Abstract
Type 2 diabetes is characterized by fasting hyperglycemia, secondary to hepatic insulin resistance and increased glucose production. Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a transcriptional coactivator that is thought to control adaptive responses to physiological stimuli. In liver, PGC-1α expression is induced by fasting, and this effect promotes gluconeogenesis. To examine whether PGC-1α is involved in the pathogenesis of hepatic insulin resistance, we generated transgenic (TG) mice with whole body overexpression of human PGC-1α and evaluated glucose homeostasis with a euglycemic-hyperinsulinemic clamp. PGC-1α was moderately (∼2-fold) overexpressed in liver, skeletal muscle, brain, and heart of TG mice. In liver, PGC-1α overexpression resulted in increased expression of hepatocyte nuclear factor-4α and the gluconeogenic enzymes phosphoenolpyruvate carboxykinase and glucose-6-phosphatase. PGC-1α overexpression caused hepatic insulin resistance, manifested by higher glucose production and diminished insulin suppression of gluconeogenesis. Paradoxically, PGC-1α overexpression improved muscle insulin sensitivity, as evidenced by elevated insulin-stimulated Akt phosphorylation and peripheral glucose disposal. Content of myoglobin and troponin I slow protein was increased in muscle of TG mice, indicating fiber-type switching. PGC-1α overexpression also led to lower reactive oxygen species production by mitochondria and reduced IKK/IκB signaling in muscle. Feeding a high-fat diet to TG mice eliminated the increased muscle insulin sensitivity. The dichotomous effect of PGC-1α overexpression in liver and muscle suggests that PGC-1α is a fuel gauge that couples energy demands (muscle) with the corresponding fuel supply (liver). Thus, under conditions of physiological stress (i.e., prolonged fast and exercise training), increased hepatic glucose production may help sustain glucose utilization in peripheral tissues.
Keywords: diabetes, phosphoenolpyruvate carboxykinase, glucose-6-phosphatase, gluconeogenesis
type 2 diabetes is characterized by insulin resistance, which becomes manifest in the early stages of the disease (7). The main tissues that become resistant to insulin are liver, skeletal muscle, and fat (38). Hepatic insulin resistance is of critical importance, since it is the primary disturbance responsible for fasting hyperglycemia (23). Liver insulin resistance results in increased flux through the glycolytic and gluconeogenic pathways (14), which normally are inhibited by insulin (4, 10, 11). The accelerated rate of hepatic glucose production (HGP) leads to a rise in fasting plasma glucose concentrations (8). Although a number of molecular/biochemical mechanisms have been suggested to account for the development of hepatic insulin resistance (3, 41), the primary defect remains undefined.
Peroxisome proliferator-activated receptor (PPAR)-γ coactivator (PGC)-1α is a transcriptional coactivator that coordinates the expression of genes that control diverse metabolic pathways in response to environmental and physiological changes (35). Under normal, ad libitum-fed conditions, PGC-1α expression is relatively low in liver compared with other tissues such as heart and brain, which rely mainly on aerobic metabolism for ATP production (26, 37). The gene expression of PGC-1α in liver increases with fasting (17, 48) and plays a central role in the regulation of gluconeogenesis (17, 22, 36, 48) by binding to and coactivating transcription factors, including hepatocyte nuclear factor (HNF)-4α, forkhead box O1A, and the glucocorticoid receptor, to coordinate the expression of rate-limiting gluconeogenic genes, such as phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) (36, 38).
PGC-1α expression in liver is upregulated in animal models of insulin resistance and diabetes (17, 45). Since PGC-1α promotes hepatic gluconeogenesis (36, 38), it has been postulated that PGC-1α may be responsible for the increases in HGP in individuals with type 2 diabetes (17, 35). Despite the large body of evidence from in vitro studies indicating that PGC-1α may play a role in the pathogenesis of insulin resistance in liver, it is not known whether increased PGC-1α signaling will cause hepatic insulin resistance in vivo. With the goal to understand the role of PGC-1α in gluconeogenesis and the development of hepatic insulin resistance, we generated transgenic (TG) mice moderately overexpressing PGC-1α and used the insulin-clamp technique to examine whole body glucose homeostasis in this model.
MATERIALS AND METHODS
Generation of PGC-1α TG mouse.
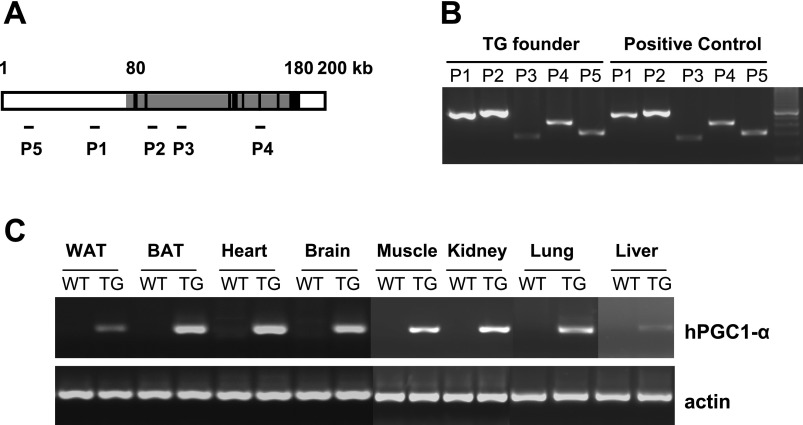
To generate TG mice that overexpress PGC-1α ubiquitously, we selected a bacterial artificial chromosome (BAC) clone (CTD2238L2) that contains endogenous human PGC-1α gene and its regulatory sequences. The human PGC-1α BAC clone has ∼100 kb of human PGC-1α gene sequence and ∼80 kb upstream and ∼20 kb downstream of regulatory sequences containing endogenous promoter and no other known genes in its range (Fig. 1A). The whole BAC clone DNA was amplified and then purified through cesium chloride. The purified PGC-1α BAC DNA was injected into the protonucleus of the fertilized eggs that were implanted into the uterus of pseudopregnant female mice. The founder PGC-1α TG mouse was generated on the C57Bl6-DBA mixed background. The mice used in this study were backcrossed to C57Bl6 two to four times. The genotype of the TG founders was determined using PCR to amplify PGC-1α BAC DNA (Fig. 1B), and expression of the human PGC-1α transgene was analyzed by RT-PCR specific for human PGC-1α mRNA (Fig. 1C).
Fig. 1.
A: structure of the human peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) gene (gray area) and its endogenous regulatory sequences in the bacterial artificial chromosome (CTD2238L2) utilized for generation of the transgenic (TG) animal. B: regions where PCR amplifications were carried out for identification of the human PGC-1α gene in the TG animal (P1–P5). C: identification of the human PGC-1α (hPGC-1α) transgene in the TG founders by PCR. RT-PCR demonstrates ubiquitous expression of hPGC-1α in white and brown adipose tissue (WAT and BAT), heart, brain, muscle, kidney, lung, and liver. WT, wild type.
Maintenance of mice.
