Table 4.
Lens and ciliary aperture diameters before and after 2 mm stretch
| Diam. increase | @ 0 mm | @ 2 mm | Diam. increase | ||||
|---|---|---|---|---|---|---|---|
| AVG* mm | STDEV*mm | AVG mm | STDEV mm | Diam. AVG mm | increase STDEV mm | ||
| Lens | Age | ||||||
| 0010-07-01 OD | 71 | 9.11 | 0.22 | 9.11 | 0.21 | 0.00 | 0.07 |
| 07-0160 OS | 60 | 9.64 | 0.02 | 9.70 | 0.01 | 0.06 | 0.01 |
| 07-0164 OS | 48 | 8.85 | 0.06 | 9.04 | 0.02 | 0.19 | 0.05 |
| Ciliary aperture | |||||||
| 0010-07-01 OD | 71 | NA | NA | ||||
| 07-0160 OS | 60 | 10.10 | 0.18 | 11.88 | 0.26 | 1.79 | 0.09 |
| 07-0164 OS | 48 | 8.57 | 0.13 | 10.85 | 0.19 | 2.28 | 0.31 |
Average and standard deviation of three repeat measurements
The ciliary aperture diameter increased by about two millimetres on average for the two millimetre radial stretch. This indicates that about half of the applied radial displacement of the shoes transfers through to the ciliary processes. The compliance of the ciliary body and to a lesser extent the sclera and bonding of the shoes, account for the remainder of the stretch distance. The widening gap between the lens and ciliary processes increases the zonular tension.
Lens 0010-07-01 OD was mounted slightly decentred and the captured images did not show the complete ciliary aperture and no diameter measurements were possible.
Cynomolgus monkey lenses
FORCE
The stretching force diagram for the monkey lenses has a similar curve to that for the human lenses (Table 5). The force increases more rapidly towards the end of the two millimetre stretch distance. The maximum stretching forces are generally lower than for the human lenses, ranging between 29 and 35 mN. For two of the samples, the measurements were repeated with all of the anterior zonules cut. This reduced the maximum force at two millimetre stretch only by about 30 per cent. The measured stretching force is the total of all the radial zonular tension plus the circumferential stretch as exerted by the ciliary body.
Table 5.
Stretching forces at 2 mm radial pull for monkey samples
| Sample | age years | zonules intact | anterior zonules cut | zonules intact – ant. zonules cut | ||
|---|---|---|---|---|---|---|
| AVG* mN | STDEV*mN | AVG* mN | AVG* mN | AVG mN | ||
| 113-217 OS | 8 | 29.2 | 1.9 | |||
