Abstract
Diabetic nephropathy, the leading cause of end-stage renal disease, is characterized by a proapoptotic and prooxidative environment. The mechanisms by which lifestyle interventions, such as exercise, benefit diabetic nephropathy are unknown. We hypothesized that exercise inhibits early diabetic nephropathy via attenuation of the mitochondrial apoptotic pathway and oxidative damage. Type 2 diabetic db/db and normoglycemic wild-type mice were exercised for an hour everyday at a moderate intensity for 7 wk, following which renal function, morphology, apoptotic signaling, and oxidative stress were evaluated. Exercise reduced body weight, albuminuria, and pathological glomerular expansion in db/db mice independent of hyperglycemic status. Changes in renal morphology were also related to reduced caspase-3 (main effector caspase in renal apoptosis), caspase-8 (main initiator caspase of the “extrinsic” pathway) activities, and TNF-α expression. A role for the mitochondrial apoptotic pathway was unlikely as both caspase-9 activity (initiator caspase of this pathway) and expression of regulatory proteins such as Bax and Bcl-2 were unchanged. Kidneys from db/db mice also produced higher levels of superoxides and had greater oxidative damage concurrent with downregulation of superoxide dismutase (SOD) 1 and 3. Interestingly, although exercise also increased superoxides, there was also upregulation of multiple SODs that likely inhibited lipid (hydroperoxides) and protein (carbonyls and nitrotyrosine) oxidation in db/db kidneys. In conclusion, exercise can inhibit progression of early diabetic nephropathy independent of hyperglycemia. Reductions in caspase-3 and caspase-8 activities, with parallel improvements in SOD expression and reduced oxidative damage, could underlie the beneficial effects of exercise in diabetic kidney disease.
Keywords: kidney, superoxide dismutase
diabetic nephropathy is the leading cause of end-stage renal disease and is an important cause of increased morbidity and mortality in type 2 diabetic patients (41). A frequently used animal model of type 2 diabetes is the db/db mouse, which has a point mutation in the leptin receptor gene that leads to hyperphagia and obesity with progressive hyperglycemia, hyperinsulinemia, and insulin resistance (35, 36). Renal abnormalities in diabetic db/db mice, such as glomerular hypertrophy, proteinuria, and glomerulosclerosis, resemble human diabetic nephropathy, making the db/db mouse a useful model for investigating the pathogenesis and therapeutic intervention in this disease (8, 30, 50).
Lifestyle modifications such as exercise and caloric restriction are the cornerstones of management of type 2 diabetes and are significantly more effective than pharmacological management of glucose reduction alone (28). Although exercise is often contraindicated in end-stage renal disease, it does attenuate early stages of nephropathy in Zucker diabetic rats (46).
Deregulated programmed cell death or apoptosis occurs in humans and animal models of diabetic renal diseases and ranges across multiple cell types including podocytes, mesangial cells, and the endothelium (9, 31, 34). Regardless of the site of occurrence, apoptosis causes changes in renal structure and function and is mediated by caspases, a family of cysteine aspartate-specific proteases, and reactive oxygen species (ROS)-mediated oxidative stress (9, 25, 43). The “intrinsic” or the mitochondrial pathway of apoptosis, involving the release of cytochrome c, and sequential activation of caspase-9 and -3, is thought to be important in the kidneys during diabetes (34). As the effects of lifestyle modification on renal proapoptotic signaling in diabetes are unknown, we examined the hypothesis that exercise prevents diabetic nephropathy via attenuation of the mitochondrial apoptotic pathway, via a reduction in caspase activities, and oxidative damage in db/db mice.
MATERIALS AND METHODS
Experimental animals and exercise protocol.
The study protocols were approved by the University of British Columbia Laboratory Animal Care Advisory Committee. Twenty 4-wk-old male diabetic db/db (BKS.cg-m +/+ Leprdb/J) and age-matched wild-type (WT) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The BKS.cg-m +/+ Leprdb/J mouse is obese and diabetic by 8 wk of age. Mice were housed five per cage with a 12:12-h light-dark cycle and temperature control (26°C). Animals had free access to food and water and were weighed weekly. At 5 wk of age, db/db and WT mice were randomly assigned to sedentary or exercised (Exe) groups as described previously (36). The exercised groups underwent 1-h exercise in a motorized exercise wheel system (Lafayette Instrument) at a speed of 5.2 meters/min. Mice were exercised for 1 h/day, 5 days a week at a set time each day for 7 wk. Sedentary WT and db/db mice were placed in nonrotating wheels for the same duration as the exercised mice.
Plasma and urine parameters.
Animals were euthanized by injection of an overdose of pentobarbital sodium (Somnotol; 50 mg/kg ip) and heparin sodium (50 U/kg ip). Blood samples were taken from the inferior vena cava and immediately dispensed into tubes (Microtainer brand tube with EDTA, Becton Dickinson) and centrifuged (8,000 rpm) for 10 min at 4°C for plasma generation. Plasma and urine samples were transferred into separate Eppendorff tubes and stored at −76°C until analysis. Plasma glucose levels were determined by Trinder assay using a commercial kit purchased from Diagnostic Chemicals Limited (Oxford, CT). Plasma insulin levels were measured using a Mercodia Ultrasensitive Mouse Insulin Assay Kit from Alpco (Salem, NH). Urinary creatinine concentration was measured by HPLC using the method of Dunn et al. (12) with the following modifications: 1) the column was Zorbax 300-SCX, 5 μm, 2.1 × 150 mm; 2) the mobile phase was 7.5 mM sodium acetate adjusted to pH 4.4 ± 0.2 with acetic acid (total acetate ∼15 mM) at a flow rate of 0.50 ml/min (isocratic); 3) the column temperature was 40°C; and 4) and the sample injection volume was 3.0 μl. Urine was diluted 1:25 and assayed for creatinine by HPLC directly without deproteinization. Urine albumin was measured using a commercial ELISA kit (Albuwell-M from Exocel, Philadelphia, PA). The ratio of concentrations of albumin (μg) to creatinine (mg) in urine specimens was used as an index of urinary albumin excretion.
