Abstract
Estrogen (E2) is reported to regulate skeletal muscle mass and contractile function; whether E2 exerts its effects through estrogen receptor-α (ERα) or -β (ERβ) is unclear. We determined the effect of ERα or ERβ elimination on muscle mass and contractile function in multiple muscles of the lower limb, muscles with different locomotor tasks and proportions of fiber types I and II: soleus (Sol), plantaris (Plan), tibialis anterior (TA), and gastrocnemius (Gast) in mature female mice. To determine E2 elimination effects on muscle, we also used aromatase (Ar) knockout (KO) and wild-type (WT) mice. ERα and ArKO body weights were ∼10 and 20% higher than WT. Although muscle mass tended to show a commensurate increase in both groups, only the TA was significantly larger in ERα (P < 0.05). Ratios of muscle mass to body mass revealed significantly lower values for Gast and TA in ArKO mice (P < 0.05). Tetanic tension (Po) per calculated anatomical cross-sectional area (aCSA) in ERα KO was lower in TA and Gast than in WT. Lower Po/aCSA in ERα KO Gast and TA was also supported histologically by significantly less Po/fiber areas (P < 0.05). ArKO mice also had lower Po/aCSA in Gast and TA compared with WT. ERβ KO and WT mice were comparable in all measures. Our results support the hypothesis that E2 effects on skeletal muscle are mediated in part via the ERα but that E2 effects may be mediated via more than one mechanism or receptor.
Keywords: muscle mass, peak tetanic tension, fiber area, myosin protein
estrogens (e2) exert diverse physical effects in many tissues, including the female reproductive tract, mammary tissues, and cardiovascular, immune, nervous, and skeletal systems (14, 41, 42). Human and animal studies (6, 8, 24) have revealed estrogen receptor (ER) involvement in female sexual development and behavior, reproductive function, immune function, regulation of the neuroendocrine and cardiovascular systems, and bone metabolism.
In contrast to other tissues, relatively little is known about the effects of E2 on skeletal muscle (3) and the effects of E2 on skeletal muscle have been controversial. Previous studies (1, 7, 23, 47) have shown that E2 reduces skeletal muscle damage, hypothetically by stabilizing the muscle membrane. Other researchers (33, 39, 48) suggest that E2 has an anabolic effect on muscle. Conversely, several groups (18, 22, 32, 44) have reported that E2 administration to previously ovariectomized (OVX) rats decreases muscle mass and fiber size and reduces the maximal isometric contraction force.
Findings from our laboratory, and one other, indicate that E2 is important (12) and necessary for the restoration of atrophic muscle (2, 31, 43). For E2 to be considered seriously as an adjunct in rehabilitation as an anabolic agent for atrophic muscle, its safety must be assured. Hence, the development of a compound with the anabolic properties of E2 without the detrimental side effects requires understanding which ER mediates its effects. The need to identify the subtype of ER associated with muscle function motivated this research.
Two ER subtypes, ERα and ERβ, mediate E2 signaling, and they function as ligand-dependent transcription factors (11, 14, 35–37, 45). In addition to ligand-dependent ER activation, the ER can also be activated independently of E2, e.g., by growth factors (14). For example, IGF-1 phosphorylation of Akt is dependent on ERα in breast cancer cells (52).
Both ERs have been identified in skeletal muscle (19–21, 26–28, 50), but their role in the control of skeletal muscle function is poorly understood. To date, there are no studies investigating the loss of ERα on skeletal muscle contractile function and only one study (13) that explored the effect of ERβ knockout (KO) on skeletal muscle. Thus the potential ERα influence on muscle mass and contractile function is unknown, and results evaluating ERβ loss on muscle tissue are limited. Consequently, the primary purpose of the present study was to examine the potential consequences of ERα and ERβ loss on muscles with different architecture, fiber type, and role: soleus (Sol), plantaris (Plan), gastrocnemius (Gast), and tibialis anterior (TA) in mature female mice.
Kahlert et al. (20) reported that skeletal myoblasts contain E2 receptors that, when stimulated by estrone, show significant growth; both estrone and 17β-estradiol induce expression of transcription factors. Accordingly, we hypothesized that ERs play a role in skeletal muscle by ligand-dependent activation. However, Kahlert et al. (20) did not identify how estrone interacted with ERs, binding either one receptor type or both. Based on the previous reports that ERβ is highly expressed in many nonclassical E2 target tissues (46) and that ERα is highly expressed at the mRNA level but not the protein level in skeletal muscle (50), we hypothesized that ERα KO may not influence skeletal muscle and that muscle contractile properties and muscle mass in ERα KO mice would be comparable with those of corresponding wild-type (WT) mice. In addition, we hypothesized that muscle mass, fiber areas, tetanic tension, and protein in ERβ KO mice would be diminished compared with the WT control mice.
It has been reported that ERα and ERβ KO animals have twofold or more serum E2 than WT mice (16). As it is possible that E2 exerts its effects on skeletal muscle via indirect pathways that bypass the ER, we also chose to examine the consequences of E2 deficiency in mature aromatase (Ar) KO and WT control mice, which have been examined only to a limited extent by one group of investigators (30). Aromatase is an enzyme of the cytochrome P450 superfamily and functions to aromatize androgens to produce E2.
METHODS
Animals.
Mature female ERα, ERβ, and ArWT and ArKO mice bred and maintained by the Lubahn laboratory in a C57BL6/J background were used for this study. Mice were 5–8 mo of age at the time of study to avoid the confound of characterizing animals in the rapid growth phase of development. The protocols used to generate the KO models have been published (17, 25, 29). Briefly, for ERα mice a Neo gene was targeted to the N-terminal of exon 2 to disrupt the reading frame of the α-receptor in embryonic stem cells. The KO mice were made on a 129 background. Because of genetic variations that result from difference in the number of backcrosses into the C57BL6 background, we always compare KO mice to WT littermates or WT mice from the same genotype. For ERβ mice, a similar process was used in that a Neo gene driven by a PGK promoter was inserted in the reverse orientation into the Pst I site in the first zinc finger in exon 3 and completely removes all E2 response element binding activity. ArKO mice were created by deletions of exons 1 and 2 of the cyp19 gene. Since this is a complete KO of all enzyme activity and there is only one aromatase gene in mice, this mouse is completely devoid of estradiol. KO and WT status was repeat verified at the end of all experiments using DNA from tail snips.
