Abstract
Acidic luminal pH and low [HCO3−] maintain sperm quiescent during maturation in the epididymis. The vacuolar H+-ATPase (V-ATPase) in clear cells is a major contributor to epididymal luminal acidification. We have shown previously that protein kinase A (PKA), acting downstream of soluble adenylyl cyclase stimulation by alkaline luminal pH or HCO3−, induces V-ATPase apical membrane accumulation in clear cells. Here we examined whether the metabolic sensor AMP-activated protein kinase (AMPK) regulates this PKA-induced V-ATPase apical membrane accumulation. Immunofluorescence labeling of rat and non-human primate epididymides revealed specific AMPK expression in epithelial cells. Immunofluorescence labeling of rat epididymis showed that perfusion in vivo with the AMPK activators 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) or A-769662 induced a redistribution of the V-ATPase into subapical vesicles, even in the presence of a luminal alkaline (pH 7.8) buffer compared with that of controls perfused without drug. Moreover, preperfusion with AICAR blocked the PKA-mediated V-ATPase translocation to clear cell apical membranes induced by N6-monobutyryl-cAMP (6-MB-cAMP). Purified PKA and AMPK both phosphorylated V-ATPase A subunit in vitro. In HEK-293 cells [32P]orthophosphate in vivo labeling of the A subunit increased following PKA stimulation and decreased following RNA interference-mediated knockdown of AMPK. Finally, the extent of PKA-dependent in vivo phosphorylation of the A subunit increased with AMPK knockdown. In summary, our findings suggest that AMPK inhibits PKA-mediated V-ATPase apical accumulation in epididymal clear cells, that both kinases directly phosphorylate the V-ATPase A subunit in vitro and in vivo, and that AMPK inhibits PKA-dependent phosphorylation of this subunit. V-ATPase activity may be coupled to the sensing of acid-base status via PKA and to metabolic status via AMPK.
Keywords: vas deferens, male reproductive tract, metabolism, soluble adenylyl cyclase, acid secretion
spermatozoa are stored along the epididymal tubule, where they are kept immotile while they mature. The maintenance of sperm in this quiescent state is due in part to the acidic epididymal luminal pH and to the low [HCO3−] of 2–7 mM in the lumen (30, 31). A subset of epithelial epididymal cells, known as clear cells, are important for the acidification capacity of the epididymis (9). The vacuolar H+-ATPase (V-ATPase) is highly expressed in these clear cells, both at their luminal plasma membrane and in intracellular sub-apical vesicles, and is responsible for net luminal acidification in the epididymal cauda and vas deferens (VD) (9). Acid secretion in proton-secreting epithelial cells such as kidney intercalated cells and epididymal clear cells is actively regulated by environmental cues that include CO2, HCO3−, and hormonal stimuli (49).
The V-ATPase is expressed ubiquitously in eukaryotes and can be found not only at the apical membrane of proton-secreting cells but also in the membrane of organelles requiring luminal acidification of lysosomes, Golgi, endosomes, and secretory vesicles (49). The regulation of V-ATPase activity is therefore relevant to many epithelial cells, and studies in clear cells could lead to important insights for other proton-secreting cells of the same developmental origin in the Wolffian duct, such as A-type intercalated cells in the distal nephron, which are important to the regulation of body acid-base homeostasis and also express V-ATPase at their apical membrane and secrete protons into the lumen (11).
The V-ATPase is a multisubunit protein complex composed of a catalytic ATP-hydrolyzing V1 domain located in the cytosol and a membrane-bound proton-translocating V0 domain (17). At the cellular level V-ATPase-mediated proton secretion can be regulated by several mechanisms, including the reversible dissociation of the membrane-associated V0 and the catalytic V1 sectors, the modulation of ATP hydrolysis coupled to proton pumping, and modulation of recycling and targeting of V-ATPase-containing vesicles (reviewed in Ref. 6). Indeed, an increase in V-ATPase surface expression and in apical surface area (including microvilli) in these proton-secreting cells correlates with an increase in proton secretion (reviewed in Ref. 44). As in other specialized V-ATPase expressing cells, V-ATPase-containing vesicles in epididymal clear cells recycle between intracellular vesicles and the apical plasma membrane, which indicates that these cells likely utilize this regulatory mechanism to control their rate of proton secretion (1, 37).
In the excurrent ducts of the male reproductive tract, we and others have shown previously that the V-ATPase accumulates in the plasma membrane of clear cells in response to the activation of soluble adenylyl cyclase by alkaline luminal pH or increases in luminal [HCO3−] (37). Our results also established that intracellular cAMP and PKA activity are needed to elicit the accumulation of V-ATPase at the apical membrane of clear cells (39). These findings of PKA effects on V-ATPase trafficking in mammalian cells are consistent with recently published reports, indicating that PKA activation via cAMP and V-ATPase C subunit phosphorylation by PKA activates the pump and induces its assembly and translocation to the apical membrane of insect epithelial cells (40, 48). The confirmed regulatory role of PKA on V-ATPase assembly, trafficking, and function suggests the potential role of other kinases on proton secretion.
AMP-activated kinase (AMPK) is a ubiquitous serine/threonine kinase that becomes activated by cellular metabolic stress (increased cellular [AMP]/[ATP] ratio) and other stress signals via phosphorylation of residue Thr-172 in its α-subunit (24). Once activated, AMPK phosphorylates many substrates leading to an inhibition of cellular ATP consumption and stimulation of ATP synthesis, thereby maintaining ATP reserves in the face of energy depletion (28). V-ATPases consume energy in the form of ATP to move protons across membranes against a gradient. The link between V-ATPases and cellular energy homeostasis is highlighted by their regulation by enzymes of the glycolytic pathway such as aldolase and phosphofructokinase-1, which makes this pump a likely target for other metabolic sensors (32, 45). However, the regulation of V-ATPase activity under conditions of energy stress has not yet been well explored. Moreover, the potential relationship between V-ATPase and the metabolic sensor AMPK is unknown. Of note, the A subunit of the V-ATPase, part of the V1 sector, did appear in a screen as a potential candidate for direct AMPK phosphorylation in mammalian cells (47). AMPK has been shown to regulate other apical membrane transport proteins such as the epithelial sodium channel (ENaC) and cystic fibrosis transmembrane conductance regulator (CFTR), potentially coupling ion transport to cellular metabolic status (14, 20, 22, 23, 50). Like the V-ATPase, the activity and trafficking of these ion channels are also regulated by protein kinase A (PKA) (3). Interestingly, in the case of CFTR, AMPK appears to play an antagonistic role with respect to PKA, inhibiting PKA-dependent activation of the channel (21–23).
In this study we present evidence that there is coregulation of V-ATPase intracellular trafficking by PKA and AMPK and that both kinases directly phosphorylate the mammalian V-ATPase A subunit. AMPK inhibits both cAMP-induced apical membrane accumulation of the V-ATPase in clear cells and PKA-dependent phosphorylation of the V-ATPase A subunit. We hypothesized that AMPK activation in clear cells, as may occur with decreased blood flow during arousal or with other metabolic stresses, may decrease V-ATPase apical membrane accumulation and thereby reduce ATP consumption, overriding and antagonizing its activation by acid-base signaling mediated by PKA (42). Complementary regulation of the V-ATPase by PKA and AMPK may afford the integration of proton secretory responses to both acid-base stimuli (via PKA) and to cellular metabolism and intracellular ATP concentration (via AMPK).
