Abstract
In the development of novel therapeutic strategies for kidney disease, new renal biomarkers for early detection and accurate evaluation of renal injury are urgently required for both acute kidney injury (AKI) and chronic kidney disease (CKD). Fatty acid-binding protein 1 (FABP1) is expressed in renal proximal tubule cells and shed into urine in response to hypoxia caused by decreased peritubular capillary blood flow. To clarify the role of urinary FABP1 in kidney disease, we established human FABP1 transgenic mice and evaluated the responses of FABP1 to several AKI and CKD models. Moreover, there are accumulating clinical data that urinary FABP1 can detect human AKI earlier than serum creatinine and can distinguish the risk population for AKI. Investigation with “humanized” FABP1 transgenic mice and measurement of clinical samples allowed us to develop urinary FABP1 as a new renal biomarker. Further clinical studies are necessary to confirm the potential of urinary FABP1 for clinical application.
Keywords: acute kidney injury, chronic kidney disease, tubular injury, hypoxia
therapeutic strategies for kidney diseases remain to be developed because there is as yet no drug that can completely reverse acute kidney injury (AKI) and chronic kidney disease (CKD), despite a number of basic and clinical investigations. AKI is a critical problem because the mortality rate of severely ill patients complicated with AKI is much higher than that of non-AKI patients (13). CKD has recently been recognized as a public health problem. Its incidence and prevalence continue to increase, with poor outcomes and high costs. CKD is frequently underdiagnosed and undertreated, thereby missing opportunities for prevention. Serum creatinine and urinary protein are widely used to detect and evaluate AKI and CKD (35a, 26), although the limitations of these conventional markers for early detection and accurate estimation of renal injury are well known. Novel renal biomarkers are indispensable to development of a new therapeutic strategy for AKI and CKD because they will allow us to detect AKI and CKD and start treatment early (6, 14). In addition to early detection, renal biomarkers should be able to estimate renal injury accurately. Recently, several new renal biomarkers have been developed and are ready to be translated into clinical settings. In this review, we summarize the findings on urinary fatty acid-binding protein 1 (FABP1) from animal experiments and clinical human studies and discuss the potential of urinary FABP1 as an emerging renal biomarker for kidney diseases.
FABP1 and the Kidney
Several decades ago, two organic anion-binding cytoplasmic proteins isolated in rat liver were demonstrated to bind to bilirubin and various dyes (20, 44). One of these proteins was called Z protein and demonstrated to bind oleic acid with high affinity (27). Now this protein has been named L-type or liver-type fatty acid-binding protein (L-FABP), or FABP1. FABP1 binds selectively to free fatty acid (FFA) but not to triolein, cholesterol, or bile salts. Therefore, FABP1 was considered to be a FFA carrier protein. FABPs are known as intracellular lipid chaperones that transport lipids to a specific component in the cell; however, little is known about their exact biological functions and mechanisms of action (11). FABPs are found in many different species including Drosophila melanogaster, Caenorhabditis elegans, mice, rats, and humans. There are several different types of FABP, and the tissue distribution of FABPs is rather ubiquitous (53). So far, nine different FABPs have been reported: liver (L), intestinal (I), muscle and heart (H), adipocyte (A), epidermal (E), ileal (Il), brain (B), myelin (M), and testis (T) (Table 1). The regulation of the tissue-specific expression pattern of each FABP is not clearly understood. The FABPs have virtually similar three-dimensional structures as a binding site, although they have a wide range of different homologies from 15% to 70%. FABP1 appears not to have so high homology with other FABPs (25–30%) (54). The FABP1 gene is located on chromosome 2p11 in the human genome, whereas the genes of four other FABPs (FABP4, 5, 8, and 9) are located in the same region, 8q21.
Table 1.
Fatty acid-binding protein (FABP) family
| Gene | Protein | Type | Expression |
|---|---|---|---|
| FABP1 | L-FABP | Liver | Liver, kidney, intestine, pancreas, lung, stomach |
| FABP2 | I-FABP | Intestinal | Intestine, liver |
| FABP3 | H-FABP (MDGI) | Heart | Heart, muscle, kidney, brain, lung, stomach |
| FABP4 | A-FABP (aP2) | Adipocyte | Adipocyte, macrophage, dendritic cell |
| FABP5 | E-FABP (mal1) | Epidermal | Skin, adipocyte, macrophage, dendritic cell, brain |
| FABP6 | Il-FABP | Ileal | Ileum, ovary, adrenal gland, stomach |
| FABP7 | B-FABP (MRG) | Brain | Brain, glial cell, retina |
| FABP8 | M-FABP (PMP2) | Myelin | Peripheral nervous system, Schwann cell |
| FABP9 | T-FABP | Testis | Testis, salivary gland, mammary gland |
MDGI, mammary-derived growth inhibitor; aP2, adipocyte P2; MRG, MDGI-related gene; PMP2, peripheral myelin protein 2.
FABP1 is expressed in hepatocytes and the crypt to villus tip of intestine from duodenum to colon. Because fatty acid is a major energy source of renal tubule epithelial cells, FABPs were thought to be involved in energy production/metabolism in renal tubule cells. With the analysis of human kidney, Veerkamp and coworkers (21) found two types of FABPs in renal tubule cells: FABP1 (L-FABP) and heart and muscle type (FABP3 or H-FABP). They investigated the characteristics of renal FABP1 and FABP3 in detail. Renal FABP1 and FABP3 showed the same Kd values for oleic acid of liver and heart. Compared with FABP1 expressed in liver, FABP1 in kidney showed a more neutral isoelectric point (pI 5.8) and had two additional tryptophan residues. Therefore, renal FABP1 is seemingly kidney specific and could be a new subtype of FABP1. In addition, a displacement study with various types of ligands demonstrated a rather promiscuous binding specificity of hepatic and renal FABP1, although percent displacement was <60% at maximum. The potentiality of kidney-directed ligand specificity was also envisaged in that study. On the other hand, renal FABP3 showed the same pI as heart FABP3 and a more ligand-specific nature. There was virtually no displacement, which is similar to heart FABP3.