The protocols used for this study complied with the guidelines of the American Physiological Society. The study was approved by the University of Missouri Institutional Animal Care and Use Committee (protocol no. 4179).
Contractile properties.
As type I fiber dominant muscles are reported to have more ER than type II dominant muscles (27), muscles with different fiber type proportions, function, and likely distribution of ER were chosen for study. To determine contractile properties, mice were anesthetized with pentobarbital sodium (0.15 ml pentobarbital with 0.85 ml saline) with 0.15 ml as first injection and anesthesia was maintained with a 0.05-ml injection given as needed. Each mouse was placed sidelying on a water-jacketed heating pad that maintained body temperature at 37°C. The left Sol, Plan, Gast, and TA muscles were surgically exposed only at their insertions. Sol, Plan, Gast, and TA muscles are uni- or multipennate; span one or more joints; vary from 40–95% type II myosin heavy chain distribution; function as locomotor, postural, or antigravity muscles; and likely have varying proportions of ER. The distal tendon of each muscle was attached in turn to Grass force transducer with 4.0 silk. Tibial and peronal nerves were isolated and placed in turn on a bipolar stimulating electrode. The exposed tendon of each muscle and the nerves were bathed continuously with 37°C mineral oil.
For contractile testing, the left hindlimb and mouse torso were rigidly immobilized and muscles were attached in the order of Sol→Plan→Gast→TA to a force transducer by the distal tendon and adjusted in length so that passive tension was 0 g. A twitch was obtained at that position with the parameters: 0.5 ms, 0.3 Hz, at 6 V; subsequently, the micromanipulator was used to progressively lengthen each muscle to the point where peak twitch was attained (Lo). At optimal length, a peak tetanic contraction (Po) was elicited by pulses delivered at 150 Hz, 300-ms duration, and an intensity of 6 V for each type muscle. Preliminary study revealed 6V to be supramaximal; the 300-ms duration was greater than what was required to achieve Po. Force curves generated at 15, 50, 75, 100, and 125 Hz revealed that all muscles were maximally recruited usually by the time 100 Hz was reached. All data were collected using Power Lab. The peak rate of tension development (+dP/dt) was obtained from the steepest linear portion of the Po curve. The duration of contractile function testing was ∼15 min.
In pilot studies, random testing of muscles was done, as well as testing in the order TA→Gast→Plan→Sol, and no differences in tension were observed, regardless of stimulation order. Repeat testing of Sol and Plan during preliminary study and subsequently during actual stimulation indicated the protocol did not result in a decrement of force.
Tissue harvest.
After contractile characteristics were obtained, left and right Sol, Plan, Gast, and TA muscles were removed, cleaned of extraneous tissue, blotted, and weighed. Left-sided muscles, those that were electrically stimulated, were pinned at their in situ length, embedded in optimum cutting temperature tissue-freezing medium, frozen slowly in chilled 2-methylbuterol, and then placed in liquid nitrogen and stored at −80°C. The right unstimulated muscles were snap frozen in liquid nitrogen and subsequently stored in a −80°C freezer until analysis.
In subsequent experiments with additional mice, Sol, Plan, Gast, and TA muscles were removed, pinned at their in situ length, and immersed in 15% nitric acid for 24 h to digest connective tissue. After being rinsed, each muscle was placed in a 50% solution of glycerol/water. Under a dissecting microscope, multiple individual muscle fibers were teased out and fiber length was measured in ∼25 fibers per muscle. Muscle lengths were averaged and used to derive anatomical cross-sectional areas (aCSA) for each muscle using the formula: aCSA = muscle mass/fiber length × muscle density (1.0562).
Histochemistry.
Left-sided optimum cutting temperature prepared muscles from ER mice were thawed to −22°C in a microtome, oriented vertically, and sectioned at 10 μm. Sections were stained using traditional hematoxylin and eosin to reveal evidence of potential muscle damage or inflammation and with NADH. Fiber areas were obtained from NADH-stained sections. Photos were taken from multiple cross-sections to obtain a minimum of 200 fibers for fiber area measures. Area determinations were done using a calibrated pen by circling each fiber. Image J software (National Institutes of Health) was used to derive area data, which were subsequently copied into an Excel spreadsheet.
Due to a complete freezer failure with loss of all frozen tissues, fiber area measures were not obtained in sufficient numbers to permit determination of Po/fiber area for all muscles. Fiber areas were obtained in sufficient sample numbers (n = 2–6 muscles) in each group for the Gast and TA, which are reported.
Total protein.
Total protein is a general indicator of muscle well-being and was determined for right unstimulated muscles. To do the assay, muscle samples were homogenized in HES buffer (20 mM HEPES, 1 mM EDTA, and 250 mM surcose, pH to 7.4) and standard samples (×3) of 10 μg were derived. Protein content was determined using the BCA protein assay (Sigma protein standard, microstandard, 1 mg BSA/ml in 0.15 M NaCl, and 0.05% NaN3) and a spectrophotometer (Bausch and Lomb; Milton Roy: Spectronic 24).
Total myosin.
Myosin content is closely associated with the contractile tension capabilities of muscle (41) and was measured for the Gast and TA because of their lower Po/CSA and for ERα Plan because of the significantly higher Po. Before the muscle samples were loaded, the protein homogenates from the BCA protein assay were diluted with equal parts of Laemmli buffer containing β-mercapthoethanol and heated to 100°C for 3 min as described by Moran et al. (34). Ten micrograms of total protein from each muscle were separated on a Bio-Rad mini gel (7.5% SDS-PAGE Tris·HCl) with an actin myosin control (Bio-Rad) as a standard. Gel electrophoresis was performed in SDS running buffer (12.1 g Tris, 11.3 g glycine, and 1 g SDS) until the tracking dye ran off the gel (Laemmli). The gel was stained overnight with Coomassie blue (0.1% Coomassie blue R 250, 40% methanol, and 10% glacial acetic acid) and destained with Coomassie destain (40% methanol and 10% glacial acetic acid). Gels were dried overnight using gel drying materials (Promega gel drying kit). Dried gels were scanned using HP Scan Jet software and subsequently analyzed using the Kodak 1D 3.6 program for densitometric analysis.
Statistical analysis.
