Abstract
Amino acid transporters at the surface of cells are in an ideal location to relay nutritional information, as well as nutrients themselves, to the cell interior. These transporters are able to modulate signaling downstream of intracellular amino acid receptors by regulating intracellular amino acid concentrations through processes of coupled transport. The concept of dual-function amino acid transporter/receptor (or “transceptor”) proteins is well established in primitive eukaryotes such as yeast, where detection of extracellular amino acid deficiency leads to upregulation of proteins involved in biosynthesis and transport of the deficient amino acid(s). The evolution of the “extracellular milieu” and nutrient-regulated endocrine controls in higher eukaryotes, alongside their frequent inability to synthesize all proteinaceous amino acids (and, hence, the requirement for indispensable amino acids in their diet), appears to have lessened the priority of extracellular amino acid sensing as a stimulus for metabolic signals. Nevertheless, recent studies of amino acid transporters in flies and mammalian cell lines have revealed perhaps unanticipated “echoes” of these transceptor functions, which are revealed by cellular stresses (notably starvation) or gene modification/silencing. APC-transporter superfamily members, including slimfast, path, and SNAT2 all appear capable of sensing and signaling amino acid availability to the target of rapamycin (TOR) pathway, possibly through PI 3-kinase-dependent mechanisms. We hypothesize (by extrapolation from knowledge of the yeast Ssy1 transceptor) that, at least for SNAT2, the transceptor discriminates between extracellular and intracellular amino acid stimuli when evoking a signal.
Keywords: SNAT2, mammalian target of rapamycin, GCN2, transporters, receptors
it is now widely accepted that, in addition to humoral and neuronal factors, changes in amino acid availability have profound effects on many aspects of cell function, including regulation of cell signaling and gene expression as well as transport and metabolism of amino acids themselves (30, 64, 93). The precise mechanisms by which changes in amino acid availability are sensed have become the focus of considerable recent interest because of the effect that this class of organic nutrients has upon signaling via the highly conserved GCN (general control nonrepressed) and TOR (target of rapamycin) pathways (9, 98, 100). Cellular amino acid deficiency enhances the abundance of uncharged tRNAs, resulting in activation of GCN2, a protein kinase that targets eukaryotic translation initiation factor (eIF)2α (104). GCN2-mediated phosphorylation of eIF2α serves to repress global mRNA translation but does not abate the synthesis of a few select proteins (e.g., the transcription factor ATF4/CREB2) that crucially support expression of genes encoding amino acid transporters and amino acid biosynthetic enzymes (64). Consequently, activation of the GCN pathway not only serves to restrain further loss of amino acids from the free intracellular pool but acts as a corrective measure aimed at restoring the normal cellular amino acid economy. By contrast, the TOR signaling complexes play an important role in the stimulatory control of cell growth and proliferation (100). For example, in mammalian cells, the mTORC1 complex is acutely responsive to amino acid sufficiency and signals to translational effectors such as the ribosomal S6 kinases (S6Ks) and eIF4E-binding protein-1 (4E-BP1) to enhance mRNA translation during periods of plentiful nutrient supply (9, 21, 97). The amino acid sensing mechanisms that lie upstream of the GCN2 and TOR pathways are beginning to be revealed (9, 98) and amino acid transporters, as the initial point of contact between cell and environment, are increasingly thought to play a pivotal role in nutrient sensing and signaling (57). Amino acid transporters exhibiting a dual transporter/receptor (“transceptor”) function have been demonstrated in lower eukaryotes such as yeast [e.g., Gap1,(25)] or Drosophila [e.g., path (43)], and there is now emerging evidence to suggest that physiologically important mammalian amino acid transporters (e.g., SNAT2) may regulate nutrient signaling not only via their capacity to modulate the free intracellular amino acid pool but through their ability to sense changes in extracellular amino acid availability (55). The capacity to monitor and distinguish between extracellular and intracellular quantity and quality of amino acids enables cells to respond appropriately to changes in nutritional state. This is particularly apparent in unicellular organisms such as yeast, but recent work indicates that cells of complex multicellular organisms, including humans, retain some of these nutrient-sensing capabilities. This review focuses on recent evidence supporting the role played by distinct amino acid transporters in nutrient sensing, highlighting their largely unacknowledged, but potentially important role as regulators of nutrient signals generated, for example, via the TOR pathway.
Impact of Amino Acid Transporters on The Intracellular Amino Acid Pool and Its Implications for Amino Acid-Dependent Signaling
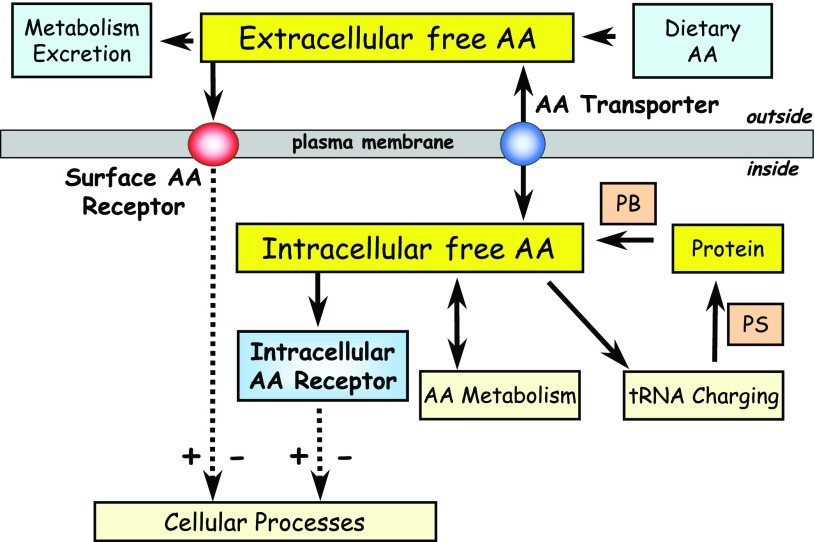
The size and composition of the free intracellular amino acid pool will depend on the relative activities of processes that remove or add amino acids to this compartment, such as the synthesis and breakdown of cellular protein, aminoacyl tRNA charging, amino acid breakdown, and biosynthesis (Fig. 1). However, it is widely accepted that the ability to maintain intracellular amino acids at concentrations greater than or equal to that found in the extracellular environment is greatly facilitated by the presence of a multitude of amino acid transporters in the plasma membrane, some of which function to actively concentrate amino acids inside cells, whereas others may facilitate cellular efflux by counterexchange mechanisms (82). In higher eukaryotes, a particular amino acid may be a substrate for several different transporters. Traditionally, many of these amino acid transporters, or “systems,” were classified on the basis of features reflecting aspects of their transport mechanism (i.e., ion dependence), substrate specificity, and regulatory characteristics, such as sensitivity to hormonal or stress-inducing factors (17). However, over the past decade, there has been significant progress in the molecular identification of the carrier proteins themselves that has led to a much greater appreciation of their functional role within cells in which they are expressed [see reviews (15, 61, 72, 80, 82, 83, 95)]. Amino acid transporters that have now been cloned have been reclassified and organized into families that form part of the solute-linked carrier (SLC) group of membrane transport proteins.
Fig. 1.
Schematic diagram showing processes that contribute to free amino acid (AA) turnover in animal cells. Note that AA transporters contribute to both influx and efflux of AAs at the cell surface. This review considers the evidence for both extracellular and intracellular amino acid “receptors” upstream of nutrient-responsive signaling mechanisms involved in control of cellular processes. PS, protein synthesis; PB, protein breakdown.
Role of Secondary Active Amino Acid Transporters
Several amino acid transporters (including members of the SLC38 System A and System N family) function as secondary active transporters, coupling the uphill transfer of amino acids to the inward movement of Na+ down its electrochemical gradient, whereas others (e.g., SLC1 System X−AG family) may also couple to gradients for K+ and H+ (61). These transporters promote the intracellular accumulation of substrate amino acids and thus impact on signaling pathways modulated by intracellular nutrient receptors. The transport cycle of many of these transporters is rheogenic (i.e., it generates an ionic current) and may produce a change in voltage across the cell membrane of sufficient magnitude to trigger the activation of voltage-sensitive signaling mechanisms. For example, the glutamine-induced secretion of glucagon-like peptide-1 from intestinal L-cells has been suggested, in part, to be driven by membrane depolarization associated with the small inward current generated by Na+-coupled glutamine uptake via the SLC38A2 (SNAT2) transporter (88). Another potentially important consequence of membrane depolarization associated with rheogenic amino acid uptake is that it may induce an increase in intracellular Ca2+. Gulati et al. (46) have recently demonstrated that amino acid supplementation increases intracellular Ca2+ in HeLa cells and that this is required to support the nutrient-induced activation of the TORC1 complex via modulation of the human vacuolar protein-sorting 34 (hVps34, a class III PI 3-kinase). However, the extent to which this mechanism plays out in other cell types and the degree to which changes in cytosolic Ca2+ are truly dependent on amino acid delivery mechanisms across the plasma membrane remain, as yet, to be explored in detail.
