Abstract
Conversion of lactate to glucose was examined in myotubes, minced muscle tissue, and rats exposed to 2H2O or 13C-enriched substrates. Myotubes or minced skeletal muscle incubated with [U-13C3]lactate released small amounts of [1,2,3-13C3]- or [4,5,6-13C3]glucose. This labeling pattern is consistent with direct transfer from lactate to glucose without randomization in the tricarboxylic acid (TCA) cycle. After exposure of incubated muscle to 2H2O, [U-13C3]lactate, glucose, and glutamine, there was minimal release of synthesized glucose to the medium based on a low level of 2H enrichment in medium glucose but 50- to 100-fold greater 2H enrichment in glucosyl units from glycogen. The 13C enrichment pattern in glycogen from incubated skeletal muscle was consistent only with direct transfer of lactate to glucose without exchange in TCA cycle intermediates. 13C nuclear magnetic resonance (NMR) spectra of glutamate from the same tissue showed flux from lactate through pyruvate dehydrogenase but not flux through pyruvate carboxylase into the TCA cycle. Carbon from an alternative substrate for glucose production that requires metabolism through the TCA cycle, propionate, did not enter glycogen, suggesting that TCA cycle intermediates do not exchange with phosphoenolpyruvate. In vivo, the 13C labeling patterns in hepatic glycogen and plasma glucose after administration of [U-13C3]lactate did not differ significantly. However, skeletal muscle glycogen was substantially enriched in [1,2,3-13C3]- and [4,5,6-13C3]glucose units that could only occur through skeletal muscle glyconeogenesis rather than glycogenesis. Lactate serves as a substrate for glyconeogenesis in vivo without exchange into symmetric intermediates of the TCA cycle.
Keywords: pyruvate kinase, phosphoenolpyruvate carboxykinase, glyconeogenesis, nuclear magnetic resonance, skeletal muscle
glycogen in skeletal muscle serves multiple well-accepted roles in systemic glucose homeostasis. Postprandial hyperglycemia normally results in insulin-stimulated transport of glucose into skeletal muscle followed by direct glucose phosphorylation and incorporation into muscle glycogen. This process, termed glycogenesis, clears glucose from the systemic circulation and is impaired among patients with type 2 diabetes (3). Skeletal muscle glycogen also serves as an energy source for muscle during anaerobic glycolysis, and lactate produced from glycogen during a fast or prolonged exercise is a major substrate for hepatic gluconeogenesis. Less clear is the capacity of skeletal muscle for de novo synthesis of glycogen from 3-carbon precursors, or glyconeogenesis. Only one pathway is generally accepted for conversion of pyruvate to phosphoenolpyruvate (PEP) in mammalian tissues: carboxylation of pyruvate to oxaloacetate followed by decarboxylation to PEP via phosphoenolpyruvate carboxykinase (PEPCK). The alternative pathway, direct conversion of pyruvate to PEP via pyruvate kinase (ATP + pyruvate → ADP + PEP), is usually held to be insignificant (4, 16, 17).
Although this conclusion is standard teaching in textbooks (21), early studies (14) reported that the reaction is reversible based on the transfer of 32P from PEP to ATP in rat muscle extracts. Later, Hiatt et al. (9) found essentially equal 14C labeling in positions 1, 2, 5, and 6 in glucosyl units from glycogen synthesized by the liver exposed to [2-14C1]pyruvate. This glucose-labeling pattern would be expected if pyruvate undergoes carboxylation to form a dicarboxylic acid in exchange with the symmetric intermediate, fumarate, before conversion to PEP. Diaphragm muscle, however, generated glycosyl units without any enrichment in glucose positions 1 or 6. These data demonstrated unequivocally that the [2-14C1]pyruvate was not converted to the symmetrical tricarboxylic acid (TCA) cycle intermediate, fumarate, in the pathway to glucose production (9). Donovan and Pagliassotti (6), studying rabbit skeletal muscle, found that glycogen synthesis from lactate was identical when measured with [1-14C1]-, [2-14C1]-, or [U-14C3]lactate. If intermediates of the TCA cycle were involved in glyconeogenesis, site-specific loss of tracer and therefore different rates of glycogen synthesis would be expected. Krimsky (13) and Dyson et al. (7) found that reverse flux through skeletal muscle pyruvate kinase is sufficient to support significant rates of glyconeogenesis. More recently, Dobson et al. (5) argued that technical limitations in early studies of pyruvate kinase coupled with the high sensitivity of the reaction to free [Mg2+] and pH interfered with initial studies of the thermodynamics of the reaction. They found that, under in vivo conditions, the mass action ratio was within a factor of three to six of the equilibrium value, and, based on this analysis, proposed that the reaction may be reversed under some conditions (5). In summary, thermodynamic studies suggest that reverse flux through pyruvate kinase may occur in vivo. Experiments examining the conversion of 14C-enriched lactate to glucose by striated muscle are consistent with two possible reaction pathways, reverse flux through the pyruvate kinase reaction or flux in the oxaloacetate/malate pool without exchange with fumarate. In this report, both pathways will be termed “direct transfer” because both pathways preserve the 14C labeling pattern of pyruvate in glucose.
The carbon tracer labeling patterns in glucose cannot distinguish the two possible direct transfer pathways, but these alternatives could be resolved by examining the 13C labeling pattern of oxaloacetate or malate. Reverse flux through the pyruvate kinase reaction bypasses the oxaloacetate/malate pool, whereas carboxylation of pyruvate to oxaloacetate/malate followed by decarboxylation to PEP without rearrangement due to exchange with fumarate will produce a unique labeling pattern in oxaloacetate. Because the concentration of oxaloacetate is low, the 13C labeling pattern in glutamate can be used to measure oxaloacetate labeling in positions 1, 2, and 3. In this study, 2H and 13C nuclear megnetic resonance (NMR) analysis of glucose, glucosyl units from glycogen, or glutamate was performed on cultured myoblast cells, isolated muscle tissue, and whole animals treated with 2H2O and/or 13C-labeled tracers. The 13C labeling pattern in glucose released in the medium from myotubes or minced, incubated muscle demonstrated small excess labeling due to direct transfer. However, the 13C labeling pattern in glycogen from minced muscle was dramatically different from that seen in glucose in the medium and demonstrated glucose production exclusively by direct transfer. The 2H NMR spectrum confirmed that very little skeletal muscle glycogen was released in the medium of incubated muscle. The 13C NMR spectrum of glutamate from the same tissue did not show evidence that oxaloacetate was an intermediate in glucose production. Finally, the 13C labeling in glycogen from skeletal muscle in vivo did not match the 13C labeling in glucose from plasma and for the first time demonstrated glyconeogenesis in skeletal muscle in vivo through a direct transfer pathway.
METHODS
Materials.
[U-13C3]lactate (98%), [U-13C3]propionate (99%), 2H2O (99.9%), and deuterated acetonitrile (99.8%) were obtained from Cambridge Isotopes (Andover, MA). Horse serum was obtained from Invitrogen (Carlsbad, CA). DSC-18 solid-phase extraction gel was obtained from Supelco (St. Louis, MO). Dulbecco's modified Eagle's medium (DMEM), FBS, Dulbecco's PBS (D-PBS), Dowex 50Wx8–200 (a cation-exchange column), Amberlite IRA-67 (an anion-exchange column), and other common chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Muscle cell culture.
C2C12 (a mouse myogenic cell line) myoblasts were proliferated in growth media consisting of DMEM and 20% FBS in 5% CO2 at 37°C. When myoblasts were grown to confluence, the media was changed to differentiation media consisting of DMEM and 2% horse serum. The differentiation media was changed every 2 days. After 5–7 days in differentiation media, myotubes with multiple nuclei were formed. These myotubes were washed with D-PBS and then further incubated for 5 h with either D-PBS containing 20 mM glucose, 5% 2H2O, and 2% FBS (protocol 1); D-PBS containing 20 mM [U-13C3]lactate, 5% 2H2O, 2% FBS, 5 mM glucose, and 2 mM glutamine (protocol 2); and D-PBS containing 20 mM [U-13C3]lactate, 5% 2H2O, 2% FBS, 5 mM glucose, and 10 mM glutamine (protocol 3).
After 5 h incubation, media from three petri dishes (150 × 25 mm) (protocol 1) or six (protocols 2 and 3) were pooled and extracted with 7% perchloric acid by volume.
