Abstract
Insulin resistance is associated with hypertension by mechanisms likely involving the kidney. To determine how the major apical sodium transporter of the thick ascending limb, the bumetanide-sensitive Na-K-2Cl cotransporter (NKCC2) is regulated by high-fat feeding, we treated young male, Fischer 344 X Brown Norway (F344BN) rats for 8 wk with diets containing either normal (NF, 4%) or high (HF, 36%) fat, by weight, primarily as lard. HF-fed rats had impaired glucose tolerance, increased urine excretion of 8-isoprostane (a marker of oxidative stress), increased protein levels for NKCC2 (50–125%) and the renal outer medullary potassium channel (106%), as well as increased natriuretic response to furosemide (20–40%). To test the role of oxidative stress in this response, in study 2, rats were fed the NF or HF diet plus plain drinking water, or water containing NG-nitro-l-arginine methyl ester (l-NAME), a nitric oxide synthase inhibitor (100 mg/l), or tempol, a superoxide dismutase mimetic (1 mmol/l). The combination of tempol with HF nullified the increase in medullary NKCC2, while l-NAME with HF led to the highest expression of medullary NKCC2 (to 498% of NF mean). However, neither of these drugs dramatically affected the elevated natriuretic response to furosemide with HF. Finally, l-NAME led to a marked increase in blood pressure (measured by radiotelemetry), which was significantly enhanced with HF. Mean arterial blood pressure at 7 wk was as follows (mmHg): NF, 100 ± 2; NF plus l-NAME, 122 ± 3; and HF plus l-NAME, 131 ± 2. Overall, HF feeding increased the abundance of NKCC2. Inappropriately high sodium reabsorption in the thick ascending limb via NKCC2 may contribute to hypertension with insulin resistance.
Keywords: kidney, renal, BSC1, renal outer medullary potassium channel, oxidative stress, insulin resistance, obesity
the metabolic syndrome is associated with higher than normal blood pressure (BP; Refs. 17, 31, 46); however, the mechanisms underlying increased BP are not fully understood. In addition to elevated BP, the metabolic syndrome is associated with an altered plasma lipid profile, increased adiposity surrounding internal organs, and insulin resistance (20). At the level of the kidney, as well as other major tissues such as liver and adipose, the altered biochemical milieu of the metabolic syndrome, in particular dyslipidemia and hyperinsulinemia, has been associated with increased tissue oxidative stress (9). It is likely that this increase in oxidative stress contributes to the rises in BP, however, via what effectors is not entirely clear. We and others have suggested that altered or increased sodium reabsorptive activity in the proximal tubule (23, 24), the thick ascending limb (TAL; Ref. 39), and/or the distal tubule (6, 44) may play a role.
High-fat (HF) feeding to rats has been utilized as a model of the human metabolic syndrome with associated weight gain (11), insulin resistance (10), and increases in BP (10, 11, 44). Dobrian et al. (9, 11) demonstrated that high levels of vascular and renal oxidative stress are associated with chronic HF feeding in obesity-prone Sprague-Dawley rats. Rats treated for 16 wk with a diet containing 32% of the calories from fat had significantly increased renal thiobarbituric acid-reactive substances and reduced urine nitrates plus nitrites (NOx) compared with rats treated with control, 10.6% kcal from fat, diet (9).
With regard to altered sodium reabsorption, the TAL of the kidney may be particularly sensitive to oxidative stress due to its high energy requirements (16, 34). Studies (18, 36, 37) conducted primarily in vitro suggest a role for upregulated TAL sodium reabsorption through the apically localized, bumetanide-sensitive Na-K-2Cl cotransporter (NKCC2) in conditions of increased oxidative stress. Furthermore, these investigators have also shown that nitric oxide (NO) can reduce NKCC2 activity and sodium reabsorptive activity in this cell type (18, 36, 37). Finally, Wangensteen et al. (50) recently found that chronic NG-nitro-l-arginine methyl ester (l-NAME) treatment of Wistar rats increased expression and activity of NKCC2 in the renal medulla. However, whether NKCC2 activity and expression are upregulated with HF feeding and its relationships to BP is not clear.
Recently, we (39) showed increased NKCC2 protein in the outer medulla from young obese Zucker rats, another model of the metabolic syndrome, compared with age-matched lean Zucker rats. However, Zucker rats do not have an intact leptin-signaling system, which might influence renal responses in the metabolic syndrome (3, 21, 38).
For the studies described here, we selected for use the Fischer 344 X Brown Norway F1 hybrid rat (F344BN). These rats are not as susceptible to renal injury with aging as other strains (15, 33, 49) and, thus, may more closely resemble human kidney response. Furthermore, we have shown that renal disease, especially renal hypertrophy, can confound the interpretation of transporter data (5). Our aim was to determine whether HF feeding in these rats results in impaired glucose utilization, an early indicator of insulin resistance, and alters NKCC2 expression and BP. To address this aim, two studies were conducted. In the first, we tested HF vs. normal fat (NF) diets. In the second study, we combined these diets with modifiers of renal oxidative stress, i.e., l-NAME, a nonspecific nitric oxide synthase (NOS) inhibitor, or 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (tempol), a superoxide dismutase mimetic. Glucose tolerance was evaluated as a measure of insulin resistance and BP measured by radiotelemetry. Expression of NKCC2 was determined by Western blotting of the outer medulla and cortex homogenates of kidney. The natriuretic response to select NKCC2 inhibition by furosemide was also determined. Finally, we measured urine and plasma levels of nitrites plus nitrates (NOx) and the renal expression of endothelial NOS (eNOS). We also examined renal protein abundance in the outer medulla homogenates of the renal outer medullary potassium channel (ROMK) and the α1-subunit of Na-K-ATPase to determine whether these proteins were also regulated. Our hypothesis was that HF feeding would increase insulin resistance, BP, and renal abundance of NKCC2 and that this would be dependent on oxidative stress.
METHODS
Animals.
Male Fischer 344 X Brown Norway F1 hybrid rats (F344BN) were obtained from Harlan Sprague-Dawley (Indianapolis, IN) at ∼3 mo of age (∼275 g). Rats were housed in microfilter-top, plastic cages (except during urine collections) with a normal 12-h light-dark cycle according to the protocols approved by the Georgetown Animal Care and Use Committee, an American Association for Accreditation of Laboratory Animal Care-approved facility.
Radiotelemetry measures of BP.
After a short (3- to 4-day) equilibration period, rats were implanted with radiotelemetry transmitters, while they were under isoflurane anesthesia (Isoflo; Abbott Laboratories, North Chicago, IL), as previously described (45) to measure BP (Data Sciences International, St. Paul, MN). Computer data acquisition software was configured to take a 10-s BP reading once every 10 min during the day (6 AM-6 PM) and night (6 PM-6 AM) periods. BP was measured 3 days per wk, with 144 measurements taken per day during those 3-day periods. These measures were averaged to obtain weekly BP mean.
