Abstract
The principal mediators of renal phosphate (Pi) reabsorption are the SLC34 family proteins NaPi-IIa and NaPi-IIc, localized to the proximal tubule (PT) apical membrane. Their abundance is regulated by circulatory factors and dietary Pi. Although their physiological importance has been confirmed in knockout animal studies, significant Pi reabsorptive capacity remains, which suggests the involvement of other secondary-active Pi transporters along the nephron. Here we show that a member of the SLC20 gene family (PiT-2) is localized to the brush-border membrane (BBM) of the PT epithelia and that its abundance, confirmed by Western blot and immunohistochemistry of rat kidney slices, is regulated by dietary Pi. In rats treated chronically on a high-Pi (1.2%) diet, there was a marked decrease in the apparent abundance of PiT-2 protein in kidney slices compared with those from rats kept on a chronic low-Pi (0.1%) diet. In Western blots of BBM from rats that were switched from a chronic low- to high-Pi diet, NaPi-IIa showed rapid downregulation after 2 h; PiT-2 was also significantly downregulated at 24 h and NaPi-IIc after 48 h. For the converse dietary regime, NaPi-IIa showed adaptation within 8 h, whereas PiT-2 and NaPi-IIc showed a slower adaptive trend. Our findings suggest that PiT-2, until now considered as a ubiquitously expressed Pi housekeeping transporter, is a novel mediator of Pi reabsorption in the PT under conditions of acute Pi deprivation, but with a different adaptive time course from NaPi-IIa and NaPi-IIc.
Keywords: brush-border membrane, inorganic phosphate, sodium-dependent transport
the principal means by which mammals achieve homeostasis of inorganic phosphate (Pi) is through control of Pi reabsorption along the renal proximal tubule (5, 28, 29). The underlying physiological signals include dietary Pi, circulating hormones such as parathyroid hormone and growth hormone, vitamin D, and phosphotonins (e.g., fibroblast growth factor 23, secreted frizzled related protein-4, matrix extracellular phosphoglycoprotein) (for review, see Refs. 4, 12, and 28). In the kidney, the functional protein targets of these factors are two gene products of the solute carrier family SLC34 (SLC34A1, SLC34A3), commonly referred to as type II Na+-coupled Pi cotransporters (NaPi-IIa, NaPi-IIc), which are expressed in the brush-border membrane (BBM) of proximal tubular epithelia. Another member of the SLC34 family, SLC34A2 (NaPi-IIb), is not expressed in the kidney, but is proposed to mediate Pi transport in the small intestine and other organs (27).
NaPi-IIa and NaPi-IIc mediate apical entry of Pi in the epithelial cell from the primary urine via a secondary-active transport mechanism that couples the movement of Na+ down their electrochemical gradient to uphill movement of Pi. A wide body of experimental evidence supports the view that the abundance of these proteins in the BBM determines the degree of renal Pi reabsorption. The electrogenic NaPi-IIa and electroneutral NaPi-IIc have been the subject of extensive kinetic studies at the molecular level (reviewed in Ref. 53) and studies on renal tubular Pi handling in vitro and in vivo (reviewed in Refs. 12, 26, and 28). Their physiological importance is underscored by the clear phenotypes observed in animal knockout studies. Mice in which the Npt2a gene coding for NaPi-IIa is knocked out exhibit Pi wasting, hypercalcuria, and skeletal abnormalities (3). Moreover, Na+-dependent Pi uptake in BBM preparations from these mice is reduced by ∼70% compared with the tissue from normal animals. However, the incomplete suppression of Pi transport activity in this and other studies (46) also implies that there must be other Pi-selective transport proteins involved. Thus, with the subsequent identification of the third member of the SLC34 family (NaPi-IIc) and its localization to the BBM of proximal tubular epithelia (40), it was generally assumed that the transport activity of NaPi-IIc could account for the remaining 30% Pi transport capacity in Npt2a−/− mice (46). This conclusion was further supported by the documentation of increased abundance of NaPi-IIc in the kidneys of Npt2a−/− mice as a compensation for the lack of NaPi-IIa (33, 46). Recently, it was reported that knockout of both NaPi-IIa and NaPi-IIc in mice results in severe hypophosphatemia, markedly reduced bone mineralization, and increased urinary Pi and Ca2+ compared with single knockout mice (41). Despite this phenotype, it is significant that, even in these animals, some renal Pi reabsorptive capacity remained. Notwithstanding passive paracellular flux of Pi driven by the transepithelial potential, this indicates that there should be other secondary-active transport proteins present in the kidney to mediate Pi transport from the nephron lumen.
Two potential candidates are gene products of the SLC17 and SLC20 families, both known to transport Pi by a secondary-active mechanism. Although originally identified through expression cloning as a Na+-dependent Pi cotransporter (30), localized in the kidney, the role of SLC17A1 in renal Pi handling has been questioned by subsequent studies (7, 28). On the other hand, the two known members of the SLC20 family, SLC20A1 (PiT-1) and SLC20A2 (PiT-2), are generally considered to fulfill a housekeeping role consistent with their ubiquitous expression (2, 14, 17, 18, 31, 45). PiT-1 and PiT-2 were originally identified as cell surface receptors for gibbon ape leukemia virus (Glvr-1) and murine amphotropic leukemia virus, respectively, and were subsequently shown to mediate Na+-coupled Pi cotransport (18, 34). Both PiT-1 and PiT-2 are electrogenic (17, 18, 36), but unlike NaPi-IIa and NaPi-IIc, they show a preference for monovalent Pi and reduced sensitivity to pH and the Pi transport inhibitor phosphonoformic acid (PFA) compared with NaPi-IIa/c (36, 51). Therefore, with such different functional characteristics compared with SLC34 proteins, one might expect them to subserve unique roles in Pi handling. For example, recent studies that address their potential roles in vascular calcification (15, 22, 23, 51) and bone mineralization (55) suggest that they may be key players in skeletal and vascular pathologies.