Animals were housed in an animal room maintained at 23°C with a 12:12-h light-dark cycle and fed standard laboratory chow and water ad libitum. Separate groups of wild-type (WT) and TG mice were fed a high-fat diet (HFD; D12331, Research Diets; 57% fat-derived calories) for 20 wk. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
RT-PCR and real-time PCR.
Total RNA from tissues was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). Two micrograms of RNA were reverse transcribed to cDNA (Ambion, Austin, TX) in 20 μl of total reaction volume. For identification of human PGC-1α mRNA expression by PCR, 1 μl of cDNA was used. The amplification was performed on a thermal cycler (Applied Biosystems, Foster City, CA) at 95°C for 5 min and then at 95°C for 30 s and 58°C for 30 s followed by 72°C for 45 s for 35 cycles. The resulting products were visualized on agarose gel. For real-time PCR, after determination by serial dilution, optimal amounts of cDNA solutions were mixed with SYBR Green PCR Master Mix (Applied Biosystems) and specific primers and run on a real-time PCR system (ABI 7900) at 50°C for 2 min, 95°C for 10 min, and then 95°C for 15 s followed by 60°C for 1 min for 40 cycles. The results were normalized to the level of β-actin expression before comparison of the relative levels of expression of TG and WT mice.
The following primers were used: 5′-gagcgccgtgtgatttatgtc-3′ (forward) and 5′-cgctgtcccatgaggtattcg-3′ (reverse) for human PGC-1α transgene expression, 5′-cctgagagagactttggaggc-3′ (forward) and 5′-gaccgaagtgcttgttcagct-3′ (reverse) for total (mouse and human) PGC-1α, 5′-gcagttagctaaaatgcacagaaa-3′ (forward) and 5′-ctagcaacaaagcaacatgactct-3′ (reverse) for PEPCK, 5-gccagctgattaaagaaaaagaac-3′ and 5′-actgaaggccaacattacctactc-3′ (reverse) for HNF-4α, 5′-ggaggagggagagtgttttatgta-3′ (forward) and 5′-tagagaaggtggtgtgagagacag-3′ (reverse) for G6Pase, 5′-tttctagataggtttggcttttgg-3′ (forward) and 5′-acagaaatgctgctatgtcacagt-3′ (reverse) for medium-chain acyl-CoA dehydrogenase (MCAD), 5′-cgctcttaggactacttgctaacc-3′ (forward) and 5′-atggtatttacatgcaatggacag-3′ (reverse) for carnitine palmitoyltransferase (CPT)-1A (liver), 5′-aagtcatggtgggcaactaactat-3′ (forward) and 5′-tgtagtgttgaacatcctctccat-3′ (reverse) for CPT-1B (muscle), 5′-gatgttgaaaaaggcaagaagatt-3′ (forward) and 5′-cctttctcccttcttcttaattcc-3′ (reverse) for cytochrome c, 5′-catacaagcacaatagatgcacaa-3′ (forward) and 5′-gtagagaggggagagcaattatga-3′ (reverse) for cytochrome c oxidase subunit II, and 5′-gggatgagaaagttcagttgtacc-3′ (forward) and 5′-tctcacttcttccactcattcttg-3′ (reverse) for cytochrome c oxidase subunit IV.
Western blot analysis.
Total cellular proteins were extracted in RIPA buffer [50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% SDS] containing 1 mM PMSF and a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). The proteins were resolved on a 4–20% SDS-polyacrylamide gradient gel under denaturing conditions and then transferred to nitrocellulose membranes. Immunoblotting was performed using antibodies against the following proteins: PGC-1α and β-actin (Calbiochem, La Jolla, CA); histone, myoglobin, and troponin I (Santa Cruz Biotechnology, Santa Cruz, CA); GAPDH (Alamo Laboratories, San Antonio, TX); phospho-Akt-Ser476, Akt, IκBα, and phospho-p38 MAPK (Cell Signaling, Danvers, MA); phospho-JNK (Promega, Madison, WI); and GLUT4 and GLUT1 (Millipore, Billerica, MA). Specific signals for the target proteins were visualized using enhanced chemiluminescence (ECL) reagent (GE Healthcare, Piscataway, NJ). The blots were analyzed and quantified using ImageQuant software.
Plasma chemistry.
Tail blood was collected for plasma chemistry. A OneTouch Ultra blood glucose meter (LifeScan, Milpitas, CA) was used to measure glucose in 3- to 5-mo-old mice fasted for 18 h. Insulin levels were determined using a radioimmunoassay kit (Sigma-Aldrich, St. Louis, MO), and serum free fatty acids (FFAs) were determined using an NEFA assay kit (Wako Chemicals, Richmond, VA).
Euglycemic-hyperinsulinemic clamp.
The euglycemic insulin clamp was performed in awake, unrestrained, chronically catheterized mice in combination with [3-3H]glucose infusion, as previously described (47). Female mice (24 wk average age) were fasted for 6 h before the insulin-clamp studies. Briefly, a prime-continuous infusion of regular insulin was administered at 18 mU·kg−1·min−1, and a variable infusion of a 10% glucose solution was started at time 0 and periodically adjusted to clamp the plasma glucose concentration at the fasting level. The insulin-clamp study included a 60-min baseline period for the assessment of basal glucose turnover rate and a 90-min euglycemic clamp period. Under steady-state conditions of fasting euglycemia, the rate of total body glucose disappearance equals the rate of total body glucose appearance and was calculated by dividing the infusion rate of [3-3H]glucose [disintegrations per minute (dpm)] by the steady-state plateau of [3-3H]glucose specific activity (dpm/mg). HGP was calculated by subtracting the exogenous glucose infusion rate from the rate of total body glucose appearance (47).
Glucose tolerance tests.
A OneTouch Ultra blood glucose meter was used to measure tail blood glucose levels before and after (15, 30, 60, and 120 min) intraperitoneal administration of glucose (1.5 mg/g body wt) in 3- to 5-mo-old fasted female mice.
Diacylglycerol and ceramide content.
Concentrations of diacylglycerol (DAG) and ceramide in mixed quadriceps muscle were measured by thin-layer chromatography as previously described (12).
Mitochondrial isolation and H2O2 production.