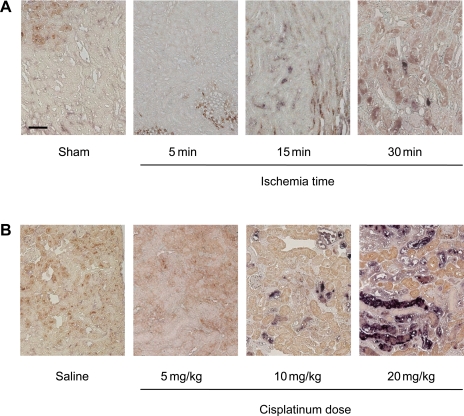
Immunohistochemical analysis elucidated the complementary localization of FABP1 and FABP3 in the kidney. FABP1 was exclusively localized to the cytoplasmic region of the proximal tubules, whereas FABP3 was localized to the cytoplasmic region of the distal tubules except for the macula densa (21, 60). Our group recently confirmed the similar localization of FABP1 and FABP3 in canine and porcine kidney (T. Yamamoto, unpublished observations). Comparison of FABP1 and FABP3 in terms of biomarkers in the kidney diseases was recently reviewed (42). It is of note that both mouse and rat kidney did not express FABP1, at least under normal conditions, because of the silencing sequence in the upstream of the promoter region as reported by Gordon and coworkers (48). We also found that mouse FABP1 protein expression in C57BL/6 wild-type kidney was absent in normal and pathophysiological conditions such as renal ischemia-reperfusion injury (I/R-AKI) or exposure to cisplatin (CP-AKI). Therefore, the kidneys of wild-type rodents are equal to those in the deficient condition. The promoter region of FABP1 contains the binding site of hepatocyte nuclear factor (HNF), hypoxia-inducible factor-1 (HIF-1), and peroxisome proliferator-activated receptors (PPARs). We have recently developed human FABP1 transgenic mice with a transgene of human FABP1 containing the whole FABP1 promoter region. The kidneys of human FABP1 transgenic mice express not rodent FABP1 but human FABP1 [“humanized” FABP1 transgenic (hFABP1-Tg) mice] (17, 36, 60) (Fig. 1). FABP1 transgenic mice can be utilized to investigate the role of FABP1 in the kidney. We have reported the role of urinary FABP1 as a novel renal biomarker with several AKI and CKD models using hFABP1-Tg mice. In addition, several clinical studies demonstrated the significance of urinary FABP1 in kidney diseases.
Fig. 1.
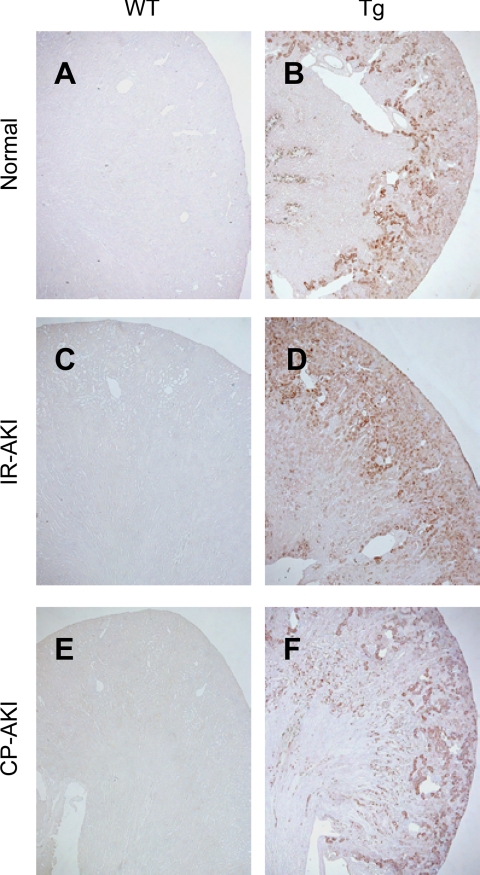
Immunohistochemical analysis of human fatty acid-binding protein 1 (FABP1) transgenic (Tg) mice. Immunohistochemical analysis was performed with the antibody that reacts with mouse and human FABP1. Because of the silencing sequence in the upstream promoter region, mouse FABP1 expression in C57BL/6 wild-type (WT) kidney was absent under normal conditions (A). Renal ischemia-reperfusion (I/R) injury (C) or exposure to cisplatin (CP; E) did not induce FABP1 expression in WT kidney. In human FABP1 Tg mouse kidney, constitutional expression of FABP1 protein was found under normal conditions (B) and FABP1 expression was amplified and expanded in the S3 segment of the cortex 24 h after renal I/R (D). In 20 mg/kg CP-injected Tg kidney (F), FABP1 expression of damaged tubules was diminished or disappeared, whereas viable tubules remained positive 72 h after CP injection. AKI, acute kidney injury.
Urinary FABP1 in Animal AKI Models
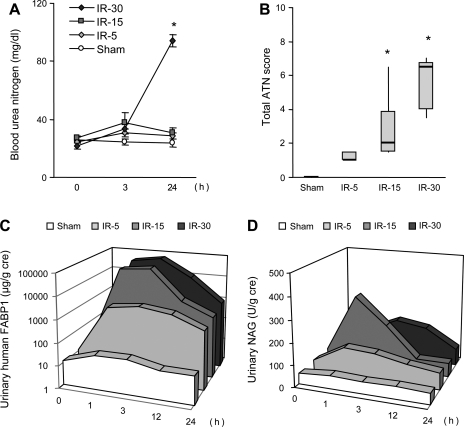
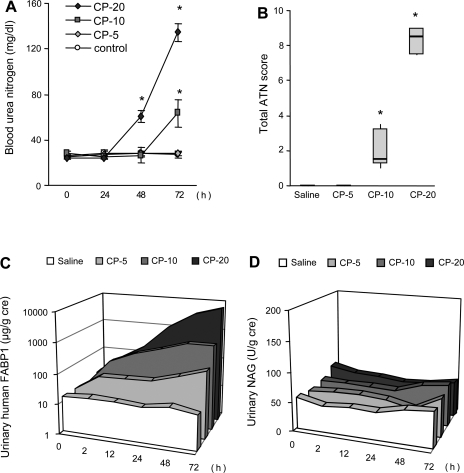
The response of urinary FABP1 in several animal AKI models has been reported. CP-induced AKI increased the shedding of urinary FABP1 in hFABP1-Tg mice, which was decreased by fibrate treatment (36, 38). We found that urinary FABP1 had a large dynamic range and could monitor the different levels of AKI in a renal I/R injury and CP model (37). The severity of histological injuries increased along with ischemia time; however, blood urea nitrogen (BUN) and urinary N-acetyl-d-glucosaminidase (NAG) could not detect renal histological injury completely. Urinary FABP1 responded more sensitively than those conventional markers (Fig. 2). Moreover, increase of urinary FABP1 was observed at 1 h after ischemia, even in the 5-min I/R group. Similarly, animals administered different doses of CP showed AKI with different severities of histological injury (Fig. 3). Correlation analysis with histological injury score and urinary FABP1 demonstrated that urinary FABP1 was superior to BUN and urinary NAG for early and accurate detection of acute tubular necrosis in different models of animal AKI.