MATERIALS AND METHODS
Reagents and chemicals.
All chemicals used in these studies were obtained from Fisher Scientific or Sigma unless otherwise noted, including the perfusion solution of PBS (10 mM sodium phosphate, 2 mM potassium phosphate, 137 mM NaCl, 2.7 mM KCl). The cell permeant, specific PKA activator N6-monobutyryl-cAMP (6-MB-cAMP) was obtained from Biomol (Plymouth Meeting, PA). Paraformaldehyde was obtained from Electron Microscopy Sciences. AICAR was purchased from Toronto Research Chemicals. Abbott compound A-769662 was purchased from the University of Dundee.
Western blot of AMPK-α in epididymis.
All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Adult male Sprague-Dawley rats were anesthetized using pentobarbital sodium (65 mg/kg body wt ip), and their epididymis and VD were harvested, dissected into epididymal caput, corpus, and cauda in PBS (pH 7.4) containing Complete Protease Inhibitor cocktail (Roche) and homogenized as previously described (27, 37). Adult rhesus monkey Macaca mulatta epididymides were provided by Dr. Tony Plant at the Univ. of Pittsburgh. After castration, the epididymides were frozen in liquid nitrogen. To prepare homogenates, the epididymides were thawed on ice, and the cauda was carefully dissected from fat and connective tissue in PBS containing Complete Protease Inhibitor cocktail. Homogenization was performed in the same solution and using the same procedure as for the rat tissues.
Electrophoresis and Western blotting were performed as previously described (27). Briefly, after quantification of protein concentration using the Bradford assay (Bio-Rad), equal amounts of protein for each sample (rhesus and rat epididymal cauda) were combined in Laemmli sample buffer and subjected to SDS-PAGE and immunoblotting using previously described protocols (36). Immunoblotting was performed at 1:2,500 dilution of anti-AMPK-α (Cell Signaling Technology) in 5% milk in TBS-Tween followed by horseradish peroxidase-conjugated secondary anti-rabbit antibody (Jackson Immunologicals) at a concentration of 1:10,000. To demonstrate anti-AMPK-α antibody specificity in these tissues, the membrane was stripped in an acidic glycine buffer after exposure and reprobed with anti-AMPK-α antibody that had been preincubated with the immunizing peptide, followed by secondary antibody as above. This membrane was exposed to film for an identical time period as the membrane incubated with the antibody alone.
Tissue fixation.
Adult rats were anesthetized as described above and perfused via the left ventricle with PBS (pH 7.4), followed by a phosphate-buffered solution containing 4% paraformaldehyde, 10 mM sodium periodate, 70 mM lysine, and 5% sucrose (PLP). The epididymis and VD were then dissected and subjected to immersion fixation overnight in the same fixative solution before processing for immunofluorescence staining as described previously (7, 8, 36, 37). Macaca mulatta epididymal cauda was fixed by immersion in PLP overnight and washed in PBS. Before cryosectioning was done, tissues were cryoprotected by immersing it in a solution of 30% sucrose in PBS, embedded in OCT (Tissue TEK), mounted on a cutting block, and frozen in a Reichert Frigocut microtome. Four-micrometer thick tissue cryosections were mounted on Fisher Superfrost Plus slides.
Immunofluorescence labeling.
Tissues cut in 4-μm cryostat sections were immunostained after SDS antigen retrieval as previously described (13). Slides were hydrated in PBS and placed in blocking solution containing 1% BSA in PBS-0.02% sodium azide for 15–30 min. All antibody dilutions were performed in DAKO background-reducing reagent (DAKO). The slides were then incubated with both the anti-AMPK-α subunit antibody used in the Western blot (raised in goat, 1:50 dilution) and an antibody against the E subunit of the V-ATPase (raised in chicken at 1:60 dilution, GenWay) for 75 min at room temperature. Sections were then washed twice for 5 min in high-salt PBS (2.7% NaCl) and once in PBS. The secondary antibody incubation (1 h) was performed with secondary antibodies raised in a donkey and coupled to FITC and donkey anti-rabbit coupled to CY3 (Jackson Immunologicals). The slides were again washed as described in the step after the primary antibody incubation. Slides were mounted on coverslips with Vectashield (Vector Labs). The immunizing peptide used to produce the AMPK-α antibody was employed for peptide inhibition controls using methods previously described (36).
In vivo perfusion of the VD and distal epididymal cauda.
Given the paucity of cell culture models to study V-ATPase regulation in epididymal clear cells, we and others have used the method of in vivo perfusion of the epididymis and VD to study V-ATPase regulation (1, 37). It has been demonstrated previously that as in kidney intercalated cells, activation of V-ATPase-dependent proton secretion in epithelial cells is proportional to the accumulation of V-ATPase in apical microvilli of these cells (10, 11, 33, 44). As a readout for V-ATPase apical accumulation in clear cells, we measured the effects of various treatments on the surface area of V-ATPase-labeled apical microvilli (1, 37). Adult male Sprague-Dawley rats were anesthetized as described above using pentobarbital sodium, and the VD lumen was cannulated (microcannula 0.4 mm OD, 0.2 mm ID; Fine Science Tools) as previously described (37). Retrograde perfusion at 2.7 ml/h was performed into the epididymis, and the perfusate exited via a small incision was made in the distal epididymal cauda. The lumen was initially washed free of sperm with PBS adjusted to pH 6.5, as indicated under results. Horseradish peroxidase was added to the perfusate at a concentration of 1–2 mg/ml to detect endocytosis in the absence or presence of inhibitors and agonists as described in the figure legends and results. At the end of the perfusion the lumen was washed free of horseradish peroxidase with ice-cold PBS for 6 min (in the continued presence of activators or inhibitors, as applicable). The perfused portions of the VD and epididymal cauda were rapidly dissected, fixed by immersion in PLP overnight at 4°C, washed, quenched, cryoprotected, and cryosectioned as described above (37). Double immunofluorescence labeling was performed using anti-sera against the E subunit of the V-ATPase raised in rabbit (1:60 dilution, Santa Cruz) combined with anti-horseradish peroxidase antibody raised in goat at a concentration of 1:300 (Jackson Immunologicals). Washes and secondary antibodies were used as previously described (37). All tissue sections in this study were SDS treated because the staining intensity increased significantly with prior SDS antigen retrieval (13).
Quantification of V-ATPase apical membrane accumulation.
V-ATPase accumulation in the apical membrane of clear cells was quantified from images obtained by confocal microscopy. This quantification method has been validated by comparison to measurements on electron micrographs in previous studies (1, 37). Briefly, confocal images were imported into Metamorph (Molecular Devices). Using this software, the microvilli positive for V-ATPase staining were outlined, and the area occupied by the microvilli was measured. The area measurement for each cell was normalized to the width of the cell at the base of the apical microvilli. At least three epididymides were perfused for each condition from different animals, and we examined a minimum of three separate immunofluorescence staining procedures for each tissue/condition. At least 10 cells per tissue were examined, and thus over 30 cells per condition were used for quantification. Two independent investigators verified the measurements. Unpaired t-tests were performed to compare the results from different treatment groups, and differences were considered significant at P < 0.05.