Citrate synthase assay.
To document the presence of an endurance-trained state, citrate synthase activity assays were performed on skeletal muscles. After the animals were killed, thigh adductor muscles were gently removed and frozen before citrate synthase activity was measured as previously described (29). Citrate synthase activity is expressed as micromoles per minute per milligram protein of the extract (measured by Bradford protein assay).
Tissue processing and histological evaluation.
Kidneys were fixed in 4% buffered formaldehyde, embedded in paraffin, and cut into 5-μm-thick sections. Sections were stained with periodic acid-Schiff (PAS) and representative renal corpuscles were photographed with an Olympus BX51 light microscope fitted with a Retiga 2000R digital camera. Using the method described by Qi et al. (40), the degree of mesangial matrix was semiquantitatively scored by a renal pathologist blinded to group identity. Fifty glomeruli per kidney per mouse were examined. The increase in mesangial matrix (also called mesangial area) was defined by PAS-positive and nuclei-free area in the mesangium. The glomerular area was calculated along the borders of capillary loop (30). The severity of matrix formation was scored from 0 to 4 where 0 = (normal glomerulus), 1 = (local, small lesions up to 25% of glomerular volume), 2 = (diffuse expansion 25–49%), 3 = (50–75%), and 4 = (greater than 75%). The number of glomeruli (out of 50 per kidney) exhibiting each kind of matrix formation was calculated and plotted.
Caspase activities.
Activities of renal caspase-3, -8, and -9 were determined using the corresponding fluorescent caspase-specific substrates Asp-Val-Glu-Asp (DEVD)-7-amino-4-trifluoromethylcoumarin (AFC; Anaspec), Ile-Glu-Thr-Asp (IETD)-AFC, and Leu-Glu-His-Asp (LEHD)-AFC (Calbiochem), respectively. Briefly, 50–75 μg of total protein were added to the reaction buffer, which contained 50 μM of the respective substrate, and incubated at 37°C for 1 h. Ac-DEVD-CHO (Anaspec), Ac-IETD-CHO, and Ac-LEHD-CHO (Calbiochem) were used as specific inhibitors of caspase-3, -8, and -9, respectively. The enzyme-catalyzed release of AFC was quantified in a fluorimeter at 380/500-nm wavelengths (14).
Superoxide generation.
Estimates of superoxide generating capacity in diabetic kidneys were performed using dihydroethidium (DHE). DHE is a blue fluorescent dye that specifically is oxidized by intracellular superoxide and then converted to a red fluorescent compound ethidium, which intercalates with double-strand DNA to create punctate nuclear staining. Briefly, flash-frozen kidneys were sectioned at 20 μm and incubated with 2 μM DHE for 30 min in a nitrogen chamber. Images were taken on a Leica inverted confocal microscope, equipped with a Ar/ArKr laser (Ex: 515 nm/Em: 605 nm). A total of four animals per group was analyzed, with two slides from each animal. Seven images were generated per slide and were analyzed with Velocity software. After image intensity per image was calculated, the signal intensity was divided by the captured area (13). The values are designated as relative DHE staining density and expressed in arbitrary units (AU).
Protein expression by Western blot.
Kidneys were cut into small pieces and homogenized in ice-cold homogenization buffer and Western blotting was performed as described elsewhere (36). Membranes were incubated with primary antibodies raised in rabbit [caspase-3 (Cell Signaling), superoxide dismutase (SOD) 3, Bax, BCl-2 (Santa Cruz Biotechnology)], sheep (SOD 1 and 2; Calbiochem, San Diego, CA), goat (TNF-α), or mouse (GAPDH, nitrotyrosine; Cayman Chemicals) for 2 h at room temperature. Goat anti-rabbit, donkey anti-sheep, donkey anti-goat, or goat anti-mouse horseradish peroxidase (HR)-conjugated antibodies were used as secondary antibodies and visualized using electrochemiluminescent (ECL) detection kit. Values were expressed as AU per milligram of protein. GAPDH was used as a loading control.
Lipid hydroperoxide assay.
Oxidative stress-induced lipid peroxidation was estimated by direct measurement of lipid hydroperoxides in kidneys using a commercially available kit (Cayman Chemicals) as described previously (14).
Protein carbonyl levels.
Protein carbonyls were assayed as an index of oxidative modification of proteins. Briefly, 50 μl of the plasma fractions were added to an equal volume of 10% trichloroacetic acid (TCA), centrifuged at 6,000 g at 4°C for 5 min, and the supernatant was discarded. The precipitated proteins were resuspended in 0.2% 2,4-dinitrophenyl hydrazine and incubated for 1 h at 37°C. Subsequently, proteins were precipitated again with TCA, centrifuged, washed with ethanol:ethyl acetate, dissolved in 6 mM guanidine hydrochloride, and the absorbance was measured spectrophotometrically at 370 nm (14).
Statistical analysis and calculations.
Results of all calculations are expressed as means ± SE. Data were analyzed using NCSS-2000 computer software. Repeated-measures ANOVA with multiple comparisons using Bonferroni's test or one-way ANOVA was performed where appropriate. GraphPad Prism (version 3.02–2000) was used for linear regression, curve fitting, and dose-response analysis. The results of statistical tests were considered significant if P values were <0.05.
RESULTS
General characteristics of mice.