Significance of Plasma Membrane Amino Acid Exchangers for Amino Acid Signaling
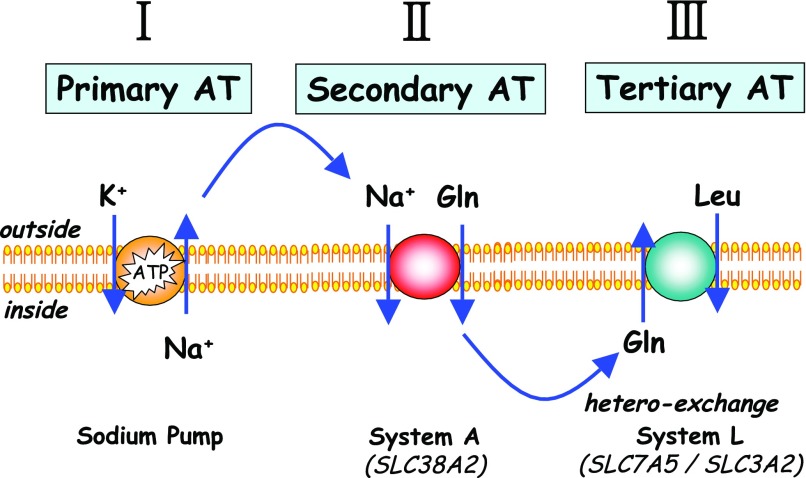
In addition to carriers that operate in a concentrative manner, cells also possess amino acid transporters that function as tertiary active exchangers, such as System L and ASC (Fig. 2). The transport activity known as System L is a heterodimeric protein complex composed of a light subunit (LAT1 or LAT2) that functions as an amino acid permease and a glycosylated heavy subunit (4F2hc/CD98) that supports translocation and insertion of the transporter complex into the plasma membrane (95). Although System L is expressed ubiquitously in mammalian tissues, it is particularly prominent in cells that exhibit rapid growth/turnover, where it mediates the cellular uptake of indispensable amino acids, most notably the branched-chain amino acids (BCAA) that exert a powerful stimulatory influence on the TOR pathway (9). In prophase-arrested Xenopus oocytes, which do not express any appreciable leucine influx, we (18) have shown that activation of the TOR-S6K1 axis is refractory to stimulation under conditions when oocytes are incubated with buffer containing a physiological amino acid mix. However, sensitivity of the TOR pathway to extracellular amino acids can be instated in this system by the expression of LAT1/CD98, strongly suggesting that System L functions essentially as a conduit for delivery of amino acids to an intracellular amino acid receptor that lies upstream of TOR (18). This concept is further consolidated by the finding that microinjection of leucine directly into the cytoplasmic compartment of oocytes, in the absence of any System L expression, is sufficient for inducing TOR-S6K1 signaling (18). We suggest that the function of System L as a conduit for leucine-dependent TOR signaling is unlikely to be restricted to amphibian oocytes, and, indeed, the leucine analog b(−)-BCH [2-aminobicyclo(2,2,1)heptane-2-carboxylic acid], used as a competitive inhibitor of leucine uptake via System L, inhibits the stimulatory effect of leucine upon S6K1 in rat cortical neurons (59). The possibility that b(−)-BCH acts in this case at a leucine-binding site distinct from the System L transporter cannot be excluded, although our studies in Xenopus oocytes did not reveal any influence of microinjected b(−)-BCH on the TOR pathway (18).
Fig. 2.
Integration of primary (I), secondary (II), and tertiary (III) active transport (AT) mechanisms may affect transmembrane distribution of particular AAs. Secondary active transporters (e.g., System A/SNAT2) generate net movement of AA from the extracellular to the intracellular pool, whereas tertiary active transport through exchangers such as LAT1 (System L) allows for redistribution of individual AAs without affecting total pool sizes.
System L operates as an obligatory 1:1 heteroexchanger facilitating uptake of leucine (and the other BCAAs) in exchange for certain cytoplasmic amino acids accumulated via secondary active transporters (e.g., SNAT2) such as glutamine (Fig. 2). Consequently, this tertiary active process may promote net accumulation or loss of System L substrates under circumstances when the activities of secondary active transporters are themselves subject to modulation (e.g., in response to hormonal or stress stimuli). Evidence supporting this functional linkage between amino acid transporters is now beginning to emerge. Sustained incubation of skeletal muscle cells with the nonmetabolizable amino acid analog methyl-aminoisobutyric acid (Me-AIB), a paradigm substrate for System A (i.e., SNAT1, SNAT2, and SNAT4), results in the competitive exclusion and intracellular diminution of amino acids that normally serve as substrates for this transporter family. Since Me-AIB itself is unable to participate in System L heteroexchange, sustained cell incubation with this amino acid analog also promotes an attendant reduction in the intracellular accumulation of BCAAs (12). In other mammalian cells, the accumulation of System L substrates can be reduced by incubation of cells with ceramide, a sphingolipid that inhibits the activity and surface expression of SNAT2 (56) and 4F2hc/CD98 (44) and which, by doing so, induces a concomitant reduction in TOR signaling and cellular protein synthesis. We now have more direct evidence of SNAT2/System L coupling that comes from experiments involving the heterologous expression of these transporters in Xenopus oocytes. Expression of System L (LAT1/CD98) alone in oocytes confers a significant increase in leucine transport that is further trans-stimulated by microinjection of glutamine (a substrate of both SNAT2 and LAT1) directly into LAT1/CD98-expressing oocytes, confirming the ability of the two amino acids to heteroexchange through System L. Significantly, the net accumulation of leucine by LAT1/CD98-expressing oocytes from a physiological amino acid mix is enhanced (∼2-fold) upon coexpression of SNAT2, coincident with a lower accumulation of glutamine than seen with oocytes expressing only SNAT2 (unpublished data). We hypothesize that such coupling may be highly relevant to amino acid signaling, whereby amino acids that are substrates for both Systems A and L (notably glutamine, due to its relatively high abundance in body fluids) provide a crucial counterdrive for the cellular uptake of leucine (and related System L substrates, including isoleucine, valine, phenylalanine, and tryptophan) via System L and thus enhance their ability to stimulate TOR signaling. In this regard, it is noteworthy that studies in cultured Jurkat cells show that activation of mTOR signaling following a period of amino acid depletion is observed only when cells are repleted using amino acid media containing both leucine and glutamine (36).
