Abstract
The incidence and prevalence of type 2 diabetes (T2D) continue to escalate at an unprecedented rate in the United States, particularly among populations with high rates of obesity. The impact of T2D on bone mass, geometry, architecture, strength, and resistance to fracture has yet to be incontrovertibly characterized because of the complex and heterogeneous nature of this disease. This study utilized skeletally mature male diabetic rats of the commonly used Zucker diabetic fatty (ZDF) and Zucker diabetic Sprague-Dawley (ZDSD) strains as surrogate models to assess alterations in bone attributable to T2D-like states. After the animals were euthanized, bone data were collected using dual-energy X-ray absorptiometry, peripheral quantitative tomography, and micro-CT imaging modalities and via three-point bending or compression mechanical testing methods. ZDF and ZDSD diabetic rats exhibited lower bone mineral densities, which coincided with declines in structural strength and increased fragility at the femoral midshaft and the L4 vertebral body in response to monotonic loading. Vertebral trabecular morphology was compromised in both diabetic rodent strains, and ZDSD diabetic rats exhibited additional phenotypic impairments to bone material properties at the spine. Because the metabolic origin of the T2D-like state that develops in the ZDSD rat strain is highly relevant to adult-onset diabetes, it is a particularly attractive novel model for future preclinical research.
Keywords: hyperglycemia, leptin, leptin receptor, Zucker diabetic fatty rat, Zucker diabetic Sprague-Dawley rat
an increasing percentage of the adult population in the United States is afflicted with chronic health complications stemming from the development and progression of non-insulin-dependent diabetes mellitus or type 2 diabetes (T2D) (2, 43). T2D is a complex heterogeneous disease; it is clinically characterized by overt hyperglycemia, which commonly develops in response to perturbing metabolic challenges. Obesity resulting from the habitual consumption of excess calories combined with a sedentary lifestyle can initiate the type of metabolic dysregulation that ultimately exerts widespread deleterious effects on the entire body (3). A large body of published research in human subjects and animals has confirmed that the sequelae of hyperglycemia associated with maturity-onset T2D predict the possibility of pathophysiological effects on multiple organ systems, most commonly the renal, circulatory, and nervous systems. Data substantiating the potential for damaging effects of T2D on the skeleton are comparatively limited. Patients with T2D present with heterogeneous bone mineral densities (BMD) that may be low (25), similar (4, 44), or high (11, 28) relative to nondiabetic control subjects and the anatomic site examined (50). Furthermore, various clinical trials that have assessed the association between T2D and fracture risk have revealed disparate findings (26). A systematic review of the published data by Janghorbani et al. (26) and a meta-analysis by Vestergaard (54) examining the association between T2D and fracture risk concluded that there was a generally higher-than-expected fracture risk in T2D patients than in nondiabetic individuals. Aside from reduced visual acuity and neuropathy, which increase the potential for falls and increase the risk for fracture, the changes that occur within bone and augment fracture risk have not been entirely elucidated and require further investigation. However, the bone architectural and mechanical properties that coincide with T2D are particularly difficult to explore in human subjects.
The effects of a diabetic-like condition on the bones of laboratory animals have been investigated in experiments that utilized various rodent models of diabetes, including 1) those that have been experimentally induced to develop a diabetic state [e.g., chemical treatment with a toxin, such as streptozotocin (STZ) or alloxan, or lesion at the hypothalamus] and 2) genetic and spontaneous models that undergo a dynamic period of disease progression (e.g., a leptin structural gene or leptin receptor defect). The male Zucker diabetic fatty (ZDF) fa/fa rat spontaneously develops a T2D-like condition as a result of a congenital leptin receptor mutation, obesity, and a relative insufficiency of insulin attributable to an inadequate compensatory response from pancreatic β-cells (21). Research related to the effects of leptin on bone has yielded inconsistent results, such that the absence of leptin signaling in rodents has been shown to increase (12, 46), decrease (47), and not change (52) bone mass. Because data pertaining to bone architectural changes and the effects of overt hyperglycemia on bone biomechanical strength in these animal models are limited, further investigation is warranted.
The general characteristics of the ZDF rats have been documented previously (34, 35). Briefly, ZDFfa/fa rats are homozygous for the Glu269 → Pro269 amino acid substitution resulting in a leptin receptor (Lepfa) mutation (32). The autosomal recessive nature of this genetic defect rapidly affects male rats, which become obese on the Purina 5008 diet (16.7% kcal fat). Male ZDFfa/fa rats also exhibit hyperlipidemia, early hyperinsulinemia, and persistent hyperglycemia by ∼12 wk of age. There are sex-related dissimilarities in the onset of this condition in ZDFfa/fa rats because of the less favorable lipid profile that develops more readily in males than in identically treated females (9).
ZDFfa/+ rats of both sexes are lean heterozygotes, rendering them genetically similar in all respects, with the exception of the absence of the fa trait encoded on the second allele of the leptin receptor gene and the associated predisposition to become hyperphagic and obese and develop a range of diabetic-like symptoms. ZDFfa/+ lean rats are considered effective controls for the ZDFfa/fa genotype. Although congenital leptin receptor defects are relatively rare in humans (16), ZDF rats rendered leptin insensitive due to a leptin receptor loss-of-function mutation are useful as a model of pseudo-leptin resistance and T2D.
Given that the onset of a T2D-like condition is quite rapid in ZDF rats compared with the more gradual onset in humans, the extent of this model's relevance in terms of simulating and predicting the types of bone changes humans might experience remains unclear. In light of such issues in bone and other organs, a new rodent strain, the Zucker diabetic Sprague-Dawley (ZDSD) rat, which may provide a more relevant animal model of T2D, was recently developed. ZDSD rats gradually develop a T2D-like condition in response to chronic dietary manipulation and their genetic predisposition to dietary-induced obesity (DIO). The key factors responsible for the onset and progression of a T2D-like condition in ZDSD rats may more closely resemble those factors that contribute to the manifestation of T2D in humans. ZDSD rats were developed by cross-breeding DIO rats derived from the Charles River Laboratory:CD (Sprague-Dawley-derived) strain with ZDFfa/+ lean rats. DIO rats exhibit insulin resistance and polygenetic obesity traits that can be manipulated by dietary regulation. Selective inbreeding produces ZDSD rats with an obese phenotype and the potential to develop overt hyperglycemia between ∼15 and 21 wk of age. ZDSD rats do not possess any leptin or leptin receptor defects and tend to deteriorate physically in response to chronic metabolic dysfunction. Consequently, the etiology of a T2D-like condition in this newly developed rodent model should better simulate human adult-onset diabetes, which develops over time and largely in response to chronic overeating combined with low levels of physical activity. A very similar T2D rat model (UCD-T2DM) with comparable characteristics to the ZDSD rats has also been developed (10).
The aim of this experiment was to focus on the effects of a T2D-like condition on bone density, bone geometry, trabecular architecture, and biomechanical properties at an axial and appendicular site in these two different diabetic rodent strains comprising 33-wk-old male ZDF rats and age-matched ZDSD diabetic rats and their respective nondiabetic controls.
MATERIALS AND METHODS
Animals.
Two different strains of male rats were used for this experiment: ZDF rats (n = 24) and ZDSD rats (n = 29). Of the ZDF rats, 12 were homozygous for a leptin receptor mutation (i.e., fa/fa) and exhibited a diabetic phenotype. The remaining 12 ZDF rats were heterozygous for the same allele mutation (fa/+); they were not overtly diabetic and served as controls. CD rats, representing one of the parental strains from which the ZDSD rats were developed, were used in this study as a normoglycemic control (referred to as ZDSD controls) for the hyperglycemic ZDSD rats. Seventeen of the ZDSD rats were diabetic and 12 were nondiabetic ZDSD controls. ZDF rats were acquired at ∼21 wk of age and were given free access to food (Purina 5008) and drinking water for 12 wk until they were euthanized at 33 wk of age. ZDSD/Pco rats were bred onsite at PreClinOmics and were maintained until 33 wk of age on the same dietary regimen used for ZDF rats. All animals were housed under standard laboratory conditions with a 12:12-h light-dark cycle and a controlled room temperature (20–21°C). The protocol and all procedures were approved by the Institutional Animal Care and Use Committee of PreClinOmics and Indiana University School of Medicine. On the day before they were killed, the rats were put on urine collection racks for collection of 24-h urine; the animals had free access to food and water. Following removal from the urine collection racks, the animals were bled from the tail vein through a nick in the skin for terminal blood analysis. The rats were killed by CO2 anesthesia. All rats were weighed at the time of death. During necropsy, organ weights were recorded for a subset of animals.