Skeletal muscle tissue incubation.
The study was approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center. After a fasting period of 24 h, male Sprague-Dawley rats (350–450 g) were anesthetized with isoflurane. Muscle from hindlimbs was dissected out and chopped into thin (less than ∼0.5-mm) pieces in saline. Muscle tissue (∼10 g, wet) was placed in a petri dish (150 × 25 mm, modified for gas tubing) with 60 ml of oxygenated Krebs-Henseleit bicarbonate buffer containing the following: 0.1% BSA, 10 mM glucose, 10 mM glutamine, 20 mM lactate (20% [U-13C3]lactate), and 5% 2H2O (protocol 4) and 0.1% BSA, 10 mM glucose, 10 mM glutamine, 20 mM lactate, 2 or 6 mM [U-13C3]propionate, and 5% 2H2O (protocol 5). The mixture was incubated by shaking at 37°C, and the mixture was gassed continuously with 95% O2-5% CO2 for 5 h. After incubation, muscle tissues were treated with 30% KOH for glycogen isolation or treated with 8% perchloric acid for glutamate isolation. The medium was treated with perchloric acid for glucose isolation.
Whole animal studies.
The study was approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center. Twenty-four-hour-fasted rats (∼430–500 g) received an intraperitoneal injection of sodium lactate (2 g/kg body wt) enriched with 10% [U-13C3]lactate under isoflurane anesthesia. The rats were placed back into their cage where they quickly awakened and were allowed free access to water. After 3 h of injection, blood was drawn from the inferior vena cava under pentobarbital sodium anesthesia, and the liver and muscle tissue from the hindlimbs were freeze-clamped and kept under −80°C for subsequent processing.
Sample processing for NMR analysis.
A 5- to 10-g portion of skeletal muscle tissue and liver tissue was used for glycogen extraction and purification (18). Isolated glycogen was dissolved in ∼5 ml of sodium acetate solution (10 mM, pH 4.8) and incubated with amyloglucosidase (50 mg glycogen/20 units) for 4 h at 50°C for hydrolysis into glucose. Blood from animals and media from myotube or tissue incubation were deproteinized by adding cold perchloric acid to a final concentration of 7% by volume. After neutralization with KOH and centrifugation, the supernatant was lyophilized. Glucose in the dried residue was purified by passing through two columns, a cation-exchange column and an anion-exchange column. Glucose was eluted through the columns with deionized water, lyophilized, and subsequently converted to monoacetone glucose (MAG, Fig. 1). This was accomplished by suspending the dried glucose in 3.0 ml of acetone containing 120 μl of concentrated sulfuric acid. The mixture was stirred for 4 h at room temperature to yield diacetone glucose. After addition of 3 ml of water, the pH was increased to 2.0 by addition of Na2CO3 (1.5 M). The mixture was stirred for 24 h at room temperature to convert diacetone glucose into MAG. The pH was then further increased to ∼8.0 by adding Na2CO3. Acetone was evaporated under a vacuum, and the sample was freeze-dried. MAG was extracted into 3 ml of hot ethyl acetate (5×) that was subsequently removed by vacuum evaporation. The resulting MAG was further purified by passage through a 3-ml DSC-18 cartridge using 5% acetonitrile as eluent. The effluent was freeze-dried and stored dry before NMR analysis. For isolation of glutamate, muscle tissue (∼5–10 g) was treated with cold perchloric acid (8%), neutralized with KOH, and freeze-dried. Glutamate was isolated by ion-exchange chromatography as described previously (11).
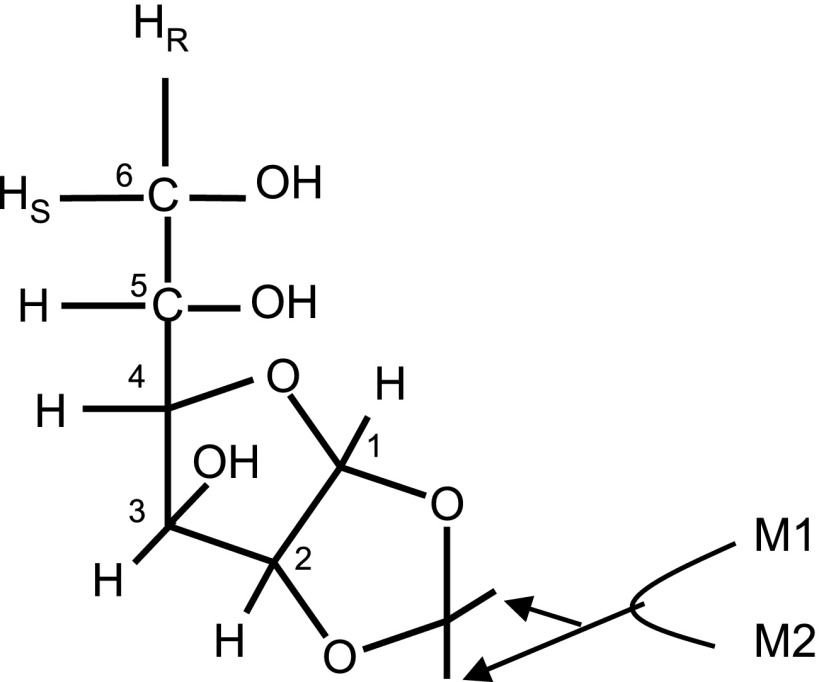
Fig. 1.
Structure of monoacetone glucose (MAG) derived from glucose. The six carbons labeled by 1–6 and seven aliphatic hydrogens attached to those carbons originated from glucose. Two methyl groups labeled by M1 and M2 are added during the derivarization of glucose, and they are independent from metabolism. The resonances of methyl groups of 2H or 13C nuclear magnetic resonance (NMR) spectra are used as references for the estimation of isotope enrichment of glucose.
For 2H NMR measurements, MAG was dissolved in a mixture of 90% acetonitrile-10% water (160 μl). After each 2H NMR acquisition, the MAG samples were lyophilized and resuspended in 90% deuterated acetonitrile-10% water for 13C NMR measurements. Glutamate was dissolved in 2H2O (160 μl) for 13C NMR measurement.
NMR spectroscopy.
All NMR spectra were collected using a Varian ANOVA 14.1 T spectrometer (Varian Instruments, Palo Alto, CA) equipped with a 3-mm broadband probe with the observe coil tuned to 2H (92.1 MHz) or 13C (150 MHz). Proton-decoupled 2H NMR spectra were acquired using a 90° pulse (12.5 μs), 920-Hz sweep width, 1,836 data points, and a 1-s acquisition time with no further delay at 50°C. Typically 20,000–80,000 scans were averaged for MAG requiring ∼5–24 h. Proton decoupling was performed using a standard WALTZ-16 pulse sequence. 13C NMR spectra of MAG were collected using 52° pulse (6.06 μs), 20,330 Hz sweep width, 60,992 data points, and a 1.5-s acquisition time with no further delay at 25°C (10). Typically, 10,000–40,000 scans were averaged requiring ∼5–18 h. Proton-decoupled 13C NMR spectra of glutamate were obtained using a 45° pulse (5.0 μs), 34,965-Hz sweep width, 104,986 data points, and a 1.5-s interpulse delay at 25°C. Spectra were averaged 7,000–30,000 scans requiring ∼6–25 h.
The notations used in 13C NMR analysis such as C5D56 represents the following: “C5” for the carbon position of glucose and “D56” for doublet arising from spin-spin coupling of carbons 5 and 6. The Q in C5Q means quartet or doublet of doublets arising from coupling of carbon-5 with both carbons-4 and -6. C5S means the singlet resonance of carbon-5. Notations for other carbon resonances are used in a similar way in this study. All NMR spectra were analyzed using the curve-fitting routine supplied with NUTS PC-based NMR spectral analysis program (Acorn NMR, Freemont, CA).
Measurement of excess isotope enrichment.
