Abstract
The goal of this study was to identify and characterize the hypothesized apical cation/H+ exchanger responsible for K+ and/or Na+ secretion in the renal (Malpighian) tubules of the yellow fever mosquito Aedes aegypti. From Aedes Malpighian tubules, we cloned “AeNHE8,” a full-length cDNA encoding an ortholog of mammalian Na+/H+ exchanger 8 (NHE8). The expression of AeNHE8 transcripts is ubiquitous among mosquito tissues and is not enriched in Malpighian tubules. Western blots of Malpighian tubules suggest that AeNHE8 is expressed primarily as an intracellular protein, which was confirmed by immunohistochemical localizations in Malpighian tubules. AeNHE8 immunoreactivity is expressed in principal cells of the secretory, distal segments, where it localizes to a subapical compartment (e.g., vesicles or endosomes), but not in the apical brush border. Furthermore, feeding mosquitoes a blood meal or treating isolated tubules with dibutyryl-cAMP, both of which stimulate a natriuresis by Malpighian tubules, do not influence the intracellular localization of AeNHE8 in principal cells. When expressed heterologously in Xenopus laevis oocytes, AeNHE8 mediates EIPA-sensitive Na/H exchange, in which Li+ partially and K+ poorly replace Na+. The expression of AeNHE8 in Xenopus oocytes is associated with the development of a conductive pathway that closely resembles the known endogenous nonselective cation conductances of Xenopus oocytes. In conclusion, AeNHE8 does not mediate cation/H+ exchange in the apical membrane of Aedes Malpighian tubules; it is more likely involved with an intracellular function.
Keywords: Na/H exchange, Malpighian tubules, Xenopus laevis oocytes, electrophysiology
the renal (malpighian) tubules of insects actively secrete fluid to form urine. In the yellow fever mosquito Aedes aegypti (referred to as Aedes hereafter), the blind-ended (distal) segments of the Malpighian tubules transport both K+ and Na+ actively from the extracellular fluid (hemolymph) to the tubule lumen, with Cl− and water following their electrochemical and osmotic gradients, respectively (6, 8, 18). The primary urine then flows through the proximal segments of the tubule and into the distal digestive tract (hindgut and rectum), where the urine may be modified before its excretion.
Urine formation in distal segments of Aedes Malpighian tubules is primarily mediated by principal cells, which are the majority cell type of the tubule in terms of both abundance and mass (6, 15, 65). In Malpighian tubules of dipteran insects, such as Aedes and Drosophila melanogaster (hereafter referred to as Drosophila), the apical membrane of principal cells forms an elaborate, luminal brush border, with each microvillus housing a mitochondrion (5, 6, 8). The brush border is enriched with the V-type H+-ATPase (24, 55, 59, 81), which is thought to establish all of the electrochemical potentials that drive transcellular and paracellular electrolyte transport (5, 7).
The mechanisms that mediate the extrusion of K+ and Na+ across the apical membrane of principal cells have not yet been elucidated. Current models propose that a monovalent cation/H+ exchanger recycles luminal protons, pumped by the V-type H+-ATPase, in exchange for the extrusion of intracellular K+ and/or Na+ (6, 8, 18, 47, 60), analogous to the model proposed for the secretion of K+ (i.e., alkalinization) across the midgut of the tobacco hornworm Manduca sexta (31). The molecular identification of this exchanger has eluded many investigators. Present hypotheses consider the exchanger to be related to the cation proton antiporter (CPA) superfamily, which includes the CPA1 or solute carrier 9 (SLC9) family of Na/H exchangers (NHEs) and the CPA2 family of Na/H antiporters (NHAs). Supporting these hypotheses are the observations that amiloride and amiloride-based compounds inhibit 1) fluid secretion in isolated Malpighian tubules of dipterans (27, 32, 56) and 2) electrogenic K+/H+ antiport in vesicles derived from highly purified apical membranes of K+-secreting cells of the Manduca midgut epithelium (83). A recent study on Aedes Malpighian tubules by Kang'ethe and colleagues (34) has proposed that the apical cation/H+ exchanger is an ortholog of mammalian NHE8 (SLC9A8), whereas a more recent study on Drosophila Malpighian tubules by Day and colleagues (21) has proposed that the exchanger is an ortholog of bacterial NHAs [i.e., the bacterial K+ efflux (Kef) family].
Parallel to the studies by Kang'ethe et al. (34) and Day et al. (21), the present study reports our efforts over the course of the past 4 years to identify and characterize the apical cation/H+ exchanger in Malpighian tubules of Aedes. We focused on the Aedes ortholog of NHE8 that in mammals resides in the apical membrane of renal proximal tubules and normal rat kidney cells (3, 28, 92), where it mediates Na/H exchange (92). Indeed, we cloned a cDNA from Aedes Malpighian tubules that is identical to AeNHE8, which was cloned from an Aedes cDNA library by Kang'ethe and colleagues (34). We detected AeNHE8 immunoreactivity in principal cells of the distal secretory segments, where it localizes to an intracellular compartment basal to, but not in, the brush border. Furthermore, the localization of AeNHE8 in principal cells was not influenced during periods of enhanced fluid secretion by the Malpighian tubules. When expressed heterologously in Xenopus laevis oocytes, AeNHE8 mediates EIPA-sensitive Na/H exchange, but not K/H exchange. In addition, the expression of AeNHE8 stimulates an endogenous nonselective cation conductance in Xenopus oocytes. Thus our data provide evidence that AeNHE8 mediates cation/H+ exchange across an intracellular membrane, but not across the apical membrane, of Malpighian tubules.
MATERIALS AND METHODS
Mosquitoes and Tissue Isolations
The mosquito colony was maintained as described by Pannabecker and colleagues (52). Tissues were dissected from adult females (3–7 days after eclosion) that were anesthetized on ice and then decapitated. Malpighian tubules and the digestive tract were isolated in Ringer solution as described by Pannabecker and colleagues (52). The Ringer solution contained (in mM) 150 NaCl, 3.4 KCl, 1.7 CaCl2, 1.8 NaHCO3, 1.0 MgSO4, 5 glucose, and 25 HEPES, pH 7.1.
The five Malpighian tubules were transferred to a 1.5-ml microcentrifuge tube (USA Scientific, Ocala, FL) containing 0.5 ml of Ringer solution on ice. The remaining gut tissue (i.e., midgut and hindgut) and the carcass (i.e., thorax and abdomen) were transferred to separate 1.5-ml microcentrifuge tubes also containing 0.5 ml of Ringer solution on ice. The dissections were performed in the time period of ∼1 h, when at least 30 female mosquitoes were processed. For whole-animal samples, anesthetized, intact mosquitoes were transferred to a 1.5-ml tube on ice.
Isolation of Total RNA and Synthesis of cDNA
All steps were performed under RNase-free conditions and at room temperature unless specified otherwise. For RNA extractions, select tissues (see above) and whole animals were centrifuged at 5,000 g for 3 min. The Ringer solution was aspirated, and the tissues or intact animals were homogenized in 1 ml of TRIzol reagent (Invitrogen, Carlsbad, CA) using a sintered-glass homogenizer. To extract total RNA, we used a phenol:chloroform phase separation with an isopropyl-alcohol precipitation (17). To minimize contamination by DNA, the resulting RNA was treated with DNase I (DNA-free, Ambion, Austin, TX) according to the manufacturer's protocol.
For RT-PCR and rapid amplification of cDNA ends (RACE), single-stranded cDNA was generated from 2 μg of total RNA, using oligo (dT)20 primers (Qbiogene, Carlsbad, CA) and the ThermoScript RT-PCR System (Invitrogen). For relative mRNA expression, 0.4 μg of total RNA was used to generate the cDNA.
RT-PCR and RACE
The initial RT-PCR experiments on Aedes Malpighian tubule cDNA were conducted before publication of the Aedes genome (45). For this reason, we used the sequence of a putative NHE (GenBank accession no. XM_307859) from the genome of the malaria mosquito Anopheles gambiae to design the first set of primers. The standard PCR protocol consisted of the following steps: 1) denaturation for 4 min at 94°C with subsequent cooling to 72°C, 2) addition of 2.5 U of DNA Taq polymerase (Qbiogene), 3) 35 amplification cycles with an optimized annealing temperature (50–60°C) and an elongation time of 1 min/kb of the predicted PCR product.
Depending on the expected size of the PCR products, they were separated on either 1 or 2% agarose gels by electrophoresis and stained with ethidium bromide for detection under UV light. The PCR products were 1) gel purified using a Qiaquick Gel Extraction Kit (Qiagen, Valencia, CA), 2) ligated into a pGEM-T vector (Promega, Mannheim, Germany), and 3) transformed into a competent Escherichia coli strain (DH5α). After selection on LB-amp agar plates, E. coli colonies containing the insert were cultured overnight in 5 ml of LB-amp liquid media at 37°C. The plasmids were isolated (Qiaprep Spin Miniprep Kit, Qiagen) and submitted for automated sequencing (Sequence Laboratories, Göttingen, Germany) using T7 and Sp6 primers (Promega).
After obtaining the partial sequence of the NHE amplified from Aedes Malpighian tubules, we amplified the 5′ and 3′ ends of the NHE cDNA using a FirstChoice RLM-RACE Kit (Ambion). Combining the sequencing data from the respective RACEs and the initial RT-PCR allowed us to assemble a full-length cDNA, which was then 1) amplified via PCR, 2) sequenced in both the 5′ and 3′ directions, and 3) submitted into GenBank (accession no. EU760347). From here, we refer to this cDNA, and the protein it encodes, as AeNHE8.
Relative mRNA Expression
To compare relative levels of AeNHE8-transcript expression, we followed previously published protocols that used a RT-PCR-based approach to analyze the accumulation of PCR products during logarithmic amplification (78–80). In brief, cDNAs derived from the following samples were used as templates for PCR: 1) 22 whole female mosquitoes, 2) 110 Malpighian tubules pooled from 22 females, 3) guts (i.e., midgut and hindgut) pooled from 22 females, and 4) thorax and abdomens pooled from 22 females. The primer pairs were designed to amplify 1) a 705-bp region of AeNHE8 (forward primer: 5′-GGT GAC ACA AAT CAC GAT GC-3′, reverse primer: 5′-GG TAT TCG GAT CGC CTG GTA-3′); and 2) a 446-bp region of Aedes ribosomal protein S3 (AeRbS3; forward primer: 5′-ATC ATC ATC ATG GCC ACC CGT A -3′, reverse primer: 5′-CCA TTT GGA TCC CAA GGC AAC A-3′). The RbS3 gene is known to be expressed equally among insect tissues (78, 93). Thus it served as an internal control for standardization.
The PCR protocol consisted of the following steps: 1) denaturation for 4 min at 90°C with subsequent cooling to 72°C, 2) addition of 2.5 U of DNA Taq polymerase (Qbiogene), and 3) 40 amplification cycles with an annealing temperature of 53°C for 30 s and an elongation temperature of 72°C for 1 min. Starting at the end of the cycle 22, and then for every other cycle afterward, the thermocycler was paused to allow for removal of 8 μl of sample.
After completion of the PCR protocol, all of the removed samples were separated by electrophoresis on a 1.5% agarose gel, which was then stained with ethidium bromide and visualized with UV light. A Fluor-S Multi-Imager (Bio-Rad, San Jose, CA) was used to digitize the gel images, and Quantity One Software (Bio-Rad) was used to quantify the optical densities of the resulting PCR products at cycle 36 (log phase) for AeNHE8 and cycle 24 (log phase) for AeRbS3. The data are presented as the ratio of the optical density of the AeNHE8 PCR product to that of the AeRbS3 PCR product (i.e., the AeNHE8/AeRbS3 ratio).
Antibodies
Charles River (Sulzfeld, Germany) was hired to raise and affinity purify a polyclonal rabbit antibody against a synthetic peptide fragment of the predicted AeNHE8 protein. The peptide corresponded to amino acid residues Ser511-Arg524 of the putative, cytosolic COOH-terminal domain of AeNHE8. Before injection of the rabbits, the peptide was covalently linked to keyhole-limpet hemocyanin. The resulting anti-sera were affinity-purified with the synthetic peptide, which was covalently linked to a HYDRA column (Squarix, Marl, Germany).
A polyclonal guinea pig antibody (C23) raised against the B-subunit of the V-type H+-ATPase from Manduca was kindly provided by the laboratory of Helmut Wieczorek (University of Osnabrück, Osnabrück, Germany). A monoclonal mouse antibody (α5) raised against the α-subunit of avian Na-K-ATPase developed by Dr. Douglas Fambrough (John Hopkins University) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA). Both the C23 and α5 antibodies recognize their respective antigens in Aedes Malpighian tubules (55, 81). In addition, a monoclonal mouse antibody (JL-8) raised against recombinant full-length Aequorea victoria green fluorescent protein was obtained from Clontech (Mountain View, CA).
Malpighian Tubule Western Blotting
Western blotting was performed as described previously (81). In brief, the proteins of a crude tubule homogenate (derived from 200 Malpighian tubules of 40 female mosquitoes) were separated by molecular mass on a denaturing 17% polyacrylamide gel using a Mini Protean 3 electrophoresis chamber (Bio-Rad, Hercules, CA). The separated proteins were transferred to a nitrocellulose membrane (SERVA Electrophoresis, Heidelberg, Germany) using a semidry apparatus (Bio-Rad).
To detect AeNHE8 immunoreactivity, the nitrocellulose membrane was 1) blocked for 60 min in a Tris-buffered saline (TBSNT; 20 mM Tris·HCl, 500 mM NaCl, 0.02% NaN3, 0.05% Tween 20, pH 7.5) plus 3% fish gelatin; 2) incubated for 60 min with the AeNHE8 antibody (diluted 1:1,000 in TBSNT plus 1% fish gelatin); and 3) washed three times (5 min each) with TBSNT. The nitrocellulose membrane was then 1) incubated for 60 min with a secondary antibody, i.e., alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma) diluted 1:10,000 in TBSNT plus 1% fish gelatin; and 2) washed three times (5 min each) with TBSNT. To visualize binding of the antibodies, colorimetric substrates of alkaline phosphatase, i.e., nitro blue tetrazolium (NBT; Sigma) and 5-bromo-4-chloro-3-indolyl phosphate p-toluidine (BCIP; Sigma), were applied to the membrane according to the manufacturer's protocol. When a protein band appeared, the membrane was rinsed with double-distilled H2O.
Malpighian Tubule Immunohistochemistry
For immunohistochemistry, 150 tubules (from 30 female mosquitoes) were isolated in Ringer solution as described above and then fixed in 1 ml of an ice-cold fixative solution containing 3% paraformaldehyde, 0.05% glutaraldehyde, and 0.05% picric acid in a 0.1 M Na-phosphate buffer, pH 7.3. In some cases, 50 isolated tubules (from 10 female mosquitoes) were incubated for 30 min at room temperature with 1 ml of Ringer solution containing 10−3 M dibutyryl-cAMP (db-cAMP), or 1 ml of Ringer alone, before they were fixed.