Dual-energy X-ray absorptiometry.
Excised femurs and L4 vertebrae were scanned using a PIXImus II small animal densitometer (Lunar, GE Medical Systems) for measurement of areal BMD (aBMD, g/cm2), bone mineral content (BMC, g), and bone area (cm2). L4 vertebrae (without the posterior processes) were scanned in two orientations: posterior side down for whole bone measures and then caudal side down to determine the cross-sectional area (CSA, cm2) for normalization of mechanical properties. Femoral midshaft analyses were conducted on the central 5.5 mm of the bone.
Peripheral quantitative CT.
Excised femurs were scanned via peripheral quantitative CT (pQCT) using an XCT Research SA+ machine (Norland-Stratec Medical Systems). A single 0.5-mm slice of bone at the diaphyseal midpoint was scanned at the minimal voxel size (70 μm), and the following variables were assessed: volumetric BMD (vBMD, mg/cm3) and BMC (mg/mm), total bone area (T.Ar, mm2), cortical area (Ct.Ar, mm2), cortical thickness (Ct.Th, mm), periosteal circumference (Ps.C, mm), endosteal circumference (Es.C, mm), polar moment of inertia (Ip, mm4), and cross-sectional moment of inertia perpendicular to the axis of mechanical testing (Iy, mm4).
Micro-CT.
Trabecular bone properties of the L4 vertebra were assessed using a high-resolution micro-CT imaging system (model μCT40, Scanco Medical, Basserdorf, Switzerland). A 2.4-mm region, directly beneath the cranial growth plate (200 slices), was scanned at 12-μm resolution. Images were segmented (σ = 0.8, support = 1 pixel) and thresholded using standard scanner software. Since the ZDF and ZDSD groups were not to be directly compared, separate thresholds were used for each strain to optimize images: ZDF rats were thresholded at 38.0% of the gray-scale value and ZDSD rats at 34.0%. Outcome parameters of trabecular bone included bone volume (BV) fraction [BV/TV (where TV is tissue volume), %], BV (mm3), TV (mm3), trabecular number (Tb.N, mm−1), trabecular thickness (Tb.Th, mm), trabecular separation (Tb.Sp, mm), connectivity density (Conn.D, mm−3), and structural model index (SMI).
Biomechanical testing.
Femoral middiaphyseal mechanical properties were measured via a three-point bending test using standard methods. Briefly, bones were equilibrated to room temperature and placed posterior side down on the bottom support (19 mm span) of a servohydraulic test system (858 Mini Bionix II, MTS Systems, Cary, NC). Bones were loaded centrally using a cross-head speed of 2 mm/min, and force vs. displacement data were collected at 10 Hz. Structural properties included yield load (defined using a 2% offset parallel to the linear elastic region of the force-displacement curve), ultimate load (maximum load during the test), failure load (load at which fracture occurred), stiffness (slope of the linear portion of the load-displacement curve), preyield energy (area under the load-displacement curve up to yield), energy to failure (area under the load-displacement curve to fracture), and postyield energy (difference between energy to failure and preyield energy). To determine material bone properties (ultimate stress, modulus, ultimate strain, and modulus of toughness), force-displacement data were converted to stress-strain data using standard beam bending equations (51).
L4 vertebral bodies were prepared for mechanical testing by removal of the posterior processes and both end plates to provide a flat parallel surface for stability during compression loading to failure (0.5 mm/min). The load-displacement data were used to directly measure the structural properties similar to those of the femoral diaphysis. Material properties (ultimate stress, modulus, ultimate strain, and modulus of toughness) were calculated using standard equations (51), and values were normalized for trabecular bone volume (from micro-CT), CSA (from DXA), and height (measured craniocaudally using digital calipers).
Biochemical methods.
Blood and urine samples were collected at the time of death to enable a number of biochemical assays on a representative subset of six rats per group to confirm the relative state of the T2D-like condition. Blood and urine were analyzed using various methods. Glucose, triglycerides, cholesterol (total, HDL, and LDL), creatinine, and glycated hemoglobin (HbA1c) were analyzed on a Beckman CX4 clinical analyzer with standard Beckman chemistries. Free fatty acids (FFAs) were measured using an FFA kit (catalog no. 990-75401, Wako Chemicals, Richmond, VA). Peptides were analyzed with ELISA kits, insulin with an Alpco-Mercodia kit (catalog no. 08-10-1137-99), leptin with Alpco 22-LEPMS-E01, and urinary microalbumin with Exocell Nephrat II NR002.
Statistical analyses.
Statistical analyses were performed with SAS software version 9.1 for Windows (SAS Institute, Cary, NC). Values are means ± SE. Two-sided comparisons were made between diabetic and nondiabetic rats within each individual strain (i.e., ZDFfa/fa vs. ZDFfa/+ rats and ZDSD diabetic rats vs. ZDSD controls). Independent group t-tests were used to compare means of the same variable between two groups when data met the requirements of equal variance (Levene's test) and normal distribution (Shapiro-Wilk test). Satterthwaite's t-test was used when variances were not equal. The Wilcoxon Mann-Whitney two-sided test was used to evaluate differences in the event that data did not approximate a normal distribution. P < 0.05 was considered significant.
RESULTS
Animal body, tissue, and organ weights.
Compared with the nondiabetic controls, the lower terminal body weights of the diabetic rats were significant for the ZDF (13%), but not the ZDSD, rats (Table 1). Organ weights were collected for the pancreas, liver, and kidneys of a subgroup of rats from each group. For comparative purposes, these data, in addition to the tissue weight of the epididymal fat pads, were normalized to body weight for diabetic vs. nondiabetic rats within each strain. Kidney and liver weights were markedly elevated in the diabetic rats of both strains vs. their respective controls. Pancreatic weight was higher for the ZDSD, but not the ZDF, strain. The weight of the epididymal fat depot was increased in the ZDF diabetic rats vs. controls, whereas there was a decrease in this fat deposit among rats of the ZDSD strain.
Table 1.