| 105-74 OD | 5 | 31.1 | 0.2 | 21.5 | 2.3 | 9.6 |
| 105-74 OS | 5 | 34.7 | 2.1 | 24.4 | 2.1 | 10.3 |
Average of three repeat measurements
POWER
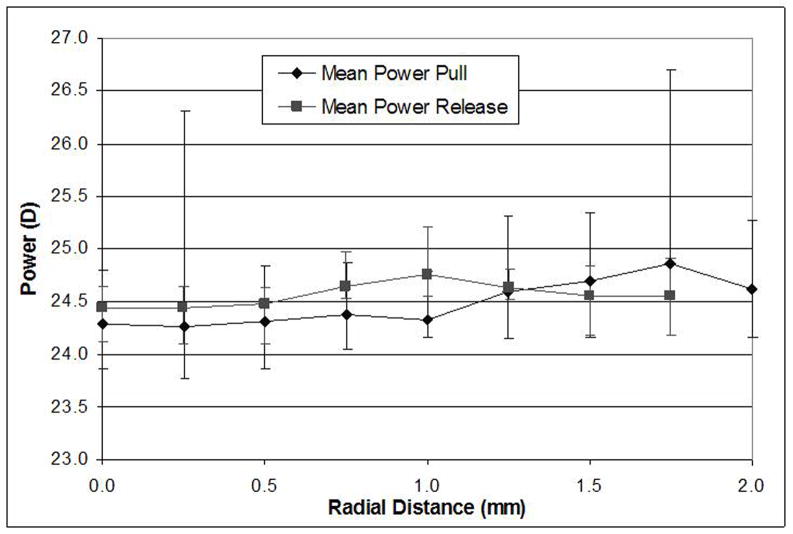
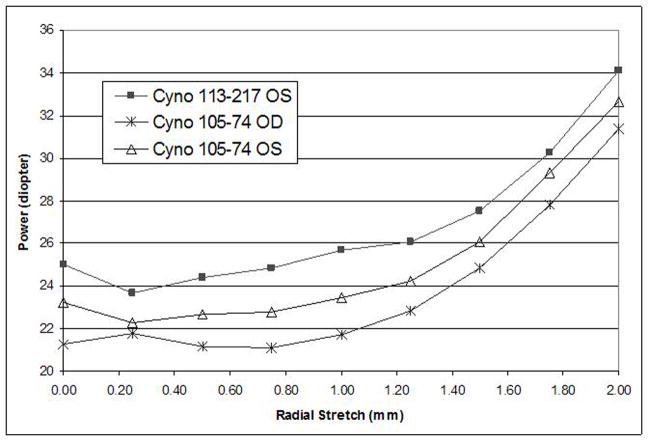
The initial, unstretched power of the monkey lenses was similar to that of the human lens samples, ranging from 21 to 25 dioptres. No significant change in lens power was observed for the first 0.5 mm stretch but then the power increased progressively to gain between 9.1 and 10.1 dioptres in power for the full two millimetre stretch (Figure 9). The standard deviation for the three repeat measurements is between 0.01 and 0.11 dioptres.
Figure 9.
Lens power versus radial stretch of monkey eyes
DIAMETER
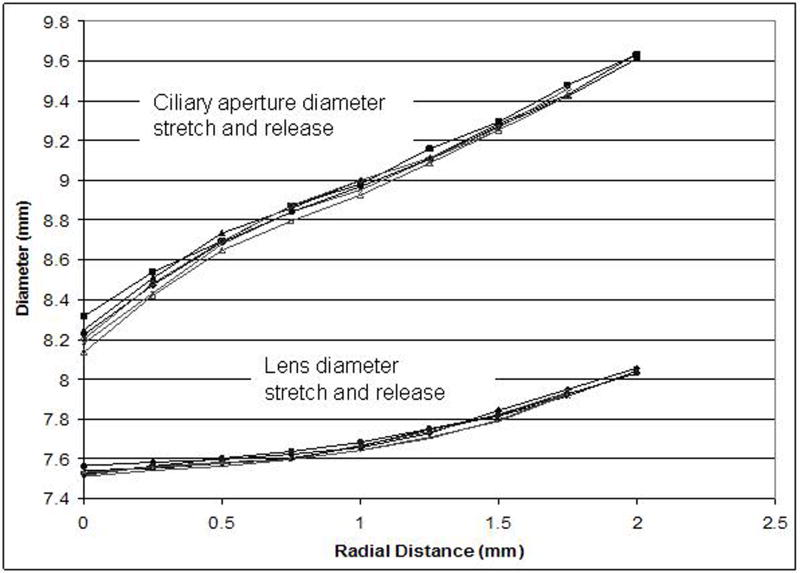
There was a measurable and consistent increase in lens diameter for all three monkey samples. For the two millimetre stretch, lens diameters increased by 0.50 mm to 0.65 mm (Table 6). Like the power increase, the increase in lens diameter was not linear in relation to the stretching movement but steepened for the larger radial displacements. Figure 10 shows a typical example of the correlation for both the lens and ciliary aperture diameters versus radial stretch. The graph also demonstrates the good repeatability and no measurable hysteresis between the pull and release cycles. The slope of the increase in ciliary aperture diameter is more linear and about twice as steep as for the lens diameter. This widens the gap between the lens and ciliary processes from which the lengthening of the zonular fibres can be estimated.
Table 6.
Lens and ciliary aperture diameters of monkey samples before and after 2 mm stretch
| @ 0 mm | @ 2 mm | Diam. increase | ||||
|---|---|---|---|---|---|---|
| AVG* mm | STDEV*mm | AVG * mm | STDEV*mm | AVG* mm | STDEV*mm | |
| Lens | ||||||
| 113-217 OS | 7.55 | 0.02 | 8.04 | 0.01 | 0.50 | 0.01 |
| 105-74 OD | 7.02 | 0.01 | 7.60 | 0.00 | 0.58 | 0.01 |
| 105-74 OS | 7.03 | 0.01 | 7.68 | 0.01 | 0.65 | 0.02 |
| Ciliary aperture | ||||||
| 113-217 OS | 8.26 | 0.05 | 9.63 | 0.01 | 1.36 | 0.04 |
| 105-74 OD | 7.61 | 0.01 | 8.81 | 0.01 | 1.20 | 0.02 |
| 105-74 OS | 7.48 | 0.04 | 8.86 | 0.02 | 1.39 | 0.02 |
Average and standard deviation of three repeat measurements
Figure 10.
