Abstract
The long-term effects of uncoupled mitochondrial respiration by uncoupling protein-2 (UCP2) in mammalian physiology remain controversial. Here we show that increased mitochondrial uncoupling activity of different tissues predicts longer lifespan of rats compared with mice. UCP2 reduces reactive oxygen species (ROS) production and oxidative stress throughout the aging process in different tissues in mice. The absence of UCP2 shortens lifespan in wild-type mice, and the level of UCP2 positively correlates with the postnatal survival of superoxide dismutase-2 mutant animals. Thus UCP2 has a beneficial influence on cell and tissue function leading to increased lifespan.
Keywords: reactive oxygen species, mitochondria, superoxide dismutase-2, longevity, aging, fatty acid oxidation
uncoupling proteins (UCPs) are inner mitochondrial membrane proteins regulating mitochondrial homeostasis. The physiological function of UCP2, which is found throughout diverse tissues, has been difficult to define. Emerging evidence suggests that UCP2 plays a positive physiological role by regulating fatty acid oxidation, mitochondrial biogenesis, substrate utilization, and reactive oxygen species (ROS) elimination (1, 3, 14, 20) and in doing so provides neuroprotective (2, 5, 10, 17) and anti-aging effects (8, 12). However, numerous studies have indicated that UCP2 has deleterious effects on cell function. For example, UCP2 negatively regulates insulin secretion and contributes to insulin resistance and the pathogenesis of type 2 diabetes (28). Moreover, inhibition of UCP2 reversed obesity- and glucose-induced β-cell dysfunction (29) and restored glucose sensing in hypothalamic proopiomelanocortin (POMC) neurons (19). Paradoxically, however, ucp2−/− mice are more sensitive to weight gain than controls with a high-fat diet (13). Furthermore, UCP2 exacerbated ischemic injury (9), suppressed inflammatory and immune responses (4), and increased sensitivity to apoptosis (25).
A role for UCP2 regulating lifespan is strongly suggested by the ability of UCP2 to reduce ROS and regulate mitochondrial function in a diverse range of tissues (1, 4), since mitochondrial dysfunction and ROS production are at the heart of the aging process. In flies, overexpressing human UCP2 (hUCP2) to adult neurons resulted in increased uncoupled respiration, decreased ROS production, decreased oxidative damage, and extended lifespan without compromising fertility or physical activity (12). These results above support the “uncoupling-to-survive” hypothesis proposed by Brand (7). This hypothesis states that increased uncoupling leads to greater oxygen consumption and reduces the proton motive force by reducing the mitochondrial membrane potential, which subsequently reduces ROS generation. Further evidence for the uncoupling-to-survive hypothesis comes from a study using outbred mice with high or low metabolic intensities (23). Mice with high metabolic intensities had greater resting oxygen consumption and lived longer than mice with low metabolic intensities. The long-lived mice with a high metabolic rate also exhibited greater mitochondrial uncoupling, suggesting that these mice reduce ROS production despite increased oxygen consumption (23).
In this study, we addressed the overall physiological effects of UCP2 on health and disease in a simplistic manner; first we investigated whether mitochondrial uncoupled respiration is inherently different in mammalian species that have different lifespans. We then analyzed the causal role of UCP2 in altering lifespan; first we examined the survival age of ucp2+/+ and ucp2−/− mice and transgenic mice overexpressing hUCP2 (hUCP2 Tg) under normal laboratory conditions.
EXPERIMENTAL PROCEDURES
Mice.
The Institutional Animal Care and Use Committee of Yale University approved all experiments. Mice and rats were kept under standard laboratory conditions with free access to standard chow food and water. The generation of ucp2−/− and transgenic UCP2 (UCP2 + Tg) mice has been previously described (2, 29). Superoxide dismutase-2 (sod2+/−) breeding pairs were purchased from Jackson Laboratory. ucp2+/− and sod2+/− mice were crossed to produce multiple genotypes for the analysis of UCP2 on lifespan of sod2 +/+ and −/− mice. Mice were back-crossed for at least eight generations to ensure a stable genetic background. C57/B6 mice at 2, 6, 15, and 24 mo of age were purchased from National Institutes of Health (NIH) Institute of Aging. Fischer rats at the same ages as above were purchased from Taconic Farms. Mice live on average 24–30 mo of age, whereas rats live on average between 36 and 48 mo of age. Thus we took mice or rats at the same age to determine whether differences in uncoupled respiration, ROS production, and lipid peroxidation could explain differences in lifespan.