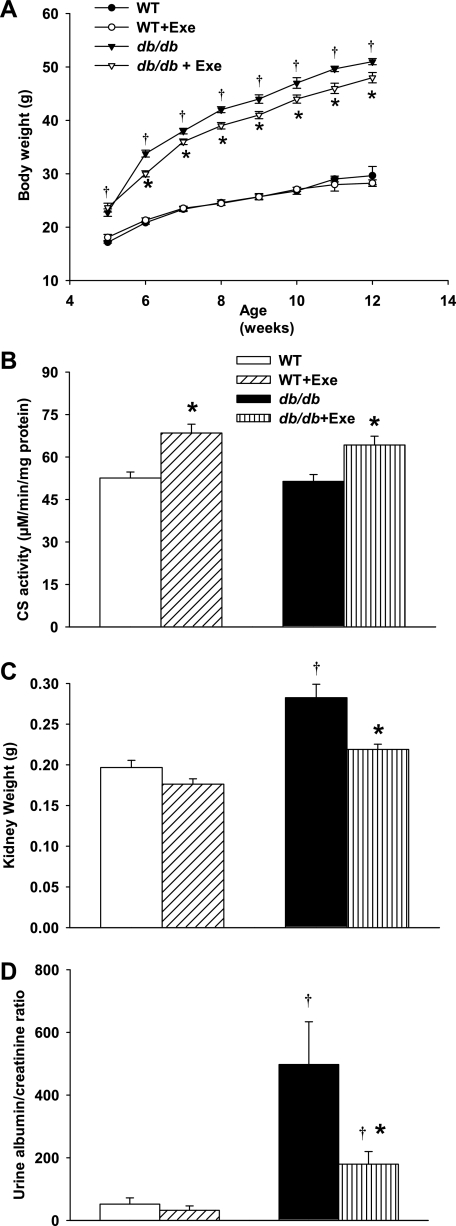
Sedentary db/db mice were obese at all time points, with a mean body weight that was significantly greater than age-matched WT mice throughout the experimental period (Fig. 1A). Exercise caused modest but significant decreases in body weight in db/db mice within 1 wk (6 wk of age) that continued for the following 6 wk and onwards (final age 12 wk). The body weights of WT mice were not affected by exercise (Fig. 1A). As an indicator of efficient exercise and higher metabolic activity, citrate synthase activity in the thigh adductor muscles of the mice (20) was increased by exercise in both WT and db/db groups (Fig. 1B). Plasma glucose and insulin concentrations in db/db mice were significantly elevated compared with WT mice (Table 1). Moderate exercise did not affect plasma glucose or insulin in either WT or db/db mice.
Fig. 1.
Exercise-related changes in body weight, citrate synthase (CS), and kidney parameters. A: db/db mice exercised with moderate intensity gained less weight compared with their sedentary littermates. This difference was apparent from 6 wk of age onward (n = 8–10 per group). Weight gain in wild-type (WT) mice was not affected by exercise. B: CS activity was used to monitor exercise-induced changes in metabolic activity in thigh adductor muscles. Both exercised db/db and WT groups demonstrated greater CS activity at all time points (n = 9–10 per group). C: sedentary db/db mice had higher kidney weights compared with WT. Exercise decreased kidney weights in db/db mice to WT levels after 7 wk. D: urinary albumin/creatinine (Cr) ratio is significantly higher in db/db mice compared with WT mice. Exercise significantly decreased this ratio in db/db mice, implicating improved renal function (n = 5–7 per group). Exe, exercise. Results are means ± SE. *Significantly different from sedentary littermate. †Significantly different from corresponding WT group, P < 0.05.
Table 1.
General parameters of animals
| Parameters | WT | WT + Exe | db/db | db/db + Exe |
|---|---|---|---|---|
| Plasma glucose, mg/dl | 185.3±6.4 | 177.5±2.4 | 834.6±83.8* | 734.8±48.6* |
| Plasma insulin, μmol/l | 2.1±0.6 | 1.1±0.3 | 3.6±0.5* | 3.7±0.7* |
Values are means ± SE for 6–8 mice in each group. Parameters were measured at 12 wk, at the time of death, and are from fed animals.
Significantly different from wild-type (WT) mice, P < 0.05. Exe, exercise.
Biochemical and morphological features in diabetic renal disease.
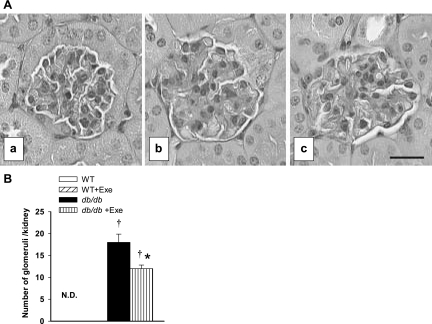
Kidney weights at 12 wk of age in db/db mice were significantly higher than in WT mice. Exercise specifically lowered kidney weights in db/db mice (Fig. 1C). To evaluate the effects of exercise on renal functional abnormalities in db/db mice, urinary albumin/creatinine ratios in urine samples were used as an index of urinary albumin excretion. The urinary albumin/creatinine ratio was significantly higher in db/db sedentary mice compared with their WT counterparts (Fig. 1D). Exercise specifically decreased the albumin/creatinine ratio in db/db mice but the difference in ratio between this group and WT mice was not significant (Fig. 1D). In the histological sections, glomerular grade III mesangial expansion was significantly greater in sedentary db/db mice compared with the WT mice (Fig. 2B). Exercise significantly improved this mesangial expansion in db/db mice (Fig. 2B). Representative renal corpuscles from sedentary WT (2Aa), sedentary db/db (2Ab), and exercised db/db (2Ac) mice are shown in Fig. 2A. Exercised WT and sedentary WT had similar renal corpuscles (data not shown).