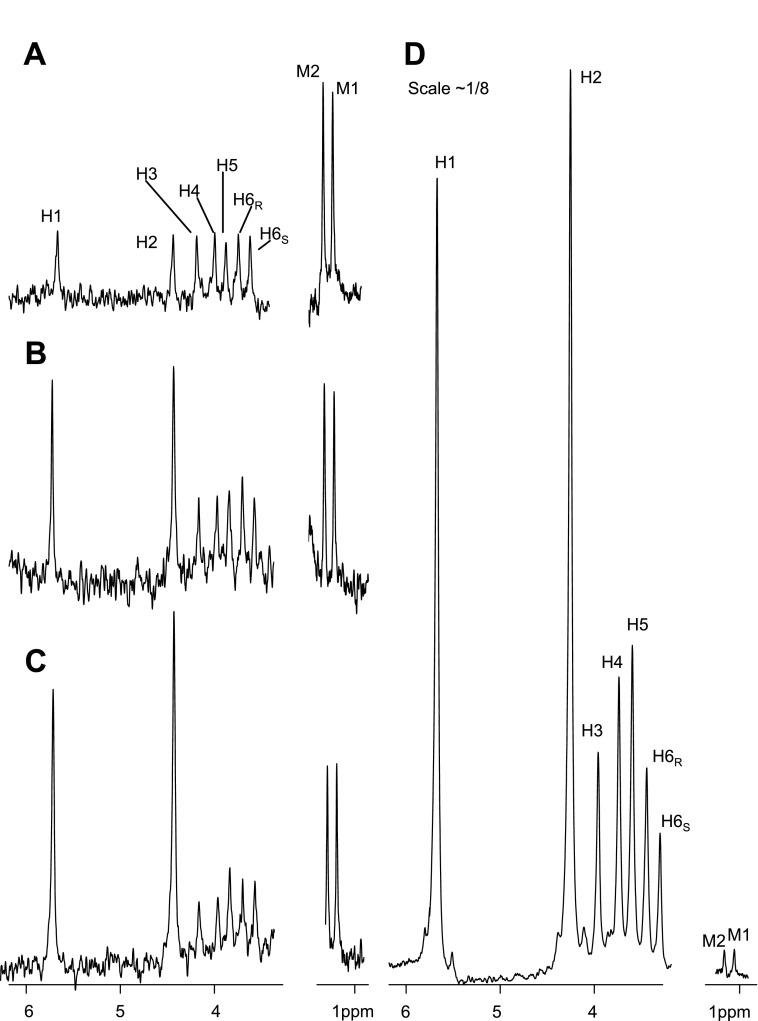
A 2H NMR spectrum of MAG derived from glucose without isotope enrichment (Fig. 2A) shows 2H resonances in the seven aliphatic hydrogens in the glucose skeleton and in the two methyl groups in the monoacetone adduct. The peak area of each 2H resonance was defined as AH1 − AH6S depending on hydrogen position in glucose. The total peak areas of two 2H natural abundance methyl resonances were defined as Amethyl. The ratio of each MAG resonance in the 2H NMR spectrum compared with Amethyl is theoretically 1:6 since there are 6 hydrogens that contribute to the two methyl groups. The measured ratios for natural abundance glucose were slightly higher (∼0.20), likely because of a longer spin-lattice relaxation time of deuterons in the methyl position (Table 1). With the use of this correction factor, the 2H enrichment above natural abundance (termed excess enrichment) could be determined by comparing the areas of each 2H resonance in MAG derived from a tissue sample with the natural abundance signals arising from the two methyl resonances, M1 and M2 (Fig. 1).
Fig. 2.
2H NMR spectra of MAG derived from natural abundance glucose (A), medium glucose from myotube culture (B, protocol 3), medium glucose from skeletal muscle tissue incubation (C, protocol 4), and glycogen from skeletal muscle tissue incubation (D, protocol 4). The scale of spectrum (D) is ∼1/8 of the others. Protocol 3 is myotube culture using Dulbecco's PBS (D-PBS) containing 20 mM [U-13C3]lactate, 5% 2H2O, 2% FBS, 5 mM glucose, and 10 mM glutamine. Protocol 4 is muscle tissue incubation using 0.1% BSA, 10 mM glucose, 10 mM glutamine, 20 mM lactate (20% [U-13C3]lactate), and 5% 2H2O.
Table 1.
2H NMR analysis of MAG
| Glucose (NA) | Myotubes |
Minced Skeletal Muscle, Protocol 4 | ||||
|---|---|---|---|---|---|---|
| Protocol 1 | Protocol 2 | Protocol 3 | Medium | Glycogen* | ||
| Hydrogen-1 | 0.21±0.01 | 0.23±0.04 | 0.36±0.05† (0.011) | 0.43±0.06† (0.016) | 0.53±0.18† (0.023) | 18.80±1.17† (1.343) |
| Hydrogen-2 | 0.21±0.01 | 0.26±0.07 | 0.37±0.09‡ (0.011) | 0.59±0.13† (0.027) | 0.75±0.23† (0.041) | 23.21±0.88† (1.658) |
| Hydrogen-3 | 0.20±0.01 | 0.18±0.02 | 0.19±0.05 | 0.21±0.02 | 0.21±0.04 | 4.64±0.75† (0.348) |
| Hydrogen-4 | 0.18±0.02 | 0.18±0.03 | 0.21±0.06 | 0.23±0.06 | 0.25±0.06§ (0.006) | 6.33±1.22† (0.527) |
| Hydrogen-5 | 0.21±0.03 | 0.18±0.03 | 0.21±0.04 | 0.23±0.07 | 0.30±0.06§ (0.006) | 7.11±0.92† (0.508) |
| Hydrogen-6R | 0.20±0.02 | 0.20±0.02 | 0.19±0.02 | 0.23±0.04 | 0.26±0.07 | 4.58±0.87† (0.343) |
| Hydrogen-6S | 0.18±0.03 | 0.18±0.04 | 0.18±0.02 | 0.18±0.05 | 0.21±0.05 | 2.75±0.53† (0.229) |
Values are means ± SE. NMR, nuclear magnetic resonance; MAG, monoacetone glucose. MAG was derived from natural abundance (NA) glucose (n = 6), from medium glucose of myotubes (protocols 1–3, n = 6 for each), and from medium glucose (n = 10) and skeletal muscle glycogen (n = 4) in tissue incubation. Each value is the ratio of peak area of each resonance normalized by areas of methyl resonances of MAG on 2H NMR spectra (Aeach resonance/Amethyl). Nos. in parentheses are excess 2H enrichment (%). Protocol 1, myotubes in Dulbecco's PBS (D-PBS) with 20 mM glucose, 5% 2H2O, 2% FBS; protocol 2, myotubes in D-PBS with 20 mM [U-13C3]lactate, 5% 2H2O, 2% FBS, 5 mM glucose, and 2 mM glutamine; protocol 3, myotubes in D-PBS with 20 mM [U-13C3]lactate, 5% 2H2O, 2% FBS, 5 mM glucose, and 10 mM glutamine; protocol 4, skeletal muscle tissue incubation in Krebs-Henseleit bicarbonate buffer containing 0.1% BSA, 10 mM glucose, 10 mM glutamine, 20 mM lactate (20% [U-13C3]lactate), and 5% 2H2O.Significantly different from NA glucose
(P < 0.001,
P < 0.01, and
P < 0.05).
Samples were pooled, if necessary, to detect the NA methyl resonances of MAG on 2H NMR.