For blood-feeding experiments, 50 tubules were isolated and fixed from 10 female mosquitoes that were fed a blood meal. These mosquitoes were placed individually in a 200-ml glass beaker covered with a mesh netting and allowed to feed on the investigator's arm until repletion, i.e., between stages 4 and 5 on the Pilitt-Jones scale (58). After mosquitoes had fed to repletion, they were allowed 5 min to process the blood meal before they were anesthetized on ice and their tubules were isolated as described above. Fifty tubules from 10 female mosquitoes that were handled as above, but not offered a blood meal, served as controls.
All of the above tubules were 1) fixed overnight at 4°C, 2) washed overnight twice in 70% ethanol at 4°C to remove the fixative, and 3) submitted to the Clinical Sciences Histology Laboratory (Cornell University, Ithaca, NY) for routine paraffin embedding and sectioning. The tubule sections (4-μm thick) were adhered to ProbeOn Plus glass slides (Fisher Scientific, Hampton, NH).
All immunohistochemical staining procedures were conducted at room temperature in the Immunopathology Research and Development Laboratory (Cornell University) unless noted otherwise. The tubule sections were 1) dewaxed with xylene; 2) rehydrated with an ethanol series (100, 95, and 70% ethanol); and 3) treated with 0.5% H2O2 in methanol for 10 min to inhibit endogenous peroxidase activity. The sections were then rinsed briefly with 70% ethanol and washed for 5 min in PBS consisting of the following (in mM): 145 NaCl, 3.2 NaH2PO4, and 7.2 Na2HPO4, pH 7.3.
To block nonspecific binding, the sections were treated for 20 min with 10% normal goat serum (Zymed, San Francisco, CA) that was supplemented with a 2× casein solution (Vector Laboratories, Burlingame, CA). The blocking solution was then removed and replaced immediately with 1) PBS supplemented with 1× casein solution (PBS-casein) as a negative control, 2) the anti-AeNHE8 antibody (diluted 1:100 in PBS-casein), 3) the C23 antibody (diluted 1:200 in PBS-casein), or 4) the α5 antibody (diluted 1:3 in PBS-casein). Incubation of the sections with the antibodies occurred overnight at 4°C in a humidified chamber. The following day, the sections were rinsed briefly three times with PBS and then washed in PBS for 5 min.
To detect binding of the primary antibodies, the sections were first incubated for 20 min with a biotinylated secondary antibody diluted 1:50 in PBS-casein; i.e., goat anti-rabbit IgG for the anti-AeNHE8 antibody, goat anti-guinea pig IgG for the C23 antibody, and goat anti-mouse IgG for the α5 antibody (all from Vector Laboratories). The sections were then 1) rinsed and washed in PBS as described above, 2) incubated for 20 min with a streptavidin/horseradish peroxidase (HRP) solution (Zymed), and 3) rinsed and washed in PBS again as described above. To visualize binding of the antibodies, a chromogenic substrate of HRP (3-amino-9-ethylcarbazole, AEC; Zymed) was applied to the sections for 10–15 min. After extensive washing with tap water the sections were dipped in hematoxylin (15–30 s) to counterstain the tissue and covered with a coverslip for viewing on an AX70 compound microscope (Olympus, Melville, NY).
Generation of AeNHE8 cRNA for Expression in Xenopus Oocytes
A cDNA containing the entire open reading frame (ORF) of AeNHE8 was subcloned into the pGH19 Xenopus expression vector (71) and then sequenced in both the 5′ and 3′ directions (Cornell DNA Sequencing Center). The resulting AeNHE8-pGH19 cDNA was used as a template to synthesize capped cRNA, encoding AeNHE8 protein, with a T7 mMessage mMachine kit (Ambion) according to the manufacturer's protocol. The AeNHE8 cRNA was purified using an RNeasy MinElute Cleanup Kit (Qiagen) and stored at −80°C in nuclease-free H2O (Integrated DNA Technologies, Coralville, IA).
The AeNHE8-pGH19 cDNA was also modified by subcloning the ORF of enhanced green-fluorescent protein (eGFP), kindly provided by the laboratory of Dr. Walter F. Boron (Case Western Reserve University), to the 3′ end of the AeNHE8 ORF. A Quikchange site-directed mutagenesis kit (Stratagene, La Jolla, CA) was then used to delete the stop codon between the ORFs of AeNHE8 and eGFP, resulting in one continuous ORF. The AeNHE8-eGFP-pGH19 cDNA was used as a template to synthesize capped cRNA (as described above) encoding an AeNHE8-eGFP fusion protein.
Heterologous Expression in Xenopus Oocytes
Stage V-VI oocytes were isolated from Xenopus as described by Romero and colleagues (63), and the following day the oocytes were injected with 28 nl of AeNHE8 cRNA (1.0 ng/nl), AeNHE8-eGFP cRNA (1.0 ng/nl), or nuclease-free H2O. The injected oocytes were cultured at 16°C in the wells of a Falcon six-well tissue culture plate (Becton Dickson, Franklin Lakes, NJ) containing OR3 media (63) for 1–14 days before any experiments commenced.
Western blotting.
To verify that Xenopus oocytes translate the AeNHE8 and AeNHE8-eGFP cRNAs into protein, Western blots were performed on membrane fractions of oocytes. All of the following steps were performed at room temperature unless noted otherwise. Seven days after injection of oocytes with 1) nuclease-free H2O, 2) 28 ng of AeNHE8 cRNA, or 3) 28 ng of AeNHE8-eGFP cRNA, 50 oocytes from each group were transferred to 1.5-ml microcentrifuge tubes (USA Scientific) containing 1 ml of solution 1 (see Table 1 for composition). After the oocytes were rinsed three times with solution 1, they were snap frozen in liquid nitrogen and stored at −80°C. On the day of analysis, the oocytes were thawed on ice in 300 μl of an ice-cold homogenization buffer consisting of solution 1 supplemented with Halt Protease Inhibitor Cocktail (Pierce Biotechnology, Rockford, IL) and EDTA (5 mM). The oocytes were then homogenized in their respective microcentrifuge tubes with a plastic pestle (Kontes, Vineland, NJ), and the volume of the homogenate was adjusted to ∼1 ml with ice-cold homogenization buffer.
Table 1.
Composition (in mM) of oocyte recording solutions
| Solution | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | 96 | 0 | 0.96 | 0.96 | 0.96 | 9.6 | 9.6 | 9.6 | 9.6 | 9.6 |
| NMDG-Cl | 9 | 105 | 104 | 9 | 9 | 95 | 9 | 9 | 9 | 9 |
| KCl | 1 | 1 | 1 | 1 | 96.04 | 1 | 1 | 87.4 | 1 | 1 |
| LiCl | 0 | 0 | 0 | 95.04 | 0 | 0 | 86.4 | 0 | 0 | 0 |
| CsCl | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 86.4 | 0 |
| RbCl | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 86.4 |
| MgCl2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| CaCl2 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| HEPES | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
The pH of all solutions was adjusted to 7.5 with the dominant cation's hydroxide salt or NMDG-OH. The osmolality of all solutions was adjusted to 210 ± 5 mosmol/kgH2O by adding double-distilled H2O or mannitol.
To clear the homogenates of cell debris, they were centrifuged at 3,000 g for 10 min at 4°C. The resulting supernatants were transferred to fresh 1.5-ml centrifuge tubes (Beckman, Fullerton, CA) and centrifuged in an OptimaMax ultracentrifuge (Beckman) at 100,000 g for 60 min at 4°C. After the supernatants were aspirated, the pellets containing the membrane protein fractions were resuspended in 50 μl of ice-cold homogenization buffer and measured for total protein using a bicinchoninic acid protein assay (Pierce Biotechnology). To the remaining volumes of the resuspended membrane fractions, an appropriate volume of a 5× Laemmli sample buffer (37) was added and the samples were denatured at 100°C for 5 min.
Approximately 25 μg of membrane protein from each sample was separated by molecular mass on a denaturing 8% polyacrylamide gel using a XCell Surelock Mini-cell electrophoresis unit (Invitrogen). The separated proteins were then transferred to an Immunoblot polyvinylidene difluoride (PVDF) membrane (Bio-Rad) using an XCell II Blot Module (Invitrogen).
To detect AeNHE8 or GFP immunoreactivity, the PVDF membrane was 1) washed three times (5 min each) with Tween-Tris-buffered saline (TTBS; 10 mM Tris·HCl, 150 mM NaCl, 0.01% Tween 20, pH 7.4); 2) blocked for 1 h with 5% nonfat dry milk dissolved in TTBS (blocking buffer); and 3) incubated overnight at 4°C with the anti-AeNHE8 antibody (diluted 1:100 in blocking buffer) or the anti-GFP antibody (diluted 1:2,500 in blocking buffer). On the following day, the PVDF membrane was 1) washed three times (5 min each) with TTBS; 2) incubated for 1.5 h with a secondary antibody, i.e., HRP-conjugated goat anti-rabbit IgG (Pierce Biotechnology) or goat anti-mouse IgG (Pierce Biotechnology) diluted 1:25,000 in blocking buffer; and 3) washed three times (5 min each) with TTBS. To visualize binding of the antibodies, a luminescent substrate of HRP (SuperSignal West Pico, Pierce Biotechnology) was applied to the PVDF membrane according to the manufacturer's protocol, and the luminescent signal was detected with X-ray film (Pierce Biotechnology).
In vivo fluorescence.
To visualize expression of AeNHE8-eGFP protein in Xenopus oocytes, in vivo fluorescence was performed as follows. One to 14 days after oocytes were injected with either 28 nl of AeNHE8-eGFP cRNA (1.0 ng/nl) or nuclease-free H2O, the oocytes were transferred to the wells of a Falcon 12-well tissue culture plate (Becton Dickson) containing 1–2 ml of solution 1. The oocytes were examined in the Cornell Microscopy and Imaging Facility with an OV100 (Olympus) fluorescence-imaging system equipped with a 150-W xenon lamp, a 460- to 490-nm excitation filter, and a 510- to 550-nm emission filter.
Oocyte Electrophysiology
The solutions used in electrophysiology experiments are described in Table 1. When required, 100 mM stock solutions of the inhibitors GdCl3, EIPA, benzamil, amiloride, diphenylamine-2-carboxylate (DPC), or heptanol (all from Sigma-Aldrich, St. Louis, MO) were diluted to their final concentrations in solution 1. All stock solutions of the inhibitors were prepared with dimethylsulfoxide, except for GdCl3 which is soluble in double-distilled H2O. For all experiments, the solutions were held in 250-ml glass Erlenmeyer flasks or 50-ml polypropylene centrifuge tubes (Fisher Scientific) and delivered by gravity to a RC-3Z oocyte chamber (Warner Instruments, Hamden, CT) at a flow rate of 4 ml/min. All oocytes were initially placed in the chamber under superfusion with solution 1. Solution changes were made with a Rheodyne Teflon eight-way rotary valve (model 5012, Rheodyne, Rohnert Park, CA).
Preliminary experiments indicated that the basic electrophysiological properties (e.g., intracellular pH, membrane potential, and membrane conductance), and their responses to lowering extracellular Na+ concentration ([Na+]o), of oocytes injected with AeNHE8 cRNA were comparable to those injected with AeNHE8-eGFP cRNA. Thus oocytes injected with 28 nl of AeNHE8-eGFP cRNA (1.0 ng/nl) were primarily used in electrophysiological experiments, because this cRNA allowed the rapid verification of heterologous expression using in vivo fluorescence. Oocytes injected with 28 nl of nuclease-free H2O served as controls. Both the AeNHE8-eGFP- and H2O-injected oocytes were used for electrophysiology experiments 5–14 days after injection.
Intracellular recordings of pH and membrane potential.
To measure intracellular pH (pHi), pH-sensitive and voltage-sensitive electrodes were fabricated from thin-walled borosilicate glass (part no. 30-0077, Harvard Apparatus, Holliston, MA) following the protocol of Romero and colleagues (63). The voltage from the pHi electrode was recorded with the high-impedance “channel A” of a Duo773 electrometer (World Precision Instruments, Sarasota, FL). The membrane voltage (Vm) was recorded with an OC-725C oocyte clamp (Warner Instruments). Inputs from the pHi and Vm electrodes were recorded digitally by a Digidata 1440A data acquisition system (Molecular Devices, Sunnyvale, CA) and the Clampex module of pCLAMP software (Version 10, Molecular Devices).
To obtain the voltage that is specific to pHi, the response of the Vm electrode was subtracted from that of the pHi electrode (“pHi − Vm”) within the Clampex module. Before an oocyte was impaled with microelectrodes, the pHi electrode was calibrated in the oocyte bath using two pH standards (6.0 and 8.0, Fisher Scientific) and solution 1 (pH 7.50). Applying a linear regression to the resulting calibration curve allowed the pHi − Vm values recorded in oocytes to be converted into actual pHi measurements, using the Clampfit module of the pCLAMP software (Version 10, Molecular Devices).
Two-electrode voltage clamping.
Whole-cell currents of oocytes were acquired with the OC-725 oocyte clamp (Warner Instruments). The data were recorded digitally by a MiniDigi-1A data acquisition system (Molecular Devices) and AxoScope software (Version 10, Molecular Devices). Both the membrane current (Im) and Vm microelectrodes were filled with 3 M KCl. For all Im recordings, the Vm was clamped to a hyperpolarizing holding potential of 30 mV. For example, if the spontaneous Vm of the oocyte was −40 mV, then the clamp voltage was set at −70 mV.
All current-voltage (I-V) relationships of oocytes were acquired and analyzed following a previously-published protocol (57). In brief, the oocytes were held at a Vm close to their spontaneous Vm in solution 1 before initiating the voltage-clamp protocol, which was controlled by the Clampex module of the pCLAMP software (Molecular Devices).
Statistics
Statistical tests were performed using Graphpad Prism 4 (Graphpad Software, San Diego, CA). Comparisons between two groups (e.g., H2O-injected vs. AeNHE8 oocytes) were evaluated with unpaired t-tests, whereas comparisons within AeNHE8 oocytes were assessed with a one-way repeated-measures ANOVA and a Bonferroni posttest. The posttest was only used to compare mean values in AeNHE8 oocytes if the means were both 1) significantly different from those of their respective H2O-injected oocytes, and 2) in the same direction (i.e., positive or negative). To compare and categorize the mean inward Im produced by various cations in AeNHE8 oocytes, a one-way unpaired ANOVA with a Newman-Keuls posttest was used. To determine whether an inhibitor had a significant effect, a one-sample t-test was used with a hypothetical value of “0.0.” The mean percent inhibitions produced by each inhibitor were compared by a one-way unpaired ANOVA and categorized with a Newman-Keuls posttest.
