Abstract
TOR complex 1 (TORC1), an oligomer of the mTOR (mammalian target of rapamycin) protein kinase, its substrate binding subunit raptor, and the polypeptide Lst8/GβL, controls cell growth in all eukaryotes in response to nutrient availability and in metazoans to insulin and growth factors, energy status, and stress conditions. This review focuses on the biochemical mechanisms that regulate mTORC1 kinase activity, with special emphasis on mTORC1 regulation by amino acids. The dominant positive regulator of mTORC1 is the GTP-charged form of the ras-like GTPase Rheb. Insulin, growth factors, and a variety of cellular stressors regulate mTORC1 by controlling Rheb GTP charging through modulating the activity of the tuberous sclerosis complex, the Rheb GTPase activating protein. In contrast, amino acids, especially leucine, regulate mTORC1 by controlling the ability of Rheb-GTP to activate mTORC1. Rheb binds directly to mTOR, an interaction that appears to be essential for mTORC1 activation. In addition, Rheb-GTP stimulates phospholipase D1 to generate phosphatidic acid, a positive effector of mTORC1 activation, and binds to the mTOR inhibitor FKBP38, to displace it from mTOR. The contribution of Rheb's regulation of PL-D1 and FKBP38 to mTORC1 activation, relative to Rheb's direct binding to mTOR, remains to be fully defined. The rag GTPases, functioning as obligatory heterodimers, are also required for amino acid regulation of mTORC1. As with amino acid deficiency, however, the inhibitory effect of rag depletion on mTORC1 can be overcome by Rheb overexpression, whereas Rheb depletion obviates rag's ability to activate mTORC1. The rag heterodimer interacts directly with mTORC1 and may direct mTORC1 to the Rheb-containing vesicular compartment in response to amino acid sufficiency, enabling Rheb-GTP activation of mTORC1. The type III phosphatidylinositol kinase also participates in amino acid-dependent mTORC1 activation, although the site of action of its product, 3′OH-phosphatidylinositol, in this process is unclear.
Keywords: mammalian target of rapamycin, Rheb, rag, FKBP38, phospholipase D, guanosine 5′-triphosphatase
TOR (target of rapamycin) is the founding member of the PI 3-kinase-related protein (Ser/Thr) kinase subfamily, identified in Saccharomyces cerevisiae (Sc) as the product of a mutant gene that confers a dominant form of resistance to inhibition of growth/proliferation by the drug rapamycin (43, 64). ScTOR is a critical regulator of the tightly coupled processes of cell growth (an increase in cell mass/size) and proliferation by virtue of its ability to control overall mRNA translation in response to nutrient availability (6). The TOR polypeptides are nearly 300 kDa and contain ∼200 kDa of amino-terminal noncatalytic sequence, composed predominantly of HEAT repeats, and in the carboxy-terminal third of the protein a bilobed kinase domain whose amino acid sequence resembles that of the PI lipid kinases more closely than that of the canonical protein kinases (58). Yeast contains two TOR genes, whereas only a single TOR gene is found in metazoans. Nevertheless, in all eukaryotes, TOR is found in two independently regulated, functionally distinct heterooligomeric complexes, TOR complexes 1 and 2 (41, 59, 69). TOR complex 1 (TORC1) is the nutrient-responsive mediator of cell growth (i.e., cell mass/size) regulation, and is composed of TOR and the WD-propeller domain containing proteins raptor and Lst8/GβL (41, 59, 60, 69). Rapamycin, in a 1:1 complex with the polypeptide FKBP12, binds directly to the TOR polypeptide in TORC1 at a regulatory (FKBP12-rapamycin binding/FRB) domain (mTOR AA 2014-2115) located slightly amino-terminal to the mTOR catalytic domain (mTOR AA 2148-2430) and inhibits the TORC1 signaling function (13, 14). The original rapamycin-insensitive ScTOR2 mutant (43, 64)contained a single point mutation located in this segment (equivalent to Ser2035 in mTOR) that eliminates the binding of the FKBP12-rapamycin complex. The mammalian TOR complex 2 (TORC2), in addition to TOR and Lst8, contains the essential polypeptides rictor and sin1 (54, 69, 96) and is concerned with the regulation of the actin cytoskeleton and of certain AGC kinase subfamilies such as the Akt/PKBs (98) and novel PKCs (27, 48, 96). The TOR polypeptide in TORC2 is unavailable to the rapamycin-FKBP12 complex, and TORC2 is therefore not susceptible to direct inhibition by rapamycin, although prolonged treatment with rapamycin can impair the assembly of TORC2 in some cells (97). Lst8 is tightly bound to the mTOR catalytic domain in both complexes; however, its function in the mTORC1 complex is unclear, inasmuch as knockout of Lst8 is accompanied by a selective loss of mTORC2 function, whereas mTORC1 function appears to be preserved (36). In contrast, raptor provides the substrate binding function to TORC1 and is thus essential; loss of raptor phenocopies loss of TORC1 (41, 73).
Surprisingly few direct substrates of the mammalian TORC1 complex are known; best characterized are the translational regulatory proteins p70 S6 kinase (S6K1), an AGC family kinase, and eukaryotic initiation factor (eIF)4E-binding protein (4E-BP), the inhibitor of the mRNA 5′ cap binding protein eIF4E. S6K1 and 4E-BP bind directly to raptor through one or more short motifs; best characterized is the TOR signaling (TOS) motif, which in S6K1 and 4E-BP has the form Phe-Ac-φ-Ac-φ (where Ac = Glu/Asp and φ = Leu/Ile/Met) (100); mutation of the Phe eliminates the ability of these polypeptides to bind to raptor and to be phosphorylated by mTORC1 in cells (82, 100, 101). In the case of S6K1, if removal/inactivation of the TOS motif is accompanied by deletion of the carboxy-terminal noncatalytic tail, this doubly mutant S6K1, now invisible in the cell to mTORC1, resembles Akt and so is instead phosphorylated at its activating site (Thr389/412) by TORC2 in an insulin-responsive but rapamycin-resistant manner (1, 11, 121). Although mTORC1 substrates other than S6K1 and 4E-BP are known (e.g., STAT3, HIF1α, PRAS40, IRS-1), the mTORC1-catalyzed phosphorylation of S6K1 and 4E-BP has been shown to underlie, at least in part, the ability of mTORC1 to promote increased cell size (31). Rapamycin is perhaps the most selective protein kinase inhibitor available and is therefore a valuable probe of TORC1 function. Rapamycin inhibits S6K1 activity by >98% in essentially all mammalian cells examined. This inhibition of S6K1 activity (IC50 ∼2 nM) is attributable to the ability of rapamycin to prevent the TORC1-catalyzed phosphorylation of the S6K1 polypeptide at Thr389/412, a regulatory site located in a hydrophobic segment carboxy-terminal to the S6K1 catalytic domain whose phosphorylation is indispensable for S6K1 activity (88). Consequently, the extent of S6K1 (Thr389/412) phosphorylation in cells is routinely used as a reflection of TORC1 kinase activity (122). It should be emphasized, however, that concentrations of rapamycin sufficient to fully inhibit this phosphorylation in intact cells have little effect when added in vitro (as a complex with FKBP12) on the ability of mTORC1 to directly phosphorylate S6K1 (Thr389/412); 50-fold or more higher concentrations of rapamycin/FKBP12 are required for inhibition of TORC1 kinase in vitro, and at these levels, the rapamycin-FKBP12 complex also promotes the dissociation of raptor from mTOR (85). Thus the major mechanism by which rapamycin inhibits mTORC1 signaling in vivo remains uncertain but probably reflects a disruption of raptor-mediated substrate presentation, instead of (or possibly, in addition to) an inhibition of mTOR catalytic function. In contrast, the TOR inhibitors LY-294002 (IC50 ∼5 μM) and wortmannin (IC50 ∼0.3 μM) act at the kinase ATP binding site, the latter through an irreversible covalent mechanism, and inhibit both TORC1 and TORC2 (7).
Regulation of TORC1: General Features
Growth factors regulate TORC1 activity via either the PI3K/Akt pathway or the Ras/MAPK pathway, which converge on the tuberous sclerosis heterodimeric complex (TSC1-TSC2) to inhibit, by phosphorylation of TSC2, the GTPase-activating function (GAP activity) toward the small GTPase Rheb (Ras homolog enriched in brain) (20, 49, 65). Rheb was identified as a gene whose expression is upregulated in rat brain by seizure and stimuli that induce long-term potentiation (35, 124). Two Rhebs exist in mammalian cells, Rheb1 and Rheb2, whereas a single Rheb gene is found in yeast and Drosophila. Drosophila Rheb was identified as a gene whose loss of function led to a decrease of cell size and whose gain of function led to cell-autonomous increase in cell size (87, 99, 113). Genetic evidence in Drosophila places Rheb downstream of the TSC complex, and biochemical studies established that the TSC complex functions as a GTPase-activating protein for Rheb (10, 33, 50, 115, 126). Elimination or inactivation of either TSC1 or TSC2 results in an increase in the fractional GTP charging of Rheb to well over 90% and in a constitutive activation of TORC1, which is not further augmented by insulin or growth factors. Little is known concerning guanyl nucleotide exchange factors acting on Rheb, although the Drosophila ortholog of the “translationally controlled tumor protein” (TCTP) has been proposed to catalyze this reaction (46). Thus, Rheb-GTP is an activator of TORC1; as with rapamycin-FKBP12, Rheb does not appear to regulate TORC2.