Data collected at death
| ZDF Comparison |
ZDSD vs. Parent Strain | |||||
|---|---|---|---|---|---|---|
| ZDFfa/+ (lean) | ZDFfa/fa (fatty) | P | Control (nondiabetic)* | ZDSD (diabetic) | P | |
| Body wt, g | 453±8 | 396±6 | <0.001 | 571±42 | 477±29 | 0.097 |
| Food intake, g/day | 14±1 | 41±2 | <0.005 | 20±3 | 50±2 | <0.050 |
| Water intake, ml/day | 16±3 | 176±11 | <0.005 | 27±9 | 215±70 | 0.320 |
| Urine volume, ml/day | 12.2±6.5 | 142.3±8.3 | <0.050 | 16.2±3.8 | 203.2±41.8 | <0.005 |
| Biochemistry | ||||||
| Fed glucose, mg/dl | 98.4±5.8 | 543.1±15.8 | <0.005 | 96.3±2.2 | 686.8±58.0 | <0.005 |
| HbA1c, % | 3.37±0.03 | 6.64±0.23 | <0.005 | 3.40±0.03 | 8.81±0.27 | <0.005 |
| Fed insulin, ng/ml | 1.44±0.17 | 3.34±1.06 | <0.050 | 1.24±0.22 | 0.37±0.05‡ | <0.010 |
| Leptin, ng/ml | 0.64±0.05 | 1.63±0.21 | <0.005 | 1.12±0.36 | 0.07±0.04 | <0.005 |
| Urinary albumin, mg/dl | 8.8±3.4 | 38.3±3.0 | <0.001 | 19.5±11.6 | 48.3±6.9 | 0.052 |
| Urinary creatinine, mg/dl | 138.6±28.5† | 14.5±0.9 | <0.005 | 133.6±30 | 18.0±2.8 | <0.005 |
| Lipid profile | ||||||
| FFA, mEq/l | 0.43±0.02 | 0.75±0.09 | <0.005 | 0.56±0.09 | 0.94±0.10 | <0.050 |
| Triglycerides, mg/dl | 86±8 | 466±46 | <0.005 | 115±13 | 829±324 | 0.065 |
| Cholesterol, mg/dl | 64±1 | 110±4 | <0.005 | 50±4 | 142±22 | <0.005 |
| LDL, mg/dl | 27±7 | 36±8 | 0.449 | 15±6 | 28±5 | 0.107 |
| HDL, mg/dl | 60±2 | 101±4 | <0.005 | 39±2 | 101±8 | <0.001 |
| Organ/tissue wt, % | ||||||
| Pancreas/body wt | 0.32±0.02 | 0.35±0.04 | 0.758 | 0.25±0.03 | 0.40±0.02 | <0.005 |
| Liver/body wt | 2.93±0.06 | 5.11±0.17 | <0.005 | 3.16±0.12 | 4.63±0.24 | <0.005 |
| Epididymal fat depot/body wt | 0.69±0.03 | 1.49±0.05 | <0.005 | 1.15±0.22 | 0.20±0.05 | <0.005 |
| Kidney (pair)/body wt | 0.61±0.02 | 1.01±0.70 | <0.005 | 0.63±0.02 | 1.19±0.05 | <0.005 |
Values are means ± SE (n = 6 unless otherwise noted). ZDF, Zucker diabetic fatty; ZDSD, Zucker diabetic Sprague-Dawley; HbA1c, glycated hemoglobin; FFA, free fatty acid.
Charles River Laboratory:CD rats were used as one of the parent strains to develop the ZDSD rats.
n = 5.
n = 5; 1 sample that was below the detectable limit of <0.125 was excluded.
Biochemical parameters of diabetes.
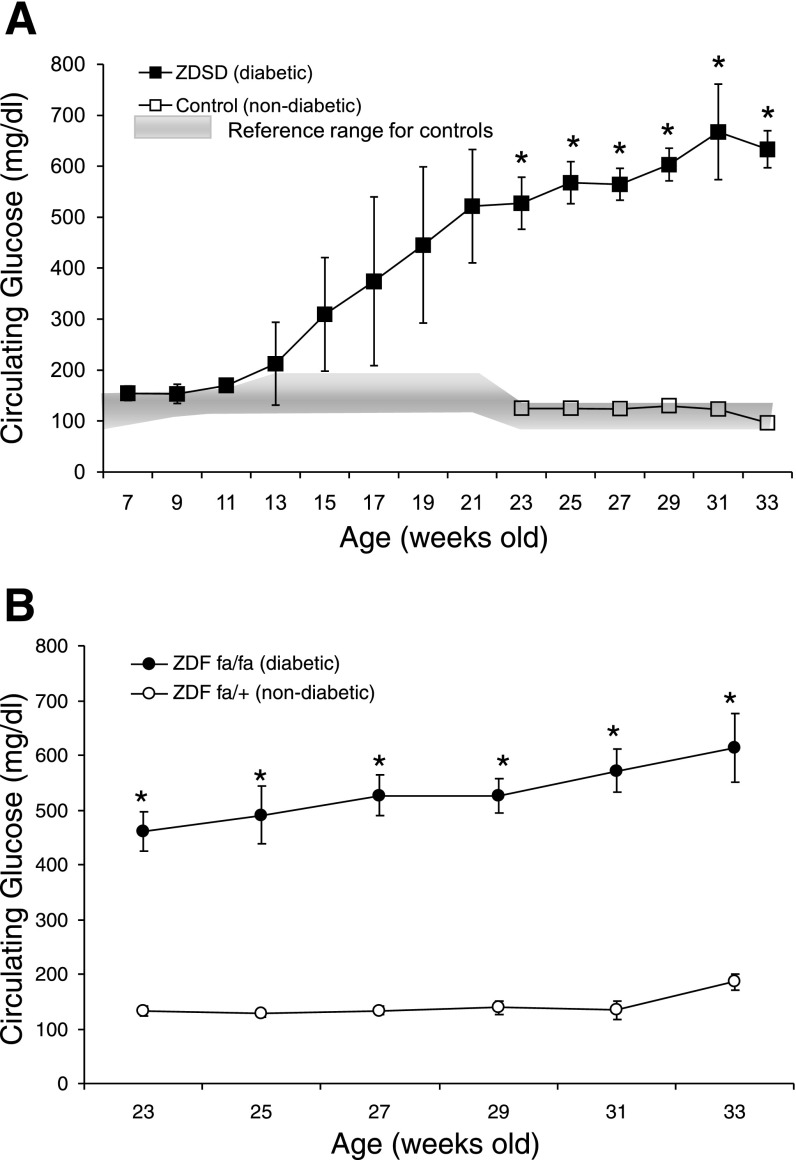
Diabetic status was confirmed in the rats via measurement of a number of biochemical parameters that are utilized in the clinical screening, diagnosis, and/or monitoring of diabetic conditions in patients (Table 1). The overall indication was that a diabetic-like condition was evident in the rats assigned to the diabetic groups and did not manifest in the control groups. Rats classified as diabetic exhibited dramatically elevated levels of blood glucose (Fig. 1.) High circulating glucose levels were instrumental in elevating the percentage of HbA1c in ZDF and ZDSD diabetic strains. HbA1c is one of the most important indicators of the degree of severity of diabetes in humans, inasmuch as it provides an average measure of ambient blood glucose exposure over the lifetime of red blood cells. In contrast to on-the-spot glucose tests, HbA1c is less prone to erratic fluctuations.
Fig. 1.
Circulating glucose concentrations vs. age. Values are means ± SD. *Significant difference between diabetic and nondiabetic rats. A: ▪, blood glucose for a subset of Zucker diabetic Sprague-Dawley (ZDSD) diabetic rats (n = 6 at 7–29 wk of age, n = 4 at 31 wk of age, n = 2 at 33 wk of age); □, blood glucose for 6 ZDSD controls; shaded area, historical serum glucose range obtained by Charles River Laboratories from 146 CD rats (i.e., the same strain as the nondiabetic controls) (18). B: blood glucose for Zucker diabetic fatty (ZDF) diabetic (ZDFfa/fa) and nondiabetic (ZDFfa/+) rats (n = 6/group).
Perturbation of lipid metabolism in diabetic ZDSD and ZDF rats was predominantly demonstrated by increased circulating triglycerides, higher levels of FFAs, and cholesterol increases compared with controls. A diabetic condition did not alter the LDL status of cholesterol in either strain of diabetic rats; however, it did increase the HDL fraction in both strains.
Diabetic rats exhibited polydipsia and polyuria, and when urinary albumin levels were normalized by urinary creatinine, the ratio was significantly increased in hyperglycemic rats. Urinary albumin concentration is dependent on hydration status and is lower in states of adequate hydration. In the early stages of kidney disease, albumin concentrations in the urine increase. Urinary creatinine is usually relatively stable from day to day and mainly reflects the amount of muscle tissue in the body. ZDF lean controls, in which the leptin receptor functions normally and signaling is effectively transduced, exhibited 43% lower plasma insulin levels than ZDF diabetic rats, in which circulating leptin levels were elevated more than twofold in relation to ZDF controls. In the ZDSD diabetic rats, which do not exhibit leptin receptor defects, mean leptin levels were substantially decreased (15-fold) and insulin levels were diminished by 70% compared with nondiabetic controls.