Mitochondrial respiration.
Mice and rats were immediately killed and perfused with cold mitochondrial isolation buffer (215 mM mannitol, 75 mM sucrose, 0.1% fatty acid-free BSA, 20 mM HEPES, 1 mM EGTA, pH adjusted to 7.2 with KOH) to preserve mitochondrial integrity while tissues were removed. The brain, liver, and kidney were homogenized in standard isolation buffer while the muscle and heart were homogenized in isolation buffer with the proteinase nagarase (Sigma) after fine chopping to ensure complete homogenization. Mitochondria were isolated using differential centrifugation as previously described (2). Protein concentrations were determined with a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). Mitochondrial respirations were assessed using a Clark-type oxygen electrode (Hansatech Instruments, Norfolk, UK) at 37°C with pyruvate and malate or succinate (with rotenone to inhibit complex 1 activity) as oxidative substrates in respiration buffer (215 mM mannitol, 75 mM sucrose, 0.1% fatty acid-free BSA, 20 mM HEPES, 2 mM MgCl, 2.5 mM KH2PO4, pH adjusted to 7.2 with KOH). After the addition of ADP and oligomycin, UCP-mediated proton conductance was measured as increased fatty acid-induced respiration (11), which was then compared with state 4 respiration induced by oligomycin, an inhibitor of H+ transporting ATP synthase.
Mitochondrial ROS production.
Mitochondria were isolated as described above. ROS production was measured in the same samples that were used for mitochondrial respiration, and ROS production was quantified using dichlorodihydrofluorescein diacetate (DCF). DCF is a cell-permeant indicator for intracellular ROS that is nonfluorescent until the acetate groups are removed by intracellular esterases and oxidation occurs within the cell. Mitochondria were suspended in respiration buffer with DCF and horseradish peroxidase with or without oligomycin, and ROS production was measured using a fluorescence plate reader (excitation 485 nm, emission 528 nm). Oligomycin induces maximal ROS production as it inhibits the ATP synthase. Data are expressed as arbitrary fluorescence units.
Thiobarbituric acid reactive substances assay.
Tissues were homogenized (1:10, wt/vol) in ice-cold aqueous 20 mM Tris·HCl buffer, pH 7.4, using a Teflon pestle motor homogenizer. Lipid peroxidation was assayed in 100 μl of homogenate after the addition of 10 μl of 100 μM Fe3+ (ammonium ferric sulfate) solution, and the mixture was incubated for 30 min at 37°C. The reaction was stopped by the addition of sodium dodecyl sulfate [100 μl of an 8.1% (wt/vol) solution] and 750 μl of 20% acetic acid (adjusted to pH 3.5 using NaOH). Precipitated proteins were then removed by centrifugation at 10,000 g for 15 min. Supernatant was heated in glass tubes capped with aluminum foil with an equal volume of TBA solution (0.8%, wt/vol; heated at 60°C to dissolve) at 95°C for 30 min. Samples were cooled on ice, 100 μl of each were pipetted into 96-well plates, and absorbance was read at 532 nm using a Ceres UV 900C microplate reader. Blank values representing samples containing 20 mM Tris·HCl were subtracted from all values. Each assay was performed in triplicate. Means of triplicates from each assay were pooled to obtain the final mean and SE. Typical thiobarbituric acid reactive substances (TBARS) formation in control brain and brain homogenates plus Fe3+ (100 μM) were calculated from the absorbance at 532 nm using 1,1,3,3-tetramethoxypropane as an external standard.
Aging studies.
The time of death (survival age) was recorded in UCP2 wild-type (WT) and −/− mice as well as UCP2 + Tg to determine whether UCP2 affects lifespan in mice. To assess the role of ROS production and UCP2 in lifespan, we generated a novel line of mice by crossing sod2 with UCP2 mice. All mice were monitored after birth at 7 AM in the morning every day until the day of death of the pups.
ROS production in UCP2/SOD mice.
To assess ROS production in UCP2/SOD2 mice, the brain was collected from embryonic day 18 mice and homogenized for ROS production as described above. A small sample was collected for genotyping.
Statistics.