Profiles of diameter change of lens and ciliary aperture versus radial stretch of typical monkey sample (113-217 OS), shown are three repeat measurements for stretch and release cycle
DISCUSSION
The simulation of the human accommodative apparatus has attracted numerous attempts to develop methods and instruments to investigate ex vivo the exact mechanisms involved in near and distance focusing.
Initially, the aim was to validate the Helmholtz theory of accommodation, followed by investigations into physiological changes due to presbyopia and more recently, efforts to restore near focusing ability to the presbyopic eye. The methods and instrumentation used varied greatly, most of them having significant deficiencies in the way they simulate accommodation or obtain results. Fisher21 was one of the first to build an accommodation simulator but he was unable to measure stretching forces. A simple lens spinning method to obtain qualitative data on lens deformation and deformation forces was used earlier by Fisher22 and more recently by Ripken.8 The isolated lens was placed on the end of a shaft and spun around its optical axis, while observing the change in cross-sectional shape. Sunderland and O’Neill23 had force sensors incorporated into their design but used only four stretching arms. A common problem with all the previous attempts to quantify stretching forces was the neglect of friction within the apparatus.13,23
The design objective for this second generation instrument was to increase force sensitivity by eliminating friction and to ensure precise control over the pulling motion. Two optical systems were added to alternatively measure changes in lens diameter and optical lens power. The Scheiner system was calibrated using a series of intraocular and ophthalmic trial lenses. With only two measurement points on the lens, no assessment of spherical, astigmatic or higher order aberrations is possible. While it is well known that the crystalline lens has significant spherical aberration,24 the measurement locations with three millimetre separation were chosen to closely match the average pupil size and effective optical power.
The ciliary muscle forms a ring, which contracts when activated to shorten its circumference and hence its diameter. Restoring forces to stretch the lens radially to its unaccommodated state are provided by elastic tissue attached between the ciliary muscle and the sclera. Our method for simulating accommodation is to move the scleral segments radially inwards and outwards. This introduces additional motion elements to the normal physiological accommodative mechanism. The preparation of the ciliary muscle and body prior to the stretching experiment can affect the measured stretching forces. To quantify the contribution of the circumferential force exerted by the ciliary body, measurements were repeated with all or part of the zonular fibres cut. The results indicate that around 30 per cent of the stretching force can be attributed to the circumferential ciliary body extension. This is an artefact of the method used to simulate accommodation but it still must be considered when drawing conclusions about the actual radial stretching forces on the lens capsule. In the live primate eye, the ciliary body is the muscle that activates accommodation. Being a circular muscle, it can only contract and reduce its diameter. In the relaxed state, elastic tissue connected to the sclera provides the tension to open the muscle and apply tension to the zonules for the unaccommodated situation.
The total pulling distance of 4.0 mm, which leads to a ciliary aperture diameter increase of between 1.2 mm and 2.3 mm, is unlikely to represent natural ciliary muscle action. Given the geometrical conditions and constraints inside the anterior of the globe, a maximum diameter change of 2.0 to 2.5 mm of the ciliary muscle under full accommodation seems realistic. Within that range, the correlations between pulling distance, lens power and lens diameter are almost linear and with very little hysteresis, indicating that no damage was done to the tissue in our simulator. Further support that stretching was done within physiological limits is that no drift in force or power results was measured over the six repeat measurements. Without muscle activation, the ciliary body tissue is fairly soft and compliant, changing its mechanical properties significantly more ex vivo than capsule, lens and zonules. To a lesser extent, zonular elongation and the bond between the shoes and the scleral tissue also contribute to the overall compliance.
To fully characterise the shape changes of the crystalline lens during accommodation, additional measurements of lens thickness, anterior and posterior surface curvatures as well as optical aberration profiles are required. Construction of a customised OCT system for thickness measurements has commenced. Once fully implemented, it will also provide data on axial motion of the lens, however, this data may not reflect actual lens movement under normal in vivo conditions, as the effects of vitreous and aqueous pressures are not included.