Cellular stresses inhibit TORC1 through a variety of mechanisms, which also converge on the TSC complex; thus energy depletion or hypoxia, for example, activate the AMPK, which (in collaboration with GSK3) phosphorylates TSC2 and activates the TSC GAP function (51, 52). Glucocorticoids (118), hypoxia (111), and other stresses induce expression of the REDD1 polypeptide, which binds 14-3-3 and thereby relieves 14-3-3 inhibition of the TSC GAP function (21). Thus, these negative regulatory inputs to TORC1 appear to operate largely through upregulation of the GTPase-activating function of the TSC complex. AMPK can engender some inhibition of TORC1 in TSC-null cells through the direct phosphorylation of raptor (38), although the potency of this inhibition is far less than in the presence of an intact TSC. The aspect of TOR regulation least well understood is the mechanism (s) by which amino acids control TORC1 activity, the focus of the discussion to follow. One major distinction between TORC1 regulation by amino acids compared with growth factors or stress is that amino acid regulation is only modestly altered in TSC-null cells (89, 110), indicating that the major site of amino acid regulation is downstream of, or on a pathway independent of, the TSC.
The concept of nutrient regulation of TOR emerged from studies in S. cerevisiae, where treatment with rapamycin or inactivation of both TOR genes elicits a profound (90%) decrease in mRNA translation, resulting in a proliferative arrest very early in the cell cycle as well as an inhibition of several other anabolic programs and concomitant activation of catabolic programs such as autophagy (6). This arrest phenotype strongly resembles Go, the state seen with nutrient deprivation or growth on very poor nitrogen or carbon sources. Thus, as a dominant regulator of cell growth and proliferation, ScTOR was proposed to be part of a nutrient-responsive pathway and perhaps was itself regulated by nutrient signals. Later studies suggested that ScTORC1 is most responsive to glutamine sufficiency, inasmuch as both glutamine depletion and rapamycin result in nuclear localization and activation of several transcription factors that mediate glutamine synthesis (19); nevertheless, the molecular components of a putative glutamine-regulatory mechanism impinging on ScTOR remain undefined. The first evidence for a role of TOR in the nutrient regulation of cell growth in an intact metazoan was provided by the finding that loss of function of Drosophila TOR arrests larval development and yields cellular phenotypes that mimic those caused by amino acid deprivation, i.e., a significant reduction in nucleolar size, substantial lipid vesicle aggregation in the larval fat body, and a cell type-specific pattern of cell cycle arrest (84, 125).
Evidence for a relatively direct regulation of TORC1 by amino acids in mammalian cells was first provided by the finding that a 1- to 2-h removal of amino acids from tissue culture medium results in the selective inhibition of S6K1 and dephosphorylation of 4E-BP, rendering these targets unresponsive to insulin; readdition of amino acids to basal levels, in the absence of serum or insulin, restores 4E-BP phosphorylation, S6K1 activity, and their responsiveness to insulin, whereas raising the amino acid concentrations further can fully activate S6K1 such that insulin elicits no further activation (42). Amino acid activation of S6K1 is inhibited by rapamycin and is mediated by phosphorylation of the same array of sites as occurs with insulin, thus reflecting the activity of TORC1. Importantly, the doubly mutant, rapamycin-resistant variant of S6K1 described above was found also to be resistant to inhibition by amino acid withdrawal, establishing that amino acid regulation of S6K1 occurs either at mTORC1 or upstream. Withdrawal of amino acids does not interfere with insulin's ability to activate Akt, an output that requires activation of the type 1A PI 3-kinase and TORC2 (98); moreover, the ability of amino acid withdrawal to inhibit S6K1 is only modestly delayed in TSC-null cells and is effected without alteration in Rheb-GTP charging (89, 110). Thus, the pathway through which amino acids regulate mTORC1 is largely independent of the type 1A PI 3-kinase and does not involve regulation of Rheb-GTP charging. Notably, however, overexpression of recombinant Rheb to very high levels, e.g., 10- to 100-fold greater than endogenous Rheb, is able to overcome fully the inhibition of mTORC1 signaling engendered by amino acid withdrawal (70–72). In vivo, overexpression of Rheb in Drosophila is sufficient to counteract the effect of amino acid starvation in tissues such as the fat body and the salivary gland (113). Removal/readdition of amino acids does not alter the kinase activity assayed in vitro of the TOR polypeptides extracted from cells exposed to these treatments (37, 42, 70; although see Ref. 37), suggesting that the amino acid regulation of TOR may not involve a stable modification of TORC1 components, but more likely a reversible inhibition of the ability of endogenous Rheb-GTP to activate mTORC1.
Regulation of TORC1: Primacy of Leucine
Withdrawal of most amino acids singly for 1–2 h inactivates TORC1 signaling to differing extents; however, withdrawal of leucine or arginine is each nearly as effective in downregulating TORC1 signaling as withdrawal of all amino acids (42), and the preeminent effect of leucine withdrawal has been consistently observed in a variety of cell types. Some cell types, e.g., hepatoma lines, are quite resistant to amino acid withdrawal, perhaps because of high rates of endogenous autophagy (107). The unique signaling function of leucine in the regulation of metabolism, in part through the regulation of mTOR signaling, has been extensively supported by studies in vivo, primarily in rodents (62, 112) but in humans as well (16, 22). Thus, it is clear that leucine regulates protein synthesis in skeletal muscle, as well as protein degradation in skeletal muscle and liver, through TORC1-dependent and -independent mechanisms. Skeletal muscle protein synthesis is stimulated by leucine uniquely among the branched-chain amino acids, and the ability of leucine to augment the increase in protein synthesis elicited by resistance training in humans and to restore this response in older individuals has been well documented. A substantial component of the effect of leucine is independent of insulin (which stimulates leucine uptake); however, leucine also promotes insulin secretion in vivo, and together leucine and insulin stimulate muscle protein synthesis synergistically (reviewed in Ref. 62). Leucine stimulation of muscle protein synthesis is inhibited by rapamycin, as is the ability of leucine to selectively promote S6K1 and 4E-BP phosphorylation and the assembly of the eIF4F complex, the primary basis for leucine-stimulated translational initiation. Leucine also acts on the central nervous system to control overall food intake through TORC1 (and food selection, probably through GCN2) (40, 77). Direct administration of l-leucine (but not l-valine) in the vicinity of the arcuate nucleus region in rat hypothalamus stimulates hypothalamic TOR signaling and results in decreased food intake (anorexia) and significant weight loss (18). Leucine-induced anorexia is inhibited by rapamycin, demonstrating that TORC1 signaling is required. TORC1 activation is also required for the ability of intracerebroventricular leptin and ciliary neurotropic factor (CNTF) to reduce food intake in mice. Both polypeptides stimulate hypothalamic S6K1/S6 phosphorylation, and the anorectic response to these agents is largely eliminated in S6K1-null mice (17). Mice fed a high-fat diet (HFD) develop resistance to the ability of leptin to reduce food intake, whereas CNTF retains its potency; similarly, a HFD eliminates the ability of leptin to promote hypothalamic S6K1/S6 phosphorylation, whereas the response to CNTF persists (17). Thus, leucine in concert with insulin stimulates TORC1 signaling in skeletal muscle to promote the translation of mRNAs that underlie cell enlargement. Leucine also promotes Leptin synthesis in the adipocyte (76, 91), and both leucine and leptin action on leptin-sensitive neurons, signals that reflect immediate and long-term adequate nutrition, activate TORC1 to suppress further food intake.
The stimulation of TORC1 by leucine appears to be initiated at an intracellular site. In Xenopus oocytes, extracellular leucine is unable to promote S6K1 phosphorylation, but expression of a recombinant system L transporter confers responsiveness to extracellular leucine and direct intracytoplasmic injection of l-leucine (or Trp, Phe, Arg, Lys, and Gly but not d-Leu, Ala, Pro, Glu, Or Gln) stimulates S6K1-P in a rapamycin-sensitive manner (15). This contrasts with S. cerevisiae, where the dominant receptor for amino acids is the plasma membrane protein Ssy1p, a nontransporting member of the amino acid permease family (123), as well as with the leucine regulation of autophagy (56, 57, 79). In rat hepatocytes, a polymer consisting of 5–8 leucines attached by amide linkage to the α- and ɛ-amino groups of a branched lysine polymer, Mr∼1900, inhibits macroautophagy without evident cellular entry and at a molar concentration comparable to that of free leucine, whereas a similar polymer of isoleucine is ineffective. Moreover, although insulin inhibition of macroautophagy in hepatocytes is reversed by rapamycin, leucine inhibition is not sensitive to rapamycin (57, 79). Thus the hepatic leucine-sensitive pathway to macroautophagy and the Ssy1p pathway are TORC1 independent, as is the activation of GCN2 induced by leucine withdrawal.