All data are presented as means ± SE. Data were analyzed using GraphPad Prism 5 for Mac OS X. All data were analyzed with two-way ANOVA followed by Bonferroni's post hoc test except analysis of survival curves. Survival curves were created using the product limit method of Kaplan and Meier and compared using the log-rank test.
RESULTS
Species-dependent mitochondrial uncoupled respiration, ROS production, and oxidative stress.
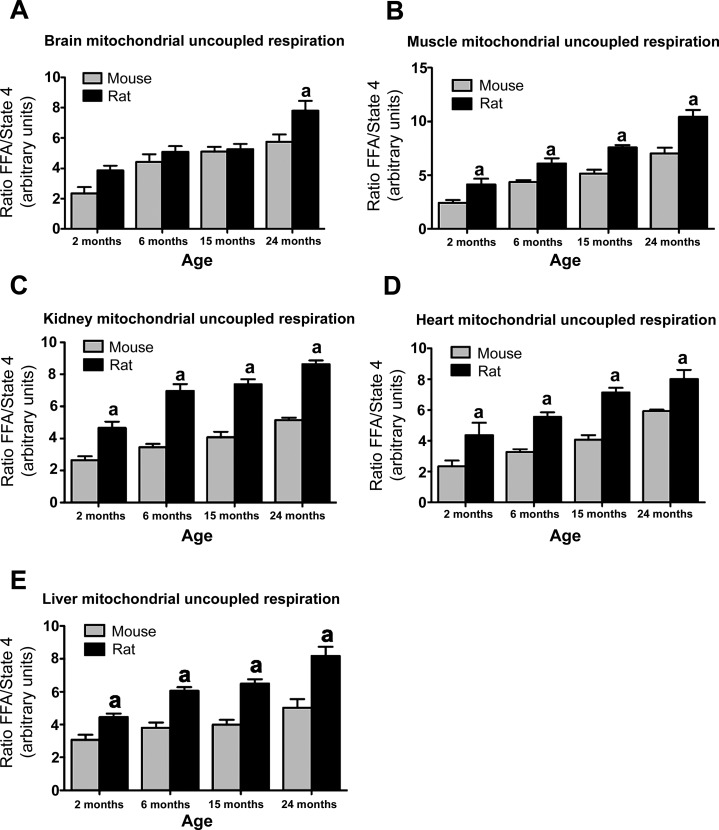
First we investigated whether mitochondrial uncoupled respiration is inherently different in mammalian species that have different lifespans. We studied mice and rats, as rats live ∼12 mo longer than mice. Mitochondrial uncoupled respiration was examined the brain, muscle, heart, kidney, and liver. In the brain, uncoupled respiration was significantly increased at 24 mo of age in rats compared with mice (24-mo-old mice 5.73 ± 0.48 vs. 24-mo-old rats 7.813 ± 0.63; n = 5, P < 0.01; Fig. 1). In the muscle, heart, kidney, and liver, uncoupled respiration was significantly elevated at all times points examined. These results indicate that aging is associated with an increase in uncoupled respiration and that this increase is greater in rats compared with mice.
Fig. 1.
Species-dependent longevity is mediated by uncoupled respiration. Mitochondrial uncoupled respiration in the brain (A), muscle (B), kidney (C), heart (D), and liver (E) increases with age across both mice and rats. However, in general, rats exhibited significantly greater levels of uncoupled respiration compared with mice in all tissues (n = 4–8, P < 0.05; A–E). Uncoupled respiration is the ratio of palmitic acid-driven respiration to state 4 oligomycin-induced respiration. FFA, free fatty acids. aSignificant with respect to mice.