RESULTS
cDNA Cloning of AeNHE8
We cloned an NHE-like cDNA from Aedes Malpighian tubules that is nearly identical to that of AeNHE8, the ortholog of mammalian NHE8 cloned from an Aedes cDNA library by Kang'ethe and colleagues (34). The AeNHE8 cDNA that we cloned consists of 2,940 bp with a predicted open-reading frame of 2,004 bp. As shown by the red bar in Supplemental Fig. 1 (all supplemental material for this article are available on the journal web site), the 5′-untranslated region (UTR) of “our” AeNHE8 cDNA (accession no. EU760347) begins 76 bp upstream of the cDNA cloned by Kang'ethe and colleagues (accession no. AY326255). The additional 76 bp are contiguous with that of the Aedes genomic data but contain two nucleotide substitutions not found in the genome (lower-case, underlined letters in line 1 of Supplemental Fig. 1). Compared with the cDNA cloned by Kang'ethe and colleagues, we find a single nucleotide substitution of an A for a G in the 5′-untranslated region (UTR; the * in line 1 of Supplemental Fig. 1). An A in this position is consistent with the genomic data.
The only difference within the ORF is a single silent substitution of an A in our AeNHE8 cDNA instead of a G (the * in line 9 of Supplemental Fig. 1). Thus the proteins encoded by both cDNAs are identical. Although the A in our AeNHE8 cDNA is inconsistent with the genomic data, this substitution may represent a polymorphism that is unique to the line of Aedes used by our laboratory, because the same substitution was found after a repetition of the RT-PCR for AeNHE8 on Malpighian tubule cDNA derived from an independent generation of mosquitoes (data not shown). The 3′-UTR of our AeNHE8 cDNA is identical to the one cloned by Kang'ethe and colleagues (data not shown).
Bioinformatic Analyses of AeNHE8 Protein
Transmembrane segments and topological prediction.
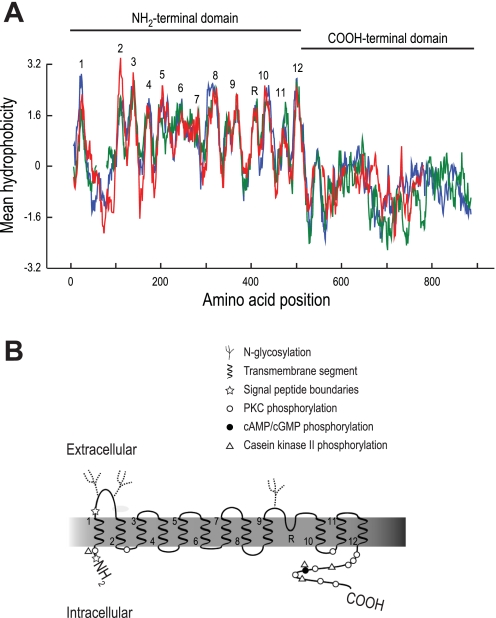
To construct a hypothetical membrane topology of the predicted AeNHE8 protein, we compared its amino acid sequence and hydrophobicity profile with those of two mammalian isoforms for which experimentally derived topology maps exist, i.e., NHE1 and NHE3 (49, 75, 94). Although the predicted AeNHE8 protein shares only ∼25% amino acid sequence identity with mammalian NHE1 and NHE3 (sequence alignment not shown), Fig. 1A shows that their hydropathic plots are similar, especially within the NH2-terminal domain that contains the transmembrane (TM) segments.
Fig. 1.
Hydropathic analysis and predicted topology of AeNHE8. A: aligned hydropathic plots of AeNHE8 (red), human NHE1 (blue), and human NHE3 (green) generated in the BioEdit Sequence Alignment Editor software, version 7 (30) using the Kyte-Doolittle algorithm (36) with a window size of 15. Breaks in the red and green lines represent gaps introduced by the sequence alignment. Numbers indicate predicted transmembrane segments, and R indicates a predicted reentrant loop. B: predicted topology map of AeNHE8 based on hydropathy plot in A. Transmembrane segments are numbered at their emerging ends. Putative posttranslational modifications and regulatory sites are also indicated (see text and Fig. 2 for details). The symbols are placed next to one another if they share a putative phosphorylation site.
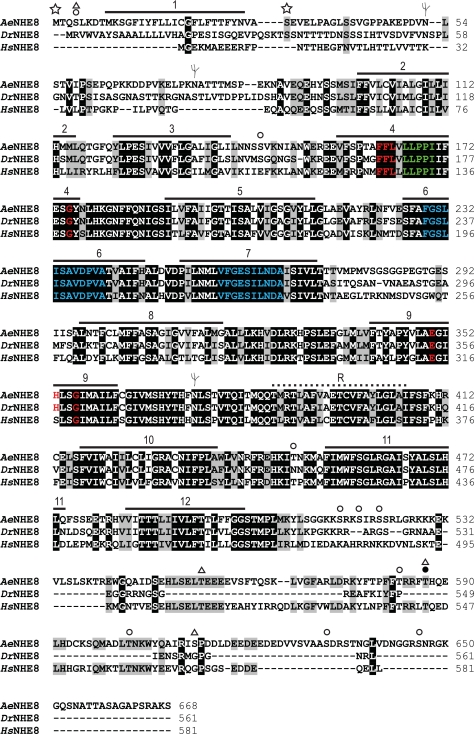
As shown in Fig. 1B, we predict that AeNHE8 consists of 1) a large NH2-terminal domain composed of 12 TM segments and a reentrant loop that are connected by several endofacial and exofacial loops, some of which are predicted to be glycosylated or phosphorylated; and 2) a cytosolic COOH-terminal domain with several predicted phosphorylation sites. The amino acid residues that compose the putative TM segments are identified in the alignment shown in Fig. 2, which also includes the amino acid sequences of NHE8 orthologs from Drosophila (DrNHE8) and H. sapiens (HsNHE8).
Fig. 2.
Annotated sequence alignment. The amino acid sequence of AeNHE8, predicted by its cDNA (accession no. EU760347), is aligned with those of NHE8 from Drosophila melanogaster (DrNHE8; accession no. AAD32689) and Homo sapiens (HsNHE8; accession no. NP_056081) by the ClustalW algorithm (39). The residue shading was performed by BioEdit Sequence Alignment software, version 7 (30) with a threshold of 100%. Identical residues are shaded in black; similar residues are highlighted in gray. Numbered horizontal bars indicate the predicted transmembrane segments, and the dotted line indicates the reentrant loop, which are depicted in Fig. 1B. Red text indicates residues associated with sensitivity to amiloride and its analogs. Green text indicates the residues conserved among all NHEs. Blue text indicates the regions of high homology. See text for details. Symbols are as in Fig. 1B.
Our topological model for AeNHE8 (Figs. 1B and 2) is similar to that proposed by Kang'ethe and colleagues (34), but with a few important modifications to accommodate the similar hydrophobicity profiles (and amino acid sequences) shared between AeNHE8 and the mammalian NHEs (i.e., NHE1 and NHE3) in the predicted TM segments. Namely, we hypothesize that residues 386-406 form a reentrant loop between TM segments 9 and 10, instead of forming TM segment 10. In addition, we propose a TM segment at residues 453-474 (i.e., TM segment 11) that is not predicted by the previous model.
Conserved and predicted features.
The amino acid sequence of AeNHE8 contains several regions that are highly conserved among NHEs. In TM segments 4 and 9, AeNHE8 contains residues (red text in Fig. 2) that are associated with sensitivity to amiloride and its analogs (11, 49). In addition, AeNHE8 contains 1) residues in TM segment 4 (green text in Fig. 2) that are highly conserved among all eukaryotic NHEs (11); and 2) the regions of high homology in TM segments 6 and 7 (blue text in Fig. 2), which are considered crucial for translocation of Na+ and H+ (51, 76).
With regard to potential posttranslational modifications and regulatory sites, Figs. 1B and 2 highlight the locations of three putative N-glycosylation sites and several phosphorylation sites as predicted by an in silico PROSCAN analysis (19). Glycosylations and phosphorylations of NHEs are common modifications associated with the maturation and acute regulation of the functional protein (reviewed by Refs 10, 23, 49, 51, 76). Note that the COOH-terminal domain of AeNHE8, which is considered the regulatory domain of most NHEs, contains three regions with predicted phosphorylation sites (i.e., Ser548-Glu558; Pro580-Phe587; Leu601-Gln607) that are highly conserved with HsNHE8. These regions are absent in DrNHE8 (Fig. 2).
An analysis of the amino acid sequence of AeNHE8 with SignalP 3.0 (4) predicts that the first 32 amino acid residues (i.e., Met1-Ser32) form a signal-anchor peptide, as indicated by the stars in Figs. 1B and 2. Signal anchors are involved with the subcellular targeting, membrane insertion, and membrane anchoring of TM proteins (41, 73). In DrNHE8, a predicted signal-peptide cleavage site occurs in a similar location (27). Thus, in contrast to AeNHE8, the first TM segment of DrNHE8 may exist as a separate entity from the rest of the molecule, as occurs for NHE3 of mammals (94). No consensus signal-anchor or signal-peptide sequences are found in HsNHE8.
Expression of AeNHE8 Transcripts in Adult Female Mosquitoes
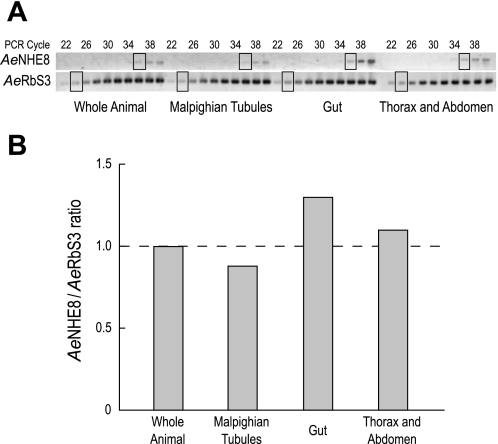
To determine whether AeNHE8 transcripts are enriched in a particular tissue of adult females, we used a RT-PCR-based approach to estimate the expression of AeNHE8 relative to that of Aedes ribosomal protein S3 (AeRbS3), which served as an internal control. In insects, RbS3 transcripts are expressed to a similar degree among a wide variety of tissues (78, 93). Expression of AeNHE8 was measured in the following samples: 1) whole female mosquitoes, 2) Malpighian tubules, 3) gut (i.e., midgut and hindgut), and 4) thorax and abdomen. As shown in Fig. 3A, expression of AeNHE8 is weak, but detectable, in all of the assays between cycles 34 and 40 of the PCR. In contrast, expression of the internal control (AeRbS3) was robust and detectable in all of the assays as early as cycle 22 of the PCR.
Fig. 3.
Relative mRNA expression of AeNHE8. A: images of RT-PCR products for AeNHE8 and ribosomal protein AeRbS3 separated by agarose gel electrophoresis. Every other PCR cycle is indicated, starting at cycle 22. The boxed areas represent those bands selected for relative expression analysis. B: ratios of AeNHE8 expression at cycle 36 to AeRbS3 expression at cycle 24. Dashed line indicates the threshold ratio for enriched or reduced expression relative to the whole animal.
Figure 3B shows the ratios of AeNHE8 to AeRbS3 expression (AeNHE8/AeRbS3 ratios) measured at the cycles indicated by the boxes. If the AeNHE8/AeRbS3 ratio is greater than that in the whole animal (dashed line in Fig. 3B), then AeNHE8 expression is enriched. Similarly, if the AeNHE8/AeRbS3 ratio is lower than that in the whole animal, then AeNHE8 expression is reduced. Expression of AeNHE8 in the Malpighian tubules is slightly below the average expression in the whole animal, whereas expression in the gut and in the thorax and abdomen are slightly above (Fig. 3B). In general, the AeNHE8/AeRbS3 ratios show that 1) expression of AeNHE8 transcripts is ubiquitous in the female mosquito, and 2) expression levels of AeNHE8 are similar among the tissues examined.
Western Blotting of AeNHE8 in Malpighian Tubules
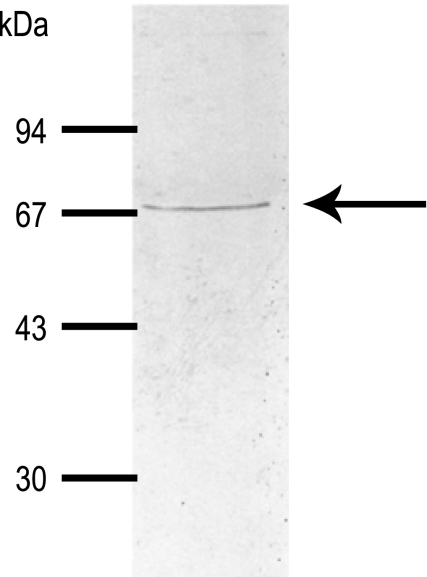
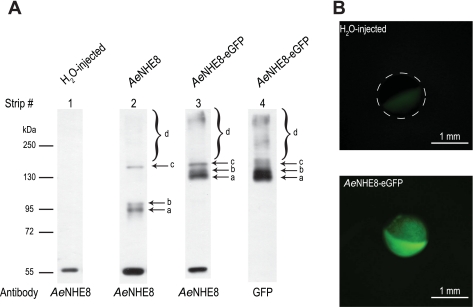
As indicated by the arrow in Fig. 4, the AeNHE8 antibody detects a single, discrete band of protein in crude extracts of Aedes Malpighian tubules. The immunoreactive band has a molecular mass of ∼67 kDa, which is slightly lower than the size of the AeNHE8 monomer predicted by its cDNA (i.e., ∼74 kDa).
Fig. 4.
Expression of AeNHE8 immunoreactivity in female Malpighian tubules. Western blotting on crude homogenates from isolated Malpighian tubules of adult Aedes females is shown. Arrow indicates the protein band displaying AeNHE8 immunoreactivity. Molecular mass markers (in kDa) are indicated.
Immunolocalization of AeNHE8 in Malpighian Tubules
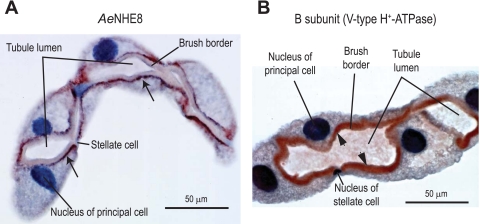
As shown in Fig. 5A, immunoreactivity for AeNHE8 occurs exclusively in principal cells of Aedes Malpighian tubules. Stellate cells show no evidence for staining. The discrete, punctate immunostaining for AeNHE8 primarily localizes to a subapical region of principal cells beneath the brush border (arrows in Fig. 5A). In some principal cells, AeNHE8 immunolabeling occurs in other intracellular regions as well (e.g., Fig. 6A and see Fig. 8). In contrast to AeNHE8, immunoreactivity for the B subunit of the V-type H+-ATPase primarily labels the brush border (arrowheads in Fig. 5B) and is occasionally found in intracellular compartments (e.g., Fig. 6B). Thus our immunohistochemical data clearly indicate that AeNHE8 is expressed primarily in intracellular compartments of principal cells. No staining was detected when normal rabbit serum or PBS was used in place of the AeNHE8 or B subunit antibodies (data not shown).
Fig. 5.
Immunoperoxidase localizations of AeNHE8 and the B subunit of the V-type H+-ATPase in female Malpighian tubules. Representative localizations are shown of AeNHE8 (A) and the B subunit of the V-type H+-ATPase (B) in isolated Malpighian tubules of adult Aedes females. Red staining identified by the arrows in A and arrowheads in B represents labeling by the respective antibodies. Sections are counterstained with hematoxylin to stain nuclei and provide contrast.
Fig. 6.
