Abstract
Colony-stimulating factor-1 (CSF1) is one of two cytokines required for normal osteoclastogenesis. There are two major isoforms of CSF1, the cell-surface or membrane-bound isoform (mCSF1) and soluble CSF1 (sCSF1). Whether these isoforms serve nonredundant functions in bone is unclear. To explore this question, we generated transgenic mice expressing human sCSF1, human mCSF1, or both (s/mCSF1) in osteoblasts using the 2.3-kb rat αI-collagen promoter. Bone density determined by peripheral quantitative computed tomography was significantly reduced in mCSF1, sCSF1, and s/mCSF1 transgenic mice compared with wild-type animals. When analyzed by sex, sCSF1, and s/mCSF1, female animals but not mCSF1 female mice were found to have greater bone loss than their male littermates (−20 vs. −9.2%; P < 0.05 for sCSF1 and −21.6 vs. −11.2% for s/mCSF1; P < 0.01). By breeding CSF1 isoform-selective transgenic mice to an op/op background, mice were generated in which a single CSF1 isoform was the only source of the cytokine (sCSF1op/op and mCSF1op/op). Unlike osteoblast-targeted overexpression of mCSF1, selective transgenic expression of sCSF1 did not completely correct the op/op phenotype in 5-mo-old animals. Interestingly, compared with sham-ovariectomized mice of the same genotype, ovariectomy in sCSF1op/op mice led to a greater loss of spinal bone mineral density (22.1%) than was seen in either mCSF1op/op mice (12.9%) or in wild-type animals (10.9%). Our findings support the conclusion that sCSF1 and mCSF1 serve nonredundant functions in bone and that sCSF1 may play a role in mediating estrogen-deficiency bone loss.
Keywords: osteoclastogenesis, estrogen deficiency, membrane-bound colony-stimulating factor
colony-stimulating factor-1 (CSF1) is a cytokine that is required through all stages of osteoclast development (4, 7, 10). Op/op mice are devoid of serum and tissue CSF1 activity and have an osteopetrotic phenotype due to a lack of osteoclasts (17, 26, 27). Osteoblasts derived from op/op mice do not support osteoclast development in vitro (23), while exogenous CSF1 induces osteoclast formation in op/op hematopoietic cells (8, 24), suggesting that the defect in osteoclastogenesis is the absence of osteoblast-derived CSF1.
Osteoblasts are an important source of CSF1 in the bone microenvironment and produce both cell-surface or membrane-bound CSF1 (mCSF1) and a soluble isoform (sCSF1) by alternative splicing in exon 6 in the CSF1 gene (28). Parathyroid hormone and TNF increase expression of both of these CSF1 isoforms (28). The roles of these two CSF1 isoforms in vivo are poorly understood. We have reported that levels of mCSF1, endogenously expressed by primary murine osteoblasts, support the formation of osteoclast-like cells in a dose-dependent manner in vitro and, at physiological concentrations, act synergistically with sCSF1 to induce osteoclast formation (29). Further, we (30) have found that selective expression of mCSF1 alone in osteoblasts rescues the osteopetrotic phenotype of op/op mice. Others (1, 21) have reported that expression of sCSF1 under the control of the endogenous CSF1 promoter or under the control of the osteoblast-restricted osteocalcin promoter also rescues the op/op skeletal phenotype in vivo. Whether these two isoforms subserve unique and nonredundant functions in vivo remains unclear.
The role of these two isoforms in pathological states of bone remodeling has not been extensively studied. One area of interest is the role of CSF1 in the accelerated bone loss that follows estrogen withdrawal. In 1996, Kimble et al. (12) reported that estrogen withdrawal was associated with increased levels of IL-1 and TNF in bone marrow that induced the formation of a stromal cell population producing high levels of sCSF1. The emergence of this population of cells correlated with increased osteoclastogenesis. Consistent with a role for CSF1 in mediating estrogen-deficiency bone loss is the observation that a neutralizing antibody to CSF1 prevents ovariectomy-induced bone loss in wild-type animals (5). In the aggregate, these data suggest a role for sCSF1 in estrogen-mediated bone loss. In contrast to these findings, using semiquantitative RT-PCR, Flanagan et al. (15, 22) reported that estrogen withdrawal selectively upregulates expression of the cell-surface CSF1 isoform in rat bone marrow cultures.
Little data are available in humans regarding the role of CSF1 in estrogen-deficiency bone loss, but consistent with a potential role for CSF1 in mediating this event, circulating levels of the cytokine rise in late menopause compared with levels observed in premenopausal women (11). Paradoxically, estrogen supplementation appears to increase serum CSF1 levels in postmenopausal women (11).
In an effort clarify the role of these two isoforms in mediating estrogen-deficiency bone loss, we engineered transgenic mice expressing mCSF1, sCSF1, or both transgenes in osteoblasts and examined their effects on bone in vivo after estrogen withdrawal.
EXPERIMENTAL PROCEDURES
Generation and identification of sCSF1 transgenic mice.
The sCSF1 transgene construct was created using the same strategy previously reported for generating the transgene used to target expression of mCSF1 to osteoblasts (30). The cDNA for human sCSF1, purchased from ATCC, was truncated at a point 50-bp 3′ to the stop codon using a unique NdeI site to remove 3′ adenosine-uridine-rich instability sequences and then cloned 3′ of the 2.3-kb rat collagen type I α-promoter. A 2.2-kb segment of the human growth hormone gene containing exons 1–5 and the intervening introns were added downstream of the cDNA to provide termination/polyadenylation signals and to increase expression efficiency.