Although leucine action on TORC1 is initiated subsequent to leucine entry, whether this is mediated by leucine itself or by some covalently liganded or metabolically transformed product is not known. Regarding the features of the leucine molecule required for TORC1 activation, modification of the α-amino group (acetylation, methylation) eliminates the ability of leucine to promote S6K1-P in H4-EII hepatoma cells; however, these derivatives are inhibitory, with an IC50 at 10-fold excess over leucine (108). In contrast, leucinamide is quite active both on H4-EII (108) and when injected into Xenopus oocytes (15), but Lynch et al. (75) observed considerable conversion of leucinamide to leucine by adipocytes. Leucinol, where an alcohol replaces the leucine carboxyl, is an inhibitor of leucyl-tRNA synthetase; microinjection of leucinol or tryptophanol into Xenopus oocytes generates a weak stimulation of S6K1-P compared with the same amount of leucine, and this response is increased further with higher doses of the alcohols (15). In Jurkat cells, however, l-leucinol is moderately inhibitory to S6K1 [other amino alcohols, e.g., histidinol, are much more inhibitory (47)], whereas it is without effect on basal or leucine-stimulated 4E-BP phosphorylation in adipocytes (74, 75). The basis for this variability in response to leucinol is unknown and leaves open the possibility that the mechanism of leucine action may vary with the cell background. Specifically, it raises the question of whether cross-regulation of the TORC1 pathway consequent to GCN2 activation occurs in a cell- or time- or otherwise conditional manner.
GCN2 is activated when the level of any amino acid diminishes sufficiently to cause the accumulation of uncharged tRNAs, which are direct activators of the GCN2 kinase (44). GCN2 phosphorylates eIF2A at Ser51, resulting in the sequestration of the eIF2A-GEF, eIF2B; this greatly reduces the rate of general translational initiation, while upregulating the translation of a subset of mRNA, e.g., the transcription factor ATF4, which promotes expression of an array of genes, including those coding for amino acid biosynthetic proteins. Mice fed a leucine-deficient diet (or any diet deficient in an essential amino acid) consume much less food (34) and exhibit semistarvation, including a suppression of protein synthesis in skeletal muscle and liver (2); GCN2-null mice fed this diet also eat less but fail to suppress hepatic protein synthesis or undergo the dephosphorylation of hepatic S6K1 and 4E-BP that occurs in wild-type mice pair-fed this deficient diet, indicating that GCN2 is required for the downregulation of hepatic mTORC1 signaling, at least in this circumstance. In contrast to the sustained liver weight in the GCN2-null mice fed the leucine-deficient diet, muscle wasting in these mice is profound, indicating major tissue-specific differences in the regulation of protein balance and perhaps TORC1 signaling (2). As to possible mechanisms by which activation of GCN2 might regulate mTORC1, one of the genes unregulated in response to the increased abundance of ATF4 is GADD34, a protein phosphatase-1 regulatory protein (83). GADD34 binds to both TSC1 and TSC2 (78, 120) and has been observed to promote the dephosphorylation of TSC2 (Thr1462), a major Akt phosphorylation site, which is claimed to disinhibit the TSC GAP function, reduce Rheb-GTP charging, and thus inhibit mTORC1. The likelihood that coordinate, reciprocal regulation of the GCN2 and TORC1 signaling occurs in response to amino acid deficiency is logical, and further examination of the tissue-specific interactions of the GCN2 and mTORC1 pathways is clearly merited.
Activation of GCN2 occurs in response to deficiency of any essential amino acid; however, regulation of mTORC1 is most responsive to specific individual amino acids. The nearly comparable inhibitory effect of leucine and arginine (42) withdrawal is especially puzzling; apart from their participation in polypeptide chain elongation through the mediation of six tRNAs, these amino acids share no commonalities in their transport or metabolic fates. The possibility, e.g., that arginine affects mTORC1 activity through the mediation of NO, is unexplored. Finally, it should be mentioned that alterations in the extracellular concentration of amino acids whose transport is Na+ linked, e.g., glutamine (but not leucine), will lead to parallel alterations in cell hydration; cell swelling induced by any mechanism is accompanied by activation of several anabolic pathways, including mTORC1, whereas cell dehydration is inhibitory (63, 102).
Mechanisms of TORC1 Regulation: Rheb
As regards the biochemical mechanism by which Rheb activates mTORC1, Rheb does not achieve this effect through modulating amino acid uptake, as overexpressing Rheb (or TSC1/2) has no effect on the steady-state concentration of individual amino acids within the cells, including the concentrations of branched-chain amino acids and arginine. In addition, although removing extracellular amino acids lowers the total intracellular amino acid level, overexpressing Rheb does not protect against the decline, nor does overexpression of TSC1/2 increase the decline (81). Similarly, in Drosophila S2 cells, Rheb overexpression activates TORC1 signaling but does not promote the import of glucose, bulk amino acids, or arginine (39). Therefore, Rheb does not activate TORC1 signaling by modulating the intracellular amino acid levels.
Considerable evidence supports the view that Rheb activates TORC1 through a direct interaction with mTOR. Rheb binds directly to the upper, small lobe of the TOR kinase domain (mTOR AA 2148-2300) in TORC1 (71). The ability of Rheb to bind directly to the mTOR catalytic domain is consistent with the occurrence of such an interaction in the normal process by which Rheb-GTP activates TORC1; however, the affinity of Rheb for TOR is rather weak, and an interaction of endogenous Rheb with endogenous mTOR has not been identified, even in TSC-null cells. Nevertheless, other physiologically important interactions between a small GTPase and its effector, e.g., ras-GTP and type 1A PI 3-kinases, exhibit comparably low affinity (86), and this may be mitigated somewhat by the substantial colocalization of Rheb and mTORC1 on overlapping endomembrane compartments. A more unusual feature of the Rheb-mTOR interaction, and a significant caveat to its physiological significance, is that the ability of recombinant Rheb to bind mTOR, in vitro and in cells, is not dependent on or stimulated by Rheb-GTP charging; nucleotide-deficient, inactive Rheb mutants actually bind mTOR more tightly than does wild-type Rheb. Nevertheless, when mTOR is coexpressed with such nucleotide-deficient Rheb mutants, the mTOR polypeptides retrieved with these Rheb mutants are essentially devoid of kinase activity when assayed in vitro (71). This finding indicates that the interaction of mTOR with a native, presumably GTP-charged Rheb is essential for the ability of mTOR (at least in TORC1) to achieve catalytic competence. The region (s) of Rheb that mediate its interaction with mTOR is not known; Rheb mutagenesis indicates that mutation of both the Rheb switch 1 domain (71), whose configuration is highly GTP dependent, and the switch 2 domain (70), which is hardly altered by GTP, both disable the ability of overexpressed Rheb to stimulate mTORC1 signaling in amino acid-deprived cells. Further evidence for the importance of a direct Rheb/TOR interaction in the activation of TOR is provided by the findings of Tamanoi et al. (117), who selected hyperactive mutants of Schizosaccharomyces pombe Rheb; in vitro, these mutants exhibit a marked decrease in affinity for GDP without altered GTP binding. Although wild-type S. pombe Rheb cannot be coprecipitated with S. pombe TOR and does not comigrate with SpTOR on sucrose density gradients, such hyperactive S. pombe Rheb mutants, expressed at wild-type levels, do coprecipitate and comigrate with endogenous S. pombe TOR. The concomitant enhancement in signaling efficacy and affinity for SpTOR exhibited by the SpTOR mutants implies that the two phenotypes are causally related; however, this remains inferential. Finally, Sancak et al. (95) reported that the direct addition of Rheb-GTP to mTORC1 in vitro results in activation of the TORC1 kinase activity; this result, if confirmed and defined in molecular terms, would provide incontrovertible support for the role of a direct Rheb/mTOR interaction in TORC1 regulation.
A ready potential explanation for the ability of amino acid withdrawal to inhibit mTORC1 signaling is provided by the finding that withdrawal of amino acids (or of just leucine or arginine) diminishes the ability of recombinant Rheb to bind to endogenous or coexpressed recombinant mTOR (72). This effect of amino acid withdrawal may also explain why substantial Rheb overexpression is needed, generating Rheb-GTP levels well above those found in TSC-null cells, to overcome the inhibition of mTORC1 engendered by amino acid withdrawal. The biochemical mechanism responsible for the ability of amino acid withdrawal to inhibit the Rheb-mTOR interaction is not known; however, amino acid regulation of the Rheb-mTOR interaction is directed entirely through mTOR and is mediated through the lower lobe of the TOR kinase domain; deletion of the lower lobe (mTOR AA 2301-2430) eliminates the ability of amino acid withdrawal to inhibit Rheb binding to the upper lobe (mTOR AA 2148-2300) without significantly altering Rheb binding per se. Thus the mechanism by which amino acid sufficiency regulates mTORC1 appears to be intimately related to the mechanism by which Rheb activates mTORC1; unfortunately, however, the mechanism (s) by which Rheb regulates mTOR is not yet clear.