Immunoreactivity for AeNHE8 and the V-type H+-ATPase in consecutive sections of female Malpighian tubules. Localizations are shown of AeNHE8 (A) and subunit B of the V-type H+-ATPase (B) in isolated Malpighian tubules of adult Aedes females. A and B: consecutive sections (4 μm apart) of the same Malpighian tubule. Red staining represents labeling by the respective antibodies. Sections are counterstained with hematoxylin to stain nuclei and provide contrast. Note that AeNHE8 is not present in every principal cell.
To examine the distribution of AeNHE8 immunoreactivity in relation to that of the V-type H+-ATPase and the Na-K-ATPase, we labeled two consecutive sections (4 μm apart) of Malpighian tubules: one section was incubated with the AeNHE8 antibody, and the other was incubated with an antibody raised against 1) the B subunit of the V-type H+-ATPase or 2) the α-subunit of the Na-K-ATPase. It is known that the B subunit is expressed by principal cells of both the distal and proximal segments of Aedes Malpighian tubules, whereas the α-subunit is expressed only by principal cells of proximal segments (55).
The AeNHE8 immunolabeling occurred in principal cells that showed brush border (and sometimes intracellular) staining for the B subunit of the V-type H+-ATPase as shown by the representative AeNHE8-positive principal cell in Fig. 6A. Not all principal cells express AeNHE8 immunoreactivity, regardless of their staining for the B subunit, even in principal cells adjacent to those expressing AeNHE8 (Fig. 6A).
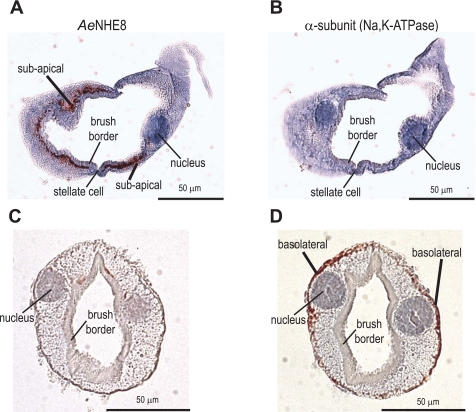
The principal cells that express AeNHE8 immunoreactivity show an absence of detectable immunolabeling for the α-subunit of the Na-K-ATPase (Fig. 7, A and B). We also observe the corollary: i.e., principal cells expressing basolateral immunoreactivity for the α-subunit of the Na-K-ATPase show an absence of detectable immunolabeling for AeNHE8 (Fig. 7, C and D).
Fig. 7.
Immunoreactivity for AeNHE8 and the Na-K-ATPase in consecutive sections of female Malpighian tubules. Localizations are shown of AeNHE8 (A and C) and the α-subunit of the Na-K-ATPase (B and D) in isolated Malpighian tubules of adult Aedes females. Consecutive sections (4 μm apart) of 1 tubule are shown, respectively, in A, B, and C, D. Red staining represents labeling by the respective antibodies. Sections are counterstained with hematoxylin to stain nuclei and provide contrast. Only principal cells of the proximal segments of Aedes Malpighian tubules express basolateral immunoreactivity for the Na-K-ATPase (55). Thus the AeNHE8-positive principal cells in A are from the distal Malpighian tubule, and the AeNHE8-negative principal cells in C are from the proximal Malpighian tubule.
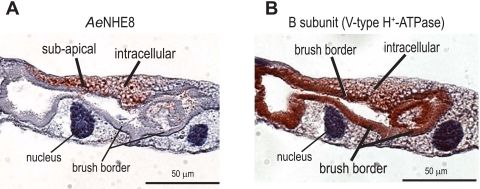
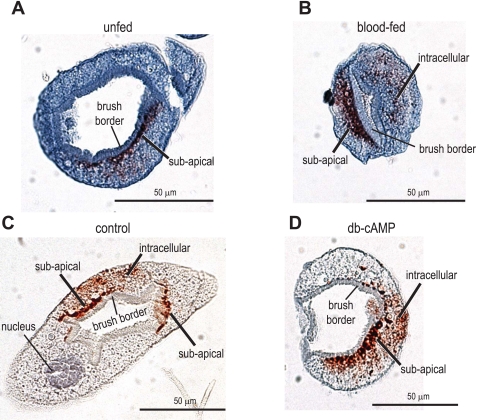
To determine whether the immunolabeling for AeNHE8 redistributes to the brush border during diuresis, we examined the localization of AeNHE8 in 1) Malpighian tubules of blood-fed mosquitoes, 5 min after they consumed a blood meal; and 2) isolated Malpighian tubules that were treated with the known secretagogue db-cAMP (10−3 M) for 30 min. As shown in Fig. 8, the immunolabeling for AeNHE8 is comparable in both control (Fig. 8, A and C) and stimulated (Fig. 8, B and D) Malpighian tubules. Staining for AeNHE8 occurs in subapical and/or intracellular compartments, but is not found in the luminal brush border (Fig. 8).
Fig. 8.
Immunoreactivity for AeNHE8 in control and stimulated female Malpighian tubules. Representative localizations are shown of AeNHE8 in Malpighian tubules of adult Aedes females. A: tubule isolated from an unfed mosquito (control). B: tubule isolated from a blood-fed mosquito, 5 min after ending a blood meal (stimulated). C: isolated Malpighian tubule incubated in Ringer solution for 30 min (control). D: isolated Malpighian tubule incubated in 10−3 M dibutyryl (db)-cAMP for 30 min (stimulated). Red staining represents labeling by the AeNHE8 antibody. Sections are counterstained with hematoxylin to stain nuclei and provide contrast. Note that AeNHE8 is not present in every principal cell (e.g., A and C).
Heterologous Expression of AeNHE8 in Xenopus Oocytes
To verify the heterologous expression of the AeNHE8 protein and the AeNHE8-eGFP fusion protein in Xenopus oocytes, we conducted Western blotting on isolated membrane fractions from oocytes 7 days after injection with H2O, AeNHE8 cRNA (28 ng), or AeNHE8-eGFP cRNA (28 ng). As shown in strips 1–3 of Fig. 9A, the AeNHE8 antibody detects three discrete protein bands (arrows a, b, and c) in membrane fractions from the AeNHE8 and AeNHE8-eGFP oocytes that are not found in those from the H2O-injected oocytes. In addition, a large band of diffuse immunoreactivity is just visible around 250 kDa in the AeNHE8 and AeNHE8-eGFP oocytes (bracket d in strips 2 and 3, Fig. 9A), but not in the H2O-injected oocytes (strip 1, Fig. 9A). This large band is more apparent when the X-ray film is overexposed to the chemiluminescent signal emitted from the PVDF membrane (Supplemental Fig. 2). The finding of multiple and diffuse immunoreactive bands in the AeNHE8 and AeNHE8-eGFP oocytes is similar to results of Western blots for mammalian NHE8 when it is overexpressed in COS-7 and yeast cells (28, 29, 43).
Fig. 9.
Immunochemical and in vivo fluorescent detection of heterologously expressed AeNHE8 in Xenopus oocytes. A: Western blotting on isolated membrane fractions from Xenopus oocytes 7 days after injection with H2O, AeNHE8 cRNA (28 ng), or AeNHE8-enhanced green fluorescent protein (eGFP) cRNA (28 ng). Labeled arrows and brackets indicate protein bands displaying AeNHE8 or GFP immunoreactivity not observed in H2O-injected oocytes. No GFP immunoreactivity was observed in membrane fractions from H2O-injected or AeNHE8 oocytes (data not shown). Molecular mass markers (in kDa) and antibodies used for each strip are indicated. Strip numbers are referred to in the text. See Supplemental Fig. 2 for overexposed versions of the same blots. B: fluorescent images of Xenopus oocytes injected with H2O or 28 ng of AeNHE8-eGFP cRNA. Dashed circle outlines the location of the H2O-injected oocyte, of which only the less-pigmented vegetal pole is visible.
As shown in strips 3 and 4 of Fig. 9A and Supplemental Fig. 2, the nearly identical patterns of immunoreactivity detected by the AeNHE8 (strip 3) and GFP (strip 4) antibodies in the AeNHE8-eGFP oocytes verifies 1) the specificity of the AeNHE8 antibody and 2) that the unique bands (relative to H2O-injected oocytes) are associated with the heterologous expression of AeNHE8 protein. One exception is a single immunoreactive band around 55 kDa that is labeled by the AeNHE8 antibody (strips 1–3, Fig. 9A), but is not labeled by the GFP antibody (strip 4, Fig. 9A). This band may represent a cross-reaction between the AeNHE8 antibody and an endogenous membrane protein of Xenopus oocytes that shares an epitope with the AeNHE8 peptide used to immunize the rabbits.
In AeNHE8 oocytes (strip 2 of Fig. 9A), bands a and b have molecular masses of 92 and 97 kDa, respectively. Both are greater than 1) the expected size of the AeNHE8 monomer (i.e., ∼74 kDa) and 2) the size of AeNHE8 immunoreactivity detected in Malpighian tubules (Fig. 4). If bands a and b indeed correspond to AeNHE8, we would expect comparable bands to appear at molecular masses that are ∼30 kDa higher in AeNHE8-eGFP oocytes, reflecting the extra mass added by the fusion to eGFP. As seen in strip 3 of Fig. 9A, we observe such bands (i.e., a and b) at molecular masses of 132 and 140 kDa, respectively. These bands are also observed in AeNHE8-eGFP oocytes with the GFP antibody (strip 4 of Fig. 9A).
In AeNHE8 oocytes, another band of immunoreactive protein appears at a molecular mass of ∼150 kDa (c in strip 2, Fig. 9A). A similarly sized band is also detected in the AeNHE8-eGFP oocytes with the AeNHE8 and GFP antibodies (c in strips 3 and 4 of Fig. 9A). Similarly, the large band of diffuse immunoreactivity in AeNHE8 oocytes (d in strip 2 of Fig. 9A and Supplemental Fig. 2) spans a similar range of molecular masses to that detected by the AeNHE8 and GFP antibodies in AeNHE8-eGFP oocytes (d in strips 3 and 4 of Fig. 9A and Supplemental Fig. 2). Thus these fractions of AeNHE8 protein and AeNHE8-eGFP fusion protein appear to migrate anomalously of their mass.
To visualize expression of the AeNHE8-eGFP fusion protein in Xenopus oocytes, we used in vivo fluorescence. Figure 9B shows in vivo fluorescence images of oocytes 6 days after injection with either H2O or AeNHE8-eGFP cRNA (AeNHE8-eGFP). Whereas no fluorescence is detectable in the H2O-injected oocyte, a robust fluorescent signal is observed over the entire surface of the AeNHE8-GFP oocyte (Fig. 9B). This fluorescent signal is detectable in AeNHE8-eGFP oocytes as early as 2 days after injection and remains detectable for at least 14–17 days after injection (data not shown).
Taken together, the data from the Western blotting and fluorescent imaging indicate that Xenopus oocytes heterologously express AeNHE8 and AeNHE8-eGFP proteins. Moreover, the data show that AeNHE8 is expressed as a protein of higher molecular mass than expected at the surface of Xenopus oocytes. In contrast, Aedes Malpighian tubules express AeNHE8 as a protein of lower molecular mass than expected in an intracellular compartment of principal cells (Figs. 4 and 5A). We explain the reasons for these differences in the discussion.
Electrophysiological Characterization of AeNHE8 in Xenopus Oocytes
Basic properties of AeNHE8-expressing and H2O-injected oocytes.
As shown in Table 2, oocytes injected with cRNA encoding AeNHE8-eGFP (hereafter referred to as AeNHE8 oocytes) have 1) a high pHi, 2) a depolarized Vm, and 3) a greater membrane conductance compared with oocytes injected with H2O. The higher pHi of AeNHE8 oocytes is consistent with the activity of an NHE expected to operate in “forward” mode, i.e., Na+ uptake in exchange for H+ extrusion in solution 1. In contrast, a depolarized Vm and markedly enhanced membrane conductance of AeNHE8 oocytes are not expected from the activity of an electroneutral NHE.
Table 2.
Properties of AeNHE8-expressing and H2O-injected oocytes in solution 1
| Oocytes | pHi | Vm, mV | gm, μS |
|---|---|---|---|
| AeNHE8 | 7.35±0.02*(n=29) | −34.1±1.4*(n=31) | 1.64±0.15*(n=36) |
| H2O-injected | 7.19±0.02 (n=25) | −52.1±1.7 (n=17) | 0.35±0.02 (n=18) |
Values are means ± SE. pHi, intracellular pH; Vm, membrane voltage; gm, membrane conductance. Measurements of gm were obtained with 2-electrode voltage clamping, where the oocyte is clamped at a holding potential that is 30 mV hyperpolarized relative to the spontaneous Vm.
Significant difference from H2O-injected oocytes (P < 0.0001).
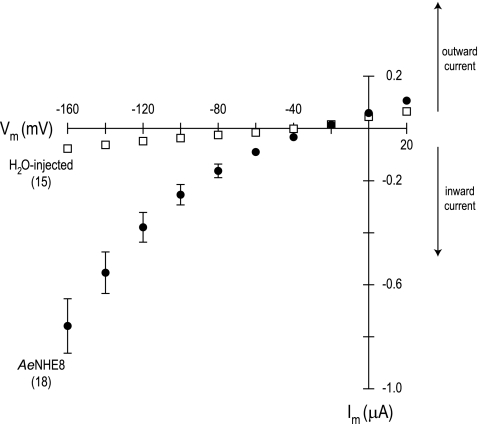
I-V relationships further illustrate the presence of the unexpected conductive pathway in AeNHE8 oocytes. As shown in Fig. 10, the I-V plot of AeNHE8 oocytes is curvilinear, increasing in voltage dependence with hyperpolarizing Vm. In contrast, the I-V plot of the H2O-injected oocytes is relatively linear and shallow throughout the range of voltages examined. At hyperpolarizing Vm, the membrane conductance is 13 times greater in AeNHE8 oocytes than in H2O-injected oocytes (Table 3). The reversal potential (Erev) of the AeNHE8 oocytes is significantly more positive by ∼10 mV compared with the H2O-injected oocytes (Table 3).
Fig. 10.
Current-voltage (I-V) plots of AeNHE8-expressing and H2O-injected oocytes. Negative membrane current (Im) values represent the net movement of positive charge into or negative charge out of the cell (inward current), whereas positive Im values represent the net movement of positive charge out of or negative charge into the cell (outward current). Data were acquired in solution 1. Values are means ± SE based on the number of oocytes in parentheses. Missing error bars indicate values too small to draw.
Table 3.
Parameters of current-voltage relationships in AeNHE8-expressing and H2O-injected oocytes
| Oocytes | Slope Conductance (μS) Between +20 and −60 mV | Slope Conductance (μS) Between −120 and −160 mV | Erev, mV |
|---|---|---|---|
| AeNHE8 (n = 18) | 2.44±0.32* | 9.47±1.22* | −28.3±1.4* |
| H2O-injected (n = 15) | 1.04±0.08 | 0.72±0.06 | −41.3±1.7 |
Values are means ± SE. Erev, reversal potential.