Because the research question of interest was focused on differences between WT and KO groups, mean differences were analyzed using the two-tail Student's t-test. Statistical significance was set at P ≤ 0.05. When muscle weights were used to derive ratio data (e.g., muscle weight-to-body weight ratios), weights from the right (unstimulated) muscle were entered.
RESULTS
Body weight and muscle mass.
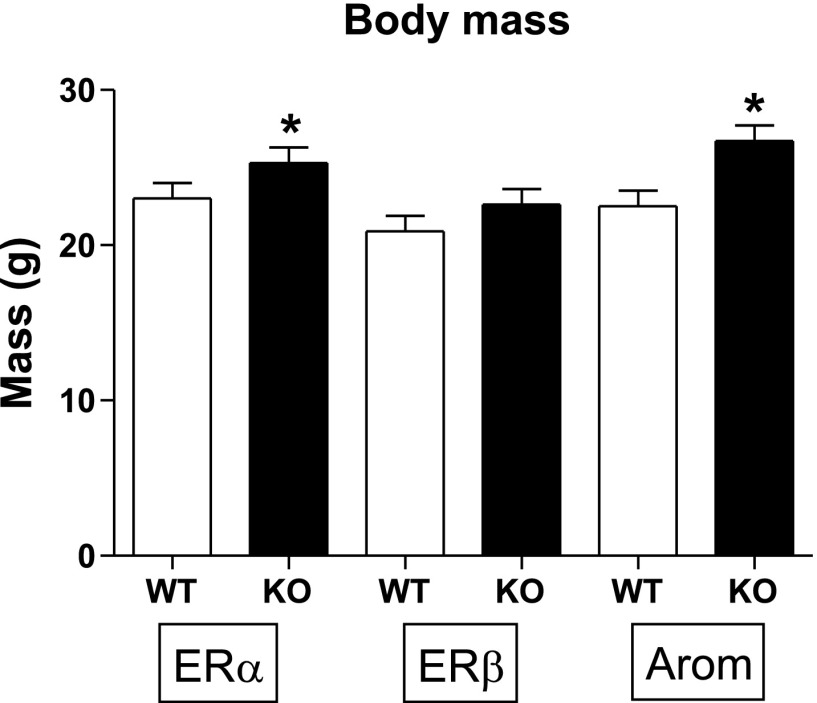
ERα KO animals had ∼10% higher body weight (P < 0.005) than WT mice (Fig. 1). ArKO mice were ∼20% larger than ArWT, which was also significant (P < 0.001).
Fig. 1.
Data are means ± SE. Body mass in grams. ER, estrogen receptor; WT, wild type; KO, knockout; Arom, aromatase (Ar). Sample sizes were as follows: ERαWT, n = 13; ERαKO, n = 17; ERβWT, n = 10; ERβKO, n = 11; ArWT, n = 8; ArKO n = 12. *P < 0.05.
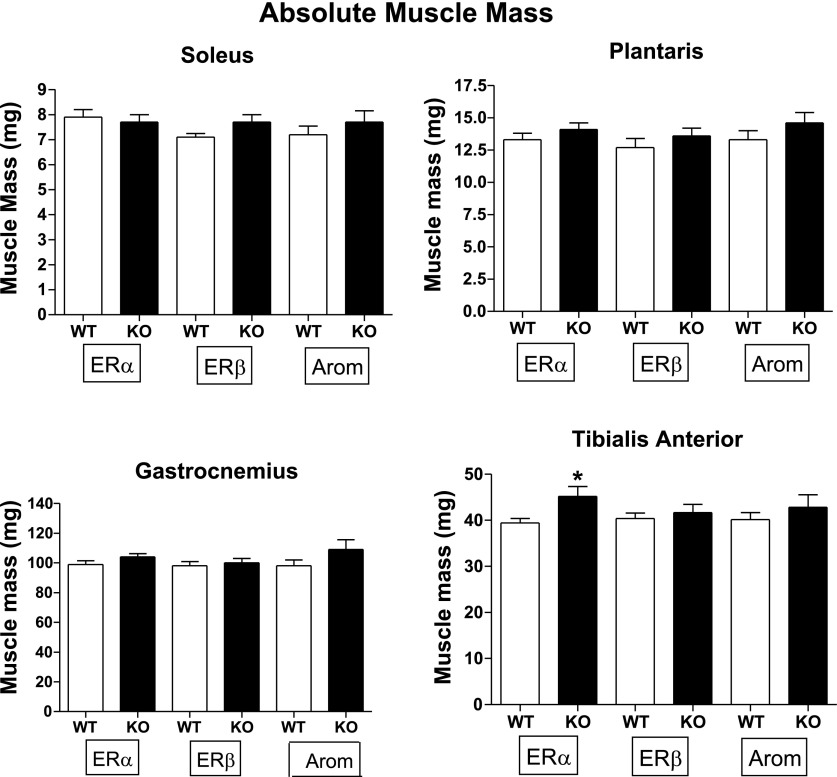
TA muscle mass was ∼15% higher in ERα KO mice (P < 0.05) than in WT mice (Fig. 2). There were no significant differences in ERβ KO Gast, TA, Plan, or Sol muscle mass compared with WT litter mates. Consistent with the larger body mass, muscles from ArKO mice tended to be larger (7–14%) but differences were not statistically significant. (Fig. 2).
Fig. 2.
Data are means ± SE. Absolute wet muscle mass (mg). Sample sizes were as follows: ERαWT, n = 13; ERαKO, n = 17; ERβWT, n = 10; ERβKO, n = 11; ArWT, n = 8; ArKO, n = 12. *P < 0.05.
Muscle weight-to-body weight ratio.