Implication of a Change in Transmembrane Amino Acid Distribution for Amino Acid Signaling
One important consequence associated with the accumulation of amino acids in the cytoplasmic pool as a result of the activity of secondary active transport mechanisms (e.g., SNAT2 and members of the SLC1 glutamate transporter family) is that their bulk flow establishes an osmotic gradient for the passive influx of water molecules that promote cell swelling. An increase in glycogen and protein synthesis as well as a reduction in protein breakdown have been linked to an increase in intracellular volume that stems from the cell swelling event in tissues such as liver (90) and skeletal muscle (70, 71). Although the increase in liver glycogen synthesis is not thought to involve mTOR signaling (90), there is some suggestion, at least in skeletal muscle cells, that the increase in cell volume may activate the TOR pathway through an adhesion/integrin-dependent mechanism (70, 71). Consequently, although the active accumulation of amino acids serves as an important stimulus to enhance TOR signaling, it is plausible that a component of the anabolic stimulus attributed to the increased intracellular amino acid pool may be derived from that associated with increases in intracellular volume. Equally, amino acid loss or deprivation would be expected to restrain TOR signaling as a result of cellular dehydration/shrinkage. Indeed, loss of glutamine, which accounts for a significant component of the free intracellular amino acid pool, not only results in a reduction in cell volume but significantly blunts the stimulation of the TOR pathway in response to extracellular leucine provision (36). The notion that suppression of mTOR signaling is linked to a reduction in the cytosolic glutamine pool may be of importance in the context of certain muscle wasting conditions. In skeletal muscle, the free intramuscular glutamine pool can fall rapidly and very significantly during a number of disease and injury states (e.g., cachexia, sepsis, uremia, surgical trauma) in which a loss of lean muscle mass is a characteristic feature (5, 96). The rapid loss in intramuscular glutamine associated with such pathologies has, in part, been attributed to the accelerated carrier-mediated efflux of the amino acid (53). Intriguingly, inducing either a “sepsis-like” state in rats by intraperitoneal injection of lipopolysaccharide (66) or muscle atrophy by spinal chord transection (26) markedly reduces the signaling capacity of the TOR pathway in skeletal muscle. Although these latter studies did not examine whether the observed defect in TOR signaling could be attributed to a loss in intramuscular glutamine, reducing the intramyocellular concentration of this amino acid by suppressing the activity or expression of SNAT2 not only enhances cellular proteolysis but impairs mTOR/S6K1 signaling in L6 muscle cells (28, 29). It follows from these observations that the fidelity with which amino acids, such as leucine, can exert an influence on cell signaling is critically dependent on not only maintaining cellular hydration but preserving the transmembrane concentration gradient of glutamine and potentially that of other SNAT2 amino acid substrates, which, although regarded as nonessential, are physiologically indispensable from the perspective of preserving the primacy of leucine in terms of TOR signaling (9).
Although a reduction in the intracellular amino acid pool has a profound suppressive effect on TOR signaling, a fall in amino acid availability increases the amount of uncharged tRNAs that induce the GCN2 pathway (11). Activation of the latter serves to selectively repress global protein synthesis but permits expression of a subset of proteins (e.g., the transcription factor ATF4) that crucially support cellular adaptation to an altered nutrient state (7, 64). The importance of GCN2 and ATF4 with respect to intracellular amino acid sensing and cellular adaptation is underscored by studies in which silencing their expression by RNA interference or expression of dominant negative mutants severely attenuates upregulation of genes encoding amino acid biosynthetic enzymes (e.g., asparagine synthetase) and amino acid transporters (e.g., ASCT1, CAT1, LAT1, SNAT2) by stimuli that induce the general amino acid control response (4, 74, 84). Intriguingly, although ATF4 is normally viewed as a stress-responsive gene (13, 49, 101), very recent work has suggested that this transcription factor can also be upregulated independently of the GCN2 pathway by insulin in a rapamycin-sensitive fashion (4, 74). Malmberg and Adams (74) recently reported that, although insulin and the GCN2 pathway are able to oppose the repressive effects of glucocorticoids on ATF4 expression in mouse L cells, their concerted action is additive with respect to increasing ATF4 mRNA and synergistic with regard to expression of ATF4-dependent genes that promote the uptake and synthesis of amino acids. These observations imply that insulin signaling associated with amino acid sufficiency on the one hand (representing a state of anabolism) and GCN2 activation associated with amino acid deficiency on the other (representing a catabolic state) utilize ATF4 as a point of convergence for the regulation of genes involved in the control of cellular amino acid supply. What remains to be established is whether the insulin-stimulated induction in ATF4 arises as a direct outcome of insulin receptor signaling to TORC1 or whether it represents an indirect effect associated with the stimulation of amino acid influx via transporters such as SNAT2 and the System L heteroexchanger.
Amino Acid Transporters as Nutrient Sensors: the Transceptor Concept
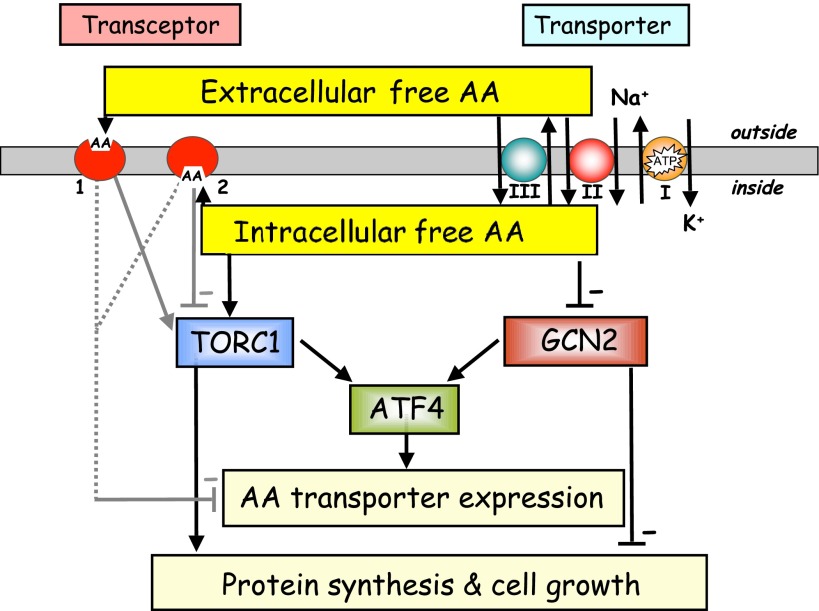
The preceding section focused on how the activity of some amino acid transporters may modulate the size and composition of the intracellular amino acid pool and the impact that this may have, both directly and by circuitous means (e.g., by affecting cellular hydration, membrane polarity, intracellular Ca2+), upon intracellular sensors that regulate signaling via nutrient-sensitive pathways. However, there is increasing recognition that binding of particular amino acids to their respective transporter proteins could also serve as an effective means of sensing amino acid availability at the cell surface if the substrate/carrier-binding event can be transduced to modulate an intracellular signaling response (Fig. 3). The presence of transceptors [a word coined to reflect the dual transport/receptor function of these plasma membrane proteins (52)] has been demonstrated in lower eukaryotes such as yeast and Drosophila, and there is now emerging evidence for their existence in the cells of higher eukaryotes. It is notable that the membrane proteins that have thus far been proposed as amino acid transceptors across the different phylogeny all belong to the superfamily of amino acid-polyamine-organocation (APC) transporters, which suggests that sensing and signaling functions associated with these transporter proteins may have been conserved through evolution.
Fig. 3.
Generalized schema of possible interactions between AA transporters/transceptors and major nutrient-responsive signaling pathways in animal cells [target of rapamycin complex 1 (TORC1), general control nonrepressed (GCN)2 pathways]. ATF, activating transcription factor. For clarity, transporter and transceptor functions are shown separately, but it should be stressed that both are incorporated within a true AA transceptor (e.g., Gap1, SNAT2). Transceptors are proposed to exist in both signaling and nonsignaling states: In the diagram, State 1 (AA bound at extracellular surface) is stimulatory, or ON, whereas State 2 (AA bound at intracellular surface) is inhibitory, or OFF, with respect to the TORC1 pathway. This may account for transient activation of the TOR pathway by external methyl-aminoisobutyric acid (Me-AIB) before it accumulates substantially in the cytoplasm. Binding of any AA substrate (for SNAT2 at least) represses the adaptive upregulation of transporter/transceptor expression in response to nutrient deprivation, although whether this requires intracellular or extracellular binding remains a matter of debate. AA transporters may modulate intracellular free AA pools by coupled transport processes at the cell surface, including primary (I), secondary (II), and tertiary (III) active transport (as detailed in Fig. 2). In discrete, unperfused extracellular spaces (e.g., synaptic clefts), AA transporter actions may also exert significant effects on extracellular AA pools.