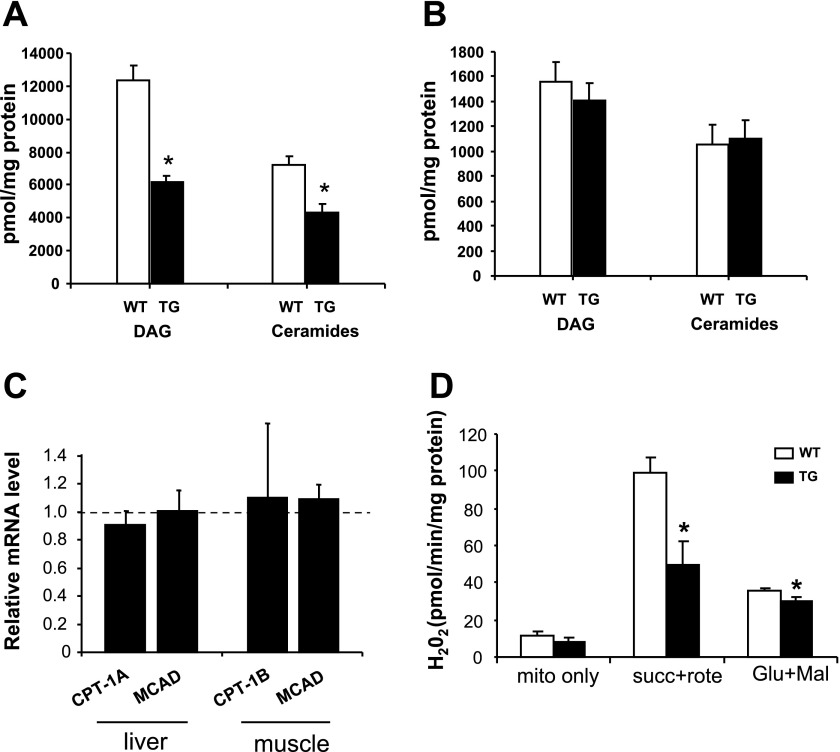
Mitochondria were isolated from skeletal muscle as described by Makinen and Lee (28). Mice were killed, and the skeletal muscles were rapidly excised and transferred to an isolation buffer [100 mM KCl, 50 mM Tris·HCl, 5 mM MgCl2, 1 mM EDTA, and 1 mM ATP (pH 7.4)]. Muscles were minced with scissors and transferred to 20 volumes of isolation buffer containing protease (0.15 mg/ml nagarse; Sigma, St. Louis, MO) for 5 min. The muscles were then homogenized in a Dounce glass homogenizer using 10–15 strokes, and the resulting homogenate was centrifuged at 600 g for 10 min. The supernatants were filtered through cheesecloth (Bellco Class, Vineland, NJ) and centrifuged at 14,000 g for 10 min. The resulting pellets were treated with washing buffer [100 mM KCl, 50 mM Tris·HCl, 1 mM MgCl2, 0.2 mM EDTA, and 0.2 mM ATP (pH 7.4)] supplemented with 0.5% BSA and centrifuged at 7,000 g for 10 min. The mitochondrial pellets were washed in washing buffer without BSA, centrifuged at 3,500 g for 10 min, and then resuspended in a buffer containing 100 mM KCl and 50 mM MOPS (pH 7.44). The mitochondrial protein concentration was determined using the Bradford protein assay reagent (Bio-Rad, Richmond, CA).
Release of H2O2 from mitochondria was utilized as a measure of reactive oxygen species (ROS) production. Mitochondrial H2O2 release was measured using Amplex Red (N-acetyl-3,7-dihydroxyphenoxazine)-horseradish peroxidase (Molecular Probes, Eugene, OR) as previously described (29). Briefly, 80 μM Amplex Red reagent and 1 U/ml horseradish peroxidase were added to the mitochondria (40 μg protein) or to the H2O2 standard solution in 100 μl of reaction buffer [50 mM MOPS and 100 mM KCl (pH 7.44)] and incubated in Falcon 96-well microplates in the dark at 30°C. Fluorescence was followed at excitation wavelength of 530 nm and emission wavelength of 590 nm for 10 min in an automatic microplate reader (Labsystems, Helsinki, Finland). The rates of H2O2 production were determined in the absence (state 1) or presence of the respiratory substrates glutamate plus malate or succinate. In some experiments, specific inhibitors for complex I (rotenone) were used in combination with respiratory substrates to block electron transport to maximally stimulate H2O2 production.
Statistical analysis.
Values are means ± SE. Data between groups were compared using unpaired Student's t-test or one-way ANOVA, as appropriate. P < 0.05 was considered statistically significant. Statistical analysis was performed using SigmaStat software.
RESULTS
Increased PGC-1α expression in TG mice.
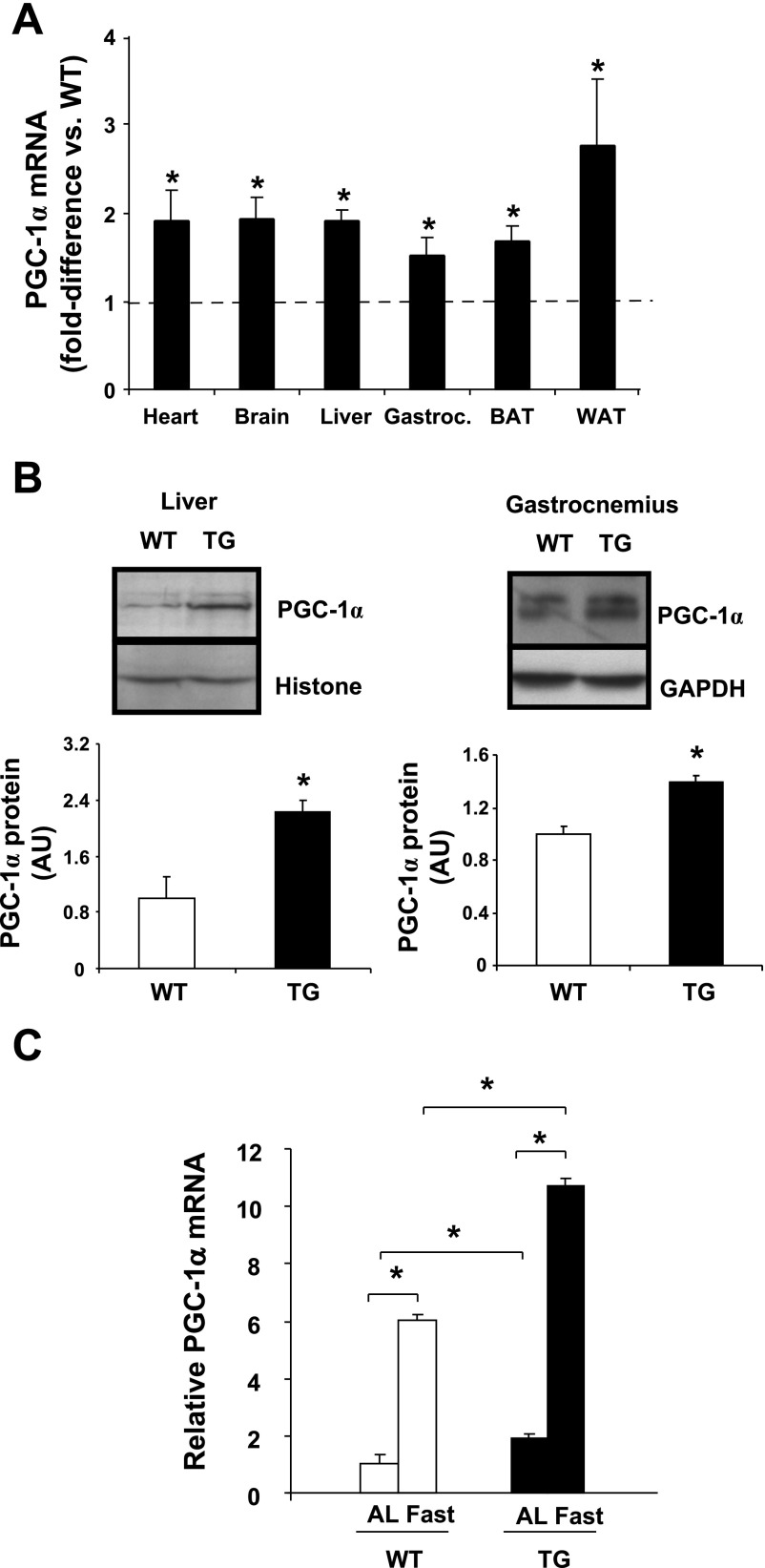
PGC-1α gene expression was measured in various tissues from ad libitum-fed mice by RT-PCR. Depending on the tissue, PGC-1α gene expression was ∼1.5- to 2.5-fold higher in TG than WT animals (Fig. 2A). An experimental goal was to achieve a PGC-1α expression level well below (≥10-fold) the levels that have been reported to produce cardiomyopathy and skeletal muscle cell necrosis (24, 32). Increases in PGC-1α expression also were reflected at the protein level in liver and muscle (Fig. 2B). As expected, fasting for 18 h led to elevated expression of PGC-1α in liver, and this effect was accentuated in TG mice (Fig. 2C).