In recent years, several publications25,26 have described the use of finite element modelling for analysing and predicting the changes in shape, optical power and zonular tension during accommodation. Many of the input parameters for these models are not well quantified and the models’ predictions have never been validated. Being able to obtain a comprehensive set of experimental data for geometrical, optical and force changes will assist with the fine tuning of numerical models. As demonstrated previously,20 it is also possible to manufacture a simplified physical model of a crystalline lens with precisely characterised shape and optical and mechanical properties. The corresponding finite element model can be well-defined in terms of input parameters and should generate closely matched results compared to the experimental stretching.
Plotting the relative change of force, power and lens diameter versus stretching distance, there appears to be a close correlation in the shape of all three curves (Figure 11). All three steepen towards the end of the two millimetre stretch distance, with rather noisy data below 0.5 mm. This inconsistent data at the beginning suggests that the presumed starting position for full accommodation is not the normal physiological state of near accommodation. Some zonular tension would be required to keep the lens centred and aligned even when fully accommodated. To shift the starting position to 0.5 mm would also lead to a more plausible increase in diameter of three millimetres in the total stretch distance. Applying this correction brings the diameter and power values into good agreement with similar data published by Glasser, Wendt and Ostrin,27 made on live rhesus monkeys. These investigators found a mean lens diameter decrease of 7.04 per cent (0.50 to 0.67 mm), almost identical to the result from the three cynomolgus eyes of 6.2, 7.6 and 8.4 per cent decrease in diameter. Glasser, Wendt and Ostrin27 also evaluated the decrease in lens diameter in relation to dioptric change of accommodation. Again, there is good agreement between the average 0.060 mm/D for the rhesus monkey eyes and the values we found for cynomolgus eyes, between 0.055 and 0.069 mm/D.
Figure 11.
Correlation between lens diameter, stretching force and lens power (cyno. 105-74 OD)
From the presbyopic human eyes, no such relationship could be determined. Further experiments with younger human lenses are in preparation to establish force, diameter and power profiles. MRI measurements by Strenk and associates28 found an average lens diameter change for 20- to 40-year-old subjects of 0.5 mm for eight dioptres of accommodative stimulus.28 If these measurements can be confirmed in ex vivo experiments, the diameter/accommodation correlation would be very similar to the monkey results.
CONCLUSIONS
A new instrument is presented to investigate ex vivo the accommodative mechanism of the mammalian eye. Sensitivity and accuracy of the instrument were demonstrated to be sufficient to make reliable measurement of changes of lens diameter, lens power and stretching forces. The concept and method of ex vivo simulation of accommodation is viable for fresh postmortem primate eyes. The crystalline lens, the lens capsule and the zonules maintain their functional integrity. The stretching amplitude for the scleral segments needs to be significantly larger than normal physiological ciliary muscle contraction to compensate for the softened elasticity of the ciliary body and to achieve zonular tension similar to that of the normal range of accommodation. The loss of muscle tonus in cadaver eyes also adds to the difficulties in determining the starting position for the fully accommodated eye. The lag in lens diameter and power change at the beginning of the stretch cycles suggests that for this series of experiments, the eye was over-accommodated at the start position. Presbyopic human eyes showed no change in lens power or diameter when stretched. For the younger monkey eyes, physiologically normal accommodation was achieved for both, lens diameter and lens power in accordance with the Helmholtz theory of accommodation.
Axial lens motion during accommodation cannot be simulated adequately with this experimental method. The lens is isolated from the normal pressure dynamics of vitreous and aqueous and the axial motion of the ciliary body itself is also neglected. MRI and OCT measurements on life primate eyes have shown that under accommodation, the posterior pole of the lens moves slightly in a posterior direction, while the forward movement of the anterior lens surface accounts for most of the lens thickening. Drexler and colleagues29 and Vilupuru and Glasser30 reported a forward movement of the lens centre of 0.05 mm for the human and 0.15 mm for the rhesus monkey eye. These axial motions correspond to about 0.2 and 0.6 dioptres power change, respectively, and are of little significance in the context of this investigation.