Mechanisms of TORC1 Regulation: Phospholipase D and Phosphatidic Acid
Two mechanisms have been proposed whereby Rheb can activate TORC1 indirectly without the requirement for a direct Rheb-mTOR interaction. One such pathway occurs through the generation of phosphatidic acid (PA), produced by Phospholipase D-catalyzed hydrolysis of phosphatidylcholine (PC). Inhibition of PA accumulation using 1-butanol (but not 2-butanol) (29) or RNAi-induced depletion of PL-D1 (28) reduces serum-stimulated S6K1 and 4E-BP phosphorylation, providing strong support to the view that PL-D1, through the generation of PA, provides a positive input to mTORC1. The site of PA action appears to be mTOR itself; salt-sensitive binding of PA-containing PC vesicles to mTOR occurs through a site within the mTOR FRB domain, mediated by several basic residues, especially Arg2109. Addition of an FKBP12-rapamycin complex displaces the PA vesicles from an isolated FRB domain, whereas the rapamycin-insensitive FRB mutant Ser2035Ile binds the PA vesicles in a rapamycin-insensitive manner. The mTOR (Arg2109Ala) mutant exhibits unaltered kinase activity in vitro, but only ∼60% the efficacy of mTOR-WT in activating coexpressed S6K1 (29). Recently Sun et al. (114) reported that Rheb, added directly in vitro, binds to and stimulates the activity of PL-D1 in a GTP-dependent manner. Rheb is thus added to the extensive array of upstream regulators of PL-D, which includes protein kinase C isoforms, other small GTPases of the ARF, Rho, and Ras families and, particularly, the phosphoinositide phosphatidylinositol 4,5-bisphosphate. In this pathway, Rheb-GTP stimulation of PL-D1 generates PA, which, through its direct binding to mTOR, contributes to TORC1 activation in vivo; stimulation of TORC1 kinase activity in vitro by addition of PA has not been observed. PA may act by directing the localization of mTORC1, enabling its proximity to Rheb or other elements, rather than by direct modulation of its catalytic function; a similar role for PA has been suggested in the regulation of the Raf kinase. Alternatively, PA may participate in mTOR regulation by FKBP38, discussed next.
Mechanisms of TORC1 Regulation: FKBP38
A second mechanism for Rheb activation of TOR that does not require a direct Rheb-TOR interaction involves the rapamycin-insensitive peptidyl prolyl cis-trans isomerase (PPI) FKBP38. Bai et al. (5) retrieved FKBP38 in a two-hybrid screen with a Rheb bait and demonstrated coprecipitation of the endogenous polypeptides from 293 cells. FKBP38 encompasses an FKBP12-like PPI domain, the site of Rheb binding, followed by three TPR repeats, a canonical Ca2+-calmodulin (CM) binding domain, and near its carboxy terminus, a transmembrane domain (67, 109). The FKBP38 polypeptide is bound to endomembranes and mitochondrial outer membrane via a carboxy-terminal transmembrane domain, so that the bulk of the polypeptide faces into the cytoplasm. Previous work had shown that FKBP38 catalytic function is activated by a calcium-calmodulin complex (EC50 ∼290 nM) binding to the Ca2+-CM binding domain, displacing it from the PPI domain (25, 26). Thereby freed, the PPI domain is able to bind and inhibit Bcl2 in a Ca2+-dependent manner, thereby contributing to Ca2+-induced apoptosis in a variety of cells. Ca2+-CM binding to FKBP38 also enables the three tetratricopeptide (TPR) repeats to bind HSP90, itself a Ca2+-CM binding protein. The ternary complex of FKBP38/Ca2+-CM/HSP90 is enzymatically inactive and unable to bind Bcl2; thus, HSP90 availability controls the ability of Ca2+ to make available the FKBP38 PPI domain to its partners (24). FKBP38 had also been identified as one of the transcripts significantly unregulated in HeLa cells overexpressing either TSC1 or TSC2, and RNAi-induced depletion of FKBP38 reversed the (10–14%) reduction in HeLa cell size (FSC) caused by overexpression of TSC1 or TSC2 (92). Bai et al. (5) demonstrated that overexpression of FKBP38 inhibits the phosphorylation of 4E-BP caused by amino acid readdition or Rheb overexpression, whereas RNAi-induced depletion of endogenous FKBP38 up regulates basal S6K1 (Thr389/412) and 4E-BP phosphorylation and ameliorates the inhibition of these phosphorylations that occurs with serum or amino acid withdrawal. Moreover, addition of FKBP38 in vitro inhibited the mTORC1 kinase activity. Thus FKBP38 appears to be an endogenous inhibitor of mTORC1. Importantly, FKBP38 binds directly to Rheb-GTP in preference to Rheb-GDP and also binds, in a rapamycin-insensitive manner, to an mTOR segment (AA 1967-2191) that encompasses the FRB domain (AA 2015-2114). The binding of FKBP38 to mTOR (AA 1967-2191), a segment that does not bind Rheb, is nevertheless inhibited by addition of Rheb-GTP (but not Rheb-GDP), presumably because the binding of Rheb-GTP to FKBP38 interferes with the ability of FKBP38 to bind mTOR. FKBP38 binds through its FKBP12-like domain to mTOR; the ability of Rheb-GTP to displace FKBP38 from mTOR is consistent with the ability of Rheb-GTP to also bind to this FKBP12-like segment. The regions of Rheb that mediate its binding to mTOR and FKBP38 are not defined; however, the nucleotide-deficient Rheb-D60K mutant, which binds both mTOR and FKBP38 more strongly than does wild-type Rheb, does not displace FKBP38 from mTOR (AA 1967-2191), suggesting that Rheb may use different domains for the binding of these two partners. Serum addition, which promotes Rheb-GTP charging, enhances the binding of Rheb to FKBP38 and reduces FKBP38 binding to mTOR, presumably reflecting the ability of Rheb-GTP to displace FKBP38 from mTOR. Amino acid withdrawal decreases the binding of Rheb to FKBP38 while increasing FKBP38 binding to mTOR; inasmuch as amino acid withdrawal does not alter Rheb-GTP charging, an explanation for this behavior is not apparent.
Caveats and Questions: PL-D/PA and FKBP38
Altogether, the data point to a significant role for FKBP38 in the regulation of mTORC1; nevertheless, questions remain regarding both the PL-D1/PA and FKBP38 mechanisms of TORC1 regulation. First, there is thus far a lack of supporting genetic evidence for either mechanism. PL-D-null flies exhibit reduced viability during cellularization but PL-D-deficient adults are overtly normal (66), inconsistent with a significant role for PL-D in Drosophila TOR regulation. Drosophila encodes an FKBP38 homolog (CG5482); however, the cell/organ screens that have revealed such elements as Rheb, TSC, REDD, etc, have not (as yet) identified CG5482 as a regulator of cell size. More definitively, SpRheb is a dominant regulator of TORC1 activity (3) despite the absence of an FKBP38 homolog in S. pombe. Conceivably, the participation of PL-D and/or FKBP38 in TORC1 regulation may be limited to higher metazoans, e.g., vertebrates. Rheb is certainly the dominant proximate regulator of mammalian TORC1 for all inputs; however, the relative contributions of Rheb's interaction with mTOR, PL-D1, or FKBP38 to the activation of TORC1 remains to be established, and several mechanisms may be operating simultaneously in a parallel or interdependent way. Inasmuch as PA binding to mTOR is competitive with the rapamycin-FKBP12 complex (29), PA may also participate in the relief of mTOR inhibition by FKBP38. Important modulators of FKBP38 action include the level of intracellular calcium and/or HSP90. The ability of amino acid withdrawal/readdition to enhance/reduce the FKBP38/mTOR interaction while reducing/enhancing the FKBP38/Rheb interaction is unexplained. Perhaps, as with the effect of amino acid withdrawal on the Rheb-mTOR interaction (72), amino acid withdrawal may act through TOR to enhance FKBP38 binding near the FRB domain, thereby diminishing FKBP38 availability to Rheb. Placing the site of action of amino acid regulation of TORC1 at TOR itself, rather than at Rheb, provides a framework for explaining TORC1 regulation in S. cerevisiae, where FKBP38 homologs do not exist and Rheb does not appear to regulate TORC1. Although it is likely that a variety of mechanisms (Fig. 1), including Rheb-independent inputs, exist for the regulation of TORC1, a more complete understanding of the mechanism by which Rheb regulates mTORC1 will be required before the mechanism of amino acid regulation is substantially clarified.