DXA imaging.
DXA data (Table 2) revealed highly significant reductions in all the aBMD, BMC, and projected area measurements of the whole femur, femoral midshaft, and L4 vertebra in diabetic ZDF rats vs. ZDF lean controls. The number of differences in DXA-generated measurements was almost as extensive among the diabetic ZDSD and ZDSD control rats as between ZDF diabetic and nondiabetic rats. The aBMD of L4 vertebra was significantly lower for the ZDSD diabetic rats than for the nondiabetic controls, despite the lack of a significant difference in the projected area of the vertebra. The lower BMC values for the femoral bones of ZDSD diabetic rats did not, however, significantly decrease aBMD after correction for the projected area.
Table 2.
Bone mineral and area parameters of the femur and L4 vertebral body assessed ex vivo using DXA
| ZDF Comparison |
ZDSD vs. Parent Strain | |||||
|---|---|---|---|---|---|---|
| ZDFfa/+ (n = 12) | ZDFfa/fa (n = 12) | P | Control (nondiabetic)* (n = 12) | ZDSD (n = 17) | P | |
| BMD, g/cm2 | ||||||
| Whole femur | 0.255±0.006 | 0.196±0.002 | <0.001 | 0.279±0.008 | 0.254±0.005 | <0.050 |
| Femur midshaft | 0.264±0.008 | 0.211±0.002 | <0.001 | 0.294±0.009 | 0.280±0.006 | 0.183 |
| L4 vertebra | 0.140±0.002 | 0.102±0.003 | <0.001 | 0.148±0.004 | 0.139±0.009 | <0.005 |
| BMC, g | ||||||
| Whole femur | 0.700±0.036 | 0.442±0.028 | <0.001 | 0.940±0.032 | 0.750±0.022 | <0.001 |
| Femur midshaft | 0.074±0.001 | 0.054±0.001 | <0.001 | 0.093±0.003 | 0.083±0.003 | <0.050 |
| L4 vertebra | 0.097±0.006 | 0.061±0.002 | <0.001 | 0.108±0.005 | 0.087±0.003 | <0.010 |
| Area, cm2 | ||||||
| Whole femur | 2.74±0.139 | 2.25±0.131 | <0.001 | 3.38±0.107 | 2.95±0.037 | <0.001 |
| Femur midshaft | 0.283±0.004 | 0.257±0.004 | <0.001 | 0.294±0.025 | 0.301±0.004 | 0.150 |
| L4 vertebra | 0.685±0.031 | 0.561±0.049 | <0.050 | 0.682±0.060 | 0.668±0.027 | 0.183 |
Values are means ± SE. DXA, dual-energy X-ray absorptiometry; BMD, bone mineral density; BMC, bone mineral content. Scanning orientation of vertebral body is the dorsal aspect resting on the imaging stage.
Charles River Laboratory:CD rats was used as one of the parent strains to develop the ZDSD rats.
pQCT and geometric measurements.
The ZDFfa/fa rats exhibited a short bone phenotype at the appendicular site, with mean reductions of 10.7% in femur length compared with ZDF lean controls (Table 3). Bone geometry was markedly compromised for the ZDF diabetic vs. ZDF lean rats, with mean decreases of 13.2% for femoral Ps.C, as well as 26.6% for Ct.Th, 24.7% for Ct.Ar, and 15.8% for T.Ar. There were also discernable differences attributable to diabetic status among the ZDSD rats in terms of femoral bone geometry, with mean decreases of 4.8% for Ps.C, 11.5% for Ct.Th, 13.8% for Ct.Ar, and 9% for T.Ar. Es.C at the femoral midshaft was not affected by chronic hyperglycemia in either rat strain. Similar to the diabetic ZDF rats, femoral bone length compared with controls was reduced in the diabetic ZDSD rats, although only by 4.1%.
Table 3.
Cross-sectional structural properties assessed ex vivo by pQCT at the midshaft of the femoral diaphysis
| ZDF Comparison |
ZDSD vs. Parent Strain | |||||
|---|---|---|---|---|---|---|
| ZDFfa/+ (n = 12) | ZDFfa/fa (n = 12) | P | Control* (n = 12) | ZDSD (n = 17) | P | |
| Femur length, mm | 41.2±0.1 | 36.8±0.2 | <0.001 | 44.1 ±0.3 | 42.3±0.2 | <0.001 |
| Geometric data | ||||||
| Ps.C, mm | 13.6±0.3 | 11.8±0.1 | <0.001 | 14.5±0.2 | 13.8±0.1 | <0.01 |
| Es.C, mm | 7.7±0.2 | 7.5±0.1 | 0.700 | 8.5±0.2 | 8.4±0.1 | 0.959 |
| Ct.Th, mm | 0.94±0.02 | 0.69±0.01 | <0.001 | 0.96±0.02 | 0.85±0.01 | <0.001 |
| T.Ar, mm2 | 13.3±0.1 | 11.2±0.2 | <0.001 | 16.7±0.4 | 15.2±0.3 | <0.010 |
| Ct.Ar, mm2 | 8.9±0.1 | 6.7±0.1 | <0.001 | 11.02±0.3 | 9.5±0.2 | <0.005 |
| Ip, mm4 | 24.45±0.48 | 16.09±0.43 | <0.001 | 39.34±1.94 | 31.17±1.27 | <0.005 |
| Iy, mm4 | 14.59±0.32 | 9.09±0.34 | <0.001 | 23.95±1.27 | 18.63±0.71 | <0.001 |
| Cortical bone mineral data | ||||||
| vBMD, mg/cm3 | 1,478±4 | 1,436±3 | <0.001 | 1,483±3 | 1,459±2 | <0.001 |
| BMC, mg/mm | 13.8±0.2 | 10.0±0.2 | <0.001 | 16.3±0.4 | 15.9±0.4 | <0.001 |
Values are means ± SE. pQCT, peripheral quantitative CT; Ps.C, periosteal circumference; Es.C, endosteal circumference; Ct.Th, cortical thickness; T.Ar, total area; Ct.Ar, cortical area; Ip, polar moment of inertia; Iy, cross-sectional moment of inertia perpendicular to axis of mechanical testing; vBMD, volumetric BMD.
Charles River Laboratory:CD rats were used as one of the parent strains to develop the ZDSD rats.
A diabetic condition impaired cortical vBMD and BMC at the midshaft of the femur to a highly significant extent in the ZDF and ZDSD rat models of diabetes compared with their respective controls. The pQCT-derived geometric indexes of bone strength, polar moment of inertia and cross-sectional moment of inertia perpendicular to the axis of mechanical testing, also appreciably diminished in response to the progression of diabetes in both rat strains.
Three-point bending of the femur.
During three-point bending tests, the femurs were mechanically loaded at the diaphyseal midpoint scanned by pQCT. The ultimate load in diabetic rats was reduced in the ZDF and ZDSD strains (Table 4). Under our test conditions, the femurs of the ZDF and ZDSD diabetic rats were only able to endure 69.8% and 80.5% of the respective ultimate load withstood by their nondiabetic counterparts. For the ZDF rats, all the femoral mechanical structural properties measured, with the exception of energy to yield, were highly significantly decreased in the presence of diabetes. Besides ultimate load, the mean percent parameter reductions for the ZDFfa/fa rats were as follows: 43% for yield force, 32% for failure load, 39% for stiffness, 36% for energy to failure, and 37% for postyield energy to failure.
Table 4.