Fig. 2.
Responses of renal biomarkers in animal ischemic AKI. AKI was induced by several different ischemia times (0, 5, 15, 30 min), and the responses of blood urea nitrogen (BUN; A), acute tubular necrosis (ATN) score (B), urinary FABP1 (C), and urinary N-acetyl-d-glucosaminidase (NAG; D) were evaluated. Urinary FABP1 levels even at 3 h could reflect the injury in ischemic AKI, whereas BUN and urinary NAG could not predict the severity of AKI. Original data from Negishi et al. (37).
Fig. 3.
Responses of renal biomarkers in animal CP-induced AKI. Different doses of CP (0, 5, 10, 20 mg/kg) were administered, and the responses of BUN (A), ATN score (B), urinary FABP1 (C), and urinary NAG (D) were evaluated. BUN and urinary FABP1 could reflect the severity of AKI; however, urinary FABP1 responded earlier than BUN. Original data from Negishi et al. (37).
Urinary FABP1 in Clinical AKI
Approximately 5 billion doses of radiocontrast medium (RCM) are annually used in the US and 1 billion shots in Japan. It is reported that the rate of RCM-induced AKI is rather small in the general population, but still high in elderly and type 2 diabetes mellitus patients (24, 25, 45). Risk evaluation is required for these high-risk populations before planning RCM administration. Nakamura and coworkers (33) recently studied urinary FABP1 in patients undergoing nonemergency coronary angiography or imaging using a nonionic, low-osmolarity type RCM. Thirteen of 66 patients had significantly high urinary FABP1 before contrast injections, and all 13 of these patients showed RCM-induced nephropathy, whereas no patient with low urinary FABP1 showed decline of renal function (Fig. 4). This patient cohort showed a serum creatinine level >1.2 mg/dl and <2.5 mg/dl, and there was no difference in serum creatinine levels between the AKI and non-AKI groups. This report strongly suggests that urinary FABP1 can identify high-risk patients with preexisting tubular injury that cannot be detected by serum creatinine, and patients with urinary FABP1 >15 μg/g Cr before radiocontrast examination should be monitored intensively for emerging AKI.
Fig. 4.
Urinary FABP1 levels in radiocontrast medium (RCM)-induced AKI. Urinary FABP1 measurement of 66 patients who had a serum creatinine level >1.2 mg/dl (>106 μmol/l) and <2.5 mg/dl (<221 μmol/l) undergoing nonemergency coronary angiography or intervention demonstrated urinary FABP1 levels before contrast injection that clearly predicted RCM-induced AKI. Original data from Nakamura et al. (33).
We reported (43) that urinary FABP1 was useful for early detection of AKI after pediatric cardiopulmonary bypass surgery (CPB-AKI) in a study in which urinary FABP1 was examined at 4 h and 12 h after the surgery. Receiver operating characteristic (ROC) curve analysis of urinary FABP1 for CPB-AKI diagnosis was performed. The area under the ROC curve of urinary FABP1 at 4 h after surgery was 0.810, which is an acceptable level for the single predictive biomarker. Univariate logistic regression analyses showed that both bypass time and urinary FABP1 were significant independent risk indicators for AKI. A stepwise multivariate logistic regression analysis excluding bypass time demonstrated that urinary FABP1 levels at 4 h after surgery were an independent risk indicator.
Pediatric cardiac surgery is recognized as an ideal clinical setting for initial studies in biomarker development in terms of minimal comorbidity and known timing of renal injury (51). Further evaluations in adult CPB-AKI or septic AKI, which include more complicated and heterogeneous patients, are necessary to confirm the possibility of clinical application of a new biomarker. For instance, urinary neutrophil gelatinase-associated lipocalin (NGAL), another biomarker for AKI, was also measured in this cohort of pediatric CPB-AKI patients. Urinary NGAL showed a significant association even in a multivariate analysis including bypass time (28) and a better prediction with area under the ROC curve (1.0). However, urinary NGAL did not show as high sensitivity and specificity in adult CPB-AKI (55). Recently, Koyner et al. (18) reported that urinary NGAL showed an area under the ROC curve of 0.691 in an adult CPB-AKI cohort. Urinary FABP1 has not yet been evaluated with an adult CPB-AKI cohort and needs to be evaluated in an adult population with comorbidities or multiple renal insults.
Sepsis and Urinary FABP1
Sepsis is the leading cause of death in critically ill patients, and the incidence of sepsis is increasing (1, 23). Sepsis causes AKI, and patients with both sepsis and AKI show an especially high mortality rate (47). In one large multicenter study involving 30,000 critically ill patients, 50% of AKI was associated with septic shock (52). Therefore, early prediction of sepsis-induced AKI will enable us to improve patient survival. However, there was virtually no effective parameter for early detection of sepsis. Recently, urinary FABP1 was examined in septic shock patients. Urinary FABP1 levels in septic shock patients were significantly higher than those in healthy subjects. In a cohort of 145 septic shock patients, a logistic regression analysis including urinary FABP1, blood endotoxin level, C-reactive protein, peripheral white blood cell count, urinary NAG, and serum creatinine revealed that only urinary FABP1 levels showed a significant association with patient survival. There was no correlation between serum creatinine and urinary FABP1 (K. Doi and T. Nakamura, unpublished observations). Forty septic shock patients in another cohort were treated by polymyxin B-immobilized fiber (PMX-F) hemoperfusion. PMX-F treatment has been performed to treat severe sepsis in more than 30,000 patients in Japan since 1994, and a meta-analysis demonstrated its efficacy on septic shock treatment (7). Of 40 septic patients, 28 survived and 12 died. Among the surviving patients, urinary FABP1 levels were reduced by treatment. However, the nonsurviving patients showed higher urinary FABP1 levels with smaller decrease after the treatment compared with the survivors. These results suggested that urinary FABP1 levels might be able to reflect the severity of sepsis, and also to monitor the effectiveness of treatment (32).