The assembled transgene was microinjected into fertilized C57BL/6XSJLF2 oocytes, and the resultant transgenic mice were identified by PCR amplification of a 171-bp sequence within exon 5 of the HGH portion of the transgene (25). The integrity of genomic DNA was assessed by coamplification of a 259-bp segment of the endogenous murine GAPDH gene (25). The integrity of the transgene was further confirmed using PCR with human sCSF1 primers as described previously (28). Four sCSF1 transgenic founders were identified. Transgenic lines were generated by mating founder animals to CD-1 wild-type mice and the transgenic animals in subsequent generations were identified by PCR. The use of animals in this study was approved by the Yale Animal Care and Use Committee.
Expression of human sCSF1 transgene in transgenic mice.
Primary murine osteoblasts were prepared from calvaria of 2- to 4-day-old mice by collagenase-dispase digestion as described previously (29). Cells were grown in MEM supplemented with 10% FBS, penicillin, streptomycin, l-glutamine, and 20 mM HEPES. At confluence, cells were switched to serum-free MEM for 24 h and the conditioned media (CM) harvested. The concentration of human sCSF1 in the CM was quantified using a human CSF1 ELISA kit (R&D Systems, Minneapolis, MN). Calvarial osteoblast-CM prepared using cells derived from neonatal animals from two of four sCSF1 transgenic founder lines contained high levels of CSF1 (320 and 500 pg sCSF1/100 μg protein). The highest expressing line was studied further. To measure circulating levels of human CSF1, blood was collected by cardiac puncture from 20 transgenic mice and 20 wild-type littermates, allowed to clot, and spun, and sera were isolated. Human sCSF1 protein levels in these sera were measured using the same human CSF1 ELISA kit used to quantitate expression in CM.
Generation and identification of CSF1op/op mice.
sCSF1op/op transgenic mice were produced by initially breeding sCSF1 transgenic mice (sCSF1+/−) with op/+ mice yielding mice with a sCSF1+ op/+ genotype as well as mice with an sCSF1− op/+ genotype. The sCSF1+ op/+ mice were then bred with op/+ mice to yield mice with the sCSF1op/op genotype used in these studies. The sCSF1− op/+ mice were used as the control mice in these experiments. Identification of op/op mice was accomplished using PCR according to published methods (16). DNA from mice identified as having the op/op genotype was then analyzed for the presence of the sCSF1 transgene (i.e., an sCSF1op/op genotype) by PCR as described above. mCSF1op/op mice were generated and genotyped as previously described (30).
Measurement of endogenous levels of sCSF1, mCSF1, osteoprotegerin, and receptor activator of NF-κB ligand by quantitative RT-PCR in transgenic animals.
Endogenous levels of mCSF1 and sCSF1 were determined by quantitative RT-PCR (qRT-PCR) using a DNA Engine Opticon 2 System from M. J. Research (Waltham, MA) and the Brilliant qRT-PCR Master Mix kit from Stratagene (La Jolla, CA). The thermal cycling conditions consisted of an initial 50°C for 30 min and a denaturation step at 95°C for 5 min, 40 cycles at 95°C for 30 s, 60°C for 1 min, and 72°C for 30 s.
The forward primer for both mCSF1 and sCSF1 was as follows: ACCAACTGGGACGATATGGAGAAGA; and the reverse primers, respectively, were as follows: mCSF1: GCTTGAGGGCAAGAGAAGTACC; and sCSF1: ATCCTTTCTATACTGGCAG. The primers used to detect murine osteoprotegerin (OPG) and receptor activator of NF-κB ligand (RANKL) have been previously described by Rucci et al. (20). Levels of OPG and RANKL transcript expression are reported as fold change compared with levels of transcript expression in wild-type littermates.
Bone density measurements.
Peripheral quantitative computed tomography (pQCT) was used to make volumetric measurements of bone density in the proximal tibial site as described by Beamer et al. (3). A Stratec XCT-960A pQCT machine (Norland, Fort Atkinson, WI) was used to acquire scans 3-mm below the tibial plateau. Total bone density measurements were made at a threshold of 1,300 mg/cm3.
In vivo bone density measurements were performed by dual-energy x-ray absorptiometry using a PIXImus densitometer (Lunar, Madison, WI). Anesthetized mice (ketamine at 30 mg/kg body wt and xylazine at 3 mg/kg body wt given ip) were placed in the prone position and scans performed with a 1.270-mm-diameter collimator, 0.762-mm line spacing, 0.380-mm point resolution, and an acquisition time of 5 min. The spine window is a rectangle spanning a length of the spine from T1 to the beginning of the sacrum. The femur window encompasses the entire right femur of each mouse. The coefficient of variation for total body bone mineral density (BMD) is ∼1.5%.
Bone histomorphometry.
Histomorphometry was performed as previously reported (2, 9, 14). At the time of death the tibias were removed, stripped of soft tissue, and fixed in 70% ethanol. Tibias were then dehydrated through graded ethanol, cleared in toluene, infiltrated with increasing concentrations of methylmethacrylate, and embedded in methylmethacrylate according to previously described methods (2, 9). Analyses were performed on 5-μM thick sections stained with toluidine blue, pH 3.7, using a Nikon microscope interfaced with the Osteomeasure system software and hardware (Osteometrics, Atlanta, GA). Measurements were obtained in an area of cancellous bone that measured ∼2.5 mm2, containing only secondary spongiosa and located 0.5- and 2.5-mm distal to the epiphyseal growth cartilage. Longitudinal sections (5-μm thick) taken in the frontal plane through the cancellous bone of the proximal tibia were prepared with a Leica RM2165 microtome, mounted on chrom-alum-coated glass slides, and stained with toluidine blue, pH 3.7. All indexes were defined according to the American Society of Bone and Mineral Research histomorphometry nomenclature (19).
Ovariectomy.