Amino Acid Sensing and Signaling by Ssy1
Initial evidence supporting the transceptor concept emerged from studies on yeast genetics in which mutations were screened to identify candidate proteins whose dysfunction disabled the uptake of BCAAs and repressed expression of not only amino acid metabolizing enzymes but several genetically distinct amino acid permeases. From the yeast mutations identified, Ssy1 was considered to function as a sensor of extracellular amino acid availability on the basis of its surface localization and the fact that, despite limited transport capability, it shared homology with other yeast amino acid permeases (23, 24, 65). Ssy1 is now known to operate in conjunction with two peripheral membrane proteins, Ptr3 and Ssy5, to form what is collectively known as the SPS sensor (35). Binding of amino acids is thought to trigger conformational changes in Ssy1 that are transduced via Ptr3 and Ssy5 to promote the NH2-terminal cleavage of two transcription factors, Stp1p and Stp2p, which enables their nuclear entry and ability to promote transcription of genes encoding numerous amino acid permeases. Recent work has suggested that Ssy5 possesses endoprotease activity and that, in response to amino acids interacting with Ssy1, it undergoes a proteolytic processing event that generates an NH2-terminal prodomain and a COOH-terminal activity domain that catalyzes the cleavage of Stp1p and Stp2p (1, 86). What has remained unclear is precisely how Ssy1 amino acid binding and/or transport promote the induction of the proteolysis-dependent signaling pathway, although some very recent studies utilizing Ssy1 mutants that exhibit hyper- or hyporesponsiveness to inducing ligands have shed some interesting insights into this issue (85). Poulsen et al. (102) have mapped the positions of these mutations with respect to their influence on Ssy1 sensor functions and rationalized them in the context of a transporter-like functional model developed using the recently solved three-dimensional structure of the bacterial leucine transporter (LeuT). On the basis of their using the LeuT template for 3D comparative modeling, as well as analysis of the apparent substrate affinity of Ssy1 mutants for inducing amino acids and the capacity to process Stp1, Poulsen et al. have suggested that binding of amino acids to Ssy1 in its outward-facing conformation confers signaling competence, whereas an inward-facing Ssy1 conformation results in a hyporesponsive amino acid sensor (85; see also Fig. 3).
The SPS-sensing pathway is also regulated by casein kinase I (CKI), which has been suggested to positively influence signaling via the SPS sensor by mechanisms that hitherto have been poorly understood. However, recent analysis of loss- and gain-of-function mutations in Ptr3 have shown that these are associated with reduced or increased phosphorylation of Ptr3, respectively (68). Intriguingly, defects in PP2A phosphatase activity also give rise to a Ptr3 hyperphosphorylated phenotype that promotes constitutive activation of SPS signaling. Given that a yeast two-hybrid analysis indicates that CKI and Ptr3 can physically interact with the NH2-terminal domain of Ssy1, this raises the possibility that CKI and PP2A may serve to coordinate SPS sensing/signaling via modulation of Ptr3 phosphorylation (68). Such a model presumes that the interaction between CKI and Ssy1 is regulated by amino acid binding to Ssy1 and that Ptr3 phosphorylation promotes activation of the associated Ssy5 endoprotease, which then permits processing of the two transcriptional factors Stp1p and Stp2p (68).
The General Amino Acid Permease (Gap1)
Although Ssy1 shares homology with other yeast amino acid permeases, it is an extremely inefficient transporter and is considered to function primarily as a sensor of extracellular amino acid availability. However, work from Thevelein's group has provided strong evidence that the general amino acid permease (Gap1), which functions as a broad-range low-affinity amino acid transporter, also functions as an amino acid sensor in Saccharomyces cerevisiae (20, 25). Yeast cells maintained in a fermentable growth media lacking a nitrogen source (e.g., amino acids) are maintained in a growth-arrested state that is also characterized by a low PKA activity phenotype. However, upon amino acid resupplementation and growth resumption, yeast cells exhibit a rapid activation of the PKA pathway that triggers a sharp fall in cellular trehalose content resulting from a PKA-dependent increase in trehelase activity. Under such circumstances, Gap1 is the principal permease expressed in S. cerevisiae and is directly responsible for initiating the amino acid-induced activation of PKA and trehalase activity. This assertion is based on a number of findings that demonstrate 1) that the rapid activation of PKA signaling does not involve metabolism of amino acids, 2) that activation of PKA and trehelase in response to amino acid supplementation is not observed in cells that are genetically deficient in Gap1, thus excluding amino acid influx via other carriers as a means of regulating PKA signaling, and 3) that although truncations of the NH2-terminal cytoplasmic domain of Gap1 have no apparent effect on transport or signaling capacity, truncating the last 46 amino acids of the COOH terminus abolishes both amino acid uptake and PKA signaling. In contrast, expression of Gap1 with smaller truncations of the COOH-terminal domain (12 or 26 amino acids) results in constitutive activation of PKA yet normal rates of amino acid uptake. This overactive PKA phenotype is not observed when Gap1 harboring the shorter truncations is expressed in cells defective in PKA (TPK1 and TPK2) subunit expression (25). Collectively, the findings that Gap1 expression can be induced or repressed depending on availability of an extracellular nitrogen (amino acid) source and that, in addition to substrate availability, activation of PKA is dependent on the COOH-terminal Gap1 domain provide compelling evidence that Gap1 functions as a transceptor. In a very exciting new development, Thevelein's group has now shown that amino acid signaling by Gap1 utilizes the same binding site as amino acid transport, but, although it is thought to involve a ligand-induced conformation change in Gap1, it does not require the complete transport cycle (94).
Role of CATs and PATs as Amino Acid Sensors
The Drosophila fat body, the vertebrate liver equivalent, has important functions associated with nutrition and tissue growth (89) that depend on the expression and function of two different classes of amino acid transporters. One of these, MDN, encoded by the minidiscs gene, resembles a subunit similar to that of heterodimeric amino acid transporters found in higher eukaryotes, including humans (77). Mutations in minidiscs give rise to larvae with nonautonomous growth defects in imaginal discs, indicating that amino acid uptake via the MDN transporter in the fat body is likely to form part of a nutrient-sensing/signaling pathway that supports the normal maturation and proliferation of imaginal discs (77). The second class of transporter, encoded by the Slimfast (Slif) gene, shares strong homology with members of the cationic amino acid transporter (CAT) family (19). Mutations in Slimfast generate larvae that exhibit systemic growth retardation and morphological changes of the fat body, a phenotype resembling that seen in response to amino acid deprivation or loss of TOR signaling (103). Intriguingly, inhibition of Slimfast within the fat body has a profound suppressive effect on PI 3-kinase directed signaling in other distant endoreplicative tissues [e.g., epidermal and salivary gland cells (19)], suggesting that influx of amino acids by Slimfast in the fat body may support expression/secretion of a humoral factor that influences signaling and growth in other tissues. The fat body does not express an insulin-like homolog but abundantly expresses a glycoprotein known as acid-labile subunit (ALS), the mammalian ortholog of which binds and stabilizes circulating insulin-like growth factor (14). Expression of dALS is markedly reduced in the fat body in response to amino acid limitation or inhibition of Slimfast, indicating that the ability to influence signaling and growth in other tissues may depend upon fat body-derived dALS stabilizing and extending the biological half-life of Drosophila insulin-like peptides (19). Although it remains unclear whether amino acid uptake by Slimfast regulates dALS expression in a dTOR-dependent manner, influx of amino acids by this transporter has been implicated in the activation of TOR signaling to support bulk endocytic uptake while preventing the targeted endocytic degradation of Slimfast (51). Thus, under circumstances of amino acid sufficiency, TOR coordinates a downregulation of endocytic events that would inhibit growth (such as the internalization of Slimfast) while simultaneously upregulating potential growth-promoting functions of endocytosis. It is noteworthy that Slimfast has also been implicated in mosquito vitellogenesis, a process involving yolk deposition during the mosquito reproductive cycle and one that is critically dependent on the expression of yolk protein precursor genes such as vitellogenin by a TOR/S6K1-dependent mechanism (8, 48). Attardo et al. (8) have identified an additional fat body cationic amino acid transporter (iCAT2), whose expression profile differs from that of Slimfast during the vitellogenic cycle but which nonetheless, like Slimfast, is required for the amino acid-induced expression of vitellogenin. Since expression of iCAT2 is elevated just following activation of vitellogenesis by blood feeding, it has been suggested that its expression is induced in the fat body in response to increased amino acid availability in the hemolymph. Whether iCAT2 is responsible for sensing the changes in circulating amino acid levels after a blood meal and for upregulating its own expression to facilitate amino acid uptake for yolk protein production remains to be determined.