SLC20 proteins have also been proposed as housekeeping transporters in basolateral membrane of renal epithelia, although immunohistochemical evidence for basolateral localization has so far been lacking (26, 28). In mouse kidney, the relative abundance of mRNA for PiT-1 and PiT-2 was reported to be >50-fold less than NaPi-IIa, and, in the same study using in situ hybridization with a probe for Glvr-1 (PiT-1), a signal was detected throughout the kidney (47) and, similarly, PiT-2 at the mRNA and protein levels has been detected in rat kidney in all regions (20). Recently, PiT-1 and PiT-2 gene expression was shown to be upregulated in Npt2a−/− mice under conditions of metabolic acidosis, which suggests a possible compensatory role for SLC20 proteins (32). Because the localization at the cellular level was not established in these studies, the relative abundance of the candidate Pi transporter mRNAs may not necessarily reflect that of their corresponding proteins or the apical Pi transport rate. Therefore, any conclusions about the roles these proteins may play in Pi handling should be treated with caution without the support of functional expression data. In this respect, several in vitro studies indicate that Pi transport mediated by SLC20 proteins is upregulated by Pi depletion. In 208F rat fibroblasts grown in Pi-free medium, increased Pi uptake and mRNA levels of both PiT-1 and PiT-2 were documented (18). Similarly, in human embryonic kidney cells, Pi deprivation stimulated PiT-1-mediated Pi uptake (11), and stimulation of PiT-2 mRNA and protein levels was reported for two osteosarcoma cell lines (8).
The aims of the present study were to investigate the kidney localization and potential regulatory roles of one member of the SLC20 family, PiT-2, in renal Pi handling under the influence of one of the key regulators of Pi reabsorption, Pi diet. Because both NaPi-IIa and NaPi-IIc show clear phenotypical adaptive responses to changes in Pi diet (24, 25, 35, 42), they were used as benchmarks against which the PiT-2 behavior was compared. In this report, we show for the first time that Pit-2 is localized to the brush border of renal proximal tubules, and its abundance is strongly regulated by Pi diet, but with a slower adaption rate compared with NaPi-IIa. Our findings should prompt a reevaluation of the role played by SLC20 proteins in Pi homeostasis and their potential as regulated targets for renal Pi handling.
MATERIALS AND METHODS
Antibody preparation.
Polyclonal antibodies were prepared against synthetic peptides from rat NaPi-IIa (rabbit), rat NaPi-IIc (chicken), and rat PiT-2 (rabbit) (Davids Biotechnologie, Regensburg, Germany). The NaPi-IIa peptide sequence was MMSYSERLGGPAV and corresponds to amino acids 1-13 in the NH2 terminal; the NaPi-IIc peptide sequence was AHCYENPQVIASQQL (50). The PiT-2 peptide sequence was HCKVGSVVAVGWIRSRKA and corresponds to amino acids 597-614, close to the COOH terminus. The peptides were conjugated to keyhole limpet hemocyanin, mixed in Freund's complete adjuvant, and injected in rabbit or chicken. Two booster injections were given to the animals before sera were collected. A monospecific antibody was affinity purified from serum using the corresponding antigenic peptide.
Animals and diets.
Specific diets and the procedure to adapt the animals to chronic diet or acute changes was performed as previously described (19). All animal handling was according to Spanish and Swiss Animal Welfare laws and were approved by the local veterinary authorities. Briefly, for chronic adaptation, male Wistar rats 6–8 wk old (Charles River Laboratories) were first stabilized on a normal diet (0.6% Pi) for 5 days and then placed on either one containing 1.2% Pi (chronic high-Pi diet) or 0.1% Pi (chronic low-Pi diet) for 5 days before being killed. Diets were otherwise identical in their mineral, electrolyte, protein, carbohydrate, fat, and calorie content. For acute experiments, animals were supplied food from 0800 to 1000 AM each day after which they had access to tap water only. They were first conditioned on the high-Pi or low-Pi diets for 3–4 days as above and then switched to the acute dietary regime (0800) [acute low-Pi (0.1%) or acute high-Pi (1.2%)], and subsequently killed in triplicates at the specified time (2, 4, 8, 24, and 48 h) after switching to the acute diet. Each animal consumed the same quantity of food.
BBM preparation and free-flow electrophoresis.
BBM were prepared according to the Mg2+ precipitation method, with one single precipitation, as previously described (6). Simultaneous isolation of BBM and basolateral membrane was achieved by free-flow electrophoretic separation of the crude membrane fraction obtained from rat kidney cortex (9, 10).
Brush-border membrane vesicle transport assays.
Brush-border membrane vesicle (BBMV) 32P uptake was performed as described elsewhere (6, 52). The protein concentration in the BBMV samples was quantitated using a Bio-Rad Protein Assay kit (Bio-Rad). BBMVs prepared from kidneys from five rats previously adapted with the chronic high-Pi diet and from five rats treated with the chronic low-Pi diet were incubated with solutions containing 300 mM mannitol, 20 mM HEPES-Tris, pH 7.4, and 125 mM NaCl. The substrate Pi was made with 0.125 mM K2HPO4 and 1 μCi/ml 32P to give a final concentration 0.1 mM close to the expected apparent affinity constant for Pi for Na+-dependent transport in BBMVs. Trisodium PFA (final concn 6 mM) was added to the same solution with 107 mM NaCl. The stop solution contained 100 mM mannitol, 5 mM Tris·HCl, pH 7.4, 150 mM NaCl, and 5 mM Pi. Na+ dependence was established by incubating BBMVs in solutions in which KCl replaced NaCl equimolarly. BBMVs were incubated for 1 or 120 min with the uptake solutions and then washed with stop solution on a 45-μm cellulose nitrate filter. 32Pi activities of individual filters were counted using a Packard Tri-Carb 2900TR scintillation counter.
Protein detection and relative quantification by Western blot.
Immunoblot assays on BBM preparations were performed as previously described (21). In brief, BBM proteins (34 μg/lane) were electrophoresed in Miniprotean III (Bio-Rad). The NaPi-IIa affinity antibody was used at 1:3,000 dilution in PBS, NaPi-IIc at 1:5,000, and Pit-2 at 1:3,000. Primary antibodies were incubated overnight in the presence of 0.01% NaNO3 and detected with horsedarish peroxidase-conjugated secondary antibodies. Chemiluminescent reaction was performed with a Millipore kit. Densitometric signals were obtained with a Gel-Doc 1000 (Bio-Rad) after exposure to an X-ray film. Specificity of signals was determined with preimmune serum and by blocking the affinity-purified antibodies with the corresponding antigenic peptide using the standard procedure, and 50-fold molar excess of peptide per IgG. NaPi-IIa and Pit-2 antibodies were used at 1:3,000 dilution, and NaPi-IIc at 1:5,000. For all immunoblots, a protein determination was performed (DC Protein Assay Kit; Bio-Rad) to ensure equal loading per lane.
Immunohistochemistry on chronically treated animals.