Bone mechanical properties at the femoral midshaft determined via three-point bending tests
| ZDF Comparison |
ZDSD vs. Parent Strain | |||||
|---|---|---|---|---|---|---|
| ZDFfa/+ (n = 12) | ZDFfa/fa (n = 12) | P | Control* (n = 12) | ZDSD (n = 17) | P | |
| Structural Properties | ||||||
| Yield force, N | 118±13 | 72±5 | <0.010 | 135±13 | 89±33 | <0.005 |
| Ultimate load, N | 212±5 | 148±4 | <0.001 | 236±10 | 190±5 | <0.001 |
| Failure load, N | 200±7 | 136±5 | <0.001 | 224±13 | 171±5 | <0.001 |
| Stiffness, N/mm | 573±18 | 349±18 | <0.001 | 636±32 | 537±15 | <0.005 |
| Energy to yield, mJ | 14±3 | 8±1 | 0.311 | 16±3 | 9±1 | <0.050 |
| Energy to failure, mJ | 107±8 | 68±3 | <0.001 | 122±5 | 114±9 | 0.444 |
| Postyield energy to failure, mJ | 93±7 | 59±3 | <0.001 | 106±6 | 105±8 | 0.974 |
| Material properties | ||||||
| Yield stress, MPa | 72±8 | 67±6 | 0.628 | 55±5 | 46±4 | 0.107 |
| Ultimate stress, MPa | 128±3 | 138±11 | 0.155 | 96±2 | 97±4 | 0.751 |
| Modulus, MPa | 5,620±159 | 5,222±327 | 0.709 | 3,834±145 | 4,210±188 | 0.153 |
| Ultimate strain | 0.042±0.002 | 0.045±0.003 | 0.477 | 0.047±0.003 | 0.054±0.003 | 0.121 |
| Postyield strain | 0.029±0.002 | 0.032±0.002 | 0.307 | 0.035±0.002 | 0.043±0.003 | 0.066 |
| Preyield toughness, mJ/mm3 | 0.53±0.11 | 0.49±0.08 | 0.560 | 0.45±0.07 | 0.28±0.04 | 0.056 |
| Toughness, mJ/mm3 | 4.03±0.34 | 3.62±0.42 | 0.559 | 3.40±0.20 | 3.77±0.28 | 0.350 |
| Postyield toughness, mJ/mm3 | 3.50±0.31 | 3.13±0.42 | 0.652 | 2.95±0.2 | 3.49±0.2 | 0.128 |
Values are means ± SE.
Charles River Laboratory:CD rats were used as one of the parent strains to develop the ZDSD rats.
ZDSD diabetic rats also exhibited significantly impaired femoral cortical bone strength. Only postyield energy to failure and energy to failure were not different between ZDSD diabetic rats and ZDSD controls. The average decreases for these parameters for ZDSD diabetic rats were as follows: 34% for yield force, 24% for failure load, 15% for stiffness, and 44% for energy to yield.
When femur size and geometry were factored out of the biomechanical test results by conversion of the load-deformation data to a stress-strain curve to measure apparent material properties, no parameters were determined to be significantly affected as a result of a diabetic condition in ZDF or ZDSD rats. However, ZDSD diabetic rats did exhibit a trend for decreased (37.8%) preyield toughness compared with ZDSD controls.
Micro-CT imaging of cancellous bone.
Micro-CT at the level of the secondary spongiosa in the cranial region of the L4 vertebra revealed numerous significant differences in trabecular bone parameters between diabetic and nondiabetic rats of each strain (Table 5). Comparisons among the two ZDF rat groups exposed multiple deficits attributable to the diabetic phenotype, including mean percent reductions in BV/TV (31%) and Tb.Th (27%). Connectivity density and SMI were significantly increased in ZDFfa/fa rats vs. lean controls. BV/TV of the diabetic ZDSD rats was diminished by >45% compared with ZDSD controls. Tb.Th was decreased by an average of 19.4%, but this did not affect Conn.D in the same way as in the ZDF diabetic rats. However, it did alter the architecture of the trabeculae considerably by significantly increasing SMI so that trabecular structures became more rodlike.
Table 5.
Three-dimensional microstructural parameters of the cancellous bone of the L4 vertebral body assessed ex vivo by micro-CT
| ZDF Comparison* |
ZDSD vs. Parent Strain† | |||||
|---|---|---|---|---|---|---|
| ZDFfa/+ (n = 11) | ZDF (n = 12) | P | Control§ (n = 12) | ZDSD (n = 17) | P | |
| L4 Vertebra‡ | ||||||
| BV/TV, % | 25.1±2.2 | 17.4±0.91 | <0.005 | 28.9±2.1 | 21.3±1.1 | <0.005 |
| Tb.N, mm−1 | 2.89±0.11 | 3.00±0.10 | 0.4776 | 3.11±0.13 | 3.01±0.08 | 0.4454 |
| Tb.Th, mm | 0.085±0.003 | 0.062±.001 | <0.0001 | 0.093±0.003 | 0.075±0.002 | <0.0001 |
| Tb.Sp, mm | 0.342±.012 | 0.326±0.010 | 0.3089 | 0.304±0.014 | 0.318±0.008 | 0.3199 |
| Conn.D, mm−3 | 38.65±2.48 | 55.44±5.76 | <0.05 | 40.25±2.32 | 44.86±2.59 | 0.1997 |
| SMI | 0.144±0.214 | 0.794±0.070 | <0.05 | 0.092±0.245 | 0.733±0.140 | <0.05 |
Values are means ± SE for male rats (33 wk old). BV/TV, bone volume (BV) fraction (where TV is tissue volume); Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation Conn.D, connectivity density; SMI, structural model index.
Thresholded at 38% of maximal gray-scale value.
Thresholded at 34% of maximal gray-scale value.
200 slices distal to the cranial growth plate at medium resolution (12 μm).
Charles River Laboratory:CD rats were used as one of the parent strains to develop the ZDSD rats.
Compression loading of the L4 vertebral body.
Structural properties deteriorated for diabetic ZDF rats vs. ZDF lean controls, with average decreases in yield force (43%) ultimate load (53%), stiffness (61%), energy to ultimate load (56%), and postyield energy (66%; Table 6). Structural strength parameters of the vertebral body for the ZDSD diabetic vs. nondiabetic controls were also adversely affected, with average decreases for yield force (35%) ultimate load (38%), stiffness (54%), energy to ultimate load (36%), and postyield energy (61%).
Table 6.
Bone mechanical properties of the L4 vertebral body determined by compression testing
| ZDF Comparison |
ZDSD vs. Parent Strain | |||||
|---|---|---|---|---|---|---|
| ZDFfa/+ (n = 11) | ZDFfa/fa (n = 8) | P | Controls* (n = 11) | ZDSD (n = 17) | P | |
| Vertebral body dimensions | ||||||
| Height, mm | 6.49±0.25 | 5.64±0.14 | <0.050 | 6.11±0.19 | 6.05±0.25 | 0.106 |
| CSA, cm2 | 0.30±0.01 | 0.25±0.02 | <0.050 | 0.32±0.01 | 0.30±0.01 | 0.073 |
| Structural properties | ||||||
| Yield force, N | 254±32 | 145±23 | <0.050 | 270±18 | 175±16 | <0.001 |
| Stiffness, N/mm | 2,401±595 | 937±225 | <0.001 | 3,574±557 | 1,657±378 | <0.005 |
| Ultimate load, N | 412±37 | 195±15 | <0.001 | 439±32 | 270±21 | <0.001 |
| Postyield displacement, mm | 0.14±0.04 | 0.08±0.02 | 0.206 | 0.07±0.02 | 0.07±0.01 | 0.886 |
| Energy to ultimate load, mJ | 70.6±8.4 | 31.2±2.5 | <0.050 | 52±7.2 | 33.2±1.7 | <0.010 |
| Preyield energy, mJ | 34±5 | 21±3 | 0.067 | 26±4 | 17±2 | 0.066 |
| Postyield energy, mJ | 41±7 | 14±4 | <0.010 | 41±10 | 16±2 | <0.010 |
| Material properties | ||||||
| Ultimate stress/(BV/TV), N/mm2 | 56.8±3.9 | 43.6±2.7 | <0.050 | 62.9±4.3 | 46.5±3.9 | <0.050 |
| Modulus/(BV/TV), N/mm2 | 1,880±304 | 1,083±149 | <0.051 | 3,036±439 | 1,679±256 | <0.050 |
| Toughness/(BV/TV), mJ/mm3 | 1.63±0.29 | 1.30±0.018 | 0.657 | 1.22±0.14 | 0.91±0.08 | <0.051 |
| Preyield toughness/(BV/TV), mJ/mm3 | 0.74±0.13 | 0.84±0.16 | 0.717 | 0.64±0.11 | 0.44±0.08 | 0.147 |
| Postyield toughness/(BV/TV), mJ/mm3 | 0.98±0.22 | 0.60±0.18 | 0.224 | 0.90±0.20 | 0.48±0.08 | 0.101 |
| Postyield strain | 0.105±0.033 | 0.085±0.024 | 0.793 | 0.057±0.008 | 0.049±0.009 | 0.566 |
Values are means ±SE. CSA, cross-sectional area. Some of the vertebral samples were inadvertently compromised as a result of movement in the fixture used for end plate removal. Because specimens that were not cut planoparallel were eliminated from this test, n was reduced for some groups of biomechanically tested vertebral specimens.