Cell culture and transfection.
The V-ATPase mouse A subunit cDNA cloned into pCMV-SPORT6 was purchased from Open Biosystems. NH2-terminal, FLAG-tagged mouse V-ATPase A subunit cDNA was generated by PCR from the above plasmid and subcloned into pTracer (Invitrogen). All plasmids were verified by DNA sequencing. Recombinant active human AMPK holoenzyme α1-T172D, β1, γ1 was synthesized in Escherichia coli using a tricistronic plasmid and purified as described (35, 41).
In vitro phosphorylation assays.
In vitro phosphorylation assays were performed essentially as described previously (4). Briefly, HEK-293 cells were transiently transfected to express FLAG-V-ATPase A subunit using Lipofectamine 2000 (Invitrogen), and cells were lysed 2 days after transfection. FLAG-V-ATPase A subunit was immunoprecipitated from cell lysates using the M2 anti-FLAG monoclonal antibody (Sigma) coupled to protein A/G beads (Pierce), as described (2, 14). In vitro phosphorylation was performed using purified active AMPK holoenzyme with [γ-32P]ATP labeling, as described (14). After SDS-PAGE and transfer to nitrocellulose membranes, immunoblotting for expression of FLAG-V-ATPase A subunit was first performed and quantified using a Versa-Doc Imager with Quantity One software (Bio-Rad). After the chemiluminescent signal had decayed, phosphorylated bands on the membrane were imaged by exposure to a phosphoscreen. The bands on the phosphoscreen were quantitated using a Bio-Rad Phosphorimager (Quantity One Software). The intensity of each phosphoscreen band was corrected by subtracting out the local background in the same lane.
Generation and characterization of HEK-293 cells stably expressing shRNA.
HEK-293 Flp-In TRex cells (Invitrogen) that stably express the tetracycline repressor were used to create stable cell lines that express short hairpin RNA (shRNA) after induction by 1 μg/ml doxycycline. These cells were transfected with pSUPERIOR.neo+GFP (Oligoengine) plasmid containing either AMPK-α1 shRNA (AMPK KD; sequence 5′-GCAGCAAUAAGCAUGCAUA-3′) or control shRNA that does not target any known mammalian gene [CON; sequence 5′-GCGCGCUUUGUAGGAUUCG-3′ (MamX), Oligoengine]. Selection of clones of cells that stably express shRNAs in a tetracycline-inducible fashion was performed as per the manufacturer's instructions by plating neomycin-resistant cells at limiting dilution. The efficacy of knockdown of individual clones was assessed by immunoblotting lysates from each for AMPK-α1 with and without doxycycline treatment for 3 days.
In vivo phosphorylation assays.
Either CON or AMPK KD cells grown on Petri dishes were transiently transfected with the pTracer-FLAG-A V-ATPase plasmid 1 day before experimentation. In vivo phosphorylation assays were performed essentially as described previously (4). For labeling, cells were washed twice in phosphate-free efflux buffer containing (in mM): 140 NaCl, 3 KCl, 1 MgSO4, 1 CaCl2, 10 glucose, and 10 HEPES (pH 7.4). Each dish of cells was then incubated at 37°C for 2 h in 1.5 ml of this buffer with 0.3 mCi of [32P]orthophosphate (MP Biomedicals). During the last 20 min of this labeling period, some filters were incubated in the presence of 6-MB-cAMP (100 μM) and IBMX (500 μM). Other cells on filters were treated with myristoylated protein kinase inhibitor (mPKI, 10 μM) for the entire labeling period. After the labeling incubation period, cells were washed once in ice-cold phosphate-free buffer before lysis in radioimmune precipitation assay buffer. After the lysates were cleared by high-speed centrifugation, FLAG-V-ATPase A subunit was immunoprecipitated, washed, eluted in sample buffer for SDS-PAGE, and immunoblotted using an anti-FLAG antibody as described above for the in vitro phosphorylation studies. After immunoblotting was completed, phosphorimager analysis of the same membrane was performed. The phosphorylation signal of the V-ATPase A subunit band under each condition was corrected for the local background phosphoscreen signal and then further normalized to the immunoblot signal in each lane, and these values were compared across conditions to derive relative phosphorylation levels.
Statistical analysis.
For immunofluorescence labeling and biochemical experiments, statistics were performed using unpaired or paired t-tests, as described for each figures. In all cases P values <0.05 were considered significant.
RESULTS
AMPK detection in epididymal homogenates from adult rat and rhesus monkey.
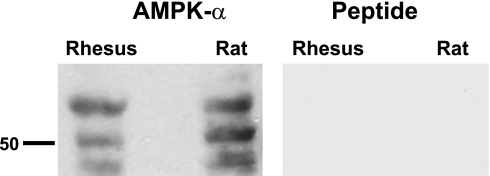
Although the tissue distribution of the metabolic sensor AMPK appears to be fairly ubiquitous (43), the presence and localization of this kinase has not been well characterized in the male reproductive tract. Immunoblotting for the catalytic α-subunit of AMPK in epididymis cauda total homogenates from adult rhesus Macaca mulatta and from rat revealed broad bands at an apparent molecular weight of 63 kDa and two lower molecular weight bands (Fig. 1, left). Lower molecular weight bands have been previously described with this affinity-purified antibody by the manufacturer. No bands were present when the immunoblot was stripped and reprobed using anti-AMPK-α antibody that had been preabsorbed with the immunizing peptide (“Peptide”, Fig. 1, right). In addition, we were able to detect AMPK-α in epididymal caput and corpus and in a VD-enriched epithelial preparation (not shown).
Fig. 1.
AMP-activated protein kinase (AMPK) is expressed in epididymal and vas deferens epithelia in rat and non-human primate by Western blot. A: Western blots of epididymal cauda homogenates from the rhesus monkey (Macaca mulatta) and rat revealed the expression of AMPK-α in these tissues. B: specificity of this antibody labeling in these tissues was demonstrated by the lack of signal when the primary antibody was preincubated with the immunizing peptide. Western blot of recombinant AMPK-α using the same antibody revealed one band at ∼63 kDa, and this band was completely competed off by preincubation of the antibody with the peptide (not shown).
AMPK immunolocalization in epididymal and VD epithelial cells of adult rat and rhesus monkey.
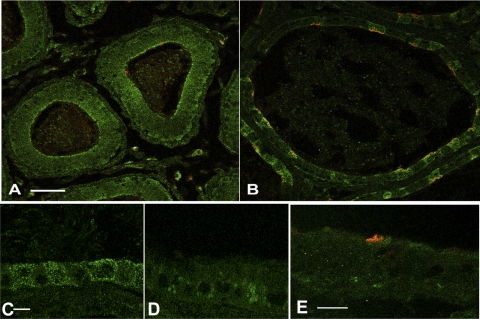
By indirect immunofluorescence labeling, AMPK-α was detected in epithelial cells of the epididymal caput and cauda (Fig. 2). More intense labeling of the cytoplasm, especially at or near the apical membrane, was observed in all epithelial cells of the epididymal caput (Fig. 2A, green). In the epididymal cauda the staining was generally more intense in clear cells, which co-immunostained with a previously characterized antibody against the E subunit of the V-ATPase (Fig. 2B; red). At higher magnification AMPK-α staining was observed throughout the cytoplasm of both epididymal clear and principal epithelial cells (Fig. 2C). This pattern of immunostaining was not detectable when the antibody was preabsorbed with the AMPK-α immunizing peptide (Fig. 2D). In the rhesus epididymal cauda epithelial cells, immunostaining for AMPK-α was diffusely cytoplasmic in both principal cells and clear cells, which express abundant V-ATPase at the apical pole (Fig. 2E; red).