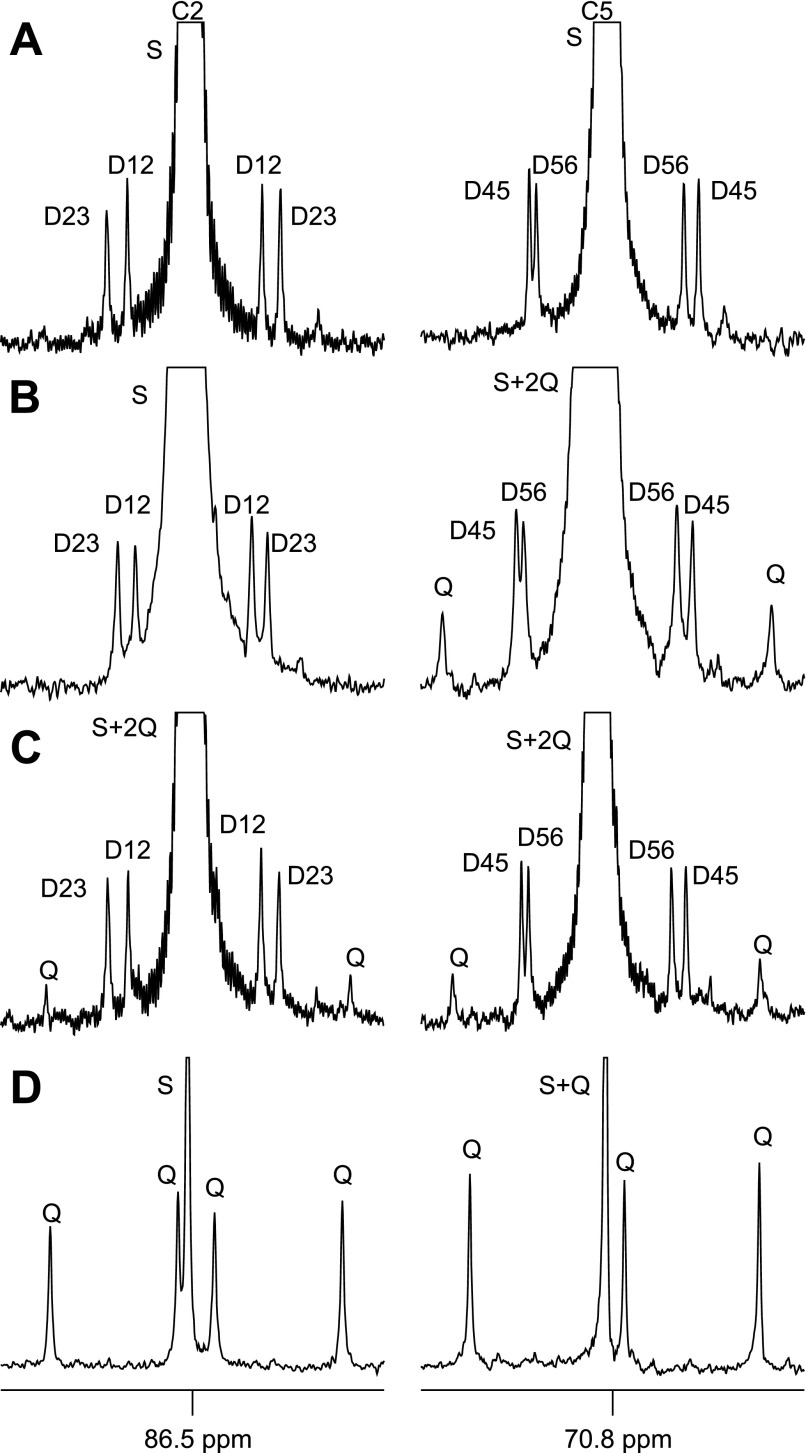
Similar considerations apply to analysis of the 13C spectra. Because each 13C is present at natural abundance levels of 1.1%, the probability of having two 13Cs as nearest neighbors in the same molecule is 0.000121 or 0.0121%. A 13C NMR spectrum of MAG containing only natural abundance levels of 13C is shown in Fig. 3A (only the carbon-2 and carbon-5 resonances are shown for clarity). The small doublets surrounding the more intense singlets in each resonance arise from 13C-13C spin-spin coupling between natural abundant levels of 13C in each neighboring carbon (D12 and D23 in the carbon-2 resonance and D45 and D56 in the carbon-5 resonance). The natural abundance 13C signal from the methyl groups of MAG was also observed. The area of the 13C singlet of each carbon was defined as AC1S − AC6S, the areas of doublets as AC1D12 − AC6D56, and the summed peak areas of two 13C natural abundance methyl resonances were defined as Amethyl. The ratio of each doublet area compared with Amethyl in the 13C NMR spectrum of natural abundance MAG should be 0.000121/(2 × 0.011) = 0.0055, exactly as observed within experimental error (Table 2). Any enrichment above this level was considered excess. The enrichment of quartet (Q, or doublet of doublets) in 13C NMR spectra cannot be estimated using natural abundance MAG directly because natural abundance Q is essentially 0% and cannot be detected.
Fig. 3.
Carbon-2 and carbon-5 regions of 13C NMR spectra of MAG derived from natural abundance glucose (A), medium glucose from myotube culture (B, protocol 3), medium glucose from skeletal muscle tissue incubation (C, protocol 4), and glycogen from skeletal muscle tissue incubation (D, protocol 4). Protocol 3 is myotube culture using D-PBS containing 20 mM [U-13C3]lactate, 5% 2H2O, 2% FBS, 5 mM glucose, and 10 mM glutamine. Protocol 4 is muscle tissue incubation using 0.1% BSA, 10 mM glucose, 10 mM glutamine, 20 mM lactate (20% [U-13C3]lactate), and 5% 2H2O. All of the doublets are natural abundance, and all quartets are excess enrichment derived from [U-13C3]lactate. The 13C excess enrichment of quartet in skeletal muscle glycogen (D) is much higher compared with the rest (see Table 2). Insets: D12, doublet from coupling of carbon-1 with carbon-2; D23, doublet from coupling of carbon-2 with carbon-3; Q, doublet of doublets, or quartet, arising from coupling of carbon-2 with both carbons-1 and -3, or from coupling of carbon-5 with both carbons-4 and -6; D45, doublet from coupling of carbon-4 with carbon-5; D56, doublet from coupling of carbon-5 with carbon-6; S, singlet.
Table 2.
13C NMR analysis of MAG derived from NA glucose, from medium glucose of myotubes, and from medium glucose and skeletal muscle glycogen in tissue incubation
| Glucose (NA) | Myotubes |
Minced Skeletal Muscle, Protocol 4 | |||
|---|---|---|---|---|---|
| Protocol 2 | Protocol 3 | Medium | Glycogen | ||
| C2D12 | 0.0049±0.0003 | 0.0052±0.0009 | 0.0053±0.0007 | 0.0049±0.0005 | ND |
| C2D23 | 0.0053±0.0005 | 0.0051±0.0003 | 0.0053±0.0004 | 0.0050±0.0005 | ND |
| C2Q | ND | ND | ND | ND (n = 8) | 0.4784±0.0788 (1.13) |
| 0.0009±0.0021 (0.0021) | |||||
| C5D45 | 0.0056±0.0004 | 0.0051±0.0007 | 0.0054±0.0006 | 0.0052±0.0005 | ND |
| C5D56 | 0.0057±0.0003 | 0.0051±0.0004* | 0.0052±0.0004* | 0.0051±0.0007 | ND |
| C5Q | ND | 0.0057±0.0014 (0.0122) | 0.0065±0.0030 (0.0139) | ND (n = 6) | 0.6469±0.0441 (1.39) |
| 0.0014±0.0026 (0.0030) | |||||
Values are means ± SE. 13C NMR analysis of MAG derived from NA glucose (n = 6), from medium glucose of myotubes (protocols 2–3, n = 6 for each), and from medium glucose (n = 10) and skeletal muscle glycogen (n = 6) in tissue incubation. Each value is the ratio of peak area of each resonance normalized by areas of methyl resonances of MAG on 13C NMR spectra (Aeach resonance/Amethyl). Nos. in parentheses are excess 13C enrichment (%). The protocols are described in the text and are summarized in the legend to Table 1. ND, not detectable.
Significantly different from NA glucose, P < 0.05.
Relative contributions of glyconeogenesis vs. direct glycogenesis in intact animals.