Urinary FABP1 as a Tubulointerstitial Marker in CKD
The prevalence of CKD is increasing all over the world, and no treatment can completely reverse the progression of CKD to end-stage renal disease (ESRD), which requires an enormous amount of medical resources. Moreover, CKD is a strong and independent risk factor for cardiovascular disease (13). Proteinuria is a predictive indicator of progressive CKD, and albuminuria is presumably an earlier predictive indicator of CKD. Proteinuria and albuminuria are considered to reflect glomerular barrier function and therefore are regarded as hallmarks of glomerular injury. It has been reported that tubulointerstitial damage plays a key role in the progression of CKD (12, 22, 35, 46). However, there is no good monitoring measurement except renal biopsy to detect damage in the tubulointerstitium accurately. Hypoxia in the tubulointerstitium caused by decreased peritubular capillary blood flow plays a crucial role in the progression of CKD (34). Urinary FABP1 is seen as a promising indicator for monitoring tubulointerstitial injury because FABP1 is exclusively expressed in proximal tubule epithelial cells in the kidney and tubular injury caused by hypoxia increases FABP1 production as described below. The combination of urinary protein/albumin and FABP1 will allow us to evaluate glomerular and tubular injury separately in clinical practice.
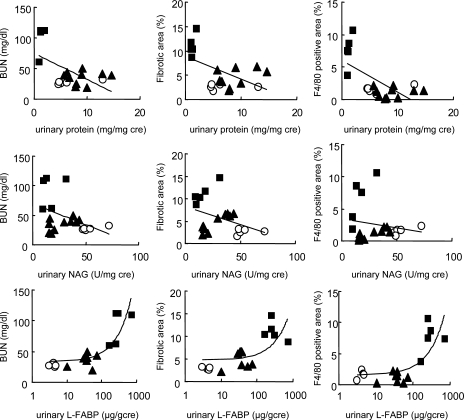
We have recently developed a mouse interstitial injury model using an excess adenine-containing diet (49). Adenine is a substrate for xanthine dehydroxygenase (XDH), which can oxidize adenine to 2,8-dihydroxyadenine (DHA) via an 8-hydroxyadenine intermediate (59). Because of the very low solubility of DHA, it is assumed that the deposition of DHA in renal tubules degenerates renal tubule epithelial cells and causes inflammatory injury with subsequent fibrotic changes (8). Severe tubulointerstitial pathological findings such as fibrosis and macrophage infiltration were found within 6 wk in this model. There was no glomerular lesion until the late stage of CKD. Urinary FABP1 increased significantly and gradually along with the progression of interstitial injury. Increase of urinary FABP1 and BUN and histological injury were attenuated by XDH inhibitor. Correlations of urinary markers of FABP1, protein, and NAG with tubulointerstitial injury of fibrotic area and macrophage infiltration were evaluated (Fig. 5). Urinary FABP1 showed the best correlations with fibrotic area and macrophage infiltration. These observations support the concept that urinary FABP1 is a good indicator to reflect tubulointerstitial injury such as fibrosis and inflammatory cell infiltration.
Fig. 5.
Correlation of urinary markers and tubulointerstitial injury. Correlations between urinary markers [protein (top), NAG (middle), and FABP1 (bottom)] and fibrotic area and F4/80 positively stained area in adenine-induced tubulointerstitial injury model are shown. •, Adenine diet group; ▴, treatment group; ○, normal diet group. Original data from Tanaka et al. (49).
Urinary FABP1 in Various CKDs
The utility of urinary FABP1 in various CKDs has been reported (16). Diabetic nephropathy is a leading cause of CKD and is rapidly increasing. Rossing and coworkers in the Steno Diabetes Center (39) recently reported that urinary FABP1 predicted the severity of diabetic nephropathy by type 1 diabetes defined by urinary albuminuria. Moreover, reduction of urinary FABP1 was found by angiotensin-converting enzyme inhibitor (ACEI) treatment. The combination of microalbuminuria with urinary FABP1 will allow us to detect high-risk diabetic nephropathy patients and to perform more aggressive treatment strategies such as increasing the dose of ACEI or angiotensin receptor blockers (ARBs) and the combined prescription of both ACEI and ARBs.
Focal glomerulosclerosis (FGS) is a crucial cause of primary glomerulonephritis; its incidence appears to be increasing, especially among the African American population. Drug treatment including glucocorticoids and calcineurin inhibitors sometimes fails to obtain a therapeutic response toward remission. When urinary FABP1 was measured in FGS, the level was significantly higher than minimal change (10.2 ± 8.4 μg/g Cr; n = 24) or healthy control (7.4 ± 4.2 μg/g Cr; n = 20). A drug-resistant FGS group showed significantly higher urinary FABP1 levels (122 ± 78.4 μg/g Cr; n = 8) compared with a sensitive group (45.9 ± 32.0 μg/g Cr; n = 9) (31). Admittedly, recent data on mouse antiglomerular basement membrane glomerulonephritis showed that lower peritubular blood flow but not proteinuria reflected the severity of tubulointerstitial change (57). These observations in CKD suggest that the target population necessary for intensive treatment should have the features of lower blood flow in peritubular capillary, subsequent hypoxic conditions, and presence of interstitial fibrosis, all of which can be monitored by urinary FABP1. Recently Nakamura and coworkers reported (30) that a new L-type calcium channel blocker decreased both urinary protein and FABP1 in mild CKD patients complicated with hypertension. They also reported that urinary FABP1 could detect histological improvement by ACEI and ARB combination treatment in normotensive IgA nephropathy (29). Further studies with large cohorts of CKD patients in prospective fashion involving multiple urinary indicators are necessary.