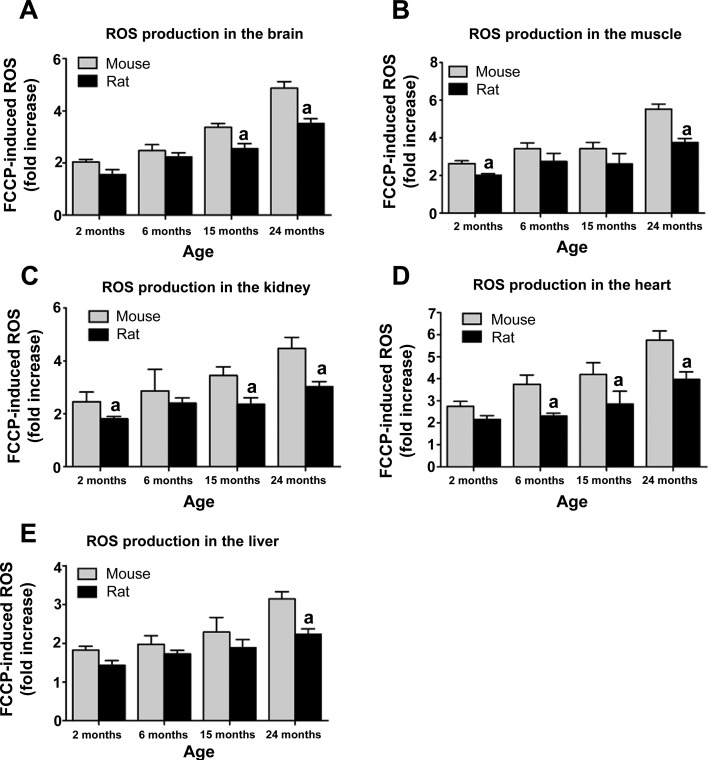
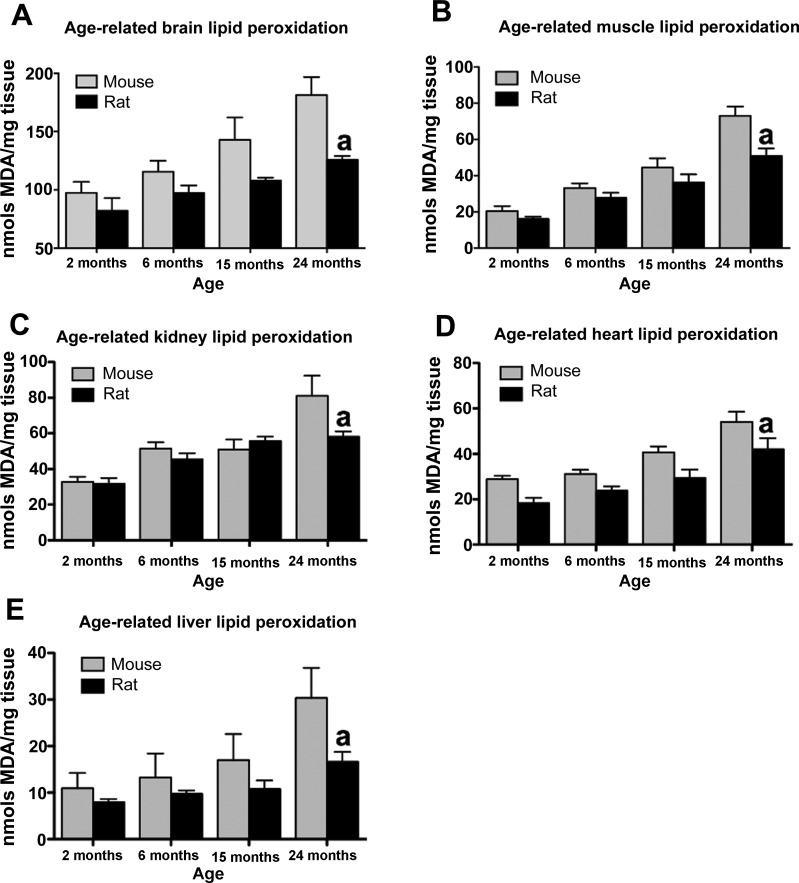
Next we tested whether greater uncoupled respiration in rats translated into altered ROS production. In all tissues examined, ROS production in rats was significantly lower at 24 mo compared with mice (Fig. 2). Furthermore, ROS production increased with age in both mice and rats, consistent with the known effects of aging on ROS production. Elevated ROS production resulted in increased lipid peroxidation in mice at 24 mo of age. Consistent with ROS production results, lipid peroxidation was significantly lower in rats compared with mice at 24 mo of age (Fig. 3). Lipid peroxidation increased with age in both species. These results collectively suggest that greater uncoupled respiration in rats leads to lower levels of mitochondrial ROS production and lipid peroxidation that may underlie differences in lifespan observed in mice and rats.
Fig. 2.