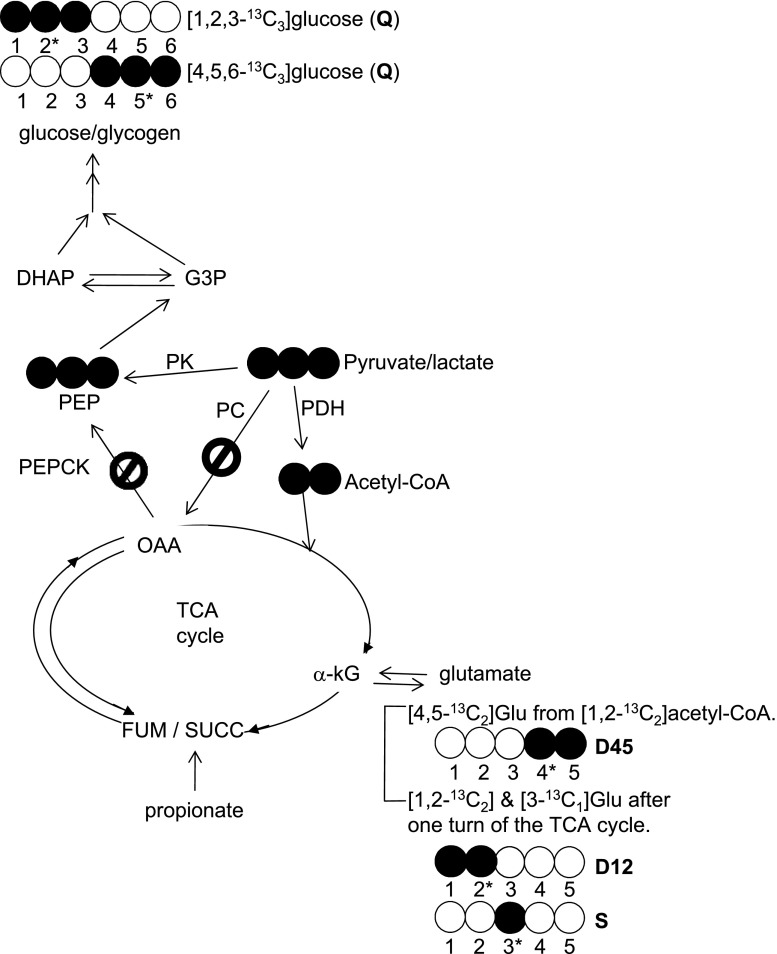
[U-13C3]lactate, provided to a fasted animal, serves as a substrate for hepatic gluconeogenesis and possibly for glyconeogenesis in skeletal muscle. Through hepatic gluconeogenesis, [U-13C3]lactate results in various glucose isotopomers because of rearrangements of 13C in the TCA cycle and pyruvate cycling. These isotopomers include both [1,2,3-13C3]- and [4,5,6-13C3]glucose plus other distinctive labeling patterns such as [1,2-13C2]glucose, etc., as described earlier (11). In contrast, the direct conversion of [U-13C3]lactate to [U-13C3]pyruvate followed by flux through a direct transfer pathway will result exclusively in [1,2,3-13C3]- or [4,5,6-13C3]glucose units in skeletal muscle glycogen without other labeling patterns (Fig. 4). In whole animals, skeletal muscle glycogen synthesis occurs through both glycogenesis (phosphorylation of blood glucose) and perhaps glyconeogenesis (de novo glycogen synthesis). Thus the labeling pattern in skeletal muscle glycogen is the weighted sum of glucose derived from liver and directly transferred to glycogen plus possible in situ glyconeogenesis. The observation of [1,2,3-13C3]- or [4,5,6-13C3]glucose units alone in skeletal muscle glycogen under these conditions cannot distinguish the two pathways.
Fig. 4.
Schematic diagram of metabolic pathways and 13C labeling patterns related to reverse flux through pyruvate kinase in skeletal muscle after [U-13C3]lactate administration. The direct conversion of [U-13C3]lactate to [U-13C3]pyruvate followed by reverse flux through pyruvate kinase results exclusively in [1,2,3-13C3]- and [4,5,6-13C3]glucose units in muscle glycogen without other labeling patterns. If phosphoenolpyruvate (PEP) formation from pyruvate occurs through a bypass (pyruvate → oxaloacetate → PEP) as seen in liver (Fig. 6, A and B), various glucose isotopomers including both [1,2,3-13C3]- and [4,5,6-13C3]glucose plus other distinctive labeling patterns such as [1,2-13C2]glucose, etc. would be observed due to rearrangements of 13C in the TCA cycle and pyruvate cycling. The glutamate analysis of the tissue showed only [4,5-13C2]-, [1,2-13C2]-, and excess [3-13C1]glutamate isotopomers. This informs that [U-13C3]pyruvate incorporated into the TCA cycle only through pyruvate dehydrogenase but not through the pyruvate carboxylase pathway. PDH, pyruvate dehydrogenase; PK, pyruvate kinase; PC, pyruvate carboxylase; PEPCK, phosphoenolpyruvate carboxykinase; OAA, oxaloacetate; G3P, glyceraldehyde 3-phosphate; DHAP, dihydroxyacetone phosphate; a-kG, alpha-ketoglutarate. Open circle, 12C; filled circle, 13C.
The 13C NMR spectrum of MAG derived from [1,2,3-13C3]glucose will produce a doublet of doublets, or a quartet, in the carbon-2 resonance while [1,2-13C2]glucose will yield a doublet in the carbon-2 resonance. The relative areas of the quartet and the doublet due to spin-spin coupling between carbon-1 and -2 (J1,2) is defined as AC2Q/AC2D12. To estimate the fraction of [1,2,3-13C3]glucose in muscle glycogen derived from glyconeogenesis, the excess 13C enrichments of C2Q and C2D12 of glucose unit in muscle glycogen and the ratio AC2Q/AC2D12 of blood glucose were used.
Enrichment of [1,2,3-13C3]glucose unit from glycogenesis (%)
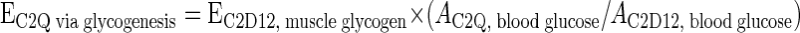 |
Enrichment of [1,2,3-13C3]glucose unit from glyconeogenesis de novo (%)
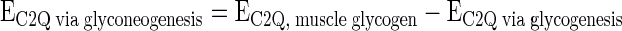 |
where EC2D12, muscle glycogen is the excess enrichment (%) of C2D12 in skeletal muscle glycogen, EC2Q, muscle glycogen is the excess enrichment (%) of C2Q in skeletal muscle glycogen, AC2Q, blood glucose is the peak area of C2Q of blood glucose, and AC2D12, blood glucose is the peak area of C2D12 of blood glucose. The same principle can be applied to estimate the origins of [4,5,6-13C3]glucose unit of muscle glycogen.
Statistical analysis.
The data are expressed as means ± SD. Comparisons between groups were performed using one-way ANOVA. Differences in mean values were considered statistically significant at a probability level of <5% (P < 0.05).
RESULTS
Glucose released by myotubes.
Myotubes were exposed to 5% 2H2O and unlabeled glucose (protocol 1). 2H enrichment in medium glucose at the hydrogen-2 position tended to be higher than natural abundance 2H, but the difference did not reach statistical significance (P = 0.08, Table 1). There was no excess enrichment in hydrogen-6 (Table 1). In a second series of experiments, myotubes were exposed to conditions designed to stimulate glucose production: 20 mM [U-13C3]lactate, 5% 2H2O, 5 mM glucose, and 2 mM glutamine (protocol 2). In these experiments, excess 2H enrichment was observed at both hydrogen-1 and hydrogen-2 positions of medium glucose, about two times natural abundance (Table 1), but again there was no excess enrichment in position 6. When the glutamine concentration was increased from 2 to 10 mM (protocol 3), the excess enrichments at hydrogen-1 and hydrogen-2 positions were even higher (Table 1 and Fig. 2B), but 2H labeling in position 6 was not significantly different from natural abundance. The presence of excess 2H label in position 2 is generally accepted as evidence of proton exchange at the level of glucose 6-phosphate isomerase, and excess 2H label in position 1 is due to equilibration with mannose-6-phosphate (2). Together, the low level of 2H enrichment in each carbon compared with the concentration of 2H in the medium suggests a low rate of gluconeogenesis.
In the 13C NMR analysis, the dominant resonances from glucose in the medium were singlets resulting from natural abundance 13C and the doublets due to adjacent natural abundance 13C nuclei (doublets due to J1,2, J2,3, J4,5, and J5,6). However, in some instances, glucose from the medium also showed a quartet due to [4,5,6-13C3]glucose at levels greater than natural abundance in protocols 2 and 3 (Fig. 3B and Table 2). The appearance of C5Q demonstrates that it originated from [U-13C3]lactate because natural abundance [4,5,6-13C3]glucose cannot be detected by 13C NMR analysis in this study (inner two components of the C5Q quartet overlap with the singlet in Fig. 3B). Although resonances due to J4,5 and J5,6 were detected, these signals were not significantly greater than natural abundance. Interestingly, there was no evidence of the existence of excess [1,2,3-13C3]glucose, which would expected under the equilibrium of the triose phosphate isomerase reaction. The 13C spectra demonstrated a small amount of glucose synthesis in myotubes by direct transfer.
Analysis of incubated, minced skeletal muscle.