Study designs.
After a 1-wk recovery from the radiotelemetry transmitter implantation, rats in study 1 were assigned to one of the following treatments (n = 6/group): 1) NF diet (4% fat by weight); or 2) HF diet (36% fat by weight). Diets were purchased from Research Diets, Open Source Diets and were D12492 (HF) and D12450B (NF). Fat was supplemented primarily as lard. Rats were treated for 8 wk and consumed food and water on an ad libitum basis. In study 2, we had six treatment groups (n = 8 total rats/treatment for physiological data, 6 for blotting, and 4 for BP data). In addition to the NF and HF groups, we had 3) NF plus l-NAME; 4) HF plus l-NAME; 5) NF plus tempol; and 6) HF plus tempol. l-NAME and tempol were provided in the drinking water at 100 mg/l and 1 mmol/l, respectively. Rats were fed and consumed fluids on an ad libitum basis. l-NAME dose was the same as used by Banks and Oyekan (2) to block NOS activity in studies examining peroxisome proliferator-activated receptor subtype-α agonist-treated rats. The dose of tempol was that effectively used by Schnackenberg and Wilcox (42) in the drinking water of spontaneously hypertensive rats to lower BP and oxidative stress. Urine was collected and food and water intake records were made periodically by housing rats singly in metabolic cages (Lab Products, Seaford, DE). Rats were euthanized by cardiac puncture and exsanguination while they were under isoflurane anesthesia. Both kidneys were rapidly harvested and prepared for Western blotting. K+/EDTA and heparin were used as anticoagulants in the separation of blood into red blood cells and plasma by centrifugation.
Furosemide-response test.
Sodium excreted in response to a single intraperitoenal injection of furosemide (12 mg·kg−1·body wt−1) was measured in rats treated for 6 wk with one of the above-described treatments. Measurement of acute natriuretic response to a single injection of furosemide has been used previously by ourselves and several other investigators to gauge in vivo activity of NKCC2 (8, 26, 27, 40, 41). The furosemide was administered at 4:00 PM. In study 1, radiotelemetry transmitters recorded BP during this period. Urine was collected in metabolic cages for 3 h after the injection. Sodium and potassium excretion in response to furosemide was determined by an ion-specific electrode system (EL-ISE; Beckman Coulter, Fullerton, CA) and the magnitude of which considered an index of the relative in vivo “activity” of NKCC2.
Glucose-tolerance test.
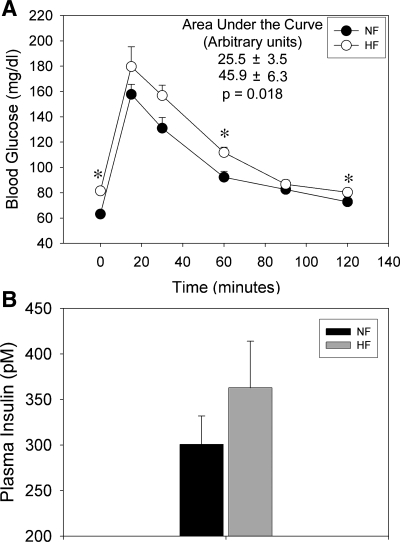
In study 1, a glucose tolerance test was given to rats at 7 wk to assess their ability to rapidly normalize blood glucose (a function of insulin sensitivity). Rats were given 50% dextrose in water (3 mg·kg−1·body wt−1) intraperitoneally. Glucose was measured in tail blood using a glucometer and pricking the tail with a 20-gauge needle after 15, 30, 60, 90, and 120 min. The blood glucose concentration over time was plotted, and the areas under the curves were calculated and statistically compared.
Immunoblotting.
For both studies, the inner stripe of the outer medulla (bright reddish portion) from all right kidneys was dissected free of the cortex (tan) and inner medulla (white) using a razor blade and sharp, curved scissors. The left kidney was processed whole. Kidneys or kidney regions were homogenized in isolation buffer (13) using a saw-tooth generator. Protein concentrations of the whole homogenates were determined by a bicinchoninic protein assay kit (Pierce, Rockford, IL). An aliquot of each sample was solubilized with Laemmli sample buffer, and Coomassie-stained “loading gels” were done to assess the quality of the proteins by sharpness of the bands and to confirm protein concentration determinations, as previously described (13, 14). Small adjustments were made in protein concentrations based on densitometry of the loading gels. For immunoblotting, 10–20 μg of protein from each sample were loaded into individual lanes of minigels of 7 or 10% polyacrylamide (precast; Bio-Rad, Hercules, CA). Blots were probed with our own polyclonal antibody against NKCC2 (44) or with commercial antibodies against eNOS (BD Transduction Laboratories, San Diego, CA), ROMK-1 (USBiological, Swampscott, MA), and α1-subunit of Na-K-ATPase. (Millipore, Billerica, MA).
Urine and plasma analyses.
Plasma aldosterone and insulin were analyzed by RIA kits as previously described (5, 43). Plasma and urinary NOx and urinary 8-isoprostane were analyzed by colorimetric assays (Cayman Chemical, Ann Arbor, MI).
Statistics.
For study 1, in general, data were analyzed by unpaired t-test (Sigma Stat, Chicago, IL). BP overtime data were analyzed by two-way (treatment × time) repeated measures regression analysis. Study 2 data were analyzed by two-way ANOVA (diet × drug) and one-way ANOVA, followed by the Holm-Sidak multiple comparisons test or the Kruskal-Wallis ANOVA on ranks followed by Dunn's multiple comparisons test (when data were not normally distributed or variance was different between groups). Multiple comparisons tests were only applied when a significant difference was determined in the ANOVA analysis. Results of multiple comparison tests are indicated on bar graphs with “A” assigned to the highest mean, followed by “B” etc (see Figs. 6–8). Bars with letters in common are not different from each other. For example, “A” is different from “B” but not from “AB.” P < 0.05 was considered significantly different for all analyses.
RESULTS
HF-feeding had little effect on general physiology but increased a measure of oxidative stress.
HF feeding had little effect on basic physiological parameters. Table 1 provides these data for study 1. There was a tendency for HF rats to be heavier and have larger kidneys, but these differences were not significant. No differences were seen between groups for kidney weight, water intake, plasma creatinine, or plasma aldosterone. However, HF feeding significantly increased urine 8-isoprostane (F2α) excretion, an indicator of oxidative stress.
Table 1.
Physiological parameters in study 1
| Treatment | Final Body Weight, g | Weight Gain, g/8 wk | Water Intake,* ml/day | Kidney Weight,† g | Urine 8-Isoprostane, ng/day | Plasma Creatinine, pmol/l | Plasma Aldosterone, nmol/l |
|---|---|---|---|---|---|---|---|
| NF | 304±12 | 97±4 | 17.8±0.7 | 0.83±0.03 | 6.2±0.9 | 23.5±2.9 | 2.28±0.75 |
| HF | 336±14 | 100±10 | 18.6±1.8 | 0.90±0.03 | 15.8±1.3 | 23.5±2.9 | 1.31±0.62 |
| P value | 0.11 | 0.81 | 0.67 | 0.11 | <0.001‡ | 1.0 | 0.36 |
Data are means ± SE; n = 5 or 6 rats/treatment. NF, normal fat; HF, high fat.