Greater longevity in rats is associated with lower reactive oxygen species (ROS) production compared with mice. Mitochondrial ROS production in the brain (A), muscle (B), kidney (C), heart (D), and liver (E) is significantly reduced at 24 mo of age in rats compared with mice (n = 4–8, P < 0.05). FCCP, carbonyl cyanide p-trifluormethoxyphenylhydrazone. aSignificant with respect to mice.
Fig. 3.
Greater longevity in rats is associated with lower lipid peroxidation at 24 mo of age. Brain (A), muscle (B), kidney (C), heart (D), and liver lipid peroxidation (E) is significantly suppressed in 24-mo-old rats relative to mice (n = 4–8, P < 0.05). MDA, malondialdehyde. aSignificant with respect to mice.
Survival proportions of ucp2−/− and UCP2 overexpressing mice.
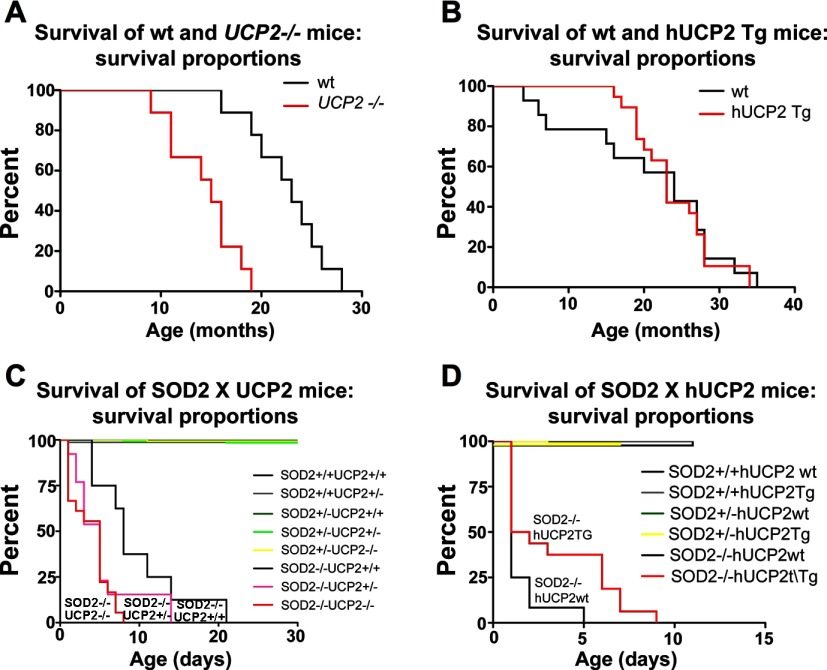
To analyze the causal role of UCP2 in altering lifespan, first we examined the survival age of ucp2+/+ and ucp2−/− mice and transgenic mice overexpressing hUCP2 (hUCP2 Tg) under normal laboratory conditions. Survival curves were generated using the product limit method of Kaplan and Meier. Comparison of survival curves, using the log-rank test, showed that survival age of ucp2−/− mice was significantly reduced compared with that of ucp2+/+ mice (n = 9 for both groups, χ2 = 14.51, df 1, P < 0.0001; Fig. 4A). Mean survival age was significantly greater in ucp2+/+ mice compared with ucp2−/− mice (22.56 ± 1.24 vs. 14.33 ± 1.13 mo; P = 0.0002). No difference between survival curves of hUCP2 Tg mice (n = 14) and WT controls was observed (n = 19, χ2 0.00085, df 1, P > 0.05; Fig. 4B), and mean survival age of hUCP2 mice was not different compared with WT controls [n = 19, 20.93 ± 2.65 vs. 23.95 ± 1.19 mo, not significant (ns)].
Fig. 4.
Uncoupling protein-2 (UCP2) mediates normal lifespan in mice. A: analysis of Kaplan-Meier survival curves indicates that ucp2−/− mice have a significantly shorter survival age compared with UCP2 wild-type (WT) mice (n = 9 for both groups, P < 0.0001, log-rank analysis of Kaplan-Meier survival curves). B: human UCP2 (hUCP2) Tg mice (n = 14) do not have an increased survival age compared with hUCP2 WT mice, although time to first death was delayed in hUCP2 Tg mice (n = 19). C: double knockout of superoxide dismutase-2 (sod2) and UCP2 (n = 7) significantly decreased survival age compared with sod2−/− mice with UCP2 (n = 30, P < 0.0001). No significant spontaneous death events were recorded in sod2 +/− or +/+ mice with or without UCP2. D: presence of hUCP2 Tg in sod2−/− mice significantly increases survival age relative to sod2−/− mice without hUCP2. No significant death events were recorded in other genotypes.