A third class of amino acid transporter that appears to modulate tissue-autonomous growth in Drosophila is one encoded by two genes, CG3424 (path) and CG1139 (43), related to the mammalian proton-coupled SLC36 amino acid transporters known as the PATs (15). Mutations in path result in flies with marked reduction in wing and eye size, whereas path overexpression induces overgrowth of the differentiating eye. Based on a number of developmental assays, the effects of path on cell and tissue growth are thought to be mediated by modulation of TOR signaling to which the Drosophila PAT transporters can be genetically linked. Intriguingly, although the transport characteristics of CG1139 are remarkably similar to those exhibited by mammalian PATs, the path transporter deviates significantly in terms of its pH dependence, substrate specificity/affinity (<3 μM), and its rather limited transport capacity (43). Moreover, while heterologous expression of path in Xenopus oocytes is associated with a modest activation of S6K1 in response to exogenous alanine supply, it seems incongruent that a carrier with very limited transport capacity, and one that is likely to be saturated at physiological amino acid concentrations, would be able to convey nutrient signals to the TOR pathway with a degree of sensitivity that might reflect changes in extracellular nutrient availability (43). Consequently, it is likely that path influences growth pathways by a transport-independent mechanism in which, for example, transporter occupancy might provide a constitutive stimulus that helps preserve basal TOR signaling to support growth during periods when nutrient levels may fluctuate significantly. Expression of human PAT transporters may also be linked to cell growth and proliferation, although their substrate preference does not interface well with known amino acid activators of the TOR pathway. PAT transporters are expressed both at the cell surface and in intracellular membranes such as lysosomes; hence, they may potentially interact with intracellular TOR signaling, perhaps in control of autophagy.
Sensing and Signaling via Excitatory Glutamate Transporters
Glutamate is a major excitatory transmitter in the mammalian central nervous system; consequently, glutamate transporters play a critical role in maintaining the integrity and sensitivity of excitatory synaptic transmission (10). Five major subtypes of excitatory amino acid transporters (EAAT1-5) have thus far been identified, which, along with ASCT1 and ASCT2, constitute the SLC1 family (61). Of these, the glial glutamate/aspartate transporter GLAST/EAAT1, which is regulated extensively by transcriptional (69), posttranslational (38), and cytoplasmic trafficking (42) mechanisms, plays a major role in extracellular glutamate clearance (6). Under circumstances of increased substrate availability, the interaction of glutamate with EAAT1 not only upregulates the activity of the transporter (27, 79) but has been reported to stimulate ERK signaling in astrocyte cultures (2). The glutamate-induced activation of ERK is thought to require amino acid transport, as it is mimicked by two transportable glutamate uptake inhibitors (dl-threo-b-hyroxyaspartate and l-trans-pyrrolidine-2,4-dicarboxylate), but not by agonists that bind to cell surface glutamate receptors or by dihydrokainate, which selectively blocks astrocytic glutamate uptake via EAAT2 (2). These findings suggest that induction of ERK signaling is closely coupled with glutamate transport mediated primarily through EAAT1 (2). The rapid substrate-induced increase in glutamate uptake is due to an increase in transport Vmax (27), which stems from a glutamate-induced redistribution of EAAT1 from a cytosolic pool to the astrocyte plasma membrane (42). Increases in extracellular glutamate/aspartate not only stimulate recruitment of EAAT1 to the plasma membrane of astrocytes, but the attendant increase in amino acid influx via this transport system also influences the surface expression and activity of ASCT2; a carrier that in addition to glutamine and certain other neutral amino acids is also able to transport protonated glutamate (41). The cellular distribution of ASCT2 is greatly influenced by glutamine generated from glutamate taken up into cells via EAAT1. Consistent with this idea, silencing the expression of glutamine synthetase by using RNA interference leads to a profound loss in glutamate's ability to promote translocation of ASCT2 to the astrocyte plasma membrane (41). While clearly implicating EAAT1 in the regulation of ASCT2, these findings serve to reinforce the indirect effect that amino acid transporters may exert on cellular functions through their capacity to modulate the intracellular availability/pool size of certain amino acids (as discussed earlier in this review). Although it is currently unknown whether activation of ERK signaling is needed for the cell surface recruitment of EAAT1, recent work demonstrating that a nontransporting EAAT1 inhibitor antagonizes the substrate-induced redistribution of the carrier provides strong support for the notion that the transporter functions both as a sensor and as an effector in a process that rapidly clears extracellular glutamate to prevent excitotoxic damage (42).
A Role for SNAT2 (System A) in Amino Acid Sensing
One of the best-documented responses to global amino acid withdrawal in mammalian cells is the upregulation of System A transport activity, a response known as “adaptive regulation” (45, 63). Adaptation of System A to amino acid withdrawal is thought to be a two-stage process: an initial acute phase involves an increase in transport activity that results from the release of transporters from substrate repression upon removal of extracellular amino acids. The second phase involves a time-dependent increase in transport activity that requires an induction of gene expression, based on the finding that it is halted by inhibitors that target transcription and translation (34). Studies from our laboratory and those of others have now demonstrated that the increase in System A activity associated with cellular amino acid withdrawal is associated with increased expression and surface localization of SNAT2 (40, 54, 67), which is the most ubiquitously expressed of the three SLC38 family members exhibiting System A-like activity (72). Despite our long-standing appreciation of the System A adaptation phenomenon, our understanding of the molecular events that permit upregulation of SNAT2 expression in response to amino acid withdrawal and those involved in its repression during amino acid sufficiency is only now beginning to develop.
The increase in SNAT2 expression in response to amino acid withdrawal will, in part, be facilitated by the activation of the GCN2 pathway and transcription of ATF4-dependent genes, of which SNAT2 is one (92). Activation of this pathway appears to depend on the overall level of cellular stress imposed and, perhaps surprisingly, is usually more pronounced when a single amino acid is withdrawn than when total amino acid starvation is imposed. Moreover, the finding that the 5′-UTR of the SNAT2 mRNA contains an internal ribosome entry sequence (IRES) suggests that SNAT2 mRNA will be efficiently translated via a cap-independent mechanism despite any global reduction in protein synthesis that would be associated with amino acid starvation (37). Notwithstanding the important contribution that these latter processes will make to an increase in System A expression/activity, there is also mounting evidence that interactions between SNAT2 and its substrates may play an important role in the adaptation response. Indeed, the notion that System A might sense substrate availability was first mooted nearly three decades ago by Gazzola et al. (39) on the basis of the observation that the adaptive increase in transport activity could be repressed by System A reactive amino acids in cultured human fibroblasts. We have recently extended this idea by demonstrating that the induction in SNAT2 protein in amino acid-deprived L6 muscle cells can be attenuated upon resupply of a single substrate amino acid to culture medium that otherwise remains amino acid deficient (55). Since the increase in uncharged tRNA levels is unlikely to subside by resupplementation of a single SNAT2 amino acid, the finding implies that the repressive signal is dominant over that inducing transporter expression via the GCN2/ATF4 signaling axis. The observed repression does not require substrate metabolism, as it is mimicked by Me-AIB, a nonmetabolizable SNAT2 substrate. Importantly, studies involving a SNAT2 reporter gene whose expression can also be induced by cellular amino acid withdrawal have revealed that the potency with which SNAT2 substrates (such as Me-AIB) repress SNAT2 expression is correlated very tightly with their transport Km for SNAT2 (55). On the basis of these findings, we have suggested that occupancy of the SNAT2 substrate-binding site (signifying an amino acid-sufficient state) invokes a repressive signal that blunts the adaptive response. In support of this idea, shRNA-mediated silencing of SNAT2 in amino acid-replete cells (an intervention that would reduce this putative repressive signal) results in the increased expression of a SNAT2-luciferase reporter gene, whereas SNAT2 overexpression reduces the reporter readout (55). Precisely how SNAT2 transporter occupancy is sensed and how this is transduced to an event that regulates distal transcriptional events still remain unknown. However, it is possible that SNAT2 may, depending on substrate binding, adopt a conformation that permits it to signal to molecules regulating its expression. One intriguing possibility is that SNAT2 signaling capability may switch between ON and OFF states depending on whether Me-AIB is bound at the outer cell surface, based on the concept of Ssy1 signaling proposed by Kielland-Brandt et al. (85). The stability of these states may conceivably be influenced by covalent modification of SNAT2, for example by phosphorylation, which might modulate the response dependent on other signals such as growth factors. Recent crystallographic studies assessing the mechanisms that govern nutrient binding and translocation within bacterial transporters such as the lactose permease (3), LeuT (91, 107), and vSGLT (the bacterial ortholog of human SGLT1) (31), have suggested that these archetypal nutrient transporters may adopt “rocker switch” or “gated pore” conformations that may also include intermediate occluded conformations in which the substrate-binding site is temporarily “closed off” to both sides of the cell membrane (62). The idea that SNAT2 may adopt such conformations is supported by the fact that this transporter is susceptible to trans-inhibition, an effect that can be rapidly imposed by the excess accumulation of System A substrates on the cytoplasmic side that suppress further amino acid uptake from the extracellular compartment. Mechanistically, the trans-inhibitory effect exerted by cytoplasmic substrates is hypothesized to “lock” or “trap” System A transporters in their inward-facing conformation by either preventing substrate unloading or inhibiting the unloaded carrier from returning to its outward-facing conformation as part of the normal transport cycle. Consequently, trans-inhibition may not only provide a rapid means for restraining overexpansion of the intracellular amino acid pool during periods of amino acid sufficiency but may also form part of a mechanism that regulates tranceptor-mediated signaling with respect to both intra- and extracellular amino acid availability.