Supplementary Material
Acknowledgments
We are grateful to Esdras Arrieta MD for surgical assistance, Dr Norma Kenyon and Dr Dora Berman-Weinberg of DRI, Dr Linda Waterman of DVR at the University of Miami Miller Medical School, and to Dr Rakhi Jain at AMO Inc for scientific support. We also thank David Borja, Noel Ziebarth, Derek Nankivil, Izuru Nose and William Lee of the Ophthalmic Biophysics Centre for their technical assistance with the experimental studies on tissues performed at the Bascom Palmer Eye Institute.
Some of the experimental work was carried out at the Ophthalmic Biophysics Center, Bascom Palmer Eye Institute, University of Miami, Miami, Florida.
GRANTS AND FINANCIAL SUPPORT
This work was supported in part by NIH Grant EY14225 and in part by the Vision Cooperative Research Centre, which is partly supported by the Australian Federal Government through the Cooperative Research Centres Program.
References
- 1.Duane A. Normal values of the accommodation at all ages . Tr Ophthalmol AMA. 1912:383. [Google Scholar]
- 2.Augusteyn RC. Growth of the human eye lens. Mol Vis. 2007;13:252–257. [PMC free article] [PubMed] [Google Scholar]
- 3.Heys KR, Cram SL, Truscott RJ. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol Vis. 2004;10:956–963. [PubMed] [Google Scholar]
- 4.Glasser A, Kaufmann P. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863–872. doi: 10.1016/S0161-6420(99)00502-3. [DOI] [PubMed] [Google Scholar]
- 5.McLeod S, Portney V, Ting A. A dual optic accommodating foldable intraocular lens. Br J Ophthalmol. 2003;87:1083–1085. doi: 10.1136/bjo.87.9.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuechle M, Nguyen N, Langenbucher A, Gusek-Schneider G, Seitz B. Zwei Jahre Erfahrung mit der akkommodativen Hinterkammerlinse 1 CU. Ophthalmologe. 2002;99:820–824. doi: 10.1007/s00347-002-0721-y. [DOI] [PubMed] [Google Scholar]
- 7.Ho A, Manns F, Pham T, Evans S, Parel J. Modelling the performance of accommodating intraocular lenses. In: Manns F, Soderberg P, Ho A, editors. Proceedings of SPIE: Ophthalmic Technologies XIV; San Jose, CA. Bellingham WA: SPIE Press; 2004. [Google Scholar]
- 8.Ripken T, Oberheide O, Ziltz C, Ertmer W, Gerten G, Lubatschowski H. Fs-laser induced elasticity changes to improve the presbyopic lens accommodation. In: Manns F, Soederberg P, Ho A, Stuck B, Belkin M, editors. Proceedings of SPIE: Ophthalmic Technologies XV; San Jose, CA. Bellingham, WA: SPIE Press; 2005. [Google Scholar]
- 9.Thornton SP. Anterior ciliary sclerotomy (ACS), a procedure to reverse the presbyopia. In: Sher N, editor. Surgery of Hyperopia and Presbyopia. Baltimore: Williams & Wilkins; 1997. pp. 33–36. [Google Scholar]
- 10.Schachar RA. Cause and treatment of presbyopia with a method for increasing the amplitude of accommodation. Ann Ophthalmol. 1992;24:445–447. [PubMed] [Google Scholar]
- 11.Mathews S. Scleral expansion surgery does not restore accommodation in human presbyopia. Ophthalmology. 1999;106:873–877. doi: 10.1016/S0161-6420(99)00503-5. [DOI] [PubMed] [Google Scholar]
- 12.Parel J-M, Holden B. Accommodating intraocular lenses and lens refilling to restore accommodation (restoring accommodation) In: Azar D, editor. Intraocular Lenses in Cataract and Refractive Surgery. Philadelphia PA: WB Saunders; 2001. pp. 313–324. [Google Scholar]
- 13.Parel J-M, Fernandez V, Billotte C, Denham DB, Lamar PD, Manns F, Milne P, Rosen A, Watling J, Ho A, Erickson P, Fantes F. Measurements of lens stretching forces during simulated accommodation in ex vivo primate eyes. XXIXth International Congress of Ophthalmology; Darling Harbour, Sydney. 2002. [Google Scholar]