2H enrichment in glucose from the medium of incubated skeletal muscle was essentially indistinguishable from the glucose produced by myotubes (Table 1). In contrast to glucose isolated from the medium, muscle glycogen from incubated, minced muscle showed very intense 2H enrichment in glucose units (Table 1 and Fig. 2, C and D). The excess enrichment in the hydrogen-2 position of glycogen was 40-fold higher than medium glucose. Because the resonances labeled M1 and M2 are the natural abundance 2H signals from MAG, these signals provide an internal standard for comparison of Fig. 2, C and D. The difference between the labeling of glucose isolated from the medium and glycogen demonstrates that the overwhelming majority of glucose 6-phosphate produced in muscle was directed toward muscle glycogen rather than released in the medium. Experiments performed with [U-13C3]propionate in the incubation medium in addition to unlabeled lactate (protocol 5) did not affect the 2H enrichment pattern in muscle glycogen and medium glucose (spectra not shown).
A very small amount of excess [1,2,3-13C3]glucose and [4,5,6-13C3]glucose was observed in the medium (Table 2 and Fig. 3C) and was qualitatively similar to results from myotubes. However, glycogen derived from [1,2,3-13C3]glucose and [4,5,6-13C3]glucose isotopomers amounted to 1.13 and 1.39%, respectively, perhaps 1,000-fold greater than that found in medium glucose. This dramatic difference is easily appreciated by comparing Fig. 3, C and D. Because only 20% of the total lactate provided in the incubation medium was [U-13C3]lactate, excess enrichment in [1,2,3-13C3]glucose or [4,5,6-13C3]glucose of 1% corresponds to 5% of the tissue glycogen. The marked difference in labeling patterns of medium glucose vs. skeletal muscle glycogen again confirmed that glucose 6-phosphate synthesized from lactate was stored almost exclusively as glycogen rather than being released into the medium.
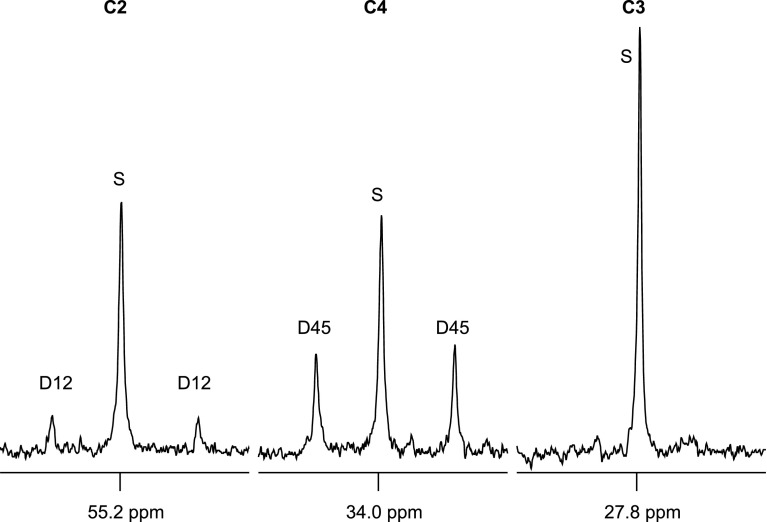
If glycogen was being synthesized via the direct transfer pathway [1,2,3-13C3]lactate → [1,2,3-13C3]pyruvate → [1,2,3-13C3]oxaloacetate → [1,2,3-13C3]PEP → [1,2,3-13C3] or [4,5,6-13C3]glucose, then the 13C labeling patterns in glucose would show a quartet but no signal due to doubly labeled glucose ([1,2-13C2]glucose, [2,3-13C2]glucose, [4,5-13C2]glucose, or [5,6-13C2]glucose). Because [1,2,3-13C3]oxaloacetate also serves as a substrate for citrate synthase to produce citrate, and subsequently isocitrate, α-ketoglutarate, and glutamate, [1,2,3-13C3]oxaloacetate would generate [2,3-13C2]glutamate. However, [2,3-13C2]glutamate was not detected (Figs. 4 and 5). Instead, 13C NMR analysis of muscle glutamate showed C4D45 plus relatively small C2D12 and excess C3S (Figs. 4 and 5). This pattern can only arise from flux of lactate to pyruvate and entry into the TCA cycle via pyruvate dehydrogenase because 13C enrichment in the acetyl group of acetyl-CoA results in [4,5-13C2]glutamate and subsequently both [1,2-13C2]glutamate and [3-13C1]glutamate after one turn of the TCA cycle. There was no evidence of pyruvate carboxylase flux from the 13C enrichment pattern in glutamate.
Fig. 5.
Carbon-2 to carbon-4 regions of 13C NMR spectra of skeletal muscle glutamate from tissue incubation with [U-13C3]lactate (protocol 4). Protocol 4 is muscle tissue incubation using 0.1% BSA, 10 mM glucose, 10 mM glutamine, 20 mM lactate (20% [U-13C3]lactate), and 5% 2H2O.
The possibility that oxaloacetate is an intermediary pool in glucose production also was tested by addition of [U-13C3]propionate to the incubation medium of minced skeletal muscle. There was no 13C excess enrichment in muscle glycogen, but labeling was observed in muscle glutamate (spectra not shown). Because there is only one pathway for propionate to enter the TCA cycle, via succinyl-CoA (Fig. 4), the absence of 13C enrichment in glycogen demonstrates that flux through PEPCK is negligible under these conditions.
13C NMR of liver glycogen, plasma glucose, and skeletal muscle glycogen from intact animals.
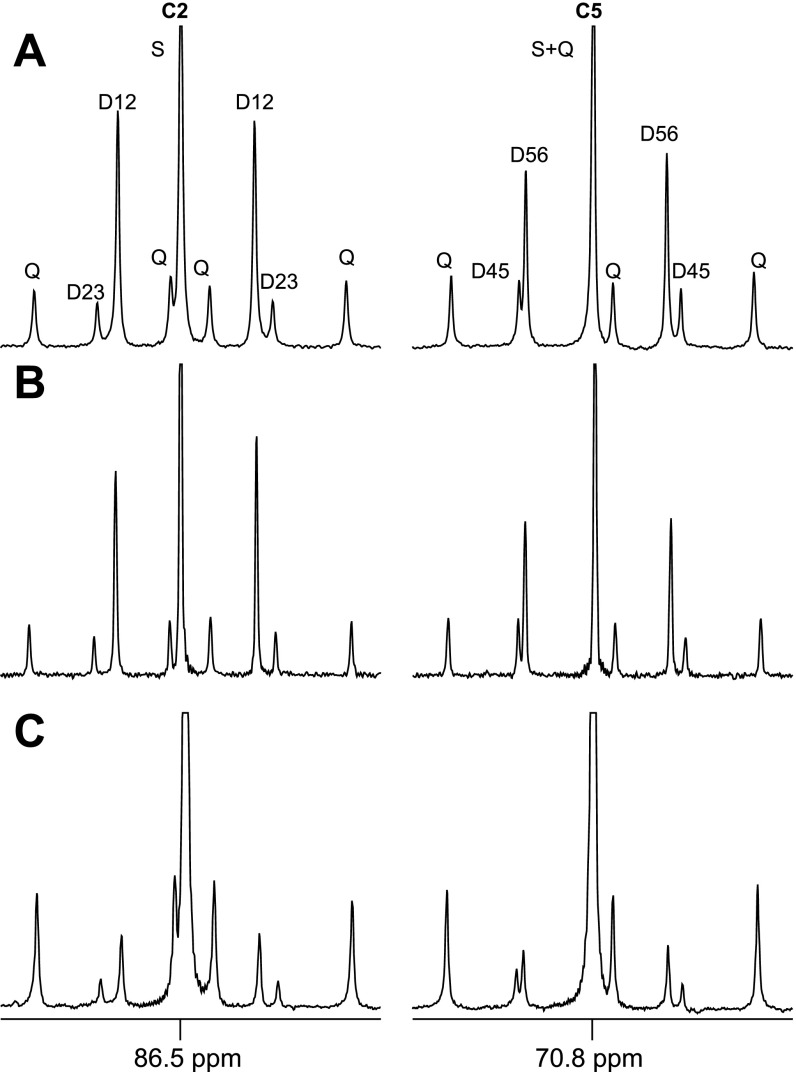
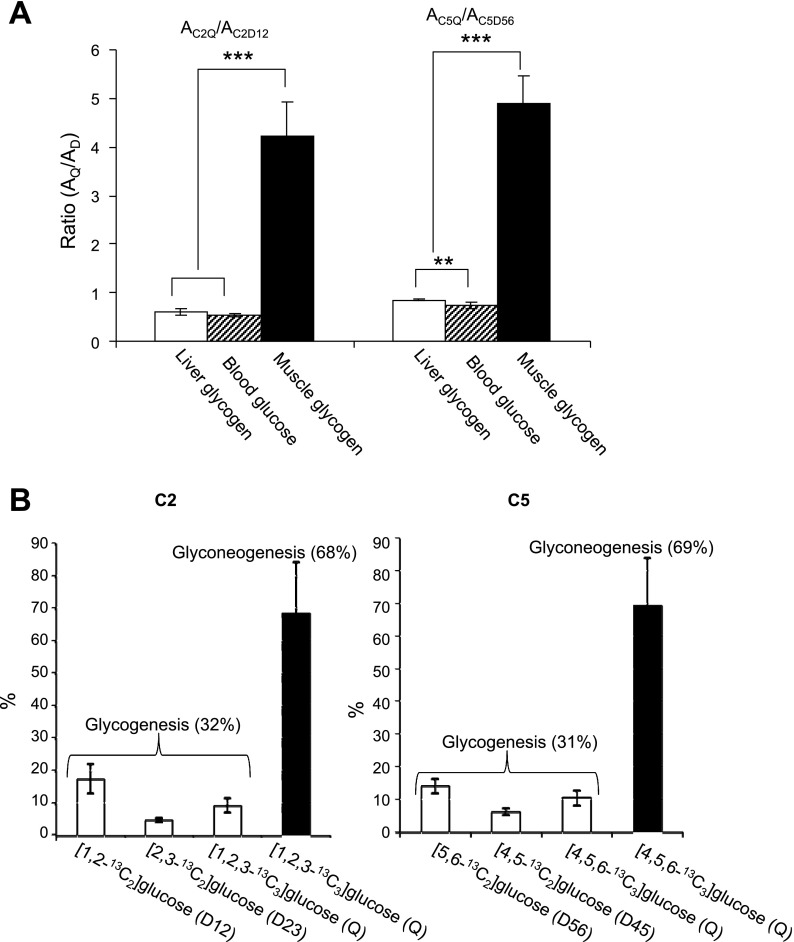