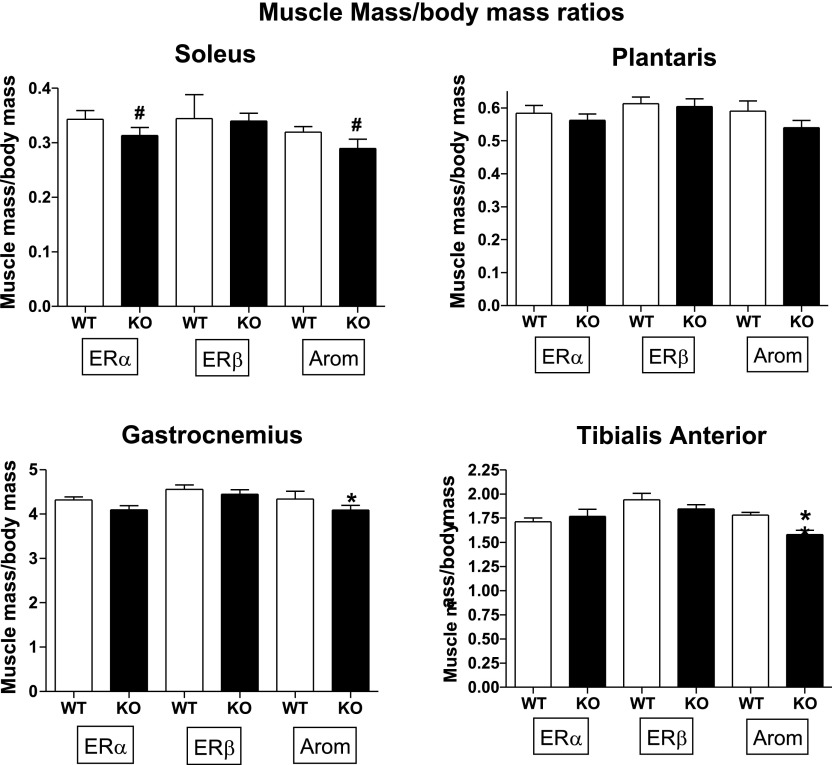
Although ERα KO status increased absolute TA muscle mass, the ratio of muscle mass to body mass eliminated the significant difference. Gast and TA in Ar KO mice had smaller ratios than WT (Fig. 3). In most animals, muscle size was a reflection of body size, but for Ar KO mice, general body growth did confer comparable muscle growth for the TA or Gast (P < 0.05).
Fig. 3.
Muscle mass normalized to body mass. Sample sizes were as follows: ERαWT, n = 13; ERαKO, n = 17; ERβWT, n = 10; ERβKO, n = 11; ArWT, n = 8; ArKO n = 12. *P < 0.05. #P = 0.08 for ERα and P = 0.06 for ArKO soleus.
In situ contractile properties.
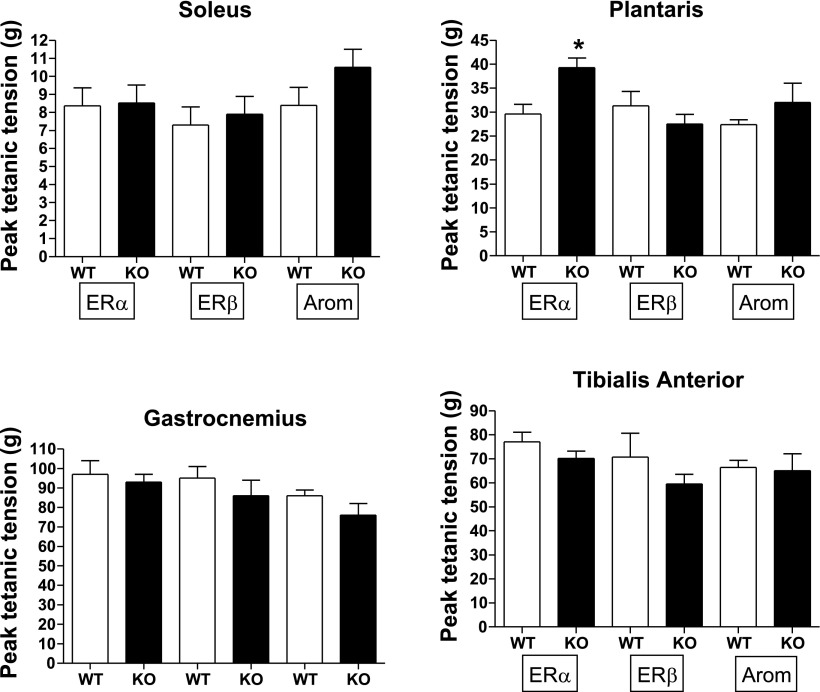
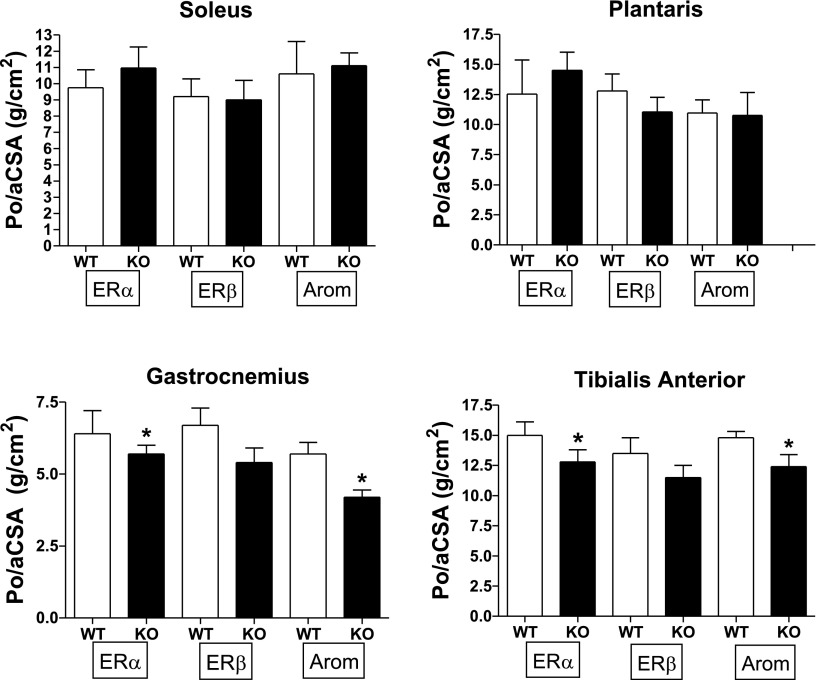
Twitch tension, +dP/dt, absolute Po, and Po/anatomical aCSA for Sol, Plan, Gast, and TA are presented in Table 1 and in Figs. 4 and 5. Plantaris Po was significantly higher in ERα KO (∼30%) than in WT controls, but this significant difference disappeared when Po/aCSA was examined. The nearly significant reduction in ERα KO Gast Po (P = 0.059) did become significant when tension was expressed per aCSA (Fig. 5) and when expressed per fiber area (Table 2). TA Po for ERα KO approached significance (P = 0.084) and became significant with the ratio of Po/aCSA (P < 0.05) and as the ratio of Po/fiber area (Table 2; Fig. 5). These findings indicate that the ERα KO Gast and TA could not produce as much force per unit area as a WT mouse. There were no additional ERα KO effects on contractile properties.