Fig. 2.
AMPK is expressed in epididymal and vas deferens epithelia in rat and non-human primate (Macaca mulatta) by immunofluorescence labeling. A and B: confocal images of immunofluorescence staining image of rat epididymal epithelium in the caput (A) and cauda (B) using an anti-AMPK-α antibody demonstrated diffuse cytoplasmic staining in epididymal epithelial cells, with a higher intensity of staining in clear cells expressing the V-ATPase (E subunit, red). Scale bar = 125 μm. C: higher magnification confocal image of immunofluorescecne staining of rat cauda epididymal epithelium using the anti-AMPK-α antibody. Scale bar = 5 μm. D: preincubation of the anti-AMPK-α antibody with its immunizing peptide substantially reduced the staining in adult rat cauda epididymis, demonstrating the specificity of this antibody for immunofluorescence labeling. E: confocal immunofluorescence labeling of epididymal cauda from adult Macaca mulatta using the same anti-AMPK-α antibody demonstrated diffuse cytoplasmic staining in epithelial cells (green). The epithelial clear cells were labeled with an antibody against the vacuolar ATPase (V-ATPase) E subunit (red). Scale bar = 10 μM.
AMPK activators inhibit pH-induced apical translocation of V-ATPase in epididymal clear cells.
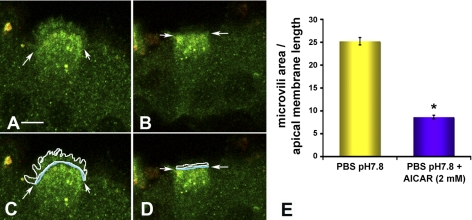
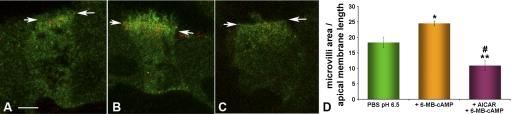
Clear cells have abundant V-ATPase at their apical pole. It has been previously established that increased apical immunofluorescence labeling using antibodies against the E subunit correlates well with increased proton secretion in epithelial proton-secreting cells (37). To test the hypothesis that AMPK activation decreases apical V-ATPase accumulation in clear cells, we used an in vivo epididymal cauda/VD perfusion system that allows the monitoring of changes in subcellular localization of the V-ATPase in response to luminal stimuli (37). We have previously established that when the epididymal/VD lumen is perfused with PBS at an alkaline pH of 7.8, a PKA-mediated translocation of the V-ATPase from subapical vesicles to the apical microvilli occurs (37, 39). To study whether AMPK activation may regulate this PKA-mediated V-ATPase translocation, we perfused epididymal cauda/VD via the lumen with PBS at pH 7.8 in the presence of the endocytic marker HRP (37) and in the presence or absence of 2 mM AICAR, a specific AMPK activator that forms an AMP-mimetic compound inside cells (16). After perfusion the tissues were fixed, and double immunofluorescence labeling for horseradish peroxidase and V-ATPase was performed, followed by confocal microscopy. Clear cells exposed to the control condition (PBS perfusion at pH 7.8 in the absence of AICAR) had V-ATPase distributed at apical microvilli, as we have shown in earlier studies (Fig. 3A) (37, 39). However, following perfusion with 2 mM AICAR, immunolabeling for the V-ATPase was distributed in intracellular apical vesicles that partially overlap in distribution with horseradish peroxidase containing endosomes (yellow staining), indicating the presence of V-ATPase in the endocytic compartment (Fig. 3B). Apical membrane length was measured at the base of the microvilli (Fig. 3, C and D, arrows). The area occupied by V-ATPase-containing microvilli was measured in the same confocal images and was normalized to the length of the apical cell membrane (Fig. 3, C and D). We observed a dramatic 60–70% decrease in the normalized area occupied by microvilli in clear cells in tissues exposed to the specific AMPK activator AICAR (Fig. 3E). These results suggest that AMPK activation inhibits or reverses alkaline-mediated V-ATPase accumulation at the apical membrane of clear cells.
Fig. 3.
AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) inhibits the pH-mediated V-ATPase accumulation at the apical membrane of clear cells. A and B: confocal images of double immunofluorescence labeling for V-ATPase distribution (green) and for the endocytic marker horseradish peroxidase (HRP, red) in clear cells perfused for 60 min with phosphate-buffered saline (PBS) pH 7.8 (A) or PBS pH 7.8 + AICAR (2 mM) (B). Arrows demarcate the base of the microvilli in clear cells. Treatment of cells with the AMPK activator AICAR prevented the elongation of apical microvilli observed upon exposure to pH 7.8 and prevented accumulation of the V-ATPase at the apical pole. C and D: level of V-ATPase accumulation in microvilli in the cells from A and B was quantified by measuring the area occupied by V-ATPase-labeled microvilli (enclosed in the white line), normalized for the width of the cells at the apical pole (blue line) for each cell. E: quantification of the surface occupied by V-ATPase-labeled microvilli of clear cells normalized by the apical width of each cell under the conditions shown in A and B. Data shown are means ± SE from three tissues and at least 30 cells per condition (*P < 0.05). Scale bar = 5 μm.
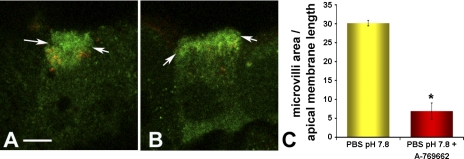
AICAR activates AMPK by forming the AMP-mimetic compound ZMP in cells, which directly binds and activates the kinase allosterically (16, 24). To further confirm that these findings resulted from AMPK activation in this epididymis/VD in vivo perfusion system, we repeated the above experiments using a second direct and specific AMPK activator, Abbott compound A-769662. This compound acts as a small molecule, nonnucleotide AMPK activator that allosterically activates AMPK independent of AMP binding (18). Again, V-ATPase immunolabeling in control tissues perfused with PBS (pH 7.8) was present largely in apical microvilli (Fig. 4A). However, following perfusion with 200 μM A-769662, V-ATPase immunolabeling shifted largely to an intracellular apical vesicular distribution similar to that obtained with AICAR treatment (Fig. 4B). The magnitude of this redistribution was similar to that observed in the presence of AICAR (Fig. 4C). Taken together, the very similar results obtained with exposure to the two mechanistically distinct AMPK activators AICAR and A-769662 indicate that AMPK activation prevents the apical membrane accumulation of V-ATPase in clear cells when exposed to an alkaline luminal pH in vivo. In our previous studies PKA inhibitors also abolished the alkaline pH-mediated V-ATPase accumulation at the apical membrane (39). We thus hypothesized that AMPK activation would prevent the PKA-mediated accumulation of V-ATPase at the apical membrane in clear cells.
Fig. 4.