Average of 2 measurements in metabolic cages;
average right and left;
significantly different (P < 0.05) as determined by unpaired t-test.
Table 2 provides detailed estimated nutrient intake in both study 1 and 2 based on two to five measurements of food intake in metabolic cages. NF rats ate more grams of food. However, when food intake was converted into kilocalories consumed (HF diets were more dense: 5.24 vs. 3.85 kcal/g for NF), no differences in energy intake were found between treatments. Fat intake and carbohydrate intake were markedly different, as was expected; however, protein intake did not differ. The HF diet contained a higher percent (by weight) of protein, mineral, and vitamin mix and fiber, than did the low-fat diet; thus few other differences were determined, except NaCl was modestly (∼10%) lower in the HF-fed rats. This difference was significant in study 2 only.
Table 2.
Estimated daily nutrient intake in studies 1 and 2
| Food Intake, g/day | Food Intake, kcal/day | Fat Intake, g/day | Carbohydrate Intake, g/day | Protein Intake, g/day | NaCl Intake, mg/day | Potassium Intake, mg/day | Fiber Intake, g/day | |
|---|---|---|---|---|---|---|---|---|
| Study 1 treatment | ||||||||
| NF | 15.6±0.6 | 60±3 | 0.67±0.03 | 10.5±0.4 | 3.00±0.13 | 39.0±1.6 | 89±4 | 0.73±0.03 |
| HF | 11.0±0.4 | 57±2 | 3.82±0.15 | 2.9±0.1 | 2.87±0.12 | 36.2±1.4 | 84±3 | 0.71±0.03 |
| P value (unpaired t-test) | <0.001§ | 0.45 | 0.002§ | 0.002§ | 0.48 | 0.22 | 0.38 | 0.62 |
| Study 2 treatment | ||||||||
| NF | 18.3±0.5 | 70±2 | 0.79±0.02 | 12.3±0.4 | 3.51±0.10 | 45.7±1.3 | 104±3 | 0.86±0.02 |
| HF | 12.1±0.8*†‡ | 64±4 | 4.24±0.27*†‡ | 3.2±0.2*†‡ | 3.18±0.21 | 40.0±2.6 | 93±6 | 0.79±0.05 |
| NFL | 18.0±0.9 | 69±3 | 0.77±0.04 | 12.1±0.6 | 3.45±0.17 | 45.0±2.2 | 102±5 | 0.84±0.04 |
| NFL | 11.5±0.4*†‡ | 60±2 | 4.01±0.13†‡ | 3.0±0.1*†‡ | 3.01±0.10 | 37.9±1.2 | 88±3 | 0.75±0.02 |
| NFT | 17.5±1.1 | 67±4 | 0.75±0.05 | 11.8±0.7 | 3.36±0.21 | 43.8±2.7 | 100±6 | 0.82±0.05 |
| HFT | 13.6±0.7*†‡ | 71±4 | 4.74±0.24*†‡ | 3.6±0.2*† | 3.56±0.18 | 44.8±2.2 | 104±5 | 0.88±0.04 |
| P values (two-way) ANOVA | ||||||||
| Diet | <0.001§ | 0.15 | <0.001§ | <0.001§ | 0.16 | 0.030§ | 0.10 | 0.28 |
| Drug | 0.55 | 0.38 | 0.085 | 0.89 | 0.38 | 0.40 | 0.39 | 0.38 |
| Interaction | 0.15 | 0.13 | 0.061 | 0.48 | 0.13 | 0.14 | 0.10 | 0.13 |
Data are means ± SE (n = 5–8 rats/treatment) and are average of 2–5 measurements of food intake in metabolic cages.
Significantly different (P < 0.05) as determined by unpaired t-test (study 1).
Significantly different from NF, NFL, or NFT, respectively, by multiple comparisons test following a significant (P < 0.05) one-way ANOVA.
Significantly different from NF, NFL, or NFT, respectively, by multiple comparisons test following a significant (P < 0.05) one-way ANOVA.
Significantly different from NF, NFL, or NFT, respectively, by multiple comparisons test following a significant (P < 0.05) one-way ANOVA.
HF-feeding led to glucose intolerance.
The ability to rapidly clear glucose from the blood (a measure of insulin sensitivity) was assessed after 7 wk on the diet. HF feeding led to significantly impaired glucose tolerance, as evident by increased blood glucose concentration at 0, 30, and 120 min after the intraperitoneal glucose challenge (Fig. 1A). Also, a significantly greater area under the curve for glucose was found for the HF rats. Fasting plasma insulin was also determined, and although it trended toward being higher, it was not significantly different between groups (P = 0.28; Fig. 1B). Thus HF feeding led to a mild state of insulin resistance in the F344BN rat.
Fig. 1.
Measures of glucose tolerance/insulin sensitivity in male F344BN rats fed normal fat (NF) or high-fat (HF) fat diets for 8 wk (n = 6 rats/group). A: blood glucose curve following a glucose tolerance test, i.e., intraperitoneal injection (3 ml·kg−1·bw−1) of 50% dextrose. Area under the curve (AUC; arbitrary units) statistics are shown at top. B: final plasma insulin levels (nonfasted). *Significant difference (P < 0.05) between treatments by unpaired t-test. Rats fed HF had significantly higher AUC (P = 0.018) and blood glucose levels at time 0, 60, and 120. Insulin levels were not significantly different between groups (P = 0.28, unpaired t-test).
HF DIET CAUSED A MODEST RISE IN MEAN ARTERIAL PRESSURE.