Table 1.
Twitch tension and −dP/dt
| ERα WT (n = 13) | ERα KO (n = 17) | ERβ WT (n = 10) | ERβ KO (n = 11) | ArWT (n = 8) | ArKO (n = 12) | |
|---|---|---|---|---|---|---|
| Soleus | ||||||
| Pt, g | 5.1±0.5 | 3.6±0.3 | 2.8±0.4 | 3.3±0.5 | 3.8±0.4 | 5.1±0.5 |
| dP/dt, g/s | 2,240±84 | 2,533±72 | 2,539±124 | 2,686±105 | 2,404±98 | 2,600±105 |
| Plantaris | ||||||
| Pt, g | 9.4±0.9 | 11.4±0.8 | 8.3±1.1 | 8.1±1.0 | 7.6±1.1 | 9.3±1.3 |
| dP/dt, g/s | 2,705±100 | 2,898±82 | 2,711±123 | 2,883±115 | 2,436±92 | 2,735±142 |
| Gastrocnemius | ||||||
| Pt, g | 29.8±3.1 | 28.1±2.2 | 23.3±3.2 | 26.4±2.3 | 22.7±2.6 | 23.4±1.2 |
| dP/dt, g/s | 4,072±214 | 3,815±167 | 3,414±251 | 3,402±251 | 3,975±215 | 4,505±268 |
| Tibialis anterior | ||||||
| Pt, g | 21.9±1.6 | 20.5±1.6 | 17.1±1.8 | 16.2±1.8 | 17.0±1.1 | 19.0±2.0 |
| dP/dt, g/s | 3,334±175 | 3,336±143 | 3,087±205 | 3,278±202 | 3,398±215 | 3,886±248 |
Values are means ± SE. ER, estrogen receptor; Ar, aromatase; Pt, twitch tension; +dP/dt, peak rate of tension development; WT, wild type; KO, knockout.
Fig. 4.
Absolute peak tetanic tension (g) in soleus, plantaris, gastrocnemius, and tibialis anterior. *P < 0.05.
Fig. 5.
Peak tetanic tension (Po) expressed per derived anatomical cross-sectional area (CSA; g/cm2). *P < 0.05.
Table 2.
Gast and TA muscle fiber area and Po/fiber areas
| ERα WT (n = 12) | ERα KO (n = 8) | ER WT (n = 6) | ERβ KO (n = 6) | ArWT (n = 3) | ArKO (n = 2) | |
|---|---|---|---|---|---|---|
| Gast | ||||||
| Muscle fiber area, μm2 | 2,207±334 | 2,576±177 | 2,760±165 | 2,237±226 | 1,674±206 | 1,377±204 |
| Po/fiber area | 0.050±0.007 | 0.037±0.005* | 0.036±0.009 | 0.038±0.012 | 0.045±0.013 | 0.047±0.011 |
| TA | ||||||
| Muscle fiber area, μm2 | 1,914±233 | 2,052±258 | 2,412±332 | 2,003±174 | 1,707±223 | 1,674±205 |
| Po/fiber area | 0.040±0.010 | 0.027±0.006* | 0.022±0.011 | 0.027±0.006 | 0.039±0.010 | 0.039±0.009 |
Values are means ± SE. Po, peak tetanic tension; Gast, gastrocnemius; TA, tibialis anterior. Area differences were not significant.
Po/fiber area: P < 0.05.
For ERβ mice, there were no significant effects of KO status on contractile measures (Table 1; Figs. 4 and 5).
Although ArKO mice had significantly higher body masses, absolute peak tensions did not reflect greater muscle demand (Fig. 4). The ArKO Gast and TA had significantly lower Po/aCSA values compared with WT, which was not reflected in Po/fiber area (Table 2).
A summary of contractile properties is given in Table 3.
Table 3.
Summary of muscle contractile function differences in KO mice compared with WT controls
| Soleus | Plantaris | Gastrocnemius | Tibialis Anterior | |
|---|---|---|---|---|
| Absolute tetanic tension | ||||
| ERα−/− | — | +↑ | — | — |
| ERß−/− | — | — | — | — |
| ArKO | — | — | — | — |
| Tetanic tension/anatomical CSA | ||||
| ERα−/− | — | — | +↓ | +↓ |
| ERß−/− | — | — | — | — |
| ArKO | — | — | +↓ | +↓ |
| +dP/dt | ||||
| ERα−/− | — | — | — | — |
| ERß−/− | — | — | — | — |
| ArKO | — | — | — | — |
CSA, cross-sectional areas; -, condition of study had no effect; +, significant condition effect; ↑ and ↓, direction of significant effect.
Muscle protein content.
There was no significant difference between KO mice and their WT controls for muscle total (g/g) protein content (data not shown).
Myosin content.