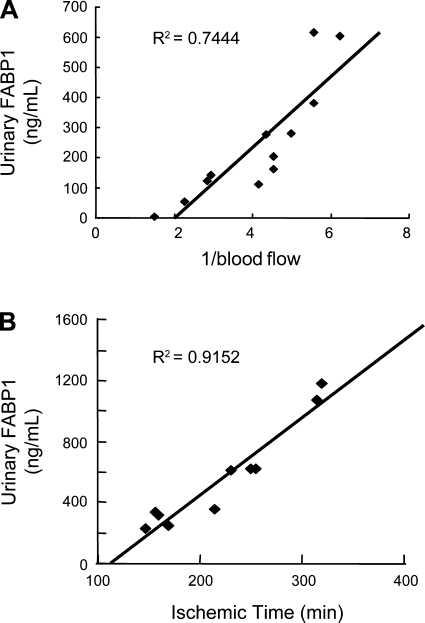
FABP1 for Renal Toxicology
Urinary FABP1 can detect drug-induced nephrotoxicity by the anticancer drug CP as described above (36, 38). We demonstrated (50) that urinary FABP1 can detect interstitial changes after the administration of cyclooxygenase 2 (COX2) inhibitors with a low-sodium diet preparation. Low sodium enhanced renin-angiotensin-aldosterone activity and reduced peritubular capillary blood flow. The additional administration of the COX2-selective inhibitor meloxicam decreased peritubular capillary blood flow significantly. Mild interstitial fibrotic region and partial cell infiltration were confirmed by histological examination. More suggestive findings of this report are the concept of responder and nonresponder for the evaluation of drug toxicology. Celecoxib, another COX2 inhibitor, induced low peritubular capillary blood flow in a certain fraction of animals (∼50%), which were categorized as responders. Animal responders showed higher urinary FABP1 compared with nonresponders. When kidneys were harvested 4 wk after celecoxib administration, kidneys derived from responders showed more interstitial fibrotic region compared with nonresponders (Fig. 6). This kind of individual variation in phenotype occurs frequently in human clinical studies. It is crucial to find drug responders and nonresponders to improve treatment strategies and reduce drug adverse effects. Urinary FABP1 may be able to distinguish responders for renal drug toxicity.
Fig. 6.
Urinary FABP1 levels of responders and nonresponders in cyclooxygenase 2 (COX2) inhibitor-induced tubulointerstitial injury model. A: COX2 inhibitor-induced low peritubular capillary blood flow determined the animals as responders or nonresponders. B: responders (urinary FABP1 >50 μg/g creatinine) showed larger interstitial fibrosis area than nonresponders (<50 μg/g creatinine). Original data from Tanaka et al. (50). Quantitative analysis was performed by the computer-aided evaluation program AIS (Fujifilm, Tokyo, Japan). Asterisk denotes P < 0.05.
FABP1 as a Marker of Renal Hypoxia
Renal tubule epithelial cells are as mitochondria rich as brain neuronal cells and therefore vulnerable under hypoxic conditions. Because of the anatomic structure of the kidney, the outer medullary region can be easily injured by hypoxia derived from decreased peritubular capillary blood flow and subsequent oxidative stress in a renal I/R model (9, 40, 61). Proximal tubules are rather susceptible to hypoxic stress compared with distal tubules. Under hypoxic conditions, proximal tubules are apt to undergo necrosis, but distal tubules show apoptosis under the same level of hypoxic stress (3). FABP1 gene is responsive to hypoxic stress because the promoter region of FABP1 gene has a hypoxia-responsive element (HRE).
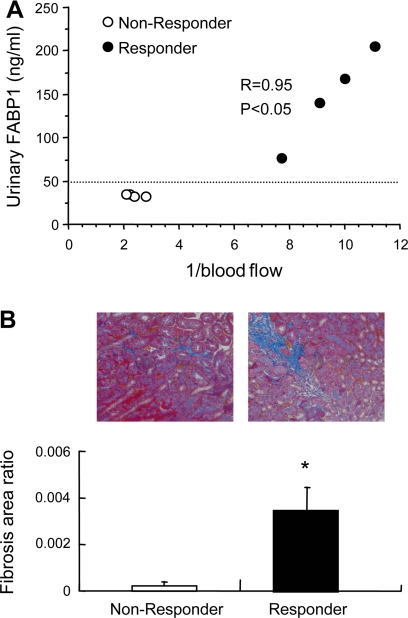
We hypothesized that simultaneous decrease of peritubular capillary blood flow might be important for FABP1 excretion into urine. Peritubular capillary blood flow of transplanted kidney was directly measured in living related kidney transplantation by intravital video analysis at different time points after the anastomosis of vessels (60). Urinary FABP1 showed an excellent reverse correlation with peritubular capillary blood flow (Fig. 7). We also found a remarkably significant correlation between urinary FABP1 and ischemia time, where ischemia time was defined as the time from clamp of the donor's renal artery to the appearance of the first urine from the recipient's ureter. These observations suggest that urinary FABP1 reflects the hypoxic condition caused by decreased peritubular capillary blood flow in the kidney.
Fig. 7.
Correlations between urinary FABP1 level and peritubular capillary blood flow and ischemia time of the transplanted kidney. Urinary FABP1 was compared with 1/blood flow (A) and ischemia time (B). Where renal ischemia is more severe, 1/blood flow is larger. The ischemic time in living-related renal transplantation was defined as the period between the clamp of the donor's renal artery and the appearance of virgin urine from the recipient's ureter. Original data from Yamamoto et al. (60).
HIF-1 is a key regulator of cellular responses to hypoxia. However, it is difficult to measure HIF-1 levels as a hallmark of hypoxic conditions because degradation of HIF-1 is very rapid and sensitive to a subtle change of reoxygenation. Urinary FABP1 is stable in urine and can be an alternative marker of renal hypoxic signatures. FABP1 in urine can be preserved at room temperature overnight and at −20°C for a year (A. Kamijo, unpublished observations).
FABP1 and Oxidative Stress and Lipotoxicity
Because urinary FABP1 increased in both AKI and CKD patients and the localization of FABP1 in human kidney was exclusively in the cytoplasmic region of proximal tubules, it is possible that urinary shedding of FABP1 from proximal tubule epithelial cells can be observed under some pathophysiological conditions. In animal models of AKI, immunohistochemistry demonstrated that a hallmark of lipid peroxidation product, 4-hydroxyhexenal (HHE), was increased in animal AKI such as I/R and CP injection models (Fig. 8). The level of HHE-modified protein increased along with CP dose and ischemia time. HHE is one of the major aldehyde products of lipid peroxidation, along with acrolein, crotonaldehyde, and 4-hydroxy-2-nonenal (HNE). HHE originates from phospholipid-bound omega-3 unsaturated fatty acids and is one of the most reliable markers of free radical-induced lipid peroxidation as well as HNE (9, 40). HHE exhibits cytopathological effects such as mitochondrial dysfunction and induction of apoptosis (4, 5, 19). Recently, Bennaars-Eiden and coworkers (2) reported the capability of E-FABP (epithelial type of FABP or FABP5) to bind HNE, and FABP5 has a structure close to that of FABP1. Severely injured tubule cells with less FABP1 expression showed HHE accumulation, whereas relatively preserved tubule cells showed more FABP1 expression and small HHE accumulation (Fig. 8). Moreover, fibrate treatment prevented tubular accumulation of HHE and lipid droplets in parallel with a favorable response of therapeutic efficacy (36). We speculate that FABP1 traps cytotoxic aldehydes like HNE and HHE and transfers them to urinary spaces for elimination.