Significant difference from H2O-injected oocytes (P < 0.0001).
In the following sections, we characterize the electrophysiological properties of the AeNHE8 oocytes in more detail using experimental manipulations of the bathing solution. Our aim is to dissect the transport events that affect the pHi of AeNHE8 oocytes from those that influence the electrical properties (e.g., Vm).
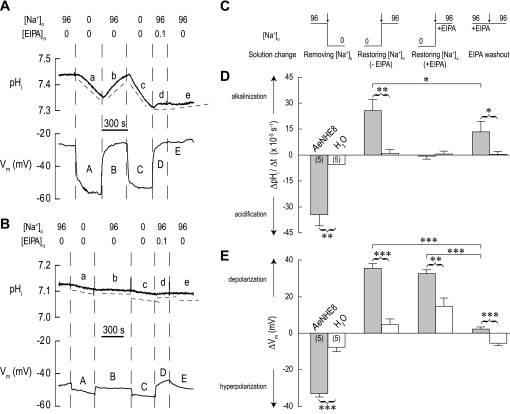
Effects of Na+ and EIPA on pHi and Vm.
A typical experiment is shown in Fig. 11A. The AeNHE8 oocyte is initially superfused with a solution containing 96 mM Na+ (solution 1). After the switch to a solution in which NMDG+ completely replaces Na+ (solution 2, Table 1), pHi begins on a declining slope (a in Fig. 11A), presumably due to AeNHE8 operating in “reverse” mode, i.e., H+ uptake and Na+ extrusion. In addition, the Na+ replacement causes an initial sharp hyperpolarization of Vm by ∼25 mV (A in Fig. 11A), followed by a more gradual hyperpolarization and stabilization of Vm, consistent with the presence of a conductive pathway. Restoring the normal [Na+]o results in 1) a reversal of the pHi trajectory and a gradual recovery of pHi to its resting level (b in Fig. 11A), and 2) a sharp repolarization of Vm (B in Fig. 11A).
Fig. 11.
Effects of removing and restoring extracellular Na+ concentration ([Na+]o) in the absence or presence of EIPA on intracellular pH (pHi) and Vm. A: representative recordings of pHi and membrane voltage (Vm) in an AeNHE8 oocyte. Extracellular concentrations (in mM) of Na+ and EIPA are indicated above the recordings. When Na+ is removed, it is replaced by NMDG+. Solution changes are indicated by dashed vertical lines. In the pHi tracing, the dashed lines (labeled a, b, c, etc.) are slopes to indicate the rates of pHi change (ΔpHi/Δt). A time bar is included. B: representative recordings of pHi and Vm in a H2O-injected oocyte, using a protocol similar to that in A. C: extracellular solution changes (arrows) after which the ΔpHi/Δt and ΔVm measurements were made in AeNHE8 and H2O-injected oocytes. The step-changes in [Na+]o associated with the solution changes are indicated (values in mM). D: summary of ΔpHi/Δt measurements. Shaded bars represent ΔpHi/Δt values of AeNHE8 oocytes (number of oocytes in parentheses) after the solution changes depicted in C. The value for removing [Na+]o represents the combined mean ΔpHi/Δt from periods a and c in A. The open bars represent H2O-injected oocytes at similar periods. E: summary of ΔVm measurements. Shaded bars represent ΔVm values of AeNHE8 oocytes measured 90 s after the solution changes depicted in C. The value for removing Na+ represents the combined mean ΔVm from A and C in A. The open bars represent H2O-injected oocytes after similar solution changes. Values are means ± SE. A missing error bar indicates a value too small to draw. Curved brackets connecting shaded and open bars represent unpaired t-tests that resulted in significant differences. Solid horizontal lines between shaded bars represent Bonferroni post hoc comparisons from a 1-way paired ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
Repeating the removal of [Na+]o repeats the effects on pHi and Vm (c and C in Fig. 11A). However, restoring the normal [Na+]o in the presence of 0.1 mM EIPA arrests the pHi recovery (d in Fig. 11A). In contrast, the Vm fully repolarizes in the presence of EIPA (D in Fig. 11A). Thus EIPA inhibits the Na+-dependent recovery of pHi, but it does not block the conductive pathway. Subsequent washout of EIPA has no effect on Vm (E in Fig. 11A), but starts a gradual recovery of pHi (e in Fig. 11A). In the oocyte shown, the recovery of pHi after EIPA washout is nominal, indicating that the effects of the inhibitor are not easily reversed. However, in other oocytes a more noticeable recovery ensued after washout of EIPA, as indicated by the data summary in Fig. 11D.
Repeating the above protocol on a H2O-injected oocyte causes minor effects on pHi (Fig. 11B) relative to those observed in the AeNHE8 oocyte. The solution changes result in similar directional changes to Vm as seen in the AeNHE8 oocyte, but with lower magnitudes (Fig. 11B). However, in the H2O-injected oocyte, restoring the normal [Na+]o in the presence of 0.1 mM EIPA causes a slow depolarization of Vm that is reversible upon the washout of EIPA (D in Fig. 11B).
Figure 11, C–E, summarizes the ΔpHi/Δt and ΔVm measurements in AeNHE8 (shaded bars) and H2O-injected (open bars) oocytes. The specific step changes in [Na+]o and EIPA are depicted in Fig. 11C. The ΔpHi/Δt of AeNHE8 oocytes is significantly greater in magnitude than that of the H2O-injected oocytes after all solution changes, except after restoration of [Na+]o in the presence of EIPA, in which case the rates are equal (Fig. 11D). In AeNHE8 oocytes, the rate of pHi recovery during the EIPA washout is significantly lower than during the restoration of normal [Na+]o without EIPA (Fig. 11D), indicating that on average the EIPA treatment has residual, but not irreversible, effects on AeNHE8 activity.
The hyperpolarization associated with removing [Na+]o (Fig. 11E) and the depolarizations associated with restoring normal [Na+]o, without or with EIPA, are significantly greater in AeNHE8 oocytes compared with H2O-injected oocytes (Fig. 11E), documenting the activation of a new membrane conductance in AeNHE8 oocytes. Again, the new membrane conductance in AeNHE8 oocytes is not affected by EIPA (Fig. 11E), in contrast to the profound effect of EIPA on ΔpHi/Δt (Fig. 11D).
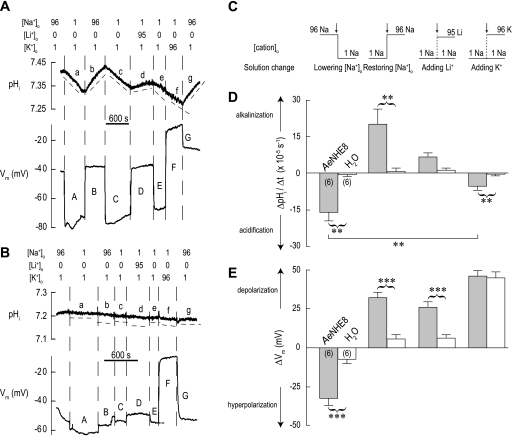
Effect of monovalent cation substitutions on pHi and Vm.
In Fig. 12A, an AeNHE8 oocyte is initially superfused with a solution containing 96 mM Na+ (solution 1). Switching to a solution in which the [Na+]o is lowered to 1 mM (solution 3) results in similar effects on pHi and Vm (a and A in Fig. 12A) as observed for removal of [Na+]o in Fig. 11A. Again, the effects are reversible upon restoration of normal [Na+]o (b and B in Fig. 12A).
Fig. 12.
Effects of replacing extracellular Na+ with Li+ or K+ on pHi and Vm. A: representative recordings of pHi and Vm in an AeNHE8 oocyte. Extracellular concentrations (in mM) of Na+, Li+, and K+ are indicated above the recordings. When Na+ is lowered, it is replaced by NMDG+, if not by Li+ or K+. Solution changes are indicated by dashed vertical lines. In the pHi tracing, the dashed lines (labeled a, b, c, etc.) are slopes to indicate the rates of pHi change (ΔpHi/Δt). A time bar is included. B: representative recordings of pHi and Vm in a H2O-injected oocyte, using a protocol similar to that in A. C: extracellular solution changes (arrows) after which the ΔpHi/Δt and ΔVm measurements were made in AeNHE8 and H2O-injected oocytes. The step-changes in extracellular cation concentration ([cation]o) associated with the solution changes are indicated (values in mM). D: summary of ΔpHi/Δt measurements. Shaded bars represent ΔpHi/Δt values of AeNHE8 oocytes (number of oocytes in parentheses) after the solution changes depicted in C. The value for lowering [Na+]o represents the combined mean ΔpHi/Δt from periods a and c in A. The value for restoring [Na+]o represents the combined mean ΔpHi/Δt from periods b and g in A. The open bars represent H2O-injected oocytes at similar periods. E: summary of ΔVm measurements. Shaded bars represent ΔVm values of AeNHE8 oocytes measured 90 s after the solution changes depicted in C. The value for lowering [Na+]o represents the combined mean ΔVm from periods A and C in A. The open bars represent H2O-injected oocytes after similar solution changes. Values are means ± SE. Curved brackets connecting shaded and open bars represent unpaired t-tests that resulted in significant differences. Solid horizontal lines between shaded bars represent Bonferroni post hoc comparisons from a 1-way paired ANOVA. **P < 0.01, ***P < 0.001.
Repeating the step reduction of [Na+]o leads again to the gradual cellular acidification and the sharp depolarization of Vm (c and C in Fig. 12A). However, in the switch to a solution in which Li+ substitutes for the lowered Na+ (solution 4, Table 1), the recovery of pHi is markedly blunted (d in Fig. 12A). Accordingly, AeNHE8 oocytes accept Li+ for exchange transport with H+, but not as effectively as Na+. In contrast, the Li+ solution repolarizes the Vm of the oocyte (D in Fig. 12A) as effectively as the Na+ solution (B in Fig. 12A), suggesting similar membrane conductances for Li+ and Na+. Again, we observe that pHi and Vm respond independently to experimental maneuvers.
Switching to a solution in which K+ replaces Na+ (solution 5) does not reverse the acidification of the oocyte (f in Fig. 12A). Instead, the cellular acidification that began upon lowering [Na+]o (e in Fig. 12A) continues unperturbed. In contrast, the Vm strongly depolarizes in the high-K+ solution (F in Fig. 12A), which reflects a membrane conductance far greater for K+ than Na+ and Li+ (B and D in Fig. 12A). Thereafter, replacing the extracellular K+ with Na+ results in 1) a profound reversal of the pHi trajectory followed by a robust recovery of pHi (g in Fig. 12A) and 2) a partial repolarization of Vm to about −22 mV (G in Fig. 12A). We assume that the AeNHE8 oocyte does not immediately repolarize to its original spontaneous Vm because it likely accumulated intracellular K+ in the previous superfusion with the high-K+ solution. Notably, this K+ loading apparently did not take place in the H2O-injected oocyte (see below and G in Fig. 12B).
In Fig. 12B, we repeated the above protocol on a H2O-injected oocyte, which elicits minor effects on pHi. The solution changes result in similar directional changes to Vm as seen in the AeNHE8 oocyte, but with lower magnitudes, except when Na+ is replaced with K+ (Fig. 12B). As in the AeNHE8 oocytes, high extracellular K+ concentration strongly depolarizes Vm (F in Fig. 12B), but the subsequent return to normal [Na+]o does not leave H2O-injected oocytes loaded with K+ (G in Fig. 12B).
Figure 12, C–E, summarizes the ΔpHi/Δt and ΔVm measurements in AeNHE8 (shaded bars) and H2O-injected (open bars) oocytes. The specific step changes in extracellular concentrations of Na+, Li+, and K+ are depicted in Fig. 12C. The ΔpHi/Δt of AeNHE8 oocytes is significantly greater in magnitude than that of the H2O-injected oocytes under all conditions except when Li+ replaces Na+, in which case the rates are similar (Fig. 12D). In the AeNHE8 oocytes, the replacement of Na+ with K+ does not recover pHi; instead, oocytes continue to acidify, albeit at a lower rate than when NMDG+ replaces Na+ (Fig. 12D). Accordingly, Li+ can partially substitute for Na+ in the maintenance of pHi, but K+ cannot.
The hyperpolarization of Vm associated with lowering [Na+]o (Fig. 12E) and the depolarizations associated with restoring the normal [Na+]o or adding extracellular Li+ (Fig. 12E) are significantly greater in AeNHE8 oocytes than in H2O-injected oocytes. Upon the replacement of extracellular Na+ with K+, the depolarization of Vm was similar in AeNHE8 and H2O-injected oocytes (Fig. 12E). These observations indicate that the new conductance in AeNHE8 oocytes is sensitive to monovalent cation concentrations of Na+, Li+, and K+.
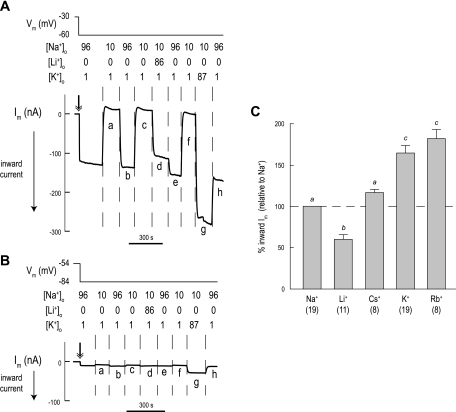
Monovalent cation preference of Im.
In view of the substantial voltage responses of AeNHE8 oocytes, together with their increased membrane conductance, we explored the cation preference of the conductive pathway using two-electrode voltage clamping. In Fig. 13A, an AeNHE8 oocyte is initially superfused with a solution containing 96 mM Na+ (solution 1), and then the oocyte is clamped at a holding potential that is 30 mV hyperpolarized to the spontaneous Vm (double-headed arrow in Fig. 13A). The inward Im of >100 nA corresponds to the conductive influx of a cation or the conductive efflux of an anion. Switching to a solution in which the [Na+]o is reduced to 10 mM (solution 6) causes the Im to reverse from a substantial inward to a small outward current (a in Fig. 13A). Restoring the normal [Na+]o restores the inward Im (b in Fig. 13A). It follows that hyperpolarizing and depolarizing currents are carried by Na+.
Fig. 13.
Effects of monovalent cations on Im of AeNHE8 oocytes. A: representative recording of Im in an AeNHE8 oocyte. Negative Im values represent the movement of positive charge into the cell or negative charge out of the cell (inward current). The double-headed arrow indicates the start of the voltage clamp. The Vm of the oocyte and extracellular concentrations of cations (in mM) of Na+, Li+, and K+ are indicated above the recording. When Na+ is lowered, it is replaced by NMDG+ if not by Li+ or K+. Solution changes are indicated by dashed vertical lines. A time bar is included. B: representative recording of Im in a H2O-injected oocyte, using a protocol similar to that in A. C: summary of relative effects of monovalent cations on inward currents of AeNHE8 oocytes. The shaded bars represent the inward current produced by replacing the lowered [Na+]o with various monovalent cations using the protocol in A. All Im values are standardized to the inward Im produced upon restoration of the normal [Na+]o (i.e., Na+ and the dashed line). Respective currents from H2O-injected oocytes have been subtracted. Values are means ± SE, based on measurements from the number of AeNHE8 oocytes in parentheses. Lower-case letters indicate grouping of means by Newman-Keuls post hoc comparisons from a 1-way unpaired ANOVA.