Immunohistochemical assays were performed with Wistar rats (Charles River Laboratories) placed on the chronic high-Pi or low-Pi diets for 5 days. On day 5, rats were induced in deep anesthesia by 20 mg xylazine and 100 mg ketamine and perfused through the abdominal aorta with 3% paraformaldehyde in PBS buffer for 5 min and with PBS buffer for a further 5 min. Kidneys were then removed, maintained in ice-cold PBS buffer, cut in 5-mm slices, and frozen using liquid propane. Consecutive 4-μm slices were cut using a Leica Cryostat Microtome, fixed on polylysine-treated slides, and allowed to dry for 2 min. After rehydration using ice-cold PBS buffer, the slices were exposed to 0.1% SDS-PBS solution for 5 min at room temperature, washed two times, 10 min each, in PBS buffer, incubated for 30 min in blocking buffer [10% normal donkey serum (Sigma), 2% BSA (Sigma), PBS buffer] at room temperature, and afterward exposed to the antibodies (dissolved in the blocking buffer) overnight at 4°C. Sections were then washed two times, 10 min each, in PBS buffer and subsequently exposed to the anti-rabbit IgG conjugated with fluorescein (Alexa Fluor 488 donkey anti-rabbit; Molecular Probes) and phalloidin (Texas Red-X; Molecular Probes), both dissolved in the blocking solution, for 1 h at room temperature. Sections were rinsed with PBS buffer and then mounted with Glycergel Mounting Medium (DakoCytomation; Denmark) supplied with DABCO and observed by a fluorescent microscope (Nikon Eclipse TE 300 plus Nikon Xenon power supply XPS-100 lamp).
Data analysis.
Data for repeated measurements are given as means ± SE. Statistical significance between groups was performed using paired Student's t-test.
RESULTS
Functional evidence that Na+/Pi transport is not mediated exclusively by SLC34 proteins in proximal tubule BBMVs.
A functional assay using BBMVs from rat renal tissue offered compelling evidence that SLC34 proteins do not exclusively mediate the Na+-coupled Pi transport capacity and the remaining transport is under dietary regulation. Here, we compared the Pi uptake of rat BBMVs in the absence and presence of the competitive inhibitor of type II Na+-coupled Pi transport, PFA (44). BBMVs were prepared from animals treated chronically with high-Pi (1.2%) and low-Pi (0.1%) diets to change the membrane abundance of SLC34 proteins (21, 42). Uptake was measured at 1 min, at which time the transport has typically peaked to give a sensitive measure of any dietary influence on transport activity. From the data (Table 1), with no PFA in the medium, BBMVs from high-Pi animals showed a 52% reduction in Pi uptake compared with vesicles from low-Pi animals, in agreement with previous findings under linear transport velocity conditions (21). BBMVs from both animal groups treated on either diet showed residual uptake with 6 mM PFA in the uptake medium. For chronic high-Pi-treated animals, we documented a 74% inhibition, whereas, for chronic low-Pi-treated animals, the inhibition was 53%, which was statistically different from the high-Pi group. In these assays, Na+-independent uptake (equimolar K+ replacement) was negligible, which confirmed that Na+-coupled Pi transport is the principal mechanism mediating Pi transport at the apical membrane of PT epithelia.
Table 1.
Uptake of BBMV prepared from rat kidney for chronic Pi diets
| BBMV uptake condition | Diet |
|
|---|---|---|
| Chronic low Pi (0.1 %) | Chronic high Pi (1.2%) | |
| Control + 0.1 mM Pi | 3,947±366 (91±1) | 1,911±348 (76±3) |
| Control + 0.1 mM Pi + 6 mM PFA | 1,860±416* | 492±172* |
| Inhibition, % | 53 | 74 |
Uptake values in pmol Pi/mg protein are given as means ± SE; n = 3 experiments. Pi concentration in uptake medium: 0.1 mM, 1 min Pi incubation. Values in parentheses are uptake data in 0 Na+ (equimolar replacement with K+, see scap≈materials and methods≈). BBMW, brush-border membrane vesicles; PFA, phosphonoformic acid.
Statistical significance between control and PFA groups for each diet determined by paired t-test (P = 0.022).
If Pi transport were mediated solely by SLC34 proteins (NaPi-IIa, NaPi-IIc), we would expect the fractional inhibition of uptake by PFA to be independent of dietary condition, since it has been shown by studies using Xenopus oocytes that both NaPi-IIc and NaPi-IIa have both comparable apparent affinities for Pi (40, 52) and inhibition constants for PFA (52). The 21% difference in uptake for BBMVs from rats conditioned under two dietary conditions therefore suggests that part of the uptake is mediated by a Na+-dependent Pi transport system that has a different sensitivity to PFA.
PiT-2 expression in rat kidney cortex.
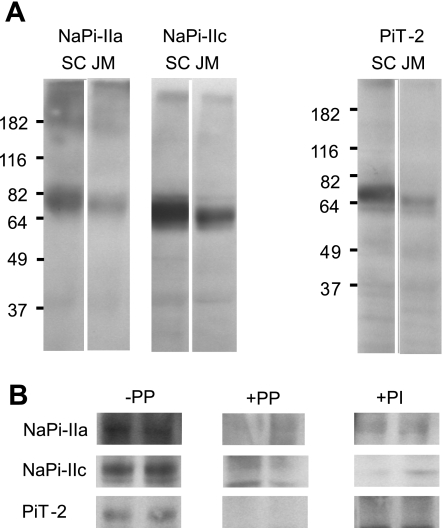
Based on the above findings and given that Pi transport by SLC20 proteins is significantly less sensitive to PFA (36, 50, 51), we focused our attention on one member of the SLC20 gene family (PiT-2, SLC20A2) that previous studies have shown is expressed in the kidney (20, 47). To confirm the specificity of our PiT-2 antibody, we first performed Western blots of HEK-293 cells transfected with the rat PiT-2 clone (data not shown). In Western blots of BBM prepared from rat superficial cortex (SC) and juxtamedullary (JM) material from the same animals under nonreducing conditions, the rat PiT-2 antibody recognized a major polypeptide fragment with an estimated molecular mass ∼70 kDa for BBMs from both SC and JM. This corresponds to the expected mass based on the amino acid sequence. NaPi-IIa and NaPi-IIc antibodies were used as positive controls (Fig. 1A). In this preparation, we confirmed the specificity of the antibody by peptide protection and by blotting with the preimmune sera of the animals (Fig. 1B). For PiT-2, the 70-kDa fragment was comparable with previously published values in basolateral liver plasma membranes (14), osteosarcoma cell lines (8), and overexpression in CHO cells (38). These data strongly suggested that PiT-2 is expressed in the rat kidney BBM. However, because of possible contamination by other membranes, we cannot exclude the possibility that PiT-2 might be expressed in other membrane fractions such as basolateral and intracellular organelles.