Ovariectomy was accomplished through a paralumbar incision in anesthetized animals as previously reported (18). The ovarian bursa opposite the ovarian hilum was incised, the ovarian hilum was exposed and clamped, and the ovary was removed. Sham ovariectomy animals underwent anesthesia and the paralumbar incision without removal of the ovaries. Four weeks after ovariectomy, bone density was determined by pQCT.
Culturing osteoblasts under estrogen-replete or estrogen-deficient conditions.
Cells were grown in either 60- or 100-mm tissue culture dishes. Primary murine osteoblasts (isolated only from female neonatal pups) or MC3T3E1 cells were prepared and grown to confluence. At confluence, all cells were switched from growth media to phenol red-free RPMI with 10% charcoal stripped FBS to which had been added either 10−9 M 17β-estradiol to mimic estrogen-replete conditions or vehicle to mimic estrogen-deficient conditions, as previously reported (6). The average concentration of 17β-estradiol in the estrogen-replete media was 1,234 and 109 pg/ml in the estrogen-deficient media. After 24 h in culture, cells were harvested, RNA was isolated, and mCSF1 and sCSF1 transcript expression was analyzed by qRT-PCR.
RESULTS
Serum and tissue levels of CSF1 in transgenic mice.
Tissue and serum levels of CSF1 have been previously reported in the mCSF1 transgenic mice (18). We confirmed that expression of the mCSF1 transgene is restricted to bone (194 pg/100 μg protein) and that, while detectable, circulating levels of CSF1 are low (64 pg/ml) in mCSF1op/op mice. In sCSF1op/op transgenic mice the mean serum level of human CSF1 was 735 ± 262 in 6-wk-old mice and 649 ± 33 pg/ml in 5-mo-old animals indicating good transgene expression. As a point of reference, serum levels of murine CSF1 in wild-type mice are 415 ± 49 pg/ml (18). Expression of the sCSF1 transgene did not change the level of expression of the endogenous mCSF1 or sCSF1 transcripts in osteoblasts as assessed by qRT-PCR (1.2- and 1.3-fold, respectively, vs. nontransgenic controls; P = NS for both). There was also no change in the levels of endogenous RANKL and OPG transcript expression in osteoblasts isolated from transgenic animals compared with the levels in wild-type littermates when measured by qRT-PCR (1.0- and 1.2-fold, respectively, for RANKL and OPG; P = NS for both).
CSF1 transgenic mice have reduced bone density.
To examine the effect of restricted expression of the CSF1 isoforms in osteoblasts on bone mass, tibial bone density was analyzed by pQCT in mCSF1, sCSF1, and mCSF1/sCSF1 double transgenic mice and their wild-type littermates. As summarized in Table 1, in 3-mo-old sCSF1 transgenic mice, tibial BMD was significantly reduced by 14% compared with wild-type animals. The m/sCSF1 double transgenic mice had a 16% lower mean tibial BMD compared with controls. We (30) have previously reported that mCSF1 mice evidenced a significant 7% reduction in bone density at this same site compared with wild-type animals (tibial BMD: 418 ± 60 vs. 450 ± 12.4 g/cm3; n = 54; P < 0.05). The mean BMD values were not statistically significant between the sCSF1 and m/sCSF1 mice.
Table 1.
Tibial BMD in 3-mo-old mCSF1 transgenic, sCSF1 transgenic, and m/sCSF1 double transgenic mice
| Genotype | No. of Mice | BMD, g/cm3 | Mean %Reduction Compared with Controls | P Value* |
|---|---|---|---|---|
| sCSF1 | 13 | 386±8.9 | 14 | < 0.01 |
| m/sCSF1 | 33 | 378±10.7 | 16 | < 0.01 |
| Wild type | 57 | 450±12.4 |
Data are means ± SE. sCSF1, soluble transgene for colony-stimulating factor-1; m/sCSF1, membrane-bound and soluble isoform double transgene for colony-stimulating factor-1, respectively; BMD, bone mineral density.
Compared to wild-type controls.
Transgenic expression of sCSF1 does not fully correct the osteopetrosis in op/op mice.
We (30) have reported that the expression of the mCSF1 transgene in osteoblasts rescues the op/op skeletal phenotype resulting in normal bone density, incisor, and molar tooth eruption and normal rates of growth.