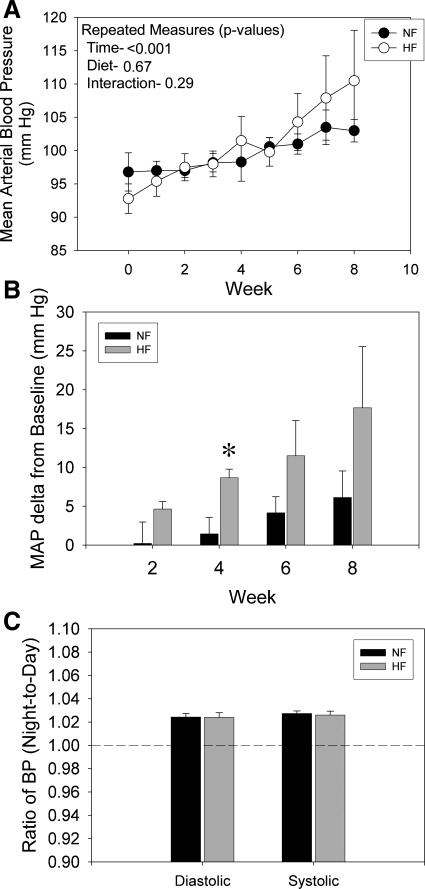
Mean arterial blood pressure (MAP), as determined by radiotelemetry, increased significantly over time in both groups (Fig. 2A) with no significant effect of diet by repeated measures analysis. As an artifact of randomization, HF rats started out with slightly lower BP (although not statistically lower). Therefore, MAP was also evaluated as “mean change from baseline” (Fig. 2B). ΔMAP was calculated individually for each rat and averaged for treatments at 2, 4, 6, and 8 wk. There was a strong tendency for HF to increase change in MAP. This change was significant after 4 wk of feeding. To evaluate the diurnal rhythm of BP, the ratio of night-to-day time diastolic and systolic BP is shown in Fig. 2B. Both treatments had slightly higher BP in the night (dark hours), as expected, and no differences between groups were seen.
Fig. 2.
Blood pressure (BP) response in male F344BN rat fed NF or HF diets for 8 wk (n = 6 rats/group). A: radiotelemetry recording of weekly mean arterial blood pressure (MAP) over the course of the study. B: ΔMAP (from baseline) after 2, 4, 6, and 8 wk of treatment. C: diurnal rhythm of diastolic and systolic BP expressed as a ratio of night-to-day recordings. *Significant difference (P < 0.05) between treatments by unpaired t-test. Both NF and HF-fed rats had significant increases in MAP with time (two-way ANOVA). ΔMAP for HF rats was significantly (P < 0.05) higher after 4 wk. No treatment differences in diurnal rhythmicity was found.
HF diet increased medullary NKCC2.
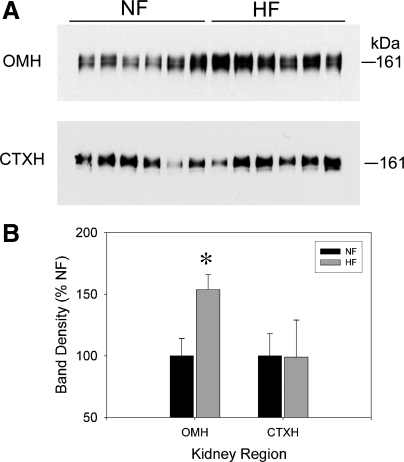
After euthanization at 8 wk of feeding, Western blots of cortex homogenates and inner stripe of the outer medulla homogenates were performed for the bumetanide-sensitive NKCC2 (Fig. 3). HF feeding led to a significant increase in NKCC2 protein levels in the inner stripe of the outer medulla. No significant change in the cortex was found.
Fig. 3.
Na-K-2Cl cotransporter (NKCC2) protein in the kidney of male F344BN rat fed NF or HF diets for 8 wk (n = 6 rats/group). A: representative Western blots from inner stripe of outer medulla (OMH; top) and cortex (CTXH, bottom) homogenates prepared from rats. Each lane was loaded with an equal amount of total protein from a different rat, as determined a priori by loading gels. B: densitometry summary. *Significant difference (P < 0.05) between treatments by unpaired t-test. HF-fed rats had significantly (P < 0.05) higher protein levels of NKCC2 in OMH.
HF diet increased outer medullary ROMK.
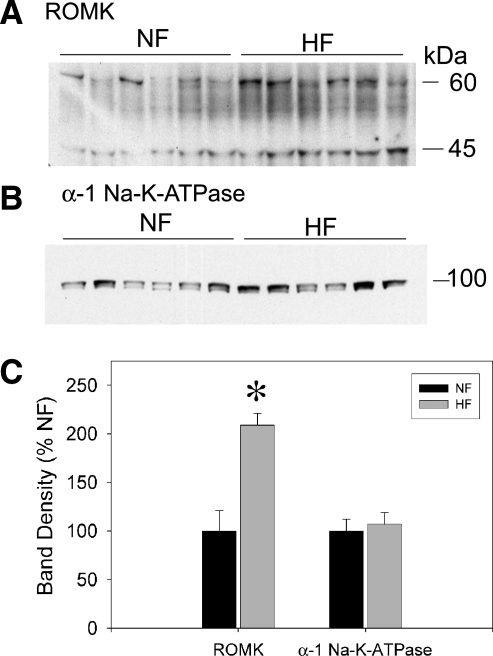
To determine whether the increase in outer medulla homogenate NKCC2 was accompanied by an increase in other TAL proteins involved in the net reabsorption of NaCl, we also performed Western blotting for ROMK (Kir1.1) and the α1-subunit of Na-K-ATPase (Fig. 4). ROMK, the apical secretory pathway for recycling of potassium in the TAL, was also significantly increased by the HF diet. ROMK, on the blot, ran as a sharp band at ∼45 kDa, as we have previously shown (5, 12), and a broader multiple band region between 50–60 kDa. Band regions were summed for densitometry, since they were identically regulated. The abundance of the α1-subunit of Na-K-ATPase was not significantly different between groups.
Fig. 4.
Renal outer medullary potassium channel (ROMK) and Na-K-ATPase (α1) protein in the outer medullary homogenates of male F344BN rat fed NF or HF diets for 8 wk (n = 6 rats/group). Representative Western blots probed with antibodies against ROMK (A) or α1-Na-K-ATPase (B). Each lane was loaded with an equal amount of total protein from a different rat, as determined a priori by loading gels. C: densitometry summary. *Significant difference (P < 0.05) between treatments by unpaired t-test. HF-fed rats had significantly (P < 0.05) higher protein levels of ROMK in the OMH.
HF increased the natriuretic response to furosemide.
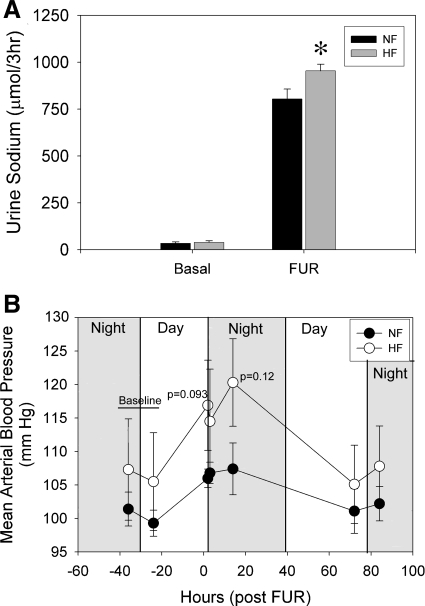
The natriuretic response to furosemide was evaluated as an index of NKCC2 activity after 6 wk of HF or NF diet (Fig. 5A). Sodium excretion in a similar 3-h period (the day before) was used to assess basal sodium excretion. Basal sodium excretion was low and not different between HF- and NF-fed rats. When rats were treated with furosemide, urine sodium increased nearly 25-fold in both treatments. However, HF rats excreted about ∼18% more than NF rats in this period (P = 0.043), suggesting relatively higher activity of NKCC2 in HF rats. We also assessed whether treatment with a single injection of furosemide would affect BP differentially in the two groups (Fig. 5B). Surprisingly, furosemide caused a transient increase in BP in both groups, which trended toward being higher in the HF group but was not significantly different (P = 0.093 and 0.12, unpaired t-test at 2 and 14 h, respectively). BP had returned to normal in both groups by 72 h.