Charles River Laboratory:CD rats were used as one of the parent strains to develop the ZDSD rats.
Of the material properties assessed, ultimate stress was reduced by 23% in diabetic ZDF rats vs. lean controls and by 26% in ZDSD rats vs. controls. Vertebral elastic modulus was decreased by 45% in ZDSD diabetic rats and demonstrated a trend toward being compromised as it decreased 42% (P = 0.0506) in the ZDF diabetic phenotype. The material toughness of the vertebral body was also on the verge of being significantly decreased (22.9%, P = .0505) in ZDSD rats with hyperglycemia compared with ZDSD controls.
DISCUSSION
Overt hyperglycemia developed in susceptible phenotypes from the ZDF and ZDSD rat strains. As a result of the progression of a T2D-like state, alterations in bone size, geometry, architecture, and mineral density impacted the fragility of excised femoral and vertebral bones under monotonic loading. The adverse effects on bone fragility attributable to diabetes in ZDF rats were in part impacted by significant growth impairment (i.e., reduced body mass, femoral length, and vertebral height and area). Because of the congenital defect in leptin receptor function, the human relevance of the types of structural changes detected in ZDFfa/fa rats are more difficult to reconcile with the effects of the onset and progression of T2D in skeletally mature human subjects. The significant increase in the fragility of the femoral midshaft and L4 vertebra of the diabetic ZDSD rats vs. ZDSD controls is less attributable to any growth impairment. As a result, the ZDSD diabetic rats may reflect changes that are more consistent with the types of osteopenic changes that might be imposed by maturity-onset T2D.
The significance of the large reductions in femoral bone size, middiaphyseal total and cortical area, and middiaphyseal periosteal circumference underlies the reduced cross-sectional moment of inertia of the middiaphyseal region. These decrements in bone size and geometry alone are expected to contribute substantially to the diminished structural properties of the femur during three-point bending tests. In fact, the ultimate load and failure load in ZDFfa/fa femurs were only 68% and 70%, respectively, of the values in controls. Similar to our results, Mathey et al. (29) revealed a comparable decrease in femoral length, a decrease in aBMD, and a femoral failure load that was 90% of that in controls during three-point bending in male ZDFfa/fa diabetic rats vs. ZDF+/+ homozygous controls. Prisby et al. (37) observed a significant reduction in the femoral and tibial length of male ZDFfa/fa vs. ZDF+/? rats at 7, 13, and 20 wk of age. Furthermore, femur midshaft BMD (mg/cm3) was also significantly decreased compared with control rats at 13 and 20 wk of age. The ultimate load of the femur was only adversely affected (81.2% of that of controls) when the rats reached 20 wk of age.
The skeleton of the rat is generally not considered mature before ∼16 wk of age (23), when a marked slowdown in weight gain (17), bone modeling, and longitudinal growth becomes evident (20). On the basis of blood glucose (34) and insulin (42) data that characterize the diabetic ZDF rat at a young age, it may be reasonable to conclude that the short bone phenotype and the less biomechanically advantageous geometry of the femur and vertebral body in male ZDFfa/fa rats may, to a large extent, be related to 1) the early prediabetic state that begins to manifest during adolescence (7–8 wk of age) as a result of the congenital leptin receptor loss of function and 2) the frank diabetic-like condition that develops as early as 10–12 wk of age. Previous studies have also demonstrated evidence of leptin's potential to influence bone size in leptin-deficient mice (45), and similar observations have been made in rare cases in which children possess congenital leptin receptor deficiencies (16).
In general, a much smaller femoral bone with a less advantageous moment of inertia at the midshaft, even in the absence of a reduced BMD, is likely to be less strong and less stiff and require less energy to fail in bending. In fact, when three-point bending data were normalized for size and geometry to generate measures of femoral material strength, the data for diabetic rats revealed no statistically significant differences for either strain, thus confirming the influential nature of the amount of bone and its distribution on the overall strength in the appendicular skeleton. The trend (P = 0.0564) for a decline in femoral preyield toughness in the ZDSD diabetic rats suggests that the bone mineral itself was showing signs of becoming less efficient at dissipating energy.
The progression of diabetic symptoms in rats has been shown to impair bone biomechanical properties. Similar to the end-point changes observed in our diabetic rats, spontaneously diabetic nonobese male WBN/Kob rats vs. Wistar (nondiabetic) controls exhibited declines in the structural mechanical properties of stiffness, ultimate load, and energy absorption (40). Reddy et al. (38) demonstrated that femoral and tibial ultimate loads were decreased by >30%, bending stiffness was increased, and energy absorption to yield and toughness were impaired by >25% in the femur and tibia of 10-wk-old Sprague-Dawley rats treated with STZ for 7 wk. Einhorn et al. (14) examined the mechanical properties of the femur under torsional loading in male Lewis rats that had been treated with STZ to develop chronic experimental diabetes. Bone strength parameters (i.e., torsional strength and energy absorption) markedly decreased; however, the material stiffness increase identified in the STZ-induced diabetic rats was not observed among the ZDF and ZDSD diabetic rats in our study at any site, likely because of the substantial decrease in bone mass compared with nondiabetic controls.
Overall, data from a number of other studies are somewhat difficult to compare with our results because of the vastly different metabolic dynamics in rats chemically induced to become diabetic. STZ- and alloxan-treated rats suddenly become hyperglycemic in the presence of abrupt insulinopenia (39) because of the toxic effects of the diabetogenic drugs. This type of diabetes is more analogous to type 1 diabetes, because it negates the cascade of metabolic dysregulation that is so integral to the development of T2D and the initial hyperinsulinemia. ZDFfa/fa rats (41) and ZDSD diabetic rats (10) become hyperglycemic while initially hyperinsulinemic and then remain hyperglycemic while, for the most part, normoinsulinemic before they succumb to the physical deterioration that leads to insulinopenia. The differences between hyperinsulinemic and insulinopenic rodent models may be substantial (50) because insulin is associated with direct and indirect anabolic effects on the skeleton. It has been observed that T2D patients usually present with insulin levels comparable to those of nondiabetic individuals, it is just that these levels are relatively ineffective at compensating for sustained high glucose levels (49).