Repeating the step reduction of [Na+]o repeats the effect on Im (c in Fig. 13A). However, switching to a solution in which Li+ replaces Na+ (solution 7) results in a blunted inward Im (d in Fig. 13A). Accordingly, the conductive pathway is less permeable to Li+ than to Na+, which is confirmed by the subsequent replacement of extracellular Li+ with Na+ (e in Fig. 13A). Thereafter, lowering Na+ reduces the inward Im again (f in Fig. 13A). Switching to a solution in which K+ replaces Na+ (solution 8) results in the largest inward Im (g in Fig. 13A).
Repeating the above protocol on a H2O-injected oocyte results in 1) a low inward Im after clamping of the oocyte (double-headed arrow in Fig. 13B); and 2) similar directional changes to Im associated with the solution changes as seen in the AeNHE8 oocyte, but with much lower magnitudes (Fig. 13B). Notably, the high-K+ solution does not produce a large inward Im in H2O-injected oocytes (g in Fig. 13B) compared with that observed in the AeNHE8 oocytes (g in Fig. 13A).
The protocol in Fig. 13A was used to also examine the effects of adding Cs+ and Rb+ in place of Na+. A typical experiment is not shown. Instead, Fig. 13C summarizes the inward Im produced by each of the monovalent cations normalized to the inward Im produced by restoring normal [Na+]o. The inward Li+ current is significantly lower (∼40%) than the inward Na+ current, whereas the inward Cs+ current is similar to the inward Na+ current. The inward K+ and Rb+ currents are significantly greater (∼75%) than the inward Na+ current.
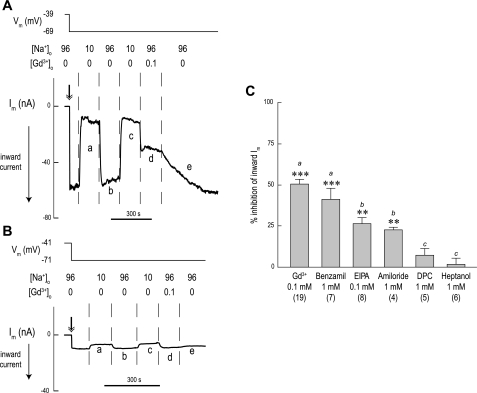
Inhibitor sensitivity of Im.
In Fig. 14A, an AeNHE8 oocyte is initially superfused with a solution containing 96 mM Na+ (solution 1), and then the oocyte is clamped at a holding potential that is 30 mV hyperpolarized to the spontaneous Vm (double-headed arrow in Fig. 14A), resulting in an inward Im. Switching to a solution in which [Na+]o is lowered to 10 mM (solution 6) causes the inward Im to approach zero and stabilize (a in Fig. 14A). Restoring the normal [Na+]o restores the inward Im (b in Fig. 14A).
Fig. 14.
Effects of inhibitors on Im of AeNHE8 oocytes. A: representative recording of Im in an AeNHE8 oocyte. In this tracing, the effects of Gd3+ on Im are shown. Negative Im values represent the movement of positive charge into the cell or negative charge out of the cell (inward current). The double-headed arrow indicates the start of the voltage clamp. The Vm of the oocyte and extracellular concentrations of Na+ and Gd3+ (in mM) are indicated above the recording. When Na+ is lowered, it is replaced by NMDG+. Solution changes are indicated by dashed vertical lines. A time bar is included. B: representative recording of Im in a H2O-injected oocyte, using a protocol similar to that in A. C: summary of the relative effects of inhibitors on the inward currents of AeNHE8 oocytes. The shaded bars represent the percent inhibition of the inward Im associated with restoration of normal [Na+]o using the protocol in A. Concentrations of the inhibitors are indicated. The inhibition is calculated by comparing the ΔIm after restoration of normal [Na+]o in the absence of an inhibitor (e.g., b in A) to that after restoration of normal [Na+]o in the presence of an inhibitor (e.g., d in A). Values are means ± SE, based on measurements from the number of AeNHE8 oocytes in parentheses. Lower-case letters indicate grouping of means by Newman-Keuls post-hoc comparisons from a 1-way unpaired ANOVA. Significant inhibition of the inward Im by the inhibitor (**P < 0.01 and ***P < 0.001).
Repeating the step reduction of [Na+]o repeats the effects on Im (c in Fig. 14A). However, restoring the normal [Na+]o in the presence of 0.1 mM Gd3+ reduces the inward Im by more than half (d in Fig. 14A). Accordingly, Gd3+ blocks the conductive influx of Na+. Washing out Gd3+ slowly restores the inward Im (e in Fig. 14A).
Repeating the above protocol on a H2O-injected oocyte (Fig. 14B) results in 1) a low inward Im after of clamping the oocyte (double-headed arrows in Fig. 14B); 2) similar directional changes to Im as seen in the AeNHE8 oocyte associated with lowering and restoring [Na+]o, but with lower magnitudes (e.g., a and b in Fig. 14B); and 3) no inhibitory effect on the small inward Im associated with restoring normal [Na+]o in the presence of Gd3+ (d in Fig. 14B).
The protocol in Fig. 14 was used to also examine the effects of other inhibitors on the inward Na+ current, including benzamil, EIPA, amiloride, DPC, and heptanol. Figure 14C summarizes the percent inhibition of the inward Im (upon restoring normal [Na+]o) by each of these compounds in AeNHE8 oocytes. As shown in Fig. 14C, Gd3+, benzamil, EIPA, and amiloride significantly inhibit ΔIm, whereas DPC and heptanol do not. The most effective inhibitors are Gd3+ and benzamil, blocking 40–50% of the inward Im, followed by EIPA and amiloride, which block ∼25% of the inward Im. In H2O-injected oocytes, none of the compounds significantly inhibit the small inward Im values (data not shown).
DISCUSSION
Cloning and Expression of the AeNHE8 cDNA
In the present study, we have cloned an ortholog of mammalian NHE8 from the Malpighian tubules of the yellow-fever mosquito Aedes aegypti. Besides a few minor differences in the 5′-UTR and the open-reading frame (Supplemental Fig. 1), the cDNA and the protein it encodes are identical to AeNHE8 cloned from an Aedes cDNA library by Kang'ethe and colleagues (34). In view of the similar hydrophobicity profiles and amino acid sequences shared between AeNHE8 and the mammalian NHEs (i.e., NHE1 and NHE3) in the predicted TM segments (Fig. 1A), we hypothesize that AeNHE8 has 12 TM segments and one reentrant loop (Figs. 1B and 2). Our predicted topological model for the AeNHE8 protein is consistent with that proposed recently for NHE isoforms from a wide variety of eukaryotic organisms (see supplemental figures in Ref. 11).
Our RT-PCR-based approach to measure AeNHE8-mRNA expression indicates that AeNHE8 transcripts are expressed ubiquitously in the female mosquito. That is, the AeNHE8 mRNA is present at a low level in all samples examined (detectable by PCR cycles 34–40, Fig. 3A) and does not appear to be substantially enriched in transporting epithelia, such as the Malpighian tubules and gut, relative to its expression in the whole animal (Fig. 3B). In other insects, such as Drosophila (21, 27) and the tobacco hornworm Manduca (Weihrauch D, unpublished observations), expression of NHE8 transcripts is also ubiquitous and not enriched within a particular tissue. Among vertebrates, ubiquitous expression of NHE8 transcripts is common (29, 43, 87, 88), but in mammals, NHE8 transcripts appear to be enriched in the kidneys and skeletal muscle (29, 43).
Expression of AeNHE8 Protein in Aedes Malpighian Tubules
Although Western blotting for AeNHE8 in Aedes Malpighian tubules indicates that the protein is expressed at a molecular mass that is ∼10 kDa lower than predicted by its cDNA (Fig. 4), the lower mass is in good agreement with that observed for AeNHE8 expressed heterologously in PS120 fibroblasts and yeast cells (34). Mammalian NHE8 also exhibits a lower than expected molecular mass (by ∼10 kDa) when expressed in intracellular compartments as an “immature” incompletely glycosylated form (28, 29, 43). The “mature” fully glycosylated form of mammalian NHE8 appears at molecular masses ∼20–30 kDa greater than expected on Western blots and reaches the plasma membrane (28, 29). In parallel, AeNHE8 in Malpighian tubules may be expressed in intracellular compartments as an immature form.
Immunohistochemical localization of AeNHE8 in Malpighian tubules verifies our interpretation of the Western blot. AeNHE8 localizes primarily to a subapical compartment of principal cells (Fig. 5A). In adult female Aedes, the subapical cytoplasm of principal cells is occupied by numerous membrane-bound concretions (6, 8). Using transmission electron microscopy, we have also observed the presence of 1) Golgi complexes, 2) vesicular-like structures (e.g., endosomes or secretory vesicles), and 3) rough endoplasmic reticulum in the subapical cytoplasm of principal cells (Piermarini PM, unpublished observations). Thus AeNHE8 may be expressed in one or more of the aforementioned organelles.
Consecutive sections of immunolabeled Malpighian tubules show that the AeNHE8-positive principal cells express the B subunit of the V-type H+-ATPase (Fig. 6), but not the α-subunit of the Na-K-ATPase (Fig. 7). Thus AeNHE8 is most likely expressed by principal cells of the distal, secretory segments of the tubules, because the V-type H+-ATPase is found in principal cells of both the proximal and distal segments of Aedes Malpighian tubules, whereas the Na-K-ATPase is only found in principal cells of the proximal segments of Aedes Malpighian tubules (55). The presence of AeNHE8 immunoreactivity in one principal cell and not in the adjacent principal cell of Aedes Malpighian tubules (e.g., Figs. 6 and 8) is consistent with observations made in Malpighian tubules of Drosophila, where principal cells express distinct genetic repertoires along the axial length of the tubule (67).
In Aedes Malpighian tubules, the localization of AeNHE8 is not influenced 5 min after mosquitoes finish a blood meal or 30 min after treatment of isolated tubules with db-cAMP (Fig. 8). Thus the transporter is not trafficked from an intracellular pool to the brush border during conditions of enhanced NaCl and fluid secretion. In contrast, the immunoreactivity for β-actin undergoes a dramatic redistribution from the cytosolic regions to the brush border 5 min after Aedes mosquitoes consume a blood meal and 15–60 min after treatment of isolated Malpighian tubules with 10−3 or 10−4 M db-cAMP (35). The redistribution of β-actin is hypothesized to participate in microvillar growth of the brush border, the trafficking of organelles to the brush border, and the assembly of the V-type H+-ATPase holoenzyme in the brush border (35), all of which are expected to promote transepithelial fluid secretion. Clearly, AeNHE8 does not participate in this promotion of diuresis.
Our localization of AeNHE8 in subapical regions of Aedes Malpighian tubules conflicts with that of Kang'ethe and colleagues (34), who report that AeNHE8 localizes to the apical membrane of principal cells. It is unlikely that the conflict arises from the different AeNHE8 antibodies used, because both were raised against similar synthetic AeNHE8 peptides (i.e., Ser511-Arg524 in our study, and Ser511-Leu525 for Kang'ethe et al.). However, on close examination, the AeNHE8 immunoreactivity in Fig. 8 of Kang'ethe et al. does not occur throughout the brush border as expected for a protein localized to the apical membrane, but is near the brush border and appears punctate, similar to what we observed. Moreover, the images of immunoreactivity taken in our study, which show greater resolution than those of Kang'ethe and colleagues, reveal a clear separation between AeNHE8 expressed in discrete subapical compartments and the B subunit of the V-type H+-ATPase expressed in the apical brush-border membrane (Fig. 5).
The intracellular localization of an NHE8 isoform is not unprecedented: 1) in Drosophila Malpighian tubules, immunolabeling for DrNHE8 localizes to intracellular compartments of principal cells, but not to the plasma membrane (21); 2) human NHE8 expressed in COS7 cells localizes to the mid- and trans-Golgi networks (43); 3) NHE8 immunoreactivity is beneath the brush border in renal proximal tubules of 1-day-old rats (3); and 4) the putative ortholog of NHE8 in C. elegans (NHX-8) appears to localize to vesicular compartments of seam cells (44). To date, the only examples in which a plasma membrane localization of an NHE8 isoform has clearly been demonstrated is in the brush border of renal proximal tubules from 10-day-old and adult rats (3, 28) and in the apical membrane of normal rat kidney (NRK) cell lines (92).
Functional Characterization of AeNHE8 in Xenopus Oocytes
To characterize the function of AeNHE8 in Xenopus oocytes using measurements of pHi and Vm, the protein must be expressed in the plasma membrane. Our immunochemical, in vivo fluorescent, and electrophysiological data in Xenopus oocytes are all consistent with the heterologous expression of AeNHE8 in the plasma membrane.
First, Western blotting in Xenopus oocytes indicates that the AeNHE8 protein and AeNHE8-eGFP fusion protein are primarily expressed at molecular masses ∼20–40 kDa greater than predicted by their cDNAs (bands a and b in Fig. 9A). In addition, we observe broad, diffuse bands of AeNHE8 (and GFP) immunoreactivity (e.g., d in Fig. 9A and Supplemental Fig. 2) that appear at even greater molecular masses than bands a and b. These findings are consistent with the expression of mature forms of AeNHE8 and AeNHE8-eGFP that reach the plasma membrane, as observed for mammalian NHE8 (28, 29). In contrast, Malpighian tubules express AeNHE8 as an immature form of lower molecular mass than expected within intracellular compartments (Figs. 4 and 5A). Second, the fluorescence associated with the expression of an AeNHE8-eGFP fusion protein is detectable over the entire surface of the oocyte (Fig. 9B), documenting the expression of AeNHE8 in the plasma membrane. Third, the display of unique physiological activities in AeNHE8 oocytes compared with H2O-injected counterparts confirms the overexpression of AeNHE8 in the plasma membrane. Most significantly, AeNHE8 oocytes exhibit large changes in pHi and Vm in response to step changes in extracellular cation concentrations. These changes are not observed in the H2O-injected oocytes.
It is important to point out that previous studies have documented endogenous NHE activity in Xenopus oocytes (12, 13, 33, 46, 64, 70). However, it is unlikely that this endogenous oocyte NHE contributes to the robust NHE activity that we observe in the AeNHE8-expressing oocytes, for the following reasons.
First, isolating oocytes from Xenopus ovaries with a collagenase digestion markedly decreases the activity of the endogenous oocyte NHE (70). In the present study, all of the oocytes were extracted from Xenopus ovaries with collagenase. Second, the activity of the endogenous oocyte NHE steadily declines within 3 days after its isolation from the ovary (13, 70). In the present study, the pHi recordings in the oocytes were conducted at least 6 days after their isolation. Third, the proton extrusion activity of the endogenous oocyte NHE is activated by a pronounced acidification of pHi (12, 14) or by cell shrinkage (33). In the present study, the AeNHE8 oocytes were evaluated near the resting pHi (typically >7.2) and the osmolalities of the experimental solutions were constant (Table 1). Fourth, although in one instance the heterologous expression of a membrane-bound protein, i.e., the Plasmodium falciparum chloroquine resistance transporter, was shown to enhance the activity of an endogenous oocyte NHE, removing and restoring [Na+]o had no detectable effects on the resting pHi of these oocytes (46). In contrast, lowering or restoring [Na+]o in AeNHE8-expressing oocytes results in noticeable effects on pHi within a few minutes of the solution change (Figs. 11A and 12A). Taken together, we attribute the pHi changes in the AeNHE8-expressing oocytes to the activity of the AeNHE8 protein.