Fig. 5.
Natriuretic and blood pressure responses to acute furosemide (FUR) in male F344BN rat fed NF or HF diets after 6 wk of treatment (n = 6 rats/group). A: rats were placed in metabolic cages at 4 PM without food but containing water, and urine was collected for 3 h after a single ip injection of furosemide (12 mg·kg−1·bw−1). A basal urine collection (left) was done in the same 3-h period on the previous day. B: MAP response to FUR with indication of light period. *Significant difference (P < 0.05) between dietary treatments by unpaired t-test. HF-fed rats excreted significantly more sodium in response to FUR. MAP rose transiently in response to FUR in both groups with no significant differences between HF and NF. MAP returned to basal levels in both groups by 72 h.
Treatment with HF plus l-NAME reduced urine NOx.
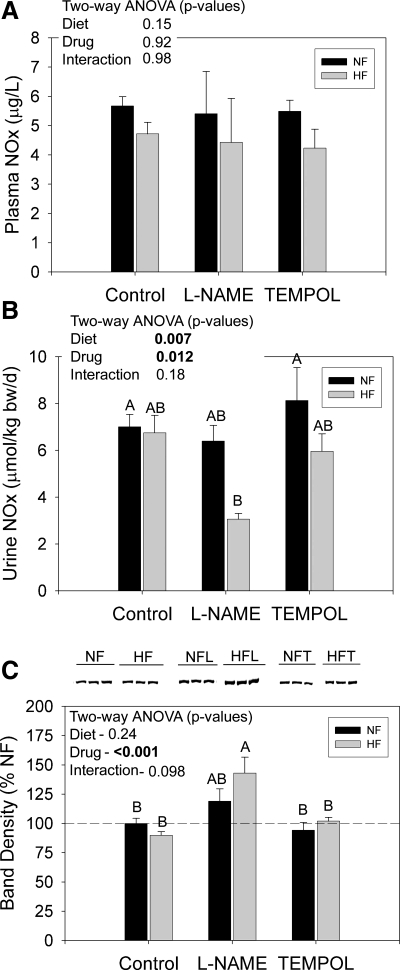
In a second study, we determined whether modifiers of oxidative stress, l-NAME and tempol, would modify NKCC2 expression and activity responses to dietary fat. We first evaluated the effect of these treatments on urine and plasma NOx. Plasma NOx was not significantly affected by treatment but trended (P = 0.15) to be reduced by HF (Fig. 6A; n = 8 rats/treatment). Urine NOx was markedly reduced (to ∼40% of other treatment groups) by the combination of HF and l-NAME (Fig. 6B). Neither HF nor l-NAME alone could significantly reduce urine NOx. Letters represent the results of the Holm-Sidak multiple comparisons test. Bars with letters in common are not different from each other. Protein expression of eNOS was also measured in whole left kidney harvested from these rats (n = 6 rats/treatment; Fig. 6C). l-NAME significantly increased eNOS expression, and this increase was slightly greater in HF-fed rats.
Fig. 6.
Plasma and urine nitric oxide and whole kidney endothelial nitric oxide synthase (eNOS) in F344BN rats fed HF or NF diets plus either plain water or water with NG-nitro-l-arginine methyl ester (l-NAME) or tempol. A: plasma NOx (nitrites plus nitrates) at 8 wk (n = 6 rats/treatment). B: urine NOx excretion in a 24-h collection at 6.5 wk (n = 8 rats/treatment). C: Western blots and densitometry summary for eNOS. Representative blot lanes are shown for whole kidney homogenates from 3 rats (n = 6 rats/group for statistics). Each lane was loaded with an equal amount of total protein from a different rat, as determined a priori by loading gels. Data were analyzed by two-way ANOVA (diet × drug) and by one-way ANOVA followed by the Holm-Sidak multiple comparisons test (MCT; only applied with a significant, P < 0.05 one-way ANOVA). Results of the MCT are shown by letters above the bars. “A” is applied to the bar with the highest mean followed by “B,” etc. Bars with letters in common are not significantly different from each other. Urine NOx was reduced and kidney eNOS protein was increased by l-NAME in the presence of HF diet. l-NAME alone increased eNOS.
Tempol reduces medullary NKCC2, while l-NAME increases it.
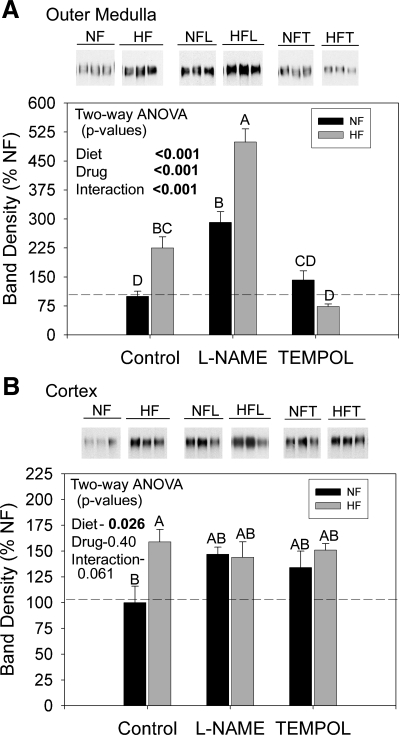
Western blots were also run for NKCC2 on cortex and outer medulla homogenates. We confirmed the medullary increase in NKCC2 with HF feeding (“control bars”; Fig. 7A). Representative lanes (3 rats/group) are shown. l-NAME dramatically exacerbated the fat-induced increase in medullary NKCC2 band density (middle gray bar) to a level ∼500% of NF controls. l-NAME also increased NKCC2 with NF diet (middle black bar). In contrast, tempol neutralized the effect of the HF diet on NKCC2 abundance. Band densities in tempol-treated HF rats were ∼80% of NF control means (right gray bar). Tempol had no effect on NKCC2 abundance with NF diet (compare right and left black bars). Cortex results are shown in Fig. 7B. In this study, HF led to a significant increase in cortical NKCC2, as well, although the percentage increase was less than that for the medulla (compare y-axes). Tempol and l-NAME did not significantly affect cortical NKCC2 abundance.
Fig. 7.