Fig. 2.
A: PGC-1α mRNA expression in heart, brain, liver, gastrocnemius (Gastroc), BAT, and WAT from TG and WT mice. B: Western blot analysis of PGC-1α protein in liver and muscle from WT and TG animals. AU, arbitrary units. C: PGC-1α mRNA expression in liver after 18 h of fasting in 2- to 4-mo-old male and female animals. AL, ad libitum. Values are means ± SE in 4–6 mice per group. *P < 0.05 vs. WT.
General characteristics of TG mice fed standard chow.
The TG mice were grossly normal, and no premature death was observed for up to 1 yr (data not shown). There was no difference in total body weight between 4-mo-old female TG and WT mice: 19.23 ± 0.48 and 18.84 ± 0.39 g, respectively. Similarly, the weights of the heart, brain, liver, adipose tissue (white and brown), and muscle (mixed gastrocnemius, soleus, and tibialis anterior) were similar in both groups (not shown). Food consumption was not affected in TG mice: average consumption was 3.45 ± 0.10 and 3.20 ± 0.26 g·day−1·mouse−1 in 4-mo-old female TG and WT animals, respectively. There was no difference in fasting plasma glucose (66.56 ± 2.11 and 62.13 ± 3.66 mg/dl for TG and WT, respectively), insulin (0.30 ± 0.04 and 0.28 ± 0.04 ng/ml for TG and WT, respectively), or FFA (1.65 ± 0.15 and 1.46 ± 0.10 mmol/l for TG and WT, respectively) concentrations between groups.
Effect of PGC-1α overexpression on insulin sensitivity.
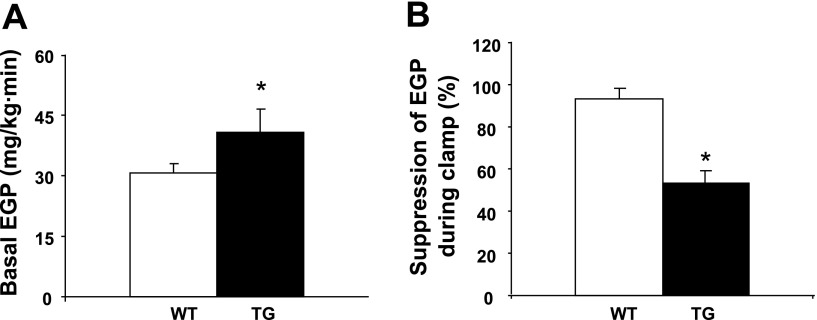
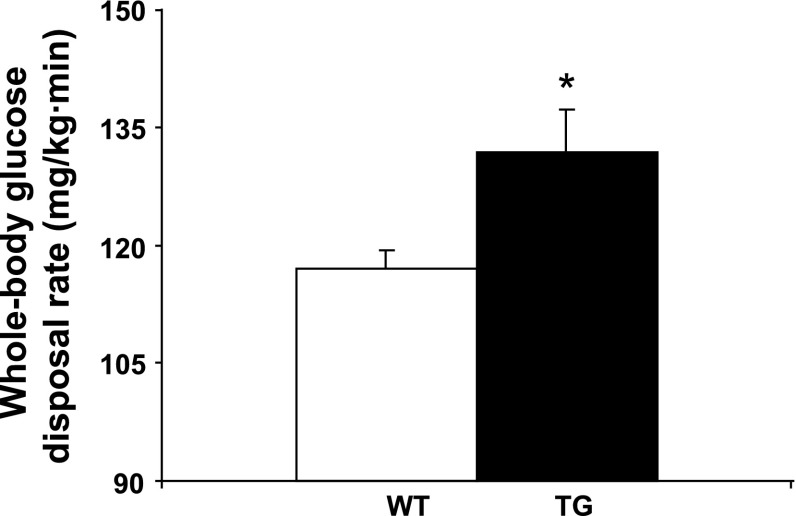
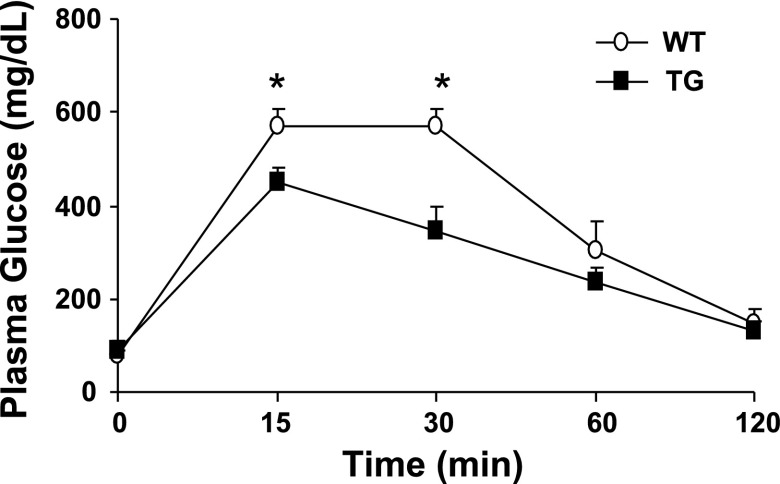
TG mice fed the standard chow diet displayed hepatic insulin resistance manifested by increased rates of basal HGP (Fig. 3A) and reduced suppression of glucose production by the liver in response to insulin (Fig. 3B). Paradoxically, PGC-1α overexpression resulted in enhanced muscle insulin sensitivity, as evidenced by an increase in insulin-stimulated peripheral (primarily muscle) glucose disposal during the clamp (Fig. 4) and improved glucose tolerance during the glucose tolerance test (Fig. 5).
Fig. 3.