The three-dimensional microstructure of the trabeculae facilitates the distribution of applied forces, making an important contribution to the capacity of each vertebra to withstand loads and resist fracture. The decrease in BV/TV and Tb.Th in diabetic rats corresponded with the impaired vertebral bone strength relative to that in nondiabetic control rats. Increased resorptive activity at this site in the ZDFfa/fa rats could explain the significant alteration in trabecular topology characterized by the increase in Conn.D. Fenestration of interposing trabecular plates transforms trabeculae into progressively more fragile rodlike structures, as evidenced by the increase in the SMI. An increase in trabecular Conn.D coinciding with a significant increase in SMI has been described previously for a minipig model of osteopenia during the earlier stages of vertebral bone loss (8) and in transilial biopsies of transmenopausal women (1). As osteopenia advances and perforations result in disconnection of the trabecular elements, Conn.D will naturally decline. ZDSD diabetic rats displayed similar morphological and topological decrements in vertebral trabeculae, although the increase in Conn.D was not significant compared with controls. The mechanical properties of the L4 vertebra appeared to be more adversely affected by compressive loading in ZDSD diabetic rats vs. ZDSD controls than in ZDF rats vs. nondiabetic controls.
The vertebral specimens that were mechanically tested in axial compression differed from the femoral midshaft, in that cortical and trabecular bone were directly subjected to loading. Significant decreases in vertebral dimensions, including height and area, likely influenced the decrease in structural strength to some extent in ZDFfa/fa rats; this situation did not apply to the ZDSD rats, in which the vertebral dimensions were comparable to controls. Extrinsic strength parameters measured in diabetic rats of both strains, with the exception of preyield energy and postyield displacement, were greatly decreased. Material (tissue) strength and modulus were also compromised in ZDF diabetic rats. Deteriorations in ultimate stress and elastic modulus were also evident in the ZDSD diabetic rats, and vertebral toughness was marginally reduced (P = 0.0505), albeit in the absence of a significant decrease in postyield toughness and postyield strain.
Chronic hyperglycemia has been implicated in the formation of advanced glycation end products (AGEs), which arise as a result of the increased availability of glucose. AGEs are a heterogeneous group of molecules formed via the nonenzymatic reaction of reducing sugars with free amino groups in proteins, lipids, and nucleic acids (33). Chronically elevated blood glucose concentrations accelerate the ordinarily slow rate of endogenous glycation and AGEs. The native structure and function of affected tissues become irreversibly altered by the accumulation of adventitious dysfunctional covalent cross-links, which are significant pathogenic mediators of almost all diabetes complications (33). Blood glucose and hemoglobin (HbA1c) data for the diabetic rats in this study attest to the increase in glycation in hyperglycemic animals of both strains compared with controls.
A component of fracture risk unexplained by bone density in T2D patients (27, 57) may be attributable to accumulated AGEs causing a stiffening of collagen fibrils and an increase in overall brittleness of the bone matrix (48). An inverse correlation between AGEs and bone toughness has been established in human bone and in bovine cortical bone specimens subjected to in vitro ribosylation (53, 56). Increased levels of the AGE pentosidine are implicated in the propensity for vertebral fracture in T2D patients (24, 58) and increased fragility of diabetic rodent bones (40). ZDSD diabetic rats exhibited a decline in the material toughness of vertebral bone that was, on average, >25% and was marginally significant (P = 0.0505). Our data also showed that the combined pre- and postyield bone toughness deficit represents an underlying decrease in bone material quality that approached statistical significance. Although postyield strain due to diabetes in ZDSD rats was reduced by an average of 14%, it was not significantly different from controls.
There is compelling evidence suggesting that the etiology of T2D in a large percentage of adults is linked to a high body mass index and the subsequent chronic dysregulation of fatty acid metabolism (30). ZDSD and ZDF diabetic rats exhibited an unfavorable blood lipid profile compared with their nondiabetic counterparts. Interestingly, in contrast to humans, an inherent lack of the cholesterol ester transferase protein in rats results in anomalously high plasma HDL cholesterol levels. This phenomenon has been observed in other ZDF rat studies (6, 55).
A continual overabundance of FFAs and triglycerides from excess energy intake and increased hyperlipolytic activity at expanded visceral fat depots eventually overwhelm the capacity of the body's regulatory hormone leptin to effectively confine the sequestration of triglycerides to adipocytes in humans (13, 19). Leptin insensitivity and hyperleptinemia (5) occur most commonly in obese individuals, predisposing their organs to the infiltration of ectopic fat and resultant metabolic derangements. In the long term, body weight per se was not an indicator of obesity in ZDFfa/fa rats with advanced T2D. Despite a lower body weight, visceral fat accumulation persisted and hepatic steatosis may have contributed to the increased liver weights of the diabetic rats, as has been reported elsewhere (22, 31). Circulating levels of leptin appeared to be influenced by body fat stores. This relationship was reflected in both rat strains; the epididymal fat depot was elevated in the ZDFfa/fa vs. ZDFfa/+ rats and leptin levels were correspondingly elevated in the diabetic phenotype, whereas the epididymal fat depot was diminished in the ZDSD diabetic rats compared with ZDSD controls and leptin levels were correspondingly severely depleted. Endogenous leptin receptor dysfunction resulting in a simulated state of leptin resistance in the ZDFfa/fa rats also contributed to the high leptin levels in this strain.
Although ectopic pancreatic lipid accumulation occurs in T2D, the increase in pancreatic weight in ZDSD diabetic rats vs. controls could also represent a compensatory mechanism described previously by Bonner-Weir (7), that is, a dynamic attempt to restore euglycemia by increasing β-cell mass in response to impaired β-cell function. Pancreatic weight was not different between the ZDF groups. Pick et al. (36) demonstrated that ZDF obese rats are afflicted with insulin secretory defects, the propensity for β-cell apoptosis to exceed β-cell renewal, and thus possess an inadequate capacity for sustained expansion of the pancreatic β-cell mass in the event of hyperglycemia. Eventual pancreatic islet exhaustion is known to lead to a progressively insulinopenic state in ZDSD diabetic rats (10), after which they deteriorate relatively quickly, with depletion of fat depots in the process. Insulin levels of the ZDSD diabetic rats were markedly diminished relative to ZDSD nondiabetic controls, resulting in extremely high blood glucose levels. The secretion of pancreatic insulin in ZDFfa/fa rats did exceed that of ZDFfa/+ rats by the end of the study (3.34 ± 1.06 vs. 1.44 ± 0.17 ng/ml). As previously observed by Etgen and Oldham (15), it appears that the higher level of insulin secretion in our ZDF diabetic rats remained insufficient to compensate for the prevailing insulin resistance that was central to the persistence of a severe hyperglycemic state in the fa/fa phenotype.
Conclusion.
The metabolic dysregulation that developed in the ZDF and ZDSD rat strains resulted in the progression of a T2D-like condition that negatively impacted BMD, bone geometry, trabecular microarchitecture, and bone structural and tissue mechanical properties. These alterations significantly increased bone fragility at the femoral midshaft and L4 vertebra during mechanical testing. The ZDFfa/fa rats represent a model of monogenic obesity characterized by a single congenital gene mutation that is rare in humans. The early age of onset of T2D in the ZDFfa/fa rats impaired bone growth, thereby introducing an inherent structural deficit that is irrelevant to adult-onset T2D. The metabolic dysregulation that causes T2D in ZDSD rats is polygenetic in origin, arising as a result of interactions between multiple genetic and environmental factors over time. The development and progression of T2D in the ZDSD diabetic rats better simulate the origin of adult-onset diabetes in Westernized societies, with high rates of T2D due to DIO. T2D likely affects bone via multiple mechanisms, and much work remains to elucidate the extent to which various factors contribute to the increase in bone fragility.
GRANTS
This study was supported by National Institutes of Health Grants AR-047838 and 1-R43-DK-074242 and by a Bridge Grant from the American Society for Bone and Mineral Research.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akhter MP, Lappe JM, Davies KM, Recker RR. Transmenopausal changes in the trabecular bone structure. Bone 41: 111–116, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. National Diabetes Education Program: Overview of Diabetes in Children, and Adolescents (Online). National Institute of Diabetes and Digestive and Kidney Diseases and Centers for Disease Control and Prevention. http://ndep.nih.gov/diabetes/youth/youth_FS.htm#Type1 [Sept. 2008].
- 3.Anonymous. Your Weight and Diabetes (Online). The Obesity Society, Silver Spring, MD. http://www.obesity.org/information/diabetes_obesity.asp [Sept. 2008].