Fig. 2.
Exercise-related changes in kidney morphology. A: light microscopic appearance of glomeruli from male nondiabetic (WT) mice (a) and diabetic (db/db) mice (b) and diabetic mice exercised with moderate intensity (c) at 12 wk of age. There is diffuse mesangial matrix expansion and evidence of arteriolar hyalinosis (b) in db/db mice. Exercise prevented mesangial matrix expansion and arteriolar hyalinosis in db/db glomerulus (c). Renal tissue was fixed with 10% neutral buffered formalin, and 4-μm sections were stained with periodic acid-Schiff. Images are taken at ×400 magnification. B: pathological grade III mesangial expansion is not detectable (N.D.) in both WT groups, whereas db/db kidneys demonstrated significant mesangial expansion. Exercise significantly reduced such expansion (n = 5 per group). Results are means ± SE. *Significantly different from sedentary littermate. †Significantly different from corresponding WT group, P < 0.05.
Factors controlling caspases.
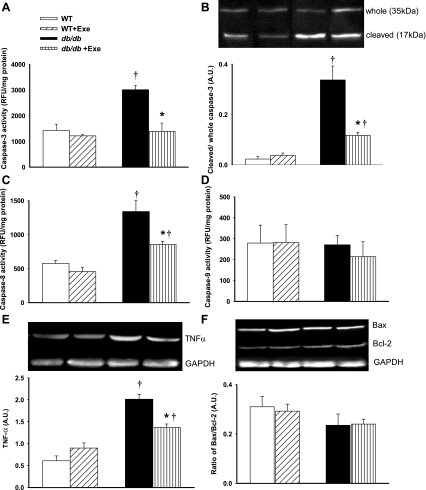
Caspase activities in the kidneys from db/db and WT mice were used to study the renal apoptotic process. Caspase-3 is the primary downstream effector caspase in the apoptotic process. Both caspase-3 activity (Fig. 3A) and processing (Fig. 3B, appearance of 17-kDa fragment) increased in db/db kidneys. These elevations were significantly reduced with exercise. Among the initiators of caspase-3 activity, caspase-8 mediates the “death receptor”-mediated pathway, whereas caspase-9 is the initiator caspase of the intrinsic mitochondrial apoptotic pathway. To our surprise, although caspase-8 activity increased in the db/db kidney (Fig. 3C), caspase-9 was unchanged (Fig. 3D). Exercise decreased caspase-8 activity in db/db mice. To further investigate factors associated with apoptosis, we examined the expression of TNF-α, Bax, and Bcl-2, which are suggested to be dysregulated in diabetic cell death (5, 7). The increased levels of the proinflammatory cytokine TNF-α in db/db kidneys were attenuated by exercise (Fig. 3E). Levels of Bax and Bcl-2, which are pro- and anti-apoptotic proteins, respectively, remained unchanged in both db/db and WT kidneys and were unaffected by exercise (Fig. 3F).
Fig. 3.
Renal apoptotic signaling in response to diabetes and exercise. A: chief effector caspase, caspase-3, activity is increased in diabetic kidneys and is reduced to WT levels with exercise. B: further evidence of renal caspase-3 activation as evidenced by enhanced caspase-3 processing (ratio of the “cleaved” 17-kDa fragment to 35-kDa whole caspase-3 expression) in diabetes. Exercise decreased caspase-3 activation in db/db kidneys. Representative Western blots are depicted in the inset. C: enhanced activity of caspase-8, the initiator caspase of the “extrinsic” pathway in diabetic kidneys. Exercise also decreases caspase-8 activity. D: caspase-9 activity, the initiator caspase of the mitochondrial “intrinsic” pathway, is unchanged by exercise in all groups. E: protein expression of TNF-α, a proinflammatory cytokine implicated in the activation of caspase-8, was increased in db/db kidneys and reduced by exercise. Representative Western blots are depicted in the inset. F: ratio between protein expression of Bax, a proapoptotic protein, and BCl-2, an anti-apoptotic protein, remained unchanged in all groups. Representative Western blots are depicted in the inset. AU, arbitrary units; RFU, relative fluorescence units. Results are means ± SE. *Significantly different from sedentary littermate. †Significantly different from corresponding WT group, P < 0.05.
Renal oxidative stress-related parameters.