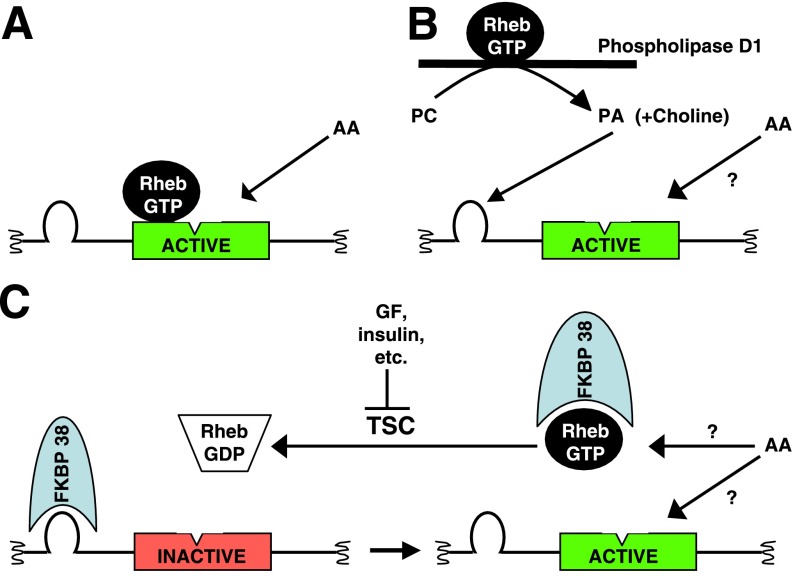
Fig. 1.
Candidate mechanisms for Rheb-GTP activation of mammalian target of rapamycin complex 1 (mTORC1). The segment of mTOR from ∼AA 1967-2500 is pictured; cleft in rectangle divides the catalytic domain into upper (AA 2147-2300) and lower (AA 2301-2430) lobes. Loop represents the FKBP12-rapamycin binding (FRB) domain (AA 2014-2115). Insulin and growth factors promote Rheb GTP charging by inhibition of the tuberous sclerosis complex (TSC), a Rheb GTPase activator, as illustrated in C. Three models for mTORC1 activation by Rheb-GTP are illustrated. A: Rheb binds to the upper lobe of the catalytic domain and when GTP charged, enables mTOR activation. Amino acid withdrawal, through an effect on the lower lobe, interferes with Rheb binding to the upper lobe. B: ability of Rheb-GTP to promote activation of phospholipase D1 generates phosphatidic acid (PA), which binds to the FRB domain, promoting activation. C: Rheb-GTP binds to mTOR inhibitor FKBP38, competing with and displacing it from mTOR, disinhibiting the mTOR kinase activity. Site of action of amino acids in B and C is unknown.
Mechanisms of TORC1 Regulation: The Rag GTPases
The rag subfamily of GTPases, Rag A–D, have recently emerged as components of the pathway by which amino acids regulate TORC1 signaling. The rags are small GTP-binding proteins that encode the GX4GKS/T and DXXG motifs; rags A and B, but not C and D, contain an appropriately situated NXXD motif, whereas all four contain an HKM/VD that might instead participate in guanosine binding. No candidate S/CA motifs are evident, and farnesylation or myristoylation motifs are lacking (103). Human rags A (313 AA) and B (346 AA) are over 98% identical, differing only by an amino-terminal extension in rag B of 33 amino acids; rag B also has a longer isoform with a 28 AA insert after AA76, into the center of what is likely the switch 1 loop. Rag C (399 AA) and D (400 AA) are 77% identical overall, differing primarily over their amino-terminal 60 and carboxy-terminal 30 amino acids. Rag A/B are only ∼20% identical to rag C/D, and each is ∼17–18% identical to Ha-ras, with long carboxy-terminal extensions beyond the Ha-ras sequence; the rag A/B polypeptides associate as heterodimers with rag C/D through these carboxy-terminal segments (103) and function as obligatory heterodimers. In the most active form, rag A/B is GTP charged, whereas rag C/D is GDP charged. Although evidence exists that ras, for example, may also function as dimers (53), the formation of stable dimers and, especially, the discordant phosphorylation state of the guanyl nucleotide in each half of the active form of the rag dimer, are entirely unique features of these small GTPases. The rag proteins are homologous to the Gtr1p and Gtr2p proteins of S. cerevisiae; human rag A[Q66L] will rescue a Gtr1 LOF mutant (45). Gtr1 was originally retrieved in mutant form in screens for suppressors of an RCC1 (Ran-GEF) mutant, and wild-type Gtr1 was shown to negatively regulate Gsp1/Ran through Gtr2 (80, 103). Recombinant rag A-GTP is predominantly cytoplasmic, whereas rag A-GDP is seen in a speckled pattern in the nucleus, and rag C/D, when coexpressed, follows rag A (103). The reported association of Gtr1/2 or rag with a variety of nuclear proteins (104, 116, 119) supports the likelihood that these GTPases cycle through the nucleus. Gao and Kaiser (32), however, identified a primarily endosomal localization for Gtr1/2; they retrieved Gtr1 and Gtr2 in a screen for genes required for the function of the general amino acid transporter GAP1, which is translocated to the cell surface in response to amino acid deficiency (12, 93). Pull-down studies with the Gtr1/2 polypeptides recovered the proteins EGO1/GSE3, EGO3/GSE2, and Ltv1, all of which were required for GAP1 function. A constitutive complex of Gtr1, Gtr2, EGO1, and EGO3 is tethered to endosomes through EGO3; EGO1/3 are small (184/162 AA) polypeptides without metazoan homologs. The ability of the complex to enable translocation of GAP1 to the PM depends on a direct interaction between Gtr2p and GAP1, GTP charging of Gtr1p, and, secondarily, GDP charging of Gtr2p (32). The EGO-Gtr complex, which remains on the endsome, probably promotes the loading of GAP1 onto a cycling vesicle for translocation to the PM. The EGO-Gtr complex is relatively specific for GAP1 among amino acid permeases, although Gtr1 was reported earlier to be important for functional expression of the phosphate transporter Pho84 (8).
The first evidence linking these small GTPases with TORC1 came from screens in S. cerevisiae. Dubouloz et al. (23) identified strains mutant for EGO1/3 and Gtr2 as deficient in their recovery from rapamycin inhibition. The EGO-Gtr complex was observed to associate with the vacuolar membrane; in response to rapamycin, the vacuole enlarges greatly in size due to fusion with autophagic vesicles. During recovery from rapamycin, the excess vacuolar membrane is endocytosed into the vacuole, a process called microautophagy; this does not occur in the EGO-Gtr mutants. Retrograde traffic of vacuolar membrane to other compartments is not impaired in the EGO mutants, indicating that this process is not sufficient to restore TORC1 activity. In contrast, mutations that promote the synthesis or inhibit the degradation of glutamate/glutamine suppress the effects of EGO LOF on recovery from rapamycin. More recently, a screen in S. pombe (127) seeking genes that enhance the growth defect of a partial TOR1 (predominantly TORC1) LOF mutation retrieved all four components of the EGO-Gtr complex, as well as vps34/vps15, many vps class C genes that are required for vesicle trafficking, along with numerous genes encoding ribosomal and mitochondrial components. As to the mechanism by which impairment of vesicle traffic synergizes with TORC1 LOF, temperature-sensitive vps11 or vps18 class C mutants shifted to the nonpermissive temperature exhibited very pronounced (50–90%) and relatively selective decreases in the intracellular levels of glutamate and/or glutamine. Taken together, these data suggest that the EGO-Gtr complex, among its various actions, controls membrane traffic to promote adequate intracellular amino acid (especially Glu/Gln) levels.
The existence of a direct physical and functional interaction between the rag GTPases and TORC1 was demonstrated by the finding that immunoprecipitates of TORC1 from mammalian cells coprecipitate rag C (94). The rags were shown to bind as heterodimers to endogenous and coexpressed TORC1, apparently through a direct association with raptor. Binding is strongly dependent on rag A/B in the GTP form is and enhanced if rag C/D is GDP charged. Independently, Kim et al. (61) generated shRNA against all 132 Drosophila small GTPases and found that depletion of the Drosophila rag A/B and C/D homologs, as well as of D-Rheb selectively reduced DS6K1[Thr398-P]. A constitutively activated mutant of rag B, expressed together with rag C is able to restore S6K1-P in amino acid deprived cells with a potency similar to Rheb, whereas stressors that inhibit TORC1 at the level of the TSC complex continue to inhibit mTORC1 despite expression of a constitutively activated rag B+C complex. Thus, depletion of endogenous Rheb-GTP greatly diminishes the stimulatory effect of recombinant rag B+C and sufficient recombinant Rheb-GTP can activate TORC1 independently of rag. Dominant inhibitory Rag A/B variants suppress the response to both amino acids and to insulin (61, 94). Although endogenous Rheb-GTP requires active rag to promote TORC1 signaling, the ability of recombinant, overexpressed Rheb to activate mTORC1 is largely unaffected if rags are depleted with RNAi. Conversely, the ability of rags, even if overexpressed in active form, to stimulate TORC1 requires Rheb-GTP. Evidence for a specific role of rags in the nutrient-dependent regulation of TORC1 comes from Drosophila; overexpression of activated variants of the rag heterodimer greatly increase cell size under starvation conditions, with little impact in the fed state. Conversely, dominant inhibitory rag mutants decrease cell size in the fed but not the fasted state (61).