UCP2 mediates survival age in SOD2 mice.
To assess the impact of UCP2 on ROS production and subsequent survival age, we crossed sod2+/− with ucp2+/− and hUCP2 Tg mice. sod2 is a critical mitochondrial enzyme that converts superoxide generated from mitochondrial respiration into oxygen and hydrogen peroxide, thereby neutralizing potential ROS-induced cellular damage. sod2−/− Mice die at ∼3 wk of age from increased mitochondrial oxidative injury mainly in the central nervous system and heart (15). We hypothesized that UCP2 regulates survival age by neutralizing ROS production, and therefore knockout of UCP2 in sod2−/− should decrease survival age, whereas overexpression of hUCP2 should increase survival age. Analysis of survival curves showed that deletion of UCP2 in sod2−/− mice (sod2−/−_ucp2−/−; n = 7) significantly reduced survival age compared with sod2−/−_ucp2+/+ mice (n = 30, χ2 1,031, df 8, P < 0.0001; Fig. 4C). Mean survival age was significantly greater in sod2−/−_ucp2+/+ mice compared with sod2−/−_ucp2−/− mice (9.4 ± 2.18 vs. 2.33 ± 0.47 days, P < 0.0001). Overexpression of UCP2 in sod−/−_hUCP2 Tg (n = 19) mice significantly increased survival age relative to sod2−/−_hUCP2 WT mice (n = 26, χ2 380.1, df 5, P < 0.0001; Fig. 4D) and increased mean survival age (2.63 ± 0.71 vs. 0.69 ± 0.21 days, P < 0.01).
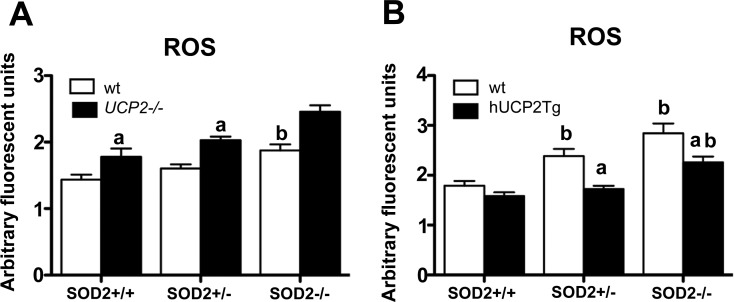
UCP2 regulates ROS production in SOD2 mice.
To analyze that UCP2 regulates ROS production regulates in sod2−/− mice, we measured ROS production in sod2 +/+, +/−, and −/− mice lacking UCP2 or in overexpressing UCP2 compared with WT controls. ROS production was significantly increased in all SOD2 genotypes lacking UCP2 relative to WT controls (sod2+/+_ucp2+/+ 1.44 ± 0.08 vs. sod2+/+_ucp2−/− 1.78 ± 0.12 arbitrary fluorescent units, n = 7 and 8, respectively, P < 0.05; sod2+/−_ucp2+/+ 1.61 ± 0.06 vs. sod2+/−_ucp2−/− 2.03 ± 0.06, n = 13 and 17, respectively, P < 0.05; sod2−/−_ucp2 WT 1.88 ± 0.09 vs. sod2−/−_ucp2−/− 2.46 ± 0.09, n = 6 and 16, respectively, P < 0.05; Fig. 5A). In addition, ROS production in sod2−/− mice relative to sod+/+ mice in both ucp2+/+ and ucp2−/− mice was significantly elevated, consistent with previous studies (15) (Fig. 5A). The role of UCP2 in mediating survival age by neutralizing ROS production is strengthened by our observations that overexpression of UCP2 significantly reduced ROS production in sod2 +/− and −/− genotypes (sod2+/+_hUCP2 WT 1.79 ± 0.09 vs. sod2+/+_hUCP2 Tg 1.59 ± 0.07, n = 6 and 9, respectively, ns; sod2+/−_hUCP2 WT 2.34 ± 0.14 vs. sod2+/−_hUCP2 Tg 1.73 ± 0.06, n = 6 and 11, respectively, P < 0.05; sod2−/−_hUCP2 WT 2.84 ± 0.19 vs. sod2−/−_hUCP2 Tg 2.26 ± 0.12, n = 6 and 6, respectively, P < 0.05; Fig. 5B). Because sod2−/− mice die within 3 wk because of excessive oxidative stress (15), the decreased survival age of sod2−/−_ucp2−/− mice suggests that the ability of UCP2 to increase lifespan is mediated by decreased ROS production and oxidative stress. Indeed, we observed increased ROS production in sod2−/−_ucp2−/− mice. These results, in which UCP2 was able to mediate ROS production and survival age in sod2−/− mice, validate previous studies showing that SOD2 overexpression enhances mitochondrial oxidative tolerance (22) and extends lifespan for sod2−/− mice treated with SOD-catalase mimetics (18).