Fig. 8.
Double immunostaining of human (h)FABP1 and 4-hydroxyhexenal (HHE) in animal AKI models. Double immunostaining of hFABP1 (brown) and HHE (purple) in I/R-AKI (A) and CP-AKI (B) is shown. HHE accumulation was found in severely damaged tubular cells, and relatively preserved tubular cells showed positive staining of FABP1. Original data partly from Negishi et al. (36).
In human CKD and an animal model of CKD, excessive urinary albumin appeared to induce proximal tubular stress and subsequently increase urinary FABP1 levels (17). Injection of bovine serum albumin (BSA) replete with FFAs in hFABP1-Tg mice caused more severe tubulointerstitial injury and higher urinary FABP1 levels than BSA depleted of FFAs, indicating that lipid binding to albumin has a crucial role for this urinary albumin-induced tubular stress. Recently, it was suggested that accumulation of fatty acids in parenchymal cells in various tissues causes chronic cellular dysfunction and injury. This process is termed “lipotoxicity,” and multiple effects of fatty acid including alternation of mitochondrial energy coupling, reactive oxygen species (ROS) production, protein kinase C activation, and NF-κB activation can cause cell dysfunction and apoptosis/necrosis and change renal tubule cells to a proinflammatory phenotype, driving progression to tubulointerstitial injury (56). Therefore, lipid peroxidation and lipid accumulation could be the primary cause for FABP1 excretion into urine, and urinary shedding of FABP1 may be protective for tubular cell injury.
Contribution of Renal FABP1 Production to Urine Level
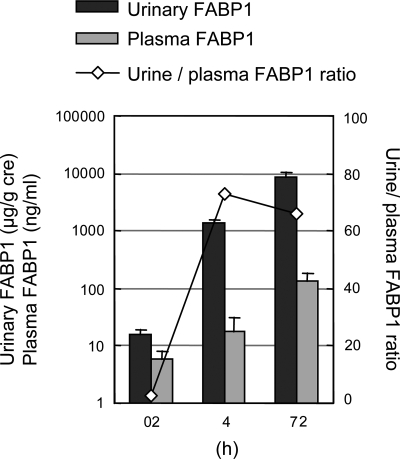
FABP1 is a 14-kDa protein produced mainly in the liver, and liver production appears to determine blood FABP1 levels. FABP1 can be filtered via glomeruli because of its small molecular size and reabsorbed in proximal tubule epithelial cells like other small proteins such as retinoic acid binding protein and α1-microglobulin. This process of tubular reabsorption is, at least in part, carried out via megalin, which is a rather promiscuous carrier protein (41). Therefore, it might be plausible that increase of cytosolic FABP1 in proximal tubule cells was derived not only from endogenous expression but also from filtering at glomeruli and reabsorbed FABP1, which comes from circulating blood. We recently compared (36) the levels of plasma and urinary FABP1 in CP-induced AKI in hFABP1-Tg mice (Fig. 9). CP-AKI increased the plasma FABP1 level, but the magnitude of the increased urine level was extremely higher than the plasma level. In human clinical CPB-AKI, there was a significant increase of urinary FABP1 levels 4 h after the surgery, whereas serum FABP1 levels started to increase at 12 h (43). These observations support the concept that the human urinary FABP1 level is mostly determined by proximal tubule injury.
Fig. 9.
Urinary FABP1, plasma FABP1, and urine-to-plasma ratio of FABP1 in CP-AKI mice. Urinary and plasma FABP1 levels were examined in CP-AKI (20 mg/kg). Urine and plasma levels of hFABP1 increased after CP injection. However, urinary level was much higher than plasma level and urine-to-plasma ratio increased >25-fold compared with 0 h. Original data from Negishi et al. (36).
Perspectives
There is accumulating evidence that several urinary indicators are effective for earlier diagnosis of AKI. In the meeting of the Acute Kidney Injury Network (AKIN) in 2008, seven markers [urinary kidney injury molecule (KIM-1), urinary NGAL, urinary IL-18, plasma IL-6, urine and plasma cystatin C, urinary NAG, urinary FABP1] were listed as being on the leading edge of predictive biomarkers of AKI; presumably they belong to the first generation of newly developed AKI biomarkers. Developing a panel of several renal biomarkers might be a good strategy. For instance, acute myocardial infarction is diagnosed with the combination of several biomarkers including white blood cell counts, creatine kinase and its MB fraction, and troponin T in addition to electrocardiogram and clinical symptoms. The panel of these indicators is helpful for clinical diagnosis in acute myocardial infarction compared with any single indicator. Han and coworkers (15) recently evaluated urinary levels of matrix metalloproteinase-9 (MMP-9), NAG, and KIM-1 in a cross-sectional study including 44 AKI patients with various etiologies and a case control study of 20 pediatric CPB-AKI and non-AKI patients. They found a perfect score in diagnosing AKI by combining all three biomarkers, whereas the combination was no better than urinary KIM-1 alone in the case control study. Further clinical studies with more biomarkers and larger sample sizes will show the effectiveness of combination of biomarkers.
High-throughput measurement is required for new biomarker development. It is ideal that multiple laboratory tests be performed simultaneously in a couple of minutes. The other aspect required for new biomarkers will be a much simpler detection system like immunochromatography. A dipstick system similar to troponin T detection will be suitable for point of care (POC) in the emergency room, intensive care unit, and operating room and immediately before RCM examination. Moreover, a POC-type diagnosis kit is suitable for diagnosis and management in the third world and in disaster areas. A urine dipstick-type kit for FABP1 has recently been developed for those purposes and awaits evaluation studies (E. Noiri and T. Sugaya, unpublished data).