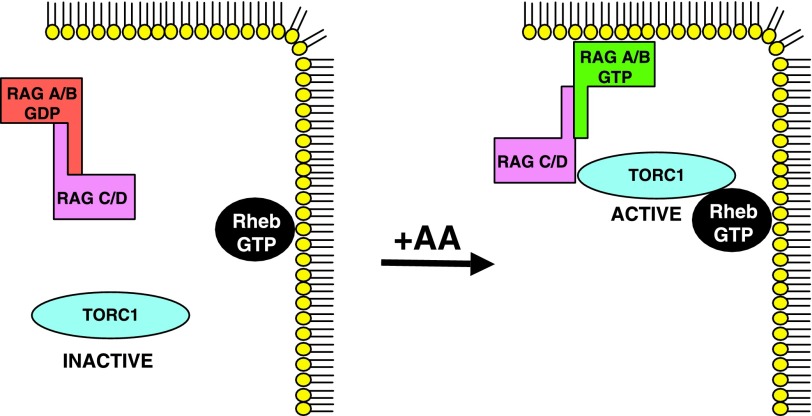
As to the mechanism of rag action in the regulation of TORC1, Sancak et al. (94) report that readdition of amino acids to deprived cells increases GTP charging of endogenous rag from 44 to 63% and increases the association of rag with TORC1, the latter demonstrable only by addition of cross-linker to the cells prior to lysis. Immunocytochemistry shows mTOR to be diffusely cytoplasmic in amino acid-deprived cells but to aggregate onto a rab7-rich vesicular compartment upon readdition of amino acids. Rag B behaves similarly, but the constitutively active rag B[Q99L] is constitutively associated with this rab7 compartment, as is Rheb. Depletion of rag A/B, rag C/D, or raptor with shRNAs prevents the recruitment of mTOR to these rab7 vesicles by amino acids. Sancak et al. (94) propose that amino acids, by promoting rag A/B GTP charging, promote rag association with raptor and the recruitment of mTORC1 to the (rab7-containing) membrane compartment that contains the mTORC1 proximal activator Rheb (Fig. 2).
Fig. 2.
Possible mechanism of action of the rag GTPases in amino acid regulation of mTORC1. The rag GTPases exist as an obligatory heterodimer. GTP charging of the rag A/B partner, possibly dependent on amino acid sufficiency, promotes association of the rag heterodimer with mTORC1 and its translocation to a membrane compartment enriched in Rheb, thereby enabling mTORC1 activation when Rheb is GTP charged. Based on data in Refs. 61 and 93; see text for details.
Caveats and questions: the rag GTPases.
The evidence indicates that rags are critical components of the pathway by which amino acids regulate TORC1 activity. The major question is whether these GTPases are situated upstream or downstream of the amino acid signal, or perhaps both. The properties of the yeast EGO-Gtr complexes described above (23, 32, 127) indicates that they function by a variety of mechanisms to promote the accumulation of intracellular amino acids. Although there is no evidence for a structural or functional equivalent of an EGO-Gtr complex in mammalian cells, it will be important to determine whether the activated mammalian rag heterodimer increases intracellular amino acid levels in deprived cells, and if so, whether its ability to activate mTORC1 is dependent on such an increase. If rag activation of TORC1 is mediated primarily by an increase in intracellular amino acids, the site of amino acid action would remain to be defined.
Several subcellular localizations and itineraries for the rag heterodimers have been described (23, 32, 94, 103); this may reflect the existence of compositionally and functionally distinct rag-containing complexes, whose function is to enable the movement of polypeptides among membrane compartments or, for TORC1, perhaps from cytosol to a membrane compartment, in response to stimuli that alter their GTP charging. The ability of amino acids to regulate rag GTP charging, if confirmed, along with ability of the GTP-activated rag heterodimer to bind raptor (23, 32, 94, 103) provide the strongest evidence for an action of the rags as mediators of amino acid signaling to mTORC1. If the function of the rag A/B GTP charging is to promote the binding of the rag heterodimer to raptor, what enables the transfer of the complex to the rab7 compartment in response to amino acid sufficiency? In any case, the mechanism by which amino acids regulate rag GTP charging is of considerable interest.
Clearly, the rag heterodimer may plausibly function both upstream and downstream of TORC1, promoting sufficient intracellular amino acids through TORC1-independent control of vesicle trafficking of relevant polypeptides while simultaneously acting directly on mTORC1 to enable its optimal activation by Rheb-GTP.
Mechanisms of TORC1 Regulation: Type III PI 3-Kinase/vps34
Several reports propose a role for the type III PI 3-kinase, the homolog of the S. cerevisiae vps34, as a signaling intermediate in the amino acid regulation of TORC1 (9, 81). Type III PI 3-kinase vps34, together with its kinase-like partner vps15, phosphorylates PI exclusively at the 3′-OH group and is the only PI kinase in S. cerevisiae. In mammalian cells, the hvps34/hvps15 heterodimer is best recognized for its many roles in endosomal trafficking and sorting and for its requirement in the initiation of autophagy (4, 68). PI3P is found primarily on early endosomes and the internal vesicles of multivesicular endosomes; PI3P-rich microdomains serve as platforms to build trafficking complexes through the interaction of PI3P with proteins that contain FYVE and PX domains. The important evidence linking type III PI 3-kinase to mTORC1 includes the finding that extracellular amino acids are capable of regulating hvps34 lipid kinase activity (9), perhaps through a Ca2+-dependent mechanism (37); withdrawal of extracellular amino acid reduces immunoprecipitable hvps34 activity by ∼50% (9). In addition, depletion of vps34 or vps15 in mammalian cells inhibits amino acid-stimulated S6K1 phosphorylation, and overexpression of recombinant vps34 and vps15 increase S6K1 phosphorylation, especially if amino acids are present. Overexpression of a dimeric FYVE domain sequesters PI3P itself and also inhibits S6K1 phosphorylation (81). Moreover, some coprecipitation of hvps34 with endogenous mTOR is detectable (37). Taken together, these findings are supportive of the conclusion that hvps34, through the generation of PI3P, participates in the activation of mTORC1. It should be noted, however, that genetic elimination of the type III PI 3-kinase in Drosophila has no impact on DTORC1 signaling (55), and we observe that RNAi depletion of vps34 or vps 15 in C. elegans recapitulates none of the phenotypes of CeTOR or CeRaptor deficiency (Ref. 90; X. Long and J. Avruch, unpublished observation). It remains possible, however, that vps34, through the generation of PI3P, participates in the amino acid signaling to TORC1 only in vertebrate or mammalian cells. Such participation might be as a direct regulator of the mTORC1 kinase (not evident in vitro) or by contributing to the generation of a membrane compartment necessary for optimal mTORC1 activation. Alternatively, however, it is also possible that the action of PI 3-kinase is much more indirect and is mediated by its contributions to vesicle trafficking, e.g., to the lysosome and thus to the provision of intracellular amino acids.
Mechanisms of TORC1 Regulation: MAP4K3/Germinal Center Kinase-Related Kinase
Findlay et al. (30) carried out an RNAi screen for Drosophila protein kinases necessary for the hyperphosphorylation of DS6K1, and identified a Ste20 family member (MAP4K3) whose depletion inhibited S6K1 and 4E-BP phosphorylation and whose overexpression increased S6K1-P in a PI 3-kinase-independent but rapamycin-sensitive manner. The activity of the recombinant MAP4K3 was reduced by amino acid withdrawal and restored by amino acid readdition but was insensitive to insulin or rapamycin. MAP4K3/Germinal center kinase-related protein kinase has been previously implicated in the TNFa and Wnt3a regulation of JNK activation in lymphocytes (105, 106). The site of action of this protein kinase and its physiological role in the regulation of TORC1 signaling remain to be defined.
GRANTS
The work of the authors cited herein was supported by National Institutes of Health Grants DK-17776 and CA-73818.
Acknowledgments
We thank Jeanette Prendable for assistance in preparation of the manuscript.