Fig. 5.
UCP2 mediates lifespan in sod2−/− mice by controlling ROS production. A: ROS production in embryonic day 18 mouse pups was significantly greater in all SOD2_ucp2−/− genotypes, indicating that the presence of UCP2 helps to neutralize ROS production and promote survival. B: ROS production is significantly suppressed in sod2 +/− and −/− mice overexpressing hUCP2. aSignificant with respect to ucp2+/+ mice. bSignificant with respect to sod2+/+ mice.
DISCUSSION
In this study, we have shown that UCP2 is involved in determining lifespan. The effect is likely mediated by increased mitochondrial uncoupled respiration, reduced ROS production, and oxidative stress.
Our results are consistent with the uncoupling-to-survive theory proposed by Brand (7) and support a growing body of recent studies in which higher mitochondrial uncoupled respiration increases lifespan in a diverse range of species (6, 12, 16, 23). Interestingly, in Saccharomuyces cerevisiae, increased respiration rates and lifespan were only observed in zero or low glucose conditions (6, 16). Indeed, in mammals, glucose suppresses the AMPK-ACC-CPT1 fatty acid oxidation pathway (27). Activation of this AMPK-ACC-CPT1 fatty acid oxidation pathway stimulated UCP2-dependent mitochondrial respiration and mitochondrial biogenesis, reduced ROS production, and enhanced cellular bioenergetic function (3). Thus increased UCP2-dependent fatty acid oxidation (3, 20) represents an attractive mechanism through which UCP2 increases mitochondrial respiration and influences survival age.
The development of aerobic metabolism and therefore fatty acid oxidation is a defining point in the evolution of biological complexity that provides the largest possible transfer of energy for a given electron transfer reaction. It is now not surprising that higher mitochondrial respiration underlies increased longevity in a diverse range of organisms (6, 12, 16, 23). Indeed, molecules that promote the most efficient use of molecular oxygen should provide the most beneficial effects on aging. However, as mitochondrial respiration increases, so too does the production of ROS (24), which facilitates the need to neutralize harmful overproduction of ROS. Intriguingly, by promoting mitochondrial uncoupled respiration, fatty acid oxidation, and mitochondrial biogenesis and reducing ROS production, UCP2 is ideally and critically positioned to positively influence biological aging by allowing the most efficient use of molecular oxygen. Furthermore, conditions that promote longevity through enhanced aerobic metabolism, such as aerobic exercise, increase expression of UCP2 in tissues (26).
In summary, UCP2 promotes survival age by reducing mitochondrial ROS production. It is hypothesized that key UCP2-dependent events such as enhancing mitochondrial respiration, fatty acid oxidation, and mitochondrial biogenesis and reducing ROS production underpin the ability of UCP2 to promote lifespan. Our results show that UCP2 has a positive influence on cell function, physiological function, and overall health. Moreover, our results question the pathological effect of UCP2 on disease states such as pancreatic dysfunction. Recent evidence has suggested that pancreatic UCP2 may have evolved to prevent excessive insulin release and act as a fuel switch to fatty acid oxidation during negative energy balance (21), thus supporting the emerging role for UCP2 in fatty acid oxidation (3, 14, 20). These studies show that global UCP2 inhibition will severely affect mitochondrial function, exaggerate both neural and peripheral pathology, and shorten lifespan. Since UCP2 is expressed in many tissues throughout the body, it may represent an attractive target to defend cells against age-related deterioration and extend lifespan.