A: increased basal endogenous (primarily hepatic) glucose production (EGP) in 6-mo-old female TG mice. B: impaired suppression of hepatic glucose production during the clamp in 6-mo-old female TG mice. Values are means ± SE in 5 TG and 7 WT mice. *P < 0.05 vs. WT.
Fig. 4.
Whole body glucose disposal is increased in 6-mo-old female TG mice. Values are means ± SE in 5 TG and 7 WT mice. *P < 0.05 vs. WT.
Fig. 5.
Glucose tolerance was improved after intraperitoneal injection of glucose in 6-mo-old female TG mice. Values are means ± SE in 8 mice per group. *P < 0.05 vs. WT.
Expression of hepatic gluconeogenic enzymes and mitochondrial genes.
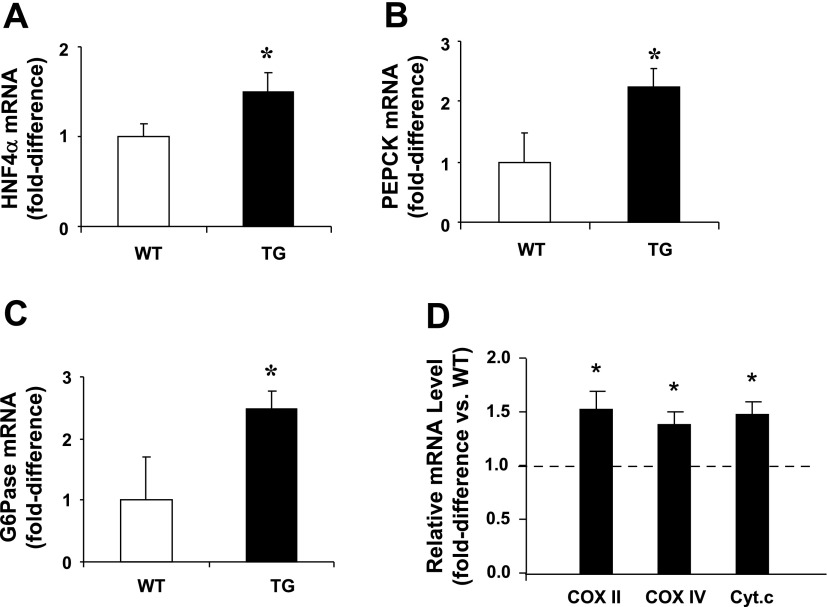
To determine the molecular basis for the increased HGP observed in TG mice, we measured the gene expression of HNF-4α, which controls the expression of gluconeogenic enzymes such as PEPCK and G6Pase. PGC-1α overexpression led to increased HNF-4α gene expression (Fig. 6A), which was accompanied by an elevation in the expression of PEPCK (Fig. 6B) and G6Pase (Fig. 6C). In addition, PGC-1α overexpression was accompanied by elevated expression of mitochondrial proteins involved in oxidative phosphorylation, such as cytochrome c oxidase subunits II and IV and cytochrome c in liver (Fig. 6D).
Fig. 6.
A–C: increased gene expression of hepatic nuclear receptor (HNF)-4α and the gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) in liver of 4- to 6-mo-old male and female ad libitum-fed PGC-1α TG mice. D: higher levels of mRNA for proteins involved in oxidative phosphorylation, such as cyclooxygenase (COX) subunits II and IV and cytochrome c (Cyt.c), in liver of 4- to 6-mo-old male and female ad libitum-fed PGC-1α TG mice. Values are means ± SE in 6–9 mice per group. *P < 0.05 vs. WT.
Effects of PGC-1α overexpression in muscle.
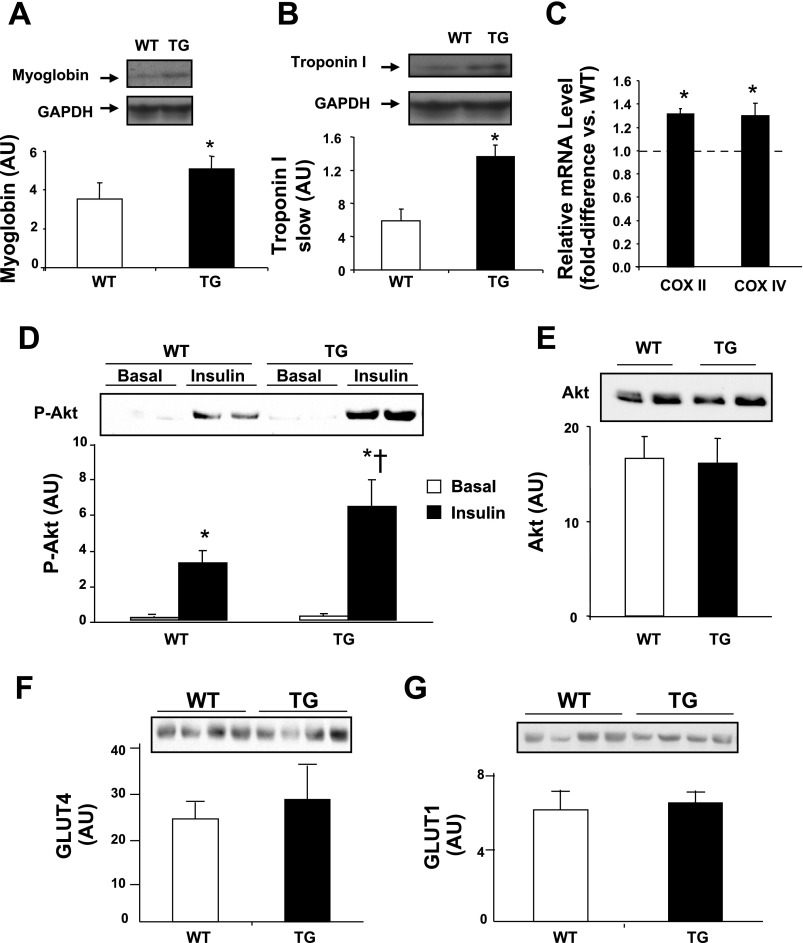
It has been postulated that PGC-1α mediates some of the adaptations that occur with physical training in muscle, such as switching from fast, more glycolytic, to slow, more oxidative, fibers (27). Consistent with this notion, content of myoglobin and troponin I slow protein, biomarkers for slow oxidative fibers, was elevated in muscle from TG mice (Fig. 7, A and B). In addition, PGC-1α overexpression resulted in increased gene expression of the oxidative phosphorylation proteins cytochrome c oxidase subunits II and IV in muscle (Fig. 7C). These changes in TG mice were accompanied by enhanced insulin signaling in muscle, manifested by elevated insulin-stimulated Akt phosphorylation (Fig. 7D). PGC-1α overexpression did not affect basal Akt (Fig. 7E), GLUT4 (Fig. 7F), and GLUT1 (Fig. 7G) protein content in muscle.
Fig. 7.