Fig. 1.
Detection of Na+-coupled Pi cotransporter (NaPi)-IIa, NaPi-IIc, and PiT-2 proteins in kidney brush-border membrane (BBM) preparations by immunoblotting. A: Western blots of BBM from superficial cortical (SC) and juxtamedullary (JM) regions for NaPi-IIa, NaPi-IIc, and PiT-2 from the same animal. In this and other Western blots, 34 μg protein were loaded per lane. NaPi-IIa affinity antibody was used at 1:3,000 dilution in PBS, NaPi-IIc at 1:5,000, and PiT-2 at 1:3,000. B: specificity of the antibodies. −PP, immunoreactive bands in control kidney cortex BBM proteins; +PP, blocking of antibodies with the immunogenic peptide. Affinity purified antibodies were incubated with 50 μg/ml peptide for 5 min at room temperature before the Western blot assay. +PI, blots obtained with preimmune sera of the corresponding animals.
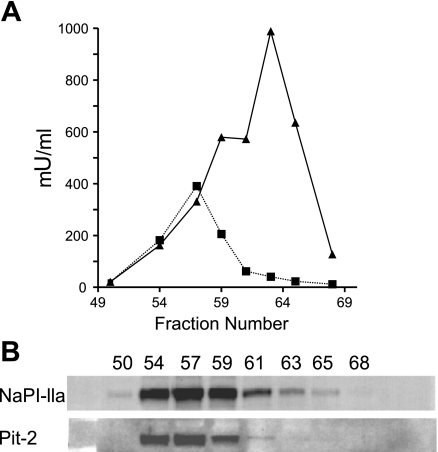
Additional confirmation of the protein localization of PiT-2 in BBM was obtained by performing free-flow electrophoresis of apical and basolateral membranes from rat kidney homogenate. Single fractions were analyzed for the enzymatic activities of leucine aminopeptidase N (an indicator of apical localization) and Na+-K+-ATPase (an indicator of basolateral localization) (Fig. 2A). Fractions were then probed with the NaPi-IIa and PiT-2 antibody (Fig. 2B). The NaPi-IIa and PiT-2 profiles follow that of leucine aminopeptidase N, which provides strong biochemical evidence that PiT-2 is mostly apically localized in the nephron, like NaPi-IIa.
Fig. 2.
Free-flow electrophoresis. Free-flow electrophoretic separation of rat kidney cortical BBM and basolateral membranes. Single fractions were analyzed for enzyme activity in leucine aminopeptidase N (▪) and Na+-K+-ATPase (▴) (A) and probed by Western blot analysis for NaPi-IIa and PiT-2 (B).
Immunohistochemistry confirms apical localization of PiT-2.
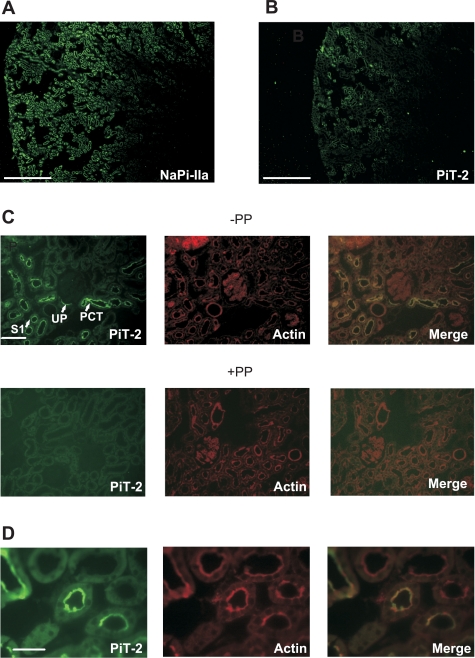
We performed immunohistochemistry on rat kidney slices staining for PiT-2 (Fig. 3A) and NaPi-IIa as a control (Fig. 3B) from the same kidney, obtained from a rat that had previously adapted on a chronic low-Pi (0.1%) diet. As expected, immunoreactive signals corresponding to NaPi-IIa were localized exclusively in the proximal tubule, in agreement with previous studies on rat kidney (9, 42). An immunoreactive signal recognized by the PiT-2 antibody was widely distributed throughout the cortex, predominantly in the early proximal tubules. At higher magnification, PiT-2 staining appears to be exclusively associated with the apical membrane of tubular epithelia and is mostly seen in the brush border of the early proximal tubules (S1 segment) (Fig. 3C, top). We also confirmed that the PiT-2 staining was absent when the slice was preincubated using the antigenic peptide (Fig. 3C, bottom). Figure 3D shows colocalization of PiT-2 with the membrane cytoskeleton protein β-actin, confirming the association of the transporter with the BBM.
Fig. 3.
PiT-2 immunohistochemistry for kidney slices from one animal fed on a chronic low-Pi (0.1%) diet. A: immunostaining with PiT-2 antibody indicates expression of PiT-2, particularly in cortical areas. Scale bar: 750 μm. B: immunostaining with NaPi-IIa antibody of material from same animal shows more widespread staining in both cortical and JM regions. Scale bar: 750 μm. C: immunostaining with PiT-2 antibody of kidney slice at higher magnification shows strong staining in the early part of the proximal tubule, including urinary pole (UP), proximal convoluted tubule (PCT), and S1 segment of PT (S1). Scale bar: 375 μm. Second row shows images with peptide protection (PP). D: higher magnification of S1 segment staining for PiT-2 (left), actin (center), and merged images (right). Scale bar: 75 μm.
Chronic Pi diet induces a redistribution of PiT-2.