REFERENCES
- 1.Ali SM, Sabatini DM. Structure of S6 kinase 1 determines whether raptor-mTOR or rictor-mTOR phosphorylates its hydrophobic motif site. J Biol Chem 280: 19445–19448, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem 279: 36553–36561, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Aspuria PJ, Sato T, Tamanoi F. The TSC/Rheb/TOR signaling pathway in fission yeast and mammalian cells: temperature sensitive and constitutive active mutants of TOR. Cell Cycle 6: 1692–1695, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Backer JM The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J 410: 1–17, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science 318: 977–980, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Barbet NC, Schneider U, Helliwell SB, Stansfield I, Tuite MF, Hall MN. TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell 7: 25–42, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J 15: 5256–5267, 1996. [PMC free article] [PubMed] [Google Scholar]
- 8.Bun-Ya M, Harashima S, Oshima Y. Putative GTP-binding protein, Gtr1, associated with the function of the Pho84 inorganic phosphate transporter in Saccharomyces cerevisiae. Mol Cell Biol 12: 2958–2966, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem 280: 33076–33082, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Castro AF, Rebhun JF, Clark GJ, Quilliam LA. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J Biol Chem 278: 32493–32496, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Cheatham L, Monfar M, Chou MM, Blenis J. Structural and functional analysis of pp70S6k. Proc Natl Acad Sci USA 92: 11696–11700, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen EJ, Kaiser CA. Amino acids regulate the intracellular trafficking of the general amino acid permease of Saccharomycescerevisiae. Proc Natl Acad Sci USA 99: 14837–14842, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Zheng XF, Brown EJ, Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA 92: 4947–4951, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi J, Chen J, Schreiber SL, Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science 273: 239–242, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Christie GR, Hajduch E, Hundal HS, Proud CG, Taylor PM. Intracellular sensing of amino acids in Xenopus laevis oocytes stimulates p70 S6 kinase in a target of rapamycin-dependent manner. J Biol Chem 277: 9952–9957, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Coffey VG, Hawley JA. The molecular bases of training adaptation. Sports Med 37: 737–763, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci 28: 7202–7208, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 312: 927–930, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Crespo JL, Powers T, Fowler B, Hall MN. The TOR-controlled transcription activators GLN3, RTG1, and RTG3 are regulated in response to intracellular levels of glutamine. Proc Natl Acad Sci USA 99: 6784–6789, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 355: 1345–1356, 2006. [DOI] [PubMed] [Google Scholar]
- 21.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev 22: 239–251, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care 11: 222–226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubouloz F, Deloche O, Wanke V, Cameroni E, De Virgilio C. The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol Cell 19: 15–26, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Edlich F, Erdmann F, Jarczowski F, Moutty MC, Weiwad M, Fischer G. The Bcl-2 regulator FKBP38-calmodulin-Ca2+ is inhibited by Hsp90. J Biol Chem 282: 15341–15348, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Edlich F, Maestre-Martinez M, Jarczowski F, Weiwad M, Moutty MC, Malesevic M, Jahreis G, Fischer G, Lucke C. A novel calmodulin-Ca2+ target recognition activates the Bcl-2 regulator FKBP38. J Biol Chem 282: 36496–36504, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Edlich F, Weiwad M, Erdmann F, Fanghanel J, Jarczowski F, Rahfeld JU, Fischer G. Bcl-2 regulator FKBP38 is activated by Ca2+/calmodulin. EMBO J 24: 2688–2699, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, Sessa WC, Qin J, Zhang P, Su B, Jacinto E. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J 27: 1932–1943, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang Y, Park IH, Wu AL, Du G, Huang P, Frohman MA, Walker SJ, Brown HA, Chen J. PLD1 regulates mTOR signaling and mediates Cdc42 activation of S6K1. Curr Biol 13: 2037–2044, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294: 1942–1945, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Findlay GM, Yan L, Procter J, Mieulet V, Lamb RF. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling. Biochem J 403: 13–20, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev 16: 1472–1487, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao M, Kaiser CA. A conserved GTPase-containing complex is required for intracellular sorting of the general amino-acid permease in yeast. Nat Cell Biol 8: 657–667, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell 11: 1457–1466, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Gietzen DW, Hao S, Anthony TG. Mechanisms of food intake repression in indispensable amino acid deficiency. Annu Rev Nutr 27: 63–78, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Gromov PS, Madsen P, Tomerup N, Celis JE. A novel approach for expression cloning of small GTPases: identification, tissue distribution and chromosome mapping of the human homolog of rheb. FEBS Lett 377: 221–226, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11: 859–871, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab 7: 456–465, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall DJ, Grewal SS, de la Cruz AF, Edgar BA. Rheb-TOR signaling promotes protein synthesis, but not glucose or amino acid import, in Drosophila. BMC Biol 5: 10, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, Gietzen DW. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 307: 1776–1778, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem 273: 14484–14494, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253: 905–909, 1991. [DOI] [PubMed] [Google Scholar]
- 44.Hinnebusch AG Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 59: 407–450, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Hirose E, Nakashima N, Sekiguchi T, Nishimoto T. RagA is a functional homologue of S. cerevisiae Gtr1p involved in the Ran/Gsp1-GTPase pathway. J Cell Sci 111: 11–21, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Hsu YC, Chern JJ, Cai Y, Liu M, Choi KW. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature 445: 785–788, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Iiboshi Y, Papst PJ, Kawasome H, Hosoi H, Abraham RT, Houghton PJ, Terada N. Amino acid-dependent control of p70 (s6k). Involvement of tRNA aminoacylation in the regulation. J Biol Chem 274: 1092–1099, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J 27: 1919–1931, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 37: 19–24, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17: 1829–1834, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126: 955–968, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Inouye K, Mizutani S, Koide H, Kaziro Y. Formation of the Ras dimer is essential for Raf-1 activation. J Biol Chem 275: 3737–3740, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6: 1122–1128, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Juhasz G, Hill JH, Yan Y, Sass M, Baehrecke EH, Backer JM, Neufeld TP. The class III PI (3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol 181: 655–666, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kadowaki M, Karim MR, Carpi A, Miotto G. Nutrient control of macroautophagy in mammalian cells. Mol Aspects Med 27: 426–443, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Kanazawa T, Taneike I, Akaishi R, Yoshizawa F, Furuya N, Fujimura S, Kadowaki M. Amino acids and insulin control autophagic proteolysis through different signaling pathways in relation to mTOR in isolated rat hepatocytes. J Biol Chem 279: 8452–8459, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Keith CT, Schreiber SL. PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science 270: 50–51, 1995. [DOI] [PubMed] [Google Scholar]
- 59.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell 11: 895–904, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10: 881–883, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr 83: 500S–507S, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Krause U, Bertrand L, Maisin L, Rosa M, Hue L. Signalling pathways and combinatory effects of insulin and amino acids in isolated rat hepatocytes. Eur J Biochem 269: 3742–3750, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Kunz J, Henriquez R, Schneider U, Deuter-Reinhard M, Movva NR, Hall MN. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell 73: 585–596, 1993. [DOI] [PubMed] [Google Scholar]
- 65.Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet 14: R251–R258, 2005. [DOI] [PubMed] [Google Scholar]
- 66.LaLonde M, Janssens H, Yun S, Crosby J, Redina O, Olive V, Altshuller YM, Choi SY, Du G, Gergen JP, Frohman MA. A role for phospholipase D in Drosophila embryonic cellularization. BMC Dev Biol 6: 60, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lam E, Martin M, Wiederrecht G. Isolation of a cDNA encoding a novel human FK506-binding protein homolog containing leucine zipper and tetratricopeptide repeat motifs. Gene 160: 297–302, 1995. [DOI] [PubMed] [Google Scholar]
- 68.Lindmo K, Stenmark H. Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci 119: 605–614, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10: 457–468, 2002. [DOI] [PubMed] [Google Scholar]
- 70.Long X, Lin Y, Ortiz-Vega S, Busch S, Avruch J. The Rheb switch 2 segment is critical for signaling to target of rapamycin complex 1. J Biol Chem 282: 18542–18551, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol 15: 702–713, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem 280: 23433–23436, 2005. [DOI] [PubMed] [Google Scholar]