A and B: Western blot analysis of myoglobin and troponin I slow protein content in quadriceps muscle of 4- to 6-mo-old male and female mice in the basal state. *P < 0.05 vs. WT. C: higher levels of mRNA for COX subunits II and IV in muscle of 4- to 6-mo-old male and female TG compared with WT mice. D: Akt-Ser476 phosphorylation (p-Akt) in quadriceps muscle in the basal state (non-insulin-infused animals) and at the end of the insulin clamp in 4- to 6-mo-old male and female mice. *P < 0.05 vs. basal. †P < 0.05 vs. WT during clamp. E–G: basal Akt, GLUT4, and GLUT1 content in quadriceps muscle of 4- to 6-mo-old male and female mice. Values are means ± SE in 6–8 mice per group.
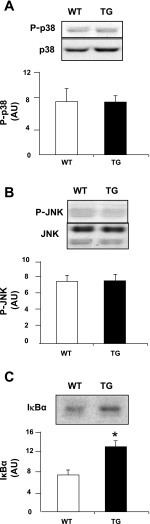
To further explore the mechanism by which PGC-1α overexpression leads to improved insulin action in muscle, we measured the phosphorylation (i.e., activity) of the MAPKs p38 and JNK, as well as the content of inhibitor κB (IκB)-α as an indication of the activity of IκB kinase (IKK) and NF-κB. Previous studies showed that the p38 MAPK (18), JNK (19), and IKK/NF-κB (6, 21, 42, 43) cascades are negative regulators of muscle insulin sensitivity. As shown in Fig. 8, p38 MAPK and JNK phosphorylation were unaffected in TG mice. In contrast, overexpression of PGC-1α resulted in elevated IκBα protein abundance (Fig. 8C). Because IκB sequesters NF-κB in the cytoplasm and because IκB abundance inversely correlates with NF-κB DNA-binding activity in muscle (1), increased IκB abundance is considered to indicate reduced IKK/NF-κB signaling.
Fig. 8.
Basal p38 MAPK phosphorylation (A), JNK phosphorylation (B), and IκBα abundance (C) in quadriceps muscle of 6-mo-old female mice. Values are means ± SE in 6 mice per group. *P < 0.05 vs. WT.
Lipid content in liver.
Considering that PGC-1α plays an important role in the regulation of lipid metabolism (25), we measured the concentrations of the lipid metabolites DAG and ceramide in the liver of standard chow-fed WT and TG mice. PGC-1α overexpression led to reduced DAG and ceramide (Fig. 9A) concentrations in the liver from TG compared with WT mice. This difference in hepatic lipid content between groups was not explained by differences in the gene expression of the fatty acid oxidative enzymes CPT-1 and MCAD (Fig. 9C).
Fig. 9.
Diacylglycerol (DAG) and ceramide content in liver (A) and quadriceps muscle (B) from standard chow-fed animals. C: carnitine palmitoyltransferase (CPT-1A) and medium-chain acyl CoA dehydrogenase (MCAD) as measured by real-time PCR in liver and muscle. D: H2O2 production by mitochondria isolated from muscle. Succinate (succ), malate (mal), and glutamate (Glu) were used as substrates for respiration. Rote, rotenone. Values are means ± SE in 6 (4- to 6-mo-old male and female) mice per group. *P < 0.05 vs. WT.
Lipid content and ROS production in muscle.
To determine the basis for reduced IKK/NF-κB signaling in muscle from TG mice, we measured the content of DAG and ceramide, as well as mitochondrial H2O2 production, because lipids and ROS can activate this pathway and, subsequently, impair insulin signaling (13). DAG and ceramide muscle content was similar between groups (Fig. 9B). However, overexpression of PGC-1α led to reduced H2O2 production by muscle mitochondria (Fig. 9D).
Effect of HFD.
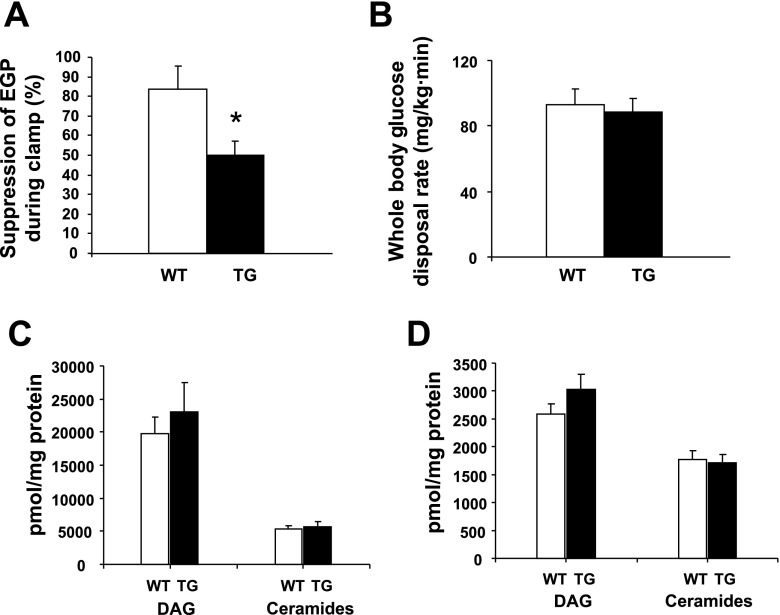
Plasma glucose concentrations (92 ± 6 and 91 ± 5 mg/dl, WT and TG, respectively) and body weights (data not shown) were similar in HFD-fed WT and TG mice. The HFD decreased hepatic PGC-1α levels by 22% and 15% in WT and TG mice, respectively (not shown). In muscle, HFD decreased PGC-1α content by 62% and 59% in WT and TG animals, respectively (not shown). Compared with WT mice fed standard chow, in which insulin caused near-total suppression of HGP (Fig. 3B), in HFD-fed WT mice, insulin reduced HGP by 86% (Fig. 10A). In addition, similar to animals fed standard chow, overexpression of PGC-1α (in TG mice) led to hepatic insulin resistance, manifested by a blunted insulin-mediated reduction in HGP (Fig. 10A). As expected, the HFD also caused peripheral insulin resistance in WT animals (Fig. 10B): whole body glucose disposal was 117 ± 4 and 93 ± 9 mg·kg−1·min−1 with standard chow and HFD, respectively (P < 0.05). However, overexpression of PGC-1α did not affect HFD-induced peripheral insulin resistance: whole body glucose disposal also decreased to 89 ± 8 mg·kg−1·min−1 with the HFD (Fig. 10B). In contrast to mice fed standard chow, in which PGC-1α overexpression led to a reduction in hepatic DAG and ceramide concentrations (Fig. 9A), upregulation of PGC-1α did not alter the content of these lipid metabolites in liver from HFD-fed TG mice (Fig. 10C). In addition, DAG and ceramide muscle content was similar in muscle from HFD-fed WT and TG mice (Fig. 10D). Similarly, IκBα content was not different in muscle from HFD-fed WT and TG mice (data not shown).
Fig. 10.
Under high-fat diet (HFD) conditions, insulin suppression of endogenous glucose production (A) and peripheral insulin sensitivity (B) during clamp are similar in TG and WT mice. DAG and ceramide levels in liver (C) and muscle (D) are similar in HFD-fed TG and WT animals. Values are means ± SE in 6 (6-mo-old male and female) mice per group.