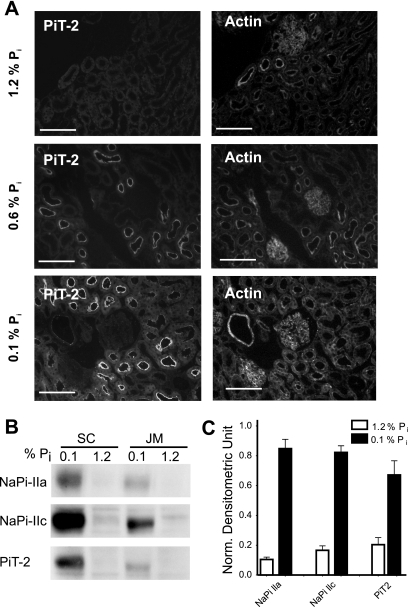
Slices of kidney from rats preconditioned with low-, normal-, or high-Pi diets show a redistribution of the immunoreactive signal of PiT-2 (Fig. 4). For animals fed chronically for 5 days on a high-Pi diet, we were unable to detect PiT-2 (Fig. 4A, top). However, for animals fed on a normal-Pi (0.6%) and low-Pi (0.1%) diets, clear evidence for PiT-2 localization in the brush-border regions of proximal tubules was obtained (Fig. 4A, center and bottom,). Under chronic low-Pi dietary conditions, the staining was stronger than under normal dietary conditions. These findings provide compelling evidence that the abundance of PiT-2 protein is regulated by Pi dietary conditions. The lack of staining for the chronic high-Pi diet condition suggests that, like NaPi-IIa (24), PiT-2 is not simply redistributed within the proximal tubule cell.
Fig. 4.
Response to chronic dietary conditions. A: immunohistochemistry of kidney slice stained for PiT-2 (left) and for actin (right). Top: chronic high-Pi (1.2%) diet; center: normal-Pi (0.6%) diet; bottom: chronic low-Pi (0.1%) diet. Scale bar: 150 μm. B: Western blots from SC and JM BBM using material from animals fed on chronic high-Pi and chronic low-Pi diets. C: quantitation of Western blots for NaPi-IIa, NaPi-IIc, and PiT-2 indicates that all three transporters respond similarly to chronic changes in Pi concentration in the diet (n = 5 experiments). The downregulation, based on densitometric analysis, of material from rats treated on low-Pi and high-Pi diet is 88% for NaPi-IIa (P < 0.0001), 84% for NaPi-IIc (P < 0.0001), and 70% for PiT-2 (P = 0.0023). Normalized densitometric units were calculated by subtracting the background from the signal and corrected using the actin signal.
To provide further confirmation of these observations, we performed Western blots of BBM preparations from both superficial and JM cortex material (Fig. 4, B and C). After 3 days of chronic low-Pi diet, clear bands in the Western blot were obtained for all three proteins in both kidney regions (Fig. 4B). Quantification of Western blots from BBM showed that they were all downregulated by >70% (Fig. 4C). Exposure to the chronic high-Pi diet led to the disappearance of a band for NaPi-IIa, NaPi-IIc, and PiT-2 in both kidney regions. The behavior of NaPi-IIa and NaPi-IIc confirmed the findings in previous studies (21, 25, 37, 42, 48). Taken together, our data provide compelling evidence that PiT-2 protein level is regulated by chronic Pi dietary manipulations.
Acute response of protein expression to change in dietary Pi.
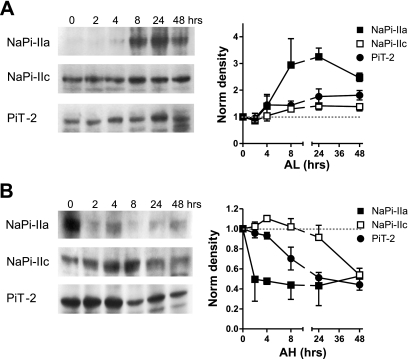
The NaPi-IIa and NaPi-IIc protein abundances in the BBM of proximal tubular epithelia are tightly regulated by acute changes to dietary Pi (21, 42). To investigate if PiT-2 protein was also affected by acute dietary manipulations, we fed rats on two complementary dietary regimes. First, for rats fed chronically with a high-Pi (1.2%) diet, and then exposed to a low-Pi (0.1%) diet for 2, 4, 8, 24, and 48 h, NaPi-IIa protein progressively upregulated in the BBM after 2 h and was maintained for 8 h (Fig. 5A). NaPi-IIc expression in BBMs followed a similar, but slower, pattern. PiT-2 protein levels in BBM paralleled the changes of NaPi-IIc, i.e., adaptation to the low-Pi diet was slower than NaPi-IIa.
Fig. 5.
Acute adaptation of NaPi-IIa, NaPi-IIc, and PiT-2 to dietary conditions. A: changes from chronic high-Pi diet to acute low-Pi diet (AL). Western blots from representative animals are shown for NaPi-IIa, NaPi-IIc, and PiT-2. In each case, densitometric quantitation of acute changes shows the mean of measurements on material taken from 3 animals killed at the indicated time point. Whereas NaPi-IIa is upregulated within 4 h of dietary change to low-Pi diet, NaPi-IIc and PiT-2 require 8 h to increase their abundance at the plasma membrane. B: changes from chronic low-Pi diet to acute high-Pi diet (AH) as in A. NaPi-IIa is eliminated from the BBM within 2 h, whereas the reduction in NaPi-IIc expression is only clearly seen at 48 h, and PiT-2 at 8 h.
In the converse experiment, animals were chronically adapted to a low-Pi diet for 3 days and then subjected to the high-Pi diet and killed at the same time points as above. As expected, NaPi-IIa showed rapid downregulation within 2 h of exposure to the high-Pi regime, in agreement with previous findings (21, 42). Both NaPi-IIc and PiT-2 were also downregulated; however, the respective time courses were slower. NaPi-IIc did not show a significant change in level until 48 h of exposure to the acute high-Pi diet, whereas the time course of PiT-2 was intermediate between NaPi-IIa and NaPi-IIc. The slow regulation of NaPi-IIc contrasts with the more rapid effect reported for NaPi-IIc (42). The discrepancy in behavior most likely lies in the different dietary conditions used in the previous study, in which animals were adapted on 0.02% Pi for the chronic low-Pi diet. This may have resulted in a higher level of NaPi-IIc protein at the start of the acute low-Pi phase compared with the present study.
DISCUSSION
We report for the first time the renal localization and dietary regulation of a member of the SLC20 family, PiT-2. We present immunohistological evidence that PiT-2 is localized to the BBM of rat kidney proximal tubules and therefore potentially offers an additional route for Pi to enter the proximal tubule epithelia from the glomerular filtrate. Moreover, we document that changes in dietary Pi markedly affect the abundance PiT-2 protein in the BBM.
The functional data presented in Table 1 also highlight the effect of diet on two components of Na+-dependent Pi transport: a “PFA-sensitive” component and a “PFA-resistant” component. In the absence of PFA, the high-Pi diet resulted in a 52% suppression of total Pi uptake relative to the low-Pi diet case, as previously established (21). In the presence of 6 mM PFA, the high-Pi diet led to a 33% suppression of the component of uptake contributed by the PFA-sensitive component (estimated for each dietary condition by subtracting the uptake with PFA from the uptake without PFA). In contrast, for the PFA-resistant component of uptake, the high-Pi diet led to a 74% suppression of Na+-dependent uptake.