GRANTS
This work was supported by a NIH Grants DK-060711 and AG-022880 to T. L. Horvath and a New Zealand FRST fellowship and a Monash fellowship to Z. B. Andrews.
Acknowledgments
We thank Dr. Brad Lowell for providing breeding pairs of UCP2 knockout mice.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci 6: 829–840, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Andrews ZB, Horvath B, Barnstable CJ, Elsworth J, Yang L, Beal MF, Roth RH, Matthews RT, Horvath TL. Uncoupling protein-2 is critical for nigral dopamine cell survival in a mouse model of Parkinson's disease. J Neurosci 25: 184–191, 2005. [Erratum. J Neurosci 25 (Feb): table of contents, 2005.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature 454: 846–851, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 26: 435–439, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bechmann I, Diano S, Warden CH, Bartfai T, Nitsch R, Horvath TL. Brain mitochondrial uncoupling protein 2 (UCP2): a protective stress signal in neuronal injury. Biochem Pharmacol 64: 363–367, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab 5: 265–277, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand MD Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol 35: 811–820, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science 314: 825–828, 2006. [DOI] [PubMed] [Google Scholar]
- 9.de Bilbao F, Arsenijevic D, Vallet P, Hjelle OP, Ottersen OP, Bouras C, Raffin Y, Abou K, Langhans W, Collins S, Plamondon J, Alves-Guerra MC, Haguenauer A, Garcia I, Richard D, Ricquier D, Giannakopoulos P. Resistance to cerebral ischemic injury in UCP2 knockout mice: evidence for a role of UCP2 as a regulator of mitochondrial glutathione levels. J Neurochem 89: 1283–1292, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Diano S, Matthews RT, Patrylo P, Yang L, Beal MF, Barnstable CJ, Horvath TL. Uncoupling protein 2 prevents neuronal death including that occurring during seizures: a mechanism for preconditioning. Endocrinology 144: 5014–5021, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature 415: 96–99, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Fridell YW, Sanchez-Blanco A, Silvia BA, Helfand SL. Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab 1: 145–152, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, Wheeler MB. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes 51: 3211–3219, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ, Jung JY, Kim HW, Park T. Anti-obesity effects of Juniperus chinensis extract are associated with increased AMP-activated protein kinase expression and phosphorylation in the visceral adipose tissue of rats. Biol Pharm Bull 31: 1415–1421, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Lebovitz RM, Zhang H, Vogel H, Cartwright J Jr, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA 93: 9782–9787, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418: 344–348, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med 9: 1062–1068, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J Neurosci 21: 8348–8353, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449: 228–232, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F, Ricquier D, Miroux B, Thompson CB. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J 22: 9–18, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Sheets AR, Fulop P, Derdak Z, Kassai A, Sabo E, Mark NM, Paragh G, Wands JR, Baffy G. Uncoulping protein-2 modulates the lipid metabolic response to fasting in mice. Am J Physiol Gastrointest Liver Physiol 294: G1017–G1024, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva JP, Shabalina IG, Dufour E, Petrovic N, Backlund EC, Hultenby K, Wibom R, Nedergaard J, Cannon B, Larsson NG. sod2 overexpression: enhanced mitochondrial tolerance but absence of effect on UCP activity. EMBO J 24: 4061–4070, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, Krol E, Jackson DM, Johnson MS, Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 3: 87–95, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Turrens JF Superoxide production by the mitochondrial respiratory chain. Biosci Rep 17: 3–8, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Voehringer DW, Hirschberg DL, Xiao J, Lu Q, Roederer M, Lock CB, Herzenberg LA, Steinman L, Herzenberg LA. Gene microarray identification of redox and mitochondrial elements that control resistance or sensitivity to apoptosis. Proc Natl Acad Sci USA 97: 2680–2685, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418–420, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Wolfgang MJ, Cha SH, Sidhaye A, Chohnan S, Cline G, Shulman GI, Lane MD. Regulation of hypothalamic malonyl-CoA by central glucose and leptin. Proc Natl Acad Sci USA 104: 19285–19290, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 105: 745–755, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Zhang CY, Parton LE, Ye CP, Krauss S, Shen R, Lin CT, Porco JA Jr, Lowell BB. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab 3: 417–427, 2006. [DOI] [PubMed] [Google Scholar]