- 14.Parel J-M, Manns F, Fernandez V, Billotte C, Denham D, Lamar P, Stoiber J, Orozco M, Ho A. Dioptric power vs zonular tension during ex vivo simulated accommodation of primate crystalline lenses before and after refiling. Invest Ophthalmol Vis Sci. 2003;44 E-Abstract 236. [Google Scholar]
- 15.Parel J-M, Fernandez V, Billotte C, Denham D, Lamar PD, Manns F, Ho A, Kenyon N, Collins BR, Erickson P. Accommodation stress-strain relation in human and non-human primate eyes ex vivo. Invest Ophthalmol Vis Sci. 2002;43 E-Abstract 406. [Google Scholar]
- 16.Denham D, Fernandez V, Billotte C, Rosen A, Lamar P, Manns F, Ho A, Erickson P, Parel J-M. Method for ex vivo assessment of accommodation forces. Invest Ophthalmol Vis Sci. 2002;43 E-Abstract 403. [Google Scholar]
- 17.Manns F, Parel J, Denham D, Billotte C, Ziebarth N, Borja D, Fernandez V, Aly M, Arrieta E, Ho A, Holden B. Optomechanical response of human and monkey Lenses in a lens stretcher. Invest Ophthalmol Vis Sci. 2007;48:3260–3268. doi: 10.1167/iovs.06-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernal A, Parel J-M, Manns F. Evidence for posterior zonular fiber attachment on the anterior hyaloid membrane. Invest Ophthalmol Vis Sci. 2006;47:4708–4713. doi: 10.1167/iovs.06-0441. [DOI] [PubMed] [Google Scholar]
- 19.Ehrmann K, Ho A, Parel J-M. Ex vivo accommodation simulator II: concept and preliminary results. In: Manns F, Soederberg P, Ho A, editors. Proceedings of SPIE: Ophthalmic Technologies XIV; San Jose, CA. Bellingham, WA: SPIE Press; 2004. [Google Scholar]
- 20.Ehrmann K, Ho A, Parel J-M. Evaluation of porcine crystalline lenses in comparison with molded polymer gel lenses with an improved ex vivo accommodation simulator. In: Manns F, Soederberg P, Ho A, Stuck B, Belkin M, editors. Proceedings of SPIE: Ophthalmic Technologies XV; San Jose, CA. Bellingham WA: SPIE Press; 2005. [Google Scholar]
- 21.Fisher R. The force of contraction of the human ciliary muscle during accommodation. J Physiol. 1977;270:51–74. doi: 10.1113/jphysiol.1977.sp011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher R. Elastic properties of the human lens. Exp Eye Res. 1971;11:143. doi: 10.1016/s0014-4835(71)80086-6. [DOI] [PubMed] [Google Scholar]
- 23.Sunderland H, O’Neill W. Functional dependence of optical parameters on circumferential forces in the cat lens. Vision Res. 1976;16:1151–1158. doi: 10.1016/0042-6989(76)90256-x. [DOI] [PubMed] [Google Scholar]
- 24.Smith G, Cox M, Calver R, Garner L. The spherical aberration of the crystalline lens of the human eye. Vision Res. 2001;41:235–243. doi: 10.1016/s0042-6989(00)00206-6. [DOI] [PubMed] [Google Scholar]
- 25.Weeber H, Martin H. Finite elements simulation of accommodation. In: Guthoff R, Ludwig K, editors. Current Aspects of Human Accommodation. Heidelberg: Kaden; 2001. pp. 135–144. [Google Scholar]
- 26.Burd H, Judge S, Flavell M. Mechanics of accommodation of the human eye. Vision Res. 1999;39:1591–1595. doi: 10.1016/s0042-6989(98)00298-3. [DOI] [PubMed] [Google Scholar]
- 27.Glasser A, Wendt M, Ostrin L. Accommodative changes in lens diameter in rhesus monkeys. Invest Ophthalmol Vis Sci. 2006;47:278–287. doi: 10.1167/iovs.05-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest Ophthalmol Vis Sci. 1999;40:1162–1169. [PubMed] [Google Scholar]
- 29.Drexler W, Baumgartner A, Findl O, Hitzenberger C, Fercher A. Biometric investigation of changes in the anterior eye segment during accommodation. Vision Res. 1997;37:2798–2800. doi: 10.1016/s0042-6989(97)00066-7. [DOI] [PubMed] [Google Scholar]
- 30.Vilupuru AS, Glasser A. The relationship between refractive and biometric changes during Edinger-Westphal stimulated accommodation in rhesus monkeys. Exp Eye Res. 2005;80:349–360. doi: 10.1016/j.exer.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.