Our findings are also supported indirectly by other functional studies of BBMVs. Consistent with the functional data that we report here for 1-min uptakes (Table 1), Villa-Bellosta et al. (50) have shown that the Na+-dependent 32P (0.05 mM total Pi) uptake rate of renal cortex BBMVs in the initial constant velocity range is reduced by ∼70% in the presence of 6 mM PFA. For this assay, the apparent inhibition constant for PFA (K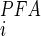 ) was estimated to be 2.2 mM, which is approximately twofold greater than values reported for assays involving SLC34 proteins heterologously expressed in Xenopus oocytes (50). If NaPi-IIa and NaPi-IIc alone were responsible for mediating Na+-dependent Pi cotransport and given the similarity of the phenomenological kinetic constants for these isoforms determined using the Xenopus oocyte expression system (52), we predict that the transport rate should be reduced by ∼80% for these uptake conditions.1
Under the tacit assumption that the oocyte data are also valid for the BBMV system, the discrepancy between measured and predicted uptake rate inhibition strongly supports the notion that a component of Pi transport capacity is present in renal BBMs that is either insensitive to PFA or has a significantly higher K
) was estimated to be 2.2 mM, which is approximately twofold greater than values reported for assays involving SLC34 proteins heterologously expressed in Xenopus oocytes (50). If NaPi-IIa and NaPi-IIc alone were responsible for mediating Na+-dependent Pi cotransport and given the similarity of the phenomenological kinetic constants for these isoforms determined using the Xenopus oocyte expression system (52), we predict that the transport rate should be reduced by ∼80% for these uptake conditions.1
Under the tacit assumption that the oocyte data are also valid for the BBMV system, the discrepancy between measured and predicted uptake rate inhibition strongly supports the notion that a component of Pi transport capacity is present in renal BBMs that is either insensitive to PFA or has a significantly higher K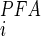 compared with that for NaPi-IIa/c. The higher value of K
compared with that for NaPi-IIa/c. The higher value of K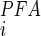 reported for renal BBMs would therefore result from a mixed inhibitory response of PFA. Moreover, our functional uptake data indicate that this transport component is regulated by Pi diet, but its abundance in the BBM does not parallel the well-characterized dietary regulation of SLC34 proteins (21, 25, 42). Given that SLC20 proteins show significantly reduced (51, 52) or no sensitivity to PFA (36), depending on the expression system and assay conditions, it is therefore tempting to speculate that PiT-2 contributes to at least part of the non-SLC34-mediated Na+-dependent Pi transport capacity. This conclusion is underscored by the correlated changes in kidney immunostaining and BBM protein abundance documented in the present study. Because no SLC20-specific inhibitor is presently available, it is not possible, based on the present data, to quantitate the relative contribution of PiT-2 to the overall Pi transport at the BBM. Furthermore, it has been reported (51, 52) that Na+-dependent Pi transport rate of SLC20 proteins is partially suppressed by high concentrations of PFA; therefore, we cannot exclude the possibility that part of the PFA-sensitive uptake results from partial inhibition of PiT-2 itself and that a PFA-insensitive component of transport is mediated by yet another transport protein.
reported for renal BBMs would therefore result from a mixed inhibitory response of PFA. Moreover, our functional uptake data indicate that this transport component is regulated by Pi diet, but its abundance in the BBM does not parallel the well-characterized dietary regulation of SLC34 proteins (21, 25, 42). Given that SLC20 proteins show significantly reduced (51, 52) or no sensitivity to PFA (36), depending on the expression system and assay conditions, it is therefore tempting to speculate that PiT-2 contributes to at least part of the non-SLC34-mediated Na+-dependent Pi transport capacity. This conclusion is underscored by the correlated changes in kidney immunostaining and BBM protein abundance documented in the present study. Because no SLC20-specific inhibitor is presently available, it is not possible, based on the present data, to quantitate the relative contribution of PiT-2 to the overall Pi transport at the BBM. Furthermore, it has been reported (51, 52) that Na+-dependent Pi transport rate of SLC20 proteins is partially suppressed by high concentrations of PFA; therefore, we cannot exclude the possibility that part of the PFA-sensitive uptake results from partial inhibition of PiT-2 itself and that a PFA-insensitive component of transport is mediated by yet another transport protein.
What could the potential role of PiT-2 in the apical membrane of PT epithelia be? First, because the time course of regulation of PiT-2 in response to dietary switching to an acute high-Pi regime is different from both NaPi-IIa and NaPi-IIc, this suggests that the regulatory pathway is different. PiT-2 might therefore play a role in fine tuning overall Pi reabsorption under different dietary conditions. Second, a possible answer may be found from kinetic studies of SLC20 proteins (36) and the related malaria plasmodium falciparum PiT (39) expressed in Xenopus oocytes. These studies show conclusively that these transporters prefer monovalent Pi (H2PO4−), whereas all three SLC34 family proteins prefer divalent Pi (HPO42−) (1, 13, 54). Moreover, the maximum electrogenic transport rate of PiT-1 shows less sensitivity to changes in external pH (36, 39) compared with NaPi-IIa (53), which makes it an ideal candidate for transporting Pi under acidic conditions. Micropuncture studies have shown that the pH in the proximal tubule can fall by up to 0.78 pH units relative to arterial blood in rats previously loaded with NH4Cl (16, 49). At pH 7.4, the ratio [HPO42−]/[H2PO4−] = 4, which decreases to 1.6 at pH 6.6, at which the NaPi-IIa/c transport rate will be reduced by ∼50% (29, 53). On the other hand, we would expect PiT-2 to be unaffected by these changes in luminal pH so that the presence of both types of transporter will assure the reuptake of the necessary amount of Pi from the primary urine.
In conclusion, until now, Pi reabsorption in the kidney was thought to be solely the responsibility of the SLC34 proteins NaPi-IIa and NaPi-IIc, whereas SLC20 proteins have been generally considered to fulfill general housekeeping roles throughout the organism. Our findings provide compelling evidence that it is now time to revise this long-standing renal Pi-handling dogma.
GRANTS
This study was supported by financial contributions from the Swiss National Science Foundation to H. Murer (31-065397/1), the Spanish Ministry of Education and Science to V. Sorribas (BFU2006-06284/BFI), a predoctoral fellowship from the Government of Aragón, Spain (B086/2007) to R. Villa-Bellosta, and the National Institute of Diabetes and Digestive and Kidney Diseases to M. Levi (R01 DK-066029).