An additional line of evidence that indicates a potential role for SNAT2 in amino acid signaling comes from the work of Bevington and coworkers, who have recently reported that incubation of muscle cells with a saturating dose of Me-AIB exerts a stimulatory effect on insulin-stimulated PI 3-kinase activity and PKB/Akt phosphorylation (29). In contrast, silencing SNAT2 expression using RNA interference had a suppressive effect on the lipid kinase activity of PI 3-kinase (29). It currently remains unclear how SNAT2 may modulate PI 3-kinase/PKB signaling, but such a response may act to synergize the effects of insulin on the TOR pathway. In preliminary work from our own laboratory using MCF7 cells (a human breast adenocarcinoma cell line), we have observed that SNAT2 may directly regulate TOR signaling on the basis that incubation of these cells with Me-AIB induces a rapamycin-sensitive phosphorylation of S6K1 despite promoting a reduction in intracellular amino acids as a result of suppressing uptake via a common carrier or one dependent on influx through a System A-coupled heteroexchanger (Pinilla-Tenas J, Hundal HS, and Taylor PM, unpublished data). It is conceivable that the positive effects associated with Me-AIB treatment upon TOR signaling are mediated by activation of nutrient sensitive intracellular kinases akin to either Ste20 (33) or hVps34 (46) rather than by SNAT2 itself; however, as yet there is no evidence in the literature that links activation of these proteins either to SNAT2 activity or indeed to the presence of synthetic amino acid analogs such as Me-AIB.
Is there any scope for the idea that SNAT2 itself may initiate an amino acid-dependent signal? Numerous proteins have been reported to interact with amino acid transporters (see Ref. 57 for examples), some of which have the potential to modulate cell signaling. Such interactions are most likely to occur via domains located within the intracellular hydrophilic regions of transporters. In the case of the Ssy1 (SPS) sensor, the extended NH2-terminal cytoplasmic domain is thought to be critical for facilitating interactions with CK1 and Ptr3 and for the conference of amino acid responsiveness of the sensor (35). Our own recent work suggests that the NH2-terminal cytoplasmic domain of SNAT2 may be important in stabilizing SNAT2 expression during amino acid deficiency (55), which may occur as a result of proteins interacting with and promoting phosphorylation or ubquitination of this part of the transporter (50, 81). Membranocytoskeletal proteins [ankyrin and fodrin (47)], as well as integrin-α3/β1 dimers (78), have been shown to interact with System A and may be important in regulating the localization and the activity of this carrier, but whether such interactions are mediated by the NH2-terminal region of the transporter and have a role in amino acid sensing and signal transduction have yet to be addressed. Recent work has reported the existence of an anion leak conductance associated with SNAT2 that is subject to differential inhibition by transported amino acid substrates (105). Although the precise significance of this SNAT2 anion conductance is currently unknown, the movement of anions via other secondary active transporters such as those for glutamate and neurotransmitters (e.g., GABA, serotonin, and dopamine) is well documented (61) and, at least in the case of the dopamine transporter, has been shown to be important in regulating presynaptic excitability (58). It is conceivable that, along with other anion conductances, that mediated by SNAT2 may contribute to maintaining the resting membrane potential or alternatively, given that it is differentially inhibited by SNAT2 substrates, that it may represent a component of the mechanism that senses carrier occupancy by different substrates.
Summary and Future Developments
In this review, we have summarized evidence for the role of amino acid transporters as sensors of both extracellular and intracellular amino acid availability. Our current interpretation, as regards signaling through the TORC1 and GCN2 pathways, is summarized in Fig. 3. In this schema, it is evident that the coupling of amino acid transport activities is likely to play an important part in determining the size and composition of the intracellular amino acid pool and, hence, signaling downstream of intracellular amino acid sensors. However, we still require a better understanding of both the kinetics of transporter coupling and the nature of the intracellular amino acid-sensing mechanisms at the molecular level if we are to fully reveal the functional importance of membrane transporters in the overall amino acid sensing process. Studies in lower eukaryotes will continue to throw light on the fundamental mechanisms of nutrient sensing and transceptor action and will provide a valuable framework for us to elucidate their functions in mammalian cells. The transceptor-like properties of mammalian proteins such as SNAT2 are likely to produce relatively subtle changes in cell metabolism under physiological circumstances compared with those described for analogous systems in yeast such as Ssy1, due largely to the homeostatic protection afforded by the extracellular fluid and the influence of endocrine factors. Continued advances in our understanding of how mammalian transceptors initiate intracellular signals are likely to involve greater use of transgenic technologies (e.g., to silence SNAT2 or LAT1 gene expression) for both in vivo and in vitro studies and, at the molecular level, will focus increasingly on the mechanism(s) of transceptor function (e.g., in relation to substrate-induced conformational changes and their effects on downstream signaling intermediates). In the long run, these advances may reveal novel approaches by which pathways influencing cell growth and proliferation can be modulated either 1) to switch on protein-anabolic signals in circumstances of cellular protein wasting (and, hence, to improve treatments for diseases including cachexia and age-related sarcopenia) or 2) to silence these signals in order to suppress the excessive cellular growth and proliferation seen in certain tumors.
GRANTS
We also acknowledge the financial support of the BBSRC, Medical Research Council (UK), the European Commission (contract LSHM-CT-20004-005272), Diabetes Research and Wellness Foundation, and Diabetes UK.
Acknowledgments
We acknowledge the valuable contribution of Dr. Russell Hyde in some of the studies and ideas that have emanated from our laboratory.