The AMPK activator A-769662 inhibits the pH-mediated V-ATPase accumulation at the apical membrane of clear cells. A and B: confocal images of double immunofluroscence labeling of the V-ATPase distribution (green) and the endocytic marker HRP (red) in clear cells perfused for 60 min with PBS pH 7.8 (A) or PBS pH 7.8 containing AMPK activator Abbott compound A-769662 (200 μM) (B). Arrows demarcate the base of the microvilli in clear cells. Treatment of cells A-769662 prevented the elongation of apical microvilli observed upon exposure to pH 7.8 and prevented accumulation of the V-ATPase at the apical pole. C: quantification of the surface occupied by V-ATPase-labeled microvilli of clear cells normalized by the apical width of each cell under the conditions shown in A and B. Data shown are means ± SE from three tissues and at least 30 cells per condition (*P < 0.05). Scale bar = 5 μm.
AICAR abolishes PKA-mediated V-ATPase translocation in vivo in clear cells.
Epididymal/VD clear cells perfused with PBS at a normal physiological luminal pH of 6.5 exhibited a V-ATPase distribution in apical microvilli and in intracellular compartments (Fig. 5A). In the presence of the specific PKA activator 6-MB-cAMP, the V-ATPase accumulated in well-developed apical microvilli (Fig. 5B) (39). However, this PKA-mediated accumulation of V-ATPase at the apical membrane by 6-MB-cAMP was inhibited by preperfusion with the AMPK activator AICAR (Fig. 5C). Quantification of the normalized area occupied by microvilli in clear cells under each condition confirmed that preperfusion of the epididymis/VD with AICAR significantly inhibited the 6-MB-cAMP-induced increase in micovilli immunostained for V-ATPase (Fig. 5D). Importantly, there was also a significant difference in V-ATPase distribution between control tissues perfused with PBS at pH 6.5 alone and those perfused with AICAR followed by 6-MB-cAMP (compare Fig. 5, A and C). These results indicate that this AMPK activator not only blocks the PKA-mediated apical translocation of the V-ATPase but also decreases the baseline presence of the V-ATPase at the apical membrane of clear cells (Fig. 5D). It is conceivable that AMPK could block PKA-mediated trafficking of the V-ATPase to the apical membrane or AMPK could enhance a competing process (e.g., retrieval of the pump from the apical membrane). To start to address the mechanism of the interaction between PKA and AMPK on the regulation of V-ATPase trafficking, we considered that one or both of these kinases could phosphorylate one or more subunits of the V-ATPase directly, as has been reported in insect cells (47), or they could phosphorylate other accessory proteins (e.g., cytoskeletal elements) that may regulate trafficking of the pump in cells (1). V1 sector subunits of the V-ATPase, such as the A subunit, form the cytoplasmic coat of V-ATPase-transporting apical vesicles in intercalated cells, which resemble epididymal clear cells (12). Given that the A subunit was recently discovered to be a direct target of AMPK using an unbiased screening method (47), we examined whether the V-ATPase A subunit could serve as a substrate for either or both of these kinases.
Fig. 5.
AMPK activator AICAR inhibits the PKA-mediated V-ATPase accumulation at the apical membrane of clear cells. A, B, and C: confocal images of the distribution of V-ATPase (green) and of the endocytic marker HRP (red) by immunofluorescence labeling in clear cells perfused with PBS pH 6.5 for 75 min (A); PBS pH 6.5 for 45 min, followed by PBS pH 6.5 containing PKA activator N6-monobutyryl-cAMP (6-MB-cAMP, 100 μM) for an additional 30 min (B); or PBS (pH 6.5) containing AICAR (2 mM) for 45 min, followed by PBS (pH 6.5) plus AICAR plus 6-MB-cAMP for an additional 30 min (C). Arrows demarcate the bases of the microvilli. Treatment of cells with AMPK activator AICAR prevented the elongation of apical microvilli observed upon exposure to a specific PKA activator 6-MB-cAMP and prevented accumulation of the V-ATPase at the apical pole. D: quantification of V-ATPase accumulation in apical microvilli for at least three independent perfusions and at least 30 cells per condition. Data shown are the means ± SE (*P < 0.05, relative to PBS, pH 6.5; #P < 0.05, relative to PBS, pH 6.5; **P < 0.05, relative to + 6-MB-cAMP). Scale bar = 7.5 μm.
PKA and AMPK phosphorylate the V-ATPase A subunit in vitro.
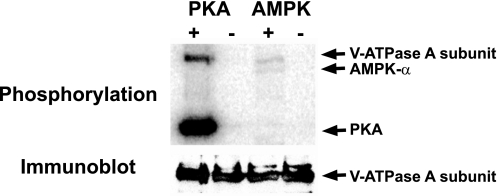
We tested whether the mammalian V-ATPase A subunit could serve as a target for PKA and/or AMPK phosphorylation by performing in vitro phosphorylation experiments of FLAG-tagged V-ATPase A subunit expressed in HEK-293 cells and then immunoprecipitated from cell lysates. As detected by [γ-32P]ATP labeling in the presence of purified active PKA catalytic subunit, PKA phosphorylated the A subunit of the V-ATPase in vitro, and PKA autophosphorylation also occurred (Fig. 6, top). The anti-FLAG immunoblot indicates equivalent protein loading in all lanes (Fig. 6, bottom). In a similar in vitro phosphorylation experiment, the V-ATPase A subunit was phosphorylated by AMPK in vitro (Fig. 6, top). The much greater relative phosphorylation of the A subunit by PKA compared with AMPK could be explained, at least in part, by the much greater specific activity of the PKA preparation, as evidenced by the greater autophosphorylation of PKA compared with AMPK. Nevertheless, these findings suggest that both PKA and AMPK could potentially directly phosphorylate the V-ATPase A subunit in a physiological setting and raise the possibility that the mechanism of V-ATPase redistribution in clear cells induced by PKA and AMPK may be through direct phosphorylation of the A subunit by these kinases.
Fig. 6.
PKA and AMPK phosphorylate the V-ATPase A subunit in vitro. Recombinant FLAG-tagged A subunit was expressed in HEK-293 cells, immunoprecipitated, and incubated with [γ-32P]ATP in the presence or absence of PKA catalytic subunit or in the presence or absence of AMPK holoenzyme. A phosphoscreen image (top) and immunoblot (bottom) of the same membrane are shown (representative of three experiments). In the top, the V-ATPase A subunit becomes phosphorylated in the presence of PKA and also in the presence of AMPK (first and third lanes). Both the PKA catalytic subunit and the AMPK-α subunit become autophosphorylated, as indicated. The immunoblot (bottom) reveals similar loading of V-ATPase A subunit in all lanes.
PKA- and AMPK-dependent phosphorylation of V-ATPase A subunit in vivo in HEK-293 cells.