Acknowledgments
We thank Drs. Beatrice Beck-Schimmer, Martin Schäpfer, B. Stieger, and Paola Capuano for assistance with the animal preparation and Drs. Nati Hernando and Carsten A. Wagner for valuable discussions throughout this project.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Assuming Michaelis-Menten kinetics and ideal competitive inhibition, the ratio of transport velocities with and without PFA can be expressed as: ([Pi] + K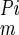 )/[[Pi] + K
)/[[Pi] + K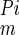 (1 + [PFA]/K
(1 + [PFA]/K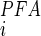 )], where K
)], where K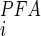 is the apparent affinity constant for Pi, K
is the apparent affinity constant for Pi, K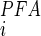 is the inhibition constant for PFA (43), and brackets denote concentration. For estimating the ratio for SLC34 proteins, we used K
is the inhibition constant for PFA (43), and brackets denote concentration. For estimating the ratio for SLC34 proteins, we used K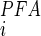 = 0.08 mM and K
= 0.08 mM and K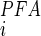 = 1 mM based on the data of Ref. 52.
= 1 mM based on the data of Ref. 52.
REFERENCES
- 1.Bacconi A, Virkki LV, Biber J, Murer H, Forster IC. Renouncing electrogenicity is not free of charge: switching on electrogenicity in a Na+-coupled phosphate cotransporter. Proc Natl Acad Sci USA 102: 12606–12611, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai L, Collins JF, Ghishan FK. Cloning and characterization of a type III Na-dependent phosphate cotransporter from mouse intestine. Am J Physiol Cell Physiol 279: C1135–C1143, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA 95: 5372–5377, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiol 69: 341–359, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Berndt TJ, Kumar R. Clinical disturbances of phosphate homeostasis. In: Seldin and Giebisch's The Kidney, edited by Alpern RJ and Hebert SC. New York, NY: Academic, 2008, p. 1989–2006.
- 6.Biber J, Stieger B, Stange G, Murer H. Isolation of renal proximal tubular brush-border membranes. Nat Protoc 2: 1356–1359, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Broer S, Schuster A, Wagner CA, Broer A, Forster I, Biber J, Murer H, Werner A, Lang F, Busch AE. Chloride conductance and Pi transport are separate functions induced by the expression of NaPi-1 in Xenopus oocytes. J Membr Biol 164: 71–77, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Chien ML, Foster JL, Douglas JL, Garcia JV. The amphotropic murine leukemia virus receptor gene encodes a 71-kilodalton protein that is induced by phosphate depletion. J Virol 71: 4564–4570, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Custer M, Lotscher M, Biber J, Murer H, Kaissling B. Expression of Na-Pi cotransport in rat kidney: localization by RT-PCR and immunohistochemistry. Am J Physiol Renal Fluid Electrolyte Physiol 266: F767–F774, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Cutillas PR, Geering B, Waterfield MD, Vanhaesebroeck B. Quantification of gel-separated proteins and their phosphorylation sites by LC-MS using unlabeled internal standards: analysis of phosphoprotein dynamics in a B cell lymphoma cell line. Mol Cell Proteom 4: 1038–1051, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes I, Beliveau R, Friedlander G, Silve C. NaPO4 cotransport type III (PiT1) expression in human embryonic kidney cells and regulation by PTH. Am J Physiol Renal Physiol 277: F543–F551, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Forster IC, Hernando N, Biber J, Murer H. Proximal tubular handling of phosphate: a molecular perspective. Kidney Int 70: 1548–1559, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Forster IC, Loo DD, Eskandari S. Stoichiometry and Na+ binding cooperativity of rat and flounder renal type II Na+-Pi cotransporters. Am J Physiol Renal Physiol 276: F644–F649, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Frei P, Gao B, Hagenbuch B, Mate A, Biber J, Murer H, Meier PJ, Stieger B. Identification and localization of sodium-phosphate cotransporters in hepatocytes and cholangiocytes of rat liver. Am J Physiol Gastrointest Liver Physiol 288: G771–G778, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Giachelli CM, Speer MY, Li X, Rajachar RM, Yang H. Regulation of vascular calcification: roles of phosphate and osteopontin. Circ Res 96: 717–722, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Gottschalk CW, Lassiter WE, Mylle M. Localization of urine acidification in the mammalian kidney. Am J Physiol 198: 581–585, 1960. [DOI] [PubMed] [Google Scholar]
- 17.Kavanaugh MP, Kabat D. Identification and characterization of a widely expressed phosphate transporter/retrovirus receptor family. Kidney Int 49: 959–963, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Kavanaugh MP, Miller DG, Zhang W, Law W, Kozak SL, Kabat D, Miller AD. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA 91: 7071–7075, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanaspa MA, Giral H, Breusegem SY, Halaihel N, Baile G, Catalan J, Carrodeguas JA, Barry NP, Levi M, Sorribas V. Interaction of MAP17 with NHERF3/4 induces translocation of the renal Na/Pi IIa transporter to the trans-Golgi. Am J Physiol Renal Physiol 292: F230–F242, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Leung JC, Barac-Nieto M, Hering-Smith K, Silverstein DM. Expression of the rat renal PiT-2 phosphate transporter. Horm Metab Res 37: 265–269, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Levi M, Lotscher M, Sorribas V, Custer M, Arar M, Kaissling B, Murer H, Biber J. Cellular mechanisms of acute and chronic adaptation of rat renal Pi transporter to alterations in dietary Pi. Am J Physiol Renal Fluid Electrolyte Physiol 267: F900–F908, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Giachelli CM. Sodium-dependent phosphate cotransporters and vascular calcification. Curr Opin Nephrol Hypertens 16: 325–328, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res 98: 905–912, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Lotscher M, Kaissling B, Biber J, Murer H, Levi M. Role of microtubules in the rapid regulation of renal phosphate transport in response to acute alterations in dietary phosphate content. J Clin Invest 99: 1302–1312, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madjdpour C, Bacic D, Kaissling B, Murer H, Biber J. Segment-specific expression of sodium-phosphate cotransporters NaPi-IIa and -IIc and interacting proteins in mouse renal proximal tubules. Pflugers Arch 448: 402–410, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto K, Ito M, Tatsumi S, Kuwahata M, Segawa H. New aspect of renal phosphate reabsorption: the type IIc sodium-dependent phosphate transporter. Am J Nephrol 27: 503–515, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Murer H, Forster I, Hilfiker H, Pfister M, Kaissling B, Lotscher M, Biber J. Cellular/molecular control of renal Na/Pi-cotransport. Kidney Int Suppl 65: S2–S10, 1998. [PubMed] [Google Scholar]
- 28.Murer H, Forster IC, Hernando N, Biber J. Proximal Tubular Handling of Phosphate: Na/Pi-Cotransporters and their Regulation. In: Seldin and Giebisch's The Kidney, edited by Alpern RJ and Hebert SC. New York, NY: Acdemic, 2008, p. 1979–1988.