AeNHE8 activity.
The Xenopus oocytes expressing AeNHE8 show changes in pHi that are consistent with the activity of an EIPA-sensitive Na/H exchanger. First, AeNHE8 oocytes have a higher pHi than H2O-injected oocytes (Table 2). Second, removing [Na+]o results in an acidification of pHi and restoring normal [Na+]o results in an alkalinization of pHi, which is blocked by EIPA (Fig. 11, A and D). These observations in oocytes agree with those made of AeNHE8 expressed in PS120 fibroblasts, where EIPA-sensitive uptake of 22Na+ is enhanced (34). However, we find that in Xenopus oocytes the proton extrusion activity of AeNHE8 has a preference for Na+ over Li+ and K+ (Fig. 12, A and D), which is in conflict with the activity of AeNHE8 expressed in yeast and characterized in reconstituted proteoliposomes (34). In proteoliposomes, the affinities of AeNHE8 for Na+ and K+ are comparable (34). A similar conflict occurs when mammalian NHE8 is studied in cells vs. proteoliposomes. For example, rat NHE8 mediates Na/H exchange, but not K/H exchange, in the apical plasma membranes of NRK cells that endogenously express the exchanger (92). In contrast, human NHE8 mediates both Na/H and K/H exchange when expressed in yeast and characterized in reconstituted proteoliposomes (43).
The above conflicts indicate that the physiological properties of NHE8 isoforms depend on the expression system and membranes in which they are characterized. In Xenopus oocytes (Fig. 9) and in NRK cells (92), NHE8 isoforms are primarily expressed as mature forms that reach the plasma membrane. However, in yeast, NHE8 isoforms are primarily expressed as immature forms in intracellular compartments (34, 43). Thus the characterization of NHE8 isoforms in Xenopus oocytes and in NRK cells reflects an activity in the plasma membrane, whereas the characterization of NHE8 in yeast proteoliposomes reflects the activity of the transporter in an intracellular compartment. A similar explanation has been offered previously by Zhang and colleagues (92). We conclude that AeNHE8 mediates EIPA-sensitive Na/H exchange in plasma membranes. Still, it is possible that AeNHE8 permits K/H exchange when expressed in the membranes of intracellular organelles, either in proteoliposomes of yeast or in the subapical compartments of Aedes Malpighian tubules.
Conductive activity.
The Xenopus oocytes expressing AeNHE8 in the present study also express a large membrane condutance, as indicated by the following. First, the AeNHE8 oocytes have a depolarized Vm and enhanced membrane conductance compared with H2O-injected oocytes (Table 2). Second, the I-V plot of AeNHE8 oocytes reveals an increasing membrane conductance at hyperpolarizing voltages that is not observed in the H2O-injected oocytes (Fig. 10). Furthermore, Erev is significantly more positive in AeNHE8 oocytes than in the H2O-injected oocytes (Table 3). Third, removing or lowering [Na+]o prominently hyperpolarizes Vm (Fig. 11, A and E) and decreases the inward flow of current (Fig. 13A). Together, these data indicate that AeNHE8 oocytes posses a conductive pathway in their plasma membranes, presumably for Na+, not found in the H2O-injected oocytes. The pathway could arise from 1) electrogenic exchange by AeNHE8 (e.g., 2Na+/H+ antiport), similar to that observed in membrane vesicles of crustaceans and echinoderms (1, 2, 66); 2) an intrinsic cation conductance within the AeNHE8 protein, as proposed for the rat electroneutral Na-HCO3 cotransporter (16, 20); or 3) an endogenous cation conductance of the oocyte that is activated by heterologous expression of AeNHE8 protein, similar to the conductances previously reported in Xenopus oocytes overexpressing heterologous membrane-bound proteins (46, 53, 72, 74).
Our data do not support a conductance due to the electrogenic 2Na+/H+ antiport by AeNHE8. First, restoring normal [Na+]o in the presence of EIPA and/or amiloride 1) fully blocks the pHi recovery of AeNHE8 oocytes, but it does not affect the repolarization of Vm (Fig. 11); and 2) does not effectively block the inward Im (Fig. 14). If AeNHE8 was an electrogenic 2Na+/H+ exchanger, then EIPA and/or amiloride would be expected to abolish the pHi recovery, repolarization of Vm, and the inward Im, as in the case of 2Na+/H+ antiport in crustaceans and echinoderms (1, 2, 66). Second, the conductive pathway of AeNHE8 oocytes favors the transport of K+ over that of Na+ and Li+ (Figs. 12 and 13), whereas the proton extrusion activity of AeNHE8 oocytes favors Na+ over Li+ and K+ (Fig. 12). An electrogenic NHE would be expected to display the same cation preferences for both the conductive pathway and the proton transport. Third, Fig. 12 shows that Vm and pHi change independently of each other in AeNHE8 oocytes, and preliminary results from our laboratory suggest that clamping the membrane potential of AeNHE8 oocytes does not influence their resting pHi (Piermarini PM, unpublished observations).
Thus our data tell of an independent conductive pathway that is activated by the heterologous expression of AeNHE8. In support of this conclusion, the relative ability of K+, Na+, and Li+ to produce inward currents in AeNHE8-expressing oocytes (Fig. 13C) is remarkably similar to that observed for various endogenous nonselective cation conductances of frog oocytes, such as those activated by membrane suction (i.e., stretch) or by exposure to maitotoxin or DIDS (9, 22, 68, 91). Furthermore, the sequence of the monovalent cations carrying inward Im (i.e., Rb+ ≈ K+ > Cs+ ≈ Na+ > Li+; Fig. 13C) suggests a type III or IV Eisenman sequence (25), indicating that the conducting pores consist of “weak” cation-binding sites that favor dehydrated permeation of ions rather than hydrated permeation. That is, the conductive pores favor larger cations that shed their hydration shells most readily. The preference for permeation of large cations with low hydration enthalpies is comparable to that observed previously for endogenous nonselective cation conductances in Xenopus oocytes (22, 91). Third, the most effective inhibitors of the inward Im associated with returning normal [Na+]o in AeNHE8 oocytes are Gd3+ and benzamil (Fig. 14). Gadolinium is a well-known blocker of endogenous nonselective cation conductances in Xenopus oocytes (9, 22, 61, 77, 90). Although the inhibition of the inward Im by Gd3+ was not complete, nonselective cation conductances in oocytes are difficult to fully block by Gd3+ once they have been activated (9, 61, 77). Furthermore, our finding that benzamil is a more effective inhibitor than amiloride is consistent with the presence of nonselective cation channels (9, 38).
Although we cannot rule out that independent parts of the AeNHE8 molecule mediate the pHi and voltage changes, we prefer the hypothesis that AeNHE8 mediates the Na+-dependent pHi changes and a protein endogenous to the oocyte mediates the voltage changes. The mechanisms that activate the endogenous conductive protein are unclear, but one study suggests that nonselective cation conductances of Xenopus oocytes are mediated by TRPC1 mechanosenstive cation channels (40).
Is AeNHE8 an Apical Cation/H+ Exchanger in Aedes Malpighian Tubules?
The study by Kang'ethe and colleagues (34) concludes that AeNHE8 may represent the EIPA-sensitive cation/H+ exchanger in the apical membrane of principal cells that is responsible for mediating the extrusion of Na+ and K+ in exchange for H+. Several lines of evidence from the present study do not support the authors' conclusion.
First, AeNHE8 transcripts are not enriched in Malpighian tubules. As discussed recently by Day and colleagues (21), one would expect to find a transcript encoding the apical cation/H+ exchanger to be enriched in epithelia enriched with the V-type H+-ATPase, i.e., Malpighian tubules and the gut. Second, our Western blotting and immunohistochemical experiments indicate that AeNHE8 is expressed as an intracellular form in Malpighian tubules. The exchanger localizes to a subapical compartment of principal cells, but not to the brush border, even after a diuresis is stimulated by a blood meal (85) or by exposing tubules to db-cAMP (84). Thus AeNHE8 does not play a direct role in the apical cation/H+ exchange of diuresis. Third, our electrophysiological characterization of AeNHE8 in oocytes demonstrates that the exchanger is EIPA sensitive but that its preferred activity in plasma membranes is Na/H exchange over K/H exchange. As noted by Day and colleagues (21), if the apical cation/H+ exchanger shows a preference, one would expect it to favor K/H exchange.
What Might Be the Role of Intracellular AeNHE8 in Principal Cells?
We hypothesize that AeNHE8 participates in regulating the pH of at least one type of organelle in principal cells, such as the membrane-bound concretions, endosomes, secretory vesicles, and/or the Golgi network. The proper sorting, targeting, and recycling of proteins and vesicles by organelles are highly dependent on the pH maintained within the lumen of organelles (42, 54). One mechanism by which the luminal pH of an organelle is regulated at a particular steady state is through the activity of a proton “leak” pathway, whose molecular identity remains unknown (54). At least in mammals, the intracellular NHEs (i.e., NHE6, NHE7, NHE8, and NHE9) are considered likely candidates that may contribute to this proton leak pathway in the various organelles of the secretory and endocytotic pathways (43, 50).
Given the prominent subapical localization of AeNHE8 in principal cells, AeNHE8 is likely to contribute to the regulation of pH in organelles that maintain and/or regulate transport processes in the brush border of principal cells. For example, it is possible that AeNHE8 is localized to endosomes or vesicles involved with the trafficking and/or recycling of membrane-bound proteins to and from the brush border. Such processes may be especially crucial to proteins in the apical membrane, because the brush border of Aedes Malpighian tubules is enriched with mitochondria that not only generate the ATP for fueling the apical V-type H+-ATPase (86) but also are likely to generate damaging free radicals via oxidative phosphorylation (i.e., ATP generation). Thus AeNHE8 could play an important role in a recycling pathway that maintains the integrity of proteins and organelles located in the brush border. Alternatively, AeNHE8 could contribute toward the regulation of pH in organelles (e.g., secretory vesicles) of principal cells that are involved with the latter stages of a basolateral-to-apical transcytotic pathway, such as those required for the excretion of xenobiotic compounds from the hemolymph (26, 69, 89) or for the secretion of mineralized concretions from within principal cells (82).
The Search for the Elusive Apical Cation/H+ Exchanger Continues
Compared with the combined 11 members of the CPA superfamily in mammals (9 NHEs and 2 NHAs), only 5 members are found in insects (3 NHEs and 2 NHAs). Of these five, orthologs of mammalian NHE3 (59) and NHE8 (the present study) have been ruled out as potential apical cation/H+ exchangers in Malpighian tubules of adult female Aedes, whereas the orthologs of mammalian NHE3, NHE6, and NHE8 have been ruled out in Malpighian tubules of Drosophila (21).
In Drosophila, transcripts encoding the two putative NHA proteins (orthologs of the bacterial K+ efflux, Kef, family) are enriched in Malpighian tubules, and both of these proteins localize to the brush border of principal cells (21). Furthermore, overexpression of these genes in Malpighian tubules increases levels of K+ and/or Na+ in the secreted fluid (21). To date, NHAs have not been studied in Aedes, but in larvae of the malarial mosquito Anopheles gambiae, NHA1 immunoreactivity is found in Malpighian tubules (48, 62). Thus the current data from Drosophila and Anopheles suggest that NHAs are the best candidates for apical cation/H+ exchangers in Malpighian tubules of Aedes. Making NHAs even more attractive is their potential for mediating K+/2H+ or Na+/2H+ exchange (62), which would enable the exchanger to be driven by the high apical Vm of principal cells that is established by the V-type H+-ATPase. A stoichiometry of K+(Na+)/nH+ is thermodynamically feasible for exporting K+ and Na+ into the tubule lumen.
GRANTS
Financial support from the following sources made this work possible: K01 DK-080194-01 awarded to P. M. Piermarini from the National Institute of Diabetes and Digestive and Kidney Diseases; SFB 431 awarded to Markus Huss from the Deutsche Forschungsgemeinschaft (DFG); WE 2868/1-2 from the DFG and a Discovery grant from the Natural Sciences and Engineering Research Council of Canada awarded to D. Weihrauch; IBN 0078058 awarded to K. W. Beyenbach from the National Science Foundation. The sabbatical support of K. W. Beyenbach with a Mercator Visiting Professorship from the DFG is gratefully acknowledged.
Acknowledgments
We thank the laboratories of Drs. William A. Horne, Huai-hu Chuang, and Robert E. Oswald at the Cornell University College of Veterinary Medicine for generously supplying the oocytes used in this study. We are also indebted to the laboratory of Dean Michael I. Kotlikoff (Cornell University College of Veterinary Medicine) for providing equipment used in molecular biology and Western blotting. Last, we thank Patricia Fisher, Kenneth Lau, and Mary Lou Norman (all of Cornell University) for excellent technical assistance.
Present address of D. Weihrauch: Dept. of Biological Sciences, Univ. of Manitoba, Winnipeg R3T 2N2, Canada
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahearn GA, Clay LP. Kinetic analysis of electrogenic 2Na+-1H+ antiport in crustacean hepatopancreas. Am J Physiol Regul Integr Comp Physiol 257: R484–R493, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Ahearn GA, Franco P. Electrogenic 2Na+/H+ antiport in Echinoderm gastrointestinal epithelium. J Exp Biol 158: 495–508, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Becker AM, Zhang J, Goyal S, Dwarakanath V, Aronson PS, Moe OW, Baum M. Ontogeny of NHE8 in the rat proximal tubule. Am J Physiol Renal Physiol 293: F255–F261, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340: 783–795, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Beyenbach KW Energizing epithelial transport with the vacuolar H+-ATPase. News Physiol Sci 16: 145–151, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Beyenbach KW Transport mechanisms of diuresis in Malpighian tubules of insects. J Exp Biol 206: 3845–3856, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Beyenbach KW, Pannabecker TL, Nagel W. Central role of the apical membrane H+-ATPase in electrogenesis and epithelial transport in Malpighian tubules. J Exp Biol 203: 1459–1468, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Beyenbach KW, Piermarini PM. Osmotic and ionic regulation in insects. In: Osmotic and Ionic Regulation: Cells and Animals, edited by Evans DH. Boca Raton, FL: CRC, 2009, p. 231–293.