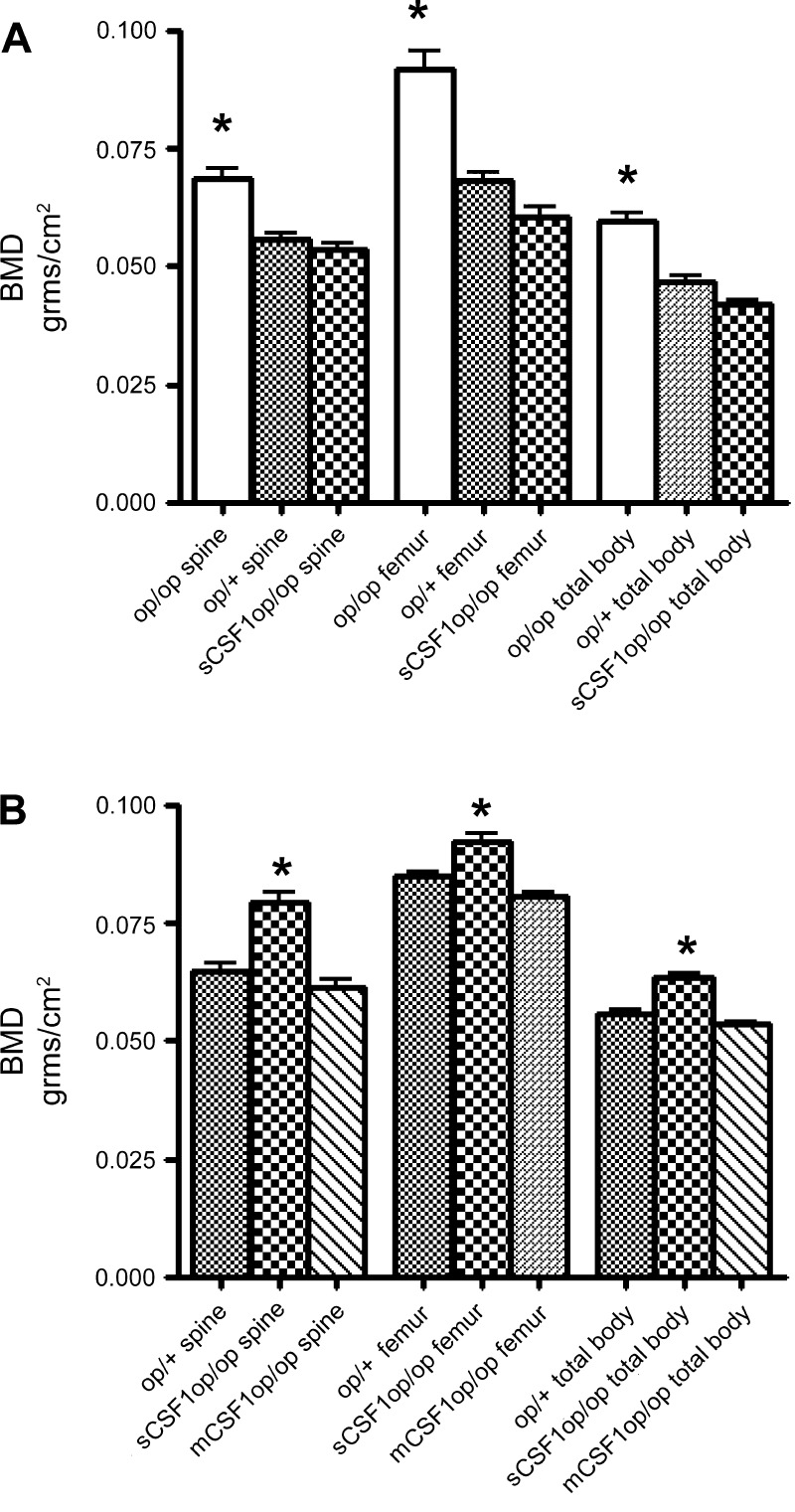
The sCSF1op/op mice also demonstrated normal incisor and molar tooth eruption. Body size, weight, and growth rate were not different in sCSF1op/op and op/+ mice. Interestingly, the skeletal phenotype of the sCSF1op/op mice was influenced by the age of the animals. At 6 wk, the mean bone density in the sCSF1op/op mice was equivalent to that in the op/+ control mice and significantly lower than the mean values in the op/op mice (Fig. 1A). However, by 5 mo, the sCSF1 transgenic animals had significantly higher regional and total body BMD values than the op/+ mice. Specifically, spinal BMD was 22% higher, femur BMD 8.7% higher, and total body BMD 13.8% higher in the sCSF1op/op mice. In contrast, BMD in the mCSF1op/op mice was indistinguishable from that in the op/+ animals at all measured skeletal sites (Fig. 1B).
Fig. 1.
A: comparison of spinal, femur, and total body bone mineral density (BMD; by PIXImus) in 6-wk-old op/op, soluble colony stimulating factor-1 (sCSF1)op/op mice, and their op/+ littermates. *P < 0.05 compared with sCSF1op/op mice and op/+ animals. B: comparison of spinal, femur, and total body BMD (by PIXImus) in 22-wk-old sCSF1op/op mice and their op/+ littermates. *P < 0.01, compared with membrane (m)CSF1op/op mice and op/+ animals.
Sex-specific effect of the CSF1 transgenes on bone mass.
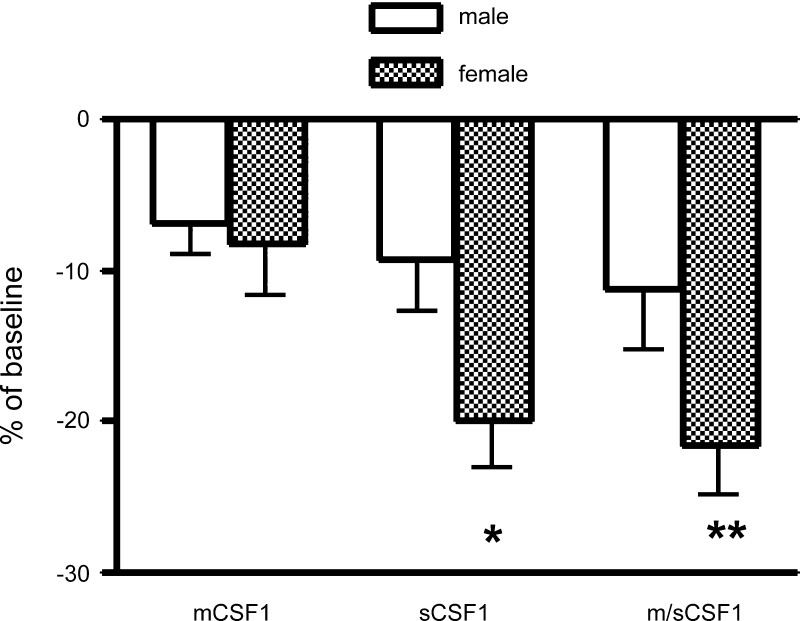
Since, as noted, there is uncertainty about the role of sCSF1 and mCSF1 in estrogen-deficiency bone loss, we further analyzed the bone density in 3-mo-old transgenic mice by sex (Fig. 2). Interestingly, females from sCSF1 and m/sCSF1 but not mCSF1 transgenic animals were found to have statistically significantly lower mean tibial bone density values compared with their male littermates (Fig. 2). A second founder line of sCSF1 transgenic mice was investigated to confirm these findings. Similar to the findings in the first transgenic line, females in this second line evidenced a proportionally greater bone loss than did the males vis à vis littermate controls (−17 vs. −10%; P < 0.05).