- 4.Ay S, Gursoy UK, Erselcan T, Marakoglu I. Assessment of mandibular bone mineral density in patients with type 2 diabetes mellitus. Dentomaxillofac Radiol 34: 327–331, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 53: 1253–1260, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Blay M, Peinado-Onsurbe J, Julve J, Rodriguez V, Fernandez-Lopez J, Remesar X, Alemany M. Anomalous lipoproteins in obese Zucker rats. Diabetes Obes Metab 3: 259–270, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bonner-Weir S Islet growth and development in the adult. J Mol Endocrinol 24: 297–302, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Boyce RW, Ebert DC, Youngs TA, Paddock CL, Mosekilde L, Stevens ML, Gundersen HJG. Unbiased estimation of vertebral trabecular connectivity in calcium-restricted ovariectomized minipigs. Bone 16: 637–642, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Corsetti JP, Sparks JD, Peterson RG, Smith RL, Sparks RL. Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female. Atherosclerosis 148: 231–241, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol 295: R1782–R1793, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int 16: 1713–1720, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100: 197–207, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Dulloo AG, Antic V, Montani JP. Ectopic fat stores: housekeepers that can overspill into weapons of lean body mass destruction. Int J Obes Relat Metab Disord 28 Suppl 4: S1–S2, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Einhorn TA, Boskey AL, Gundberg CM, Vigorita VJ, Devlin VJ, Beyer MM. The mineral and mechanical properties of bone in chronic experimental diabetes. J Orthop Res 6: 317–323, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Etgen GJ, Oldham BA. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism 49: 684–688, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O'Rahilly S. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356: 237–247, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferry EL The rate of growth of the albino rat. Anat Rec 7: 433–441, 2005. [Google Scholar]
- 18.Giknis MLA, Clifford CB. Clinical Laboratory Parameters for Crl:CD (SD) Rats. Wilmington, MA: Charles River Laboratories, 2006.
- 19.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9: 367–377, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansson LI, Menander-Sellman K, Stenstrom A, Thorngren KG. Rate of normal longitudinal bone growth in the rat. Calcif Tissue Int 10: 238–251, 1972. [DOI] [PubMed] [Google Scholar]
- 21.Hirose H, Lee YH, Inman LR, Nagasawa Y, Johnson JH, Unger RH. Defective fatty acid-mediated beta-cell compensation in Zucker diabetic fatty rats. J Biol Chem 271: 5633–5637, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Huang THW, Peng G, Kota BP, Li GQ, Yamahara J, Roufogalis BD, Li Y. Pomegranate flower improves cardiac lipid metabolism in a diabetic rat model: role of lowering circulating lipids. Br J Pharmacol 145: 767–774, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes PCR, Tanner JM. The assessment of skeletal maturity in the growing rat. J Anat 106: 371–402, 1970. [PMC free article] [PubMed] [Google Scholar]
- 24.Insogna K Can serum pentosidine levels predict risk of vertebral fracture in patients with type 2 diabetes mellitus? Nat Clin Pract Endocrinol Metab 4: 366–367, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Isaia G, Bodrato L, Carlevatto V, Mussetta M, Salamano G, Molinatti CM. Osteoporosis in type II diabetes. Acta Diabetol Lat 24: 305–310, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166: 495–505, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Yano S, Sugimoto T. Combination of obesity with hyperglycemia is a risk factor for the presence of vertebral fractures in type 1 diabetic men. Calcif Tissue Int 83: 324–331, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Kao WHL, Kammerer CM, Schneider JL, Bauer RL, Mitchell BD. Type 2 diabetes is associated with increased bone mineral density in Mexican-American women. Arch Med Res 34: 399–406, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Mathey J, Horcajada-Molteni MN, Chanteranne B, Picherit C, Puel C, Lebecque P, Cubizoles C, Davicco MJ, Coxam V, Barlet JP. Bone mass in obese diabetic Zucker rats: influence of treadmill running. Calcif Tissue Int 70: 305–311, 2002. [DOI] [PubMed] [Google Scholar]
- 30.McGarry JD Banting Lecture 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51: 7–18, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Milagro FI, Campión J, Martínez JA. Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obesity 14: 1118–1123, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Packer CS Estrogen protection, oxidized LDL, endothelial dysfunction and vasorelaxation in cardiovascular disease: new insights into a complex issue. Cardiovasc Res 73: 6–7, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Peppa M, Uribarri J, Vlassara H. Glucose, advanced glycation end products, and diabetes complications: what is new and what works. Clin Diabetes 21: 186–187, 2003. [Google Scholar]
- 34.Peterson RG The Zucker diabetic fatty (ZDF) rat. In: Animal Models of Diabetes—A Primer, edited by Sima AFE, Shafir E. Newark, NJ, Harwood Academic Publishers, 2000, p. 109–128.
- 35.Peterson RG, Shaw WN, Neel MA, Little LA, Eichberg J. Zucker diabetic fatty rat as a model for non-insulin-dependent diabetes mellitus. ILAR J 32: 16–19, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky KS. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes 47: 358–364, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Prisby R, Swift J, Bloomfield S, Hogan H, Delp M. Altered bone mass, geometry and mechanical properties during the development and progression of type 2 diabetes in the Zucker diabetic fatty rat. J Endocrinol 199: 379–388, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Reddy GK, Stehno-Bittel L, Hamade S, Enwemeka CS. The biomechanical integrity of bone in experimental diabetes. Diabetes Res Clin Pract 54: 1–8, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Reed MJ, Meszaros LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism 49: 1390–1394, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int 17: 1514–1523, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt RE, Dorsey DA, Beaudet LN, Peterson RG. Analysis of the Zucker diabetic fatty (ZDF) type 2 diabetic rat model suggests a neurotrophic role for insulin/IGF-I in diabetic autonomic neuropathy. Am J Pathol 163: 21–28, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata T, Takeuchi S, Yokata S, Kakimoto K, Yonemori F, Wakitani K. Effects of peroxisome proliferator-activated receptor-α and γ agonist, JTT-501, on diabetic complications in Zucker diabetic fatty rats. Br J Pharmacol 130: 495–504, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skyler JS, Oddo C. Diabetes trends in the USA. Diabetes Metab Res Rev 18: S21–S26, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Sosa MDM, Navarro MC, Segarra MC, Hernandez D, de Pablos P, Betancor P. Bone mineral metabolism is normal in non-insulin-dependent diabetes mellitus. J Diabetes Complications 10: 201–205, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept 92: 73–78, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell 111: 305–317, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Tamasi JA, Arey BJ, Bertolini DR, Feyen JHM. Characterization of bone structure in leptin receptor-deficient Zucker (fa/fa) rats. J Bone Miner Res 18: 1605–1611, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone 40: 1144–1151, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 140: 1630–1638, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Thrailkill KM, Lumpkin CK Jr, Bunn RC, Kemp SF, Fowlkes JL. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab 289: E735–E745, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner C, Burr DB. Experimental techniques for bone mechanics In: Bone Mechanics Handbook, edited by Cowin SC. Boca Raton, FL: CRC, 2001, p. 7–1.
- 52.Unger RH, Zhou YT, Orci L. Regulation of fatty acid homeostasis in cells: novel role of leptin. Proc Natl Acad Sci USA 96: 2327–2332, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 28: 195–201, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Vestergaard P Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int 18: 427–444, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Wang P, Chatham JC. Onset of diabetes in Zucker diabetic fatty (ZDF) rats leads to improved recovery of function after ischemia in the isolated perfused heart. Am J Physiol Endocrinol Metab 286: E725–E736, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone 31: 1–7, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Bone mineral density is not sensitive enough to assess the risk of vertebral fractures in type 2 diabetic women. Calcified Tiss Int 80: 353–358, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 93: 1013–1019, 2008. [DOI] [PubMed] [Google Scholar]