- 9.Bielfeld-Ackermann A, Range C, Korbmacher C. Maitotoxin (MTX) activates a nonselective cation channel in Xenopus laevis oocytes. Pflügers Arch 436: 329–337, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Bobulescu IA, Moe OW. Na+/H+ exchangers in renal regulation of acid-base balance. Semin Nephrol 26: 334–344, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol 288: C223–C239, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Burckhardt BC, Kroll B, Fromter E. Proton transport mechanism in the cell membrane of Xenopus laevis oocytes. Pflügers Arch 420: 78–82, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Busch S, Burckhardt BC, Siffert W. Expression of the human sodium/proton exchanger NHE-1 in Xenopus laevis oocytes enhances sodium/proton exchange activity and establishes sodium/lithium countertransport. Pflügers Arch 429: 859–869, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Busch S, Rosskopf D, Lang HJ, Weichert A, Siffert W. Expression, functional characterization and tissue distribution of a Na+/H+ exchanger cloned from Xenopus laevis oocytes (XL-NHE). Pflügers Arch 436: 828–833, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Cabrero P, Pollock VP, Davies SA, Dow JA. A conserved domain of alkaline phosphatase expression in the Malpighian tubules of dipteran insects. J Exp Biol 207: 3299–3305, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405: 571–575, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Coast G The endocrine control of salt balance in insects. Gen Comp Endocrinol 152: 332–338, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Combet C, Blanchet C, Geourjon C, Deleage G. NPS@: network protein sequence analysis. Trends Biochem Sci 25: 147–150, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Cooper DS, Saxena NC, Yang HS, Lee HJ, Moring AG, Lee A, Choi I. Molecular and functional characterization of the electroneutral Na/HCO3 cotransporter NBCn1 in rat hippocampal neurons. J Biol Chem 280: 17823–17830, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Day JP, Wan S, Allan AK, Kean L, Davies SA, Gray JV, Dow JA. Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J Cell Sci 121: 2612–2619, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Diakov A, Koch JP, Ducoudret O, Muller-Berger S, Fromter E. The disulfonic stilbene DIDS and the marine poison maitotoxin activate the same two types of endogenous cation conductance in the cell membrane of Xenopus laevis oocytes. Pflügers Arch 442: 700–708, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Donowitz M, Li X. Regulatory binding partners and complexes of NHE3. Physiol Rev 87: 825–872, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Du J, Kean L, Allan AK, Southall TD, Davies SA, McInerny CJ, Dow JA. The SzA mutations of the B subunit of the Drosophila vacuolar H+ ATPase identify conserved residues essential for function in fly and yeast. J Cell Sci 119: 2542–2551, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Eisenman G, Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol 76: 197–225, 1983. [DOI] [PubMed] [Google Scholar]
- 26.Evans JM, Allan AK, Davies SA, Dow JA. Sulphonylurea sensitivity and enriched expression implicate inward rectifier K+ channels in Drosophila melanogaster renal function. J Exp Biol 208: 3771–3783, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Giannakou ME, Dow JA. Characterization of the Drosophila melanogaster alkali-metal/proton exchanger (NHE) gene family. J Exp Biol 204: 3703–3716, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Goyal S, Mentone S, Aronson PS. Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol 288: F530–F538, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Goyal S, Vanden Heuvel G, Aronson P. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol 284: F467–F473, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Hall TA BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41: 95–98, 1999. [Google Scholar]
- 31.Harvey WR, Wieczorek H. Animal plasma membrane energization by chemiosmotic H+ V-ATPases. J Exp Biol 200: 203–216, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Hegarty JL, Zhang B, Carroll MC, Cragoe EJJ, Beyenbach KW. Effects of amiloride on isolated Malpighian tubules of the yellow fever mosquito (Aedes aegypti). J Insect Physiol 38: 329–337, 1992. [Google Scholar]
- 33.Humphreys BD, Jiang L, Chernova MN, Alper SL. Hypertonic activation of AE2 anion exchanger in Xenopus oocytes via NHE-mediated intracellular alkalinization. Am J Physiol Cell Physiol 268: C201–C209, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Kang'ethe W, Aimanova KG, Pullikuth AK, Gill SS. NHE8 mediates amiloride-sensitive Na+/H+ exchange across mosquito Malpighian tubules and catalyzes Na+ and K+ transport in reconstituted proteoliposomes. Am J Physiol Renal Physiol 292: F1501–F1512, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Karas K, Brauer P, Petzel D. Actin redistribution in mosquito Malpighian tubules after a blood meal and cyclic AMP stimulation. J Insect Physiol 51: 1041–1054, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol 157: 105–132, 1982. [DOI] [PubMed] [Google Scholar]
- 37.Laemmli UK Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 38.Lane JW, McBride DW Jr, Hamill OP. Structure-activity relations of amiloride and its analogues in blocking the mechanosensitive channel in Xenopus oocytes. Br J Pharmacol 106: 283–286, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. ClustalW and ClustalX version 2. Bioinformatics 23: 2947–2948, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol 7: 179–185, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Martoglio B, Dobberstein B. Signal sequences: more than just greasy peptides. Trends Cell Biol 8: 410–415, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem 280: 1561–1572, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Nehrke K, Melvin JE. The NHX family of Na+-H+ exchangers in Caenorhabditis elegans. J Biol Chem 277: 29036–29044, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O'Leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316: 1718–1723, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nessler S, Friedrich O, Bakouh N, Fink RH, Sanchez CP, Planelles G, Lanzer M. Evidence for activation of endogenous transporters in Xenopus laevis oocytes expressing the Plasmodium falciparum chloroquine resistance transporter, PfCRT. J Biol Chem 279: 39438–39446, 2004. [DOI] [PubMed] [Google Scholar]
- 47.O'Donnell MJ, Ianowski JP, Linton SM, Rheault MR. Inorganic and organic anion transport by insect renal epithelia. Biochim Biophys Acta 1618: 194–206, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Okech BA, Boudko DY, Linser PJ, Harvey WR. Cationic pathway of pH regulation in larvae of Anopheles gambiae. J Exp Biol 211: 957–968, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflügers Arch 447: 549–565, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Orlowski J, Grinstein S. Emerging roles of alkali cation/proton exchangers in organellar homeostasis. Curr Opin Cell Biol 19: 483–492, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orlowski J, Grinstein S. Na+/H+ exchangers of mammalian cells. J Biol Chem 272: 22373–22376, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Pannabecker TL, Hayes TK, Beyenbach KW. Regulation of epithelial shunt conductance by the peptide leucokinin. J Membr Biol 132: 63–76, 1993. [DOI] [PubMed] [Google Scholar]
- 53.Parker MD, Young MT, Daly CM, Meech RW, Boron WF, Tanner MJ. A conductive pathway generated from fragments of the human red cell anion exchanger AE1. J Physiol 581: 33–50, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paroutis P, Touret N, Grinstein S. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology (Bethesda) 19: 207–215, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Patrick ML, Aimanova K, Sanders HR, Gill SS. P-type Na+/K+-ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. J Exp Biol 209: 4638–4651, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Petzel DH Na+/H+ exchange in mosquito Malpighian tubules. Am J Physiol Regul Integr Comp Physiol 279: R1996–R2003, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Piermarini PM, Choi I, Boron WF. Cloning and characterization of an electrogenic Na/HCO3− cotransporter from the squid giant fiber lobe. Am J Physiol Cell Physiol 292: C2032–C2045, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Pilitt DR, Jones JC. A qualitative method for estimating the degree of engorgement of Aedes aegypti adults. J Med Entomol 9: 334–337, 1972. [DOI] [PubMed] [Google Scholar]
- 59.Pullikuth AK, Aimanova K, Kang'ethe W, Sanders HR, Gill SS. Molecular characterization of sodium/proton exchanger 3 (NHE3) from the yellow fever vector, Aedes aegypti. J Exp Biol 209: 3529–3544, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Pullikuth AK, Filippov V, Gill SS. Phylogeny and cloning of ion transporters in mosquitoes. J Exp Biol 206: 3857–3868, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Reifarth FW, Clauss W, Weber WM. Stretch-independent activation of the mechanosensitive cation channel in oocytes of Xenopus laevis. Biochim Biophys Acta 1417: 63–76, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Rheault MR, Okech BA, Keen SB, Miller MM, Meleshkevitch EA, Linser PJ, Boudko DY, Harvey WR. Molecular cloning, phylogeny and localization of AgNHA1: the first Na+/H+ antiporter (NHA) from a metazoan, Anopheles gambiae. J Exp Biol 210: 3848–3861, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol 274: F425–F432, 1998. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki S, Ishibashi K, Nagai T, Marumo F. Regulation mechanisms of intracellular pH of Xenopus laevis oocyte. Biochim Biophys Acta 1137: 45–51, 1992. [DOI] [PubMed] [Google Scholar]
- 65.Satmary WM, Bradley TJ. The distribution of cell types in the Malpighian tubules of Aedes taeniorhynchus Diptera Culicidae. Int J Insect Morphol Embryol 13: 209–214, 1984. [Google Scholar]
- 66.Shetlar RE, Towle DW. Electrogenic sodium-proton exchange in membrane vesicles from crab (Carcinus maenas) gill. Am J Physiol Regul Integr Comp Physiol 257: R924–R931, 1989. [DOI] [PubMed] [Google Scholar]
- 67.Sozen MA, Armstrong JD, Yang M, Kaiser K, Dow JA. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc Natl Acad Sci USA 94: 5207–5212, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taglietti V, Toselli M. A study of stretch-activated channels in the membrane of frog oocytes: interactions with Ca2+ ions. J Physiol 407: 311–328, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Torrie LS, Radford JC, Southall TD, Kean L, Dinsmore AJ, Davies SA, Dow JA. Resolution of the insect ouabain paradox. Proc Natl Acad Sci USA 101: 13689–13693, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Towle DW, Baksinski A, Richard NE, Kordylewski M. Characterization of an endogenous Na+/H+ antiporter in Xenopus laevis oocytes. J Exp Biol 159: 359–369, 1991. [DOI] [PubMed] [Google Scholar]
- 71.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269: 92–95, 1995. [DOI] [PubMed] [Google Scholar]
- 72.Tzounopoulos T, Maylie J, Adelman JP. Induction of endogenous channels by high levels of heterologous membrane proteins in Xenopus oocytes. Biophys J 69: 904–908, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Geest M, Lolkema JS. Membrane topology and insertion of membrane proteins: search for topogenic signals. Microbiol Mol Biol Rev 64: 13–33, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wagner CA, Broer A, Albers A, Gamper N, Lang F, Broer S. The heterodimeric amino acid transporter 4F2hc/LAT1 is associated in Xenopus oocytes with a non-selective cation channel that is regulated by the serine/threonine kinase sgk-1. J Physiol 526: 35–46, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wakabayashi S, Pang T, Su X, Shigekawa M. A novel topology model of the human Na+/H+ exchanger isoform 1. J Biol Chem 275: 7942–7949, 2000. [DOI] [PubMed] [Google Scholar]
- 76.Wakabayashi S, Shigekawa M, Pouyssegur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiol Rev 77: 51–74, 1997. [DOI] [PubMed] [Google Scholar]
- 77.Weber WM, Popp C, Clauss W, Van Driessche W. Maitotoxin induces insertion of different ion channels into the Xenopus oocyte plasma membrane via Ca2+ stimulated exocytosis. Pflügers Arch 439: 363–369, 2000. [DOI] [PubMed] [Google Scholar]
- 78.Weihrauch D Active ammonia absorption in the midgut of the Tobacco hornworm Manduca sexta L.: transport studies and mRNA expression analysis of a Rhesus-like ammonia transporter. Insect Biochem Mol Biol 36: 808–821, 2006. [DOI] [PubMed] [Google Scholar]
- 79.Weihrauch D, Kanchanapoo J, Ao M, Prasad R, Piyachaturawat P, Rao MC. Weanling, but not adult, rabbit colon absorbs bile acids: flux is linked to expression of putative bile acid transporters. Am J Physiol Gastrointest Liver Physiol 290: G439–G450, 2006. [DOI] [PubMed] [Google Scholar]
- 80.Weihrauch D, Ziegler A, Siebers D, Towle DW. Molecular characterization of V-type H+-ATPase (B-subunit) in gills of euryhaline crabs and its physiological role in osmoregulatory ion uptake. J Exp Biol 204: 25–37, 2001. [DOI] [PubMed] [Google Scholar]
- 81.Weng XH, Huss M, Wieczorek H, Beyenbach KW. The V-type H+-ATPase in Malpighian tubules of Aedes aegypti: localization and activity. J Exp Biol 206: 2211–2219, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Wessing A, Zierold K. The formation of type-I concretions in Drosophila Malpighian tubules studied by electron microscopy and X-ray microanalysis. J Insect Physiol 45: 39–44, 1999. [DOI] [PubMed] [Google Scholar]
- 83.Wieczorek H, Putzenlechner M, Zeiske W, Klein U. A vacuolar-type proton pump energizes K+/H+ antiport in an animal plasma membrane. J Biol Chem 266: 15340–15347, 1991. [PubMed] [Google Scholar]
- 84.Williams JC, Beyenbach KW. Differential effects of secretagogues on the electrophysiology of the Malpighian tubules of the yellow fever mosquito. J Comp Physiol 154: 301–309, 1984. [Google Scholar]
- 85.Williams JC, Hagedorn HH, Beyenbach KW. Dynamic changes in flow rate and composition of urine during the post blood meal diuresis in Aedes aegypti. J Comp Physiol 153: 257–266, 1983. [Google Scholar]
- 86.Wu DS, Beyenbach KW. The dependence of electrical transport pathways in Malpighian tubules on ATP. J Exp Biol 206: 233–243, 2003. [DOI] [PubMed] [Google Scholar]
- 87.Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36–G41, 2005. [DOI] [PubMed] [Google Scholar]
- 88.Yan JJ, Chou MY, Kaneko T, Hwang PP. Gene expression of Na+/H+ exchanger in zebrafish H+ -ATPase-rich cells during acclimation to low-Na+ and acidic environments. Am J Physiol Cell Physiol 293: C1814–C1823, 2007. [DOI] [PubMed] [Google Scholar]
- 89.Yang J, McCart C, Woods DJ, Terhzaz S, Greenwood KG, Ffrench-Constant R, Dow JA. A Drosophila systems approach to xenobiotic metabolism. Physiol Genomics 2007. [DOI] [PubMed]
- 90.Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science 243: 1068–1071, 1989. [DOI] [PubMed] [Google Scholar]
- 91.Yang XC, Sachs F. Characterization of stretch-activated ion channels in Xenopus oocytes. J Physiol 431: 103–122, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, Bobulescu IA, Goyal S, Aronson PS, Baum MG, Moe OW. Characterization of Na+/H+ exchanger NHE8 in cultured renal epithelial cells. Am J Physiol Renal Physiol 293: F761–F766, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu YC, Specht CA, Dittmer NT, Muthukrishnan S, Kanost MR, Kramer KJ. Sequence of a cDNA and expression of the gene encoding a putative epidermal chitin synthase of Manduca sexta. Insect Biochem Mol Biol 32: 1497–1506, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Zizak M, Cavet ME, Bayle D, Tse CM, Hallen S, Sachs G, Donowitz M. Na+/H+ exchanger NHE3 has 11 membrane spanning domains and a cleaved signal peptide: topology analysis using in vitro transcription/translation. Biochemistry 39: 8102–8112, 2000. [DOI] [PubMed] [Google Scholar]