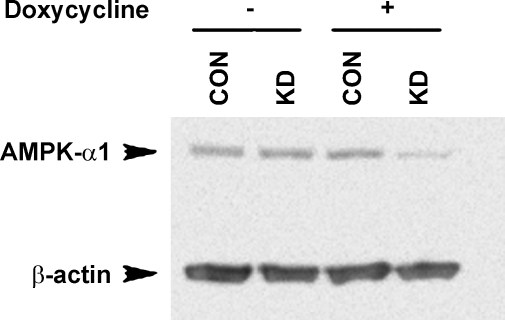
There are currently no available cell lines that recapitulate the characteristics of epididymal clear cells in vivo. Thus, for in vivo phosphorylation labeling studies as an expression system, we have developed and used HEK-293 cells that are stably transfected with either a control shRNA that does not target any known mammalian gene (CON) or an shRNA designed specifically to knock down AMPK-α1 in a tetracycline-inducible fashion (AMPK KD). AMPK-α1 knockdown cells express ∼50% less AMPK-α protein following doxycycline induction (Fig. 7). Immunoblotting for the active form of AMPK-α using an antibody directed against the phosphorylated Thr-172 residue of AMPK-α demonstrated a similar reduction in the active form of AMPK (not shown).
Fig. 7.
Tetracycline-inducible knockdown of AMPK-α1 in HEK-293 cells. Immunoblots for AMPK-α1 (top band) and β-actin (bottom band) of lysates derived from HEK-293 cells that were stably transfected with either a control short hairpin RNA (shRNA) that does not silence any known mammalian gene (CON) or an shRNA designed to specifically knock down AMPK-α (KD). Upon exposure to doxycycline for 3 days a ∼50% decrease in AMPK-α1 protein expression normalized to β-actin was observed.
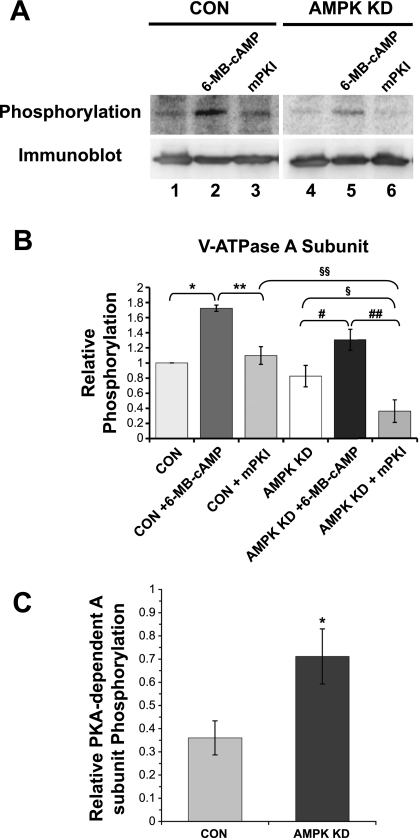
To determine whether PKA- and AMPK-dependent phosphorylation of the A subunit occurs in an intact cellular milieu, we compared [32P]orthophosphate labeling normalized to protein expression of the A subunit under PKA-activated versus -inhibited conditions and in CON versus AMPK KD HEK-293 cells (Fig. 8A). Quantification of the data from several replicate experiments demonstrates that PKA-dependent phosphorylation of the A subunit occurred in vivo in cells stably transfected with an irrelevant shRNA (Fig. 8B, CON). A-subunit phosphorylation significantly increased in the presence of PKA activator (6-MB-cAMP or “cAMP”, lane 2) relative to unstimulated cells (lane 1), yet it remained at control levels in the presence of the PKA inhibitor mPKI (lane 3). The lack of significant difference in A subunit phosphorylation intensity between unstimulated cells and cells treated with mPKI (lanes 1 and 3) suggests that there was little baseline PKA activity in these cells under our experimental conditions and that other kinase(s) are involved in phosphorylating the A subunit at baseline. In addition, we found that when AMPK activity was knocked down (AMPK KD, lanes 4–6), the relative magnitude of PKA-dependent A-subunit phosphorylation was greater. Specifically, the phosphorylation signal with mPKI treatment (lane 3) was reduced by 36 ± 7% (means ± SE) relative to that with cAMP treatment (lane 2) in CON cells, whereas the similar PKA-dependent phosphorylation signal was reduced by 71 ± 12% in AMPK KD cells (lanes 5 and 6). These data are summarized in Fig. 8C, where it is shown that the range of PKA-dependent A subunit phosphorylation is greater when AMPK is knocked down. These results suggest that there was AMPK activity present at baseline in these cells, which dampened the PKA-dependent phosphorylation of the A subunit in vivo.
Fig. 8.
PKA- and AMPK-dependent in vivo phosphorylation of the V-ATPase A subunit in HEK-293 cells. FLAG-tagged A subunit was transfected into HEK-293 cells expressing an irrelevant mammalian shRNA (Fig. 7, “CON” cells) or in AMPK KD cells expressing an shRNA for AMPK-α1. Cells were then incubated with [32P]orthophosphate for 2 h in the presence of a PKA activator (6-MB-cAMP, last 20 min of labeling period) or in the presence of PKA inhibitor myristoylated protein kinase inhibitor (mPKI) (for entire labeling period), followed by lysis of the cells, immunoprecipitation using an anti-FLAG antibody, SDS-PAGE, and immunoblotting using an anti-FLAG antibody. A: typical phospho-screen image (top) revealing the signal of phosphorylated A subunit under the indicated conditions in CON or AMPK KD cells. The Western blot (bottom) confirms similar protein expression and loading of the gel for the different conditions. B: quantification of V-ATPase A subunit phosphorylation signal normalized for protein expression in vivo (*P = 1.6 × 10−5; **P = 1.3 × 10−4; #P = 3.7 × 10−4; ##P = 3.2 × 10−6; §P = 4.9 × 10−6, and §§P = 0.016) indicates that the differences between the indicated conditions were statistically significant by analysis of variance; (n = 4 experiments). C: relative PKA-dependent A subunit phosphorylation in CON and AMPK KD cells as calculated by the difference in phosphorylation signal between 6-MB-cAMP- and mPKI-treated cells over the total phosphorylation signal with 6-MB-cAMP treatment (comparing lanes 2 and 3 for CON and lanes 5 and 6 for AMPK KD). *P < 0.05, unpaired t-test.
Under conditions when PKA was inhibited by mPKI, AMPK knockdown significantly reduced the A-subunit phosphorylation level by 67 ± 17% relative to control cells (compare Fig. 8B, lanes 3 and 6), suggesting that AMPK-dependent phosphorylation of the A subunit occurs in vivo in these cells. The lack of significant difference in phosphorylation between vehicle-treated CON and AMPK KD cells (lanes 1 and 4) is consistent with the idea that AMPK knockdown enhances PKA-dependent phosphorylation of the A subunit, thereby compensating for the decreased AMPK-dependent phosphorylation. In summary, these results suggest that both PKA- and AMPK-dependent phosphorylation of the V-ATPase A subunit occur in vitro and in vivo and that AMPK inhibits PKA-dependent phosphorylation of the A subunit in vivo.