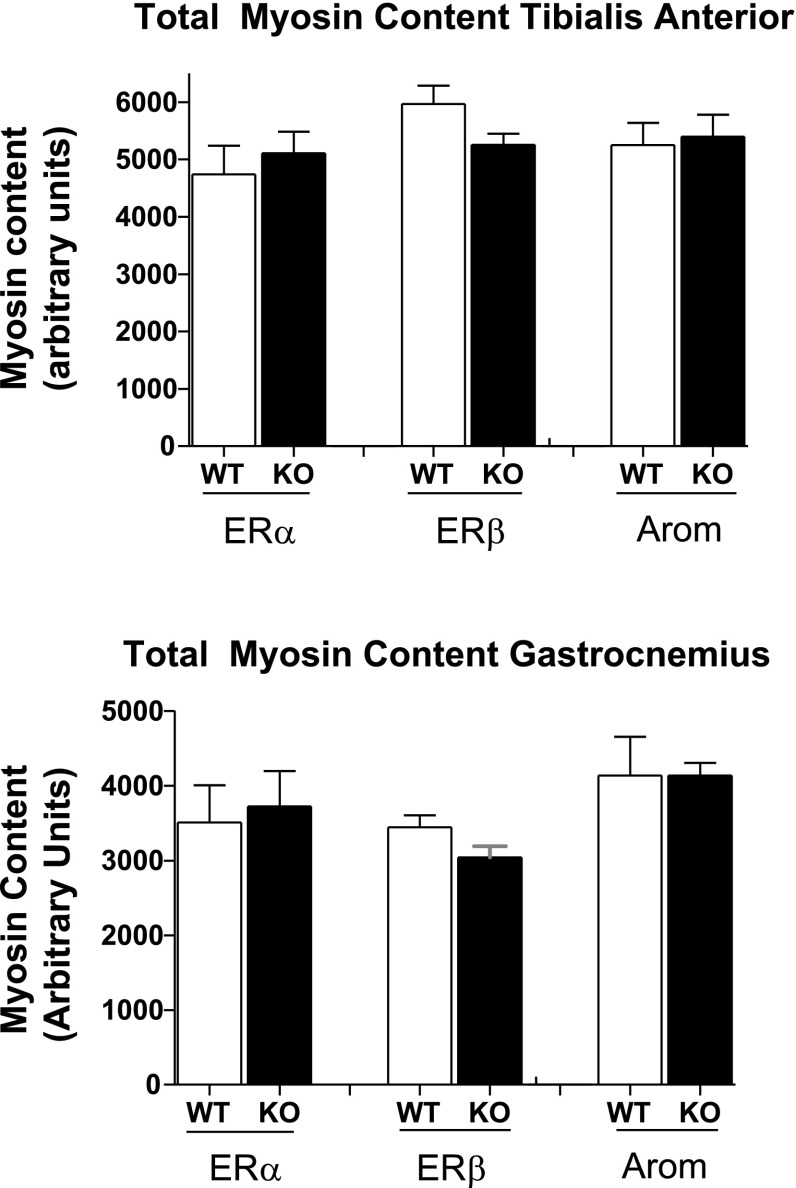
Even though there was a decline in tension/aCSA for ERα TA and Gast and ArKO Gast and an increase in ERα KO Pla, there were no significant differences in myosin content for any of the KO groups compared with WT (Fig. 6).
Fig. 6.
Total myosin protein in arbitrary units. There were no differences in total myosin content between groups.
Gast and TA muscle fiber area.
Fiber CSA was determined for Gast and TA as tetanic tension/aCSA was lower in these muscles. Average fiber CSA for each group is presented in Table 2. There was no significant difference in Gast or TA fiber area between KO and WT groups.
DISCUSSION
Several investigators (12, 30, 34) have found a loss in muscle mass and/or contractile tension in E2-deficient animals, suggesting that E2 may affect quality and quantity of the contractile proteins actin and myosin. Findings from this study support these results in that lack of E2 had a detrimental effect on muscle contractile quality but not for every muscle studied. We expected to find clear differences in muscle function among one or more groups of KO mice, but the interpretation of our current results shows that the story is more complex.
Recently, Moran et al. (34) demonstrated in the mouse extensor digitorum muscle that the loss of E2 diminished Po, the consequence of altered actin:myosin interaction. Subsequently, Moran et al. (33) restored Po and actin-myosin binding characteristics in OVX E2-deficient mice by giving them estradiol. These findings strongly support the need for E2 to maintain optimal muscle contractile function. Our results also indicate that E2 has effects on peak tension that may be mediated through the α-receptor.
The presence of ER in skeletal muscle suggests that skeletal muscle is a target tissue for E2 (9), but whether E2 impacts skeletal muscle tissue through ERα or ERβ or through other indirect pathways has been unclear. The results suggest the probability that the α-receptor mediates E2 effects and compensates for a missing β-receptor. At least in Sol and Plan, the ERβ may compensate for the missing α-receptor. Findings also suggest that there are ligand-dependent and ligand-independent effects of ER that are muscle specific.
ERα KO effects on skeletal muscle tissue.
Our major findings in the ERα group were that ERα KO resulted in 1) an increase in whole body weight and TA muscle mass; 2) a reduction in Po/aCSA in Gast and TA muscles; and 3) a Plan Po that was significantly greater in ERα KO than in WT female mice but not when expressed per aCSA.
ERα KO mice had an ∼13% higher body weight compared with WT animals. This finding is in agreement with previous reports (16, 49) demonstrating an ∼14% increase in adult body weight in ERα KO female mice. There was also a concomitant increase in muscle TA mass. However, muscle mass-to-body weight ratios were not different, suggesting that the increase in muscle size was the consequence of general body growth or occurred in response to increased demand (larger body size). Fisher et al. (12) demonstrated that mature OVX rats had an ∼18% increase in body mass and about the same percentile increase in both type I and type II myosin heavy chain dominant muscle mass compared with intact rats. Fisher et al. (12) found the increase in body size and muscle mass in rats was significantly correlated to IGF-1 values, which increased ∼35%. IGF-1 reportedly does not increase with OVX in the mouse, indicating that a different mechanism (e.g., increased growth hormone) for the increase in body and muscle mass exists in the two species (49). When IGF-1 levels are low, there may be a compensatory increase in growth hormone as evidenced by Yakar et al. (51). However, muscle is an autocrine organ producing its own IGF-1; potentially, within tissue IGF-1, levels regulate muscle mass independently of whole body growth or growth hormone. Nonetheless, the exact mechanism by which muscle mass is regulated and the discovery of the molecular effects of ERs within muscle are a long-term goals of our laboratories.
There are still other factors that may be responsible for the observed effects on contractile function in ERα KO female mice. Changes in ERα-to-ERβ ratios or lacking ERα and ERβ heterodimer formation (15) in ERα KO mice could have effects on Po. However, these may not be the only factors explaining the decreased Po/aCSA in ERα KO mice, as only two muscles of four were different between the ERα KO and WT groups (Table 3). Potentially, the elevated circulating estradiol levels in ERα KO female mice (4, 5) may generate signaling via the remaining ERβ or through other ERs (10, 38) to regulate skeletal muscle contractile tension.
Although loss of ERα decreased Gast and TA muscle Po/aCSA and Po/fiber area, total protein and myosin content were similar in ERα KO and WT mice, which is consistent with the findings of Moran et al. (34). They revealed in OVX rats a lower extensor digitorum longus Po resulting from a smaller fraction of strongly bound myosin, but total myosin levels were comparable for OVX and intact mice. Their finding suggests that loss of ERα in our current study may have affected the fraction of strong-binding myosin in the fast-fibered Gast and TA muscles. Alternatively, the loss of ERα may have altered calcium dynamics in muscle, an entirely different mechanism that could affect muscle function, particularly myosin binding (33).