NKCC2 protein in kidney of F344BN rats fed HF or NF diets plus either plain water or water with l-NAME or tempol. Representative Western blots from the inner stripe of the outer medulla (A) and the cortex homogenates (B). Densitometry summaries for each region are below the blots (n = 6 rats/treatment). Each lane was loaded with an equal amount of total protein from a different rat, as determined a priori by loading gels. Data were analyzed by two-way ANOVA (diet × drug) and by one-way ANOVA followed by the Holm-Sidak MCT (only applied with a significant, P < 0.05 one-way ANOVA). Results of the MCT are shown by letters above the bars. “A” is applied to the bar with the highest mean followed by “B,” etc. Bars with letters in common are not significantly different from each other. HF-fed rats had significantly higher protein levels of NKCC2 in the cortex and outer medulla. l-NAME markedly increased medullary NKCC2 abundance and tempol reduced it. The increase in medullary NKCC2 with l-NAME was significantly greater in HF rats.
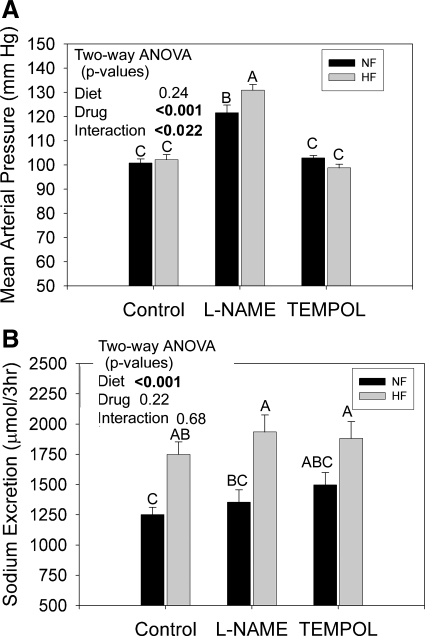
HF exacerbates BP increase observed with l-NAME.
MAP was also recorded in a subset (n = 4 rats/treatment) in study 2. MAP at week 7 is shown in Fig. 8A. l-NAME caused a marked and significant increase in BP regardless of diet. However, the combination of l-NAME plus HF led to a significant rise in BP above l-NAME alone (as determined by multiple comparisons test). No significant effect of tempol on MAP was observed, relative to control rats.
Fig. 8.
MAP and FUR-natriuretic response in F344BN rats fed HF or NF diets plus either plain water or water with l-NAME or tempol. A: MAP after 7 wk. B: FUR-natriuretic response (3 h post-FUR) conducted at 6 wk. Data were analyzed by two-way ANOVA (diet X drug) and by one-way ANOVA followed by the Holm-Sidak multiple comparisons test (MCT, only applied with a significant, P < 0.05 one-way ANOVA). Results of the MCT are shown by letters above the bars. “A” is applied to the bar with the highest mean followed by “B,” etc. Bars with letters in common are not significantly different from each other. MAP was significantly increased by l-NAME and this effect was significantly enhanced by HF diet. HF increased natriuretic response to FUR, which was not modifiable by either l-NAME or tempol.
Furosemide natriuresis is increased by HF but not altered by tempol or l-NAME.
Finally, we determined whether the increased and decreased medullary expression of NKCC2 with l-NAME and tempol, respectively, resulted in increased natriuretic response to furosemide (Fig. 8B). Consistent with our first study, HF feeding increased the natriuretic response to furosemide in this instance by ∼50%. Moreover, l-NAME-treated HF rats had the highest natriuretic response in agreement with the highest MAP and NKCC2 protein levels. However, it was not markedly higher than HF alone (compare left and middle gray bars). Tempol had no effect on HF-induced natriuresis, which did not agree with reduced NKCC2 protein levels.
DISCUSSION
Diets high in saturated fats have numerous convincing detrimental influences on overall energy metabolism, tissue insulin sensitivity, as well as renal, cardiac, and vascular function. In the current studies, we demonstrate that a HF (high-saturated fat) diet fed for 8 wk to male F344BN rats increases expression and furosemide-induced natriuresis of the renal bumetanide-sensitive Na-K-2Cl cotransporter (NKCC2 or BSC1), the major apical reabsorptive protein of the TAL. In the outer medulla, we showed that this increase (in protein level at least) was accompanied by the increase in ROMK, the apically expressed, inwardly rectifying potassium channel (Kir1.1), another critical determinant of overall NaCl reabsorption in the TAL. It was also exacerbated by cotreatment with the nonspecific NOS inhibitor l-NAME and abrogated by cotreatment with the superoxide dismutase mimetic tempol. Finally, we demonstrate that HF-feeding in combination with l-NAME produces an elevation in MAP significantly higher than l-NAME alone. These findings are discussed in greater detail below.
Role of oxidative stress in the regulation of NKCC2 abundance.
Our findings provide strong evidence that upregulated NKCC2 protein levels in the outer medullary TAL with HF feeding are at least partly the result of altered medullary oxidative stress. At the level of the kidney, HF diets have been shown to result in increased production of reactive oxygen species (9, 10), e.g., superoxide (O2−) produced, for example, from membranous NAD(P)H oxidase. The fact that the O2− scavenging tempol was able to abolish HF-induced NKCC2-elevated expression in the medulla suggests that increased levels of O2− with HF feeding are an important determinant of NKCC2 expression. In contrast, reduced NO bioavailability, the expected consequence of chronic l-NAME treatment, was associated with marked increased levels of medullary NKCC2. This is in agreement with Wangensteen et al. (50) who demonstrated increased NKCC2 protein levels in the kidney harvested from Wistar rats treated with l-NAME at three different doses. Interestingly, the combination of HF with l-NAME led to an even greater increase in NKCC2 than l-NAME alone. This suggests either that HF has NO-independent effects on NKCC2 expression (see more on this below) or that HF had an additive effect to further reduce NO bioavailability levels. The second possibility is supported by our finding of substantially reduced urine NOx in rats treated with both HF and l-NAME. l-NAME alone did not markedly affect urine NOx. In addition, plasma NOx was marginally reduced (although not significantly, P = 0.15) by HF but not affected by l-NAME.
Regulation of NKCC2 activity by HF and modifiers of oxidative stress.
For the most part, the increase in whole cell protein levels observed with the HF diet correlated with increased natriuretic response to furosemide, the common diuretic Lasix. HF fed rats had significantly increased 3-h natriuretic responses to furosemide, suggesting that the increased TAL expression of NKCC2 led to increased sodium reabsorptive activity in this segment. However, they also had a marginally (but not significant) greater increase in BP in response to the furosemide, than did NF rats, and it is possible that this may have resulted in increased pressure natriuresis. It is unclear what causes the increase in BP with furosemide. BP eventually returned to basal levels (within 3 days). Using furosemide-mediated natriuresis as a means of determining NKCC2 activity has certain limitations and assumptions associated with it. First, it is unclear whether the treatments themselves might affect the distribution, activity, or half-life of furosemide. Second, treatment with furosemide is likely to lead to compensatory responses at other tubule sites that may affect overall sodium excretion, and the ability of these sites to compensate may vary due to treatments. Nonetheless, variations of this test have been used by several different groups to noninvasively assess NKCC2 activity (7, 8, 26, 27, 29, 41).