Blood glucose, liver glycogen, and skeletal muscle glycogen were isolated from rats after an intraperitoneal injection of [1,2,3-13C3]lactate. The 13C enrichment pattern of skeletal muscle glycogen shown in Fig. 6C is quite different from that of liver glycogen or blood glucose. The C2Q and C5Q are dominant in muscle glycogen while C2D12 and C5D56 are dominant in liver glycogen and blood glucose. Thus [1,2,3-13C3]- and [4,5,6-13C3]glucose isotopomers are abundant in muscle glycogen while [1,2-13C2] and [5,6-13C2]glucose are abundant in liver glycogen and blood glucose. The area of the quartet relative to the doublet due to J1,2 (AC2Q/AC2D12) in carbon-2 resonance was 0.59, 0.53, and 4.23 in liver glycogen, blood glucose, and skeletal muscle glycogen, respectively. The area of the quartet in carbon-5 relative to the doublet due to J5,6 (AC5Q/AC5D56) was 0.85, 0.73, and 4.90, respectively (Fig. 7A). Based on the disproportionate amplitude of the quartet signal in muscle glycogen, it was estimated that 88% of [1,2,3-13C3]glucose in muscle glycogen originated from glyconeogenesis and the remainder from blood glucose. Similarly, 87% of [4,5,6-13C3]glucose units originated from glyconeogeneisis based on carbon-5 analysis. According to the calculation based on these percentages and the enrichments of glucose isotopomers (Table 3), ∼70% of multiple-labeled glucose units in skeletal muscle glycogen was synthesized through a direct transfer pathway from [U-13C3]lactate in whole animals while the remaining 30% was from blood glucose phosphorylation (Fig. 7B).
Fig. 6.
Carbon-2 and carbon-5 regions of 13C NMR spectra of MAG derived from liver glycogen (A), blood glucose (B), and skeletal muscle glycogen (C) of whole animals treated with [U-13C3]lactate. Quartet is dominant in skeletal muscle glycogen, which is different from blood glucose or liver glycogen.
Fig. 7.
A: graph of peak area ratios, AC2Q/AC2D12 and AC5Q/AC5D56, based on 13C NMR analysis of MAG derived from liver glycogen, blood glucose, and skeletal muscle glycogen of whole animals treated with [U-13C3]lactate (spectra shown in Fig. 6). B: graphs showing the relative distributions of multiply labeled glucose units in skeletal muscle glycogen originated from blood glucose (∼30%) and from de novo glycogen synthesis (∼70%) of the whole animals (spectra shown in Fig. 6C). **P < 0.01 and ***P < 0.001.
Table 3.
13C NMR analysis of MAG derived from liver glycogen, blood glucose, and skeletal muscle glycogen of whole animals treated with [U-13C3]lactate
| Liver Glycogen | Blood Glucose | Muscle Glycogen | |
|---|---|---|---|
| C2D12 | 0.5735±0.1052*(1.4040) | 0.4279±0.0325 (1.0446) | 0.0453±0.0116† (0.0999) |
| C2D23 | 0.1197±0.0164† (0.2611) | 0.0843±0.0078 (0.1805) | 0.0174±0.0024† (0.0277) |
| C2Q | 0.3343±0.0465† (0.7932) | 0.2237±0.0100 (0.5306) | 0.1882±0.0438 (0.4466) |
| C5D45 | 0.1612±0.0110† (0.3362) | 0.1159±0.0123 (0.2384) | 0.0208±0.0057† (0.0329) |
| C5D56 | 0.4735±0.0758*(0.9931) | 0.3675±0.0182 (0.7679) | 0.0408±0.0114† (0.0745) |
| C5Q | 0.4006±0.0611† (0.8579) | 0.2686±0.0341 (0.5751) | 0.1967±0.0424*(0.4212) |
Values are means ± SE. 13C NMR analysis of MAG derived from liver glycogen (n = 6), blood glucose (n = 6), and skeletal muscle glycogen (n = 6) of whole animals treated with [U-13C3]lactate. Each value is the ratio of peak area of each resonance normalized by areas of methyl resonances of MAG on 13C NMR spectra (Aeach resonance/Amethyl). Nos. in parentheses are excess 13C enrichment (%). Significantly different from blood glucose
(P < 0.01 and
P < 0.001).
DISCUSSION
This study demonstrated direct transfer of lactate to glucose in skeletal muscle both in vitro and in vivo. It is the first demonstration of this pathway in vivo. Surprisingly, under these conditions, ∼70% of multiple-labeled glucosyl units of skeletal muscle glycogen in whole animals originated through reverse flux of pyruvate kinase while ∼30% originated from phosphorylation of blood glucose. Nevertheless, the physiological relevance of these observations is not known. In particular, it is already well-established that direct transfer of blood glucose into muscle glycogen plays an essential role in systemic glucose homeostasis, and it seems very unlikely that reverse flux could play any meaningful role under postprandial conditions where the concentration of lactate is low. It is conceivable that reverse flux could be relevant in glycogen replenishment after intense exercise when the concentration of lactate is increased up to 10–12 mM (8, 22), which is closer to the concentration of lactate used in the minced muscle studies, 20 mM (Fig. 3D). This hypothesis remains to be examined.
The strongest evidence of the direct transfer comes from exposure of minced muscle tissues to 20% [U-13C3]lactate. If [1,2,3-13C3]oxaloacetate was produced, then exchange with fumarate must produce equal concentrations of [1,2,3-13C3]oxaloacetate and [2,3,4-13C3]oxaloacetate. The former must produce [1,2,3-13C3]- or [4,5,6-13C3]glucose, and the latter must produce [1,2-13C2]glucose or [5,6-13C2]glucose. These doubly labeled glucose isotopomers are easily distinguished from triply labeled glucose isotopomers in a NMR spectrum, and their absence in Fig. 3D absolutely excludes exchange of the gluconeogeneic precursor with fumarate in the TCA cycle. These data from the incubated skeletal muscle are fully consistent with the radiotracer studies by Hiatt et al. (9) in isolated rat diaphragm and by Donovan and Pagliassotti (6) in perfused rabbit skeletal muscle.