REFERENCES
- 1.Abdel-Sater F, El Bakkoury M, Urrestarazu A, Vissers S, Andre B. Amino acid signaling in yeast: casein kinase I and the Ssy5 endoprotease are key determinants of endoproteolytic activation of the membrane-bound Stp1 transcription factor. Mol Cell Biol 24: 9771–9785, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe K, Saito H. Possible linkage between glutamate transporter and mitogen-activated protein kinase cascade in cultured rat cortical astrocytes. J Neurochem 76: 217–223, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science 301: 610–615, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Adams CM Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J Biol Chem 282: 16744–16753, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Alvestrand A, Fürst P, Bergström J. Plasma and muscle free amino acids in uremia: influence of nutrition with amino acids. Clin Nephrol 18: 297–303, 1982. [PubMed] [Google Scholar]
- 6.Amara SG, Fontana AC. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int 41: 313–318, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol 40: 14–21, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Attardo GM, Hansen IA, Shiao SH, Raikhel AS. Identification of two cationic amino acid transporters required for nutritional signaling during mosquito reproduction. J Exp Biol 209: 3071–3078, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab (September 2, 2008). doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed]
- 10.Beart PM, O'shea RD. Transporters for l-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol 150: 5–17, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berlanga JJ, Santoyo J, de Haro C. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur J Biochem 265: 754–762, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Bevington A, Brown J, Butler H, Govindji S, Khalid K, Sheridan K, Walls J. Impaired system A amino acid transport mimics the catabolic effects of acid in L6 cells. Eur J Clin Invest 32: 590–602, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Blais JD, Filipenko V, Bi M, Harding HP, Ron D, Koumenis C, Wouters BG, Bell JC. Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol 24: 7469–7482, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol 170: 63–70, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Boll M, Daniel H, Gasnier B. The SLC36 family: proton-coupled transporters for the absorption of selected amino acids from extracellular and intracellular proteolysis. Pflügers Arch 447: 776–779, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem 280: 33076–33082, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Christensen HN Role of amino-acid transport and counter-transport in nutrition and metabolism. Physiol Rev 70: 43–77, 1990. [DOI] [PubMed] [Google Scholar]
- 18.Christie GR, Hajduch E, Hundal HS, Proud CG, Taylor PM. Intracellular sensing of amino acids in Xenopus laevis oocytes stimulates p70 S6 kinase in a TOR-dependent manner. J Biol Chem 277: 9952–9953, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell 114: 739–749, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Crauwels M, Donaton MC, Pernambuco MB, Winderickx J, de Winde JH, Thevelein JM. The Sch9 protein kinase in the yeast Saccharomyces cerevisiae controls cAPK activity and is required for nitrogen activation of the fermentable-growth-medium-induced (FGM) pathway. Microbiology 143: 2627–2637, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Dann SG, Thomas G. The amino acid sensitive TOR pathway from yeast to mammals. FEBS Lett 580: 2821–2829, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Deken SL, Beckman ML, Boos L, Quick MW. Transport rates of GABA transporters: regulation by the N-terminal domain and syntaxin 1A. Nat Neurosci 3: 998–1003, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Didion T, Grausland M, Kielland-Brandt C, Andersen HA. Amino acids induce expression of BAP2, a branched-chain amino acid permease gene in Saccharomyces cerevisiae. J Bacteriol 178: 2025–2029, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Didion T, Regenberg B, Jorgensen MU, KiellandBrandt MC, Andersen HA. The permease homologue Ssy1p controls the expression of amino acid and peptide transporter genes in saccharomyces cerevisiae. Mol Microbiol 27: 643–650, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Donaton MC, Holsbeeks I, Lagatie O, Van Zeebroeck G, Crauwels M, Winderickx J, Thevelein JM. The Gap1 general amino acid permease acts as an amino acid sensor for activation of protein kinase A targets in the yeast Saccharomyces cerevisiae. Mol Microbiol 50: 911–929, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Dreyer HC, Glynn EL, Lujan HL, Fry CS, DiCarlo SE, Rasmussen BB. Chronic paraplegia-induced muscle atrophy downregulates the mTOR/S6K1 signaling pathway. J Appl Physiol 104: 27–33, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan S, Anderson CM, Stein BA, Swanson RA. Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J Neurosci 19: 10193–10200, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans K, Nasim Z, Brown J, Butler H, Kauser S, Varoqui H, Erickson JD, Herbert TP, Bevington A. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol 18: 1426–1436, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Evans K, Nasim Z, Brown J, Clapp E, Amin A, Yang B, Herbert TP, Bevington A. Inhibition of SNAT2 by metabolic acidosis enhances proteolysis in skeletal muscle. J Am Soc Nephrol 19: 2119–2129, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fafournoux P, Bruhat A, Jousse C. Amino acid regulation of gene expression. Biochem J 351: 1–12, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faham S, Watanabe A, Besserer GM, Cascio D, Specht A, Hirayama BA, Wright EM, Abramson J. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science 321: 810–814, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature 390: 81–85, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Findlay GM, Yan L, Procter J, Mieulet V, Lamb RF. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling. Biochem J 403: 13–20, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fong AD, Handlogten ME, Kilberg MS. Substrate-dependent adaptive regulation and trans-inhibition of System A-mediated amino acid transport. Studies using rat hepatoma plasma membrane vesicles. Biochim Biophys Acta 1022: 325–332, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Forsberg H, Ljungdahl PO. Genetic and biochemical analysis of the yeast plasma membrane Ssy1p-Ptr3p-Ssy5p sensor of extracellular amino acids. Mol Cell Biol 21: 814–826, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fumarola C, La Monica S, Guidotti GG. Amino acid signaling through the mammalian target of rapamycin (mTOR) pathway: role of glutamine and of cell shrinkage. J Cell Physiol 204: 155–165, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Gaccioli F, Huang CC, Wang C, Bevilacqua E, Franchi-Gazzola R, Gazzola GC, Bussolati O, Snider MD, Hatzoglou M. Amino acid starvation induces the SNAT2 neutral amino acid transporter by a mechanism that involves eukaryotic initiation factor 2alpha phosphorylation and cap-independent translation. J Biol Chem 281: 17929–17940, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Gamboa C, Ortega A. Insulin-like growth factor-1 increases activity and surface levels of the GLAST subtype of glutamate transporter. Neurochem Int 40: 397–403, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Gazzola GC, Dall'Asta V, Guidotti GG. Adaptive regulation of amino acid transport in cultured human fibroblasts. Sites and mechanism of action. J Biol Chem 256: 3191–3198, 1981. [PubMed] [Google Scholar]
- 40.Gazzola RF, Sala R, Bussolati O, Visigalli R, Dall'Asta V, Ganapathy V, Gazzola GC. The adaptive regulation of amino acid transport system A is associated to changes in ATA2 expression. FEBS Lett 490: 11–14, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Gegelashvili M, Rodriguez-Kern A, Pirozhkova I, Zhang J, Sung L, Gegelashvili G. High-affinity glutamate transporter GLAST/EAAT1 regulates cell surface expression of glutamine/neutral amino acid transporter ASCT2 in human fetal astrocytes. Neurochem Int 48: 611–615, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Gegelashvili M, Rodriguez-Kern A, Sung L, Shimamoto K, Gegelashvili G. Glutamate transporter GLAST/EAAT1 directs cell surface expression of FXYD2/gamma subunit of Na,K-ATPase in human fetal astrocytes. Neurochem Int 50: 916–920, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Goberdhan DC, Meredith D, Boyd CA, Wilson C. PAT-related amino acid transporters regulate growth via a novel mechanism that does not require bulk transport of amino acids. Development 132: 2365–2375, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci USA 105: 17402–17407 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guidotti GG Hormonal and adaptive control of amino acid transport in muscle. Proc Nutr Soc 31: 179–184, 1972. [DOI] [PubMed] [Google Scholar]
- 46.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab 7: 456–465, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Handlogten ME, Dudenhausen EE, Yang W, Kilberg MS. Association of hepatic system A amino acid transporter with the membrane-cytoskeletal proteins ankyrin and fodrin. Biochim Biophys Acta 1282: 107–114, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Hansen IA, Attardo GM, Roy SG, Raikhel AS. Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. J Biol Chem 280: 20565–20572, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Hatanaka T, Hatanaka Y, Setou M. Regulation of amino acid transporter ATA2 by ubiquitin ligase Nedd4–2. J Biol Chem 281: 35922–35930, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Hennig KM, Colombani J, Neufeld TP. TOR coordinates bulk and targeted endocytosis in the Drosophila melanogaster fat body to regulate cell growth. J Cell Biol 173: 963–974, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holsbeeks I, Lagatie O, Van Nuland A, Van d V, Thevelein JM. The eukaryotic plasma membrane as a nutrient-sensing device. Trends Biochem Sci 29: 556–564, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Hundal HS Role of membrane transport in the regulation of skeletal muscle glutamine turnover. Clin Nutr 10 Suppl: 33–42, 1991. [DOI] [PubMed] [Google Scholar]