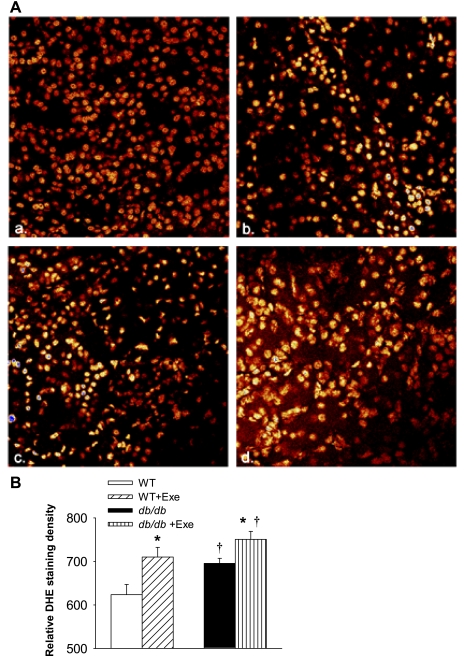
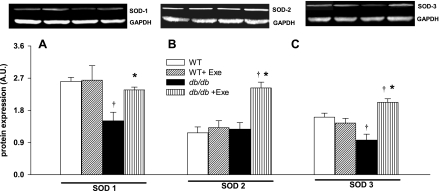
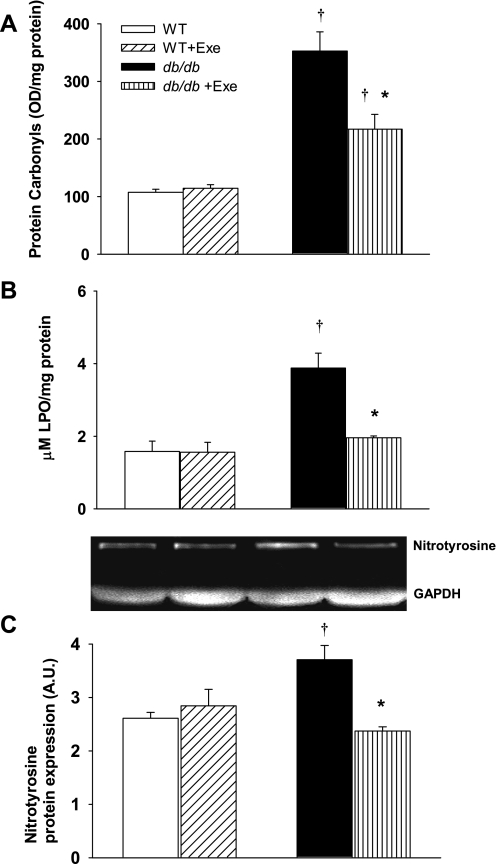
Renal superoxide generation was determined in WT and db/db kidneys using DHE staining. Compared with sedentary WT (red nuclei in micrographs in Fig. 4Aa), db/db mice had higher in vitro renal superoxide generation, and exercise increased ROS production in both groups (yellow nuclei in micrographs in Fig. 4A, b, c, d) and quantitatively expressed as staining density in Fig. 4B. Superoxides are neutralized in vivo by three SODs, with SOD 1 (cytoplasmic) and SOD 3 (extracellular) both requiring Cu/Zn as cofactors while mitochondrial SOD 2 utilizes manganese as a cofactor. Protein expression of SOD 1 and 3 was specifically decreased in db/db mice (Fig. 5, A and C). Exercise did not affect SOD levels in WT kidneys, but increased the expression of all three SOD isoforms in db/db kidneys (Fig. 5). Levels of protein carbonyls and lipid hydroperoxides, products of protein and lipid peroxidation due to oxidative stress, respectively, were increased in db/db kidneys (Fig. 6, A and B). Exercise further increased renal protein and lipid hydroperoxides in db/db but not WT kidneys (Fig. 6, A and B). Peroxynitrite, which is a combination product of nitric oxide and superoxide, is an important mediator of diabetic oxidative kidney damage (2, 39) in part by causing nitration of tyrosine residues within cellular proteins leading to the formation of nitrotyrosine (39, 48). Levels of nitrotyrosine (a biomarker for peroxynitrite) were increased in the kidneys of sedentary db/db mice while exercise normalized nitrotyrosine expression in db/db mice (Fig. 6C).
Fig. 4.
Exercise and diabetes induced changes in ex vivo superoxide generation. Renal superoxide generation as measured by dihydroethidium (DHE) staining of nuclei. A: representative micrographs from WT (a), WT + Exe (b), db/db (c), and db/db + Exe (d) are depicted. Cells with mild reactive oxygen species (ROS) have red-stained nuclei, whereas cells with intense ROS have yellow-stained nuclei. Sedentary WT mice (a) have minimal ROS production (mostly red nuclei), whereas exercise in WT mice (b) increases renal ROS generation (increased number of yellow nuclei). Sedentary db/db mice (c) also have higher renal ROS generation than sedentary WT, with exercise further increasing ROS generation in db/db mice (intense yellow nuclei in d). Images were taken on an inverted confocal microscope, equipped with a Ar/ArKr laser (Ex: 515 nm/Em: 605 nm). A total of 4 animals per group was analyzed, with 2 slides from each animal and 7 images per slide. After image intensity per image was calculated, the signal intensity was divided by the captured area and expressed (B) as DHE staining intensity. Results are means ± SE. *Significantly different from sedentary littermate. †Significantly different from corresponding WT group, P < 0.05.
Fig. 5.
Differential regulation of superoxide dismutase (SOD) isoforms following diabetes and exercise. Representative Western blots are depicted in the inset. Cytosolic SOD 1 (A), mitochondrial SOD 2 (B), and extracellular SOD 3 (C) were evaluated. SOD 1 and 3 decreased in db/db kidneys. Exercise increased protein expression of all SODs in db/db kidneys. Results are means ± SE. *Significantly different from sedentary littermate. †Significantly different from corresponding WT group, P < 0.05.
Fig. 6.
Exercise and diabetes-related changes on renal oxidative stress. A: protein carbonyl levels as a measure of nonspecific protein oxidation. B: lipid hydroperoxide levels as a measure for lipid peroxidation. C: peroxinitrite-induced protein nitration as measured by nitrotyrosine protein expression. Diabetes increased oxidative lipid, protein, and nitrotyrosine formation, all of which were reduced with exercise. Representative Western blots are depicted in the inset. LPO, lipid hydroperoxide. Results are means ± SE. *Significantly different from sedentary littermate. †Significantly different from corresponding WT group, P < 0.05.
DISCUSSION
In this study, we provide novel evidence on the differential regulation of kidney caspases and oxidative stress-related parameters during exercise-induced improvements in kidney morphology and function. These changes occurred without alterations in plasma glucose and insulin levels in db/db mice, as we reported in related studies on the vascular benefits of exercise in diabetic mice (35, 36).