The current study extends earlier work by showing that the 13C enrichment patterns in skeletal muscle glycogen in vivo is also due predominantly to direct transfer from lactate to glucose. The in vivo experiment is somewhat more difficult to interpret than incubated skeletal muscle because hepatic gluconeogenesis from lactate will produce plasma glucose with complex labeling patterns due to recycling of 13C in the liver. However, analysis of 13C isotopomers in plasma glucose allowed for correction of the effects of hepatic gluconeogenesis followed by glycogen synthesis in skeletal muscle. The amount of [1,2,3-13C3]glucose (or [4,5,6-13C3]glucose) in skeletal muscle glycogen was far greater than could be contributed by plasma glucose and therefore must be due to in situ skeletal muscle glyconeogenesis.
Although these experiments confirmed a direct transfer pathway for glucose production by skeletal muscle, the 13C labeling pattern in glucose could in principle be consistent with flux into oxaloacetate followed by conversion to PEP without exchange with fumarate. This seems unlikely for a number of reasons. First, TCA cycle enzymes in skeletal muscle mitochondria are active, and “reverse flux” into fumarate from malate and oxaloacetate is well-studied and complete or nearly so in the liver (12, 15, 20). Second, the possibility that oxaloacetate was involved in glyconeogenesis was also tested by adding [U-13C3]propionate. Entry of [U-13C3]propionate in the TCA cycle via propionyl-CoA, methylmalonyl-CoA, and succinyl-CoA must produce a mixture of 13C isotopomers in PEP. If glycogen is derived from oxaloacetate through PEPCK, one would expect many more isotopomers to appear in glycogen. Because 13C enrichment was not observed in glycogen under these conditions, this eliminates the possibility of flux coming through PEPCK. Third, 13C NMR analysis of muscle glutamate during exposure to [U-13C3]lactate confirmed flux through pyruvate dehydrogenase, but negligible flux through pyruvate carboxylase. Finally, the absence of [1,2-13C2]glucose (or [5,6-13C2]glucose) in the incubated skeletal muscle exposed to [U-13C3]lactate demonstrates the absence of reverse exchange with fumarate. The other doublet in position 2 or 5, due to [2,3-13C2]- or [4,5-13C2]glucose, could arise from “forward” flux through the cycle. The absence of these isotopomers could be attributed to dilution of TCA cycle intermediates by very high flux through anaplerotic pathways, which is typically at least 10 times TCA cycle flux. However, very high anaplerotic flux with unlabeled substrates is not consistent with the observed 13C spectrum of glutamate.
These results also bear on the functional significance of the newly discovered glucose-6-phosphatase β in muscle (19). The presence of this phosphatase would seem to allow glucose release in the circulation from skeletal muscle glycogen. However, in this study, the 2H and 13C labeling patterns in medium glucose were quite different from glycogen, and the whole animal data showed no evidence of glucose release from muscle. Based on both the 2H and 13C NMR data, glucose 6-phosphate formed from lactate is directed almost exclusively into glycogen. Although 2H labeling of glucose from 2H2O is a rich source of information, little work has been done using 2H NMR analysis of skeletal muscle metabolism.
In summary, the 13C distribution in glucose produced by glyconeogenesis in skeletal muscle from [U-13C3]lactate was not consistent with flux through a “well-mixed” TCA cycle. If lactate is converted to glucose in muscle via a TCA cycle intermediate, then other rather implausible conditions must be invoked. The alternative interpretation is significant reverse flux through the pyruvate kinase reaction. Despite early thermodynamic studies indicating that the reaction is essentially irreversible under cellular conditions (4, 16, 17), both tracer and subsequent thermodynamic studies in isolated systems (5–7, 9, 14) reported significant reverse flux. The current study demonstrated this pathway in vivo. Because skeletal muscle glycogen plays numerous important roles in carbohydrate homeostasis, it will be important to properly quantify all pathways feeding glycogen production and the physiological relevance of each.
GRANTS
This study was supported by National Institutes of Health Grants RR-02584, DK-16194, HL-34557, and DK-78933.
Acknowledgments
We thank Charles Storey and Angela Milde for excellent work in animal experiments and Zheng Yan for technical support.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Inve 93: 2438–2446, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandramouli V, Ekberg K, Schumann WC, Wahren J, Landau BR. Origins of the hydrogen bound to carbon 1 of glucose in fasting: significance in gluconeogenesis quantitation. Am J Physiol Endocrinol Metab 277: E717–E723, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, Inzucchi S, Dresner A, Rothman DL, Shulman GI. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med 341: 240–246, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Connett RJ Glyconeogenesis from lactate in frog striated muscle. Am J Physiol Cell Physiol 237: C231–C236, 1979. [DOI] [PubMed] [Google Scholar]
- 5.Dobson GP, Hitchins S, Teague WE Jr. Thermodynamics of the pyruvate kinase reaction and the reversal of glycolysis in heart and skeletal muscle. J Biol Chem 277: 27176–27182, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Donovan CM, Pagliassotti MJ. Quantitative assessment of pathways for lactate disposal in skeletal muscle fiber types. Med Sci Sports Exerc 32: 772–777, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Dyson RD, Cardenas JM, Barsotti RJ. The reversibility of skeletal muscle pyruvate kinase and an assessment of its capacity to support glyconeogenesis. J Biol Chem 250: 3316–3321,1975. [PubMed] [Google Scholar]
- 8.Fairchild TJ, Armstrong AA, Rao A, Liu H, Lawrence S, Fournier PA. Glycogen synthesis in muscle fibers during active recovery from intense exercise. Med Sci Sports Exerc 35: 595–602, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Hiatt HH, Goldstein M, Lareau J, Horecker BL. The pathway of hexose synthesis from pyruvate in muscle. J Biol Chem 231: 303–307, 1958. [PubMed] [Google Scholar]
- 10.Jin ES, Jones JG, Merritt M, Burgess SC, Malloy CR, Sherry AD. Glucose production, gluconeogenesis, and hepatic tricarboxylic acid cycle fluxes measured by nuclear magnetic resonance analysis of a single glucose derivative. Anal Biochem 327: 149–155, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Katz J, Wals P, Lee WNP. Isotopomer studies of gluconeogenesis and the Krebs cycle with 13C-labeled lactate. J Biol Chem 268: 25509–25521, 1993. [PubMed] [Google Scholar]
- 12.Krebs HA, Hems R, Weidemann MJ, Speake RN. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J 101: 242–249, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krimsky I Phosphorylation of pyruvate by the pyruvate kinase reaction and reversal of glycolysis in a reconstructed system. J Biol Chem 234: 232–236, 1959. [PubMed] [Google Scholar]
- 14.Lardy HA, Ziegler JA. The enzymatic synthesis of phosphopyruvate from pyruvate. J Biol Chem 159: 343–351, 1945. [Google Scholar]
- 15.Magnusson I, Schumann WC, Bartsch GE, Chandramouli V, Kumaran K, Wahren J, Landau BR. Noninvasive tracing of Krebs cycle metabolism in liver. J Biol Chem 266: 6975–6984, 1991. [PubMed] [Google Scholar]
- 16.McQuate JT, Utter MF. Equilibrium and kinetic studies of the pyruvic kinase reaction. J Biol Chem 234: 2151–2157, 1959. [PubMed] [Google Scholar]
- 17.Meyerhof O, Ohlmeyer P, Gentner W, Maier-Leibnitz H. Studies on the intermediate reactions of glycolysis with the aid of radioactive phosphorus. Biochem Z 298: 396–406, 1938. [Google Scholar]
- 18.Moriwaki T, Landau BR. Sources of the carbon atoms of liver glycogen formed by cortisol administration to rats in vivo. Endocrinology 72: 134–145, 1963. [Google Scholar]
- 19.Shieh JJ, Pan CJ, Mansfield BC, Chou JY. Potential new role for muscle in blood glucose homeostasis. J Biol Chem 279: 26215–26219, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Shulman GI, Rossetti L, Rothman DL, Blair JB, Smith D. Quantitative analysis of glycogen repletion by nuclear magnetic resonance spectroscopy in the conscious rat. J Clin Invest 80: 387–393, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stryer WH Biochemistry (4th ed.). New York, NY: Freeman, 1995, p. 571.
- 22.Tucker R, Kayser B, Rae E, Raunch L, Bosch A, Noakes T. Hyperoxia improves 20 km cycling time trial performance by increasing muscle activation levels while perceived exertion stays the same. Eur J Appl Physiol 101: 771–781, 2007. [DOI] [PubMed] [Google Scholar]