Fig. 2.
Percent reduction in BMD in male and female mCSF1 transgenic, sCSF1 transgenic, and m/sCSF1 double transgenic animals compared with wild-type littermates. Mean tibial BMD values (g/cm2) in these groups were as follows: male mCSF1 = 402 ± 9, female mCSF1 = 437 ± 14, male sCSF1 = 393 ± 16, female sCSF1 = 381 ± 14, male m/sCSF1 = 384 ± 13, female m/sCSF1 = 374 ± 10, male wild type = 432 ± 13, and female wild type = 476 ± 31. **P < 0.01, compared with male m/sCSF1. *P < 0.05, compared with male sCSF1.
In general, bone turnover tended to be higher in the sCSF1 transgenic animals (when considered as a group) with a trend toward higher numbers of osteoblasts per bone surface, numbers of osteoblasts per total area, and higher cellular parameters of osteoclast activity (Table 2). When these parameters were separately analyzed for male and female sCSF1 transgenic animals, it was found that while none of the osteoblast parameters were significantly different between the sexes, all parameters of osteoclast activity were significantly higher in the female sCSF1 animals. This was true for the number of osteoclasts per bone surface, the number of osteoclasts per total area, and the number of osteoclasts per bone perimeter. In the wild-type animals, none of the measures of osteoblast activity were different between male and female animals. In contrast to the finding in the sCSF1 transgenic animals, the resorptive activity of osteoclasts as assessed by the number of osteoclasts per bone surface and the number of osteoclasts per total area were not different in the two groups of wild-type animals. There was an increase in the number of osteoclasts per bone perimeter in the female wild-type animals.
Table 2.
Histomorphometric data in 8- to 12-wk-old male and female transgenic and wild-type animals
| n | BV/TV | BS/BV | ObS/BS | OcS/BS | NOb/TAR | NOc/TAR | NOc/Bpm | |
|---|---|---|---|---|---|---|---|---|
| sCSF1 mice | ||||||||
| Male | 10 | 19±1 | 113±3 | 18±1 | 6.7±0.5 | 529±31 | 54±3 | 3.2±0.2 |
| Female | 8 | 22±1 | 102±2 | 21±1 | 9.5±0.7 | 625±43 | 79±5 | 4.6±0.3 |
| P value* | NS | 0.04 | NS | 0.04 | NS | 0.01 | 0.02 | |
| WT mice | ||||||||
| Male | 12 | 20±1.5 | 89±9 | 17±3 | 3.4±1 | 453±128 | 33±8 | 2.6±0.7 |
| Female | 16 | 17±2 | 78±5 | 16±2 | 4.1±1 | 289±78 | 50±7 | 6.0±1.0 |
| P value* | NS | NS | NS | NS | NS | NS | 0.01 | |
Data are means ± SE. WT, wild-type littermates of transgenic mice; BV/TV, total trabecular bone volume; BS/BV, bone surface/bone volume; ObS/BS, osteoblast surface/bone surface; OcS/BS, osteoclast surface/bone surface; NOb/TAR, number of osteoblasts per total area; NOc/TAR, number of osteoclasts/per total area; NOc/Bpm, number of osteoclasts/per bone perimeter.
*Male vs. female mice of a given genotype.
sCSF1op/op mice lose bone after ovariectomy to a greater extent than mCSF1op/op mice.
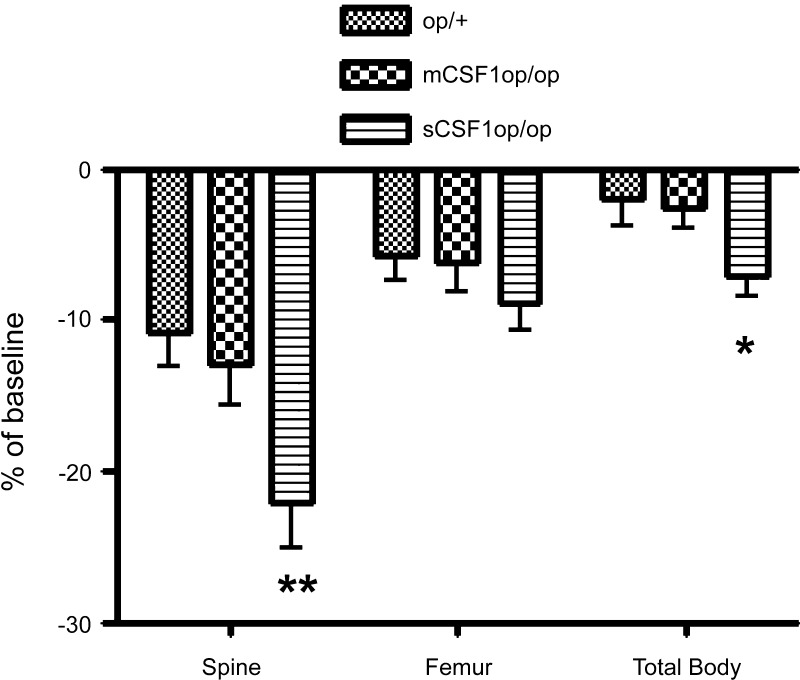
In an effort to determine if expression of sCSF1 or mCSF1 alone was enough to sensitize the skeleton to the effects of estrogen withdrawal, we ovariectomized or sham-ovariectomized 5-mo-old sCSF1op/op and mCSF1op/op female mice and quantified bone mass 1 mo later. Ovariectomized or sham-ovariectomized op/+ animals were used as controls. Very surprisingly, although the sCSF1 transgene did not fully restore BMD to normal in the op/op mice, ovariectomy in the sCSF1op/op animals led to a greater loss of bone than was observed when the mCSF1op/op mice were ovariectomized (Fig. 3). This differential effect was not due to a change in the level of sCSF1 transgene expression, since the mean serum level of human CSF1 in the sCSF1op/op ovariectomized transgenic mice was not different from that in the sCSF1op/op sham-ovariectomized animals (683 ± 55 vs. 622 ± 32 pg/ml; P = NS).