- 29.Murer H, Hernando N, Forster I, Biber J. Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol Rev 80: 1373–1409, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Murer H, Werner A, Reshkin S, Wuarin F, Biber J. Cellular mechanisms in proximal tubular reabsorption of inorganic phosphate. Am J Physiol Cell Physiol 260: C885–C899, 1991. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab Pharmacokinet 23: 22–44, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Nowik M, Picard N, Stange G, Capuano P, Tenenhouse HS, Biber J, Murer H, Wagner CA. Renal phosphaturia during metabolic acidosis revisited: molecular mechanisms for decreased renal phosphate reabsorption. Pflugers Arch 457: 539–549, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Ohkido I, Segawa H, Yanagida R, Nakamura M, Miyamoto K. Cloning, gene structure and dietary regulation of the type-IIc Na/Pi cotransporter in the mouse kidney. Pflugers Arch 446: 106–115, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Olah Z, Lehel C, Anderson WB, Eiden MV, Wilson CA. The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J Biol Chem 269: 25426–25431, 1994. [PubMed] [Google Scholar]
- 35.Pfister MF, Hilfiker H, Forgo J, Lederer E, Biber J, Murer H. Cellular mechanisms involved in the acute adaptation of OK cell Na/Pi-cotransport to high- or low-Pi medium. Pflugers Arch 435: 713–719, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Ravera S, Virkki LV, Murer H, Forster IC. Deciphering PiT transport kinetics and substrate specificity using electrophysiology and flux measurements. Am J Physiol Cell Physiol 293: C606–C620, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Ritthaler T, Traebert M, Lotscher M, Biber J, Murer H, Kaissling B. Effects of phosphate intake on distribution of type II Na/Pi cotransporter mRNA in rat kidney. Kidney Int 55: 976–983, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Salaun C, Rodrigues P, Heard JM. Transmembrane topology of PiT-2, a phosphate transporter-retrovirus receptor. J Virol 75: 5584–5592, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saliba KJ, Martin RE, Broer A, Henry RI, McCarthy CS, Downie MJ, Allen RJ, Mullin KA, McFadden GI, Broer S, Kirk K. Sodium-dependent uptake of inorganic phosphate by the intracellular malaria parasite. Nature 443: 582–585, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, Tatsumi S, Miyamoto K. Growth-related renal type II Na/Pi cotransporter. J Biol Chem 277: 19665–19672, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Segawa H, Onitsuka A, Aranami F, Tomeo Y, Kaneko I, Furutani J, Ito M, Matsumoto M, Li M, Amizuka N, Kuwahata M, Miyamoto KI. Npt2a and Npt2c in mice play distinct and synergistic roles in inorganic phosphate metabolism and skeletal development. J Amer Soc Nephrol SA-FC101, 2007. [DOI] [PubMed]
- 42.Segawa H, Yamanaka S, Ito M, Kuwahata M, Shono M, Yamamoto T, Miyamoto K. Internalization of renal type IIc Na-Pi cotransporter in response to a high-phosphate diet. Am J Physiol Renal Physiol 288: F587–F596, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Segel IH Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-state Enzyme Systems. New York, NY: Wiley, 1975.
- 44.Szczepanska-Konkel M, Yusufi AN, VanScoy M, Webster SK, Dousa TP. Phosphonocarboxylic acids as specific inhibitors of Na+-dependent transport of phosphate across renal brush border membrane. J Biol Chem 261: 6375–6383, 1986. [PubMed] [Google Scholar]
- 45.Tatsumi S, Segawa H, Morita K, Haga H, Kouda T, Yamamoto H, Inoue Y, Nii T, Katai K, Taketani Y, Miyamoto KI, Takeda E. Molecular cloning and hormonal regulation of PiT-1, a sodium-dependent phosphate cotransporter from rat parathyroid glands. Endocrinology 139: 1692–1699, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Tenenhouse HS, Martel J, Gauthier C, Segawa H, Miyamoto K. Differential effects of Npt2a gene ablation and X-linked Hyp mutation on renal expression of Npt2c. Am J Physiol Renal Physiol 285: F1271–F1278, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Tenenhouse HS, Roy S, Martel J, Gauthier C. Differential expression, abundance, and regulation of Na+-phosphate cotransporter genes in murine kidney. Am J Physiol Renal Physiol 275: F527–F534, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Traebert M, Lotscher M, Aschwanden R, Ritthaler T, Biber J, Murer H, Kaissling B. Distribution of the sodium/phosphate transporter during postnatal ontogeny of the rat kidney. J Am Soc Nephrol 10: 1407–1415, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Vieira FL, Malnic G. Hydrogen ion secretion by rat renal cortical tubules as studied by an antimony microelectrode. Am J Physiol 214: 710–718, 1968. [DOI] [PubMed] [Google Scholar]
- 50.Villa-Bellosta R, Barac-Nieto M, Breusegem SY, Barry NP, Levi M, Sorribas V. Interactions of the growth-related, type IIc renal sodium/phosphate cotransporter with PDZ proteins. Kidney Int 73: 456–464, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villa-Bellosta R, Bogaert YE, Levi M, Sorribas V. Characterization of phosphate transport in rat vascular smooth muscle cells: implications for vascular calcification. Arterioscler Thromb Vasc Biol 27: 1030–1036, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Villa-Bellosta R, Sorribas V. Role of rat sodium/phosphate cotransporters in the cell membrane transport of arsenate. Toxicol Appl Pharmacol 232: 125–134, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Virkki LV, Biber J, Murer H, Forster IC. Phosphate transporters: a tale of two solute carrier families. Am J Physiol Renal Physiol 293: F643–F654, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Virkki LV, Forster IC, Biber J, Murer H. Substrate interactions in the human type IIa sodium-phosphate cotransporter (NaPi-IIa). Am J Physiol Renal Physiol 288: F969–F981, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Yoshiko Y, Candeliere GA, Maeda N, Aubin JE. Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol Cell Biol 27: 4465–4474, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]