- 73.Long X, Spycher C, Han ZS, Rose AM, Muller F, Avruch J. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Curr Biol 12: 1448–1461, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Lynch CJ Role of leucine in the regulation of mTOR by amino acids: revelations from structure-activity studies. J Nutr 131: 861S–865S, 2001. [DOI] [PubMed] [Google Scholar]
- 75.Lynch CJ, Fox HL, Vary TC, Jefferson LS, Kimball SR. Regulation of amino acid-sensitive TOR signaling by leucine analogues in adipocytes. J Cell Biochem 77: 234–251, 2000. [DOI] [PubMed] [Google Scholar]
- 76.Lynch CJ, Gern B, Lloyd C, Hutson SM, Eicher R, Vary TC. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am J Physiol Endocrinol Metab 291: E621–E630, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Maurin AC, Jousse C, Averous J, Parry L, Bruhat A, Cherasse Y, Zeng H, Zhang Y, Harding HP, Ron D, Fafournoux P. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab 1: 273–277, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Minami K, Tambe Y, Watanabe R, Isono T, Haneda M, Isobe K, Kobayashi T, Hino O, Okabe H, Chano T, Inoue H. Suppression of viral replication by stress-inducible GADD34 protein via the mammalian serine/threonine protein kinase mTOR pathway. J Virol 81: 11106–11115, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mordier S, Deval C, Bechet D, Tassa A, Ferrara M. Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubes through a mammalian target of rapamycin-independent signaling pathway. J Biol Chem 275: 29900–29906, 2000. [DOI] [PubMed] [Google Scholar]
- 80.Nakashima N, Noguchi E, Nishimoto T. Saccharomyces cerevisiae putative G protein, Gtr1p, which forms complexes with itself and a novel protein designated as Gtr2p, negatively regulates the Ran/Gsp1p G protein cycle through Gtr2p. Genetics 152: 853–867, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA 102: 14238–14243, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem 278: 15461–15464, 2003. [DOI] [PubMed] [Google Scholar]
- 83.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 153: 1011–1022, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oldham S, Montagne J, Radimerski T, Thomas G, Hafen E. Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev 14: 2689–2694, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oshiro N, Yoshino K, Hidayat S, Tokunaga C, Hara K, Eguchi S, Avruch J, Yonezawa K. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells 9: 359–366, 2004. [DOI] [PubMed] [Google Scholar]
- 86.Pacold ME, Suire S, Perisic O, Lara-Gonzalez S, Davis CT, Walker EH, Hawkins PT, Stephens L, Eccleston JF, Williams RL. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell 103: 931–943, 2000. [DOI] [PubMed] [Google Scholar]
- 87.Patel PH, Thapar N, Guo L, Martinez M, Maris J, Gau CL, Lengyel JA, Tamanoi F. Drosophila Rheb GTPase is required for cell cycle progression and cell growth. J Cell Sci 116: 3601–3610, 2003. [DOI] [PubMed] [Google Scholar]
- 88.Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, Thomas G. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J 14: 5279–5287, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roccio M, Bos JL, Zwartkruis FJ. Regulation of the small GTPase Rheb by amino acids. Oncogene 25: 657–664, 2006. [DOI] [PubMed] [Google Scholar]
- 90.Roggo L, Bernard V, Kovacs AL, Rose AM, Savoy F, Zetka M, Wymann MP, Muller F. Membrane transport in Caenorhabditis elegans: an essential role for VPS34 at the nuclear membrane. EMBO J 21: 1673–1683, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roh C, Han J, Tzatsos A, Kandror KV. Nutrient-sensing mTOR-mediated pathway regulates leptin production in isolated rat adipocytes. Am J Physiol Endocrinol Metab 284: E322–E330, 2003. [DOI] [PubMed] [Google Scholar]
- 92.Rosner M, Hofer K, Kubista M, Hengstschlager M. Cell size regulation by the human TSC tumor suppressor proteins depends on PI3K and FKBP38. Oncogene 22: 4786–4798, 2003. [DOI] [PubMed] [Google Scholar]
- 93.Rubio-Texeira M, Kaiser CA. Amino acids regulate retrieval of the yeast general amino acid permease from the vacuolar targeting pathway. Mol Biol Cell 17: 3031–3050, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell 25: 903–915, 2007. [DOI] [PubMed] [Google Scholar]
- 96.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302, 2004. [DOI] [PubMed] [Google Scholar]
- 97.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22: 159–168, 2006. [DOI] [PubMed] [Google Scholar]
- 98.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005. [DOI] [PubMed] [Google Scholar]
- 99.Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol 5: 566–571, 2003. [DOI] [PubMed] [Google Scholar]
- 100.Schalm SS, Blenis J. Identification of a conserved motif required for mTOR signaling. Curr Biol 12: 632–639, 2002. [DOI] [PubMed] [Google Scholar]
- 101.Schalm SS, Fingar DC, Sabatini DM, Blenis J. TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr Biol 13: 797–806, 2003. [DOI] [PubMed] [Google Scholar]
- 102.Schliess F, Richter L, vom Dahl S, Haussinger D. Cell hydration and mTOR-dependent signalling. Acta Physiol (Oxf) 187: 223–229, 2006. [DOI] [PubMed] [Google Scholar]
- 103.Sekiguchi T, Hirose E, Nakashima N, Ii M, Nishimoto T. Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. J Biol Chem 276: 7246–7257, 2001. [DOI] [PubMed] [Google Scholar]
- 104.Sekiguchi T, Todaka Y, Wang Y, Hirose E, Nakashima N, Nishimoto T. A novel human nucleolar protein, Nop132, binds to the G proteins, RRAG A/C/D. J Biol Chem 279: 8343–8350, 2004. [DOI] [PubMed] [Google Scholar]
- 105.Shi CS, Huang NN, Harrison K, Han SB, Kehrl JH. The mitogen-activated protein kinase kinase kinase kinase GCKR positively regulates canonical and noncanonical Wnt signaling in B lymphocytes. Mol Cell Biol 26: 6511–6521, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi CS, Leonardi A, Kyriakis J, Siebenlist U, Kehrl JH. TNF-mediated activation of the stress-activated protein kinase pathway: TNF receptor-associated factor 2 recruits and activates germinal center kinase related. J Immunol 163: 3279–3285, 1999. [PubMed] [Google Scholar]
- 107.Shigemitsu K, Tsujishita Y, Hara K, Nanahoshi M, Avruch J, Yonezawa K. Regulation of translational effectors by amino acid and mammalian target of rapamycin signaling pathways. Possible involvement of autophagy in cultured hepatoma cells. J Biol Chem 274: 1058–1065, 1999. [DOI] [PubMed] [Google Scholar]
- 108.Shigemitsu K, Tsujishita Y, Miyake H, Hidayat S, Tanaka N, Hara K, Yonezawa K. Structural requirement of leucine for activation of p70 S6 kinase. FEBS Lett 447: 303–306, 1999. [DOI] [PubMed] [Google Scholar]
- 109.Shirane M, Nakayama KI. Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and inhibits apoptosis. Nat Cell Biol 5: 28–37, 2003. [DOI] [PubMed] [Google Scholar]
- 110.Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem 280: 18717–18727, 2005. [DOI] [PubMed] [Google Scholar]
- 111.Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol 25: 5834–5845, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stipanuk MH Leucine and protein synthesis: mTOR and beyond. Nutr Rev 65: 122–129, 2007. [DOI] [PubMed] [Google Scholar]
- 113.Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol 5: 559–565, 2003. [DOI] [PubMed] [Google Scholar]
- 114.Sun Y, Fang Y, Yoon MS, Zhang C, Roccio M, Zwartkruis FJ, Armstrong M, Brown HA, Chen J. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci USA 105: 8286–8291, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 13: 1259–1268, 2003. [DOI] [PubMed] [Google Scholar]
- 116.Todaka Y, Wang Y, Tashiro K, Nakashima N, Nishimoto T, Sekiguchi T. Association of the GTP-binding protein Gtr1p with Rpc19p, a shared subunit of RNA polymerase I and III in yeast Saccharomyces cerevisiae. Genetics 170: 1515–1524, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Urano J, Comiso MJ, Guo L, Aspuria PJ, Deniskin R, Tabancay AP Jr, Kato-Stankiewicz J, Tamanoi F. Identification of novel single amino acid changes that result in hyperactivation of the unique GTPase, Rheb, in fission yeast. Mol Microbiol 58: 1074–1086, 2005. [DOI] [PubMed] [Google Scholar]
- 118.Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem 281: 39128–39134, 2006. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Nakashima N, Sekiguchi T, Nishimoto T. Saccharomyces cerevisiae GTPase complex: Gtr1p-Gtr2p regulates cell-proliferation through Saccharomyces cerevisiae Ran-binding protein, Yrb2p. Biochem Biophys Res Commun 336: 639–645, 2005. [DOI] [PubMed] [Google Scholar]
- 120.Watanabe R, Tambe Y, Inoue H, Isono T, Haneda M, Isobe K, Kobayashi T, Hino O, Okabe H, Chano T. GADD34 inhibits mammalian target of rapamycin signaling via tuberous sclerosis complex and controls cell survival under bioenergetic stress. Int J Mol Med 19: 475–483, 2007. [PubMed] [Google Scholar]
- 121.Weng QP, Andrabi K, Kozlowski MT, Grove JR, Avruch J. Multiple independent inputs are required for activation of the p70 S6 kinase. Mol Cell Biol 15: 2333–2340, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weng QP, Kozlowski M, Belham C, Zhang A, Comb MJ, Avruch J. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. J Biol Chem 273: 16621–16629, 1998. [DOI] [PubMed] [Google Scholar]
- 123.Wu B, Ottow K, Poulsen P, Gaber RF, Albers E, Kielland-Brandt MC. Competitive intra- and extracellular nutrient sensing by the transporter homologue Ssy1p. J Cell Biol 173: 327–331, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamagata K, Sanders LK, Kaufmann WE, Yee W, Barnes CA, Nathans D, Worley PF. rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J Biol Chem 269: 16333–16339, 1994. [PubMed] [Google Scholar]
- 125.Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev 14: 2712–2724, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol 5: 578–581, 2003. [DOI] [PubMed] [Google Scholar]
- 127.Zurita-Martinez SA, Puria R, Pan X, Boeke JD, Cardenas ME. Efficient Tor signaling requires a functional class C Vps protein complex in Saccharomyces cerevisiae. Genetics 176: 2139–2150, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]