- 54.Hyde R, Christie GR, Litherland GJ, Hajduch E, Taylor PM, Hundal HS. Subcellular localization and adaptive up-regulation of the System A (SAT2) amino acid transporter in skeletal-muscle cells and adipocytes. Biochem J 355: 563–568, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hyde R, Cwiklinski EL, MacAulay K, Taylor PM, Hundal HS. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability. J Biol Chem 282: 19788–19798, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J 19: 461–463, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J 373: 1–18, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci 5: 971–978, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Ishizuka Y, Kakiya N, Nawa H, Takei N. Leucine induces phosphorylation and activation of p70S6K in cortical neurons via the system L amino acid transporter. J Neurochem 106: 934–942, 2008. [DOI] [PubMed] [Google Scholar]
- 60.Jackson M, Song W, Liu MY, Jin L, Dykes-Hoberg M, Lin CI, Bowers WJ, Federoff HJ, Sternweis PC, Rothstein JD. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature 410: 89–93, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflügers Arch 447: 469–479, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Karpowich NK, Wang Structural biology DN. Symmetric transporters for asymmetric transport. Science 321: 781–782, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kilberg MS, Han H, Barber EF, Chiles TC. Adaptive regulation of neutral amino acid transport System A in rat H4 hepatoma cells. J Cell Physiol 122: 290–298, 1985. [DOI] [PubMed] [Google Scholar]
- 64.Kilberg MS, Pan YX, Chen H, Leung-Pineda V. Nutritional control of gene expression: how mammalian cells respond to amino acid limitation. Annu Rev Nutr 25: 59–85, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klasson H, Fink GR, Ljungdahl PO. Ssy1p and Ptr3p are plasma membrane components of a yeast system that senses extracellular amino acids. Mol Cell Biol 19: 5405–5416, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lang CH, Frost RA. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E-BP1, and S6K1 in skeletal muscle. J Cell Physiol 203: 144–155, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Ling R, Bridges CC, Sugawara M, Fujita T, Leibach FH, Prasad PD, Ganapathy V. Involvement of transporter recruitment as well as gene expression in the substrate-induced adaptive regulation of amino acid transport system A. Biochim Biophys Acta 1512: 15–21, 2001. [DOI] [PubMed] [Google Scholar]
- 68.Liu Z, Thornton J, Spirek M, Butow RA. Activation of the SPS amino acid-sensing pathway in Saccharomyces cerevisiae correlates with the phosphorylation state of a sensor component, Ptr3. Mol Cell Biol 28: 551–563, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez-Bayghen E, Ortega A. Glutamate-dependent transcriptional regulation of GLAST: role of PKC. J Neurochem 91: 200–209, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Low SY, Rennie MJ, Taylor PM. Involvement of integrins and the cytoskeleton in modulation of skeletal muscle glycogen synthesis by changes in cell volume. FEBS Lett 417: 101–103, 1997. [DOI] [PubMed] [Google Scholar]
- 71.Low SY, Rennie MJ, Taylor PM. Signaling elements involved in amino acid transport responses to altered muscle cell volume. FASEB J 11: 1111–1117, 1997. [DOI] [PubMed] [Google Scholar]
- 72.Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflügers Arch 447: 784–795, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Maier S, Reiterer V, Ruggiero AM, Rothstein JD, Thomas S, Dahm R, Sitte HH, Farhan H. GTRAP3-18 serves as a negative regulator of Rab1 in protein transport and neuronal differentiation. J Cell Mol Med 2008. [DOI] [PMC free article] [PubMed]
- 74.Malmberg SE, Adams CM. Insulin signaling and the general amino acid control response. Two distinct pathways to amino acid synthesis and uptake. J Biol Chem 283: 19229–19234, 2008. [DOI] [PubMed] [Google Scholar]
- 75.Marie H, Attwell D. C-terminal interactions modulate the affinity of GLAST glutamate transporters in salamander retinal glial cells. J Physiol 520: 393–397, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marie H, Billups D, Bedford FK, Dumoulin A, Goyal RK, Longmore GD, Moss SJ, Attwell D. The amino terminus of the glial glutamate transporter GLT-1 interacts with the LIM protein Ajuba. Mol Cell Neurosci 19: 152–164, 2002. [DOI] [PubMed] [Google Scholar]
- 77.Martin JF, Hersperger E, Simcox A, Shearn A. minidiscs encodes a putative amino acid transporter subunit required non-autonomously for imaginal cell proliferation. Mech Dev 92: 155–167, 2000. [DOI] [PubMed] [Google Scholar]
- 78.McCormick JI, Johnstone RM. Identification of the integrin alpha 3 beta 1 as a component of a partially purified A-system amino acid transporter from Ehrlich cell plasma membranes. Biochem J 311: 743–751, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munir M, Correale DM, Robinson MB. Substrate-induced up-regulation of Na(+)-dependent glutamate transport activity. Neurochem Int 37: 147–162, 2000. [DOI] [PubMed] [Google Scholar]
- 80.O'Mara M, Oakley A, Broer S. Mechanism and putative structure of B(0)-like neutral amino acid transporters. J Membr Biol 213: 111–118, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127: 635–648, 2006. [DOI] [PubMed] [Google Scholar]
- 82.Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev 78: 969–1054, 1998. [DOI] [PubMed] [Google Scholar]
- 83.Palacin M, Kanai Y. The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflügers Arch 447: 490–494, 2004. [DOI] [PubMed] [Google Scholar]
- 84.Palii SS, Thiaville MM, Pan YX, Zhong C, Kilberg MS. Characterization of the amino acid response element within the human sodium-coupled neutral amino acid transporter 2 (SNAT2) System A transporter gene. Biochem J 395: 517–527, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poulsen P, Gaber RF, Kielland-Brandt MC. Hyper- and hyporesponsive mutant forms of the Saccharomyces cerevisiae Ssy1 amino acid sensor. Mol Membr Biol 25: 164–176, 2008. [DOI] [PubMed] [Google Scholar]
- 86.Poulsen P, Lo LL, Kielland-Brandt MC. Mapping of an internal protease cleavage site in the Ssy5p component of the amino acid sensor of Saccharomyces cerevisiae and functional characterization of the resulting pro- and protease domains by gain-of-function genetics. Eukaryot Cell 5: 601–608, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quick MW Substrates regulate gamma-aminobutyric acid transporters in a syntaxin 1A-dependent manner. Proc Natl Acad Sci USA 99: 5686–5691, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reimann F, Ward PS, Gribble FM. Signalling mechanisms underlying the release of glucagon-like peptide-1. Diabetes 55: S78–S85, 2006. [Google Scholar]
- 89.Schlegel A, Stainier DY. Lessons from “lower” organisms: what worms, flies, and zebrafish can teach us about human energy metabolism. PLoS Genet 3: e199, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schliess F, Richter L, vom DS, Haussinger D. Cell hydration and mTOR-dependent signalling. Acta Physiol (Oxf) 187: 223–229, 2006. [DOI] [PubMed] [Google Scholar]
- 91.Shi L, Quick M, Zhao Y, Weinstein H, Javitch JA. The mechanism of a neurotransmitter:sodium symporter–inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol Cell 30: 667–677, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thiaville MM, Dudenhausen EE, Awad KS, Gjymishka A, Zhong C, Kilberg MS. Activated transcription via mammalian amino acid response elements does not require enhanced recruitment of the Mediator complex. Nucleic Acids Res 36: 5571–5580, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Sluijters DA, Dubbelhuis PF, Blommaart EF, Meijer AJ. Amino-acid-dependent signal transduction. Biochem J 351: 545–550, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Zeebroeck G, Bonini BM, Versele M, Thevelein JM. Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor. Nat Chem Biol 5: 45–52, 2008. [DOI] [PubMed] [Google Scholar]
- 95.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflügers Arch 447: 532–542, 2004. [DOI] [PubMed] [Google Scholar]
- 96.Vinnars E, Bergström J, Fürst P. Influence of the postoperative state on the intracellular free amino acids in human muscle tissue. Ann Surg 182: 665–671, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 21: 362–369, 2006. [DOI] [PubMed] [Google Scholar]
- 98.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34: 7–11, 2006. [DOI] [PubMed] [Google Scholar]
- 99.Woodlock TJ, Chen X, Young DA, Bethlendy G, Lichtman MA, Segel GB. Association of HSP60-like proteins with the L-system amino acid transporter. Arch Biochem Biophys 338: 50–56, 1997. [DOI] [PubMed] [Google Scholar]
- 100.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471–484, 2006. [DOI] [PubMed] [Google Scholar]
- 101.Yamaguchi S, Ishihara H, Yamada T, Tamura A, Usui M, Tominaga R, Munakata Y, Satake C, Katagiri H, Tashiro F, Aburatani H, Tsukiyama-Kohara K, Miyazaki J, Sonenberg N, Oka Y. ATF4-mediated induction of 4E-BP1 contributes to pancreatic beta cell survival under endoplasmic reticulum stress. Cell Metab 7: 269–276, 2008. [DOI] [PubMed] [Google Scholar]
- 102.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl–dependent neurotransmitter transporters. Nature 437: 215–223, 2005. [DOI] [PubMed] [Google Scholar]
- 103.Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev 14: 2712–2724, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, Jefferson LS, Cavener DR. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol 22: 6681–6688, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Z, Grewer C. The sodium-coupled neutral amino acid transporter SNAT2 mediates an anion leak conductance that is differentially inhibited by transported substrates. Biophys J 92: 2621–2632, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zharikov SI, Block ER. Association of l-arginine transporters with fodrin: implications for hypoxic inhibition of arginine uptake. Am J Physiol Lung Cell Mol Physiol 278: L111–L117, 2000. [DOI] [PubMed] [Google Scholar]
- 107.Zhou Z, Zhen J, Karpowich NK, Goetz RM, Law CJ, Reith ME, Wang DN. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science 317: 1390–1393, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]