In this study, we report reductions in renal mass with exercise in diabetes, which may be related to amelioration of renal hypertrophy reported in the db/db mice (8). Furthermore, albuminuria, associated with progressive diabetic nephropathy (8), was also decreased as evidenced by the normalization of the urinary albumin/creatinine ratio in exercised db/db mice. Next, we investigated the hypothesis that exercise attenuates mesangial matrix expansion in diabetic mice. Mesangial matrix expansion is considered a pathologic feature of diabetic nephropathy in both humans and the db/db mice (8). It was previously reported that in db/db mice, increased albumin excretion appears soon after the establishment of hyperglycemia and is followed several weeks later by pronounced glomerular mesangial matrix expansion (11). Hyperglycemia leads to glomerular hypertrophy and diffuse glomerulosclerosis characterized by increases in mesangial matrix and thickening of the glomerular basement membrane (6). In db/db mice, mesangial matrix expansion starts at 8 wk of age, is increased by twofold at 12 wk of age, and by 16 wk of age, it has increased by threefold (8). Mesangial expansion reduces the surface area of glomerular capillaries available for filtration and leads to decreased glomerular filtration (42). We show that moderate levels of exercise significantly decreased grade III matrix mesangial expansion evident in db/db mice.
Loss of podocytes via apoptosis and oxidative stress triggers mesangial expansion and underlies the etiology of diabetic kidney disease in the db/db mouse (43). Podocytes are specialized cells on the glomerular basement membrane that control the structure and function of the glomerular filtration apparatus. Podocyte loss by apoptosis is a predictor of albuminuria in both humans and animal models of diabetes (37, 43). Thus, the loss of podocytes by apoptosis not only leads to abnormal leakage of protein in the urine (proteinuria), but it also triggers mesangial expansion in an attempt to reduce filtration surface area (47). However, this simplistic view of podocyte apoptosis is complicated by findings that mesangial cells (25), proximal tubule epithelial cells (18), and endothelial cells (4) all exhibit apoptosis under hyperglycemic conditions. Thus, we monitored the impact of exercise on the global proapoptotic environment in the diabetic kidney, as determined by caspase activities. In our study, the inhibition of mesangial expansion and normalization of albuminuria suggest that exercise was able to at least partially reduce the proapoptotic environment in diabetic kidneys.
Accurate estimation of apoptosis in the diabetic kidney is complicated by the high clearance rates and loss of apoptotic cells in the urine (33). Thus, estimation of caspase-3 activity/processing may have higher specificity for the prediction of an apoptotic environment than the more traditional nuclear fragmentation (TUNEL) method, which also detects necrotic cell populations (33). We report that both caspase-3 processing and caspase-3 activity were increased in diabetic kidneys, both of which were substantially reduced by moderate levels of exercise. This is the first report of a beneficial role of exercise on caspase-3 activity, the primary executioner caspase involved in apoptosis in diabetic mouse kidneys (33, 34).
A number of pathways modulate the activation of caspase-3 (26). The first involves activation of caspase-3 via death receptor-mediated activation of upstream caspases such as caspase-8 (“extrinsic” pathway) (3, 26). In our study, caspase-8 activity was increased in parallel with caspase-3 activity and was downregulated by exercise. We measured the protein expression levels of TNF-α, an activator of the death receptor pathway commonly found in diabetic kidneys (24). Apart from causing sodium retention and renal hypertrophy in the kidney, TNF-α also directly activates caspase-8 (26, 45). The upregulation of TNF-α during diabetes and its subsequent reduction by exercise may be due to the anti-inflammatory effects of a loss in body weight in exercised db/db mice (38).
The second pathway of caspase-3 activation is the intrinsic or the mitochondrial pathway of apoptosis (15, 26) and involves damage to the mitochondria, followed by the release of apoptogenic proteins such as cytochrome c, which then facilitate apoptosome-mediated caspase-9 activation. Bax upregulation and translocation to mitochondria promote cytochrome c release while overexpression of Bcl-2 prevents cytochrome c release and subsequently caspase-9 activation. Their relative abundance via homo- or heterodimerization at the mitochondrial membrane determines the probability of cytochrome c release from the intermembrane space and activation of caspase-9 and, ultimately, caspase-3 (10, 15). This pathway is activated in mesangial cells under hyperglycemic conditions (34). However, in our study, caspase-9 activity was not altered in the kidneys of either db/db or WT mice. This could be due to a number of reasons. First, we detected appreciable caspase-9 activity and cleavage only in 16-wk-old db/db kidneys, which could be the result of caspase-8 directly activating caspase-9 in the presence of prolonged exposure to TNF-α (17). Second, the Western blots in some studies were normalized against β-actin (34), which is known to be degraded by caspase-3 under proapoptotic conditions to artificially augment caspase-9 cleavage (49). Third, unlike our findings, Bax levels were augmented at 16 wk in some studies (34), which in itself can lead to the release of cytochrome c and activation of caspase-9.
Irrespective of the caspases or cell types involved, oxidative stress plays a central role in diabetic kidney disease (2, 9, 33, 39, 43, 48). Oxidative stress results from an imbalance between the production of free radicals such as superoxide and their removal by available antioxidants. Increased free radical producion may lead to lipid and protein oxidation, which we measured in this study via evaluation of lipid hydroperoxides and protein carbonyls, respectively. We used DHE staining of db/db kidneys in vitro to determine ex vivo superoxide generation capacity. Superoxide generation was increased in sedentary db/db kidneys, as previously reported in rodent models of this disease (21, 32). Cellular antioxidants such as SODs quench such free radical bursts and reduce superoxide generation in vivo. Lower levels of cytosolic SOD 1 levels contribute to diabetic nephropathy (1) and may explain the increased oxidative tissue damage we observed in sedentary db/db kidneys (which generate more superoxides). On a similar note, a drop in SOD 3, which is an extracellular form of Cu/Zu SOD sharing ∼50% sequence homology with SOD 1 within its catalytic site, could also be partially responsible for such oxidative kidney damage (19, 27).