There are other factors that could affect contractile quality that should be examined in future studies, such as diminished extraceullar matrix proteins, alterations in the structural framework of the sarcolemma or Z-line, and an increase in intramuscular fat and/or connective tissue.
ERβ KO effects on skeletal muscle tissue.
No variables were affected by the loss of ERβ. The only muscle of the four we examined that has been studied heretofore in ERβ mice is the Sol. Contractile function results from the present study are compatible with the previous report of Glenmark et al. (13), who indicated that there were no Sol contractile tension differences between WT and ERβ KO female mice. The lack of ERβ effect suggests that ERα can compensate for the loss and/or muscle ERβ is involved in other functions. Further studies are needed to verify these possibilities.
Aromatase−/− deficiency effects on skeletal muscle.
Of the three conditions studied, loss of aromatized E2 had the greatest effect on body and muscle mass, which is consistent with our prior and other prior findings for OVX rats (12, 40). Our findings are not, however, consistent with those of MacLean et al. (30), who reported no differences for female ArKO mice in either body or muscle mass, when KO mice were compared with WT controls. It is probable that the 9-wk-old mice used in the Maclean et al. (30) study had not yet reached their mature body or muscle mass. It is unclear whether the cre-lox method used by MacLean et al. (30) to produce an ArKO mouse had a differential mass effect than the breeding process used in this study.
Our mice exhibited an increase in body and muscle size consistent with general body growth and suggestive of increased IGF-1 (12). Although ERα KO mice were found to have lower IGF-1 values (49), IGF-1 values for ArKO mice were unchanged (30). Regardless of the mechanism for the increase in size, there was no advantage conferred by this increase, as muscle mass normalized to body mass in ArKO was comparable with WT mice.
Why the Gast and TA muscles were more sensitive to E2 loss than the other muscles examined is unknown, but possibilities include a higher ER density, greater dependence on E2 for contractile protein synthesis (e.g, actin, myosin) than other hormones, and higher susceptibility to E2 mediated actin-myosin interaction. We expected to observe greater deficits in contractile tension in ArKO mice than we did, which suggests that growth factors other than E2 may have affected the study outcomes.
In summary (see Table 3), loss of ERα resulted in increased body mass and TA muscle mass, a higher force production in Pla, and a reduction in Gast and TA muscle force per anatomical CSA. Knocking out ERβ had no effects on any of the variables studied. Loss of aromatized E2 resulted in a large body mass increase with a concomitant increase in muscle size such that muscle mass normalized to body mass was comparable with WT mice. ArKO Gast and TA had lower Po/aCSA. There were no changes in total protein or myosin, but contractile tension results for TA and Gast suggest altered cross-bridge mechanics.
Taken together, our results indicate a complex effect of the removal of ERα and aromatase on skeletal muscle with a clear need for further investigation. Our data support the hypothesis that ligand-dependent and ligand-independent effects of ERs are responsible for the complex outcomes observed. Pharmacological intervention to attenuate the loss of muscle mass and strength with menopause will be complex.
Acknowledgments
Funding from this study came from the Missouri Spinal Injuries Research Board, the University of Missouri Research Board, National Institute of Child Health and Human Development Grant HD-058834, National Institute of Environmental Health Sciences Grant P01 ES-10535, the National Center for Complementary and Alternative Medicine RO1 AT-002978, and the University of Missouri Center for Phytonutrient and Phytochemical Studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bar PR, Amelink GJ, Oldenburg B, Blankenstein MA. Prevention of exercise induced muscle membrane damage by oestradiol. Life Sci 42: 2677–2681, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Brown M, Foley AM, Ferreira JA. Ovariectomy, hind limb unweighting, and recovery effects on skeletal muscle in adult rats. Aviat Space Environ Med 76:1012–1018, 2005. [PubMed] [Google Scholar]
- 3.Ciocca DR, Roig LM. Estrogen receptors in human nontarget tissues: biological and clinical implications. Endocr Rev 16: 35–62, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned, and where will they lead us? Endocr Rev 20: 358–417, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach K. Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild type and ERa-knockout mouse. Endocrinology 138: 4613–4621, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Couse JF, Curtis Hewitt S, Korach KS. Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. J Steroid Biochem Mol Biol 74: 287–296, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Cooke VE, Carlisle RT, Talbot RT, Boswell T, Mitchell MA. Oestrogen receptor alpha (ERα) and beta (ERβ) in chicken skeletal muscle: mediators of myo-protection? Br Poult Sci 44, Suppl: S12–S13, 2003. [Google Scholar]
- 8.Curran EM, Berghaus LJ, Vernetti NJ, Saporita AJ, Lubahn DB, Estes DM. Natural killer cells express estrogen receptor-alpha and estrogen receptor-beta and can respond to estrogen via a non-estrogen receptor-alpha mediated pathway. Cell Immunol 214: 12–20, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Dahlberg E Characterization of the cytosolic estrogen receptor in rat skeletal muscle. Biochim Biophys Acta 16: 65–75, 1982. [DOI] [PubMed] [Google Scholar]
- 10.Das SK, Taylor JA, Korach KS, Paria BC, Dey SK, Lubahn DB. Estrogenic responses in estrogen receptor-alpha deficient mice reveal a distinct estrogen signaling pathway. Proc Natl Acad Sci USA 94: 12786–91, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 127: 4277–4291, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JS, Hasser EM, Brown M. Effects of ovariectomy and hindlimb unloading on skeletal muscle. J Appl Physiol 85: 1316–1321, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Glenmark B, Nilsson M, Gao H, Gustafsson JA, Wright KD, Westerblad H. Difference in skeletal muscle function in male vs. female: role of estrogen receptor-β. Am J Physiol Endocrinol Metab 287: E1125–E1131, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med 356: 340–352, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Hall JM, McDonnell DP. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcription activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140: 5566–5578, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Heine PA, Taylor JA, Lwamoto GA, Lubahn DB, Cook PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA 97: 12729–12734, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honda SJ, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase deficient mice lacking exons 1 and 2 of the cyp 19 gene. Biochem Biophys Res Commun 252: 445–449, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Ihemelandu EC Comparison of effect of oestrogen on muscle development of male and female mice. Acta Anat (Basel) 110: 311–317, 1981. [DOI] [PubMed] [Google Scholar]