Tempol and l-NAME had little effect on the HF-induced increased in the natriuretic response to furosemide. That is, rats treated with HF plus tempol still had a significantly elevated natriuresis to the HF, despite normalized (or even below normal) levels of the protein in outer medulla. Furthermore, l-NAME did not cause a further increase in the natriuretic response (over what was observed in control or tempol HF rats), despite substantially higher whole cell medullary protein levels. We do not know the reasons underlying this disconnect between putative activity and medullary expression in our study. It may be that we are not getting an accurate reading on activity (see above assumptions). For example, it would not be surprising if the distal tubule sodium reabsorption was affected by either tempol or l-NAME, which might thus modify the furosemide-natriuretic response. On the other hand, our activity measure may be accurate and strongly influenced by cortical NKCC2. Cortical NKCC2 expression, like activity, was not sensitive to tempol or l-NAME. This would agree with the medulla being an environment experiencing the greatest degree of oxidative stress. Finally, activity may be accurate and NKCC2 is being regulated by a means other than whole cell abundance, e.g., phosphorylation or trafficking (19), i.e., in this the case increased whole cell levels of the protein are not proportionally on the surface or active with l-NAME. Nonetheless, other investigators (18, 22, 28, 37, 50) have clearly shown a relationship between oxidative stress and NKCC2 activity in isolated medullary TAL. For example, Garvin and Juncos (28) showed increased furosemide-sensitive uptake of sodium in TAL that had been treated with xanthine oxidase and hypoxanthine, chemicals that increase the production of O2−. They (37) also showed that the application of an NO donor, spermine, to isolated TAL decreased NKCC2 sodium uptake. Wangensteen et al. (50) showed that isolated TAL from rats treated with various doses of l-NAME had increased furosemide-sensitive rubidium uptake (marker for potassium) that was linearly associated with dose of l-NAME. In conclusion, we suggest that modifiers of oxidative stress, i.e., tempol and l-NAME, are more influential in the regulation of medullary NKCC2, while HF may have some effect along the entire TAL to enhance sodium reabsorption.
HF exacerbates l-NAME-induced BP elevation.
HF diet alone (for 8 wk) did not substantially affect BP compared with rats fed NF diet. Both groups of rats had a significant increase in BP over time, possibly due to continual aging and maturation of the rats. This is not that surprising given that relatively hypertensive-resistant strains of rats have multiple, redundant mechanisms to try to normalize BP in the face of changes in physiology that might predispose them to an increase. However, HF coupled to l-NAME increased BP to a greater level than l-NAME alone. This was somewhat surprising since l-NAME alone is such a potent elevator of BP and its administration is commonly used as a model for hypertension (30, 32) Wangensteen et al. (50) showed that NKCC2 abundance and activity in l-NAME-treated rats correlated with BP rises (by tail-cuff). We, likewise, found the highest BP in rats with the highest protein levels of NKCC2. We speculate that the combination of HF with l-NAME may make it particularly difficult for rats to compensate and suggest that this may be due to the fact that HF in combination with l-NAME causes a further reduction in NO bioavailability. This again is supported by the markedly reduced urine NOx levels found in the HF plus l-NAME-treated rats, relative to all other groups.
Attempts at compensation and NO-independent regulation of the kidney by HF.
Rats treated with l-NAME try to compensate for the drug by increasing protein levels of eNOS; the elevation in this protein, in whole kidney, was greatest in the HF plus l-NAME group, suggesting the greatest attempt at compensation. However, this was not reflected in any rise in plasma or urine NOx with HF, which suggested that the elevation in eNOS protein was not a very effective means of compensation.
HF feeding has been reported to impair glycolytic pathways and glucose oxidation due to a high degree of lipid oxidation (35). This results in poor utilization of circulating glucose and insulin resistance of the liver and skeletal muscle. Thus it is also possible for HF diets to have additional effects on BP independent of oxidative changes. For example, HF feeding has been demonstrated to increase renal sympathetic nerve activity in the rat, possibly via leptin stimulation of afferent pathways (25, 47). Both increased oxidative stress and activation of the SNA have been associated with increased activity of NKCC2 (26, 28, 37).
In another model of insulin resistance, the fructose-fed Sprague-Dawley rat, we found a significant decrease (rather than increase) in whole kidney levels of renal NKCC2, relative to rats fed normal chow (44). The fructose-fed rat is a commonly used model in which insulin resistance has been linked to elevated BP (1, 4, 48). However, we did not evaluate the outer medullary NKCC2 separately from the whole kidney. We have reported differences in the regulation of NKCC2 in cortex vs. medulla in another strain of insulin-resistant rodents, i.e., the obese Zucker rat. Obese Zucker rats (at 5–6 mo of age) have significantly increased outer medullary NKCC2 but significantly reduced cortical NKCC2 (39).
In summary, we demonstrate increased outer medullary protein abundance of NKCC2 and furosemide-induced natriuresis in F344BN rats fed a HF diet for 8 wk. Increased renal oxidative stress likely plays some role in this increase, as tempol abolished the increase in protein expression with HF. Chronically elevated TAL sodium reabsorption may eventually impact on BP, when other BP normalizing compensatory mechanisms begin to fail.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-074142 (to C. Ecelbarger) and HL-093173 and the American Heart Association Established Investigator Award (to C. Ecelbarger).