Our study also indicates that exercise increases renal superoxide production in both WT and db/db mice, similar to findings reported in skeletal muscle (23). However, oxidative stress in exercised WT kidneys was unaffected by increased superoxide generation, possibly due to the sustained SOD levels which are capable of quenching excess superoxide. Diabetes led to modest increases in superoxide generation, but a large increase in all oxidized metabolites such as lipid hydroperoxides, protein carbonyls, and nitrotyrosine. This could be partly attributed to a loss in SOD 1 and SOD 3 protein expression in the kidneys. Similarly, although superoxide generation may have increased further with exercise in db/db mice, the amelioration of oxidative stress in this group could be attributed to increased expression of all three isoforms of SOD; which would provide an increased capacity to mitigate oxidative challenges due to any changes in either extra- or intracellular free radical generation (32). The increase in SODs occurring with exercise is supported by other studies linking greater SOD expression in response to an increased generation of superoxide (16, 23). Increased levels of superoxides scavenge nitric oxide to form peroxynitrite, which causes extensive oxidative damage in diabetic kidneys (21, 39, 48). Nitrotyrosine, a biomarker for peroxynitrite activity, was increased in the kidneys of sedentary db/db mice (21) and was reduced to control levels by exercise, an effect that can also be attributed to the increased expression of SODs in exercised db/db mice.
In conclusion, we demonstrate that exercising db/db mice at a moderate intensity for 7 wk reduced mesangial matrix expansion and albuminuria, lowered caspase-3 and -8 activities, while also improving SOD expression and blunting renal oxidative damage. Importantly, normalization of plasma glucose levels in diabetic patients with hypoglycemic treatment also increases platelet SOD levels in humans (22) with similar effects in the kidneys of rodent models of diabetic nephropathy (44). In this study, exercising diabetic animals also improved renal function and increased renal SOD levels without changes in plasma glucose or insulin status. Thus, low-intensity exercise augments antioxidant status independent of changes in hyperglycemia. This effect of exercise on renal SOD and downregulation of caspases may underlie beneficial effects via the attenuation of early diabetic kidney disease by lifestyle modifications (16).
GRANTS
This work was funded in part through grants and fellowships from the Heart and Stroke Foundation of Canada (I. Laher, D. J. Granville), Canadian Institutes for Health Research (D. J. Granville, L. S. Ang, S. Ghosh), Canadian Diabetes Association (C. B. Verchere, S. Ghosh), Michael Smith Foundation for Health Research (L. S. Ang, S. Ghosh), and National Institute of Diabetes and Digestive and Kidney Diseases Grants U01-DK-076133 and R01-DK-53867 (K. Sharma).
Acknowledgments
The technical assistance of G. Qiu is much appreciated.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Al-Kateb H, Boright AP, Mirea L, Xie X, Sutradhar R, Mowjoodi A, Bharaj B, Liu M, Bucksa JM, Arends VL, Steffes MW, Cleary PA, Sun W, Lachin JM, Thorner PS, Ho M, McKnight AJ, Maxwell AP, Savage DA, Kidd KK, Kidd JR, Speed WC, Orchard TJ, Miller RG, Sun L, Bull SB, Paterson AD. Multiple superoxide dismutase 1/splicing factor serine alanine 15 variants are associated with the development and progression of diabetic nephropathy: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Genetics study. Diabetes 57: 218–228, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen DA, Harwood S, Varagunam M, Raftery MJ, Yaqoob MM. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J 17: 908–910, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Baba K, Minatoguchi S, Sano H, Kagawa T, Murata I, Takemura G, Hirano T, Ohashi H, Takemura M, Fujiwara T, Fujiwara H. Involvement of apoptosis in patients with diabetic nephropathy: a study on plasma soluble Fas levels and pathological findings. Nephrology (Carlton) 9: 94–99, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner-Parzer SM, Wagner L, Pettermann M, Grillari J, Gessl A, Waldhausl W. High-glucose-triggered apoptosis in cultured endothelial cells. Diabetes 44: 1323–1327, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol 172: 1411–1418, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilous RW, Mauer SM, Sutherland DE, Steffes MW. Mean glomerular volume and rate of development of diabetic nephropathy. Diabetes 38: 1142–1147, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Bojunga J, Nowak D, Mitrou PS, Hoelzer D, Zeuzem S, Chow KU. Antioxidative treatment prevents activation of death-receptor- and mitochondrion-dependent apoptosis in the hearts of diabetic rats. Diabetologia 47: 2072–2080, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Breyer MD, Bottinger E, Brosius FC 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Catherwood MA, Powell LA, Anderson P, McMaster D, Sharpe PC, Trimble ER. Glucose-induced oxidative stress in mesangial cells. Kidney Int 61: 599–608, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 18: 157–164, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MP, Sharma K, Jin Y, Hud E, Wu VY, Tomaszewski J, Ziyadeh FN. Prevention of diabetic nephropathy in db/db mice with glycated albumin antagonists. A novel treatment strategy. J Clin Invest 95: 2338–2345, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn SR, Qi Z, Bottinger EP, Breyer MD, Sharma K. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int 65: 1959–1967, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Elmi S, Sallam NA, Rahman MM, Teng X, Hunter AL, Moien-Afshari F, Khazaei M, Granville DJ, Laher I. Sulfaphenazole treatment restores endothelium-dependent vasodilation in diabetic mice. Vascul Pharmacol 48: 1–8, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, Pulinilkunnil T, Yuen G, Kewalramani G, An D, Qi D, Abrahani A, Rodrigues B. Cardiomyocyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am J Physiol Heart Circ Physiol 289: H768–H776, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Gobe G, Zhang XJ, Cuttle L, Pat B, Willgoss D, Hancock J, Barnard R, Endre RB. Bcl-2 genes and growth factors in the pathology of ischaemic acute renal failure. Immunol Cell Biol 77: 279–286, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44: 126–131, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Gyrd-Hansen M, Farkas T, Fehrenbacher N, Bastholm L, Hoyer-Hansen M, Elling F, Wallach D, Flavell R, Kroemer G, Nylandsted J, Jaattela M. Apoptosome-independent activation of the lysosomal cell death pathway by caspase-9. Mol Cell Biol 26: 7880–7891, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harwood SM, Allen DA, Raftery MJ, Yaqoob MM. High glucose initiates calpain-induced necrosis before apoptosis in LLC-PK1 cells. Kidney Int 71: 655–663, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Hjalmarsson K, Marklund SL, Engstrom A, Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci USA 84: 6340–6344, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holloszy JO, Oscai LB, Don IJ, Mole PA. Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun 40: 1368–1373, 1970. [DOI] [PubMed] [Google Scholar]
- 21.Ishii N, Patel KP, Lane PH, Taylor T, Bian K, Murad F, Pollock JS, Carmines PK. Nitric oxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus. J Am Soc Nephrol 12: 1630–1639, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Jennings PE, Belch JJ. Free radical scavenging activity of sulfonylureas: a clinical assessment of the effect of gliclazide. Metabolism 49: 23–26, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Ji LL Exercise-induced modulation of antioxidant defense. Ann NY Acad Sci 959: 82–92, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Kalantarinia K, Awad AS, Siragy HM. Urinary and renal interstitial concentrations of TNF-α increase prior to the rise in albuminuria in diabetic rats. Kidney Int 64: 1208–1213, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Kang BP, Frencher S, Reddy V, Kessler A, Malhotra A, Meggs LG. High glucose promotes mesangial cell apoptosis by oxidant-dependent mechanism. Am J Physiol Renal Physiol 284: F455–F466, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kaushal GP, Basnakian AG, Shah SV. Apoptotic pathways in ischemic acute renal failure. Kidney Int 66: 500–506, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Kimura F, Hasegawa G, Obayashi H, Adachi T, Hara H, Ohta M, Fukui M, Kitagawa Y, Park H, Nakamura N, Nakano K, Yoshikawa T. Serum extracellular superoxide dismutase in patients with type 2 diabetes: relationship to the development of micro- and macrovascular complications. Diabetes Care 26: 1246–1250, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korzick DH, Laughlin MH, Bowles DK. Alterations in PKC signaling underlie enhanced myogenic tone in exercise-trained porcine coronary resistance arteries. J Appl Physiol 96: 1425–1432, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa R. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J 14: 439–447, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem 259: 67–70, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Lin CL, Wang FS, Kuo YR, Huang YT, Huang HC, Sun YC, Kuo YH. Ras modulation of superoxide activates ERK-dependent fibronectin expression in diabetes-induced renal injuries. Kidney Int 69: 1593–1600, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Menini S, Iacobini C, Oddi G, Ricci C, Simonelli P, Fallucca S, Grattarola M, Pugliese F, Pesce C, Pugliese G. Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia 50: 2591–2599, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Mishra R, Emancipator SN, Kern T, Simonson MS. High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int 67: 82–93, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Moien-Afshari F, Ghosh S, Elmi S, Khazaei M, Rahman MM, Sallam N, Laher I. Exercise restores coronary vascular function independent of myogenic tone or hyperglycemic status in db/db mice. Am J Physiol Heart Circ Physiol 295: H1470–H1480, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Moien-Afshari F, Ghosh S, Khazaei M, Kieffer TJ, Brownsey RW, Laher I. Exercise restores endothelial function independently of weight loss or hyperglycemic status in db/db mice. Diabetologia 51: 1327–1337, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol 98: 1154–1162, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Prabhakar S, Starnes J, Shi S, Lonis B, Tran R. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J Am Soc Nephrol 18: 2945–2952, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Ruggenenti P, Remuzzi G. Nephropathy of type 2 diabetes mellitus. J Am Soc Nephrol 9: 2157–2169, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Steffes MW, Osterby R, Chavers B, Mauer SM. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes 38: 1077–1081, 1989. [DOI] [PubMed] [Google Scholar]
- 43.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006. [PubMed] [Google Scholar]
- 44.Tang Z, Shou I, Wang LN, Fukui M, Tomino Y. Effects of antihypertensive drugs or glycemic control on antioxidant enzyme activities in spontaneously hypertensive rats with diabetes. Nephron 76: 323–330, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell 133: 693–703, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Ward KM, Mahan JD, Sherman WM. Aerobic training and diabetic nephropathy in the obese Zucker rat. Ann Clin Lab Sci 24: 266–277, 1994. [PubMed] [Google Scholar]
- 47.Wiggins RC The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Xu S, Jiang B, Maitland KA, Bayat H, Gu J, Nadler JL, Corda S, Lavielle G, Verbeuren TJ, Zuccollo A, Cohen RA. The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes 55: 110–119, 2006. [PubMed] [Google Scholar]
- 49.Yang F, Sun X, Beech W, Teter B, Wu S, Sigel J, Vinters HV, Frautschy SA, Cole GM. Antibody to caspase-cleaved actin detects apoptosis in differentiated neuroblastoma and plaque-associated neurons and microglia in Alzheimer's disease. Am J Pathol 152: 379–389, 1998. [PMC free article] [PubMed] [Google Scholar]
- 50.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA 97: 8015–8020, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]