DISCUSSION
PGC-1α is an important regulator of the gluconeogenic metabolic pathway (17, 48). In this study, we provide direct in vivo evidence that upregulation of PGC-1α increases HGP. Rates of HGP are controlled by gluconeogenic enzymes such as PEPCK and G6Pase, and expression of these enzymes was elevated in the TG mice. This effect is likely due to PGC-1α-mediated increases in HNF-4α expression, because PGC-1α loses its capacity to stimulate PEPCK and G6Pase when HNF-4α is absent (38). Some insulin-resistant animal models, including ob/ob (48), liver-specific insulin receptor-knockout (LIRKO) (48), and db/db (45) mice, have been shown to have elevated PGC-1α expression in the liver (17, 45). However, it is not known (to the best of our knowledge) whether an elevation in PGC-1α expression in the liver causes hepatic insulin resistance in vivo, as assessed by a method such as the insulin clamp with tritiated glucose, which provides direct, quantitative measurements of HGP. The present finding that upregulation of PGC-1α per se can cause hepatic insulin resistance suggests that increased expression of this transcriptional coactivator, as seen in ob/ob (48), LIRKO (48), and db/db (45) mice, may be responsible for the higher rates of HGP and liver insulin resistance in individuals with type 2 diabetes. Nevertheless, in the present study, we found that the HFD decreased PGC-1α expression in the liver. This finding argues against a role for PGC-1α in the pathogenesis of hepatic insulin resistance, at least in the HFD mouse model. Because PGC-1α expression is sensitive to fuel availability, the decrease in PGC-1α expression caused by the HFD could be caused by nutrient oversupply. Future studies are needed to clarify why the HFD differs from other mouse models of insulin resistance with regard to hepatic PGC-1α expression. Most importantly, it remains to be determined whether PGC-1α expression/function is abnormal in the liver of insulin-resistant individuals.
In contrast to the development of insulin resistance in the liver, TG mice overexpressing PGC-1α displayed improved peripheral (muscle) insulin sensitivity and signaling. In rodents (2, 15, 34, 46) and humans (33, 44), acute exercise induces PGC-1α gene expression in muscle. It has been proposed that this increase in muscle PGC-1α expression after prolonged exercise mediates the insulin-sensitizing effects (16). Consistent with the findings of Lin et al. (27), upregulation of PGC-1α increased the content of myoglobin I and troponin I slow protein in muscle, an indication of fast-to-slow fiber switching. However, we did not observe an increase in glucose transporter content (another typical adaptation to training) that occurred when PGC-1α was overexpressed in cultured muscle cells with use of an adenoviral vector (29). This discrepancy is likely due to differences in experimental systems (in vitro vs. in vivo) and the much higher levels of PGC-1α expression that were achieved with the viral vector than the modest (i.e., physiological) elevation observed in our TG mice.
To examine further the molecular basis for the improvement in insulin sensitivity/signaling in muscle from the TG mice, we examined whether PGC-1α upregulation affected the p38 MAPK, JNK, and IKK/NF-κB cascades. TG mice had increased abundance of the inhibitory protein IκBα, which is an indication of decreased IKK/NF-κB signaling. Because the IKK/NF-κB pathway inhibits insulin action (6, 21, 42, 43), it is possible that the lower IKK/NF-κB activity contributed to the improvement in insulin action in the PGC-1α TG mice. We also measured the content of the intramyocellular lipid metabolites DAG and ceramide, since increased muscle content of these lipid metabolites has been shown to activate IKK/NF-κB signaling (5, 21). We had predicted lower DAG and ceramide content in muscle of TG mice; however, we did not observe differences in the content of these lipid metabolites between WT and TG mice. The IKK/NF-κB pathway is activated by other stimuli, including ROS (13). Indeed, PGC-1α overexpression decreased ROS production by muscle mitochondria, which may be the cause of lower IKK/NF-κB signaling.
Overexpression of PGC-1α leads to a reduction in the content of DAG and ceramides in the liver. Considering that PGC-1α plays a role in the regulation of mitochondrial fatty acid oxidative enzyme gene expression (25), we compared the expression of CPT-1 and MCAD in the liver of WT vs. TG mice. However, expression of these key fatty acid oxidative enzymes was similar between groups. It is possible that elevated PGC-1α expression leads to a reduced DAG and ceramide content through a decrease in the rate of synthesis of these metabolites, as opposed to an increase in the rate of oxidation. Further experimentation is required to determine the mechanism of action of PGC-1α on the lipid metabolic effects we have observed.
Another important observation derived from this study is that overexpression of PGC-1α decreased insulin sensitivity of the liver, despite a reduction in the content of DAG and ceramides, agents that are generally thought to be important mediators of insulin resistance (18, 40). Other investigators also have observed such a dissociation between the cellular content of these lipid metabolites and insulin sensitivity. Monetti et al. (31) described a mouse model in which overexpression of acyl-CoA:diacylglycerol acyltransferase-2 led to increases in the hepatic content of triacyglycerol, DAG, and ceramides without affecting the insulin sensitivity of the liver. It is also possible that the upregulation of gluconeogenic genes in the PGC-1α TG mice could have overcome any potential beneficial effect on insulin action caused by the reduction of DAG and ceramides.
The PGC-1α TG mice were challenged with the HFD to test whether overexpression of this transcriptional coactivator would ameliorate the deleterious effect of the HFD on peripheral insulin sensitivity. Nonetheless, parameters of insulin sensitivity (Fig. 10B) and signaling (not shown) were not different between HFD-fed WT and TG animals. Furthermore, PGC-1α overexpression did not affect DAG and ceramide levels in liver (Fig. 10C) and muscle (Fig. 10D) from HFD-fed mice. Similarly, PGC-1α overexpression did not alter phospho-JNK, phospho-p38, or IκB content (not shown) in liver and muscle from HFD-fed mice. Because the degree of PGC-1α was modest, it is possible that the metabolic/cellular changes caused by the HFD, which is a fairly robust challenge, overwhelmed the improvements in insulin action in the TG mice.
By examining whole body glucose homeostasis in the PGC-1α TG mice using the insulin clamp-tritiated glucose technique, we found that overexpression of this transcriptional coactivator had a negative effect on hepatic insulin sensitivity but simultaneously improved insulin action in muscle. Although these results might appear to be somewhat paradoxical, they are consistent with the notion that PGC-1α exerts control of metabolic pathways in a tissue-specific manner (16, 35). Thus, PGC-1α acts as a fuel sensor that couples energy demands with the corresponding fuel supply. For example, under conditions of increased metabolic demand, such as fasting and exercise training, increased HGP would help sustain increased glucose utilization in peripheral tissues, primarily muscle. The dichotomous effect of PGC-1α expression on liver and muscle insulin sensitivity also underscores the difficulty of targeting PGC-1α for the treatment of human disease, such as type 2 diabetes. Tissue-specific modulators would be required to stimulate PGC-1α signaling specifically in skeletal muscle, where enhanced insulin action is desired, without activating this pathway in liver, where it would have a deleterious effect on glycemia by promoting glucose production.