Acknowledgments
We would like to thank Xinqun Hu, Lina Nordquist, and Veerendra K. Madala Halagappa for technical assistance with care and testing of animals, BP determinations, and Western blotting.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anuradha CV, Balakrishnan SD. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can J Physiol Pharmacol 77: 749–754, 1999. [PubMed] [Google Scholar]
- 2.Banks T, Oyekan A. Peroxisome proliferator-activated receptor alpha activation attenuated angiotensin type 1-mediated but enhanced angiotensin type 2-mediated hemodynamic effects to angiotensin II in the rat. J Hypertens 26: 468–477, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Beltowski J Effect of hyperleptinemia on endothelial nitric oxide production. Atherosclerosis 178: 403–404, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bhanot S, McNeill JH, Bryer-Ash M. Vanadyl sulfate prevents fructose-induced hyperinsulinemia and hypertension in rats. Hypertension 23: 308–312, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Bickel CA, Knepper MA, Verbalis JG, Ecelbarger CA. Dysregulation of renal salt and water transport proteins in diabetic Zucker rats. Kidney Int 61: 2099–2110, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Bickel CA, Verbalis JG, Knepper MA, Ecelbarger CA. Increased renal Na-K-ATPase, NCC, and β-ENaC abundance in obese Zucker rats. Am J Physiol Renal Physiol 281: F639–F648, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Canessa CM, Schafer JA. AVP stimulates Na+ transport in primary cultures of rabbit cortical collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 262: F454–F461, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Cantone A, Yang X, Yan Q, Giebisch G, Hebert SC, Wang T. Mouse model of type II Bartter's syndrome. I. Upregulation of thiazide-sensitive Na-Cl cotransport activity. Am J Physiol Renal Physiol 294: F1366–F1372, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension 37: 554–560, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Dobrian AD, Schriver SD, Khraibi AA, Prewitt RL. Pioglitazone prevents hypertension and reduces oxidative stress in diet-induced obesity. Hypertension 43: 48–56, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Dobrian AD, Schriver SD, Lynch T, Prewitt RL. Effect of salt on hypertension and oxidative stress in a rat model of diet-induced obesity. Am J Physiol Renal Physiol 285: F619–F628, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Ecelbarger CA, Kim GH, Knepper MA, Liu J, Tate M, Welling PA, Wade JB. Regulation of potassium channel Kir 1.1 (ROMK) abundance in the thick ascending limb of Henle's loop. J Am Soc Nephrol 12: 10–18, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol 279: F46–F53, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Ecelbarger CA, Knepper MA, Verbalis JG. Increased abundance of distal sodium transporters in rat kidney during vasopressin escape. J Am Soc Nephrol 12: 207–217, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Eiam-Ong S, Sabatini S. Age-related changes in renal function, membrane protein metabolism, and Na,K-ATPase activity and abundance in hypokalemic F344 x BNF(1) rats. Gerontology 45: 254–264, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Evans RG, Fitzgerald SM. Nitric oxide and superoxide in the renal medulla: a delicate balancing act. Curr Opin Nephrol Hypertens 14: 9–15, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287: 356–359, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Garvin JL, Ortiz PA. The role of reactive oxygen species in the regulation of tubular function. Acta Physiol Scand 179: 225–232, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Gimenez I, Forbush B. Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem 278: 26946–26951, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM Metabolic syndrome: therapeutic considerations. Handb Exp Pharmacol: 107–133, 2005. [DOI] [PubMed]
- 21.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertens 14: 103S–115S, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol 292: F993–F998, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Hussain T, Becker M, Beheray S, Lokhandwala MF. Dopamine fails to inhibit Na,H-exchanger in proximal tubules of obese Zucker rats. Clin Exp Hypertens 23: 591–601, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Hussain T, Beheray SA, Lokhandwala MF. Defective dopamine receptor function in proximal tubules of obese zucker rats. Hypertension 34: 1091–1096, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Iwashita S, Tanida M, Terui N, Ootsuka Y, Shu M, Kang D, Suzuki M. Direct measurement of renal sympathetic nervous activity in high-fat diet-related hypertensive rats. Life Sci 71: 537–546, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Jonassen TE, Brond L, Torp M, Graebe M, Nielsen S, Skott O, Marcussen N, Christensen S. Effects of renal denervation on tubular sodium handling in rats with CBL-induced liver cirrhosis. Am J Physiol Renal Physiol 284: F555–F563, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Jonassen TE, Marcussen N, Haugan K, Skyum H, Christensen S, Andreasen F, Petersen JS. Functional and structural changes in the thick ascending limb of Henle's loop in rats with liver cirrhosis. Am J Physiol Regul Integr Comp Physiol 273: R568–R577, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol 288: F982–F987, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Karalliedde J, Buckingham R, Starkie M, Lorand D, Stewart M, Viberti G. Effect of various diuretic treatments on rosiglitazone-induced fluid retention. J Am Soc Nephrol 17: 3482–3490, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Kopkan L, Majid DS. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension 46: 1026–1031, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Kopp W Pathogenesis and etiology of essential hypertension: role of dietary carbohydrate. Med Hypotheses 64: 782–787, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Kunes J, Hojna S, Kadlecova M, Dobesova Z, Rauchova H, Vokurkova M, Loukotova J, Pechanova O, Zicha J. Altered balance of vasoactive systems in experimental hypertension: the role of relative NO deficiency. Physiol Res 53, Suppl 1: S23–S34, 2004. [PubMed] [Google Scholar]
- 33.Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway x Fischer 344, and Fischer 344 x brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci 51: B54–B59, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori T, Cowley AW Jr. Renal oxidative stress in medullary thick ascending limbs produced by elevated NaCl and glucose. Hypertension 43: 341–346, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Oakes ND, Cooney GJ, Camilleri S, Chisholm DJ, Kraegen EW. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes 46: 1768–1774, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol Renal Physiol 282: F777–F784, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na(+)-K(+)-2Cl(−) cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Rahmouni K, Haynes WG. Leptin and the cardiovascular system. Recent Prog Horm Res 59: 225–244, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Riazi S, Khan O, Tiwari S, Hu X, Ecelbarger CA. Rosiglitazone regulates ENaC and Na-K-2Cl cotransporter (NKCC2) abundance in the obese Zucker rat. Am J Nephrol 26: 245–257, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Riazi S, Madala Halagappa VK, Hu X, Ecelbarger CA. Sex and body-type interactions in the regulation of renal sodium transporter abundance, urinary excretion, and activity in lean and obese Zucker rats. Gend Med 3: 309–327, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension 33: 424–428, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Song J, Hu X, Khan O, Tian Y, Verbalis JG, Ecelbarger CA. Increased blood pressure, aldosterone activity, and regional differences in renal enac protein during vasopressin-escape. Am J Physiol Renal Physiol 287: F1076–F1083, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Song J, Hu X, Shi M, Knepper MA, Ecelbarger CA. Effects of dietary fat, NaCl, and fructose on renal sodium and water transporter abundances and systemic blood pressure. Am J Physiol Renal Physiol 287: F1204–F1212, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Song J, Knepper MA, Hu X, Verbalis JG, Ecelbarger CA. Rosiglitazone activates renal sodium- and water-reabsorptive pathways and lowers blood pressure in normal rats. J Pharmacol Exp Ther 308: 426–433, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Sowers JR, Frohlich ED. Insulin and insulin resistance: impact on blood pressure and cardiovascular disease. Med Clin North Am 88: 63–82, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Tanida M, Iwashita S, Ootsuka Y, Terui N, Suzuki M. Leptin injection into white adipose tissue elevates renal sympathetic nerve activity dose-dependently through the afferent nerves pathway in rats. Neurosci Lett 293: 107–110, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Tay A, Ozcelikay AT, Altan VM. Effects of l-arginine on blood pressure and metabolic changes in fructose-hypertensive rats. Am J Hypertens 15: 72–77, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 54: B492–B501, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Wangensteen R, Rodriguez-Gomez I, Moreno JM, Vargas F, Alvarez-Guerra M. Chronic nitric oxide blockade modulates renal Na-K-2Cl cotransporters. J Hypertens 24: 2451–2458, 2006. [DOI] [PubMed] [Google Scholar]