Fig. 3.
Percent reduction in BMD (as determined by PIXImus) from baseline after ovariectomy in op/+, mCSF1op/op, and sCSF1op/op mice. **P < 0.001, compared with mCSF1op/op. *P < 0.05, compared with mCSF1op/op.
Estrogen withdrawal selectively upregulates sCSF1 production.
To determine if estrogen-withdrawal directly affected CSF1 isoform expression in bone, we cultured primary murine osteoblasts and MC3T3E1 cells under estrogen-replete or -deficient conditions, isolated RNA from these cells, and quantified sCSF1 and mCSF1 transcript expression.
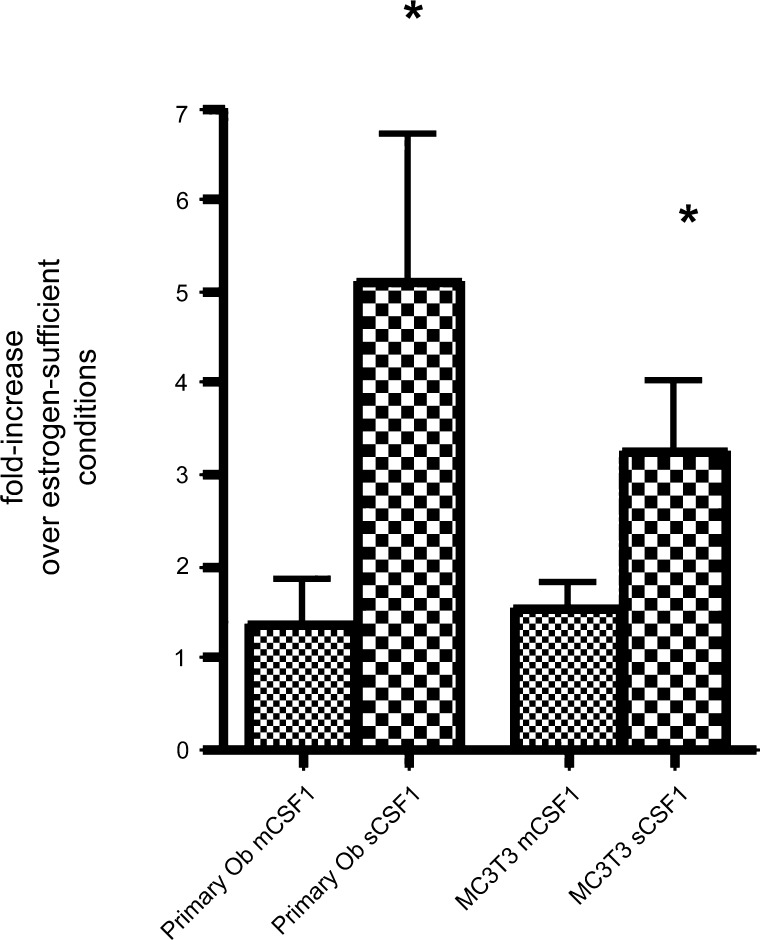
As shown in Fig. 4, estrogen-withdrawal induced significant 5.1 ± 1.6- and 3.3 ± 0.77-fold increases in the expression of sCSF1 in cultured primary murine osteoblasts and MC3T3E1 cells, respectively. In contrast, the level of mCSF1 expression was either unchanged or changed minimally after estrogen withdrawal.
Fig. 4.
Effect of estrogen withdrawal for 24 h on levels of mCSF1 and sCSF1 transcript expression in primary murine osteoblasts isolated from the calvariae of female pups (left two bars) and in MC3T3E1 cells (right two bars). Levels of CSF-1 isoform expression were quantified by quantitative RT-PCR and expressed as fold change compared with estrogen-replete parallel cultures. Data reflect mean results from 4–7 experiments. *P < 0.05, compared with estrogen-replete culture conditions. +P < 0.05, compared to wild-type controls.
DISCUSSION
While an essential role has been established for CSF1 in the process of normal osteoclastogenesis, the role of this cytokine in mediating states of pathological bone modeling and remodeling is unclear. Recent evidence has suggested a contribution of CSF1 to inflammatory arthritis. In particular, Kitaura et al. (13) have reported that inhibiting CSF1 in vivo attenuates TNF-dependent inflammatory arthritis in a model of immune joint destruction. As noted in the Introduction, Lea et al. (5) have shown that a neutralizing antibody to CSF1 prevents estrogen-deficiency bone loss, suggesting a role for CSF1 in this process. The current work seeks to further define the role of the two CSF1 isoforms in estrogen-deficiency bone loss. As also noted in the Introduction, prior work has led to contradictory conclusions about the relative importance of sCSF1 and mCSF1 to this process. The work from Kimble et al. (12) suggested that sCSF1 was upregulated in ovariectomized mice, while the findings of Flanagan et al. (15) in rat marrow indicated that mCSF1 was selectively upregulated after ovariectomy. In the current work, we used mCSF1 and sCSF1 transgenic animals as models of constitutive high-level, osteoblast-restricted, isoform-specific expression of CSF1 to examine the modulating effect this would have on rates of bone loss after ovariectomy.