GRANTS
This study was supported by grants from the American Diabetes Association (N. Musi, R. A. DeFronzo, and R. M. O'Doherty), National Institutes of Health (AG-028294 to W. F. Ward, AG-013319 and DK-080157 to N. Musi, AG-26557 to A. Richardson, DK-24092 to R. A. DeFronzo, and DK-058855 to R. M. O'Doherty), San Antonio Nathan Shock Aging Center (1P30-AG-13319), South Texas Health Research Center (N. Musi), US Department of Veterans Affairs (R. A. DeFronzo), Endocrine Fellows Foundation (B. Balas), and Thai Ministry of Public Health (P. Tantiwong). N. Musi is a recipient of a Paul B. Beeson Award on Aging Research.
Acknowledgments
The authors acknowledge the valuable consultative input of Dr. Qitao Ran (Barshop Institute for Longevity and Aging Studies) during development of the PGC-1α transgenic mouse.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agusti A, Morla M, Sauleda J, Saus C, Busquets X. NF-κB activation and iNOS upregulation in skeletal muscle of patients with COPD and low body weight. Thorax 59: 483–487, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj M, DeFronzo RA. Metabolic and molecular basis of insulin resistance. J Nucl Cardiol 10: 311–323, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bergman RN New concepts in extracellular signaling for insulin action: the single gateway hypothesis. Recent Prog Horm Res 52: 359–385, 1997. [PubMed] [Google Scholar]
- 5.Bhatt BA, Dube JJ, Dedousis N, Reider JA, O'Doherty RM. Diet-induced obesity and acute hyperlipidemia reduce IκBα levels in rat skeletal muscle in a fiber-type dependent manner. Am J Physiol Regul Integr Comp Physiol 290: R233–R240, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med 11: 183–190, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeFronzo RA Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 88: 787–835, ix, 2004. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism 38: 387–395, 1989. [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76: 149–155, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Hendler R, Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes 31: 795–801, 1982. [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Simonson D, Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia 23: 313–319, 1982. [DOI] [PubMed] [Google Scholar]
- 12.Dube JJ, Bhatt BA, Dedousis N, Bonen A, O'Doherty RM. Leptin, skeletal muscle lipids, and lipid-induced insulin resistance. Am J Physiol Regul Integr Comp Physiol 293: R642–R650, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23: 599–622, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Giaccari A, Morviducci L, Pastore L, Zorretta D, Sbraccia P, Maroccia E, Buongiorno A, Tamburrano G. Relative contribution of glycogenolysis and gluconeogenesis to hepatic glucose production in control and diabetic rats. A re-examination in the presence of euglycaemia. Diabetologia 41: 307–314, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T. cDNA coning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun 274: 350–354, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27: 728–735, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413: 179–183, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Heydrick SJ, Ruderman NB, Kurowski TG, Adams HB, Chen KS. Enhanced stimulation of diacylglycerol and lipid synthesis by insulin in denervated muscle. Altered protein kinase C activity and possible link to insulin resistance. Diabetes 40: 1707–1711, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Ho RC, Alcazar O, Fujii N, Hirshman MF, Goodyear LJ. p38γ MAPK regulation of glucose transporter expression and glucose uptake in L6 myotubes and mouse skeletal muscle. Am J Physiol Regul Integr Comp Physiol 286: R342–R349, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Hotamisligil GS Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes 54, Suppl 2: S73–S78, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκBα. Diabetes 51: 2005–2011, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J, Montminy M. PGC-1 promotes insulin resistance in liver through PPAR-α-dependent induction of TRB-3. Nat Med 10: 530–534, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 40: 1397–1403, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Madeiros DM, Kelly DP. Peroxisome proliferator-activated receptor γ coactivator 1α promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehman JJ, Boudina S, Banke NH, Sambandam N, Han X, Young DM, Leone TC, Gross RW, Lewandowski ED, Abel ED, Kelly DP. The transcriptional coactivator PGC-1α is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol 295: H185–H196, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin J, Tarr PT, Yang R, Rhee J, Puigserver P, Newgard CB, Spiegelman BM. PGC-1β in the regulation of hepatic glucose and energy metabolism. J Biol Chem 278: 30843–30848, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Makinen MW, Lee CP. Biochemical studies of skeletal muscle mitochondria. I. Microanalysis of cytochrome content, oxidative and phosphorylative activities of mammalian skeletal muscle mitochondria. Arch Biochem Biophys 126: 75–82, 1968. [DOI] [PubMed] [Google Scholar]
- 29.Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA 98: 3820–3825, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura S, Tomitsuka E, Kamei Y, Yamazaki T, Kai Y, Tamura M, Kita K, Nishino I, Ezaki O. Overexpression of peroxisome proliferator-activated receptor-γ co-activator-1α leads to muscle atrophy with depletion of ATP. Am J Pathol 169: 1129–1139, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV Sr, Hevener AL, Farese RV Jr. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab 6: 69–78, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279: 49064–49073, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T. PGC-1α mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol 96: 189–194, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol 546: 851–858, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puigserver P Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1α. Int J Obes (Lond) 29, Suppl 1: S5–S9, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423: 550–555, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. Regulation of hepatic fasting response by PPARγ coactivator-1α (PGC-1): requirement for hepatocyte nuclear factor 4α in gluconeogenesis. Proc Natl Acad Sci USA 100: 4012–4017, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414: 799–806, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274: 24202–24210, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Shulman GI Cellular mechanisms of insulin resistance in humans. Am J Cardiol 84: 3J–10J, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Sinha S, Perdomo G, Brown NF, O'Doherty RM. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor-κB. J Biol Chem 279: 41294–41301, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Sriwijitkamol A, Christ-Roberts C, Berria R, Eagan P, Pratipanawatr T, DeFronzo RA, Mandarino LJ, Musi N. Reduced skeletal muscle inhibitor of κBβ content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes 55: 760–767, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes 56: 836–848, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamura Y, Ogihara T, Uchida T, Ikeda F, Kumashiro N, Nomiyama T, Sato F, Hirose T, Tanaka Y, Mochizuki H, Kawamori R, Watada H. Amelioration of glucose tolerance by hepatic inhibition of nuclear factor-κB in db/db mice. Diabetologia 50: 131–141, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Taylor EB, Lamb JD, Hurst RW, Chesser DG, Ellingson WJ, Greenwood LJ, Porter BB, Herway ST, Winder WW. Endurance training increases skeletal muscle LKB1 and PGC-1α protein abundance: effects of time and intensity. Am J Physiol Endocrinol Metab 289: E960–E968, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Balas B, Christ-Roberts CY, Kim RY, Ramos FJ, Kikani CK, Li C, Deng C, Reyna S, Musi N, Dong LQ, DeFronzo RA, Liu F. Peripheral disruption of the Grb10 gene enhances insulin signaling and sensitivity in vivo. Mol Cell Biol 27: 6497–6505, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413: 131–138, 2001. [DOI] [PubMed] [Google Scholar]