- 19.Kalbe C, Mau M, Wollenhaupt K, Rehfeldt C. Evidence for estrogen receptor alpha and beta expression in skeletal muscle of pigs. Histochem Cell Biol 127: 95–107, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kahlert S, Grohe C, Karas RH, Lobbert K, Neyses L, Vetter H. Effects of estrogen on skeletal myoblast growth. Biochem Biophys Res Commun 232: 373–378, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Knutsson A, Glenmark B, Bodin K, Jansson E, Enmark E. Estrogen receptor alpha and beta in human skeletal muscle (Abstract). FASEB J 16: A396, 2002. [Google Scholar]
- 22.Kobori M, Yamamuro T. Effects of gonadectomy and estrogen administration on rat skeletal muscle. Clin Orthop 243: 306–311, 1989. [PubMed] [Google Scholar]
- 23.Koot RW, Amelink CG, Blankenstein MA, Bar PR. Tamoxifen and oestrogen both protect the rat muscle against physiological damage. J Steroid Biochem Mol Biol 40: 689–695, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Korach KS, Emmen JM, Walker VR, Hewitt SC, Yates M, Hall JM, Swope DL, Harrell JC, Couse JF. Update on animal models developed for analyses of estrogen receptor biological activity. J Steroid Biochem Mol Biol 86: 387–391, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95: 15677–15682, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larionov AA, Vasyliev DA, Mason JI, Howie AF, Bersterin LM, Miller WR. Aromatase in skeletal muscle. J Steroid Biochem Mol Biol 84: 485–492, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Lemoine S, Granier P, Tiffoche C, Berthon PM, Thieulant ML, Carre F, Delamarche P. Effect of endurance training on estrogen receptor alpha expression in different rat skeletal muscle type. Acta Physiol Scand 175: 211–217, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Lemoine S, Granier P, Tiffoche C, Rannou-Bekono F, Thieulant ML, Delamarche P. Estrogen receptor alpha mRNA in human skeletal muscles. Med Sci Sports Exerc 35: 439–443, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90: 11162–11166, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacLean HE, Chiu WSM, Notini AJ, Axell AM, Davey RA, McManus JF, Ma C, Plant DR, Lynch GS, Zajac JD. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J 22: 2676–2689, 2008. [DOI] [PubMed] [Google Scholar]
- 31.McClung JM, Davis JM, Wilson MA, Goldsmith EC, Carson JA. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol 100: 2012–2023, 2006. [DOI] [PubMed] [Google Scholar]
- 32.McCormick KM, Burn KL, Piccone CM, Gosselin LE, Brazeau GA. Effects of ovariectomy and estrogen on skeletal muscle function in growing rats. J Muscle Res Cell Motil 25: 21–27, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reveres ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol 102: 1387–1392, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Moran AL, Warren GL, Lowe DA. Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol 100: 548- 559, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson S, Gustafsson JA. Estrogen receptor action. Crit Rev Eukaryot Gene Exp 12: 237–257, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mouse estrogen receptor β forms estrogen response element-binding heterodimers with estrogen receptor α. Mol Endocrinol 11: 1486–1496, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Pfaffl MW, Lange IG, Daxenberger A, Meyer HH. Tissue-specific pattern of estrogen receptors (ER): quantification of ERα and ERβ mRNA with real-time RT-PCR. APMIS 109: 345–355, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Phillips BJ, Ansell PJ, Newton LG, Harada N, Honda S, Ganjam VK, Rottinghaus GE, Welshons WV, Lubahn DB. Estrogen receptor-independent catechol estrogen binding activity: protein binding studies in wild type, estrogen receptor-α KO and aromatase KO tissues. Biochemistry 43: 6698–6708, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci 84: 95–98, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Santollo J, Wiley MD, Eckel LA. Acute activation of ERα decreases food intake, meals size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 293: R2194–R2201, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76: 371–342, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Simpson ER, Misso M, Hewitt KN, Hill RA, Boon WC, Jones ME, Kovacic A, Zhou J, Clyne CD. Estrogen–the good, the bad, and the unexpected. Endocrine Reviews 26: 322–330, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Sitnick M, Foley AM, Brown M, Spangenburg EE. Ovariectomy prevents the recovery of atrophied gastrocnemius muscle mass. J Appl Physiol 100: 286–293, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki S, Yamamuro T. Long-term effects of estrogen on rat skeletal muscle. Exp Neurol 87: 291–299, 1985. [DOI] [PubMed] [Google Scholar]
- 45.Swope DL, Korach KS. Estrogen receptor biology and lessons from knockout mice. In: Encylopedia of Hormones, edited by J Henry and A Norman. Riverside, CA: University of California Press, 2003, vol. 1, pp. 608–614, 2003. [Google Scholar]
- 46.Tayler AH, Al-Azzawi F. Immnunolocalization of estrogen receptor beta in human tissues. J Mol Endocrinol 24: 145–155, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Tiidus PM Influence of estrogen on skeletal muscle damage, inflammation, and repair. Exerc Sport Sci Rev 31: 40–44, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Trenkle A The anabolic effect of estrogens on nitrogen metabolism of growing and finishing cattle and sheep. Environ Qual Saf Suppl 5: 79–88, 1976. [PubMed] [Google Scholar]
- 49.Vidal O, Lindberg M, Savendahl L, Lubahn DB, Ritzen EM, Gustafsson JA, Ohlsson C. Disproportional body growth in female estrogen receptor-alpha-inactivated mice. Biochem Biophys Res Commun 19: 569–571, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Wiik A, Glenmark B, Ekman M, Esbjörnssoon-Liljedahl M, Johansson O, Bodin K, Enmark E, Jansson E. Oestrogen receptor β is expressed in adult human skeletal muscle both at the mRNA and protein level. Acta Physiol Scand 179: 381–387, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor. Proc Natl Acad Sci USA 96: 7324–7329, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Li X, Burghardt R, Smith R III, Safe SH. Role of estrogen receptor (ER) in insulin-like growth factor (IGF)-1-induced responses in MCF-7 breast cancer cells. J Mol Endocrinol 35: 433–437, 2005. [DOI] [PubMed] [Google Scholar]