In genetically normal animals, transgenic expression of each of the CSF1 isoforms individually or together was associated with a reduction in bone mass. This suggests that when added to endogenous levels of CSF1, overexpression of either isoform leads to bone loss. The histomorphometric data support the conclusion that the basis for the reduction in bone mass is an increase in osteoclast-mediated resorption. This is consistent with the known stimulatory effect of CSF1 on osteoclastogenesis. A direct effect of CSF1 overexpression on osteoblasts is less likely since normal osteoblasts do not express a functional CSF1 receptor. The fact that mean values for total trabecular bone volume were similar in the two groups of animals suggests that changes in cortical bone mass may make a more significant contribution to the skeletal phenotype in the sCSF1 transgenic animals.
In op/op mice, restricted expression of sCSF1 in osteoblasts led to normal tooth eruption and normal rates of growth. At 6 wk, BMD was similar in the sCSF1op/op and sCSF1op/+ animals. This finding is consistent with the report of Kitaura et al., who also reported a complete rescue of the op/op phenotype by osteoblast-restricted sCSF1 transgene expression in 5-wk-old animals (13). Importantly, expression of the sCSF1 transgene under the control of the collagen I αI promoter in our animals led to circulating levels of human CSF1 that were not dissimilar to levels of the endogenous cytokine found in wild-type mice. Thus it is reasonable to assume that the systemic exposure of our sCSF1op/op mice to bioactive CSF1 approximates that in wild-type mice. Of course, in the setting of the transgenic animal, the sole source of CSF1 is that the osteoblast and the expression levels of sCSF1 are constant.
In contrast to the findings at 6 wk, sCSF1op/op mice showed partial but not complete correction of the osteopetrotic phenotype at 5 mo. We have shown that transgenic expression of mCSF1 in osteoblasts completely rescued the op/op phenotype, suggesting the possibility of different biological activities of these two isoforms, at least in the bone microenvironment.
The reason for the delayed appearance of the skeletal phenotype in the sCSF1op/op animals is not clear. It is not due to an increase in the expression level of the sCSF1 transgene, since the serum level of human CSF1 in 6-wk-old sCSF1op/op mice was actually slightly higher than that in 5-mo-old mice of the same genotype. As noted, we have direct evidence of expression of our transgene as early 2–4 days after birth. We therefore assume that skeletal exposure to sCSF1 is constant throughout early postnatal development. A more likely explanation for this finding is that as the skeleton matures, remodeling rather than modeling becomes the dominant cellular process in bone. This means that if the remodeling cycle is differentially affected by sCSF1 and mCSF1, then this difference would become more manifest as the animal ages, consistent with our findings.
Further supporting the possibility that these two isoforms have different biological activities in bone in vivo are the findings of a sex-specific effect of the sCSF1 but not the mCSF1 transgene on bone mass in animals with a wild-type background and on rates of bone loss after ovariectomy in op/op transgenic animals (Figs. 2 and 3). Interestingly, estrogen withdrawal in vitro resulted in an increase in the expression of sCSF1 but not mCSF1 in primary murine osteoblasts and MC3T3E1 cells (Fig. 4). Since estrogen levels fluctuate in the female mice, the skeleton of our sCSF1 transgenic animals would periodically be exposed to the combined effect of increased endogenous sCSF1 production (due to low tissue estrogen) and tonic sCSF1 production from the transgene. The fact that this putative “additive or synergistic” effect was observed with the sCSF1 but not with the mCSF1 transgene again supports the notion of differential actions of the two isoforms on certain skeletal envelopes. Finally, the differential rate of bone loss in the ovariectomized animals also suggests distinct effects of the CSF1 isoforms in the setting of low estrogen levels. Since ovariectomy did not affected the level of transgene expression, the finding that the sCSF1op/op transgenic female mice showed greater rates of bone loss after ovariectomy than did mCSF1op/op transgenic animals raises the possibility that osteoclastogenesis becomes more sensitized to high levels of sCSF1 in the estrogen-depleted, bone microenvironment than to mCSF1. The reason for this differential effect after ovariectomy is unclear. One possibility is that sCSF1 more effectively reaches a greater pool of osteoclast precursors, since it is secreted into the bone microenvironment, or that this expanded pool of cells is uniquely sensitive to sCSF1. Since mCSF1 is quite effective at rescuing the op/op phenotype, it is hard to envision that greater access is the explanation, and we therefore favor the notion that somehow estrogen changes the sensitivity to sCSF1 to a greater extent than it does to mCSF1.
In summary, our findings establish that mCSF1 and sCSF1, as well as the double transgene s/mCSF1, exert important biological effects on the skeleton. Findings in the sCSF1 and s/mCSF1 transgenic animals, as well as in the sCSF1op/op mice, all suggest that sCSF1 may have a uniquely important role in the process of estrogen-deficiency bone loss. Since recent studies suggest that inhibiting sCSF1 may restrain pathological, without affecting physiological bone loss, sCSF1 may be a target for drug discovery.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-45228 and National Institute of Dental and Craniofacial Research Grant DE-12459 (to K. Insogna) and in part by the Yale Core Center for Musculoskeletal Disorders (P30; AR46032).