In this review we have summarized our investigation with human FABP1 transgenic mice and human urinary FABP1 measurement by ELISA. We evaluated urinary FABP1 in a wide variety of acute and chronic kidney injuries in detail. This system consisting of humanized mice and measurement of human samples is a good strategy for biomarker development (Fig. 10). Moreover, this system will be better applied to nephrotoxicologic screening in drug development before clinical studies; it is more effective for future drug discovery processes. The FDA started a Critical Path Initiative in 2004, intending to improve drug development and reduce uncertainty by applying new scientific tools to the development process. Our proposed system of urinary FABP1 will be satisfactory for FDA requirements (58).
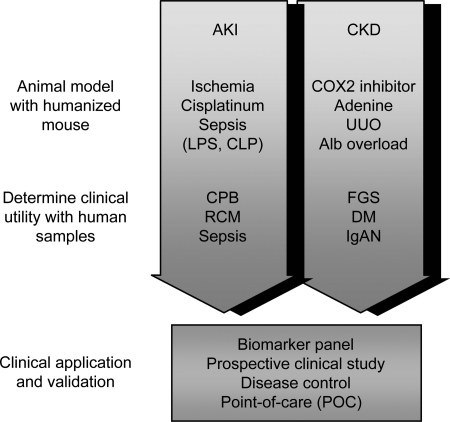
Fig. 10.
A strategy for biomarker development. CKD, chronic kidney disease; LPS, lipopolysaccharide; CLP, cecal ligation and puncture; CPB, cardiopulmonary bypass; UUO, unilateral ureter obligation; Alb, albumin; FGS, focal glomerulosclerosis; DM diabetes mellitus; IgAN, IgA nephropathy.
In conclusion, a number of animal and human preliminary studies suggest a possible role of urinary FABP1 in clinical diagnosis of renal disease and early judgment of therapeutic intervention. Development of urinary FABP1 as a new renal biomarker has moved to the next stage. To confirm the potential of urinary FABP1 for clinical application, sensitivity and specificity in a wide range of kidney diseases should be evaluated. In addition, evaluating the renal biomarker panel containing urinary FABP1 in human clinical studies is necessary, and a more rapid and easier measurement system for POC is indispensable.
GRANTS
Part of this study was supported by Special Coordination Funds for Promoting Science and Technologies no. 1200015, Ministry of Education, Culture, Sports, Science, and Technology-Japan (MEXT) (E. Noiri, T. Sugaya) and KAKEN-HI no. 19590935, MEXT (E. Noiri, T. Sugaya) and by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-075976 and a Department of Veterans Affairs Merit Award (D. Portilla).
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Bennaars-Eiden A, Higgins L, Hertzel AV, Kapphahn RJ, Ferrington DA, Bernlohr DA. Covalent modification of epithelial fatty acid-binding protein by 4-hydroxynonenal in vitro and in vivo. Evidence for a role in antioxidant biology. J Biol Chem 277: 50693–50702, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Chen JJ, Bertrand H, Yu BP. Inhibition of adenine nucleotide translocator by lipid peroxidation products. Free Radic Biol Med 19: 583–590, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Choudhary S, Zhang W, Zhou F, Campbell GA, Chan LL, Thompson EB, Ansari NH. Cellular lipid peroxidation end-products induce apoptosis in human lens epithelial cells. Free Radic Biol Med 32: 360–369, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int 73: 1008–1016, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Cruz DN, Perazella MA, Bellomo R, de Cal M, Polanco N, Corradi V, Lentini P, Nalesso F, Ueno T, Ranieri VM, Ronco C. Effectiveness of polymyxin B-immobilized fiber column in sepsis: a systematic review. Crit Care 11: R47, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vries A, Sperling O. Implications of disorders of purine metabolism for the kidney and the urinary tract. Ciba Found Symp 48: 179–206, 1977. [DOI] [PubMed] [Google Scholar]
- 9.Doi K, Suzuki Y, Nakao A, Fujita T, Noiri E. Radical scavenger edaravone developed for clinical use ameliorates ischemia/reperfusion injury in rat kidney. Kidney Int 65: 1714–1723, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7: 489–503, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int 56: 1627–1637, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein SL, Devarajan P. Progression from acute kidney injury to chronic kidney disease: a pediatric perspective. Adv Chronic Kidney Dis 15: 278–283, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int 73: 863–869, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamijo-Ikemori A, Sugaya T, Kimura K. Urinary fatty acid binding protein in renal disease. Clin Chim Acta 374: 1–7, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kamijo A, Sugaya T, Hikawa A, Okada M, Okumura F, Yamanouchi M, Honda A, Okabe M, Fujino T, Hirata Y, Omata M, Kaneko R, Fujii H, Fukamizu A, Kimura K. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol 165: 1243–1255, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, Kasza KE, O'Connor MF, Konczal DJ, Trevino S, Devarajan P, Murray PT. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int 74: 1059–1069, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristal BS, Park BK, Yu BP. 4-Hydroxyhexenal is a potent inducer of the mitochondrial permeability transition. J Biol Chem 271: 6033–6038, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Levi AJ, Gatmaitan Z, Arias IM. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest 48: 2156–2167, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maatman RG, Van Kuppevelt TH, Veerkamp JH. Two types of fatty acid-binding protein in human kidney. Isolation, characterization and localization. Biochem J 273: 759–766, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackensen-Haen S, Bader R, Grund KE, Bohle A. Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules and impairment of the glomerular filtration rate. Clin Nephrol 15: 167–171, 1981. [PubMed] [Google Scholar]
- 23.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546–1554, 2003. [DOI] [PubMed] [Google Scholar]
- 24.McCullough PA Contrast-induced acute kidney injury. J Am Coll Cardiol 51: 1419–1428, 2008. [DOI] [PubMed] [Google Scholar]
- 25.McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med 103: 368–375, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishkin S, Stein L, Gatmaitan Z, Arias IM. The binding of fatty acids to cytoplasmic proteins: binding to Z protein in liver and other tissues of the rat. Biochem Biophys Res Commun 47: 997–1003, 1972. [DOI] [PubMed] [Google Scholar]