DISCUSSION
This study implicates a novel role for the metabolic sensor AMPK in the regulation of V-ATPase trafficking in epithelial proton-secreting cells in vivo. AMPK activators inhibited both PKA- and alkaline pH-mediated apical accumulation of the V-ATPase in epididymal/VD clear cells. However, the molecular and cellular mechanisms of both PKA- and AMPK-dependent regulation of V-ATPase trafficking are currently unknown. Of note, the A subunit in the cytoplasmic V1 sector of the V-ATPase was recently identified as a potential direct phosphorylation target in an unbiased substrate screen for AMPK (47). Here we have shown that the V-ATPase A subunit can serve a phosphorylation target for both PKA and AMPK in vitro and in vivo. Moreover, AMPK appears to inhibit the PKA-dependent phosphorylation of the A subunit in vivo. These phosphorylation results are consistent with our observation that AMPK inhibits the PKA-dependent activation and accumulation of the V-ATPase at the apical membrane in clear cells in vivo. However, it will be important in the future to identify the site(s) of phosphorylation for both PKA and AMPK in the A subunit of the V-ATPase and to test the hypothesis that the AMPK-dependent inhibition of V-ATPase trafficking is mediated through modulation of PKA-dependent phosphorylation of the A and/or other subunits of the pump. Specifically, future studies are needed to test whether the phosphorylation events observed here translate into the V-ATPase translocation responses induced by these kinases.
Sperm stored in the epididymal lumen mature in an acidic, very low [HCO3−] environment (31). The low luminal pH is essential for maintaining sperm immotile while they mature and are stored (5, 9, 38, 52). The clear cell V-ATPase is one of the epithelial cellular transport systems that contributes to epididymal/VD luminal acidification (9). Under the influence of hormones released during sexual arousal, such as oxytocin and vasopressin, and adrenergic agonists, such as norepinephrine, epididymal/VD epithelial cells secrete anions, including HCO3−, which alkalinizes luminal pH (19). These changes in luminal composition before ejaculation are essential for proper sperm capacitation and fertilization of the oocyte (15, 29). Among other factors, the entry of HCO3− into sperm via CFTR is essential for capacitation (51). The epididymal/VD lumen can undergo wide pH oscillations depending on the presence or absence of different agonists on both the luminal and interstitial sides. The V-ATPase in clear cells responds to alkaline luminal pH, activation of soluble adenylyl cyclase by HCO3−, and to cAMP-PKA by accumulating at the apical membrane of clear cells (37). We have postulated that the V-ATPase activation in response to alkalinization of the lumen occurs in an effort to normalize luminal pH to prevent premature capacitation of the sperm during sexual arousal. However, if the adrenergic stimulation continues, there eventually will be reduced blood perfusion and oxygenation of this epithelium (42). It is thus likely that AMPK, which is exquisitely sensitive to even minor metabolic perturbations, becomes activated before ejaculation when there is reduced epididymal epithelial perfusion (25, 34). It is also likely that epithelial hypoperfusion and AMPK activation occur once capacitation of the sperm is already well underway by adrenergic stimulation of HCO3− secretion. In this setting, AMPK-mediated V-ATPase retrieval from the apical membrane of clear cells would further contribute to sperm capacitation, just before sperm delivery into the female reproductive tract. The findings of this study that AMPK inhibits the alkaline pH- and PKA-mediated V-ATPase accumulation to the apical membrane of clear cells are consistent with this paradigm. In addition, pharmacological AMPK activation significantly decreases V-ATPase localization in apical microvilli even under normal physiological conditions at luminal pH 6.5 (Fig. 5).
The C subunit in insect epithelia is phosphorylated by PKA, leading to assembly and trafficking of the V-ATPase holoenzyme with translocation of the C subunit to the apical membrane, as well as to activation of the pump, and to luminal proton secretion (40, 48). The C subunit protein sequence, however, is not well conserved among species (∼35% homology) and in mammalian epithelial cells C1, C2a, and C2b isoforms have been identified. The C1 isoform is ubiquitously expressed while the C2a isoform is expressed in lung and the C2b isoform is expressed in kidney and epididymal epithelium (46). Investigating the potential role of PKA and AMPK phosphorylation of the C subunit in mammalian epithelial cells is thus an important area for future study.
We have recently found that AMPK regulates several ion transport proteins, including CFTR and ENaC (4, 14, 21–23). The regulation of membrane transport proteins by AMPK fits well with its role as a regulator of cellular energy balance. Specifically, AMPK-dependent inhibition of ion transport proteins like CFTR, a channel gated by ATP binding and hydrolysis, and the V-ATPase should act to limit cellular energy expenditure by directly decreasing ATP consumption. In addition, AMPK inhibition of CFTR and other downhill ion transport proteins such as ENaC should also indirectly inhibit ATP consumption by preventing the dissipation of ion gradients maintained by the Na+-K+-ATPase and other cellular pumps, thereby limiting their activities (20). Importantly, we have shown previously that AMPK phosphorylates and inhibits the PKA-dependent activation of CFTR gating in epithelial cells (22). The results of this study raise the possibility that AMPK and PKA may have a similar antagonistic regulatory relationship with respect to V-ATPase trafficking in clear cells. Moreover, additional regulatory relationships between PKA and AMPK function have been reported. Specifically, cAMP-dependent signaling systems can regulate AMPK activity via phosphorylation of the catalytic α-subunit (26). More specifically, it appears that PKA-mediated phosphorylation of AMPK can directly attenuate AMPK activity (R. Tuerk and D. Neumann, unpublished results).
In conclusion, we postulate that the complementary regulation of the V-ATPase by PKA and AMPK may afford the integration of proton secretory responses to both acid-base stimuli (via PKA) and to cellular metabolism and intracellular ATP concentration (via AMPK). Potential mechanisms for the inter-regulation of the V-ATPase by these kinases could involve various scenarios regarding endocytosis and exocytosis of V-ATPase-containing vesicles. For example, AMPK could inhibit PKA-driven V-ATPase apical membrane accumulation (e.g., by inhibiting exocytosis), and/or AMPK could stimulate V-ATPase intracellular accumulation (e.g., by stimulating endocytosis). Of note, the in vivo phosphorylation assay results (Fig. 8) demonstrating that PKA-dependent phosphorylation of the V-ATPase A subunit is inhibited by AMPK suggest that AMPK directly blocks the PKA-induced apical accumulation of the pump in clear cells, although direct support for this hypothesis will require further study. Indeed, further characterization of the cellular and molecular mechanisms of V-ATPase regulation by these kinases are important goals for future studies. Such work will hopefully lead to the identification of novel targets for the treatment of male infertility, as it may relate to V-ATPase dysfunction, or for the development of a male contraceptive. In addition, follow-up studies may have relevance to other proton-secreting cells of Wolffian duct origin, such as kidney intercalated cells.
GRANTS
This work was supported by National Institutes of Health Grants P30 DK-079307 “Pittsburgh Kidney Research Center” and R01 DK-075048 (to K. R. Hallows), K08 HD-045524 (to N. M. Pastor-Soler), and The Eunice Kennedy Shriver National Institute for Child Health and Human Development through cooperative agreement U54 HD 08160 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (Pilot Project to N. M. Pastor-Soler). R. Alzamora was supported by American Heart Association Postdoctoral Fellowship award (AHA-0825540D). D. Neumann was supported by grants from the European Union (EU) FP6 contract LSHM-CT-2004-005272 (EXGENESIS) and the Swiss National Science Foundation (SNSF) Grant No. 3100A0-11437/1.