Acknowledgments
Present address of S. Ovadia: Faculty of Health Sciences in the Negev, Ben-Gurion University of the Negev, Beer-Sheva, Israel, 84105.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abboud SL, Woodruff K, Liu C, Shen V, Ghosh-Choudhury N. Rescue of the osteopetrotic defect in op/op mice by osteoblast-specific targeting of soluble colony-stimulating factor-1. Endocrinology 143: 1942–1949, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Baron R, Vignery A, Neff L, Silvergate A, Santa Maria A. Bone histomorphometry. In: Techniques and Interpretation, edited by Recker R. Boca Raton, FL: CRC, 1983, p. 31–32.
- 3.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone 18: 397–403, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 423: 337–342, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Cenci S, Weitzmann MN, Gentile MA, Aisa MC, Pacifici R. M-CSF neutralization and egr-1 deficiency prevent ovariectomy-induced bone loss. J Clin Invest 105: 1279–1287, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eielson C, Kaplan D, Mitnick MA, Paliwal I, Insogna K. Estrogen modulates parathyroid hormone-induced fibronectin production in human and rat osteoblast-like cells. Endocrinology 135: 1639–1644, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Fuller K, Owens JM, Jagger CJ, Wilson A, Moss R, Chambers TJ. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J Exp Med 178: 1733–1744, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hattersley G, Owens J, Flanagan AM, Chambers TJ. Macrophage colony stimulating factor (M-CSF) is essential for osteoclast formation in vitro. Biochem Biophys Res Commun 177: 526–531, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Insogna KL, Stewart AF, Vignery AM, Weir EC, Namnum PA, Baron RE, Kirkwood JM, Deftos LM, Broadus AE. Biochemical and histomorphometric characterization of a rat model for humoral hypercalcemia of malignancy. Endocrinology 114: 888–896, 1984. [DOI] [PubMed] [Google Scholar]
- 10.Jimi E, Akiyama S, Tsurukai T, Okahashi N, Kobayashi K, Udagawa N, Nishihara T, Takahashi N, Suda T. Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J Immunol 163: 434–442, 1999. [PubMed] [Google Scholar]
- 11.Kamada M, Irahara M, Maegawa M, Ohmoto Y, Takeji T, Yasui T, Aono T. Postmenopausal changes in serum cytokine levels and hormone replacement therapy. Am J Obstet Gynecol 184: 309–314, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Kimble RB, Srivastava S, Ross FP, Matayoshi A, Pacifici R. Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1and tumor necrosis factor-mediated stimulation of macrophage colony-stimulating factor production. J Biol Chem 271: 28890–28897, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Kitaura H, Zhou P, Kim HJ, Novack DV, Ross FP, Teitelbaum SL. M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest 115: 3418–3427, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knopp E, Troiano N, Bouxsein M, Sun BH, Lostritto K, Gundberg C, Dziura J, Insogna K. The effect of aging on the skeletal response to intermittent treatment with parathyroid hormone. Endocrinology 146: 1983–1990, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Lea CK, Sarma U, Flanagan AM. Macrophage colony stimulating-factor transcripts are differentially regulated in rat bone-marrow by gender hormones. Endocrinology 140: 273–279, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Lieschke GJ, Stanley E, Grail D, Hodgson G, Sinickas V, Gall JA, Sinclair RA, Dunn AR. Mice lacking both macrophage- and granulocyte-macrophage colony-stimulating factor have macrophages and coexistent osteopetrosis and severe lung disease. Blood 84: 27–35, 1994. [PubMed] [Google Scholar]
- 17.Marks SC, Lane PW. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. J Hered 67: 11–18, 1976. [DOI] [PubMed] [Google Scholar]
- 18.Ovadia S, Insogna K, Yao GQ. The cell-surface isoform of colony stimulating factor 1 (CSF1) restores but does not completely normalize fecundity in CSF1-deficient mice. Biol Reprod 74: 331–336, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2: 595–610, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Rucci N, Rufo A, Alamanou M, Teti A. Modeled microgravity stimulates osteoclastogenesis and bone resorption by increasing osteoblast RANKL/OPG ratio. J Cell Biochem 100: 464–473, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Ryan GR, Dai XM, Dominguez MG, Tong W, Chuan F, Chisholm O, Russell RG, Pollard JW, Stanley ER. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse [Csf1(op)/Csf1(op)] phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood 98: 74–84, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Sarma U, Edwards M, Motoyoshi K, Flanagan AM. Inhibition of bone resorption by 17beta-estradiol in human bone marrow cultures. J Cell Physiol 175: 99–108, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi N, Udagawa N, Akatsu T, Tanaka H, Isogai Y, Suda T. Deficiency of osteoclasts in osteopetrotic mice is due to a defect in the local microenvironment provided by osteoblastic cells. Endocrinology 128: 1792–1796, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatsu T, Stanley ER, Kurokawa T, Suda T. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest 91: 257–263, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weir EC, Philbrick WM, Amling M, Neff LA, Baron R, Broadus AE. Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc Natl Acad Sci USA 93: 10240–10245, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA 87: 4828–4832, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiktor-Jedrzejczak WW, Ahmed A, Szczylik C, Skelly RR. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med 156: 1516–1527, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao GQ, Sun B, Hammond EE, Spencer EN, Horowitz MC, Insogna KL, Weir EC. The cell-surface form of colony-stimulating factor-1 is regulated by osteotropic agents and supports formation of multinucleated osteoclast-like cells. J Biol Chem 273: 4119–4128, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Yao GQ, Sun BH, Weir EC, Insogna KL. A role for cell-surface CSF-1 in osteoblast-mediated osteoclastogenesis. Calcif Tissue Int 70: 339–346, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Yao GQ, Wu JJ, Sun BH, Troiano N, Mitnick MA, Insogna K. The cell surface form of colony-stimulating factor-1 is biologically active in bone in vivo. Endocrinology 144: 3677–3682, 2003. [DOI] [PubMed] [Google Scholar]