- 28.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Inoue T, Sugaya T, Kawagoe Y, Suzuki T, Ueda Y, Koide H, Node K. Beneficial effects of olmesartan and temocapril on urinary liver-type fatty acid-binding protein levels in normotensive patients with immunoglobin A nephropathy. Am J Hypertens 20: 1195–1201, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Sugaya T, Kawagoe Y, Suzuki T, Ueda Y, Koide H, Inoue T, Node K. Azelnidipine reduces urinary protein excretion and urinary liver-type fatty acid binding protein in patients with hypertensive chronic kidney disease. Am J Med Sci 333: 321–326, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Osada S, Koide H. Urinary liver-type fatty acid-binding protein levels for differential diagnosis of idiopathic focal glomerulosclerosis and minor glomerular abnormalities and effect of low-density lipoprotein apheresis. Clin Nephrol 65: 1–6, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T, Sugaya T, Koide H. Urinary liver-type fatty acid-binding protein in septic shock: effect of polymyxin B-immobilized fiber hemoperfusion. Shock (October 6, 2008); doi: 10.1097/SHK.0b013e3181891131. [DOI] [PubMed]
- 33.Nakamura T, Sugaya T, Node K, Ueda Y, Koide H. Urinary excretion of liver-type fatty acid-binding protein in contrast medium-induced nephropathy. Am J Kidney Dis 47: 439–444, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Nangaku M Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Nath KA Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992. [DOI] [PubMed] [Google Scholar]
- 35a.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: S1–S201, 2003. [PubMed] [Google Scholar]
- 36.Negishi K, Noiri E, Maeda R, Portilla D, Sugaya T, Fujita T. Renal L-type fatty acid-binding protein mediates the bezafibrate reduction of cisplatin-induced acute kidney injury. Kidney Int 73: 1374–1384, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Negishi K, Noiri E, Maeda R, Tanaka T, Okamoto K, Sugaya T, Fujita T. A sensitive biomarker of AKI: monitoring of urinary L-type fatty acid binding protein (L-FABP) (Abstract). J Am Soc Nephrol S18: S643A, 2007. [Google Scholar]
- 38.Negishi K, Noiri E, Sugaya T, Li S, Megyesi J, Nagothu K, Portilla D. A role of liver fatty acid-binding protein in cisplatin-induced acute renal failure. Kidney Int 72: 348–358, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen S, Sugaya T, Tarnow L, Schjoedt KJ, Astrup AS, Baba T, Parving HH, Rossing P. Urinary liver-type fatty acid-binding protein increases with levels of albuminuria in type 1 diabetic patients, and is reduced with ACE inhibition. European Association for the Study of Diabetes, Rome, Italy, 2008.
- 40.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol 281: F948–F957, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Oyama Y, Takeda T, Hama H, Tanuma A, Iino N, Sato K, Kaseda R, Ma M, Yamamoto T, Fujii H, Kazama JJ, Odani S, Terada Y, Mizuta K, Gejyo F, Saito A. Evidence for megalin-mediated proximal tubular uptake of L-FABP, a carrier of potentially nephrotoxic molecules. Lab Invest 85: 522–531, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Pelsers MM Fatty acid-binding protein as marker for renal injury. Scand J Clin Lab Invest Suppl 241: 73–77, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, Noiri E, Devarajan P. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int 73: 465–472, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Reyes H, Levi AJ, Gatmaitan Z, Arias IM. Studies of Y and Z, two hepatic cytoplasmic organic anion-binding proteins: effect of drugs, chemicals, hormones, and cholestasis. J Clin Invest 50: 2242–2252, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105: 2259–2264, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2: 363–366, 1968. [DOI] [PubMed] [Google Scholar]
- 47.Russell JA, Singer J, Bernard GR, Wheeler A, Fulkerson W, Hudson L, Schein R, Summer W, Wright P, Walley KR. Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit Care Med 28: 3405–3411, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Sweetser DA, Lowe JB, Gordon JI. The nucleotide sequence of the rat liver fatty acid-binding protein gene. Evidence that exon 1 encodes an oligopeptide domain shared by a family of proteins which bind hydrophobic ligands. J Biol Chem 261: 5553–5561, 1986. [PubMed] [Google Scholar]
- 49.Tanaka T, Noiri E, Negishi K, Maeda R, Sugaya T, Fujita T. Urinary L-type fatty acid binding protein (L-FABP) is a sensitive biomarker in adenine induced cardiorenal anemia syndrome (CRAS) model (Abstract). J Am Soc Nephrol S18: S187A, 2007. [Google Scholar]
- 50.Tanaka T, Noiri E, Yamamoto T, Sugaya T, Negishi K, Maeda R, Nakamura K, Portilla D, Goto M, Fujita T. Urinary human L-FABP is a potential biomarker to predict COX-inhibitor-induced renal injury. Nephron Exp Nephrol 108: e19–e26, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Thurman JM, Parikh CR. Peeking into the black box: new biomarkers for acute kidney injury. Kidney Int 73: 379–381, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Veerkamp JH, Paulussen RJ, Peeters RA, Maatman RG, van Moerkerk HT, van Kuppevelt TH. Detection, tissue distribution and (sub)cellular localization of fatty acid-binding protein types. Mol Cell Biochem 98: 11–18, 1990. [DOI] [PubMed] [Google Scholar]
- 54.Veerkamp JH, Peeters RA, Maatman RG. Structural and functional features of different types of cytoplasmic fatty acid-binding proteins. Biochim Biophys Acta 1081: 1–24, 1991. [DOI] [PubMed] [Google Scholar]
- 55.Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 105: 485–491, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Weinberg JM Lipotoxicity. Kidney Int 70: 1560–1566, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Wong MG, Suzuki Y, Tanifuji C, Akiba H, Okumura K, Sugaya T, Yamamoto T, Horikoshi S, Tan SY, Pollock C, Tomino Y. Peritubular ischemia contributes more to tubular damage than proteinuria in immune-mediated glomerulonephritis. J Am Soc Nephrol 19: 290–297, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu Rev Med 59: 1–12, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Wyngaarden JB, Dunn JT. 8-Hydroxyadenine as the intermediate in the oxidation of adenine to 2,8-dihydroxyadenine by xanthine oxidase. Arch Biochem Biophys 70: 150–156, 1957. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, Nakamura K, Goto M, Shinozaki N, Ohshima S, Sugaya T. Renal L-type fatty acid binding protein in acute ischemic injury. J Am Soc Nephrol 18: 2894–2902, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto T, Tada T, Brodsky SV, Tanaka H, Noiri E, Kajiya F, Goligorsky MS. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am J Physiol Renal Physiol 282: F1150–F1155, 2002. [DOI] [PubMed] [Google Scholar]