Acknowledgments
We thank Dr. Thomas Kleyman and Dr. John P. Johnson for helpful discussions and guidance. We thank Dr. Tony Plant, Dr. Stefan Schlatt and Dr. Suresh Ramaswami for kindly providing us with the adult Macaca mulatta epididymal tissues.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+-ATPase recycling. J Biol Chem 280: 8452–8463, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bhalla V, Daidie D, Li H, Pao AC, LaGrange LP, Wang J, Vandewalle A, Stockand JD, Staub O, Pearce D. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4–2 by inducing interaction with 14-3-3. Mol Endocrinol 19: 3073–3084, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J Biol Chem 281: 26159–26169, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Blomqvist SR, Vidarsson H, Soder O, Enerback S. Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. EMBO J 25: 4131–4141, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breton S, Brown D. New insights into the regulation of V-ATPase-dependent proton secretion. Am J Physiol Renal Physiol 292: F1–F10, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Breton S, Hammar K, Smith PJ, Brown D. Proton secretion in the male reproductive tract: involvement of Cl−-independent HCO-3 transport. Am J Physiol Cell Physiol 275: C1134–C1142, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Breton S, Nsumu NN, Galli T, Sabolic I, Smith PJ, Brown D. Tetanus toxin-mediated cleavage of cellubrevin inhibits proton secretion in the male reproductive tract. Am J Physiol Renal Physiol 278: F717–F725, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Breton S, Smith PJ, Lui B, Brown D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med 2: 470–472, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Breton S, Wiederhold T, Marshansky V, Nsumu NN, Ramesh V, Brown D. The B1 subunit of the H+ATPase is a PDZ domain-binding protein. Colocalization with NHE-RF in renal B-intercalated cells. J Biol Chem 275: 18219–18224, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Brown D, Breton S. Mitochondria-rich, proton-secreting epithelial cells. J Exp Biol 199: 2345–2358, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Brown D, Gluck S, Hartwig J. Structure of the novel membrane-coating material in proton-secreting epithelial cells and identification as an H+ATPase. J Cell Biol 105: 1637–1648, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown D, Lydon J, McLaughlin M, Stuart-Tilley A, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS). Histochem Cell Biol 105: 261–267, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 280: 17608–17616, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558–565, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Forgac M Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8: 917–929, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem 282: 32549–32560, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagedorn TM, Carlin RW, Schultz BD. Oxytocin and vasopressin stimulate anion secretion by human and porcine vas deferens epithelia. Biol Reprod 77: 416–424, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Hallows KR Emerging role of AMP-activated protein kinase in coupling membrane transport to cellular metabolism. Curr Opin Nephrol Hypertens 14: 464–471, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol 284: C1297–C1308, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J Biol Chem 278: 998–1004, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest 105: 1711–1721, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardie DG AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 32, Suppl 4: S7–S12, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays 23: 1112–1119, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem 281: 36662–36672, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Isnard-Bagnis C, Da Silva N, Beaulieu V, Yu AS, Brown D, Breton S. Detection of ClC-3 and ClC-5 in epididymal epithelium: immunofluorescence and RT-PCR after LCM. Am J Physiol Cell Physiol 284: C220–C232, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans 31: 162–168, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439: 737–740, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Levine N, Kelly H. Measurement of pH in the rat epididymis in vivo. J Reprod Fertil 52: 333–335, 1978. [DOI] [PubMed] [Google Scholar]
- 31.Levine N, Marsh DJ. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol 213: 557–570, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu M, Sautin YY, Holliday LS, Gluck SL. The glycolytic enzyme aldolase mediates assembly, expression, and activity of vacuolar H+-ATPase. J Biol Chem 279: 8732–8739, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Madsen KM, Verlander JW, Kim J, Tisher CC. Morphological adaptation of the collecting duct to acid-base disturbances. Kidney Int Suppl 33: S57–S63, 1991. [PubMed] [Google Scholar]
- 34.Mount PF, Hill RE, Fraser SA, Levidiotis V, Katsis F, Kemp BE, Power DA. Acute renal ischemia rapidly activates the energy sensor AMPK but does not increase phosphorylation of eNOS-Ser1177. Am J Physiol Renal Physiol 289: F1103–F1115, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Neumann D, Woods A, Carling D, Wallimann T, Schlattner U. Mammalian AMP-activated protein kinase: functional, heterotrimeric complexes by co-expression of subunits in Escherichia coli. Protein Expr Purif 30: 230–237, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Pastor-Soler N, Bagnis C, Sabolic I, Tyszkowski R, McKee M, Van Hoek A, Breton S, Brown D. Aquaporin 9 expression along the male reproductive tract. Biol Reprod 65: 384–393, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem 278: 49523–49529, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pastor-Soler N, Pietrement C, Breton S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology (Bethesda) 20: 417–428, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294: C488–C494, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rein J, Voss M, Blenau W, Walz B, Baumann O. Hormone-induced assembly and activation of V-ATPase in blowfly salivary glands is mediated by protein kinase A. Am J Physiol Cell Physiol 294: C56–C65, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Riek U, Scholz R, Konarev P, Rufer A, Suter M, Nazabal A, Ringler P, Chami M, Muller SA, Neumann D, Forstner M, Hennig M, Zenobi R, Engel A, Svergun D, Schlattner U, Wallimann T. Structural properties of AMP-activated protein kinase: dimerization, molecular shape, and changes upon ligand binding. J Biol Chem 283: 18331–18343, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Setchell BP, Maddocks S, Brooks DE. Anatomy, vasculature, innervation, and fluids of the male reproductive tract. In: The Physiology of Reproduction, edited by Knobil E and Neil JD. New York: Raven, 1994, p. 1063–1175.
- 43.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase subfamily. J Biol Chem 271: 611–614, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Steinmetz PR Cellular organization of urinary acidification. Am J Physiol Renal Fluid Electrolyte Physiol 251: F173–F187, 1986. [DOI] [PubMed] [Google Scholar]
- 45.Su Y, Zhou A, Al-Lamki RS, Karet FE. The a-subunit of the V-type H+-ATPase interacts with phosphofructokinase-1 in humans. J Biol Chem 278: 20013–20018, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Sun-Wada GH, Murata Y, Namba M, Yamamoto A, Wada Y, Futai M. Mouse proton pump ATPase C subunit isoforms (C2-a and C2-b) specifically expressed in kidney and lung. J Biol Chem 278: 44843–44851, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Tuerk RD, Thali RF, Auchli Y, Rechsteiner H, Brunisholz RA, Schlattner U, Wallimann T, Neumann D. New candidate targets of AMP-activated protein kinase in murine brain revealed by a novel multidimensional substrate-screen for protein kinases. J Proteome Res 6: 3266–3277, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Voss M, Vitavska O, Walz B, Wieczorek H, Baumann O. Stimulus-induced phosphorylation of vacuolar H(+)-ATPase by protein kinase A. J Biol Chem 282: 33735–33742, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar-ATPase. Physiol Rev 84: 1263–1314, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Woollhead AM, Scott JW, Hardie DG, Baines DL. Phenformin and 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (AICAR) activation of AMP-activated protein kinase inhibits transepithelial Na+ transport across H441 lung cells. J Physiol 566: 781–792, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu WM, Shi QX, Chen WY, Zhou CX, Ni Y, Rowlands DK, Yi Liu G, Zhu H, Ma ZG, Wang XF, Chen ZH, Zhou SC, Dong HS, Zhang XH, Chung YW, Yuan YY, Yang WX, Chan HC. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc Natl Acad Sci USA 104: 9816–9821, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci USA 98: 14132–14137, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]